Abstract
Introduction
Bladder cancer is the second most common genitourinary malignancy in the United States. The incidence of bladder cancer rises with age, and it is two times more common in Caucasians than in African-Americans (23.1 vs. 12.6 cases/100,000 persons). We aimed to investigate the racial and age-related differences in the distribution of metastasis in a large, contemporary cohort of metastatic bladder cancer patients.
Material and methods
Within the National Inpatient Sample database (2008–2015) we identified 5,767 patients with metastatic bladder cancer. Trend test, Chi-square test and multivariable logistic regression models were used to evaluate the relationship between ethnicity, age, and site of metastasis.
Results
Of 5,767 patients with metastatic bladder cancer, 598 (10.4%) were African-American. Lung was the most common metastatic site in African-Americans (28.6%) vs. bone in Caucasians (21.7%). Overall, African-Americans showed higher rates of lung (+10.2%), liver (+7.5%) and bone (+5.2%) metastases, compared to Caucasians (all p <0.01). Brain metastases were rare in both ethnicities (3.3 vs. 2.4%; p = 0.2). Rates of exclusive bone, lung or liver metastases increased with age, but were higher in African-Americans, regardless of age strata. In the multivariable logistic regression models, African-American ethnicity independently predicted higher risk of lung (Odds ratio: 1.69), liver (odds ratio: 1.50) and bone (odds ratio: 1.27) metastases, relative to Caucasians. Moreover, a dose-response effect was found after combining the three main risk factors for developing bone metastases, namely African-American ethnicity, younger age and male gender.
Conclusions
Racial differences exist in the distribution of metastatic bladder cancer metastasis. Moreover, based on higher risk of bone metastases in African-American patients, bone imaging may be warranted in this patient population, especially in the presence of other risk factors for bone metastases, namely male gender or younger age.
Keywords: advanced bladder cancer, bladder cancer, epidemiology, location of disease, National Inpatient Sample database
INTRODUCTION
Bladder cancer is the second most common genitourinary malignancy in the United States, with estimated incidence rates of 19.2 cases per 100,000 persons. The incidence of bladder cancer rises with age, and it is two times more common in Caucasians than in African-Americans (23.1 vs. 12.6 cases/100,000 persons) [1, 2, 3]. Approximately, 8 to 18% of bladder cancer patients present metastatic disease, [4] and its incidence is on the rise. [5] Several studies demonstrated racial differences in bladder cancer oncologic outcomes, due to genetic, societal, and environmental factor differences that may be operational between Caucasians and African-Americans [6, 7, 8]. However, to date very few studies described the site-specific location of metastasis in bladder cancer patients [9, 10], and no studies tested for variability in the distribution of metastasis according to ethnicity. Historically, Bianchi et al. [10] reported different patterns of metastatic distribution according to age. However, Bianchi et al. [10] relied on a historical cohort of metastatic bladder cancer patients (1998–2007) and did not evaluate racial differences in the distribution patterns. We addressed this limitation in a contemporary cohort of patients with metastatic bladder cancer. Specifically, we hypothesized that the distribution of metastasis may vary according to age and ethnicity.
MATERIAL AND METHODS
Study population
The current study relied on the National Inpatient Sample database (2008–2015) [11, 12] that is composed of longitudinal hospital inpatient databases from the Healthcare Cost and Utilization Project family and includes 20% of United States inpatient hospitalizations [13, 14]. Within the National Inpatient Sample database we focused on patients older than 18 years, with the primary diagnosis of metastatic bladder cancer. (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 188.x) from 2008 to 2015. Metastatic sites were identified using ICD-9-CM codes, as follows: lung (197.0), bone (198.5), regional lymph nodes (196.2, 196.6), distant lymph nodes (196.0, 196.1, 196.3, 196.5, 196.8, 196.9), liver (197.7), adrenal (198.7), brain (198.3), peritoneum and retroperitoneum (197.6), other digestive system (esophagus, gallbladder, pancreas, stomach) and spleen (197.8), pleura (197.2), mediastinum (197.1), kidney (198.0), colorectum (197.5), duodenum (197.4), skin (198.2), bronchus and trachea (197.3), ovary (198.6). Overall, 5,767 (5,169 Caucasians and 598 African-Americans) assessable patients were identified.
Variables definition
Ethnicity was defined based on the National Inpatient Sample database ethnicity categories. Due to very low numbers of other ethnic groups, without any possibility of meaningful conclusions, our analyses focused on differences between Caucasian and African-American ethnicities. Age was categorized according to quartiles: <63, 64–72, 73–79 and >80. According to previously established methodology, [10] thoracic metastatic sites included lung, distant lymph nodes, pleura, mediastinum, bronchus and trachea. Similarly, [15] abdominal metastatic sites included liver, regional lymph nodes, adrenal, peritoneum and retroperitoneum, kidney, colorectum, duodenum, ovary, spleen, other digestive system and urinary tract. Conversely, brain, as well as bone were considered as two independent sites. Other variables included gender (male, female), number of metastatic sites (1 site, >2 sites) and Charlson Comorbidity Index ([CCI] 0-1 vs. >2).
Statistical analyses
Descriptive statistics included frequencies and proportions for categorical variables. Means, medians, and ranges were reported for continuously coded variables. Chi-square test, t-test and Kruskal-Wallis tests examined the statistical significance in proportions’, means’ and medians’ differences. Five sets of analyses were performed. First, we investigated rates of site-specific metastasis, within each ethnic group. Second, we tested for differences in the distribution of site-specific metastasis between Caucasians and African-Americans. Third, trends in proportions tested the effect of age on distribution of metastatic sites, according to ethnicity. Fourth, multivariable logistic regression models investigated predictors of bone, lung, liver and brain metastases. Covariates in multivariable logistic regression models were ethnicity (African-Americans vs. Caucasians), age (<63 vs. >63 years), gender (male vs. female) and CCI (>2 vs. 0–1). Finally, multivariable logistic regression models were used to test for dose-response effect [16] after combining independent predictors of bone metastases, namely ethnicity, age, and gender.
All multivariable models relied on weighting using a Generalized Estimating Equation function to provide more accurate national estimates based on National Inpatient Sample database-provided weights [17]. For all statistical analyses, R software environment for statistical computing and graphics (version 3.4.3) was used. All tests were two sided with a level of significance set at p <0.05.
RESULTS
General characteristics of the study population
Overall, 5,767 patients with metastatic bladder cancer were identified between 2008 and 2015. Of those, 5,169 (89.6%) were Caucasians and 598 (10.4%) were African-Americans. African-American patients were younger (68 vs. 72; p <0.001) and more frequently female (34.4 vs. 24.9%; p <0.001), compared to Caucasians. No differences on the Charlson Comorbidity Index distribution were found (p = 0.8).
Location of metastasis according to ethnicity
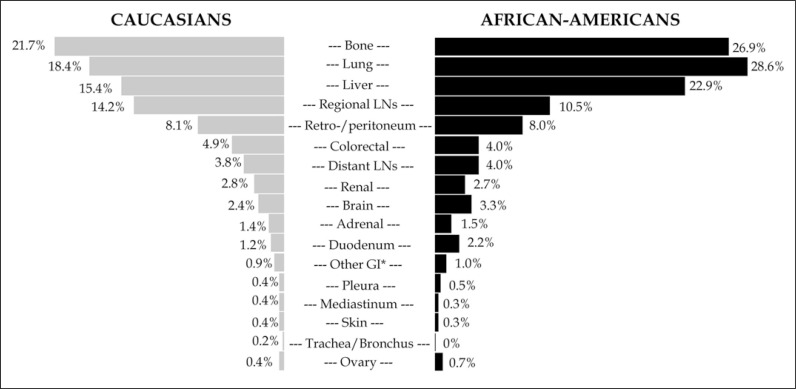
Figure 1 depicts rates of site-specific metastasis according to ethnicity. Bone was the most common metastatic site in Caucasians (21.7%), followed by lung (18.4%) and liver (15.4%). Conversely, lung was the most common metastatic site in African-Americans (28.9%), followed by bone (26.9%) and liver (22.9%). Brain metastases were rare in both Caucasians and in African-Americans (2.4 vs. 3.3%; p = 0.2). Overall, African-American patients showed higher rates of bone, lung and liver metastases (all p <0.01), compared to Caucasians.
Figure 1.
Bar plots depicting rates of metastatic sites in 5,767 metastatic bladder cancer patients (5,169 Caucasians [gray] and 598 African-Americans [black]) identified within the National Inpatient Sample database, between 2008 and 2015, stratified according to ethnicity.
Metastasis in uncommon metastatic sites, namely those different than lung, bone, liver, brain and lymph nodes were present in 17.4 vs. 18.5% of Caucasian vs. African-American patients (p = 0.5). Retro-/peritoneum (8.1 vs. 8.0%), colorectum (4.9 vs. 4.0%) and kidney (2.8 vs. 2.7%) represented the most frequent metastatic sites in both ethnicities (all p>0.05), among the less common sites of metastasis.
Location of metastasis according to ethnicity and age
Table 1 illustrates rates of site-specific metastasis according to ethnicity and age. Rates of thoracic metastases increased with age from 18% to 22.4% (p <0.001) in Caucasians, and were stable in African-Americans (p = 0.1). However, rates of thoracic metastases were higher in African-Americans and remained so, regardless of age strata. Age did not affect rates of abdominal metastases in Caucasians (p = 0.1). Conversely, rates of abdominal metastases decreased with age in African-Americans, from 47 to 31% (p <0.01). Regarding brain metastases, we recorded a decrease with age in Caucasians (from 3.2 to 1.7%; p <0.01) but not in African-Americans (p = 0.06). Finally, rates of bone metastases were higher in African-American patients, regardless of age strata, but age did not affect such rates, neither in Caucasians (p = 0.1) nor in African-Americans (p = 0.4).
Table 1.
Site-specific rates of metastases in 5,767 metastatic bladder cancer patients (5,169 Caucasians and 598 African–Americans) identified within the National Inpatient Sample, between 2008 and 2015
| Variable | Caucasians n = 5,169 (89.6%) | African–Americans n = 598 (10.4%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | ≤63 n = 1,293; 25.0% | 64–72 n = 1,314; 25.4% | 73–79 n = 1,156; 22.4% | ≥80 n = 1,406; 27.2% | p-value | Overall | ≤63 n = 236; 39.5% | 64–72 n = 133; 22.2% | 73–79 n = 116; 19.4% | ≥80 n = 113; 18.9% | p-value | ||
| Metastatic site, n (%) | Thoracic | 981 (19.0) | 233 (18.0) | 215 (16.4) | 218 (18.9) | 315 (22.4) | <0.001 | 174 (29.1) | 65 (27.5) | 33 (24.8) | 36 (31.0) | 40 (35.4) | 0.1 |
| Lung | 950 (18.4) | 225 (17.4) | 208 (15.8) | 208 (18.0) | 309 (22.0) | <0.001 | 171 (28.6) | 64 (27.1) | 32 (24.1) | 36 (31.0) | 39 (34.5) | 0.1 | |
| Other thoracic | 47 (0.9) | 14 (1.1) | 11 (0.8) | 14 (1.2) | ≤10 | 0.3 | ≤10 | ?≤10 | ≤10 | ≤10 | ≤10 | 1 | |
| Abdominal | 2,290 (44.3) | 581 (44.9) | 611 (46.5) | 496 (42.9) | 602 (42.8) | 0.1 | 263 (44.0) | 111 (47.0) | 71 (53.4) | 46 (39.7) | 35 (31.0) | <0.01 | |
| Liver | 798 (15.4) | 216 (16.7) | 189 (14.4) | 157 (13.6) | 236 (16.8) | 1 | 137 (22.9) | 56 (23.7) | 34 (25.6) | 25 (21.6) | 22 (19.5) | 0.3 | |
| Digestive system | 340 (6.6) | 91 (7.0) | 95 (7.2) | 72 (6.2) | 82 (5.8) | 0.1 | 39 (6.5) | 17 (7.2) | 14 (10.5) | ≤10 | ?≤10 | 0.04 | |
| Retro-/Peritoneum | 418 (8.1) | 131 (10.1) | 113 (8.6) | 91 (7.9) | 83 (5.9) | <0.001 | 48 (8.0) | 21 (8.9) | 13 (9.8) | ≤10 | ≤10 | 0.1 | |
| Other abdominal | 211 (4.1) | 40 (3.1) | 58 (4.4) | 45 (3.9) | 68 (4.8) | 0.05 | 25 (4.2) | 12 (5.1) | ≤10 | ≤10 | ≤10 | 0.1 | |
| Lymph node | 914 (17.7) | 293 (22.7) | 258 (19.6) | 203 (17.6) | 160 (11.4) | <0.001 | 85 (14.2) | 45 (19.1) | 17 (12.8) | 17 (14.7) | ≤10 | <0.01 | |
| Regional | 732 (14.2) | 231 (17.9) | 205 (15.6) | 173 (15.0) | 123 (8.7) | <0.001 | 63 (10.5) | 32 (13.6) | 14 (10.5) | 13 (11.2) | ≤10 | 0.01 | |
| Distant | 197 (3.8) | 68 (5.3) | 56 (4.3) | 31 (2.7) | 42 (3.0) | <0.001 | 24 (4.0) | 14 (5.9) | ≤10 | ≤10 | ≤10 | 0.1 | |
| Bone | 1,120 (21.7) | 298 (23.0) | 287 (21.8) | 241 (20.8) | 294 (20.9) | 0.1 | 161 (26.9) | 73 (30.9) | 27 (20.3) | 31 (26.7) | 30 (26.5) | 0.4 | |
| Brain | 124 (2.4) | 42 (3.2) | 38 (2.9) | 20 (1.7) | 24 (1.7) | <0.01 | 20 (3.3) | 12 (5.1) | ≤10 | ≤10 | ≤10 | 0.06 | |
| Number of metastatic sites, n (%) | Single site | 2,595 (50.2) | 583 (45.1) | 666 (50.7) | 592 (51.2) | 754 (53.6) | <0.001 | 316 (52.8) | 119 (50.4) | 69 (51.9) | 61 (52.6) | 67 (59.3) | 0.2 |
| 2 sites | 1,481 (28.6) | 389 (30.1) | 364 (27.7) | 339 (29.3) | 389 (27.6) | 142 (23.7) | 58 (24.5) | 31 (23.3) | 24 (20.7) | 29 (25.6) | |||
| 3 sites | 929 (18) | 270 (20.9) | 240 (18.3) | 188 (16.3) | 231 (16.4) | 117 (19.6) | 47 (19.9) | 27 (20.3) | 29 (25) | 14 (12.4) | |||
| ≥4 sites | 164 (3.2) | 51 (3.9) | 44 (3.3) | 37 (3.2) | 32 (2.3) | 23 (3.8) | 12 (5.1) | ≤10 | ≤10 | ≤10 | |||
Bold values indicate statistical significance
Distribution of exclusive single organ metastases according to ethnicity and age
Overall, 2,574 (49.8%) Caucasians and 282 (47.2%) African-Americans had ≥2 metastatic sites. Increasing age was associated with lower rates of multiple metastatic sites in Caucasians (from 54.9 to 46.4%; p <0.001) but not in African-Americans (p = 0.2).
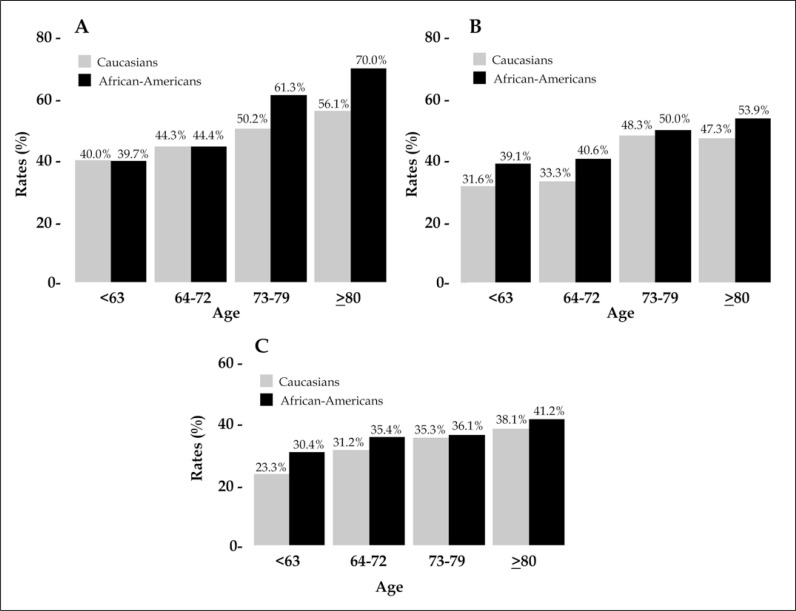
Detailed stratification of the distribution of exclusive A) bone, B) lung, or C) liver metastases were subsequently tabulated according to ethnicity, as well as to age categories. (Figure 2A-C). Overall, the rates of exclusive single organ metastases increased with age and were consistently higher in African-American patients. Specifically, the rates of exclusive bone metastases increased from 40 to 56.1% in Caucasians vs. 39.7 to 70% in African-Americans. The rates of exclusive lung metastases increased from 31.6 to 47.3% in Caucasians vs. 39.1 to 53.9% in African-Americans. Finally, the rates of liver metastases increased from 23.3 to 38.1% vs. 30.4 to 41.2% in Caucasians vs. African-Americans, respectively.
Figure 2.
Bar plots depicting rates of exclusive organ metastasis, stratified according to the presence of bone (A), lung (B) or liver (C) metastases, in addition to tabulation for ethnicity (Caucasians [gray] vs. African-Americans [black]) and age categories (<63 vs. 64–72 vs. 73–79 vs. >80) in 5,767 metastatic bladder cancer patients identified within the National Inpatient Sample database, between 2008 and 2015.
Multivariable analyses predicting lung, bone, liver and brain metastases
Finally, we corroborated our observations within multivariable analyses (Table 2). At multivariable logistic regression models, African-American ethnicity achieved an independent predictor status for higher risk of lung (Odds Ratio: 1.69, 95%CI 1.39–2.07; p <0.001), liver (odds ratio: 1.50, 95%CI 1.22–1.83; p <0.01) or bone metastases (OR: 1.27, 95%CI 1.04–1.55; p = 0.01), but not for brain metastases (p = 0.4). Younger age (odds ratio: 1.18, 95%CI; p <0.01) and male gender (odds ratio: 1.36, 95%CI 1.17–1.58; p <0.001) were also associated with increased risk of bone metastases.
Table 2.
Multivariable logistic regression models predicting lung, liver, bone and brain metastases in 5,767 metastatic bladder cancer patients identified within the National Inpatient Sample database, between 2008 and 2015
| Predictors | Lung metastases | Liver metastases | Bone metastases | Brain metastases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | p–value | Odds ratio | 95% Confidence interval | p–value | Odds ratio | 95% Confidence interval | p–value | Odds ratio | 95% Confidence interval | p–value | ||
| Ethnicity | Caucasian | Reference | Reference | Reference | Reference | ||||||||
| African–American | 1.69 | 1.39–2.07 | <0.001 | 1.50 | 1.22–1.83 | <0.01 | 1.27 | 1.04–1.55 | 0.01 | 1.23 | 0.74–2.05 | 0.4 | |
| Age | >63 | Reference | Reference | Reference | Reference | ||||||||
| ≤63 | 0.94 | 0.81–1.08 | 0.4 | 1.14 | 0.98–1.33 | 0.07 | 1.18 | 1.03–1.35 | 0.01 | 1.65 | 1.17–2.32 | <0.01 | |
| Gender | Female | Reference | Reference | Reference | Reference | ||||||||
| Male | 0.83 | 0.73–0.95 | <0.01 | 0.99 | 0.85–1.15 | 0.9 | 1.36 | 1.17–1.58 | <0.001 | 0.87 | 0.60–1.25 | 0.4 | |
| Charlson Comorbidity Index | 0–1 | Reference | Reference | Reference | Reference | ||||||||
| ≥2 | 1.05 | 0.87–1.26 | 0.6 | 1.12 | 0.93–1.37 | 0.2 | 0.98 | 0.83–1.18 | 0.9 | 0.85 | 0.52–1.37 | 0.5 | |
Bold values indicate statistical significance
Interestingly, when independent predictors of bone metastases were combined, a dose-response effect was shown. Specifically, African-American ethnicity, in the presence of both risk factors for bone metastases, namely younger age (<63) and male gender, was associated with a 2-fold higher risk of developing bone metastases, relative to no risk factors (odds ratio: 2.15, 95%CI 1.47–3.16; p <0.001). Lower odds ratios were observed, when African-American ethnicity was combined with only one risk factor, namely male gender (odds ratio: 1.61, 95%CI 1.16–2.24; p <0.01) or younger age (odds ratio: 1.67, 95%CI 1.02–2.72; p = 0.04) (Table 3).
Table 3.
Multivariable logistic regression models predicting bone metastases in 5,767 metastatic bladder cancer patients identified within the National Inpatient Sample database, between 2008 and 2015. Dose-response effect after combination of age, ethnicity and gender
| Predictors | Bone metastases | ||
|---|---|---|---|
| Odds ratio | 95% Confidence interval | p–value | |
| Caucasian >63 Female |
Reference | ||
| African–American <63 Male |
2.15 |
1.47–3.16 |
<0.001 |
| African–American <63 Female |
1.67 |
1.02–2.72 |
0.04 |
| African–American >63 Male |
1.61 |
1.16–2.24 |
<0.01 |
| Caucasian <63 Male |
1.52 |
1.23–1.88 |
<0.01 |
| Caucasian >63 Male |
1.29 |
1.08–1.55 |
<0.01 |
| Caucasian <63 Female |
1.01 |
0.74–1.39 |
0.9 |
| African–American >63 Female |
0.95 |
0.59–1.56 |
0.9 |
| Charlson Comorbidity Index (>2 vs. 0–1) | 0.99 | 0.83–1.19 | 0.9 |
DISCUSSION
Approximately, 8 to 18% of bladder cancer patients harbour metastatic disease [4]. However, while the effect of age on location of metastasis was historically analyzed [10], to date no studies investigated the impact of ethnicity on distribution of metastasis in patients with metastatic bladder cancer. Based on this unmet need, we relied on a contemporary population-based dataset and hypothesized that racial differences may exist in the distribution of metastatic bladder cancer metastasis. Our study showed several important observations.
First, we identified 5,767 patients with metastatic bladder cancer. Of these, only 598 (10.4%) were African-Americans. Moreover, it was impossible to analyze data regarding ethnic minorities other than African-Americans, due to there being a small number of such patients. On one hand, such low numbers within one of the largest currently available population-based datasets clearly demonstrates that testing for racial differences in the context of metastatic bladder cancer may be difficult. On the other hand, the nature of the National Inpatient Sample database allowed us to investigate rates of very uncommon metastatic sites, such as adrenal, pleural or duodenal metastases, as previously reported in other urological malignancies [15, 18], within a large contemporary cohort of metastatic bladder cancer patients and even according to patient ethnicity. To the best of our knowledge, we are not aware of similar studies.
Second, lung and bone represented the most common metastatic sites, both in Caucasians and in African-Americans. Specifically, from one out of five to one out of three patients harboured bone or lung metastases.
These observations indicate that lung and bone should invariably be imaged in all bladder cancer patients, in whom metastasis is suspected. Currently, the role of chest imaging in bladder cancer is well established and international guidelines recommend to perform chest imaging for staging and follow-up in all bladder cancer patients [19, 20, 21]. Conversely, evaluation of bone metastasis is recommended in the presence of specific criteria, such as bone symptoms, high-risk disease or in patients with laboratory indicators of bone lesions [20]. However, our analyses demonstrated that up to 30% of bladder cancer patients harbour bone metastases. Moreover, the rates of exclusive bone metastases increase with age, up to 70% in African-American patients. Although the presence of bone metastases in bladder cancer represent an indicator of poor oncologic outcomes [22], it is of note that early diagnosis may lead to timely medical or surgical intervention and skeletal-related events prevention [23]. In consequence, our findings suggest that selection criteria for bone imaging should be revised and a bone scan should be offered to a wider cohort of bladder cancer patients. In that regard, bone surveys that include long bones may be advocated. Such an approach may result in higher detection of lytic lesions than if bone scintigraphy is performed, where such lesions may go unnoticed [24].
Third, we identified important racial differences in the distribution of metastasis between Caucasian and African-American patients. Specifically, bone represented the most common metastatic site in Caucasians (21.7%), followed by lung (18.4%). Conversely, lung was the most common metastatic site in African-Americans (28.6%), followed by bone (26.9%). Moreover, overall rates of the three most common metastatic sites, namely bone (+5.2%), lung (+10.2%) and liver (+7.5%) were consistently higher in African-American patients, compared to Caucasians (all p <0.01), even after stratification according to patient age. We corroborated these findings in multivariable analyses, where African-American ethnicity achieved an independent predictor status for higher risk of bone (odds ratio: 1.27), lung (odds ratio: 1.69), or liver metastases (odds ratio: 1.50). Moreover, further stratification according to the number of metastatic sites involved demonstrated overall higher rates of multiple sites in African-American vs. Caucasian patients. Interestingly, African-Americans were more likely to harbour metastases in different locations, compared to Caucasians.
Multiple factors may have contributed to these racial differences. Specifically, workers in high-risk occupations, such as mechanic industries, involving exposure to dyes, paints or well-known carcinogens (i.e. 2-naphthylamine, benzidine, and 4-aminobiphenyl) have increased risk of bladder cancer [25]. In this regard, Burns et al. [26] demonstrated an increased risk of bladder cancer in African-Americans with a typical occupation of automobile mechanic. Parkin et al. [27] found higher incidence of the squamous cell carcinoma variant in bladder cancer African-American patients. Of note, squamous cell carcinoma is a more aggressive histological variant than urothelial, which is associated with higher stage at presentation and poor survival [28, 29, 30]. It is also very important to emphasize the existence of differences in referral patterns between Caucasian and African-American patients. For instance, Schrag et al. [31] revealed a 1.6-fold higher risk of inadequate surveillance after bladder cancer diagnosis in African-Americans, relative to Caucasians. Finally, reduced access to healthcare for African-American patients [32, 33] may lead to a delay in bladder cancer diagnosis, and may further contribute to a higher risk of developing metastasis. Nonetheless, racial differences in the distribution of metastatic sites may be only partially explained by disparities in access to healthcare or routine cancer screening. Indeed, important biological factors, such as molecular, biochemical or genetic factors [34, 35] may contribute to racial differences, and may predispose African-American patients to faster tumor growing and spreading, as well as to different metastatic profiles. Additionally, several comorbidities, such as obesity or diabetes, may induce an altered metabolic and/or inflammatory status, which have been associated with adverse cancer outcomes [36, 37]. Of note, such conditions are more common among African-Americans [36, 37]. However, further studies are needed to assess the biological rationale that may run behind our observations.
Fourth, we investigated the presence of metastasis in uncommon metastatic sites, namely those different than lung, bone, liver, brain or lymph nodes. Interestingly, we found that roughly one out of five bladder cancer patients harbour metastases in rare metastatic sites. Although no major racial differences were found, prevalence of uncommon metastatic sites may be of importance in the design of clinical trials, where presence of specific metastatic sites may guide clinicians. To the best of our knowledge, we are the first to investigate rates of uncommon metastatic sites according to ethnicity in metastatic bladder cancer. In consequence, we cannot compare our results with similar analyses in metastatic bladder cancer patients. Nonetheless, our study is in agreement with Stolzenbach et al. [38], who addressed this topic in metastatic prostate cancer, and did not find any meaningful difference according to patient ethnicity.
Fifth, our analyses revealed that age significantly affects rates of exclusive bone, lung or liver metastases, both in Caucasian and in African-American patients. Bianchi et al. [10] previously demonstrated an age-related distribution of metastasis in metastatic bladder cancer. However, the authors did not evaluate racial-related differences. In this regard, stratification according to patient ethnicity revealed important differences between Caucasians and African-Americans. Specifically, rates of single organ involvement were higher in African-Americans, compared to Caucasians, regardless of site of metastasis and patient age. Moreover, rates of exclusive bone metastases recorded the highest increase with age in African-Americans vs. Caucasians, +30.3% vs. +15.6%, respectively.
These observations provide additional proof of racial differences in the distribution of bladder cancer metastasis. Moreover, these findings further support the use of bone imaging for investigation of bone lesions, especially in elderly African-American patients, where the rates of exclusive bone metastases are extremely high and may be missed during patient follow-up, when only chest, but not bone imaging is recommended [19, 20, 21].
Sixth, in the last part of our analyses, we focused on bone metastases. Specifically, multivariable analyses revealed that younger age and male gender, in addition to African-American ethnicity, are independent predictors of bone metastases in metastatic bladder cancer. Interestingly, we found a dose-response effect, after combination of the three risk factors. Indeed, African-American ethnicity associated with one risk factor showed lower odds ratios than if associated with both risk factors (1.61 vs. 1.67 vs. 2.15), relative to the absence of other risk factors.
These findings are important, and further support the need to revise the current guideline recommendations for performing bone imaging.
Last but not least, brain metastases were particularly rare, regardless of ethnicity and age. These findings are in agreement with guideline recommendations, [20] and confirm that brain imaging may possibly be left up to the discretion of the clinician, based on clinical suspicious.
Taken together, our analyses revealed that racial differences exist in the distribution of metastatic bladder cancer metastasis. From a clinical prospective, our findings indicate that bladder cancer African-American patients deserve more attentive evaluation and stricter follow-up, compared to Caucasians, due to overall higher rates of bone, lung and liver metastases. Moreover, careful evaluation of bone metastases is needed in this patient population, due to extremely high rates of exclusive bone metastases, especially in elderly patients, which may be missed during patient counseling. Finally, other specific racial and age-related differences are more applicable to the design and conduct of clinical trials, where the prevalence and the distribution of metastatic sites may be of particular importance.
Despite its novelty, our study is not devoid of limitations. First, the National Inpatient Sample database does not include more granular information about tumor characteristics or tumor histology. Similarly, the number of metastases in each metastatic site was not available. In consequence, our analyses could not account for variability according to patient ethnicity. However, our analyses focused on the distribution of metastasis, and did not investigate survival outcomes, where the role of these important variables is well established [11, 39]. Second, information regarding diagnostic methodology used to evaluate the presence of metastasis was not available. Third, the National Inpatient Sample database is based on hospitalized patients. In consequence, the patterns of metastatic sites described in our study may not exactly reflect those of all metastatic bladder cancer patients. Nonetheless, the National Inpatient Sample database estimates are considered to be accurate and precise, [40] and were used to evaluate the distribution of metastasis in other urological malignancies, such as kidney cancer or prostate cancer [15, 18].
CONCLUSIONS
Racial differences exist in the distribution of metastatic bladder cancer metastasis. Moreover, based on the higher risk of bone metastases in African-American patients, bone imaging may be warranted in this patient population, especially in the presence of other risk factors for bone metastases, namely male gender or younger age.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 2.Sloan FA, Yashkin AP, Akushevich I, Inman BA. The Cost to Medicare of Bladder Cancer Care. Eur Urol Oncol. 2019:1–8. doi: 10.1016/j.euo.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Chang Q, Li Y. Racial differences in Urinary Bladder Cancer in the United States. Sci Rep. 2018;8:1–8. 4. doi: 10.1038/s41598-018-29987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koll T, Fang X, Subbiah S. Trends in metastatic bladder cancer incidence and prognosis by histologic subtypes. J Clin Oncol. 2012;30:322. [Google Scholar]
- 5.Abdollah F, Gandaglia G, Thuret R, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: A trend analysis. Cancer Epidemiol. 2013;37:219–225. doi: 10.1016/j.canep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Hollenbeck BK, Dunn RL, Ye Z, Hollingsworth JM, Lee CT, Birkmeyer JD. Racial differences in treatment and outcomes among patients with early stage bladder cancer. Cancer. 2010;116:50–56. doi: 10.1002/cncr.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallin K, David KA, Carroll PR, Milowsky MI, Nanus DM. Transitional cell carcinoma of the bladder: Racial and gender disparities in survival (1993 to 2002), stage and grade (1993 to 2007) J Urol. 2011;185:1631–1666. doi: 10.1016/j.juro.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Bjurlin MA, Cohn MR, Freeman VL, Lombardo LM, Hurley SD, Hollowell CMP. Ethnicity and smoking status are associated with awareness of smoking related genitourinary diseases. J Urol. 2012;188:724–758. doi: 10.1016/j.juro.2012.04.110. [DOI] [PubMed] [Google Scholar]
- 9.Sengeløv L, Kamby C, Von Der Maase H. Pattern of metastases in relation to characteristics of primary tumor and treatment in patients with disseminated urothelial carcinoma. J Urol. 1996;155:111–114. [PubMed] [Google Scholar]
- 10.Bianchi M, Roghmann F, Becker A, et al. Age-stratified distribution of metastatic sites in bladder cancer: A population-based analysis. J Can Urol Assoc. 2014;8:E148–158. doi: 10.5489/cuaj.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaffuto E, Bandini M, Moschini M, et al. Location of Metastatic Bladder Cancer as a Determinant of In-hospital Mortality After Radical Cystectomy. Eur Urol Oncol. 2018;1:169–175. doi: 10.1016/j.euo.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Barashi NS, Pearce SM, Cohen AJ, Pariser JJ, Packiam VT, Eggener SE. Incidence, Risk Factors, and Outcomes for Rectal Injury During Radical Prostatectomy: A Population-based Study. Eur Urol Oncol. 2018;1:501–506. doi: 10.1016/j.euo.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 13.National (Nationwide) Inpatient Sample (NIS) n.d. https://www.hcup-us.ahrq.gov/news/exhibit_booth/nis_brochure.jsp. (accessed May 19, 2020) [DOI] [PMC free article] [PubMed]
- 14.Rosiello G, Palumbo C, Knipper S. Preoperative frailty predicts adverse short-term postoperative outcomes in patients treated with radical prostatectomy. Prostate Cancer Prostatic Dis. 2020;23:573–580. doi: 10.1038/s41391-020-0225-3. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann Oncol. 2012;23:973–980. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 16.Rosiello G, Palumbo C, Deuker M, et al. Preoperative frailty predicts adverse short-term postoperative outcomes in patients treated with radical nephroureterectomy. J Surg Oncol. 2020;121:688–696. doi: 10.1002/jso.25840. [DOI] [PubMed] [Google Scholar]
- 17.HCUP-US NIS Overview n.d. https://www.hcup-us.ahrq.gov/nisoverview.jsp. (accessed May 20, 2020)
- 18.Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate. 2014;74:210–216. doi: 10.1002/pros.22742. [DOI] [PubMed] [Google Scholar]
- 19.Chang SS, Bochner BH, Chou R, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. 2017;198:552–559. doi: 10.1016/j.juro.2017.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flaig TW, Spiess PE, Chair V, Agarwal N, Bangs R, Patient Advocate M, et al. NCCN Guidelines Version 6.2020 Bladder Cancer. 2020. [Google Scholar]
- 21.Witjes JA, Bruins M, Cathomas R, et al. Muscle-invasive and metastatic Bladder Cancer 2020. EAU Guideline. https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/ [Google Scholar]
- 22.Zhang C, Liu L, Tao F, et al. Bone metastases pattern in newly diagnosed metastatic bladder cancer: A population-based study. J Cancer. 2018;9:4706–4711. doi: 10.7150/jca.28706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel V, Lorduy AC, Stern A, et al. Survival after metastasectomy for metastatic urothelial carcinoma: A systematic review and meta-analysis. Bladder Cancer. 2017;3:121–132. doi: 10.3233/BLC-170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quddus FF, Espinola D, Kramer SS, Leventhal BG. Comparison between X-ray and bone scan detection of bone metastases in patients with rhabdomyosarcoma. Med Pediatr Oncol. 1983;11:125–129. doi: 10.1002/mpo.2950110211. [DOI] [PubMed] [Google Scholar]
- 25.Swanson GM. Cancer prevention in the workplace and natural environment. A review of etiology, research design, and methods of risk reduction. 1988;62(8 Suppl):1725–1746. doi: 10.1002/1097-0142(19881015)62:1+<1725::aid-cncr2820621310>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Burns PB, Swanson GM. Risk of urinary bladder cancer among Blacks and Whites: the role of cigarette use and occupation. Cancer Causes Control. 1991;2:371–379. doi: 10.1007/BF00054297. [DOI] [PubMed] [Google Scholar]
- 27.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 28.Bandini M, Pederzoli F, Madison R, et al. Unfavorable Cancer-specific Survival After Neoadjuvant Chemotherapy and Radical Cystectomy in Patients With Bladder Cancer and Squamous Cell Variant: A Multi-institutional Study. Clin Genitourin Cancer. 2020;18:e543–556. doi: 10.1016/j.clgc.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krasnow RE, Drumm M, Roberts HJ, et al. Clinical Outcomes of Patients with Histologic Variants of Urothelial Cancer Treated with Trimodality Bladder-sparing Therapy. Eur Urol. 2017;72:54–60. doi: 10.1016/j.eururo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Zahoor H, Elson P, Stephenson A, et al. Patient Characteristics, Treatment Patterns and Prognostic Factors in Squamous Cell Bladder Cancer. Clin Genitourin Cancer. 2018;16:e437–442. doi: 10.1016/j.clgc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Schrag D, Hsieh LJ, Rabbani F, Bach PB, Herr H, Begg CB. Adherence to surveillance among patients with superficial bladder cancer. J Natl Cancer Inst. 2003;95:588–597. doi: 10.1093/jnci/95.8.588. [DOI] [PubMed] [Google Scholar]
- 32.Riley WJ. Health disparities: gaps in access, quality and affordability of medical care. Trans Am Clin Climatol Assoc. 2012;123:164–167. [PMC free article] [PubMed] [Google Scholar]
- 33.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 34.Dookeran KA, Dignam JJ, Ferrer K, et al. P53 as a marker of prognosis in African-American women with breast cancer. Ann Surg Oncol. 2010;17:1398–1405. doi: 10.1245/s10434-009-0889-3. [DOI] [PubMed] [Google Scholar]
- 35.Mehrotra J, Ganpat MM, Kanaan Y, et al. Estrogen Receptor/Progesterone Receptor-Negative Breast Cancers of Young African-American Women Have a Higher Frequency of Methylation of Multiple Genes than Those of Caucasian Women. Clin Cancer Res. 2004;10:2052–2057. doi: 10.1158/1078-0432.ccr-03-0514. [DOI] [PubMed] [Google Scholar]
- 36.Bandera EV, Maskarinec G, Romieu I, John EM. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: A global perspective. Adv Nutr. 2015;6:803–819. doi: 10.3945/an.115.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura T, Inagawa S, Hisakura K, Enomoto T, Ohkohchi N. Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. J Gastroenterol Hepatol. 2012;27:1635–1640. doi: 10.1111/j.1440-1746.2012.07189.x. [DOI] [PubMed] [Google Scholar]
- 38.Stolzenbach LF, Rosiello G, Deuker M, et al. The Impact of Race and Age on Distribution of Metastases in Patients with Prostate Cancer. J Urol. 2020;204:962–968. doi: 10.1097/JU.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 39.Rosiello G, Pecoraro A, Palumbo C, et al. Radical cystectomy plus chemotherapy in patients with pure squamous cell bladder carcinoma: a population-based study. World J Urol. 2020 doi: 10.1007/s00345-020-03247-3. [DOI] [PubMed] [Google Scholar]
- 40.Measures KR, Report M. HCUP Methods Series 2012.