Abstract
Introduction
Transitional cell carcinoma recurrence within an intestinal urinary diversion (TCCUD) after radical cystectomy (RC) is a rare condition with unknown origin, prognosis and treatment. The aim of this study was to describe treatment options and oncologic outcomes of this understudied site of recurrence in a multi-institutional case series.
Material and methods
TCCUD relapse cases after RC were investigated in a retrospective, multi-institutional study. Surgical approach and adjuvant chemotherapy were discussed. Early and late complications were described according to the Clavien-Dindo classification. Kaplan-Meier method was used to assess progression-free and cancer-specific survival.
Results
A total of 19 patients were selected. The most common presentation was gross hematuria. The median interval between RC and TCCUD was 51.2 months. Fifteen patients (78.9%) underwent surgical excision, and two underwent concomitant radical nephroureterectomy. In 12 (63.1%) cases the site of TCCUD was the uretero-ileal anastomosis. Tumor invading the muscularis of the intestinal diversion was described in 10 (52.6%) cases. Surgical complications occurred in 7/15 (46.6%) patients, of these two with Clavien-Dindo Grade III. Four patients (21.0%) underwent adjuvant chemotherapy and two (10.5%) both chemotherapy and radiation therapy. During follow-up 15 patients (78.9%) presented with other sites of recurrence, with lymph nodes (21.0%) and liver (15.7%) being the most common localizations. Recurrence free and overall survival rates were 36.8% and 15.8%, and 56.5% and 24.2%, respectively at 12 and 18 months.
Conclusions
Most patients with TCCUD have invasive disease and a substantial percentage experience upper tract cancer during their disease course. TCCUD is often the herald of advanced disease and systemic progression, with poor progression-free and overall survival rates.
Keywords: bladder cancer, radical cystectomy, urinary diversion, recurrence, transitional cell carcinoma, urinary diversion/adverse effects, undiversion
INTRODUCTION
After radical cystectomy (RC) for bladder cancer, well-known sites for local recurrence of transitional cell carcinoma (TCC) are the surgical bed and pelvic lymph nodes, while distant recurrences can be found in juxta-regional lymph nodes above the aortic bifurcation, lungs, liver and bones [1]. TCC is highly recurrent, metastatic and heterogeneous, thereby resulting in poor prognosis.
Pelvic recurrence is seen in 5–15% of patients treated with cystectomy. Most recurrences manifest during the first 24 months, often within 6 to 18 months after surgery. However, late recurrence can occur up to five years after cystectomy with poor prognosis. Patients with pelvic recurrence have poor prognosis. Even after treatment, median survival ranges from four to eight months following diagnosis. Possible treatments are systemic chemotherapy, local surgery, or radiation therapy (RT) [2]. Although these therapies may prolong survival, they mostly provide significant palliation of symptoms.
Distant recurrence is seen in up to 50% of patients treated with cystectomy [3]. Systemic recurrence is more common in locally advanced disease (pT3/4), ranging from 32 to 62%, and in patients with lymph node involvement (range 52–70%) [4].
Nearly 90% of distant recurrences appear within the first three years after RC, mainly in the first two years, although late recurrence has been described after 10 years. Median survival of patients with progressive disease treated with platinum-based chemotherapy is 9–26 months [5, 6].
Transitional cell carcinoma recurrence impacting intestinal urinary diversion (TCCUD) after RC is a very rare condition. Only a few case reports are described in the literature, thus the management of these patients is uncertain due to the unstudied prognostic impact of this particular site of recurrence. Therefore, understanding the way TCC may manifest after cystectomy for bladder cancer is critical to gain insights into the mechanisms responsible for TCC progression.
Thus, the aim of the present study is to describe the clinical presentation, treatment options, surgical complications and oncologic outcomes of this rare site of recurrence.
MATERIAL AND METHODS
Study population
After obtaining institutional ethics approval, a retrospective chart review was conducted to identify patients with relapse on an ileal conduit or orthotopic ileal neobladder after open RC, between 1990 to 2014. Data collection was conducted in two secondary referral centers (Mayo Clinic, Rochester, USA and University of Padua, Italy). For each patient we collected: demographic data, clinical data (history of upper urinary tract urothelial carcinoma [UTUC], UTUC treatment, pTNM stage for the primary tumor), symptoms presentation, site of urinary recurrence, management of urinary diversion recurrence, histology of recurrence, early and late complications (according to the Clavien-Dindo classification) and use of adjuvant treatments.
Procedural specifics of the primary tumor
Radical cystectomy with bilateral lymphadenectomy was performed using standard techniques by various surgeons over the time frame of the study. Negative frozen sections of the distal ureter were obtained before anastomosis. Type of continent and incontinent diversions were decided upon according to patients’ characteristics and surgeon preference. Nephroureterectomy concomitant to cystectomy was performed in the presence of a high-risk upper urinary tract tumor (TCC).
Follow-up after treatment of the primary tumor
Postoperative follow-up was not standardized but patients were generally evaluated every 3–4 months postoperatively for the first year, semi-annually for the second year, and annually thereafter. After a clinical evaluation, a CT or MRI was performed in all patients.
Evaluation of transitional cell carcinoma recurrence impacting intestinal urinary diversions
Loopography/pouchography were used to examine the conduit or the pouch, ureters, and pelvicalyceal system, particularly if a recurrent tumor was suspected (gross hematuria, positive urine cytology). To perform loopography/pouchography, water-soluble contrast material was injected in a retrograde fashion (typically by means of gentle hand injection or instilled under gravity pressure) through a 12–14-F Foley catheter inserted into the conduit/pouch. The catheter balloon was inflated with 5–10 mL of contrast material or fluid. Fluoroscopic spot images of the conduit/pouchography, ureters, and collecting systems were obtained in multiple projections. Endoscopic study was used to evaluate and biopsy the intestinal segment of the urinary diversion in order to assess the presence of TCC recurrence. During the endoscopic evaluation, normal intestinal mucosa appeared pinkish and velvety. The procedure may present some challenges in the passage of the cystoscope due to sacculation of the urinary diversion, narrow communications and difficult angulations. Furthermore, mucus production by the intestinal wall may reduce the view. TCCUD appears as swollen mucosa with papillary, exophytic lesions. Less commonly, they may be sessile or ulcerated. Carcinoma in situ (CIS) is recognizable as a flat, velvety patch of anaplastic epithelium.
Surgical managements of recurrence impacting intestinal urinary diversion
When patients’ comorbidities were favorable, the entire neobladder/ileal conduit with its subsequent mesentery and the distal part of the ureters were resected for final pathology. To reestablish urinary continuity, an ileal conduit or ureterocutaneostomy was created.
In some cases, to reduce the total amount of normal ileum that was resected, the proximal ileal conduit including the recurrence was resected and the ureters were anastomosed to the remaining part of the ileal conduct with negative margins.
If the recurrence involved the ureter or the remaining urethra, a nephroureterectomy or urethrectomy were respectively performed.
Patients with severe comorbidities were treated with an endoscopic fulguration of the lesion.
Outcomes and statistical analyses
Descriptive statistics were used to report clinical and pathological characteristics. The Kaplan–Meier method was used to compare progression-free survival and cancer-specific survival rates. Statistical analyses were performed using SPSS v. 20.0 (IBM Corp., Armonk, NY, USA).
Literature review
A complete literature review was carried out for discussion. The findings of the most representative case reports were summarized in a non-systematic review. The Medline and Web of Knowledge/Google Scholar databases were used with the following key words for articles only in English: ‘transitional cell carcinoma’ OR ‘bladder cancer’ OR ‘urothelial carcinoma’ OR ‘urothelial cell carcinoma’ AND ‘Neoplasm Recurrence, Local/diagnosis’ OR ‘recurrence’ AND ‘Urinary Reservoirs, Continent’, 'urothelial carcinoma' and 'urothelial cell carcinoma'.
The references of articles and reviews found in the literature search were also examined to find additional reports that met the inclusion criteria. The following items were searched for in each of these series: number of patients reported, age, sex, histology at cystectomy, type of urinary diversion, recurrence site, clinical symptoms at presentation, time from RC until presentation of TCCUD, history of upper tract transitional cell carcinoma, surgical treatment, additional therapy, final pathology of TCCUD, death at follow-up.
Abstracts and case reports in which non transitional cell carcinoma recurrence impacting intestinal urinary diversion was assessed at the final pathology were excluded.
Descriptive statistics were also used to report clinical and pathological characteristics defined in the case reports from the literature. The Kaplan-Meier method was used to describe overall mortality. Statistical analyses were performed using SPSS v. 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
A total of 19 patients with TCCUD were selected. Table 1 describes patients’ characteristics, while Table 2 describes patients’ treatment and follow-up.
Table 1.
Patients characteristics with transitional cell carcinoma recurrence within an intestinal urinary diversion (TCCUD)
| Total Patients Male Female |
19 14 (73.6) 5 (26.3) |
| Age at radical cystectomy (SD) | 66.7 ±6.5 |
| ASA 2 3 Missing |
6 (31.5) 8 (15.7) 5 (26.3) |
| Neoadjuvant chemotherapy before radical cystectomy | 2 (12.5) |
| BCG before cystectomy | 9 (56.2) |
| Presence of CIS at radical cystectomy | 10 (52.6) |
| Bladder cancer multifocality | 11 (57.8) |
| pT stage Bladder pT1 pT2 pT3 PT4 CIS |
4 (21.0) 5 (26.3) 4 (21.0) 1 (5.2) 5 (26.3) |
| Type of urinary diversion Ileal conduct Neobladder |
10 (52.6) 9 (47.3) |
| pN+ at RC | 3 (15.7) |
| Negative surgical margins at radical cystectomy | 18 (94.8) |
| Adjuvant chemotherapy post radical cystectomy | 5 (26.3) |
| History of Upper tract TCC | 9/19 (47.3) |
| Site of upper tract TCC Renal pelvis only Ureter and renal pelvis Ureter alone |
2 (10.5) 5 (26.3) 2 (10.5) |
| Nephroureterectomy after RC, before diagnosis of TCCUD Nephroureterectomy after salvage surgery for TCCUD Nephroureterectomy concomitant to salvage surgery for TCCUD |
6/9 (66.6) 1/9 (11.1) 2/9 (22.2) |
| pT stage nephroureterectomy pT1 pT2 CIS |
1 (5.2) 4 (21.0) 4 (21.0) |
| Presentation Gross hematuria Urine cytology Imaging abnormalities on follow-up Fecaluria |
9 (47.3) 5 (26.3) 3 (15.7) 2 (10.5) |
| Time from radical cystectomy to urinary diversion recurrence, months (IQR) | 51.2 (15.7–111.4) |
ASA – American Society of Anesthesiologists physical status classification; IQR – interquartile range, SD – standard deviation; TCC – transitional cell carcinoma; TCCUD – transitional cell carcinoma recurrence within an intestinal urinary diversion; RC – radical cystectomy; BCG – Bacillus Calmette–Guérin; CIS – carcinoma in situ
Table 2.
Transitional cell carcinoma recurrence within intestinal urinary diversion (TCCUD) treatment and follow-up
| Time from radical cystectomy to treatment for TCCUD, months (IQR) | 51.2 (15.7–111.4) |
| Type of treatment for TCCUD Observation Chemotherapy Endoscopic fulguration Urinary undiversion Excision of proximal ileal conduct |
1 (5.2) 1 (5.2) 2 (10.4) 5 (26.3) 10 (52.6) |
| Site of urinary recurrence Neobladder Ileal-urethra anastomosis Uretero-ileal anastomosis Missing |
2 (10.5) 2 (10.5) 12 (63.1) 3 (15.7) |
| Stage of TCC Invasive TCC Superficial TCC TCC not better specified |
10 (52.6) 3 (15.7) 6 (31.5) |
| Histology Transitional cell carcinoma |
19/19 (100%) |
| Surgical complications after urinary undiversion/ intestinal resection No complications Clavien-Dindo 1 Clavien-Dindo 2 Clavien-Dindo 3a Clavien-Dindo 3b Clavien-Dindo 4 Missing |
9 (47.3) 1 (5.2) 4 (21.0) 1 (5.2) 1 (5.2) 1 (10.5) 2 (15.7) |
| Adjuvant therapy after surgery for TCCUD None Chemotherapy Chemotherapy and radiation therapy Missing |
10 (52.6) 4 (21.0) 2 (10.5) 3 (15.7) |
| Urethrectomy | 3 (15.7) |
| Time from radical cystectomy to systemic recurrence, months (IQR) | 19 (1–20.3) |
| Follow-up, months (IQR) | 19 (5.9–30.8) |
| Follow-up Patients alive Patients dead from other causes Patients dead from TCC recurrence |
9/19 0/19 10/19 |
The most common presentation was gross hematuria. However, positive urine cytology, imaging abnormalities on follow-up and fecaluria were also found at the time of diagnosis. Concomitant upper tract TCC was present in nine patients (47.3%). The median interval between RC and TCCUD was 51.2 months (IQR 15.7–111.4). 15/19 patients (78.9%) underwent surgical excision, and two of them underwent concomitant radical nephroureterectomy. All recurrences were confirmed to be transitional cell carcinomas. A tumor invading the muscularis of the intestinal diversion was described in 10 (52.6%) cases. Complications occurred respectively in one (5.2%) patient with grade I (fever), four patients (21.0%) with grade II (anemia requiring transfusion), one (5.2%) patient with grade IIIa (anemia requiring arterial embolization), one (5.2%) patient with grade IIIb (5.2%) (ileus requiring open surgery) and one (5.2%) patient with grade IV (pulmonary embolism). In the majority of the cases the site of recurrence was the ureteroileal anastomosis (12 patients, 63.1%). Four patients (21.0%) underwent adjuvant chemotherapy and two (10.5%) chemotherapy and radiation therapy. Median follow-up time after TCCUD finding was 19 (5.9–30.8) months. During this time, other sites of recurrence developed in 15 patients (78.9%), with small bowel mesentery lymph nodes (four patients – 21.0%), liver (three patients – 15.7%) and liver + lungs (two patients – 10.5%) being common sites of tumor relapse. Median systemic progression-free survival was 10 months, while median cancer-specific survival was 23 months.
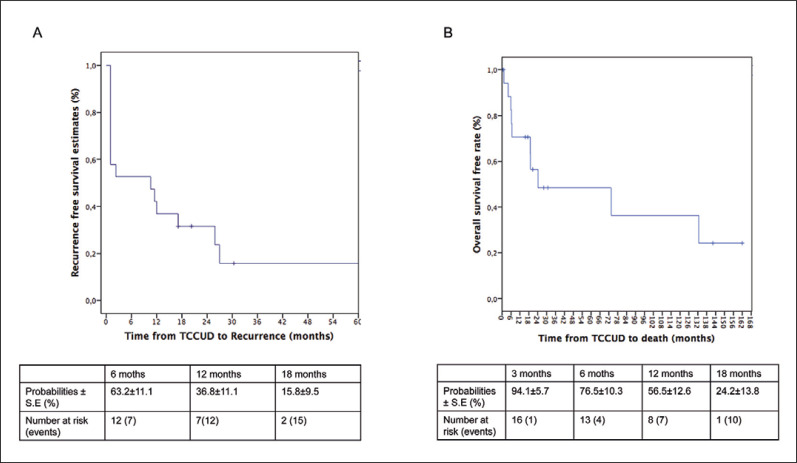
Recurrence-free rates were 36.8% and 15.8% respectively at 12 and 18 months (Figure 1A).
Figure 1.
A. Recurrence-free survival of patients with transitional cell carcinoma recurrence impacting intestinal urinary diversion (TCCUD) from the case series. B. Overall survival from the case series. All patients died from transitional cell recurrence.
Overall survival rates were 56.5% and 24.2%, respectively at 12 and 18 months (Figure 1B). All patients died of disease recurrence.
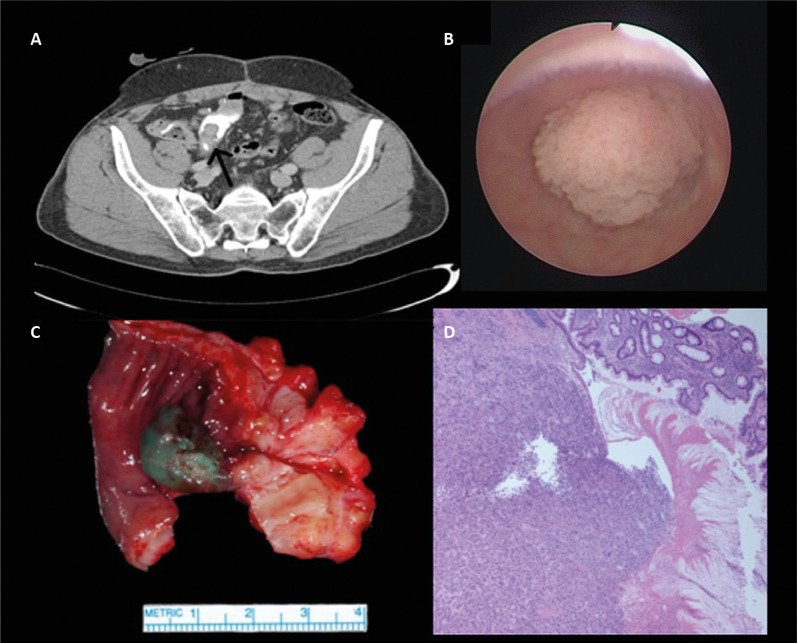
Figure 2 illustrates how TCCUD may appear in a CT scan (A), endoscopically (B) macroscopically (C) and microscopically (D).
Figure 2.
Transitional cell carcinoma recurrence within an intestinal urinary diversion (TCCUD). appearance in a CT scan (A), endoscopically (B) macroscopically (C) and microscopically (D).
Literature review
A total of 30 patients were described in 29 case reports identified in the literature [7–35] (Table 3). Twenty-six patients (86.7%) were male with a median age at initial presentation of 63.3 years (IQR 57–69). Median time interval between cystectomy and TCCUD was 63.9 months (IQR 14–108).
Table 3.
Summary of case reports found in the literature with transitional cell carcinoma recurrence within an intestinal urinary diversion (TCCUD) after radical cystectomy
| Author | Journal | Age/sex | Histology at cystectomy | Urinary diversion | Recurrence site | Symptoms at presentation | Months after cystectomy | Upper Tract TCC | Treatment of the recurrence | Additional therapy | Final pathology of TCCUD | Death | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soloway et al. [7] | J Urol. 1972 | 71/F | N/A | IC | UIA | GE and PC | 19 | Yes | RT | N/A | TCC | Yes | 24 |
| Soloway et al. [7] | J Urol. 1972 | 57/M | N/A | IC | UIA | PC | 13 | Yes | PR and formation of a new conduit + NUT | N/A | TCC | Yes | 15 |
| Grabstald [8] | J Urol. 1974 | 58/M | pT3N0 | IC | Around the ileal stoma | Tumor formation around the ilea! stoma | 48 | No | PR | No | TCC infiltrating grade 2 carcinoma with deep invasion into the muscularis of the ileal conduit. | No | 18 |
| Wajsman et al. [9] | Urology. 1975 | 57/M | N/A | IC | UD | GE | 108 | Yes | PR | CHT | TCC grade II | No | 12 |
| Banigo et al. [10] | J Urol. 1975 | 69/M | N/A | IC | UIA | N/A | 36 | Yes | PR + Resection of distal left ureter | N/A | TCC | No | 9 |
| Allan DM et al. [11] | Br J Urol. 1976 | 56/M | N/A | IC | UIA | abscess | 15 | Yes | TR + NUT | None performed | TCC | N/A | N/A |
| Rubin et al. [12] | Urol Radiol. 1979 | 68/F | N/A | IC | UD | GE and PC | 78 | No | PR and reanastomosis of ileal conduit | None performed | TCC | Yes | 1 day post surgery |
| Curran et al. [13] | Postgrad Med J. 1986 | 66/F | T1G2 | IC | UD | GE | 48 | Yes | PR + NUT | None performed | TCC noninvasive grade I and II | N/A | N/A |
| Moskovitz et al. [14] | Urol Int. 1986 | 52/M | N/A | IC | UIA | N/A | 15 | Yes | NUT+ resection of the ureter with the cuff of ileum. Reanastomosis of the loop. | None performed | TCC | N/A | N/A |
| Roberts et al. [15] | J Urol. 1987 | 69/F | T3bNoMx | IC | UD | UTI and upper tract obstruction | 4 | No | PR + distal portion of the conduit was fashioned into a new stoma | CHT | TCC poorly differentiated transitional | Yes | 4 |
| Rosvanis et al. [16] | Cancer. 1989 | 73/M | CIS | IC | UD | GE | 60 | Yes | NUT + PR | None performed | TCC noninvasive grade 2 | Yes | 12 |
| Mulholland et al. [17] | BrJ Urol. 1993 | 54/M | N/A | IC | UD | GE | N/A | Yes | PR + Resection of 3 cm of ureter | None performed | Grade II TCC without a direct invasion of the submucosa N0M0 | N/A | N/A |
| Garcia et al. [18] | Br J Urol 1993 | 77/M | pT2G3L1 | IC | UD | GE | 36 | No | Not performed | CHT | TCC | N/A | N/A |
| Corral et al. [19] | J Urol. 1993 | 54/M | pT3aNo | IC | UD | GE | 12 | No | PR | Preoperative and postoperative CHT | Poorly differentiated TCC with transmural extension into serosal adipose tissue with marked angiolymphatic invasion. | No | 54 |
| Carter et al. [20] | Eur Urol. 1996 | 67/M | PT3. No Mo G2 | IC | UD | GE, UTI, PC | 24 | No | TR + new conduit fashioned away from the original stoma | CHT | TCC invading the full thickness of the bowel wall to the surrounding retroperitoneal | Yes | N/A |
| Inobe T et al. [21] | Int J Urol. 1999 | 66/M | pT3bG3pN0 | IC | UD | No symptoms | 4 | No | Not performed | Palliative RT | TCC | Yes | 7 |
| Sanchez Zalabardo et al. [22] | Actas Urol Esp. 2001 | 57/M | pT3aN0M0G3 | OIN | UD | N/A | 108 | Yes | PR | CHT and RT | TCC | No | 3 |
| Shioji et al. [23] | Urol Nephrol. 2001 | 67/M | pT3bN2M0 | IC | UIA + UD | GE | 11 | Yes | PR + resection of the ureteroileal junction | None performed | TCCG2 | Yes | 19 |
| Hara et al.[24] | Urology. 2003 | 67/M | Invasive bladder cancer | SCN | UIA + UD | N/A | 96 | Yes | TR +NUT | None performed | TCC, grade 3 + CIS | N/A | N/A |
| Herawi et al. [25] | Urology. 2006 | 60/M | N/A | OIN | UD | PC | 3 | Yes | Biopsies of the ileocolonic neobladder | None performed | TCC noninvasive low- grade | N/A | N/A |
| Ide et al. [26] | Urology. 2007 | 73/M | Grade 3, Stage pT1 | OIN | UIA | PC | 144 | Yes | TR+ NUT+ conversion to ileal conduit + urethrectomy | None performed | TCC pT2, grade 3. CIS, | No | 6 |
| Moore et al. [27] | Urology. 2007 | 62/M | pT3N0M0 | OIN | UD | GE | 12 | No | TR+ conversion to ileal conduit + urethrectomy + mesenteric lymphadenectomy. | CHT | TCC high-grade. Positive metastatic lymph nodes | Yes (multiple metastasis liver, lung, and adrenal gland) | 15 |
| Kotb et al.[28] | Ecancermedicalscience. 2012 | 59/F | pT2N0, with squamoid differentation | OIN | UD | GE | 156 | No | TR+ continent reservoir Urethrectomy | None performed | TCC pT3 | No | 3 |
| Hadzi-Djokic et al. [29] | Vojnosanit Pregl. 2013 | 65/M | pT2G2N0M0 | OIN | UIA | GE | 144 | Yes | PR + NUT | None performed | TCC pT2bG2 | Yes, for laryngeal carcinoma | 12 |
| Yamashita et al. [30] | Int J Urol. 2014 | 74 /M | pT3aN0M0 + CIS | OIN | UD | GE and PC | 72 | Yes | Endoscopic resection | BCG instillation for 8 months | TCC pTa + CIS | N/A | N/A |
| Cakmak et al. [31] | Case Rep Urol. 2014 | 51/M | grade 3, stage pT1N0M0 | OIN | UD | GE | 132 | No | Endoscopic resection | None performed | TCC low-grade | Yes | 18 |
| Kawamoto et al. [32] | Urology Case Reports. 2016 | 61/M | cT2 pTIS,G3N0 | OIN | UIA | PC | 108 | Yes | TR+ conversion to ileal conduit + NUT | CHT | TCC pT2b, grade 3 | No | 20 |
| Cherbanyk et al. [33] | Case Rep Urol. 2016 | 66/M | pT3a pN1 (1/13) cM0, G3, L1 | OIN | UD | GE | 108 | No | Endoscopic resection | CHT | TCC tumor invaded the muscularis propria of the ileal neobladder | N/A | Recurrence in few months in right frontal cerebral mass |
| Groen, et al. [34] | BMJ Case Rep, 2017 | 65/M | cT4aN2M1 (pT0N0Mx after neoadjuvant chemotherapy) | OIN | UIA | N/A | 108 | No | TR+ conversion to ileal conduit | CHT and retroperitoneal lymph one year after urinary diversion | TCC pT2b, N1, grade 3 | No | 24 |
| Doshi et al. [35] | Case Rep Urol. 2019 | 71/M | pT3aN0 + CIS | OIN | UD | PC | 132 | No | TR+ conversion to ileal conduit | CHT (after cystectomy) | TCC HG extending into the surrounding fat | N/A | N/A |
OIN – orthotopic ileal neobladder; SCN – sigmoid colon neobladder; IC – Ileal Conduit; GE – gross hematuria; PC – positive urine cytology; UTI – urinary tract infection; N/A – not available; UIA – ureteroileal anastomosis; UD – recurrence with the urinary diversion, away from both ureteroileal anastomoses; CHT – chemotherapy, RT – radiation therapy; NUT– nephroureterectomy; TR – total resection of the neobladder (with the mesentery); PR – partial resection; HG – high-grade; TCC – transitional cell carcinoma
The most common initial presentation was gross hematuria 16/30 (53.3%), although positive urine cytology, radiographic evidence of recurrence, or urinary tract infection had also been reported. Concomitant or previous upper tract tumors were found in 17 cases (56.7%) [7, 9, 10, 11, 13, 14, 16, 17, 22-26, 29, 30, 32]. Interestingly, 12/30 (40%) occurred in the absence of TCC in the upper urinary tract or at the ureteroileal anastomosis.
TCCUD was described in only one sigmoid colon neobladder [24]. All of the other 29 cases were recurrences in the ileum. Of these, 17 involved ileal conduits (56.7%), and the other 13 cases (43.3%) involved the continent urinary division.
In 10/30 (33.3%) cases a tumor was identified arising in the intestinal mucosa adjacent to the ureteroileal anastomosis [7, 10, 11, 23, 24, 26, 29, 32, 34]. The unique instance of an invasive TCC at the ileal conduit stoma had been described as well [8]. Tumor invasion into the muscularis of the intestinal diversion had been described in nine cases in which depth of tumor invasion was reported. Two CIS of the ileum had been reported [24, 26]. Treatment of the TCCUD was radiation therapy in 1/30 [7], endoscopic resection/biopsy in 4/30 [25, 30, 31, 33], partial resection of the urinary diversion in 12/30 [8, 9, 10, 12, 14, 15, 17, 19, 20, 22, 23, 29] and a complete resection of the urinary diversion in 11/30 [7, 11, 13, 16, 24, 26-28, 32, 34, 35]. Only two cases were not surgically treated and underwent palliation with RT/chemotherapy [18, 21].
Additional therapy to surgery were the instillation of Bacillus Calmette-Guerin (BCG) into the ileal reservoir [30], systemic chemotherapy [9, 15, 18, 19, 20, 27, 32, 33], radiation therapy [21], combination of chemotherapy + radiation therapy [22] or chemotherapy + retroperitoneal lymphadenectomy [34].
Median time of follow-up after recurrence was 14.4 months (IQR 6–19). Only one case of Clavien-Dindo V was described [12].
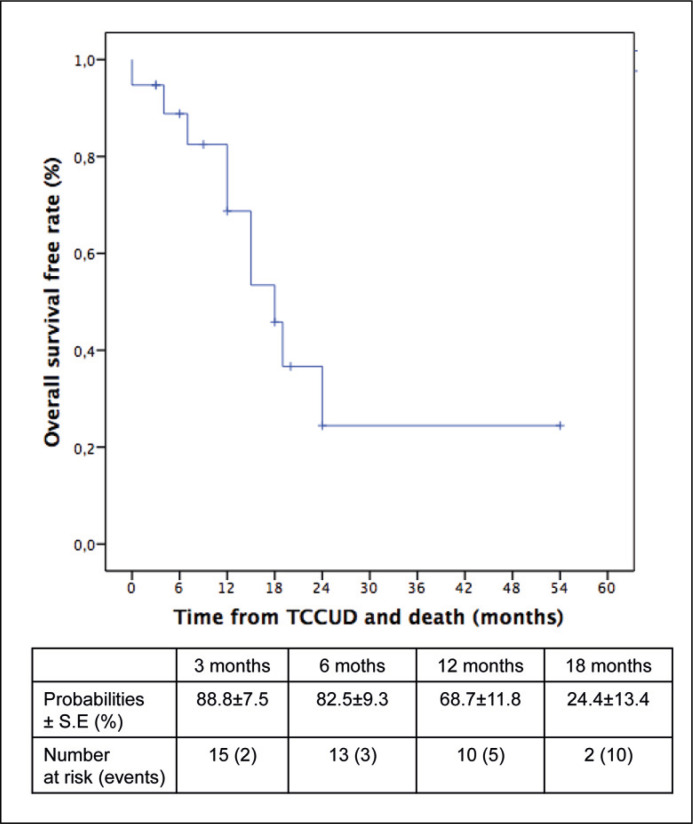
Overall survival-free rates calculated from case reports were 68.7 % and 24.4 %, respectively at 12 and 18 months (Figure 3).
Figure 3.
Overall survival in patients from the literature with transitional cell carcinoma recurrence impacting intestinal urinary diversion (TCCUD).
DISCUSSION
TCCUD following RC is a rare event. Zincke et al. [36] have recommended periodic surveillance with urine cytology and retrograde ileography following cystectomy which, when combined with urine microscopy to detect hematuria, appears to be sufficient to detect recurrences in the upper urinary tract as well as the urinary diversion. Indeed, as described in the literature and also in the present study, hematuria and positive urine cytology may suggest a TCC recurrence in the urinary diversion.
Several surgical treatment options such as endoscopic fulguration, urinary undiversion, and excision of the proximal ileal conduct have been used in the present series and in the literature.
Furthermore, some patients in the presented case series were offered adjuvant platinum-based chemotherapy. Alternative adjuvant chemotherapy regimens [15, 19, 37] as well as adjuvant radiation therapy [7, 38] have been described in the management of similar cases.
Unfortunately, the survivorship at 18 months is poor in all cases regardless of the surgical treatment or the systemic therapy used. Indeed, comparable results have been found in both the present case series and in the literature with an overall survival around 24%. This could be explained by an increased ability of the carcinoma to metastasize to distant organs like the liver and lung caused by intestinal absorption of urothelial cancer cells. In our series metastases happened in 26.3% of cases.
Interestingly, the staging of TCCUD has been poorly addressed in the literature. While muscular invasion in the bladder or ureter is considered stage T2, we feel that any level of invasion of bowel should be considered stage IV disease, despite the fact that the ileal conduit or neobladder is a part of the urinary tract. In fact, invasion of bowel wall carries a significant risk of systemic diffusion and is strongly associated with concurrent or subsequent systemic diffusion.
Wajsman et al. [37], who described a tumor arising at the ureteroileal junction, proposed that such a tumor might arise from one of two mechanisms; first, from a residual tumor left unidentified on the distal ureter that could then invade ileal mucosa by direct extension; or second, implantation of exfoliated tumor cells in the ileal mucosa at the time of surgery. The authors concluded that the second possibility was more likely as their surgical margins at the time of initial cystoprostatectomy were negative. Another possibility would be the development of a new TCC not associated with the original tumor and arising in urothelium at the anastomotic site, as has been proposed by Rosvanis et al. [16]. Others have hypothesized that a discrete TCC lesion arising from ileal mucosa in the setting of recurrent, synchronous tumors in the ureter or renal pelvis, was due to ileal seeding of tumor cells from the upper tract [7, 13, 16, 39, 40]. TCC in the ileum not adjacent to urothelium and in the absence of a tumor in the upper tract is more difficult to explain. A plausible mechanism appears to be spillage of tumor cells at the time of cystectomy, however, tumor cell seeding of the urinary diversion from an unrecognized upper tract CIS may also be a possible mechanism. A solitary ileal tumor may also represent a hematogenous metastasis from the primary bladder tumor. Molecular genetic studies [41, 42, 43] support the theory that multifocal TCC in the urinary tract arises from a single transformed cell that, following clonal expansion, is able to ‘seed’ other areas of the urinary tract. A similar study to establish clonality between a ureteral tumor and an ileal tumor was inconclusive [23]. A final possibility to consider is malignant transformation of metaplastic intestinal epithelium, although histological analysis of intestinal urinary conduits in place up to 10 years showed no evidence of epithelial metaplasia [44]. If some of these plausible mechanisms may facilitate the presence of TCCUD, its uncommonness may be justified by an intrinsic mechanism of resistance of the ileum such as:
the presence of two different types of tissue (transitional cells vs epithelial cells);
the mechanical properties of intraluminal bolus progression due to its intrinsic contraction;
the physiologic presence of intestinal intraluminal mucus; it may trap the TCC and facilitate their expulsion;
cell adhesion and proliferation may be inhibited by an intraluminal adverse pH and osmolality; the ileal tissue still possesses absorption capabilities with fluid and ion exchange with the intraluminal environment;
Resistance to TCCUD can be attributed to immunological factors with antitumor properties. Tumor-infiltrating innate and adaptive immune cells play a key role in cancer blockage; immune cells and an immunosuppressive microenvironment can recognize and kill recently transformed malignant cells. In this context, mucosa-associated lymphoid tissue may enhance and improve antitumor effector cells. A better understanding of how the bowel microenvironment shapes the immune response to TCC is, however, needed to identify mechanisms of resistance and a possible genetic predisposition to guide the development of novel therapeutics.
Almost 56.2% of the patients in our cohort received BCG before cystectomy with concomitant CIS at the time of cystectomy. Clearly, CIS and progression of T1 (failure of BCG), seems to be a significant risk factor of upper tract recurrence, and local conduit recurrence. Similarly, almost 47.3% of the cohort had a history of upper tract TCC, mainly from the terminal ureters.
From our study and from the literature review, we may summarize that TCCUD following RC occurs in half of the patients with upper tract tumors, supporting the idea of extension or seeding of this tumor.
Since the seeding of exfoliated tumor cells at the time of cystectomy may explain all of the other cases, this may suggest the importance to have a clean surgical field and to exchange surgical instruments from the excision phase to the reconstruction phase.
Our study is not devoid of limitations. First of all, the absence of a control group prevented us the design of comparative analysis and the ability to find risk factors. Moreover, the small number of patients is certainly a limitation, however, we described the biggest case series in the literature with such a site of recurrence.
Due to the retrospective nature of the study, certain biases may have been present in the analysis. Examples of that are the changes in treatments and imaging technologies, the presence of different operating surgeons involved in the cystectomy, and the differences in follow-up schedule. All of these variables could not be properly controlled for and may have impacted the outcomes. Finally, survival analysis calculated with data from the literature may have all of the bias of retrospective studies for patients treated in different time periods, in most cases lacking data on long-term survival.
CONCLUSIONS
TCCUD following RC is a rare phenomenon that necessitates prompt diagnosis and aggressive multimodal management due to the high probability of recurrence and death. The possibility of recurrence within a conduit should be considered in any patient who presents with hematuria following a resection for malignant disease. In our study, the most common site of recurrence was at the uretero-ileal anastomosis supporting the idea that TCCUD are not de novo tumors in a diversion. However, since a clear mechanism explaining such recurrences is not known, particular attention should be paid during surgery to avoid spillage of TCC cells, as well as in patients with a history of upper tract tumors.
CONFLICT OF INTEREST
All authors declare that have no conflict of interest.
ACKNOWLEDGMENTS
Author contributions: Fabio Zattoni had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Study concept and design: Fabio Zattoni, Jeffey R. Karnes
Acquisition of data: Alessandro Morlacco, Fabio Zattoni, Paolo Beltrami, Giovanni Motterle
Drafting of the manuscript: Fabio Zattoni, Iliana Bednarova
Analysis and interpretation of data: Fabio Zattoni, Iliana Bednarova, Fabrizio Dal Moro
Statistical analysis: Fabio Zattoni, Alessandro Morlacco
Critical revision of the manuscript for important intellectual content: Iliana Bednarova, Fabrizio Dal Moro
Administrative, technical, or material support: Fabrizio Dal Moro
Supervision: Jeffey R Karnes, Fabizio Dal Moro
Language editing: Iliana Bednarova
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
INFORMED CONSENT
Informed consent was obtained from all individuals included in the study
References
- 1.Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486–494. doi: 10.1016/j.eururo.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 2.Soukup V, Babjuk M, Bellmunt J, et al. Follow-up after surgical treatment of bladder cancer: a critical analysis of the literature. Eur Urol. 2012;62:290–302. doi: 10.1016/j.eururo.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Ghoneim MA, Abdel-Latif M, el-Mekresh M, et al. Radical cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5 years later. J Urol. 2008;180:121–127. doi: 10.1016/j.juro.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Donat SM. Staged based directed surveillance of invasive bladder cancer following radical cystectomy: valuable and effective? World J Urol. 2006;24:557–564. doi: 10.1007/s00345-006-0117-8. [DOI] [PubMed] [Google Scholar]
- 5.Vrooman OP, Witjes JA. Follow-up of patients after curative bladder cancer treatment: guidelines vs. practice. Curr Opin Urol. 2010;20:437–442. doi: 10.1097/MOU.0b013e32833cf10e. [DOI] [PubMed] [Google Scholar]
- 6.Cagiannos I, Morash C. Surveillance strategies after definitive therapy of invasive bladder cancer. Can Urol Assoc J. 2009;3:S237–242. doi: 10.5489/cuaj.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soloway MS, Myers GH, Jr, Burdick JF, et al. Ileal conduit exfoliative cytology in the diagnosis of recurrent cancer. J Urol. 1972;107:835–839. doi: 10.1016/s0022-5347(17)61154-6. [DOI] [PubMed] [Google Scholar]
- 8.Grabstald H. Carcinoma of ileal bladder stoma. J Urol. 1974;112:332–334. doi: 10.1016/s0022-5347(17)59722-0. [DOI] [PubMed] [Google Scholar]
- 9.Wajsman Z, Baumgartner G, Merrin C. Transitional cell carcinoma of ileal loop following cystectomy. Urology. 1975;5:255–256. doi: 10.1016/0090-4295(75)90025-4. [DOI] [PubMed] [Google Scholar]
- 10.Banigo OG, Waisman J, Kaufman JJ. Papillary (transitional) carcinoma in an ileal conduit. J Urol. 1975;114:626–627. doi: 10.1016/s0022-5347(17)67103-9. [DOI] [PubMed] [Google Scholar]
- 11.Allan DM. Recurrent transitional cell carcinoma complicating ileal conduit. Br J Urol. 1976;48:60. doi: 10.1111/j.1464-410x.1976.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 12.Rubin BE, Rodriguez E, Mangasarian R, et al. Recurrent transitional cell carcinoma in an ileal conduit. Urol Radiol. 1979;1:61–62. doi: 10.1007/BF02926601. [DOI] [PubMed] [Google Scholar]
- 13.Curran FT, Fuggle WJ. Transitional cell carcinoma in an ileal conduit. Postgrad Med J. 1986;62:769–771. doi: 10.1136/pgmj.62.730.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moskovitz B, Levin DR. Recurrent transitional cell carcinoma in an ileal conduit. Urol Int. 1986;41:225–227. doi: 10.1159/000281204. [DOI] [PubMed] [Google Scholar]
- 15.Roberts SD, Williams HJ, Resnick MI. Metastatic transitional cell carcinoma in an ileal conduit following cystectomy. J Urol. 1987;137:734–735. doi: 10.1016/s0022-5347(17)44194-2. [DOI] [PubMed] [Google Scholar]
- 16.Rosvanis TK, Rohner TJ, Abt AB. Transitional cell carcinoma in an ileal conduit. Cancer. 1989;63:1233–1236. doi: 10.1002/1097-0142(19890315)63:6<1233::aid-cncr2820630633>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Mulholland C, McCallion WA, Biggart JD, et al. Transitional cell carcinoma in an ileal conduit. Br J Urol. 1993;71:618–619. doi: 10.1111/j.1464-410x.1993.tb16044.x. [DOI] [PubMed] [Google Scholar]
- 18.Garcia D, Porcel JM, Castro-Salomo A, et al. Transitional cell carcinoma in a Camey enterocystoplasty. Br J Urol. 1993;71:491. doi: 10.1111/j.1464-410x.1993.tb16006.x. [DOI] [PubMed] [Google Scholar]
- 19.Corral DA, Bahnson RR. Recurrent transitional cell carcinoma in an ileal conduit treated by sandwich chemotherapy and surgical resection. J Urol. 1993;150:471–472. doi: 10.1016/s0022-5347(17)35518-0. [DOI] [PubMed] [Google Scholar]
- 20.Carter A, Gillatt DA. Recurrent transitional cell carcinoma arising within an ileal conduit following cystectomy. A case report and review of the literature. Eur Urol. 1996;30:519–520. doi: 10.1159/000474227. [DOI] [PubMed] [Google Scholar]
- 21.Inobe T, Kanda K, Murakami Y, et al. Recurrent bladder adenocarcinoma in an ileal conduit stoma: a case report. Int J Urol. 1999;6:467–470. doi: 10.1046/j.1442-2042.1999.00094.x. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez Zalabardo D, Lopez Ferrandis J, Arocena Garcia-Tapia J, et al. Recurrent urothelial tumor in orthotopic neobladder. Actas Urol Esp. 2001;25:600–602. doi: 10.1016/s0210-4806(01)72681-2. [DOI] [PubMed] [Google Scholar]
- 23.Shioji Y, Morita T, Tokue A. Transitional cell carcinoma in the ileal conduit following radical cystectomy and nephroureterectomy. Scand J Urol Nephrol. 2001;35:416–417. doi: 10.1080/003655901753224495. [DOI] [PubMed] [Google Scholar]
- 24.Hara I, Hara S, Miyake H, et al. Carcinoma in situ spread to mucosa of sigmoid colon neobladder. Urology. 2003;62:145. doi: 10.1016/s0090-4295(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 25.Herawi M, Leppert JT, Thomas GV, et al. Implants of noninvasive papillary urothelial carcinoma in peritoneum and ileocolonic neobladder: support for ‘seed and soil’ hypothesis of bladder recurrence. Urology. 2006;67:746–750. doi: 10.1016/j.urology.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Ide H, Kikuchi E, Shinoda K, et al. Carcinoma in situ developing in an ileal neobladder. Urology. 2007;69:576.e9–11. doi: 10.1016/j.urology.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 27.Moore CD, Iczkowski KA, Blue KM, et al. Urothelial carcinoma recurrence in ileal orthotopic neobladder: urethrectomy and creation of ileal conduit. Urology. 2007;184:e11–13. doi: 10.1016/j.urology.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Kotb A, Alkosiry M, Abdelkawy N, et al. Late recurrent urothelial carcinoma in the Studer neobladder: conversion to continent reservoir. Ecancermedicalscience. 2012;6:268. doi: 10.3332/ecancer.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadzi-Djokic J, Pejcic T, Andrejevic V, et al. Transitional cell carcinoma in orthotopic ileal neobladder 12 years after radical cystectomy. Vojnosanit Pregl. 2013;70:1062–1064. doi: 10.2298/vsp1311062h. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita R, Matsuzaki M, Niwakawa M, et al. Bacillus Calmette-Guerin treatment of urothelial carcinoma arising in the ileal neobladder after radical cystectomy. Int J Urol. 2014;21:333–334. doi: 10.1111/iju.12268. [DOI] [PubMed] [Google Scholar]
- 31.Cakmak O, Tarhan H, Celik O, et al. Transitional cell carcinoma in orthotopic ileal neobladder. Case Rep Urol. 2014;2014:218615. doi: 10.1155/2014/218615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamoto B, Honda M, Iwamoto H, et al. Urothelial Carcinoma Recurrence at an Ileal Orthotopic Neobladder and Unilateral Lower Ureter After Surgery. Urol Case Rep. 2016;9:27–29. doi: 10.1016/j.eucr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherbanyk F, Prod'homme M, Pezzetta E, et al. Urothelial Carcinoma Recurrence in an Ileal Neobladder Nine Years after Primary Surgery with Muir-Torre Syndrome. Case Rep Urol. 2016;2016:4762514. doi: 10.1155/2016/4762514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groen VH, Lock T, Keizer B, et al. Urothelial carcinoma in an orthotopic neobladder: an unusual pattern of recurrence and metastasis. BMJ Case Rep. 2017:2017. doi: 10.1136/bcr-2017-221052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doshi CP, Barkan GA, Quek ML. Urothelial Carcinoma Recurrence in an Orthotopic Neobladder without Urethral or Upper Urinary Tract Involvement. Case Rep Urol. 2019;2019:8458706. doi: 10.1155/2019/8458706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zincke H, Garbeff PJ, Beahrs JR. Upper urinary tract transitional cell cancer after radical cystectomy for bladder cancer. J Urol. 1984;131:50–52. doi: 10.1016/s0022-5347(17)50195-0. [DOI] [PubMed] [Google Scholar]
- 37.Wajsman GBaCM. Transitional cell carcinoma of ileal loop following cystectomy. Urology. 1975;5:61–62. doi: 10.1016/0090-4295(75)90025-4. [DOI] [PubMed] [Google Scholar]
- 38.Tsujimura A, Miki T, Takayama H, et al. Recurrence of transitional cell carcinoma in bilateral upper urinary tracts and ileal conduit with invasion in the abdominal wall around nephrostomy after total cystectomy: a case report. Hinyokika Kiyo. 1995;41:383–386. [PubMed] [Google Scholar]
- 39.Hashimoto J, Takashi M, Kinjo T, et al. Recurrence of transitional cell carcinoma in the left pelvis and ureter, and ileal conduit after total cystectomy: a case report. Hinyokika Kiyo. 1987;33:1450–1454. [PubMed] [Google Scholar]
- 40.Zalabardo DS, Ferrandis JL, Arocena García-Tapia J, Sanz Pérez G, Zudaire Bergera J, Berían Polo JM. Recurrent urothelial tumor in orthotopic neobladder. Actas Urol Espanola. 2001;25:332–334. doi: 10.1016/s0210-4806(01)72681-2. [DOI] [PubMed] [Google Scholar]
- 41.Habuchi T. Origin of multifocal carcinomas of the bladder and upper urinary tract: molecular analysis and clinical implications. Int J Urol. 2005;12:709–716. doi: 10.1111/j.1442-2042.2005.01155.x. [DOI] [PubMed] [Google Scholar]
- 42.Pycha A, Mian C, Hofbauer J, et al. Multifocality of transitional cell carcinoma results from genetic instability of entire transitional epithelium. Urology. 1999;53:92–97. doi: 10.1016/s0090-4295(98)00461-0. [DOI] [PubMed] [Google Scholar]
- 43.Sidransky D, Frost P, Von Eschenbach A, et al. Clonal origin bladder cancer. N Engl J Med. 1992;326:737–740. doi: 10.1056/NEJM199203123261104. [DOI] [PubMed] [Google Scholar]
- 44.Garner JW, Goldstein AM, Cosgrove MD. Histologic appearance of the intestinal urinary conduit. J Urol. 1975;114:854–857. doi: 10.1016/s0022-5347(17)67159-3. [DOI] [PubMed] [Google Scholar]