Much of virus fate, both in the environment and in physical/chemical treatment, is dependent on electrostatic interactions. Developing an accurate means of predicting virion isoelectric point (pI) would help to understand and anticipate virus fate and transport, especially for viruses that are not readily propagated in the lab.
KEYWORDS: capsid, DNA binding, electrostatic, modeling, polynucleotide, prediction, RNA binding, virion, DNA-binding proteins, RNA-binding proteins, colloid, predictive model, surface charge, virion structure
ABSTRACT
Much of virus fate, both in the environment and in physical/chemical treatment, is dependent on electrostatic interactions. Developing an accurate means of predicting virion isoelectric point (pI) would help to understand and anticipate virus fate and transport, especially for viruses that are not readily propagated in the lab. One simple approach to predicting pI estimates the pH at which the sum of charges from ionizable amino acids in capsid proteins approaches zero. However, predicted pIs based on capsid charges frequently deviate by several pH units from empirically measured pIs. Recently, the discrepancy between empirical and predicted pI was attributed to the electrostatic neutralization of predictable polynucleotide-binding regions (PBRs) of the capsid interior. In this paper, we review models presupposing (i) the influence of the viral polynucleotide on surface charge or (ii) the contribution of only exterior residues to surface charge. We then compare these models to the approach of excluding only PBRs and hypothesize a conceptual electrostatic model that aligns with this approach. The PBR exclusion method outperformed methods based on three-dimensional (3D) structure and accounted for major discrepancies in predicted pIs without adversely affecting pI prediction for a diverse range of viruses. In addition, the PBR exclusion method was determined to be the best available method for predicting virus pI, since (i) PBRs are predicted independently of the impact on pI, (ii) PBR prediction relies on proteome sequences rather than detailed structural models, and (iii) PBR exclusion was successfully demonstrated on a diverse set of viruses. These models apply to nonenveloped viruses only. A similar model for enveloped viruses is complicated by a lack of data on enveloped virus pI, as well as uncertainties regarding the influence of the phospholipid envelope on charge and ion gradients.
INTRODUCTION
Electrostatic forces play a critical role in virus fate and transport in engineered and natural systems. Because the charge of organic particles in aqueous solutions is dependent on the ionic environment, notably the hydrogen ion concentration, it is convenient to determine the virus’ isoelectric point (pI), i.e., the pH at which the virion’s net charge is 0 (neutral), when assessing the probable effects of electrostatic forces. Above their pI, organic macromolecules (such as virions) have a net negative charge due to deprotonated carboxyl groups, while below the pI, protonated amine groups confer a net positive charge. Independent of charge magnitude, knowing even the sign of a viral particle’s charge can inform water treatment, such as coagulation (1, 2), disinfection (especially in conditions of virus aggregation) (3, 4), or membrane filtration (5), as well as modeling virus transport through porous media (6) and virus sampling and concentration (7–10).
Electrostatic forces are not the sole determiner of virus fate and transport; other interactions, such as van der Waals forces, the hydrophobic effect, cation bridging, and steric interactions, also play a prominent role in virus interactions with the surrounding environment (1, 5, 11). In turn, pI is not a perfect indicator of electrostatic forces under all conditions. Electrophoretic mobility away from the pI is highly dependent on environmental conditions, e.g., conductivity (5). The pI cannot indicate whether a given virus may alternate definitively between strong positive and negative surface charges below and above the pI or meander near zero charge over a broad pH range. While electrostatic forces are not a perfect predictor of virus physical/chemical interactions, and pI is not a perfect indicator of electrostatic forces under all conditions, pI provides a reliable and quantitative benchmark for comparing environmental interactions of different viruses across a range of conditions and experimental methods. In addition, focusing on pI allows us to address the greatest disparities between theoretical and empirical results before proposing a more refined model to estimate the magnitudes of surface charge and potential.
Many attempts have been made to model the pI of nonenveloped viruses based on ionizable residues within capsid proteins (12–16). However, major discrepancies arise between predicted pIs based on capsid proteins and empirically determined virus pIs. While empirical pIs are commonly reported in the acidic range (pH 2 to 5) (17), capsid proteome sequences overwhelmingly contain balanced concentrations of amino acids reflecting predicted pIs near neutral (pH 5.5 to 8) (18, 19). Therefore, capsid amino acid composition alone cannot account for virus pI.
Several researchers have proposed electrostatic models of the virion to explain the poor predictive value of ionizable amino acids. Based on a “soft colloid” model proposed by Duval and Ohshima (20), Langlet et al. (21, 22) and Dika et al. (5, 23) suggested that nucleic acids at the core of the virus capsid contribute to overall virus surface charge. Schaldach et al. (16) developed a similar permeable virion model that weighted the influence of capsid moieties based on electrostatic screening of the surrounding medium. Both models suggest that with increasing permeability, buried components of the virion have a greater impact on the overall pI (16, 21, 23). In contrast, Penrod et al. (15) and Armanious et al. (13) suggested that only exterior residues contribute to the surface charge; thus, heterogeneous distribution of positive and negative amino acid charges within the capsid coat results in higher or lower pI values. Božič et al. (14) also evaluated a one- or two-shell model of virion surface charge to account for heterogeneity in ionizable amino acid distribution, though the model was specifically applied to empirical pI values only for bacteriophage PP7 (24). While the debate around fundamental hypotheses can be polarizing, not all elements of these models are contradictory.
Recently, Heffron and Mayer (25) suggested a divergent approach to modeling nonenveloped virion pI based not on a single electrostatic model of the virion but rather on the variable extent of electrostatic interactions between the capsid and the viral genome. Since several of the viruses with the greatest discrepancy between predicted and empirical pIs featured large capsid regions devoted to binding the viral polynucleotide, Heffron and Mayer hypothesized that the charges of these polynucleotide-binding regions (PBRs) and bound sections of the viral polynucleotide itself are mutually neutralized. The authors also predicted the location of PBRs from virus capsid proteome sequences to predict the pI of viruses whose detailed capsid structures were unknown. This approach supported the observations of Šiber and Podgornik (26) that the two-shell model of Božič et al. (14) was appropriate for spontaneously assembling viruses with strong, nonspecific interactions between capsid proteins and single-stranded RNA (ssRNA). However, the PBR exclusion approach showed improvement in pI prediction for double-stranded DNA (dsDNA) viruses as well as ssRNA viruses (25).
The goal of this review was to evaluate the potential of polynucleotide influence and exterior residue theories for developing a model of nonenveloped, icosahedral virus pIs, compared to the newly proposed hypothesis of PBR exclusion. In “Polynucleotide charge contribution,” models suggesting polynucleotide influence are discussed in light of empirical evidence. In “Surface-weighted capsid models,” the theory that external capsid residues contribute disproportionately to overall charge is investigated using three-dimensional (3D) capsid structures for 26 viruses with known (empirical) pIs. In “Polynucleotide-binding regions,” Heffron and Mayer’s approach of excluding PBRs is discussed, as is the model of virion charge structure that arises from the PBR exclusion approach. Finally, the potential of these competing theories for developing a predictive pI model is discussed in “Further considerations for a predictive isoelectric point model,” as are the impediments to applying such a model to enveloped viruses.
POLYNUCLEOTIDE CHARGE CONTRIBUTION
Some researchers (16, 21, 23, 27) have accounted for the differences between theoretical and empirical virion pI by developing models of virions as permeable colloids. A permeable virion implies that interior charges can affect overall virion charge. The main, hypothesized interior charge contribution comes from the densely packaged polynucleotide core. Polynucleotide phosphodiester groups have a pKa near pH 1 and would therefore contribute a negative charge even at very low pH. Below approximately pH 5, this charge would be moderated by positive charges from amino groups on adenine, cytosine, and guanine (28). Still, the overall charge of the polynucleotide would be net negative at pH >1, since all nucleotides have a phosphate group, regardless of base.
However, the polynucleotide folding necessary for virion packaging is mediated by a cloud of counterions that to some degree negates electrostatic repulsion (29–32). While many of these counterions may be released in mature virions (33), the viral core likely retains a relatively high concentration of divalent cations (34). Thus, the presence of an overwhelming negative charge at the virion core is not a foregone conclusion. If the nucleic acid core impacts virion charge, the effect should be empirically demonstrable via (i) comparison of whole virions to virus-like particles (VLPs) and/or (ii) comparison of virion pI in various ionic strength solutions.
VLPs are viral capsids lacking all or most of the internal genome. As shown in Table 1, VLPs have pIs extremely similar to those of the corresponding intact virus, even when the predicted pI differs greatly. In interpreting these results, Dika et al. (23, 35) hypothesized that some negatively charged host material was trapped within the VLPs during propagation or that virus purification by polyethylene glycol (PEG) precipitation may coat the capsid surface and cause insensitivity to charge contributions from the virion core. However, the comparatively empty state of VLPs was confirmed in most experiments by a variety of quality control methods, as listed in Table 1. A demonstrably lower core content would be expected to have some impact on pI. Yet, VLPs had the same pI as whole virions in tests using a variety of protocols for both purification and assessment of polynucleotide content of VLPs. In their study comparing purification protocols, Dika et al. (35) also did not use a solvent extraction phase (e.g., washing with chloroform or Vertrel), which typically follows the concentration phase to remove the PEG and promote monodispersion (1). To accept the hypothesis that the viral polynucleotide influences pI, we should see a distinct increase in the pI of VLPs compared to that of whole virions, which was not observed.
TABLE 1.
Studies comparing pIs of VLPs and whole virionsa
| Virus species | Virus pI | VLP pI | Theoretical VLP pIb | Concn/purification method | VLP method | VLP quality control | Reference |
|---|---|---|---|---|---|---|---|
| Adeno-associated virus 4 | 2.6 | 2.6 | 5.8 | Centrifugation, dialysis | Naturally occurring | Differential sedimentation | Salo and Mayor, 1978 (102) |
| Enterobacteria phage MS2 | 3.9 | 4.0 | 7.4 | Chloroform lysis, centrifugal filtration | In situ degradation | Fluorometric RNA assay | Armanious et al., 2016 (13) |
| Enterobacteria phage MS2 | 3.5 | 3.4–3.8 | 7.4 | Centrifugation, dialysis | Clonal plasmid expression | Electron microscopy | Dika et al., 2011 (23) |
| Enterobacteria phage MS2 | 3.3 | 3.3 | 7.4 | PEG precipitation | In situ degradation | Electron microscopy | Nguyen et al., 2011 (103) |
| Feline panleukopenia virus | 5.0–5.3 | 5.3 | 5.4 | PEG precipitation, ultracentrifugation, dialysis | Unknown | Differential sedimentation | Weichert et al., 1998 (104) |
VLP, virus-like particle (capsid lacking the viral genome).
Theoretical VLP pI calculated here is based on the total charge of ionizable amino acids in capsid proteins.
The influence of the polynucleotide on virion charge should also be evident by tests at various ionic strengths. If capsids are permeable, electrostatic screening due to the electrolyte solution should influence the distance from the exterior surface at which buried charges can influence the surface charge. Since Debye lengths in freshwater are on the order of 10 to 100 Å (36), and virus capsids are typically 20 to 40 Å thick (14), the virion components contributing to surface charge could be expected to vary depending on ionic strength. The impact of electrolyte screening on depth of charge influence was the hypothesis behind the modeling approach used by Schaldach et al. (16), which weights the influence of ionizable amino acids based on depth within the capsid as a function of solution ionic strength, I. Thus, capsid residues are progressively weighted based on their proximity to the exterior capsid surface. For simplicity, the virion is typically modeled as a perfect sphere, with the exterior surface defined as the outer radius, as shown in Fig. 1C. However, this simplification is likely not appropriate for viruses with a high degree of crenulation. The Schaldach et al. model (16) also assumes a negatively charged virion core. Results closely matched electrophoretic mobility measurements of bacteriophage MS2 and norovirus VLPs at an I of 0.01 M and bacteriophage Qβ in solution with an I of 0.1 M. However, the team determined that the absence of polynucleotide influence did not impact the fit of the norovirus model to empirical data using VLPs. Nap et al. (24) used a contrasting model developed by Božič et al. (14) for dividing capsids into inner and outer shells to explain the impact of I on the pI of bacteriophage PP7 as reported by Brorson et al. (8) (see Table 2).
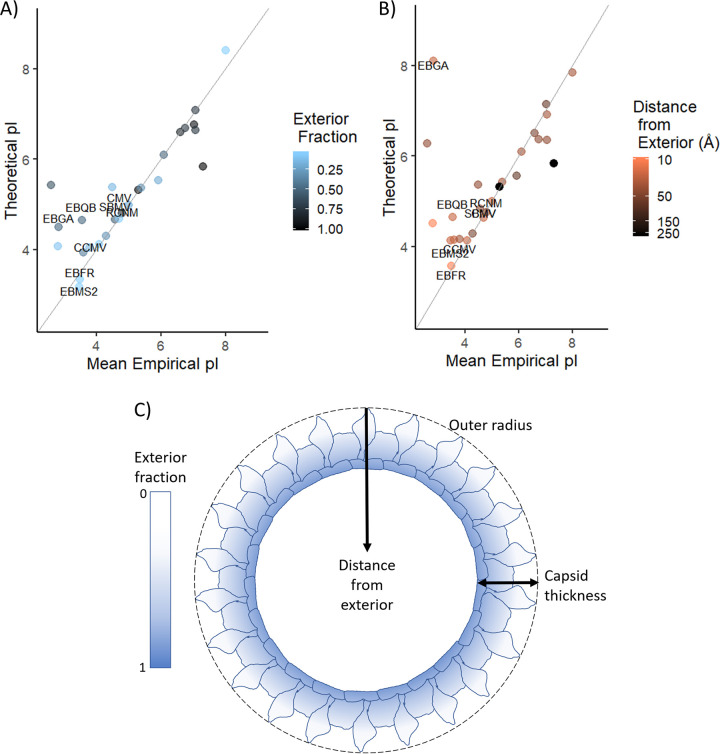
FIG 1.
Impact of including only exterior residues in predicted pI calculation using 3D capsid structures. (A to C) The mean empirical pI value for each unique virus is shown in comparison to the virus’ predicted pI calculated from exterior capsid residues. Exterior residues represent increasingly narrow shells determined by fraction of outermost amino acids (A) and distance from the exterior surface (B), as illustrated in panel C. Distances in panel B are displayed on a log color scale to clearly show the impact on all viruses despite large disparities in capsid size and thickness. In both panels A and B, a lighter tint indicates a narrower “slice” of the capsid, while a darker tint indicates that a larger portion of the capsid was considered in the pI calculation. The diagonal line represents equivalent theoretical and empirical pIs; to accept either method of calculating pI based on exterior residues, points of similar tint should be clustered along this line. Two groups that appeared to benefit most from including only exterior residues are labeled in the figure: ssRNA Leviviridae phages with basic, interior beta sheets (bacteriophages fr [EBFR], GA [EBGA], MS2 [EBMS2], and Qβ [EBQB]) and ssRNA viruses with basic, interior N termini (cowpea chlorotic mosaic virus [CCMV], cucumber mosaic virus [CMV], red clover necrotic mosaic virus [RCNM], and southern bean mosaic virus [SBMV]).
TABLE 2.
Studies comparing pIs at various ionic strengthsa
| Reference and virus | Capsid outer radiusb (nm) (reference) | Measured pI | Ionic strength, I (mM) | Estimated Debye lengthc (nm) | Electrolyte | Methodd |
|---|---|---|---|---|---|---|
| Brorson et al., 2008 (8) | NaCl plus buffer | CF | ||||
| Enterobacteria phage PP7 | 15 | 4.9 | <1 | >9.6 | ||
| 4.7 | 40 | 1.5 | ||||
| 4.3 | 100 | 1.0 | ||||
| Enterobacteria phage PR772 | ∼29 (105) | 4.4 | <1 | >9.6 | ||
| 4.3 | 5 | 4.3 | ||||
| 4.3 | 20 | 2.2 | ||||
| 4.2 | 40 | 1.5 | ||||
| Enterobacteria phage ΦX174 | 17 | 7 | <1 | >9.6 | ||
| 7.3 | 5 | 4.3 | ||||
| 7.5 | 10 | 3.1 | ||||
| 7.8 | 20 | 2.2 | ||||
| Langlet et al., 2008 (22) | NaNO3 | EM | ||||
| Enterobacteria phage GA | 14 | 2.1 | 1 | 9.6 | ||
| 2.3 | 100 | 1.0 | ||||
| Enterobacteria phage MS2 | 14 | 3.1 | 1 | 9.6 | ||
| 3.9 | 100 | 1.0 | ||||
| Enterobacteria phage Qβ | 15 | 2.7 | 1 | 9.6 | ||
| 1.9 | 100 | 1.0 | ||||
| Enterobacteria phage SP | ∼15e | 2.1 | 1 | 9.6 | ||
| 2.6 | 100 | 1.0 | ||||
| Molodkina et al., 1986 (37) | NaCl | EM | ||||
| Influenza A virus H1N1 | ∼50 (106) | 4.5 | 0.2 | 21 | ||
| 4.35 | 0.4 | 15 | ||||
| 4.25 | 2 | 6.8 | ||||
| 4 | 10 | 3.1 | ||||
| Taylor and Bosmann, 1981 (38) | NaCl | EM | ||||
| Mammalian orthoreovirus 3 | 43 | 3.8 | 1 | 9.6 | ||
| 3.8 | 10 | 3.1 | ||||
| 3.8 | 100 | 1.0 |
Summarized from a report by Michen and Graule (17).
Outer radius values were obtained from the ViperDB database (107), except as noted.
Debye length in 1:1 electrolyte approximated by the formula Debye length (nm) ≈ 0.305(I (M))−1/2, as described by Otterstedt and Brandreth (108).
CF, chromatofocusing; EM, electrophoretic mobility.
Approximate radius based on Qβ, which is in the same genus (Allolevivirus) and shares 80% similarity in coat protein amino acid sequence (109).
Many investigators (8, 22, 37, 38) have measured variations in pI over various ionic strengths. Table 2 provides a summary of experiments in which a single researcher evaluated virus pI at multiple ionic strengths. To support the hypothesis that the core polynucleotide contributes significantly to overall capsid charge, measured pI should increase at higher ionic strength. Overall, however, the pIs did not increase uniformly with I to reflect a substantial charge contribution from the core, and changes were not on the scale expected from the difference in pI of capsid proteins (pI ∼ 5.5 to 8) and nucleic acids (pI ∼ 1) (18, 28). Even when I varied by 2 orders of magnitude (Debye lengths from ∼10 nm to 1 nm), these dramatic differences were not seen between pIs. Rather, virus pIs increased, decreased, or remained constant with increasing I, indicating a virus-specific response expected from heterogeneous charge distributions.
The precision of pI measurements decreases with increasing I due to reduction of surface charge from electrostatic shielding (39). Therefore, even the minor variation observed in pIs at low and high I could be explained by this lack of precision. For the empirical pIs referenced in Michen and Graule’s review (17), I ranged from ∼0.5 mM, typical of isoelectric focusing in ampholyte buffers (40), to ≥100 mM in concentrated electrolyte solutions (22, 38, 41–43). In addition, viruses are more likely to aggregate at high ionic strength, while the models discussed here assume monodispersion. Differences in I and electrolyte composition are likely responsible for some variation in reported pIs (17). Nonetheless, there remains broad agreement between empirical pIs where multiple experiments are available, despite widely differing solution compositions and measurement techniques (17).
Overall, empirical evidence does not support the hypothesis that the polynucleotide contributes strongly to net virion charge. This lesser contribution may be due to counterions retained within the capsid (34), resulting in a lesser negative-charge magnitude. The ionic composition of the virion core may play a major role in overall virion charge. However, more research is needed to determine the composition and impact of counterions around the polynucleotide. The main support for polynucleotide charge contribution comes from theoretical models to account for the discrepancy between theoretical and empirical pIs of Leviviridae phages (21–23). However, Leviviridae phages have distinctive thin capsids with large, positively charged interior regions devoted to polynucleotide binding (44–46) and are therefore poor exemplars of virion structure.
SURFACE-WEIGHTED CAPSID MODELS
Some electrostatic models have relied on detailed 3D virus structures to attempt pI prediction, either instead of or in addition to supposing a polynucleotide charge contribution. Presuming an impermeable capsid, Penrod et al. (15) accounted for the measured pI of enterobacteria phage MS2 by evaluating only those charged structures exposed on the surface of the capsid. Armanious et al. (13) also successfully employed this method to account for the pI of MS2 and three other bacteriophages of the family Leviviridae. Many other viruses with acidic pIs feature a concentration of basic amino acids toward the capsid interior. Therefore, the exclusion of interior residues decreases the predicted pI of these viruses and may better approximate some acidic empirical pIs. As further discussed in “Polynucleotide-binding regions,” this concentration of basic residues is not applicable to all viruses. Božič et al. (14) noted that this concentration of basic charges within virus capsids defied any simple pattern or classification based on virus structure. The asymmetrical distribution of capsid charges was instead attributed to nonspecific electrostatic interactions involved in virus self-assembly (26). In addition, experimental evidence contradicts the theory of a capsid that is completely impermeable to electrolytes (16). From a practical perspective, not only is manually selecting exterior-exposed residues labor-intensive, but the definition of “capsid exterior” also begins to blur for thicker capsids with extensive crenulations. Given the subjectivity and tedium of manually selecting individual capsid residues, a method to separate the capsid into exterior (charge-contributing) and interior (noncontributing) shells would be beneficial. Božič et al. (14) suggested that such a model could be applied for some viruses, though many viruses do not show a two-shell charge distribution. The applicability of the two-shell model was later determined to be dependent on interactions between capsid and polynucleotide (47). Thus, Božič et al. did not use the two-shell model to predict empirical pIs for a range of viruses.
Based on a set of 26 viruses with available detailed 3D structures and empirical pI values, we attempted to define an interior and exterior shell based on (i) relative distribution within the capsid and (ii) absolute distance from the capsid surface. (Further details of this analysis can be found in the supplemental material [Section S1, “Methods for figure generation”].) As shown in Fig. 1, inclusion of only exterior residues did not improve pI prediction for all viruses, regardless of how exterior residues were defined. (These data are also presented in detail in Fig. S1 in the supplemental material for the entire range of exterior fractions and distances.) Of the viruses evaluated, two groups appeared to benefit most from including only exterior residues: ssRNA Leviviridae phages with basic, interior beta sheets (bacteriophages fr [EBFR], GA [EBGA], MS2 [EBMS2], and Qβ [EBQB]) and ssRNA viruses with basic, interior N termini (cowpea chlorotic mosaic virus [CCMV], cucumber mosaic virus [CMV], red clover necrotic mosaic virus [RCNM], and southern bean mosaic virus [SBMV]), as labeled in Fig. 1. However, no one method of slicing the capsid was optimal even for these eight viruses. For the remaining viruses, predicted pI was slightly more likely to trend away from the range of empirical pIs when only exterior residues were considered (Fig. S1). Therefore, the strategy of selecting only exterior residues cannot be used indiscriminately to predict unknown pIs.
Schaldach et al. (16) used a more nuanced 3D model, in which electrostatic screening was modeled by inversely weighting residues by distance from the capsid surface as a function of Debye length. By this model, calculating theoretical charge with all capsid residues better matched empirical electrophoretic mobility measurements than using surface residues only. However, the Schaldach model required a theoretical polynucleotide charge to account for the charge of Leviviridae phages MS2 and Qβ, whereas the polynucleotide influence was irrelevant to the norovirus model (16).
Heffron and Mayer (25) determined that removing only the interior surface-exposed residues resulted in the best fit between theoretical and empirical pIs for a diverse set of 21 viruses, whereas models using only the exterior surface residues showed no apparent correlation to overall capsid pI. The interior surface of many viruses is involved in viral polynucleotide binding (48–52). Because capsid surfaces are irregular, identifying and removing interior-accessible residues would most selectively remove structures like the interior beta sheets of Leviviridae and arginine-rich regions in the disordered N termini of many ssRNA plant viruses, two groups that benefitted greatly from excluding interior residues (Fig. 1). As further discussed in “Polynucleotide-binding regions,” these basic, interior capsid features are noncovalently bound to the viral RNA (46, 48, 52, 53), and therefore their positive contribution to virion charge is likely negated by the negatively charged polynucleotide.
Some authors (13) have also excluded from pI calculation residues whose surface area is buried by the folding of the polypeptide. Practically, buried residues may be defined as having a relative solvent-accessible surface area less than 20% that of the corresponding amino acid, based on models with an approximate resolution of 3 Å (13). Using this cutoff did not improve the overall pI prediction for the whole capsid or outer capsid residues (Fig. S2) nor did attempts to weight amino acid influence by solvent-accessible surface area alone or in combination with relative or absolute distance from the exterior (data not shown). Excluding buried residues selects against beta sheets, the least solvent-accessible protein structures (54). Ignoring beta sheets may work well for Leviviridae phages, in which most large beta sheets are involved in RNA binding (46). However, beta sheets are major components of many virus capsids (e.g., “jelly roll” folds) and should not be discounted from charge calculations without strong justification.
POLYNUCLEOTIDE-BINDING REGIONS
PBRs are a feature of many virus capsids. While some viruses (e.g., many dsDNA viruses and picornaviruses) feature genomes that are covalently bound, often to a single, small capsid protein (55–58), many virus polynucleotides are noncovalently bound via electrostatic interactions with residues on the capsid interior (PBRs). The two methods of polynucleotide binding reflect different packaging strategies; dsDNA and dsRNA polynucleotides are typically spooled into a previously formed capsid, while capsids that are formed spontaneously by assembly of subunits require more extensive bonding between the polynucleotide and capsid (34, 44, 59). PBRs may occur in a single region of a capsid protein sequence, as in the disordered, arginine-rich terminal domains in many positive-stranded RNA viruses (e.g., the ssRNA plant viruses mentioned in “Surface-weighted capsid models”) (52, 59–62) or along broader, positively charged regions (“clefts”) that are contiguous on the protein surface but not necessarily continuous in the primary sequence (e.g., negative-stranded ssRNA viruses and Leviviridae phages) (46, 63). In either case, the predominantly basic charges of the interior capsid PBR residues would be countered via this electrostatic interaction with the polynucleotide. The polynucleotide segment and PBR would therefore not contribute to overall virion charge, and these regions should be excluded from theoretical charge calculations.
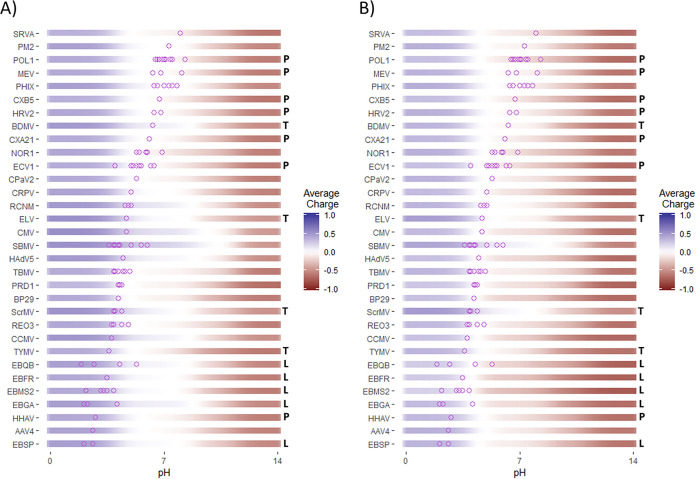
Heffron and Mayer (25) evaluated the effect of excluding predicted PBR regions from capsid charge calculations and reported an overall improvement in accuracy of the modified pI predictions compared to the unmodified predictions, from a deviation of 2.1 ± 2.4 to 0.1 ± 1.7 pH units. This difference was significant to a high degree of confidence (P = 4 × 10−8) (25). (A list of the viruses evaluated in this study is provided in Table 3.) A comparison of capsid charge predictions with and without PBRs is shown in Fig. 2, based on the empirical pIs and predicted PBRs presented by Heffron and Mayer (25). (Section S1 details how this plot was generated.) Compared to the original predictions without modification (Fig. 2A), far more empirical pIs fall within the range of theoretical net-neutral charge after modification (Fig. 2B). Predicting pH ranges of low net charge (as visualized here) may be more valuable than a single, predicted pI, as the virion may function similarly over a pH region around the pI, including in behaviors relied on for empirical pI measurements (e.g., aggregation and electrophoresis). Although Heffron and Mayer did not advance a model for surface charge magnitude, the analysis in Fig. 2 provides a qualitative account of how surface charge varies with pH. Knowing the breadth of these regions is valuable for predicting the effect of pH on phenomena such as aggregation and surface adhesion.
TABLE 3.
Classification and abbreviations for virusesa
| Abbreviation | Species | Genus | Nucleic acid | NCBI taxonb | PDB IDc (reference) | Resolution (Å) |
|---|---|---|---|---|---|---|
| AAV4 | Adeno-associated virus 4 | Dependoparvovirus | ssDNA | 57579 | 2G8G (110) | 3.2 |
| BDMV | Belladonna mottle virus | Tymovirus | ssRNA | 12149 | ||
| BP29 | Bacillus phage Φ29 | Salasvirus | dsDNA | 10756 | ||
| CCMV | Cowpea chlorotic mottle virus | Bromovirus | ssRNA | 12303 | 1CWP (111) | 3.2 |
| CMV | Cucumber mosaic virus | Cucumovirus | ssRNA | 12307 | 1F15 (112) | 3.2 |
| CPaV2 | Canine parvovirus 2 | Protoparvovirus | ssDNA | 10790 | ||
| CPaV2d | Feline panleukopenia virus | Protoparvovirus | ssDNA | 10787 | 1C8G (113) | 3.0 |
| CRPV | Cottontail rabbit papillomavirus | Kappapapillomavirus | dsDNA | 31553 | ||
| CRPVd | Human papillomavirus 16 | Alphapapillomavirus | dsDNA | 333760 | 5KEQ (114) | 4.3 |
| CXA21 | Coxsackievirus A21 | Enterovirus | ssRNA | 12070 | 1Z7S (115) | 3.2 |
| CXB5 | Human coxsackievirus B5 | Enterovirus | ssRNA | 103907 | ||
| CXB5d | Human coxsackievirus B3 | Enterovirus | ssRNA | 103904 | 1COV (116) | 3.5 |
| EBFR | Enterobacteria phage fr | Levivirus | ssRNA | 12017 | 1FRS (117) | 3.5 |
| EBGA | Enterobacteria phage GA | Levivirus | ssRNA | 12018 | 1GAV (118) | 3.4 |
| EBMS2 | Enterobacteria phage MS2 | Levivirus | ssRNA | 329852 | 2MS2 (119) | 2.8 |
| EBQB | Enterobacteria phage Qβ | Allolevivirus | ssRNA | 39803 | 5VLY (120) | 3.3 |
| EBSP | Enterobacteria phage SP | Allolevivirus | ssRNA | 12027 | ||
| ECV1 | Echovirus 1 | Enterovirus | ssRNA | 103908 | 1EV1 (121) | 3.6 |
| ELV | Erysimum latent virus | Tymovirus | ssRNA | 12152 | ||
| HAdV5 | Human adenovirus 5 | Mastadenovirus | dsDNA | 28285 | 4V4U (122) | 10 |
| HHAV | Hepatitis A virus | Hepatovirus | ssRNA | 12098 | 4QPI (123) | 3.0 |
| HRV2 | Human rhinovirus 2 | Enterovirus | ssRNA | 12130 | 1FPN (124) | 2.6 |
| MEV | Mengo encephalomyocarditis virus | Cardiovirus | ssRNA | 12107 | 2MEV (125) | 3.0 |
| NOR1 | Norwalk virus | Norovirus | ssRNA | 524364 | 1IHM (126) | 3.4 |
| PHIX | Enterobacteria phage ΦX174 | Sinsheimervirus | ssDNA | 10847 | 2BPA (127) | 3.0 |
| PM2 | Pseudoalteromonas phage PM2 | Corticovirus | dsDNA | 10661 | 2W0C (75) | 7.0 |
| POL1 | Poliovirus | Enterovirus | ssRNA | 12081 | 1HXS (128) | 2.2 |
| PRD1 | Enterobacteria phage PRD1 | Alphatectivirus | dsDNA | 10658 | 1W8X (76) | 4.2 |
| RCNM | Red clover necrotic mosaic virus | Dianthovirus | ssRNA | 12267 | 6MRM (129) | 2.9 |
| REO3 | Reovirus 3 | Orthoreovirus | dsRNA | 10886 | 2CSE (130) | 7.0 |
| SBMV | Southern bean mosaic virus | Sobemovirus | ssRNA | 652938 | 4SBV (131) | 2.8 |
| ScrMV | Scrophularia mottle virus | Tymovirus | ssRNA | 312273 | ||
| SRVA | Simian rotavirus A | Rotavirus | dsRNA | 450149 | 4V7Q (132) | 3.8 |
| TBMV | Tobacco mosaic virus | Tobamovirus | ssRNA | 12243 | ||
| TYMV | Turnip yellow mosaic virus | Tymovirus | ssRNA | 12154 | 1AUY (133) | 3.0 |
As previously used by Heffron and Mayer (25).
NCBI Taxon, National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov) taxonomical ID (134).
PDB ID, Protein Data Bank (https://rcsb.org) ID used for 3D structural comparisons (135).
Alternate species/strain used for 3D structure only.
FIG 2.
Theoretical average charge of virus proteomes before (A) and after (B) modification by removing predicted polynucleotide-binding regions, as reported by Heffron and Mayer (25). Empirical pI values from the literature are shown as purple circles. Good fit between theoretical and empirical pIs is indicated when the purple circles fall within the white space (net-neutral virion surface charge) of the colored bars. Theoretical charge was calculated based on the sum of ionizable amino acids in capsid proteome sequences. Highly represented virus families (>2 representatives) are noted by letters to the right of each graph: L, Leviviridae; P, Picornaviridae; and T, Tymoviridae. A key to the virus abbreviations (y axis) is provided in Table 3.
Although ssRNA viruses showed some of the greatest improvements from PBR exclusion in the report of Heffron and Mayer (25), the need for PBR exclusion was not predictable based on genome type. Several enteroviruses showed slightly poorer pI prediction after PBR exclusion, despite having ssRNA genomes. This result can be seen for the enteroviruses poliovirus 1 (POL1), coxsackieviruses A21 and B5 (CXA21, CXB5), human rhinovirus 2 (HRV2), and echovirus 1 (ECV1), as well as the closely related Mengo encephalomyocarditis virus (MEV) (Fig. 2). Enteroviruses and other picornaviruses form capsids primarily though protein-protein binding, rather than protein-RNA binding (64), and thus provide an example of ssRNA viruses with no need for PBR exclusion. Furthermore, the dsDNA viruses human adenovirus 5 (HAdV5) and cottontail rabbit papillomavirus (CRPV) showed notable improvement after PBR exclusion. HAdV5 contains histone-like proteins and core proteins for dsDNA packaging (65–67), while papillomavirus major and minor capsid proteins have DNA-binding C and N termini, respectively (68, 69). All of these regions were identified as arginine-rich regions via PBR prediction in the report of Heffron and Mayer (25). Therefore, the usefulness of the PBR exclusion method is not restricted to only ssRNA viruses. Greater specificity in PBR prediction will likely also improve pI prediction for a wider range of viruses.
To summarize the picture of virion charges developed thus far, (i) the polynucleotide does not show evidence of contributing a strong negative charge and may be coulombically neutralized by a cloud of counterions (34) and (ii) the best 3D model that does not suppose polynucleotide influence suggests that only charges on the interior surface should be omitted, regardless of capsid dimensions. Omitting interior surface charges showed the greatest improvement in pI prediction for ssRNA viruses with interior concentrations of basic residues devoted to polynucleotide binding. If the unbound polynucleotide retains a cloud of counterions after folding and packaging, the impact of the polynucleotide on overall virion charge would be observed primarily as neutralization of these PBR charges. Finally, attempts to predict pI by excluding known and predicted PBR regions showed significant improvement in pI prediction for a wide range of viruses (25). Figure 3 presents a hypothesized conceptual model of virion charges that arises from the PBR exclusion approach. This conceptual model does not account for either (i) quantitative charge (except net-neutral charge at the pI) or (ii) nonelectrostatic forces within the virion, such as osmotic pressure and polymer elasticity (47). However, we hope this model can serve as a basis for further conversation and refinements.
FIG 3.
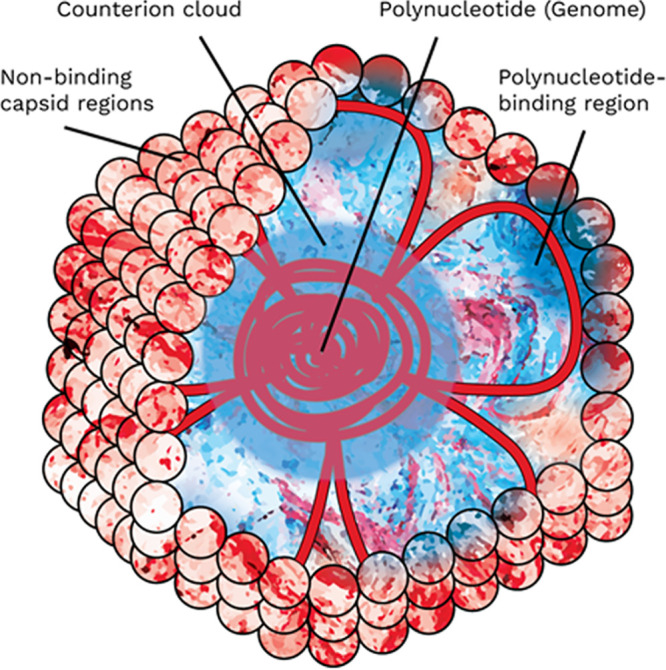
Hypothesized electrostatic model of the virion including polynucleotide-binding regions. Capsid proteins as a whole contain a balance of acidic and basic residues. At a given pH, these residues range across a broad spectrum of charge from strongly negative (dark red), to neutral (white), to strongly positive (dark blue). However, some viruses have a high concentration of basic residues on the capsid interior which are electrostatically bound to the polynucleotide. The charges of both the polynucleotide-binding regions of the capsid and associated polynucleotide segments are mutually negated. The charge of the polynucleotide core is screened by a hypothesized cloud of counterions retained in the virion core. The overall charge arises from the nonbinding portions of the capsid, which have an acidic pI due to a disproportionately low concentration of basic residues.
FURTHER CONSIDERATIONS FOR A PREDICTIVE ISOELECTRIC POINT MODEL
Previous capsid charge models have been based on theoretically provable assumptions of polynucleotide charge contribution or capsid impermeability/nonpermittivity. As discussed in “Surface-weighted capsid models” and “Polynucleotide-binding regions,” empirical evidence tends to contradict the core principles behind both of these assumptions. Regardless of the reality of virion charge structure, these two approaches to calculating theoretical virion pI have a serious practical impediment to developing a predictive pI model, in that both must be fit to empirical pI data. A polynucleotide contribution model must determine the extent of core contribution, as well as account for differences in capsid size, geometry, and apparent importance of polynucleotide influence (e.g., between leviviruses and enteroviruses). A surface residue-only model requires a universal criterion for defining surface residues for capsids of various sizes and structures. Pending a dramatic push to expand and verify virus pI data, current empirical pIs are few, poorly corroborated, and overrepresentative of a few virus genera (e.g., Levivirus, Enterovirus, and Tymovirus) (17, 25).
The overrepresentation of Leviviridae in the literature is a particular problem, as these ssRNA bacteriophages have been the exceptions around which models were built (13, 15, 22, 70). A large amount of the interior capsid surface of Leviviridae phages is devoted to polynucleotide binding (∼57% of the MS2 capsid protein) (44–46). The predicted pI of these phages can be brought into accordance with empirical pI by (i) excluding these predominantly basic residues on the basis of capsid impermeability or polynucleotide binding or (ii) proposing a strong negative charge from the virion core. However, the same model must also be applicable to a wide range of viruses that do not share these features.
Among these approaches, the PBR exclusion method is unique in that it is nonarbitrary, i.e., the inclusion or exclusion of a residue in the charge calculation is predicted according to an independent criterion (whether or not a residue occurs in a PBR), rather than directly from the impact of that residue on charge. No additional fitting is required to translate the predicted PBR into a weighted charge contribution; predicted PBR residues are simply excluded. Therefore, PBR exclusion is less likely than previous models to overfit pI prediction to the limited empirical data available. The PBR prediction method outlined by Heffron and Mayer (25) also relied on proteome sequences alone, obviating the need for detailed 3D capsid models, another research bottleneck. In addition, neither polynucleotide influence nor impermeable colloid models have been applied to a wide range of diverse viruses. When applied to a diverse set of viruses, the PBR exclusion approach accounted for very acidic pIs without sacrificing prediction of circumneutral pIs for viruses lacking PBRs.
The PBR exclusion method also reconciles the lack of empirical evidence for a strong charge contribution from the capsid interior. After PBR exclusion, differences between empirical and predicted pIs for the virus set evaluated by Heffron and Mayer (25) were distributed around a mean of 0.1, whereas viruses without modification had predicted pIs on average 2.1 pH units higher than empirical values. Therefore, the PBR exclusion method agreed with empirical data suggesting at most a minor charge contribution from the polynucleotide, as discussed in “Polynucleotide charge contribution.” When comparing pIs in solutions of various ionic strengths, the extremely basic regions neutralized via nucleotide binding would not impact virion charge, regardless of I and location within the capsid structure.
Also, PBRs may be neutralized even in VLPs. As suggested by Dika et al. (23), VLPs may retain some nucleic acid or host cell material (23, 71). Since electrostatic PBR-polynucleotide interactions are nonspecific (26, 52), the extent of capsid charge largely determines the amount of encapsidated material (59). However, ssRNA viruses typically contain roughly twice the charge equivalent of polynucleotide compared to PBR charge (59, 72). If the polynucleotide influenced overall virion charge, the decreased density of the VLP core should impact overall virion charge, whereas a lesser amount of material may be sufficient to neutralize PBRs. For viruses that rely on electrostatic interactions for assembly, inclusion of a threshold of negatively charged material may be required for intact VLPs.
While the PBR exclusion method somewhat vindicates the approach of negating interior residues, this approach was beneficial only for viruses with substantial PBRs on the capsid interior. A few predictions were dramatically worse when removing interior residues (Fig. 1 and Fig. S1). Even for bacteriophage GA (EBGA), a levivirus with RNA-binding beta sheets, most methods of defining exterior residues resulted in a prediction several pH units from the empirical pI (Fig. S1). Therefore, a physical model that incorporates the permeable and charge-permitting nature of the capsid appears more valid than an impermeable capsid model. However, such a model should build from the insights in the study of Heffron and Mayer (25) showing that the charge contribution of PBRs should be excluded. This avoids imposing arbitrary variables for a negative core charge or virion permeability without experimental support.
Considerations for enveloped viruses.
Current models of virion charge are limited to nonenveloped, icosahedral virions. Environmental persistence of viruses with phospholipid envelopes, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is generally considered insufficient to be relevant to transport or water and wastewater treatment (73, 74). For this reason, only nonenveloped viruses were considered here, except for bacteriophages PM2 and PRD1, which contain an internal lipid membrane (75, 76). However, some enveloped viruses, especially those transmitted via the fecal-oral route (e.g., avian influenza virus), may persist for months in aqueous environments (77, 78). In addition, the electrostatic charge of enveloped viruses may inform virus removal via air filtration and deposition on surfaces.
Unfortunately, enveloped viruses present unique challenges to pI prediction. Envelope phospholipids may contribute substantially to surface charge, and the low dielectric constant of phospholipid bilayers may decrease their apparent pKa by as much as one pH unit (79). The diversity of phospholipids in virus envelopes may also defy efforts for a predictive model. Ivanova et al. (80) quantified over 125 different phospholipids from three strains of influenza virus and found that the composition of lipids in the virion envelopes differed not only from the host cell membrane but also between virus strains. Since these phospholipids are acquired from the host, the complex lipid profiles are not predictable from the viral genome. Virions may acquire other materials from the host as well. For example, human papillomavirus acquires histones from the host that stabilize the polynucleotide within the capsid (81). These structures are likewise not coded for in the viral genome yet may impact overall capsid charge by neutralizing the polynucleotide charge.
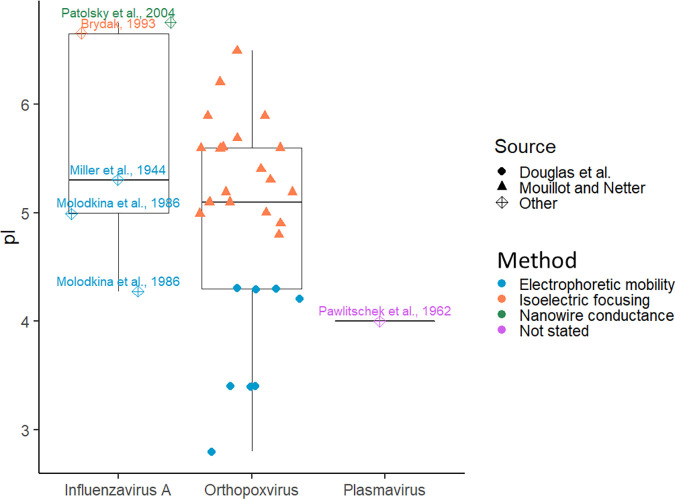
Perhaps most damning is that empirical pI data for enveloped viruses are particularly sparse, with poor agreement between sources, as summarized in Fig. 4. Unfortunately, only three genera are represented in Michen and Graule’s exhaustive review of empirical pI data (17), although pIs of isolated proteins (and especially glycoproteins) from enveloped viruses are more common (82–86). Empirical pIs for several strains of Orthopoxvirus are available, but much of the data come from two research groups with poor agreement, even when the same virus strains are being compared (87). As previously observed for nonenveloped viruses (25), pI measurements based on electrophoretic mobility were more acidic than measurements made by isoelectric focusing or other methods. However, the method of measurement was confounded by the source. Douglas et al. (88, 89) performed the majority of enveloped virus electrophoretic mobility measurements, whereas Mouillot and Netter (90) were responsible for the majority of isoelectric focusing measurements (17, 87). Douglas et al. used a more rigorous purification process than Mouillot and Netter (17). However, Douglas et al. performed experiments in molar sucrose, which may have impacted virion charge, aggregation, and electrophoretic mobility (89). Therefore, it is difficult to determine if there is a true difference between the two methods. In addition, poxviruses may have multiple infectious forms and numerous membrane-embedded proteins (91). For development of a theory of enveloped virus pI, the priority should be collecting empirical pI values for strains of viruses with one or two well-defined membrane proteins (e.g., coronaviruses or influenza A virus [92, 93]). However, the wide diversity in envelope proteins between strains may still present a challenge to extrapolation of a model to novel viruses.
FIG 4.
Distribution of empirical pI values for enveloped viruses referenced by Michen and Graule (17). Box plots summarizing the pIs for each virus genus are overlaid with individual pI values. Individual pI values are distinguished by method of determination (color) and literature source (shape). Two teams, Douglas et al. (88, 89) and Mouillot and Netter (90), were responsible for all Orthopoxvirus empirical pIs in this plot; all other sources (37, 98–101) are labeled in the figure. Points are horizontally scattered within each group for clarity only.
Interactions between viruses and the surrounding environment.
Ions in the water matrix may bind to moieties on the capsid surface, thereby altering surface charge. This is especially true of multivalent ions such as calcium and phosphate (17, 38), which may even be retained after viruses are transferred from the propagation/storage solution (6). In addition to ions from the surrounding medium, polyvalent cations are integral to the structure of many viruses. These ions may significantly alter pI and may be so integral as to be removed only through denaturation (94). Of the viruses shown in Fig. 2, five viruses (Bacillus phage Φ29 [BP29], canine parvovirus 2 [CPaV2], Pseudoalteromonas phage PM2 [PM2], reovirus 3 [REO3], simian rotavirus A [SRVA]) had zinc, magnesium, and/or calcium binding sites listed in the UniProt database (95). SRVA, in particular, had several cation-binding sites that may contribute to the higher than predicted pI. These integral ions, in addition to polyvalent counterions retained in the core, might have a dramatic impact on the overall charge of some viruses. However, the degree to which these cations alter surface charge, as well as the irreversibility of many cation binding sites, remains to be determined.
Virions may also have a more nuanced permeability than models of soft or hard colloids. For example, some viruses (e.g., human rhinovirus, southern bean mosaic virus, and Mengo encephalomyocarditis virus) have selective cation channels located at capsid vertices (96, 97). Bacteriophage MS2 also has pores at its 5-fold axes that are ringed by disordered loops with a single glutamic acid at the apex (21). The negative charge of these loops above pH 4 may aid in selective diffusion of cations into the virion core and may help recruit and retain counterions to stabilize the negatively charged polynucleotide. Such a mechanism would further explain the lack of influence of the viral genome on virion charge.
All of the above factors might complicate a predictive model of virus pI. Whether a model can successfully incorporate or safely ignore these virion complexities constitutes important future research. However, every confounding factor for a single model of virion charge lends support for an approach like PBR exclusion, which identifies functional virion structures rather than universally applying a simplified physical model. With expanded empirical pI data, more accurate pI prediction may be possible based on conserved virion structures. The PBR exclusion model has applications for researchers in water and wastewater treatment, as well as virus transport and microbial source tracking. As a general heuristic, viruses relying on electrostatic interactions between the polynucleotide and capsid proteins are more likely to have acidic pIs outside the circumneutral range expected from the sum of ionizable capsid residues. Thus, researchers may make use of the insights of the PBR exclusion method, even without identifying known PBRs or using the PBR prediction method developed by Heffron and Mayer (25). Future research should incorporate PBR exclusion into a quantitative model for virus surface charge. In addition, the PBR exclusion method gives rise to a conceptual electrostatic model of the virion that better unifies empirical evidence of virion structure and morphogenesis. This conceptual model is not an ab ovo assumption to account for a small subset of aberrant viruses. Instead, the PBR model follows from the success of the PBR exclusion method in accounting for both empirical pIs that align with capsid residue composition and empirical pIs that vary significantly. Further confirmation and refinement of this electrostatic model, particularly regarding the ionic composition of the virion core, could have far-reaching importance for structural virology in general.
Supplementary Material
Biographies

Joe Heffron is a returned Peace Corps volunteer who received his Ph.D. in Civil Engineering from Marquette University (2019). He is currently a postdoctoral researcher with the USDA Agricultural Research Service investigating quantitative microbial risk assessment of zoonotic pathogens in drinking water. Dr. Heffron first became interested in virion modeling as a Ph.D. student researching viral surrogates in water treatment processes. His research interests include waterborne pathogens, environmental electrochemistry, and distributed water treatment and reclamation. Dr. Heffron is especially motivated to pursue high-design, appropriate-tech water and wastewater solutions, and he is currently seeking a permanent position aligned with this research goal.

Brooke K. Mayer is an Associate Professor in the Department of Civil, Construction and Environmental Engineering at Marquette University. She graduated from Arizona State University (B.S. in 2004, M.S. in 2006, Ph.D. in 2008) with an emphasis in environmental engineering. Dr. Mayer’s teaching and research interests focus on physical-chemical treatment processes for water and wastewater applications, including the mitigation of viral pathogens, nutrients, and disinfection byproducts. Her research emphasizes improved public health and safety as well as advancing the waste-to-resource paradigm. For her work in these areas, Dr. Mayer was recognized with an NSF CAREER award as well as Marquette University’s Opus College of Engineering Outstanding Researcher Award.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Heffron J, Mayer BK. 2016. Emerging investigators series: virus mitigation by coagulation: recent discoveries and future directions. Environ Sci (Camb) 2:443–459. doi: 10.1039/C6EW00060F. [DOI] [Google Scholar]
- 2.Heffron J, McDermid B, Maher E, McNamara P, Mayer BK. 2019. Mechanisms of virus mitigation and suitability of bacteriophages as surrogates in drinking water treatment by iron electrocoagulation. Water Res 163:114877. doi: 10.1016/j.watres.2019.114877. [DOI] [PubMed] [Google Scholar]
- 3.Mattle MJ, Crouzy B, Brennecke M, Wigginton KR, Perona P, Kohn T. 2011. Impact of virus aggregation on inactivation by peracetic acid and implications for other disinfectants. Environ Sci Technol 45:7710–7717. doi: 10.1021/es201633s. [DOI] [PubMed] [Google Scholar]
- 4.Gerba CP, Betancourt WQ. 2017. Viral aggregation: impact on virus behavior in the environment. Environ Sci Technol 51:7318–7325. doi: 10.1021/acs.est.6b05835. [DOI] [PubMed] [Google Scholar]
- 5.Dika C, Duval JFL, Francius G, Perrin A, Gantzer C. 2015. Isoelectric point is an inadequate descriptor of MS2, Phi X 174 and PRD1 phages adhesion on abiotic surfaces. J Colloid Interface Sci 446:327–334. doi: 10.1016/j.jcis.2014.08.055. [DOI] [PubMed] [Google Scholar]
- 6.Yuan B, Pham M, Nguyen TH. 2008. Deposition kinetics of bacteriophage MS2 on a silica surface coated with natural organic matter in a radial stagnation point flow cell. Environ Sci Technol 42:7628–7633. doi: 10.1021/es801003s. [DOI] [PubMed] [Google Scholar]
- 7.Horká M, Kubíček O, Růžička F, Holá V, Malinovská I, Šlais K. 2007. Capillary isoelectric focusing of native and inactivated microorganisms. J Chromatogr A 1155:164–171. doi: 10.1016/j.chroma.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Brorson K, Shen H, Lute S, Pérez JS, Frey DD. 2008. Characterization and purification of bacteriophages using chromatofocusing. J Chromatogr A 1207:110–121. doi: 10.1016/j.chroma.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 9.Cashdollar JL, Wymer L. 2013. Methods for primary concentration of viruses from water samples: a review and meta-analysis of recent studies. J Appl Microbiol 115:1–11. doi: 10.1111/jam.12143. [DOI] [PubMed] [Google Scholar]
- 10.Polaczyk AL, Roberts JM, Hill VR. 2007. Evaluation of 1MDS electropositive microfilters for simultaneous recovery of multiple microbe classes from tap water. J Microbiol Methods 68:260–266. doi: 10.1016/j.mimet.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez L, Mylon SE, Nash B, Nguyen TH. 2010. Deposition and aggregation kinetics of rotavirus in divalent cation solutions. Environ Sci Technol 44:4552–4557. doi: 10.1021/es100120k. [DOI] [PubMed] [Google Scholar]
- 12.Mayer BK, Yang Y, Gerrity DW, Abbaszadegan M. 2015. The impact of capsid proteins on virus removal and inactivation during water treatment processes. Microbiol Insights 8:15–28. doi: 10.4137/MBI.S31441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armanious A, Aeppli M, Jacak R, Refardt D, Sigstam T, Kohn T, Sander M. 2016. Viruses at solid-water interfaces: a systematic assessment of interactions driving adsorption. Environ Sci Technol 50:732–743. doi: 10.1021/acs.est.5b04644. [DOI] [PubMed] [Google Scholar]
- 14.Božič AL, Šiber A, Podgornik R. 2012. How simple can a model of an empty viral capsid be? Charge distributions in viral capsids. J Biol Phys 38:657–671. doi: 10.1007/s10867-012-9278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penrod SL, Olson TM, Grant SB. 1996. Deposition kinetics of two viruses in packed beds of quartz granular media. Langmuir 12:5576–5587. doi: 10.1021/la950884d. [DOI] [Google Scholar]
- 16.Schaldach CM, Bourcier WL, Shaw HF, Viani BE, Wilson WD. 2006. The influence of ionic strength on the interaction of viruses with charged surfaces under environmental conditions. J Colloid Interface Sci 294:1–10. doi: 10.1016/j.jcis.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 17.Michen B, Graule T. 2010. Isoelectric points of viruses. J Appl Microbiol 109:388–397. [DOI] [PubMed] [Google Scholar]
- 18.Kozlowski LP. 2017. Proteome-pI: proteome isoelectric point database. Nucleic Acids Res 45:D1112–D1116. doi: 10.1093/nar/gkw978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiraga J, Mackiewicz P, Mackiewicz D, Kowalczuk M, Biecek P, Polak N, Smolarczyk K, Dudek MR, Cebrat S. 2007. The relationships between the isoelectric point and: length of proteins, taxonomy and ecology of organisms. BMC Genomics 8:163–116. doi: 10.1186/1471-2164-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duval JFL, Ohshima H. 2006. Electrophoresis of soft particles. Langmuir 22:3533–3546. doi: 10.1021/la0528293. [DOI] [PubMed] [Google Scholar]
- 21.Langlet J, Gaboriaud F, Gantzer C, Duval JFL. 2008. Impact of chemical and structural anisotropy on the electrophoretic mobility of spherical soft multilayer particles: the case of bacteriophage MS2. Biophys J 94:3293–3312. doi: 10.1529/biophysj.107.115477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langlet J, Gaboriaud F, Duval JFL, Gantzer C. 2008. Aggregation and surface properties of F-specific RNA phages: implication for membrane filtration processes. Water Res 42:2769–2777. doi: 10.1016/j.watres.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Dika C, Duval JFL, Ly-Chatain HM, Merlin C, Gantzer C. 2011. Impact of internal RNA on aggregation and electrokinetics of viruses: comparison between MS2 phage and corresponding virus-like particles. Appl Environ Microbiol 77:4939–4948. doi: 10.1128/AEM.00407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nap RJ, Božič AL, Szleifer I, Podgornik R. 2014. The role of solution conditions in the bacteriophage pp7 capsid charge regulation. Biophys J 107:1970–1979. doi: 10.1016/j.bpj.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heffron J, Mayer BK. 2020. Improved virus isoelectric point estimation by exclusion of known and predicted genome-binding regions. Appl Environ Microbiol 86:e01674-20. doi: 10.1128/AEM.01674-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Šiber A, Podgornik R. 2008. Nonspecific interactions in spontaneous assembly of empty versus functional single-stranded RNA viruses. Phys Rev E Stat Nonlin Soft Matter Phys 78:051915. doi: 10.1103/PhysRevE.78.051915. [DOI] [PubMed] [Google Scholar]
- 27.Duval JFL, Merlin J, Narayana PAL. 2011. Electrostatic interactions between diffuse soft multi-layered (bio)particles: beyond Debye-Hückel approximation and Deryagin formulation. Phys Chem Chem Phys 13:1037–1053. doi: 10.1039/c004243a. [DOI] [PubMed] [Google Scholar]
- 28.Blackburn GM. 2006. Nucleic acids in chemistry and biology, 3rd ed Royal Society of Chemistry, Cambridge, United Kingdom. [Google Scholar]
- 29.Gao T, Zhang W, Wang Y, Yang G. 2019. DNA compaction and charge neutralization regulated by divalent ions in very low pH solution. Polymers (Basel) 11:337. doi: 10.3390/polym11020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murthy VL, Rose GD. 2000. Is counterion delocalization responsible for collapse in RNA folding? Biochemistry 39:14365–14370. doi: 10.1021/bi001820r. [DOI] [PubMed] [Google Scholar]
- 31.Todd BA, Rau DC. 2008. Interplay of ion binding and attraction in DNA condensed by multivalent cations. Nucleic Acids Res 36:501–510. doi: 10.1093/nar/gkm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuller DN, Rickgauer JP, Jardine PJ, Grimes S, Anderson DL, Smith DE. 2007. Ionic effects on viral DNA packaging and portal motor function in bacteriophage φ29. Proc Natl Acad Sci U S A 104:11245–11250. doi: 10.1073/pnas.0701323104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angelescu DG, Bruinsma R, Linse P. 2006. Monte Carlo simulations of polyelectrolytes inside viral capsids. Phys Rev E Stat Nonlin Soft Matter Phys 73:049121. [DOI] [PubMed] [Google Scholar]
- 34.Angelescu DG, Linse P. 2008. Modelling of icosahedral viruses. Curr Opin Colloid Interface Sci 13:389–394. doi: 10.1016/j.cocis.2007.10.004. [DOI] [Google Scholar]
- 35.Dika C, Gantzer C, Perrin A, Duval JFL. 2013. Impact of the virus purification protocol on aggregation and electrokinetics of MS2 phages and corresponding virus-like particles. Phys Chem Chem Phys 15:5691–5700. doi: 10.1039/c3cp44128h. [DOI] [PubMed] [Google Scholar]
- 36.Appelo CAJ, Postma D. 2004. Geochemistry, groundwater and pollution, 2nd ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 37.Molodkina LM, Molodkin VM, Vostryukhina OA, Kolikov VM, Golikova EV, Chernoberezhskii YM. 1986. Study of the electrophoretic mobility of A1 (Leningrad) and A3 (Leningrad) influenza-viruses. Colloid J USSR 48:66–70. [Google Scholar]
- 38.Taylor DH, Bosmann HB. 1981. The electrokinetic properties of reovirus type 3: electrophoretic mobility and zeta potential in dilute electrolytes. J Colloid Interface Sci 83:153–162. doi: 10.1016/0021-9797(81)90020-5. [DOI] [Google Scholar]
- 39.Trilisky EI, Lenhoff AM. 2007. Sorption processes in ion-exchange chromatography of viruses. J Chromatogr A 1142:2–12. doi: 10.1016/j.chroma.2006.12.094. [DOI] [PubMed] [Google Scholar]
- 40.Righetti PG. 1980. Molarity and ionic strength of focused carrier ampholytes in isoelectric focusing. J Chromatogr 190:275–282. doi: 10.1016/s0021-9673(00)88230-8. [DOI] [PubMed] [Google Scholar]
- 41.Magdoff-Fairchild BS. 1967. Electrophoretic and bouyant density variants of southern bean mosaic virus. Virology 31:142–153. doi: 10.1016/0042-6822(67)90018-9. [DOI] [PubMed] [Google Scholar]
- 42.Overby LR, Barlow GH, Doi RH, Jacob M, Spiegelman S. 1966. Comparison of two serologically distinct ribonucleic acid bacteriophages. II. Properties of the nucleic acids and coat proteins. J Bacteriol 92:739–745. doi: 10.1128/JB.92.3.739-745.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubio V, Salas M, Viñuela E, Usobiaga P, Saiz JL, Llopis JF. 1974. Biophysical properties of bacteriophage phi29. Virology 57:112–121. doi: 10.1016/0042-6822(74)90112-3. [DOI] [PubMed] [Google Scholar]
- 44.Toropova K, Basnak G, Twarock R, Stockley PG, Ranson NA. 2008. The three-dimensional structure of genomic RNA in bacteriophage MS2: implications for assembly. J Mol Biol 375:824–836. doi: 10.1016/j.jmb.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 45.Rolfsson Ó, Middleton S, Manfield IW, White SJ, Fan B, Vaughan R, Ranson NA, Dykeman E, Twarock R, Ford J, Cheng Kao C, Stockley PG. 2016. Direct evidence for packaging signal-mediated assembly of bacteriophage MS2. J Mol Biol 428:431–448. doi: 10.1016/j.jmb.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rumnieks J, Tars K. 2014. Crystal structure of the bacteriophage Qβ coat protein in complex with the RNA operator of the replicase gene. J Mol Biol 426:1039–1049. doi: 10.1016/j.jmb.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 47.Šiber A, Božič AL, Podgornik R. 2015. Energies and pressures in viruses: contribution of nonspecific electrostatic interactions. Phys Chem Chem Phys 14:3746–3765. doi: 10.1039/C1CP22756D [DOI] [PubMed] [Google Scholar]
- 48.Lee SK, Hacker DL. 2001. In vitro analysis of an RNA binding site within the N-terminal 30 amino acids of the southern cowpea mosaic virus coat protein. Virology 286:317–327. doi: 10.1006/viro.2001.0979. [DOI] [PubMed] [Google Scholar]
- 49.Grieger JC, Snowdy S, Samulski RJ. 2006. Separate basic region motifs within the adeno-associated virus capsid proteins are essential for infectivity and assembly. J Virol 80:5199–5210. doi: 10.1128/JVI.02723-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ford RJ, Barker AM, Bakker SE, Coutts RH, Ranson NA, Phillips SEV, Pearson AR, Stockley PG. 2013. Sequence-specific, RNA-protein interactions overcome electrostatic barriers preventing assembly of satellite tobacco necrosis virus coat protein. J Mol Biol 425:1050–1064. doi: 10.1016/j.jmb.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.García-García C, Draper DE, 2003. Electrostatic interactions in a peptide-RNA complex. J Mol Biol 331:75–88. doi: 10.1016/S0022-2836(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 52.Belyi VA, Muthukumar M. 2006. Electrostatic origin of the genome packing in viruses. Proc Natl Acad Sci U S A 103:17174–17178. doi: 10.1073/pnas.0608311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basnak G, Morton VL, Rolfsson Ó, Stonehouse NJ, Ashcroft AE, Stockley PG. 2010. Viral genomic single-stranded RNA directs the pathway toward a T=3 capsid. J Mol Biol 395:924–936. doi: 10.1016/j.jmb.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lins L, Thomas A, Brasseur R. 2003. Analysis of accessible surface of residues in proteins. Protein Sci 12:1406–1417. doi: 10.1110/ps.0304803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oram M, Black LW. 2011. Mechanisms of genome packaging, p 203–219. In Agbandje-McKenna M, McKenna R (ed), Structural virology. Royal Society of Chemistry, Cambridge, United Kingdom. [Google Scholar]
- 56.Goetschius DJ, Parrish CR, Hafenstein S. 2019. Asymmetry in icosahedral viruses. Curr Opin Virol 36:67–73. doi: 10.1016/j.coviro.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Ahi YS, Mittal SK. 2016. Components of adenovirus genome packaging. Front Microbiol 7:1503. doi: 10.3389/fmicb.2016.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong C, Oksanen HM, Liu X, Jakana J, Bamford DH, Chiu W. 2014. A structural model of the genome packaging process in a membrane-containing double stranded DNA virus. PLoS Biol 12:e1002024. doi: 10.1371/journal.pbio.1002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perlmutter JD, Hagan MF. 2015. Mechanisms of virus assembly. Annu Rev Phys Chem 66:217–239. doi: 10.1146/annurev-physchem-040214-121637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Annamalai P, Apte S, Wilkens S, Rao ALN. 2005. Deletion of highly conserved arginine-rich RNA binding motif in cowpea chlorotic mottle virus capsid protein results in virion structural alterations and RNA packaging constraints. J Virol 79:3277–3288. doi: 10.1128/JVI.79.6.3277-3288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park S-H, Sit TL, Kim K-H, Lommel SA. 2013. The red clover necrotic mosaic virus capsid protein N-terminal amino acids possess specific RNA binding activity and are required for stable virion assembly. Virus Res 176:107–118. doi: 10.1016/j.virusres.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Park SH, Sit TL, Kim KH, Lommel SA. 2012. The red clover necrotic mosaic virus capsid protein N-terminal lysine-rich motif is a determinant of symptomatology and virion accumulation. Mol Plant Pathol 13:744–754. doi: 10.1111/j.1364-3703.2011.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruigrok RWH, Crépin T, Kolakofsky D. 2011. Nucleoproteins and nucleocapsids of negative-strand RNA viruses. Curr Opin Microbiol 14:504–510. doi: 10.1016/j.mib.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y, Wang C, Mueller S, Paul AV, Wimmer E, Jiang P. 2010. Direct interaction between two viral proteins, the nonstructural protein 2CATPase and the capsid protein VP3, is required for enterovirus morphogenesis. PLoS Pathog 6:e1001066. doi: 10.1371/journal.ppat.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burg JL, Schweitzer J, Daniell E. 1983. Introduction of superhelical turns into DNA by adenoviral core proteins and chromatin assembly factors. J Virol 46:749–755. doi: 10.1128/JVI.46.3.749-755.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fedor MJ, Daniell E. 1980. Acetylation of histone-like proteins of adenovirus type 5. J Virol 35:637–643. doi: 10.1128/JVI.35.3.637-643.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chroboczek J, Bieber F, Jacrot B. 1992. The sequence of the genome of adenovirus type 5 and its comparison with the genome of adenovirus type 2. Virology 186:280–285. doi: 10.1016/0042-6822(92)90082-z. [DOI] [PubMed] [Google Scholar]
- 68.Schäfer F, Florin L, Sapp M. 2002. DNA binding of L1 is required for human papillomavirus morphogenesis in vivo. Virology 295:172–181. doi: 10.1006/viro.2002.1361. [DOI] [PubMed] [Google Scholar]
- 69.Wang JW, Roden RBS. 2013. L2, the minor capsid protein of papillomavirus. Virology 445:175–186. doi: 10.1016/j.virol.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dika C, Ly-Chatain MH, Francius G, Duval JFL, Gantzer C. 2013. Non-DLVO adhesion of F-specific RNA bacteriophages to abiotic surfaces: importance of surface roughness, hydrophobic and electrostatic interactions. Colloids Surf A Physicochem Eng Asp 435:178–187. doi: 10.1016/j.colsurfa.2013.02.045. [DOI] [Google Scholar]
- 71.Hirth L, Givord L. 1988. Tymoviruses, p 163–212. In Koenig R. (ed), The plant viruses. Plenum Press, New York, NY. [Google Scholar]
- 72.Hu T, Zhang R, Shklovskii BI. 2008. Electrostatic theory of viral self-assembly. Phys A Stat Mech Its Appl 387:3059–3064. doi: 10.1016/j.physa.2008.01.010. [DOI] [Google Scholar]
- 73.Kotwal G, Cannon JL. 2014. Environmental persistence and transfer of enteric viruses. Curr Opin Virol 4:37–43. doi: 10.1016/j.coviro.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 74.Firquet S, Beaujard S, Lobert PE, Sané F, Caloone D, Izard D, Hober D. 2015. Survival of enveloped and non-enveloped viruses on inanimate surfaces. Microbes Environ 30:140–144. doi: 10.1264/jsme2.ME14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abrescia NGA, Grimes JM, Kivelä HM, Assenberg R, Sutton GC, Butcher SJ, Bamford JKH, Bamford DH, Stuart DI. 2008. Insights into virus evolution and membrane biogenesis from the structure of the marine lipid-containing bacteriophage PM2. Mol Cell 31:749–761. doi: 10.1016/j.molcel.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 76.Abrescia NGA, Cockburn JJB, Grimes JM, Sutton GC, Diprose JM, Butcher SJ, Fuller SD, San Martín C, Burnett RM, Stuart DI, Bamford DH, Bamford JKH. 2004. Insights into assembly from structural analysis of bacteriophage PRD1. Nature 432:68–74. doi: 10.1038/nature03056. [DOI] [PubMed] [Google Scholar]
- 77.Wigginton KR, Ye Y, Ellenberg RM. 2015. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ Sci (Camb) 1:735–746. doi: 10.1039/C5EW00125K. [DOI] [Google Scholar]
- 78.Ye Y, Ellenberg RM, Graham KE, Wigginton KR. 2016. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ Sci Technol 50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- 79.Tsui FC, Ojcius DM, Hubbell WL. 1986. Intrinsic pKa values for phosphatidylethanolamine phosphatidylcholine bilayers. Biophys J 49:459–468. doi: 10.1016/S0006-3495(86)83655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ivanova PT, Myers DS, Milne SB, McClaren JL, Thomas PG, Brown HA. 2015. Lipid composition of viral envelope of three strains of influenza virus—not all viruses are created equal. ACS Infect Dis 1:399–452. doi: 10.1021/acsinfecdis.5b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim HJ, Kwag HL, Kim HJ. 2016. Characterization of human papillomavirus type 16 pseudovirus containing histones. BMC Biotechnol 16:63. doi: 10.1186/s12896-016-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kapil S, Richardson KL, Maag TR, Goyal SM. 1999. Characterization of bovine coronavirus isolates/from eight different states in the USA. Vet Microbiol 67:221–230. doi: 10.1016/s0378-1135(99)00042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siddell S, Wege H, Barthel A, Ter Meulen V. 1981. Coronavirus JHM: intracellular protein synthesis. J Gen Virol 53:145–155. doi: 10.1099/0022-1317-53-1-145. [DOI] [PubMed] [Google Scholar]
- 84.Murray MJ, Kabat D. 1979. Genetic and sialylation sources of heterogeneity of the murine leukemia virus membrane envelope glycoproteins gp69/71. J Biol Chem 254:1340–1348. [PubMed] [Google Scholar]
- 85.Tran NT, Taverna M, Chevalier M, Ferrier D. 2000. One-step capillary isoelectric focusing for the separation of the recombinant human immunodeficiency virus envelope glycoprotein glycoforms. J Chromatogr A 866:121–135. doi: 10.1016/s0021-9673(99)01045-6. [DOI] [PubMed] [Google Scholar]
- 86.Dalrymple JM, Schlesinger S, Russell PK. 1976. Antigenic characterization of two Sindbis envelope glycoproteins separated by isoelectric focusing. Virology 69:93–103. doi: 10.1016/0042-6822(76)90197-5. [DOI] [PubMed] [Google Scholar]
- 87.Zerda KS, Gerba CP. 1984. Agarose isoelectrofocusing of intact virions. J Virol Methods 9:1–6. doi: 10.1016/0166-0934(84)90077-6. [DOI] [PubMed] [Google Scholar]
- 88.Douglas HW, Williams BL, Rondle CJM. 1969. Micro-electrophoresis of pox viruses in molar sucrose. J Gen Virol 5:391–396. doi: 10.1099/0022-1317-5-3-391. [DOI] [PubMed] [Google Scholar]
- 89.Douglas HW, Rondle CJ, Williams BL. 1966. Micro-electrophoresis of cowpox and vaccinia viruses in molar sucrose. J Gen Microbiol 42:107–113. doi: 10.1099/00221287-42-1-107. [DOI] [PubMed] [Google Scholar]
- 90.Mouillot L, Netter R. 1977. Identification of orthopox virus by isoelectrofocusing in a granulated gel. Ann Microbiol (Paris) 128:417–419. [PubMed] [Google Scholar]
- 91.Moss B. 2012. Poxvirus cell entry: how many proteins does it take? Viruses 4:688–707. doi: 10.3390/v4050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wasilewski S, Calder LJ, Grant T, Rosenthal PB. 2012. Distribution of surface glycoproteins on influenza A virus determined by electron cryotomography. Vaccine 30:7368–7373. doi: 10.1016/j.vaccine.2012.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schoeman D, Fielding BC. 2019. Coronavirus envelope protein: current knowledge. Virol J 16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schäfer R, Hinnen R, Franklin RM. 1974. Structure and synthesis of a lipid‐containing bacteriophage: properties of the structural proteins and distribution of the phospholipid. Eur J Biochem 50:15–27. doi: 10.1111/j.1432-1033.1974.tb03868.x. [DOI] [PubMed] [Google Scholar]
- 95.The UniProt Consortium. 2019. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalko SG, Cachau RE, Silva AM. 1992. Ion channels in icosahedral virus: a comparative analysis of the structures and binding sites at their fivefold axes. Biophys J 63:1133–1145. doi: 10.1016/S0006-3495(92)81693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silva AM, Cachau RE, Goldstein DJ. 1987. Ion channels in southern bean mosaic virus capsid. Biophys J 52:595–602. doi: 10.1016/S0006-3495(87)83249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brydak L. 1993. Studies on the adaptation of influenza virus replicated at low temperature. V. Isoelectric focusing studies. Acta Microbiol Pol 42:29–33. [PubMed] [Google Scholar]
- 99.Miller GL, Lauffer MA, Stanley WM. 1944. Electrophoretic studies on the PR8 influenza virus. J Exp Med 80:549–559. doi: 10.1084/jem.80.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patolsky F, Zheng G, Hayden O, Lakadamyali M, Zhuang X, Lieber CM. 2004. Electrical detection of single viruses. Proc Natl Acad Sci U S A 101:14017–14022. doi: 10.1073/pnas.0406159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pawlitschek W, Laue F, Völz H. 1962. Einige physikalische Eigenschaften des O1-Phagen. Naturwissenschaften 49:526–526. doi: 10.1007/BF00636366. [DOI] [Google Scholar]
- 102.Salo RJ, Mayor HD. 1978. Isoelectric focusing of parvoviruses. Intervirology 10:87–93. doi: 10.1159/000148972. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen TH, Easter N, Gutiérrez L, Huyett L, Defnet E, Mylon SE, Ferri JK, Viet NA. 2011. The RNA core weakly influences the interactions of the bacteriophage MS2 at key environmental interfaces. Soft Matter 7:10449–10456. doi: 10.1039/c1sm06092a. [DOI] [Google Scholar]
- 104.Weichert WS, Parker JSL, Wahid ATM, Chang SF, Meier E, Parrish CR. 1998. Assaying for structural variation in the parvovirus capsid and its role in infection. Virology 250:106–117. doi: 10.1006/viro.1998.9352. [DOI] [PubMed] [Google Scholar]
- 105.Lute S, Aranha H, Tremblay D, Liang D, Ackermann H, Chu B, Moineau S, Brorson K. 2004. Characterization of coliphage PR772 and evaluation of its use for virus filter performance testing. Appl Environ Microbiol 70:4864–4871. doi: 10.1128/AEM.70.8.4864-4871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noda T. 2012. Native morphology of influenza virions. Front Microbiol 2:269. doi: 10.3389/fmicb.2011.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ho PT, Montiel-Garcia DJ, Wong JJ, Carrillo-Tripp M, Brooks CL, Johnson JE, Reddy VS. 2018. VIPERdb: a tool for virus research. Annu Rev Virol 5:477–488. doi: 10.1146/annurev-virology-092917-043405. [DOI] [PubMed] [Google Scholar]
- 108.Otterstedt JE, Brandreth DA. 1998. Small particles technology. Springer US, New York, NY. [Google Scholar]
- 109.Inokuchi Y, Jacobson AB, Hirose T, Inayama S, Hirashima A. 1988. Analysis of the complete nucleotide sequence of the group IV RNA coliphage SP. Nucleic Acids Res 16:6205–6221. doi: 10.1093/nar/16.13.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Govindasamy L, Padron E, McKenna R, Muzyczka N, Kaludov N, Chiorini JA, Agbandje-McKenna M. 2006. Structurally mapping the diverse phenotype of adeno-associated virus serotype 4. J Virol 80:11556–11570. doi: 10.1128/JVI.01536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Speir JA, Munshi S, Wang G, Baker TS, Johnson JE. 1995. Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopy. Structure 3:63–78. doi: 10.1016/s0969-2126(01)00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith TJ, Chase E, Schmidt T, Perry KL. 2000. The structure of cucumber mosaic virus and comparison to cowpea chlorotic mottle virus. J Virol 74:7578–7586. doi: 10.1128/jvi.74.16.7578-7586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simpson AA, Chandrasekar V, Hébert B, Sullivan GM, Rossmann MG, Parrish CR. 2000. Host range and variability of calcium binding by surface loops in the capsids of canine and feline parvoviruses. J Mol Biol 300:597–610. doi: 10.1006/jmbi.2000.3868. [DOI] [PubMed] [Google Scholar]
- 114.Guan J, Bywaters SM, Brendle SA, Ashley RE, Makhov AM, Conway JF, Christensen ND, Hafenstein S. 2017. Cryoelectron microscopy maps of human papillomavirus 16 reveal L2 densities and heparin binding site. Structure 25:253–263. doi: 10.1016/j.str.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 115.Xiao C, Bator-Kelly CM, Rieder E, Chipman PR, Craig A, Kuhn RJ, Wimmer E, Rossmann MG. 2005. The crystal structure of coxsackievirus A21 and its interaction with ICAM-1. Structure 13:1019–1033. doi: 10.1016/j.str.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 116.Muckelbauer JK, Kremer M, Minor I, Tong L, Zlotnick A, Johnson JE, Rossmann MG. 1995. Structure determination of coxsackievirus B3 to 3.5 Å resolution. Acta Crystallogr D Biol Crystallogr 51:871–887. doi: 10.1107/S0907444995002253. [DOI] [PubMed] [Google Scholar]
- 117.Liljas L, Fridborg K, Valegård K, Bundule M, Pumpens P. 1994. Crystal structure of bacteriophage fr capsids at 3.5 A resolution. J Mol Biol 244:279–290. doi: 10.1006/jmbi.1994.1729. [DOI] [PubMed] [Google Scholar]
- 118.Tars K, Bundule M, Fridborg K, Liljas L. 1997. The crystal structure of bacteriophage GA and a comparison of bacteriophages belonging to the major groups of Escherichia coli leviviruses. J Mol Biol 271:759–773. doi: 10.1006/jmbi.1997.1214. [DOI] [PubMed] [Google Scholar]
- 119.Golmohammadi R, Valegard K, Fridborg K, Liljas L. 1993. The refined structure of bacteriophage MS2 at 2.8 A resolution. J Mol Biol 234:620–639. doi: 10.1006/jmbi.1993.1616. [DOI] [PubMed] [Google Scholar]
- 120.Cui Z, Gorzelnik KV, Chang JY, Langlais C, Jakana J, Young R, Zhang J. 2017. Structures of Qβ virions, virus-like particles, and the Qβ-MurA complex reveal internal coat proteins and the mechanism of host lysis. Proc Natl Acad Sci U S A 114:11697–11702. doi: 10.1073/pnas.1707102114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Filman DJ, Wien MW, Cunningham JA, Bergelson JM, Hogle JM. 1998. Structure determination of echovirus 1. Acta Crystallogr D Biol Crystallogr 54:1261–1272. doi: 10.1107/s0907444998002790. [DOI] [PubMed] [Google Scholar]
- 122.Fabry CMS, Rosa-Calatrava M, Conway JF, Zubieta C, Cusack S, Ruigrok RWH, Schoehn G. 2005. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J 24:1645–1654. doi: 10.1038/sj.emboj.7600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X, Ren J, Gao Q, Hu Z, Sun Y, Li X, Rowlands DJ, Yin W, Wang J, Stuart DI, Rao Z, Fry EE. 2015. Hepatitis A virus and the origins of picornaviruses. Nature 517:85–88. doi: 10.1038/nature13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Verdaguer N, Blaas D, Fita I. 2000. Structure of human rhinovirus serotype 2 (HRV2)11. J Mol Biol 300:1179–1194. doi: 10.1006/jmbi.2000.3943. [DOI] [PubMed] [Google Scholar]
- 125.Krishnaswamy S, Rossmann MG. 1990. Structural refinement and analysis of Mengo virus. J Mol Biol 211:803–844. doi: 10.1016/0022-2836(90)90077-Y. [DOI] [PubMed] [Google Scholar]
- 126.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 127.McKenna R, Xia D, Willingmann P, Ilag LL, Krishnaswamy S, Rossmann MG, Olson NH, Baker TS, Incardona NL. 1992. Atomic structure of single-stranded DNA bacteriophage phi X174 and its functional implications. Nature 355:137–143. doi: 10.1038/355137a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller ST, Hogle JM, Filman DJ. 2001. Ab initio phasing of high-symmetry macromolecular complexes: successful phasing of authentic poliovirus data to 3.0 Å resolution. J Mol Biol 307:499–512. doi: 10.1006/jmbi.2001.4485. [DOI] [PubMed] [Google Scholar]
- 129.Sherman MB, Guenther R, Reade R, Rochon D, Sit T, Smith TJ. 2019. Near-atomic-resolution cryo-electron microscopy structures of cucumber leaf spot virus and red clover necrotic mosaic virus: evolutionary divergence at the icosahedral three-fold axes. J Virol 94:e01439-19. doi: 10.1128/JVI.01439-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X, Ji Y, Zhang L, Harrison SC, Marinescu DC, Nibert ML, Baker TS. 2005. Features of reovirus outer capsid protein μ1 revealed by electron cryomicroscopy and image reconstruction of the virion at 7.0 Å resolution. Structure 13:1545–1557. doi: 10.1016/j.str.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Silva AM, Rossmann MG. 1985. The refinement of southern bean mosaic virus in reciprocal space. Acta Crystallogr B Struct Sci 41:147–157. doi: 10.1107/S0108768185001781. [DOI] [Google Scholar]
- 132.Settembre EC, Chen JZ, Dormitzer PR, Grigorieff N, Harrison SC. 2011. Atomic model of an infectious rotavirus particle. EMBO J 30:408–416. doi: 10.1038/emboj.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Canady MA, Larson SB, Day J, McPherson A. 1996. Crystal structure of turnip yellow mosaic virus. Nat Struct Biol 3:771–781. doi: 10.1038/nsb0996-771. [DOI] [PubMed] [Google Scholar]
- 134.Sayers EW, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, Dicuccio M, Edgar R, Federhen S, Feolo M, Geer LY, Helmberg W, Kapustin Y, Landsman D, Lipman DJ, Madden TL, Maglott DR, Miller V, Mizrachi I, Ostell J, Pruitt KD, Schuler GD, Sequeira E, Sherry ST, Shumway M, Sirotkin K, Souvorov A, Starchenko G, Tatusova TA, Wagner L, Yaschenko E, Ye J. 2010. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 38:D5–D15. doi: 10.1093/nar/gkp967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. 2000. The Protein Data Bank. Nucleic Acids Res 28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.