Abstract
Background
Coronavirus disease 2019 (COVID-19) is an infection with possible serious consequences. The plasma of recovered patients might serve as treatment, which we aim to assess in the form of a prospective meta-analysis focusing on mortality, multi-organ failure, duration of intensive care unit stay, and adverse events.
Methods
A systematic search was conducted to find relevant registered randomized controlled trials in five trial registries.
A comprehensive search will be done continuously on a monthly basis in MEDLINE (via PubMed), Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science to find the results of previously registered randomized controlled trials. The selection will be done by two independent authors. Data extraction will be carried out by two other independent reviewers. Disagreements will be resolved by a third investigator.
An update of the search of the registries and the first search of the databases will be done on the 21st of July.
Data synthesis will be performed following the recommendations of the Cochrane Collaboration. In the case of dichotomous outcomes (mortality and organ failure), we will calculate pooled risk ratios with a 95% confidence interval (CI) from two-by-two tables (treatment Y/N, outcome Y/N). Data from models with multivariate adjustment (hazard ratios, odds ratio, risk ratio) will be preferred for the analysis. P less than 0.05 will be considered statistically significant. In the case of ICU stay, weighted mean difference with a 95% confidence interval will be calculated. Heterogeneity will be tested with I2, and χ2 tests. Meta-analysis will be performed if at least 3 studies report on the same outcome and population.
Discussion
Convalescent plasma therapy is a considerable alternative in COVID-19, which we aim to investigate in a prospective meta-analysis.
Keywords: COVID-19, SARS-COV-2, nCOV-2019, Convalescent plasma, Prospective meta-analysis
Background
There is currently an outbreak of respiratory disease caused by a novel coronavirus. The virus has been named “SARS-CoV-2,” and the disease it causes has been named “coronavirus disease 2019” (COVID-19). The outbreak has affected almost every country of the world, and as of 5 July 2020, a total of 11,046,917 confirmed cases and 526,465 deaths had been reported (www.who.int). Recently, two papers have been published reporting on efficient and safe vaccines, [1, 2] and several others are under investigation and approval in phase III. Since widespread vaccination takes time, alternative treatments are still needed in the early stage of the disaese. International randomized, controlled trials investigating the effect of treatments in patients hospitalized with COVID-19 have been launched (recovery and solidarity). The RECOVERY trial so far demonstrated the efficacy of dexamethasone in patients receiving either invasive mechanical ventilation or oxygen alone [3]. Among the treatment strategies under investigation is the administration of convalescent plasma collected from individuals who have recovered from COVID-19 [4–7]. Use of convalescent plasma was studied in outbreaks of other respiratory infections, including the 2003 SARS-CoV-1 epidemic, the 2009–2010 H1N1 influenza virus pandemic, and the 2012 MERS-CoV epidemic [8–10]. The effectiveness of convalescent plasma was highlighted in these studies, and none of the studies demonstrated adverse events. Therefore, it is essential to study the safety and efficacy of COVID19 convalescent plasma in clinical trials. Multiple published and unpublished studies have now reported on the use of convalescent plasma to treat severely or critically ill COVID-19 patients, without unexpected or severe adverse events. In the sole randomized controlled trial reported to date, critically ill but not intubated patients, receiving convalescent plasma showed more frequent and faster clinical improvement compared to controls. However, the trial was terminated early due to a lack of eligible patients at the study sites in China [11]. The results from this RCT, and many other systematic works, which were not conducted in a prospective manner support the concept that convalescent plasma should be used before COVID-19 is life-threatening to clear the virus more rapidly and avoid further tissue damage, rather than using this approach to treat patients with inflammatory end-stage organ failure [12–14]. Although none of these works aimed to include only randomized controlled trials in a prospective manner, but they provide quantitative synthesis of the available data. Multiple ongoing clinical trials are investigating the use of convalescent plasma in patients with less severe infection, or prophylactically in highly susceptible individuals, such as exposed health care workers or family caregivers of COVID-19 patients, situations predicted to result in more potential benefit from passive antibody transfer.
COVID-19 is a newly emerging disease, and there is not much evidence on its treatment. Thus, applying a prospective approach of a comprehensive evaluation of novel therapies is desirable, which can be achieved by the use of prospective meta-analysis (PMA). As the question requires sufficient statistical power, PMA also proves to be beneficial. Furthermore, as the hypotheses, selection criteria, and intended analyses are stated before the availability of the results of the actual randomized trials, it overcomes the limitations of traditional, retrospective meta-analyses [15].
We aim to assess the efficacy of convalescent plasma treatment of COVID-19 patients for the outcomes of mortality, multi-organ failure, and duration of intensive care unit (ICU) stay in a prospective meta-analysis.
Methods
The protocol is based on the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) statement [16]. Throughout the review process, the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions will be followed [17].
This protocol has been registered at PROSPERO International prospective register of systematic reviews in advance under the number of CRD42020197442.(https://www.crd.york.ac.uk/prospero/).
Systematic search and selection of trial registries
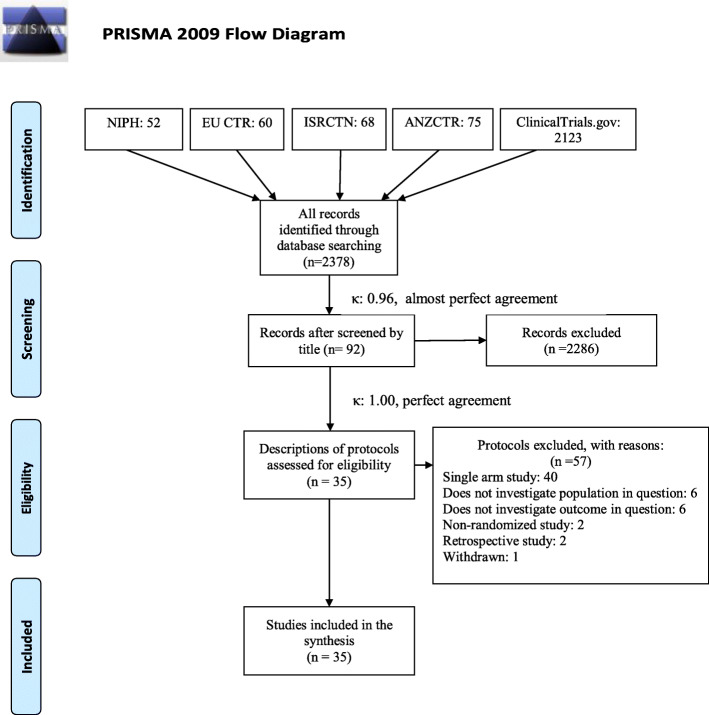
A systematic search was carried out on 15 June 2020 with the following search key: COVID 19 OR “SARS-CoV2” OR “2019-nCoV” in the ClinicalTrials.gov, EU Clinical Trial Register, International Standard Randomised Controlled Trial Number (ISRCTN) registry, Australia and New Zealand Clinical Trial Registry (ANZCTR), and NIPH Clinical Trials Search registry to find eligible, registered randomized controlled trials. Two independent review authors performed the selection first based on the title, then based on the full protocol individually. In the case of disagreements, a third investigator was involved. A trial protocol proved to be eligible by title if it contained the term “plasma” in the context of intervention. A protocol was included in the level of full-text selection if it was a two-arm, randomized trial reporting on at least one of the populations and outcomes in question. All included patients should be PCR-confirmed COVID-19 cases, which will be divided into four subpopulations: P1: respiratory involvement (hypoxia, pneumonia, acute respiratory distress syndrome, requirement of oxygenation or ventilation); P2: patients admitted to the intensive care unit or are critically ill; P3: hospitalized patients without restriction on the severity, including mild, moderate, severe, and critically ill patients; P4: severe condition, defined as following the most recent World Health Organization (WHO) classification; intervention (I): convalescent plasma; control (C): placebo or any other active control; outcomes (O): mortality at any points of time after baseline, intensive care unit stay, multi-organ failure, adverse events. To quantify the level of agreement, Cohen’s kappa of both stages of the selection was calculated. The selection process and Cohen’s kappa results are presented in Fig. 1. Details of the included protocols are shown in Table 1. Reasons for exclusion on the level of full-text protocol are presented in Table 2.
Fig. 1.
Prisma flowchart of the selection process and the results of Cohen’s kappa
Table 1.
Basic characteristics of identified trial protocols
| Registration number | Anticipated completion | Sample size | Plasma therapy details (dose, titer, etc.) | Comparison(s) | Outcomes | ||
|---|---|---|---|---|---|---|---|
| Mortality | MOF | ICU stay | |||||
| Adult COVID-19 patients with respiratory involvement (mechanical ventilation, pneumonia, ARDS, etc.) | |||||||
| NCT04405310 | July 2020 | 80 | 250 mL of CP | Placebo | + | + | + |
| NCT04421404 | April 2021 | 30 | 250 mL of CP | Non-immune control plasma | + | − | − |
| NCT04415086 | May 2022 | 120 | 400 mL (300–600 mL) CP + SMT | 200 mL (150–300 mL) CP + SMT | + | − | − |
| SMT | |||||||
| NCT04418518 | December 2021 | 1200 | 500 mL of CP | No-CP | + | − | + |
| NCT04397757 | November 2020 | 80 | 2 units of CP | SMT | + | + | − |
| NCT04374487 | May 2021 | 100 | 200 mL of CP | SMT | + | + | − |
| NCT04428021 | December 2021 | 180 | 3 units of CP on day 1,3,5 + SMT | 3 units of standard plasma + SMT | + | + | + |
| SMT | |||||||
| NCT04356534 | June 2020 | 40 | 400 mL of CP given as 200 mL over 2 h in 2 consecutive days + SMT | SMT | + | − | + |
| NCT04393727 | October 2020 | 126 | 200 mL of CP | SMT | + | − | + |
| NCT04358783 | May 2021 | 30 | 200 mL of CP | SMT | + | − | − |
| NCT04340050 | November 2020 | 80 | 2 units of CP | SMT | + | + | − |
| NCT04385199 | August 2020 | 30 | 200 mL of CP transfusion over 3 h | SMT | − | − | + |
| NCT04385186 | December 2020 | 60 | 2 × 200 mL of CP | SMT | + | + | + |
| NCT04380935 | August 2020 | 60 | CP + SMT | SMT | + | + | + |
| Adult COVID-19 patients admitted to the intensive care unit | |||||||
| NCT04391101 | December 2021 | 231 | 2 units of CP (between 400 and 500 mL) | SMT | + | − | − |
| NCT04342182 | July 2020 | 426 | 300 mL of CP | SMT | + | − | − |
| NCT04347681 | April 2021 | 40 | 10–15 mL/kg CP daily, up to 5 days | No-plasma | + | − | + |
| NCT04381858 | September 2020 | 500 | 400 mL (2 units) of CP | Human Ig | + | + | + |
| Adult, severe COVID-19 patients | |||||||
| NCT04385043 | May 2021 | 400 | CP + SMT | SMT | + | − | − |
| NCT04403477 | October 2020 | 20 | 400 mL of CP + SMT | 200 mL of CP + SMT | + | + | + |
| SMT | |||||||
| NCT04364737 | April 2023 | 300 | 250-500 mL of CP | Placebo | + | − | + |
| NCT04346446 | May 2020 | 29 | CP + SMT | Placebo + SMT | + | + | + |
| NCT04425915 | May 2021 | 400 | 2 units of CP for 3 days + SMT | SMT | + | − | + |
| NCT04359810 | April 2021 | 105 | 200–250 mL of CP | Standard plasma | + | − | − |
| Adult, hospitalized COVID-19 patients | |||||||
| NCT04425837 | February 2021 | 236 | 400 mL of CP + SMT | SMT | + | + | + |
| NCT04345991 | June 2020 | 120 | 2 units of CP | SMT | + | − | − |
| NCT04388410 | December 2020 | 250 | 2 units of CP | Placebo | + | − | − |
| NCT04366245 | December 2021 | 72 | CP | SMT | + | + | − |
| NCT04348656 | December 2020 | 1200 | 500 mL of CP | SMT | + | − | + |
| NCT04345523 | July 2020 | 278 | CP | SMT | + | − | − |
| NCT04392414 | September 2020 | 60 | 2 units of CP in 24 h | 2 units of standard plasma in 24 h | + | − | + |
| NCT04383535 | September 2020 | 333 | 10–15 mL/kg of CP (5–10 mL/h infusion rate) | Placebo + SMT | + | + | + |
| NCT04377568 | May 2022 | 100 | 10 mL/kg (max 500 mL) of CP + SMT | SMT | + | + | + |
| NCT04395170 | June 2021 | 75 | 2 units of CP in 3 days | Anti-COVID-19 human Ig | + | − | + |
| SMT | |||||||
| NCT04344535 | August 2021 | 500 | 450–550 mL of CP | 450–550 mL of standard plasma | + | − | − |
MOF multiorgan failure, ICU intensive care unit, COVID-19 coronavirus disease 2019, ARDS acute respiratory distress syndrome, CP convalescent plasma, SMT standard medical therapy, Ig immunoglobulin
Table 2.
Excluded protocols and the reason for exclusions
| Protocol number | Reason for exclusion |
|---|---|
| NCT04354831 | Non-randomized study |
| NCT04353206 | Single-arm study |
| NCT04345679 | Single-arm study |
| NCT04372368 | Single-arm study |
| NCT04333355 | Single-arm study |
| NCT04412486 | Single-arm study |
| NCT04355897 | Single-arm study |
| NCT04390178 | Single-arm study |
| NCT04321421 | Single-arm study |
| NCT04343755 | Single-arm study |
| NCT04397523 | Single-arm study |
| NCT04384497 | Single-arm study |
| NCT04388527 | Single-arm study |
| NCT04344015 | Single-arm study |
| NCT04383548 | Single-arm study |
| NCT04420988 | Single-arm study |
| NCT04363034 | Single-arm study |
| NCT04389710 | Single-arm study |
| NCT04338360 | Single-arm study |
| NCT04397900 | Retrospective study |
| NCT04409184 | Retrospective study |
| NCT04360278 | Single-arm study |
| NCT04358211 | Single-arm study |
| NCT04352751 | Single-arm study |
| NCT04408209 | Single-arm study |
| NCT04389944 | Single-arm study |
| NCT04360486 | Single-arm study |
| NCT04348877 | Single-arm study |
| NCT04343261 | Single-arm study |
| NCT04392232 | Single-arm study |
| NCT04384588 | Single-arm study |
| NCT04356482 | Single-arm study |
| NCT04374565 | Single-arm study |
| NCT04384588 | Single-arm study |
| NCT04374565 | Single-arm study |
| NCT04377672 | Single-arm study |
| NCT04332380 | Single-arm study |
| NCT04357106 | Single-arm study |
| NCT04354766 | Non-randomized prospective cohort |
| NCT04327349 | Single-arm study |
| NCT04407208 | Single-arm study |
| NCT04361253 | Children included |
| NCT04365439 | Single-arm study |
| NCT04411602 | Single-arm study |
| NCT04325672 | Single-arm study |
| NCT04373460 | Outpatient care |
| NCT04390503 | Does not investigate population in question |
| NCT04375098 | Does not investigate population in question |
| NCT04374526 | Does not investigate population in question |
| NCT04323800 | Does not investigate population in question |
| NCT04325672 | Withdrawn |
| NCT04361253 | Does not investigate the outcome in question |
| NCT04333251 | Does not investigate the outcome in question |
| NCT04374370 | Does not investigate the outcome in question |
| NCT04365439 | Does not investigate the outcome in question |
| NCT04372979 | Does not investigate the outcome in question |
| NCT04355767 | Does not investigate the outcome in question |
Systematic search and selection of databases
A systematic search will be performed on 21 July 2020 in four scientific databases, MEDLINE (via PubMed), Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science, for randomized controlled trials (RCT). The following query will be used in all databases without any filters or restrictions: ((COVID 19 OR “SARS-CoV2” OR “2019-nCoV”) AND (plasma OR serotherapy OR “passive immun*”)). Reference lists of eligible articles and citing articles (via Google Scholar search engine) will also be screened to capture all relevant studies.
After the automatic and manual removal of duplicates using a reference management software (EndNote X9, Clarivate Analytics), two review authors will independently screen titles, abstracts, and full-texts against predefined eligibility criteria. A third review author will resolve any disagreements at each level of the selection process.
Inclusion criteria specified any RCTs that are reporting on the population and outcomes (mortality, multi-organ failure, duration of intensive care unit stay, adverse events) in question, as stated above. We will exclude non-randomized clinical trials and trials not reporting on the population and outcomes in question. In the case of overlapping study populations and updates, we will include the study with a higher patient number.
Updates on the systematic searches
Regarding the trial registries and scientific databases, we intend to extract all records every month with the same methodology. The first systematic search and update on the trial registries will be on 21 July 2020.
Data extraction
A standardized data extraction form will be developed a priori and will be piloted by the authors performing the data extraction. Two independent reviewers will extract data from all included studies. The following data will be extracted: first author, year of publication, study location, study design, study population, the type and details of interventions received, mean age, sex, number of patients in each group, inclusion criteria, and outcomes. Outcomes will include mortality, multi-organ failure, and intensive care unit stay. Discrepancies will be resolved by consensus and the involvement of a third author. All data will be compiled in an Excel spreadsheet (Office 365, Microsoft, Redmond, WA, USA) for analysis.
Statistical analysis
Data synthesis will be performed using the methods recommended by the working group of the Cochrane Collaboration [11]. Data from models with multivariate adjustment (hazard ratios, odds ratio, risk ratio) will be preferred for the analysis. In the case of dichotomous outcomes (mortality and organ failure), we will calculate pooled risk ratios with a 95% confidence interval from two-by-two tables (treatment Y/N, outcome Y/N), if multivariate results are not available. P less than 0.05 will be considered statistically significant. Statistical analysis will be performed using random effects model. In the case of ICU stay, weighted mean difference with a 95% confidence interval will be calculated. Heterogeneity will be tested with I2, and χ2 tests, p less than 0.1, will indicate significant heterogeneity.
Meta-analysis will be performed using STATA v.16 (StataCorp. 2019, College Station, TX: StataCorp LLC.), Comprehensive Meta-Analysis v.3 (Biostat 2013, Englewood NJ), and R v.4.0.0 (R Core Team 2020, Vienna, Austria) software if at least three studies of same outcomes, assessed at the same point of time after the baseline are available.
A Trial Sequential Analysis (TSA 0.9.5.10.) will also be performed to quantify the statistical reliability and to estimate the optimal information size (OIS), if it is possible. We plan to perform the TSA for every included outcome. In the case of the Z curve, a p value less than 0.05 will be considered significant.
The presence of publication bias will be assessed visually by examining a funnel plot, as well as statistically by using Egger’s regression method if at least 8 studies are available. We will also use the trim and fill method to address this question [18].
If possible, subgroups of treatment modalities (dose and administration of plasma), age (< 18 years, 18–65 year, > 65 years), gender, and comorbidities will be presented. In the case of missing data, the corresponding authors will be contacted, or if individual patient data is available the missing variables will be calculated.
Risk of bias and certainty of the evidence
The quality of all the included studies will be independently assessed by two reviewers using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [19]. Bias will be evaluated in five distinct domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. Within each domain, one or more signaling questions will be answered, which will lead to the judgments of the level of risk of bias: low (low for all domains), some concerns (some concerns in at least two domains), and high (at least one domain or some concerns for multiple domains) risk of bias. The results of the risk of bias assessment will be summarized narratively with full assessments, furthermore, a figure describing the results will be also published. We plan to perform the risk of bias assessment for every included outcome. Any disagreements will be solved by discussion and the involvement of a third reviewer if necessary.
The quality of evidence will be assessed by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. The certainty of evidence will be classified into four levels: high, moderate, low, or very low. Evidence is downgraded by concerns about the risk of bias, imprecision, inconsistency, indirectness, or publication bias. Two independent reviewers will decide the overall quality of the evidence. A third review author will resolve disagreements.
Patient and public involvement
No patients were or will be involved in the design, conduction, or interpretation of our review.
Discussion
Convalescent plasma therapy might be a good alternative to prevent the negative effects of COVID-19, but the clear benefits remain unclear. A prospective meta-analysis from randomized controlled trials can fill this void in terms of mortality, need and duration of intensive care unit stay, and organ failure. Furthermore, this protocol might serve as a basis for the not widely used methodology of prospective meta-analysis. Although prospective meta-analyses might include individual patient data, we do not intend to do so, corresponding authors will be only contacted in the case of missing data.
Acknowledgments
Publication policy
Based on the continuous nature of our work, we plan to publish our results after the statistical analysis is possible for at least one subpopulation and outcome based on the predefined requirements above. We plan to publish our findings in peer-reviewed journals.
Trials status
Not yet started, the first systematic search is scheduled to 21 July.
Protocol amendments
Protocol amendments will be documented in the published article of results.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-COV-2
Severe acute respiratory syndrome coronavirus 2
- nCOV-2019
Novel coronavirus 2019
- CI
Confidence interval
- Y/N
Yes/no
- ICU
Intensive care unit
- PRISMA-P
Preferred Reporting Items for Systematic review and Meta-Analysis Protocols
- SARS-CoV-1
Severe acute respiratory syndrome coronavirus 1
- MERS-CoV
Middle East respiratory syndrome coronavirus
- RCT
Randomized controlled trial
- PMA
Prospective meta-analysis
- ISRCTN
International Standard Randomised Controlled Trial Number
- WHO
World Health Organization
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
Authors’ contributions
Lajos Szakó: conduction of the study, selection of the trial protocols, drafting the manuscript, Nelli Farkas: writing of the statistical analysis plan, drafting the manuscript. Szabolcs Kiss: conduction of the study, resolve of disagreements during the protocol selection process, drafting the manuscript. Szilárd Váncsa: conduction of the study, data extraction from trial protocols, drafting the manuscript. Noémi Zádori: conduction of the study, selection of the trial protocols, drafting the manuscript. Nóra Vörhendi: conduction of the study, data extraction. Bálint Erőss: resolved the disagreements of data extraction, provided methodological supervision, critical revision of the manuscript. Péter Hegyi: provided methodological supervision, critical revision of the manuscript. Hussain Alizadeh: an expert in the field of apheresis and plasma therapy, who supervised the conduction of the study, provided key knowledge and critical revision. The authors read and approved the final manuscript.
Funding
This study was supported by the Economic Development and Innovation Operative Program Grant (GINOP 2.3.2-15-2016-00048) and by the Human Resources Development Operational Program Grant (EFOP-3.6.2-16-2017-00006, EFOP-3.6.2-16-2017-00009), both co-financed by the European Union (European Regional Development Fund) within the framework of Széchenyi 2020 Program. The sponsor or the funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Availability of data and materials
Data is available from the corresponding author upon reasonable request. We plan to publish our results in peer-reviewed journals.
Ethics approval and consent to participate
No ethical approval was required for this protocol, and neither will be necessary to the review, as all data were or will be published in peer-reviewed journals.
Consent for publication
All authors provided critical conceptual input, interpreted the data analysis, and critically revised and approved the final version of the manuscript.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lajos Szakó, Email: szaklaj@gmail.com.
Nelli Farkas, Email: farkas.nelli@gmail.com.
Szabolcs Kiss, Email: kisszabolcs1995@gmail.com.
Szilárd Váncsa, Email: vancsaszilard@gmail.com.
Noémi Zádori, Email: znoeemi@gmail.com.
Nóra Vörhendi, Email: vorinocci@gmail.com.
Bálint Erőss, Email: eross.balint@pte.hu.
Péter Hegyi, Email: hegyi2009@gmail.com.
Hussain Alizadeh, Email: alizadeh.hussain@pte.hu.
References
- 1.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. 2020;383(27):2603-15. [DOI] [PMC free article] [PubMed]
- 3.The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 4.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv. 2020; 2020.03.16.20036145.
- 6.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roback JD, Guarner J. Convalescent Plasma to Treat COVID-19: Possibilities and Challenges. JAMA. 2020;323(16):1561-62. [DOI] [PubMed]
- 8.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leider JP, Brunker PA, Ness PM. Convalescent transfusion for pandemic influenza: preparing blood banks for a new plasma product? Transfusion. 2010;50(6):1384–1398. doi: 10.1111/j.1537-2995.2010.02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arabi YM, Hajeer AH, Luke T, Raviprakash K, Balkhy H, Johani S, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22(9):1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020. [DOI] [PMC free article] [PubMed]
- 12.Piechotta V, Chai KL, Valk SJ, Doree C, Monsef I, Wood EM, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;7:Cd013600. doi: 10.1002/14651858.CD013600.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun M, Xu Y, He H, Zhang L, Wang X, Qiu Q, Sun C, Guo Y, Qiu S, Ma K. A potentially effective treatment for COVID-19: A systematic review and meta-analysis of convalescent plasma therapy in treating severe infectious disease. Int J Infect Dis. 2020;98:334-46. [DOI] [PMC free article] [PubMed]
- 14.Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol. 2020;92(9):1475-83. [DOI] [PMC free article] [PubMed]
- 15.Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet. 1998;351(9095):47–52. doi: 10.1016/S0140-6736(97)08461-4. [DOI] [PubMed] [Google Scholar]
- 16.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. 2015;349:g7647. [DOI] [PubMed]
- 17.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. 2. Chichester (UK): John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine. 2019;98(23):e15987. [DOI] [PMC free article] [PubMed]
- 19.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available from the corresponding author upon reasonable request. We plan to publish our results in peer-reviewed journals.