This study established a logarithmic relationship between the thermal death time (D-value) of S. Enteritidis PT 30 and the moisture content (XW) of the bacterial cells by conducting thermal inactivation tests on freeze-dried S. Enteritidis PT 30.
KEYWORDS: moisture content, bacterial cells, thermal resistance, Salmonella enterica serotype Enteritidis PT 30, low-moisture foods
ABSTRACT
Salmonella spp. are resilient bacterial pathogens in low-moisture foods. There has been a general lack of understanding of critical factors contributing to the enhanced thermal tolerance of Salmonella spp. in dry environments. In this study, we hypothesized that the moisture content (XW) of bacterial cells is a critical intrinsic factor influencing the resistance of Salmonella spp. to thermal inactivation. We selected Salmonella enterica serotype Enteritidis PT 30 to test this hypothesis. We first produced viable freeze-dried S. Enteritidis PT 30, conditioned the bacterial cells to different XWs (7.7, 9.2, 12.4, and 15.7 g water/100 g dry solids), and determined the thermal inactivation kinetics of those cells at 80°C. The results show that the D-value (the time required to achieve a 1-log reduction) decreased exponentially with increasing XW. We further measured the water activities (aw) of the freeze-dried S. Enteritidis PT 30 as influenced by temperatures between 20 and 80°C. By using those data, we estimated the XW of S. Enteritidis PT 30 from the published papers that related the D-values of the same bacterial strain at 80°C with the aw of five different food and silicon dioxide matrices. We discovered that the logarithmic D-values of S. Enteritidis PT 30 in all those matrices also decreased linearly with increasing XW of the bacterial cells. The findings suggest that the amount of moisture in S. Enteritidis PT 30 is a determining factor of its ability to resist thermal inactivation. Our results may help future research into fundamental mechanisms for thermal inactivation of bacterial pathogens in dry environments.
IMPORTANCE This study established a logarithmic relationship between the thermal death time (D-value) of S. Enteritidis PT 30 and the moisture content (XW) of the bacterial cells by conducting thermal inactivation tests on freeze-dried S. Enteritidis PT 30. We further verified this relationship using literature data for S. Enteritidis PT 30 in five low-moisture matrices. The findings suggest that the XW of S. Enteritidis PT 30, which is rapidly adjusted by microenvironmental aw, or relative humidity, during heat treatments, is the key intrinsic factor determining the thermal resistance of the bacterium. The quantitative relationships reported in this study may help guide future designs of industrial thermal processes for the control of S. Enteritidis PT 30 or other Salmonella strains in low-moisture foods. Our findings highlight a need for further fundamental investigation into the role of water in protein denaturation and the accumulation of compatible solutes during thermal inactivation of bacterial pathogens in dry environments.
INTRODUCTION
Free water in foods is required to support the growth of yeasts, molds, and bacteria (1). Water activity (aw) is an indirect measure of the free water available to the microorganism in food systems (2). Generally, low-moisture foods have an aw at or below 0.6 at room temperature (3). The low-aw environment in low-moisture foods, such as milk powder, chocolate, peanut butter, and cereal, inhibits the growth of microorganisms (4, 5). However, pathogens such as Salmonella can survive in low-moisture foods during storage and frequently cause worldwide outbreaks (1, 3, 6–8).
Salmonella bacteria are among the most common pathogens causing severe foodborne illness for humans (7, 8). These resilient microorganisms can easily adapt to environmental conditions beyond their optimal growth range, including temperature, pH and aw (1, 5, 9). The minimum aw for the growth of Salmonella is 0.94, but it can survive and remain viable in low-moisture foods for long periods (4). In particular, Salmonella has enhanced thermal resistance under dry conditions. Studies have shown that its thermal resistance, as evaluated by the D-value (the time required to inactivate 90% of target bacteria at a fixed temperature), increases sharply with decreasing aw in various low-moisture foods (3, 5, 10–13).
Early researchers attempted to establish a direct correlation between the thermal resistance of foodborne pathogens at certain treatment temperatures and the aw of the inoculated foods at room temperature (10). Recent studies, however, have shown that the aw of food systems at room temperature does not accurately reflect the microenvironment to which foodborne pathogens are exposed in a thermal treatment. For instance, when wheat flour, whey protein, and almond flour are conditioned to an aw of 0.45 at room temperature and then heated to 80°C in sealed containers (without moisture loss), their aw values changed to 0.68, 0.62, and 0.54, respectively (11). The D-values at 80°C (D80°C-values) of Salmonella enterica serotype Enteritidis PT 30 in these foods were about 5, 11, and 21 min, respectively (11). Thus, the aw of foods measured at room temperature should not be considered a process control parameter in the design of thermal inactivation processes. Our most recent studies have demonstrated that the aw of food systems measured at treatment temperatures (aw, treatment temperature) should be considered a critical extrinsic factor determining the D-values of microorganisms during thermal treatment (11, 13–16).
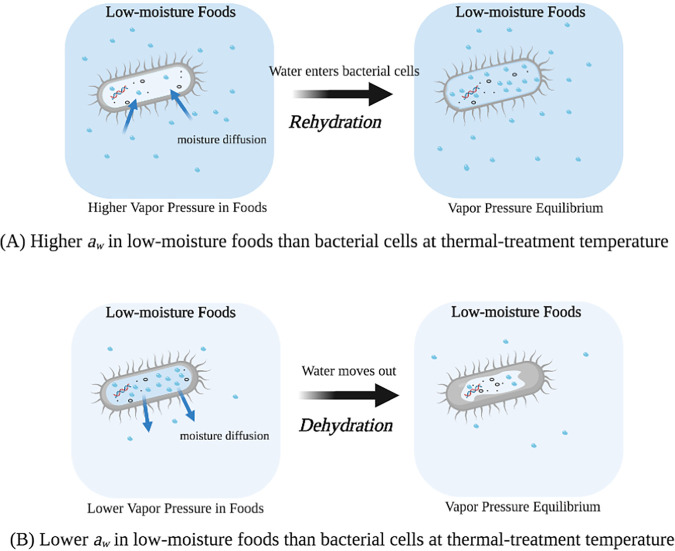
The aw of a biomass is expressed as the ratio of its water vapor pressure to the saturated water pressure at a given temperature. It is a thermodynamic parameter that generally changes with temperature in food systems (11, 15, 17–19). Thus, it is an extrinsic property of a biomaterial. In a high-moisture environment, water accounts for 60% to 90% of the mass of bacterial cells (20). In low-moisture foods, however, bacterial cells respond to the aw of the food matrices in multiple ways, and a direct reaction is the adjustment of the moisture content (XW, expressed as grams of water per 100 g of dry solids) of bacterial cells through moisture diffusion (17, 21). The moisture diffusion between bacterial cells and their surrounding food matrices in a thermal process is illustrated in Fig. 1. Even though the biomass of bacterial cells and the contaminated food have the same aw at room temperature, their aw may change when heated to a high temperature. The differences in aw between bacterial cells and the food creates a moisture vapor pressure gradient that drives moisture diffusion in or out of the bacterial cells until an equilibrium is reached. In industrial operations, the food matrices have much larger masses than the bacteria that potentially contaminate the foods. Thus, the aw of the bacterial cells should be equal to that of the foods at equilibrium. As shown in Fig. 1A, bacterial cells will gain moisture from their microenvironment when the aw of the food is higher than that of bacterial cells at thermal-treatment temperatures. On other hand, the bacterial cells would be dehydrated if a lower aw is provided by a food system (Fig. 1B). The moisture diffusion between bacterial cells and their food environment can be rapid (within seconds) because of the smaller characteristic size of bacterial cells (17). Therefore, we hypothesize that the XW of bacteria might be the dominant intrinsic parameter that determines their thermal resistance in low-moisture foods in a thermal process.
FIG 1.
Illustration of moisture exchange between Salmonella cells and different microenvironments during heating (created with BioRender).
The relationship between the XW and aw of a biomass at different temperatures is commonly referred to as the moisture sorption isotherms. Extensive data on the moisture sorption isotherms for foods exist in the literature. But no research had been published on the isotherms for bacteria until the work of Syamaladevi et al. (17), who reported the moisture sorption isotherms for a biomass of freeze-dried Enterococcus faecium, a commonly used surrogate for Salmonella. There has been no published research on aw changes of the biomass of Salmonella cells with temperature.
The goal of this research was to test the hypothesis that the moisture content of bacterial cells is the intrinsic parameter determining the thermal resistance of Salmonella in low-moisture environments. The specific objectives of this study were to (i) determine the thermal resistance of freeze-dried S. Enteritidis PT 30 at 80°C with different XW values (7.7, 9.2, 12.4. and 15.7 g water/100 g dry solids), (ii) establish the relationship between the XW and the aw of freeze-dried S. Enteritidis PT 30 biomass at different temperatures, and (iii) study the correlation between XW and the thermal resistance of S. Enteritidis PT 30 in low-moisture matrices.
We selected S. Enteritidis PT 30 as the target bacterium in this study because its thermal resistance in different low-moisture matrices has been reported (10, 11, 22–24). We used freeze-drying, also known as lyophilization, to produce desiccated S. Enteritidis PT 30 samples with high vitality (22). The XW of a porous mass of freeze-dried S. Enteritidis PT 30 could easily be adjusted by exposing it to different relative humidities. We conducted thermal inactivation tests on freeze-dried S. Enteritidis PT 30 at different values of XW using improved thermal-death-time (TDT II) cells and investigated the relationship between D80°C values and XW. We also measured temperature-dependent changes in the aw of freeze-dried S. Enteritidis PT 30 cells at different XW values in order to establish the relationships between the XW and aw of S. Enteritidis PT 30 biomass at different temperatures (isotherms). Finally, we estimated the XW of S. Enteritidis PT 30 in five low-aw systems reported in the literature, generated the linear regression for the logarithmic D80°C values against the XW in these matrices, and compared it to that of freeze-dried S. Enteritidis PT 30.
RESULTS AND DISCUSSION
Thermal inactivation kinetics of freeze-dried S. Enteritidis PT 30.
The population of the concentrated S. Enteritidis PT 30 inoculum was ∼12 log CFU/ml. The initial viability of freeze-dried S. Enteritidis PT 30 was 10.72 ± 0.34 log CFU/g, and a <2 log reduction in population was caused by freeze-drying. The samples were pre-equilibrated under different relative humidities or equivalent aw values at room temperature (∼21°C). In the thermal inactivation tests, the come-up time (CUT, expressed in minutes) for the core temperature of freeze-dried S. Enteritidis PT 30 samples to reach 79.5°C was 1.5 min when samples were heated to 80°C in TDT II test cells. The representative thermal inactivation curves for freeze-dried S. Enteritidis PT 30 with different XW values (7.7, 9.2, 12.4, and 15.7 g water/100 g dry solids) are shown in Fig. 2. The population reduction followed the typical first-order linear regression relationship with the treatment time (equation 1), with an R2 value between 0.84 and 0.98.
| (1) |
where t is the thermal treatment time (in minutes), N> is the bacterial population (in CFU per gram) at time t, N0 is the initial bacterial population at the come-up time (in CFU per gram), and D is the time (in minutes) required to inactivate the microbial population by 90% at a given temperature.
FIG 2.
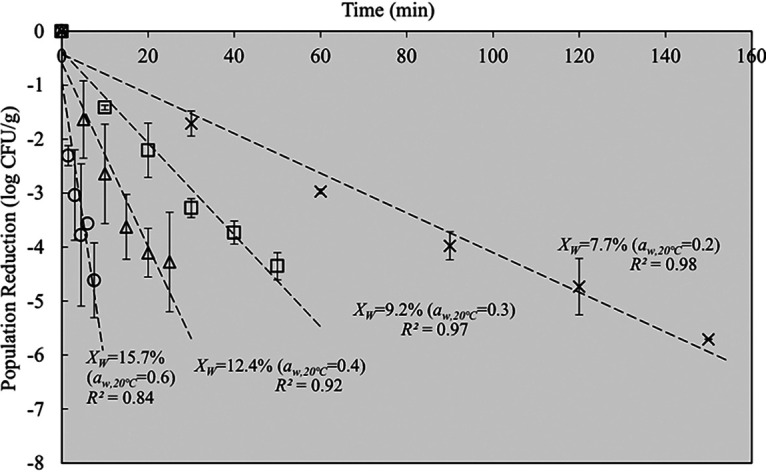
Representative thermal inactivation curves of freeze-dried S. Enteritidis PT 30 at 80°C. XW, moisture content, expressed in grams of water per 100 g dry solids; aw,20°C, equivalent target water activity at room temperature after relative-humidity-controlled equilibration.
More-rapid microbial reductions [] were observed at higher XW values, as shown in Fig. 2, a finding consistent with the thermal inactivation trends of Salmonella and Enterococcus faecium in several low-aw systems (10, 11, 14, 25, 26).
Changes in D-value with XW of bacterial cells.
Microbial survival from thermal inactivation was analyzed using the log-linear model (equation 1) and the Weibull model:
| (2) |
where α is the scale parameter (in minutes) and β is the shape parameter. The Weibull model showed a better goodness of fit than the log-linear model. Given that the Weibull model is performed by two parameters during analysis, and the purpose of this study was to investigate the relationship associated with D-values, thus, the log-linear model was used for subsequent analyses and comparison. The D80°C-values of freeze-dried S. Enteritidis PT 30, obtained from the slope of the trend lines in Fig. 2, were 27.7 ± 0.7, 11.9 ± 0.2, 6.5 ± 1.1, and 1.9 ± 0.6 min at XW values of 7.7, 9.2, 12.4, and 15.7 g water/100 g dry solids, respectively (Table 1). The D80°C-values as influenced by bacterial cell XW are shown in Fig. 3. In general, the D80°C-values decreased exponentially with increasing XW of bacterial cells. This suggests that the XW of bacterial cells is a critical factor that determines the thermal resistance of Salmonella in thermal treatments.
TABLE 1.
D80°C-values of freeze-dried S. Enteritidis PT 30 at different moisture contentsa
| aw,20°C ± 0.02 | Measured XW (g water/100 g dry solids) | aw,80°C ± 0.02 | Linear model |
Weibull model |
|||
|---|---|---|---|---|---|---|---|
| D80°C (min) | R2 | α (min) | β | R2 | |||
| 0.20 | 7.7 ± 0.5 | 0.36 | 27.7 ± 0.7 | 0.98 | 13.8 ± 0.0 | 0.73 ± 0.11 | 0.99 |
| 0.30 | 9.2 ± 0.0 | 0.49 | 11.9 ± 0.2 | 0.97 | 6.1 ± 2.0 | 0.69 ± 0.10 | 0.96 |
| 0.40 | 12.4 ± 0.3 | 0.60 | 6.5 ± 1.1 | 0.92 | 1.9 ± 1.2 | 0.57 ± 0.06 | 0.98 |
| 0.60 | 15.7 ± 1.2 | 0.78 | 1.9 ± 0.6 | 0.84 | 0.4 ± 0.4 | 0.48 ± 0.07 | 0.95 |
Values are means ± standard deviations. The values of aw,80°C were predicted using the CCE (equation 3). R2, coefficient of determination; higher values indicate a better fit of the model.
FIG 3.
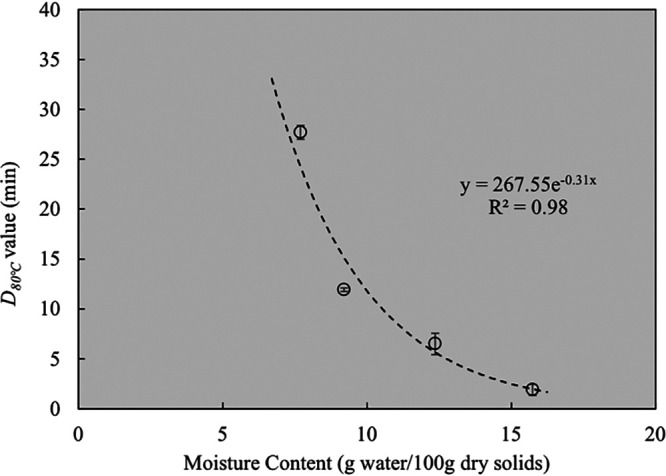
D80°C-values of freeze-dried S. Enteritidis PT 30 at different moisture contents (XW, expressed in grams of water per 100 g dry solids). An exponential trend was observed in D80°C-values as a function of XW.
Moisture sorption isotherms of freeze-dried S. Enteritidis PT 30.
The temperature-dependent changes in the aw of freeze-dried S. Enteritidis PT 30 with different XW values as measured by high-temperature cells were analyzed by the Clausius-Clapeyron equation (CCE) according to the method of Tadapaneni et al. (19), as follows:
| (3) |
where aw1 and aw2 are the water activity values of a sample with the same Xw at temperatures T1 and T2 (in kelvins), respectively; R is the universal gas constant (8.314 joules per mole kelvin); and qst is the net isosteric heat of sorption (in joules per mole), which can be determined from the slope of plotted data (ln aw versus 1/T). These changes in aw are presented in Fig. 4. Generally, the aw of freeze-dried S. Enteritidis PT 30 increased linearly with increasing temperature at a specific XW. This trend is similar to that of high-protein and high-starch food systems, including corn starch, soy protein, and wheat flour (12, 18). However, it dramatically differs from that of high-oil and sugar-rich foods. Specifically, the aw values of peanut oil, coconut milk powder, and almond flour do not increase, and may even decrease, with increasing temperature (14, 16, 18).
FIG 4.
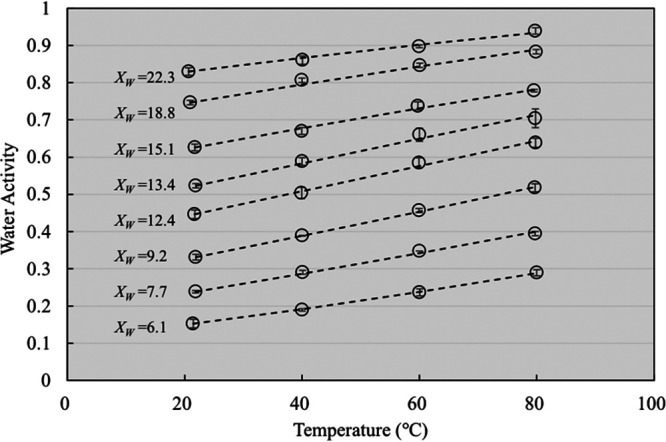
Temperature-dependent changes in the water activity of freeze-dried S. Enteritidis PT 30 at different moisture contents (XW, expressed in grams of water per 100 g dry solids) (n = 3). Open circles represent the average aw values as measured by high-temperature-cells, and dashed lines are derived from equation 3.
The effect of temperature on the aw changes of freeze-dried S. Enteritidis PT 30 was more obvious in samples with a relatively lower XW. For instance, at the XW of 9.2 g water/100 g dry solids, the aw of freeze-dried S. Enteritidis PT 30 at 20°C (aw,20°C) was 0.33. When freeze-dried S. Enteritidis PT 30 was heated to 80°C, its aw (aw,80°C) was increased to 0.52. The aw increased by 0.19. When the XW of the bacterial cells was 22.3 g water/100 g dry solids, the aw of freeze-dried S. Enteritidis PT 30 increased from 0.83 to 0.94 as the temperature was elevated from 20°C to 80°C; thus, a smaller increment in aw (0.11) was obtained. Similar results have also been reported in multiple low-moisture food systems, including wheat flour, almond flour, nonfat milk powder, corn starch, soy protein, cheddar cheese powder, and coconut milk powder (18, 19).
The relationship between the XW of freeze-dried S. Enteritidis PT 30 biomass and its aw at different temperatures, commonly referred to as moisture sorption isotherms, was determined by the Guggenheim-Anderson-de Boer (GAB) model, as follows:
| (4) |
where Xm is the monolayer moisture content (in grams of water per 100 g dry solids) in bacterial cells, aw is the water activity value at Xw, C is a heat constant, ranging from 1 to 20, and K is a multilayer factor, ranging from 0.70 to 1. The results are shown in Fig. 5. The specific parameters for GAB equations (equation 4) at different temperatures in this study are shown in Table 2. The derived monolayer moisture content (Xm) of freeze-dried S. Enteritidis PT 30 ranged from 10.0 g water/100 g dry solids at 20°C to 7.5 g water/100 g dry solids at 80°C (Table 2). These values are much larger than the Xm of most protein-rich systems. For example, Perez-Reyes at al. recently reported Xm of 6 g water/100 g dry solids at 20°C and 5 g water/100 g dry solids at 80°C for egg white powders (84.3% protein, 0.6% fat, dry basis [d.b.]) and 4 g water/100 g dry solids at 20°C and 3 g water/100 g dry solids at 80°C for whole egg powder (46.4% protein, 55.1% fat, d. b.) (27). This suggests that the biomass of freeze-dried S. Enteritidis PT 30 had much larger water binding capacity than those protein-rich powders.
FIG 5.
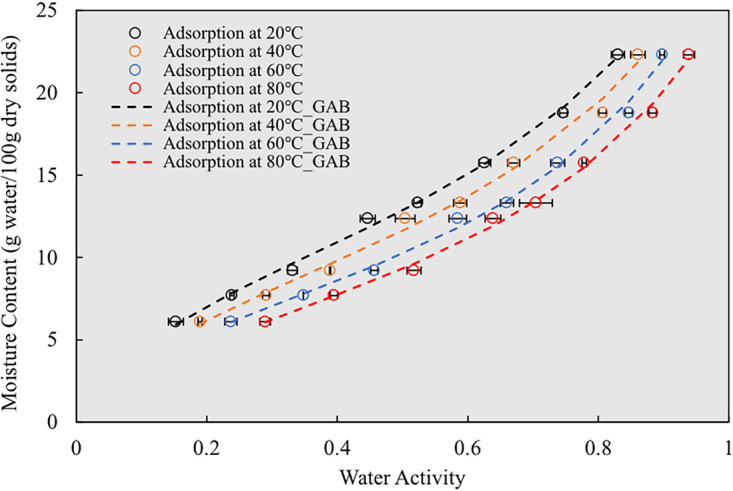
Moisture adsorption isotherms of freeze-dried S. Enteritidis PT 30 at different temperatures. The scattered data were generated using high-temperature cells, and the dashed lines represent the curves fitted by the GAB model (equation 4).
TABLE 2.
Experimental values for the parameters in the GAB model (equation 4) at different treatment temperatures
| Treatment temp (°C) | GAB model parameter |
||
|---|---|---|---|
| Xm (g water/100 g dry solids) | C | K | |
| 20 | 10.0 | 9.42 | 0.70 |
| 40 | 9.1 | 8.43 | 0.71 |
| 60 | 7.6 | 9.00 | 0.75 |
| 80 | 7.5 | 6.67 | 0.73 |
In general, the XW of freeze-dried S. Enteritidis PT 30 increased with increasing aw at any given temperature (Fig. 5). For example, the XW of freeze-dried S. Enteritidis PT 30 was 9.2 g water/100 g dry solids when conditioned to an aw of 0.3 at 20°C. The XW increased to 15.0 g water/100 g dry solids when conditioned to an aw of 0.6 at the same temperature. The aw values of freeze-dried S. Enteritidis PT 30 at a fixed XW increased significantly with heating from 20°C to 80°C, as shown in Fig. 5. The sorption isotherms of freeze-dried S. Enteritidis PT 30 resemble a part of the type II isotherm (S-shaped), exhibiting a sigmoid curve. The two slightly bending areas were observed at aw values below 0.3 and above 0.7. However, J-shaped isotherms were reported for freeze-dried E. faecium cells (17). The difference might be attributed to the differences in cell wall structures and compositions between Gram-positive and Gram-negative bacteria. Salmonella bacteria are Gram negative; the cell wall consists of an outer membrane, a thin layer of peptidoglycan, and a cytoplasmic membrane. E. faecium, on the other hand, is a Gram-positive bacterium; its cell wall consists of a thicker layer of peptidoglycan and one cytoplasmic membrane (29).
High-protein-content or starch-rich food systems, such as corn starch, soy protein, and wheat flour, usually show the same type of isotherm curves as freeze-dried S. Enteritidis PT 30 (6, 18). But for high-sugar-content and oil-rich food systems, the moisture sorption isotherms generally show a type III isotherm (J-shaped), which is concave upward due to more moisture gain from the surface crystalline dissolution at a higher aw (28). The aw of peanut butter also increased sharply with an extremely small increment of XW at room temperature, and this increase was more significant at higher treatment temperatures (6).
Net isosteric heat of sorption for freeze-dried S. Enteritidis PT 30.
The net isosteric heat of sorption (qst), defined as the total enthalpy change for sorption minus the specific latent heat of vaporization of liquid water (30), is a unique parameter for different biomasses. The value of qst reflects the bond energy between water molecules and solid substances, which can be obtained from an empirical relation at a specific XW (31, 32). In this study, qst was related to the XW (expressed in grams of water per 100 g dry solids) of bacterial cells through an exponential relation, as follows:
| (5) |
where the goodness of fit (R2) was >0.99. A comparison of qst for freeze-dried S. Enteritidis PT 30 and several representative low-aw systems is shown in Fig. 6.
FIG 6.
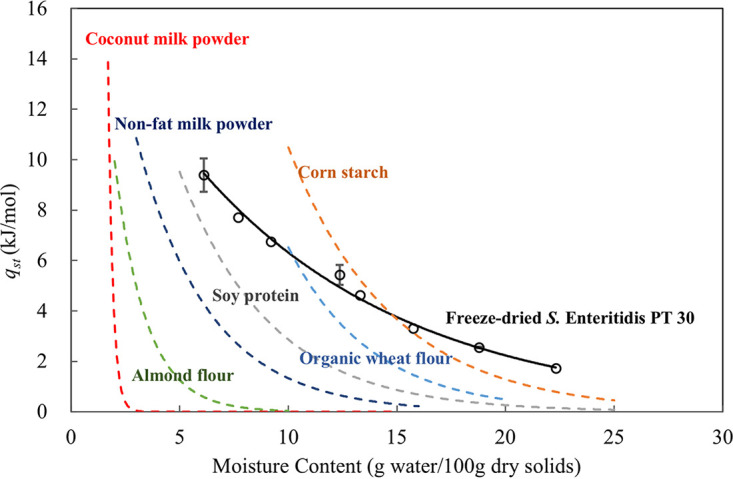
Net isosteric heat of sorption for freeze-dried S. Enteritidis PT 30 and several representative food systems. Open circles represent data calculated on the basis of measurements for freeze-dried S. Enteritidis PT 30. Both the solid curve and the dashed curves were generated by fitting the qst equations. The dashed curves were generated using the data from Jin et al. (18) and Tadapaneni et al. (19).
As shown in Fig. 6, qst correlates negatively with XW. The higher qst values obtained at lower XW values indicate stronger bonds between water molecules and solid matrices (33). That is, more energy is required to break the bonds at a lower XW. The relationship between the qst and XW of freeze-dried S. Enteritidis PT 30 differs sharply from that for the foods shown in Fig. 6. The qst value of freeze-dried S. Enteritidis PT 30 is, in general, higher than that of the high-protein- and high-oil-content foods at XW values between 6 and 10 g water/100 g dry solids and higher than that of all the selected foods when the XW is above 15 g water/100 g dry solids. This observation is consistent with the high values of monolayer moisture contents (Xm) of freeze-dried S. Enteritidis PT 30 discussed above.
A sharp drop in qst with increasing XW was observed for all the foods for which data are shown in Fig. 6. The deepest drop was obtained for oil-rich foods, such as coconut milk powder (consisting of 63.8% fat, d.b. [18]) and almond flour (consisting of 50.7% fat, d.b. [11]), followed by intermediate foods and protein-rich foods (nonfat milk powder and soy protein), and finally high-carbohydrate foods, such as organic wheat flour (86.1% carbohydrate, d.b. [11]) and corn starch (98.0% carbohydrate, d.b. [18]). Lipids are hydrophobic. The hydrophobic interaction between lipids and water is weaker than hydrogen bonds, resulting in a decreased enthalpic demand to break the bonds between water molecules and the solids (34). Carbohydrates are hydrophilic macromolecules, and polysaccharides (such as starch) have strong affinity for water molecules because of multihydroxyl (-OH) groups (35). Combined interactions of hydrogen bonds and glycosidic bonds in carbohydrates are energetically favorable, leading to large amounts of energy required to break the bonds between water and the solids. Thus, high-carbohydrate foods, such as corn starch, have the highest qst of all foods at a specific XW. Proteins are polymers with complex structures, and multiple bonds are present, including peptide bonds (primary structure), hydrogen bonds (secondary and tertiary structure), and ionic bonds and disulfide bonds (tertiary structure) (36). Proteins have less affinity for water than polysaccharides, so the qst of protein-rich foods is lower than that of carbohydrate-rich foods.
Bacterial cells consist of more complex chemical compounds, including proteins, RNA, phospholipids, and polysaccharides (37). The chemical bonds with water molecules in bacterial cells are also complex; the cells may have unique abilities to retain moisture compared to the food matrices included in Fig. 6. Although it is difficult to directly connect the D80°C-values with the qst of freeze-dried S. Enteritidis PT 30, it is noteworthy that they both decrease exponentially with the XW of the bacterial cells (Fig. 3 and 6).
Comparison of log D-values with the XW of S. Enteritidis PT 30 in different matrices.
The log D80°C-values of S. Enteritidis PT 30 in silicon dioxide, wheat flour, whey protein, honey powder, and almond flour (11, 14, 23) are summarized in Table 3. In those studies, the liquid S. Enteritidis PT 30 inoculum was inoculated into the matrices mentioned (wet inoculation) and the inoculated matrices were then conditioned for 3 to 5 days to different aw values at room temperature. The D-values of the bacterium at 80°C were reported against the aw values of the matrices measured at the treatment temperature, 80°C (Table 3). We estimated the XW of S. Enteritidis PT 30 in these low-aw systems using the GAB model (equation 4), and the values are included in Table 3. With those data, we developed a linear regression line between the log D80°C-values and the estimated XW for S. Enteritidis PT 30 in low-aw systems, as shown in Fig. 7 (red dashed line; R2 = 0.88). The linear regression for log D80°C-values of freeze-dried S. Enteritidis PT 30 against its XW obtained from this study is also plotted as a dark dashed line (R2 = 0.98), in Fig. 7, along with 95% confidence intervals (represented by the shaded area). Interestingly, the log D80°C-values of S. Enteritidis PT 30 in silicon dioxide and different low-moisture foods are scattered mostly within the 95% confidence interval area for freeze-dried S. Enteritidis PT 30. The regression line derived from those data is found to fall entirely within the 95% confidence intervals. Moreover, the regression lines for freeze-dried S. Enteritidis PT 30 and low-aw systems are almost overlapping. This suggests that the thermal resistance of S. Enteritidis PT 30 was largely determined by its XW. This is reasonable, because the aw of the microenvironment uniquely controlled the XW of the bacterial cells according to the relationships in Fig. 5, regardless of the matrices.
TABLE 3.
Estimated moisture contents of S. Enteritidis PT 30 using the GAB model at 80°C with reported D-values and aw,80°C in different low-aw systemsa
| Low-aw system | aw,80°C | D80°C-value (min) | Estimated XW of S. Enteritidis PT 30 (g water/100 g dry solids) |
|---|---|---|---|
| Wheat flour (11) | 0.47 | 12.2 ± 0.7 | 8.8 |
| 0.68 | 4.9 ± 0.5 | 12.8 | |
| 0.78 | 1.2 ± 0.2 | 15.5 | |
| Almond flour (11) | 0.43 | 27.3 ± 0.3 | 8.2 |
| 0.54 | 21.2 ± 0.9 | 10.0 | |
| 0.63 | 11.1 ± 0.8 | 11.7 | |
| 0.81 | 0.8 ± 0.1 | 16.5 | |
| Whey protein (11) | 0.41 | 17.5 ± 1.3 | 7.9 |
| 0.62 | 10.6 ± 0.2 | 11.5 | |
| 0.74 | 5.1 ± 0.4 | 14.4 | |
| 0.87 | 1.5 ± 0.1 | 18.7 | |
| Honey powder (23) | 0.18 | 35.6 ± 2.3 | 4.3 |
| 0.31 | 27.3 ± 3.2 | 6.4 | |
| 0.40 | 19.6 ± 1.8 | 7.7 | |
| 0.50 | 14.4 ± 1.1 | 9.3 | |
| Silicon dioxide (14) | 0.18 | 159.3 ± 5.8 | 4.1 |
| 0.27 | 64.0 ± 0.2 | 5.7 | |
| 0.37 | 30.7 ± 1.0 | 7.3 | |
| 0.47 | 21.3 ± 1.4 | 8.8 | |
| 0.55 | 10.4 ± 0.3 | 10.1 | |
| 0.63 | 6.8 ± 0.3 | 11.8 | |
| 0.72 | 1.8 ± 0.1 | 13.8 |
FIG 7.
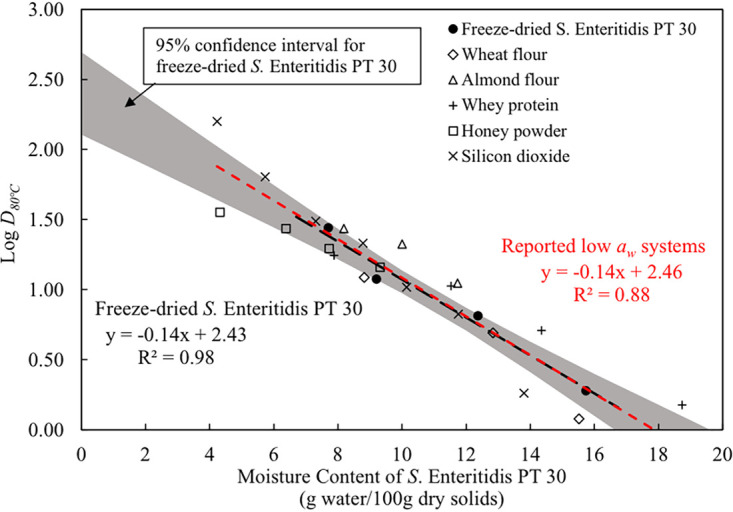
Comparison of logarithmic D80°C-values of S. Enteritidis PT 30 in this study and in other reported low-aw systems as influenced by the moisture content of bacterial cells. The shaded area represents the 95% confidence interval of log D80°C-values for freeze-dried S. Enteritidis PT 30. The dark dashed line represents the linear regression of logarithmic D80°C-values for freeze-dried S. Enteritidis PT 30; the red dashed line represents that of S. Enteritidis PT 30 for other reported low-aw systems.
Interpretation of the thermal resistance of Salmonella in low-aw systems.
The aw directly reflects the moisture vapor pressure within the food systems where bacteria are embedded. As demonstrated in Fig. 1, moisture diffusion occurs between the bacterial cells and the microenvironment when their aw values are not the same during heating. In general, the trends of aw changes in protein- or starch-rich low-moisture foods are similar to that of bacterial cells when heated to a high temperature (11, 17–19), and the XW of the bacterial cells does not change much during a thermal treatment. However, the aw of oil-rich foods is stable or even decreased when they are heated to high temperatures (15, 18, 19). The bacterial cells in oil and oil-rich foods may be dehydrated during thermal treatment (15). For instance, when the wheat flour (rich in protein and starch) or almond flour (oil rich) in which S. Enteritidis PT 30 is embedded is equilibrated to an aw of 0.45 at room temperature and then heated to 80°C, the aw of freeze-dried S. Enteritidis PT 30 would increase to 0.65 as predicted by the CCE (equation 3) of this study. The aw,80°C values of wheat flour and almond flour would increase to 0.68 and 0.54, respectively (Table 4). According to the GAB modeled moisture sorption isotherms at 80°C (Table 2), the XW of freeze-dried S. Enteritidis PT 30 at an aw,20°C of 0.45 would be 12.2 g water/100 g dry solids. Due to moisture diffusion, the XW of the bacterial cells would increase to 12.8 g water/100 g dry solids (gaining 0.6 g water/100 g dry solids) in wheat flour but decrease to 10.0 g water/100 g dry solids in almond flour (losing 2.2 g water/100 g dry solids) (Table 4). According to the exponential relation shown in Fig. 3, the predicted D80°C-value of freeze-dried S. Enteritidis PT 30 in the wheat flour would be 4.9 min when the XW of the bacterial cells is 12.8 g water/100 g dry solids, which is not significantly different from the reported D80°C-value of S. Enteritidis PT 30 in wheat flour (4.9 min; Table 3) (11). In contrast , the predicted D80°C-value of S. Enteritidis PT 30 in almond flour would be 11.9 min, which is lower than the reported one (21.2 min; Table 3). Similar observations can be made when two foods are preconditioned to an aw of 0.6 at room temperature and heated to 80°C (as summarized in Table 4). It is shown from the examples presented above that the equilibrium XW of S. Enteritidis PT 30 in heated food matrices is the dominant factor determining this organism’s resistance in thermal treatments. Dehydration in bacterial cells shows its contribution to enhancing heat resistance (larger D-values).
TABLE 4.
Predicted D80°C-values for S. Enteritidis PT 30 in low-aw systems
| aw,20°C | Low-aw system | aw,80°Ca | Estimated XW of S. Enteritidis PT 30b (g water/100 g dry solids) | D80°C-valuec (min) |
|---|---|---|---|---|
| 0.45 | Wheat flour | 0.68 | 12.8 | 4.9 ± 1.0 |
| Almond flour | 0.54 | 10.0 | 11.9 ± 1.8 | |
| 0.60 | Wheat flour | 0.78 | 15.5 | 2.1 ± 0.6 |
| Almond flour | 0.63 | 11.7 | 6.9 ± 1.3 |
The aw,80°C values of wheat flour and almond flour were derived from the work of Xu et al. (11).
The XW values of S. Enteritidis PT 30 were obtained from the GAB model (equation 4) at 80°C.
The D80°C-values of freeze-dried Salmonella were estimated from experiment-based regression as shown in Fig. 3.
The results of this study allow prediction of the XW of S. Enteritidis PT 30 in a low-moisture food by using the GAB model (equation 4 and Table 2) and make it possible to estimate the corresponding D-value of S. Enteritidis PT 30 by using the exponential equation in Fig. 3. Taking peanut oil as another example: if the aw of peanut oil is 0.53 at room temperature, it will decrease to 0.21 when the peanut oil is heated to 80°C, according to Yang et al. (39). The estimated XW of S. Enteritidis PT 30 in the peanut oil will be 4.8 g water/100 g dry solids, and the corresponding D80°C-value will be 59.4 min. That is, it would take 6 h of heating at 80°C to achieve a 6-log reduction of S. Enteritidis PT 30 in peanut oil with an initial aw of 0.53.
It is reasonable to speculate that the thermal inactivation of S. Enteritidis PT 30 is caused mainly by irreversible stereochemical structural alterations of the hydrophilic protein components of the cells at 80°C (40). Aggregation of cytoplasmic proteins (including ribosomes) and denaturation of DNA in high-moisture S. Enteritidis PT 30 cells were observed from transmission electron micrographs after a 10-min thermal treatment at 80°C, while no visible aggregates were found in dried cells within 60 min of heating at 80°C (40). Indeed, it has been reported that the thermal inactivation of bacterial cells with high XW is attributed mainly to the loss of functionality of proteins (40–42). Cytoplasmic proteins, DNA, and rRNA of bacterial cells are considered to be the major cellular targets in microbial inactivation induced by heat (43). It has also been reported that the thermal denaturation of proteins is more effective in water than in dry air, since the preferential hydration of solutes under low-moisture conditions provides protection against protein denaturation (29, 44, 45). Dehydration of proteins would induce conformational transitions (46); the absence of water may prevent proteins from deformation (47, 48) and produce more-compatible solutes to counteract the environmental stress (45) and thus may somehow stabilize aggregate-structured cytoplasmic proteins, DNA, and ribosomal units against thermally induced damages (40, 48). More systematic studies are needed to investigate the roles of water molecules in structural alterations of key cellular components, in particular in the rate of denaturation of functional proteins, during thermal inactivation of microbial organisms.
Conclusion.
This study established a quantitative relationship between the XW and D-values of freeze-dried S. Enteritidis PT 30. The moisture sorption isotherms provided the bridge for comparing our results with previously reported data on the thermal resistance of S. Enteritidis PT 30 in multiple low-aw matrices. The linear relationship between the log D-values of S. Enteritidis PT 30 and its XW can be used to predict its thermal resistance in different low-moisture foods and thus to design effective industrial thermal processes for the control of Salmonella in low-moisture foods. Our study provided experimental evidence to support the hypothesis that the thermal resistance of bacteria is intrinsically determined by the amount of moisture in the bacterial cells, which is adjusted by the aw of low-moisture foods or environmental relative humidity at the treatment temperature. Food systems, such as wheat flour, that have large increases in aw during heating will cause hydration of bacterial cells (increase in cell XW), making them more vulnerable to thermal treatment. On the other hand, bacterial cells in oil-rich foods are more difficult to inactivate because of desiccation. Similarly, in an open environment, bacterial cells are more difficult to inactivate in low-relative-humidity environments than at high relative humidities. This work also demonstrates the importance of predicting aw of food matrices at treatment temperatures in thermal inactivation of pathogens. This study was limited to S. Enteritidis PT 30. It will be interesting to explore direct connections between the moisture contents of other serotypes or strains and their thermal resistance. Validation of the quantitative relations between the moisture content of bacterial cells and their thermal resistance may help further investigation into the fundamental roles that water molecules play in the denaturation of key protein components, leading to thermal inactivation of bacterial pathogens.
MATERIALS AND METHODS
Preparation of freeze-dried S. Enteritidis PT 30.
The stock culture of Salmonella Enteritidis PT 30 (ATCC 1045) was acquired from Linda Harris at the University of California, Davis. It was stored at –80°C in tryptic soy broth supplemented with 0.6% (wt/vol) yeast extract (TSBYE; Difco, Detroit, MI, USA) and 20% (vol/vol) glycerol.
The procedure to produce the biomass of freeze-dried of S. Enteritidis PT 30 was modified based on a previous study (22). Briefly, a loop of thawed stock was inoculated into 9 ml TSBYE with two consecutive transfers and was incubated at 37°C for 24 h. Three hundred microliters of this aliquot was transferred to a centrifuge tube with 30 ml TSBYE and was incubated at 37°C for 24 h. Then 4 ml of the previous culture broth was further transferred to a sterile conical flask with 400 ml TSBYE and was incubated at 37°C for 24 h with constant shaking at 230 rpm. The enlarged bacterial culture was further washed twice with sterile double-distilled water (ddH2O) by centrifuging at 6,000 × g for 10 min at 4°C. The supernatant was discarded, and the washed pellets from a total of 1.2 liters of bacterial broth (3 conical flasks) were pooled and resuspended in 6 ml sterile ddH2O. Washing caused a reduction in population of ∼0.06 log10 CFU/ml, and the population in the concentrated bacterial suspension was ∼12.3 log10 CFU/ml. One milliliter of the above bacterial suspension (∼12 log10 CFU/ml) was distributed into each sterile clear serum vial (5 ml; outer diameter, 22 mm; Wheaton; DWK Life Sciences, Millville, NJ, USA) and then loosely sealed with a 2-leg lyophilization stopper (DWK Life Sciences, Millville, NJ, USA). Vials were prefrozen in liquid nitrogen for a few minutes and then lyophilized at –90°C and 0.6 Pa for 48 h in a freeze-dryer (FreeZone Plus 4.5-liter cascade benchtop freeze-dry system, Labconco Corporation, Kansas City, MO, USA).
Generally, about 2 g of freeze-dried S. Enteritidis PT 30 was harvested from the vials after each batch of freeze-drying. All solids from each batch were collected into a 4-oz sterilized Whirl-Pak bag (Nasco, Modesto, CA, USA), hand mixed for 5 min to eliminate clumps, and further homogenized using a stomacher at 230 rpm for 3 min (Stomacher model 400 circulator; Seward Laboratory Systems Inc., Norfolk, UK). Then this homogeneous freeze-dried S. Enteritidis PT 30 powder (10 to 11 log10 CFU/g) was kept at –80°C until use (within 1 week). Each batch of the sample was used in an individual thermal treatment. All repeated batches were derived from the same cold stock and were cultivated independently.
Viability test of freeze-dried S. Enteritidis PT 30.
The viability and population of freeze-dried S. Enteritidis PT 30 were determined immediately by placing 0.100 ± 0.010 g in a 2-ml sterile snap-lock microtube (Axygen, Union City, CA, USA) and then mixing with 0.9 ml buffered peptone water (BPW; Difco, Detroit, MI, USA) to obtain a 10-fold dilution. The freeze-dried S. Enteritidis PT 30 was fully mixed with BPW by subsequent vortexing (Fisherbrand pulsing vortex mixer; Thermo Fisher Scientific, Waltham, MA, USA). Serial dilutions were performed to proper levels, and the diluted material was then spread in duplicate for enumeration at 37°C for 48 h on modified TSAYE plates (49), which were made up of tryptic soy agar (Difco, Detroit, MI, USA), 0.6% (wt/vol) yeast extract (Difco, Detroit, MI, USA), 0.05% (wt/vol) ferric ammonium citrate (Sigma-Aldrich, St. Louis, MO, USA) and 0.03% (wt/vol) sodium thiosulfate (Sigma-Aldrich, St. Louis, MO, USA). Typical Salmonella cells exhibited colonies that resembled dark solid circles. The average number of viable colonies was expressed as the CFU count per gram based on two technical replicates.
Conditioning freeze-dried S. Enteritidis PT 30 biomass to different aw values.
The initial aw of the above homogeneous freeze-dried S. Enteritidis PT 30 powder was <0.025 at room temperature (∼21°C), as measured with a water activity meter (Aqualab; Meter Group, Inc., Pullman, WA, USA). The XW of freeze-dried S. Enteritidis PT 30 was determined by the oven-drying method according to AOAC official method 925.10 (50).
In order to obtain freeze-dried S. Enteritidis PT 30 powder samples with different aw or XW levels, freeze-dried S. Enteritidis PT 30 was evenly spread in each sample cup (∼300 mg for high-temperature cells) or petri dish (∼2 g for thermal treatment) and conditioned at room temperature for 2 to 3 days in airtight jars with a saturated salt solution under various relative humidity levels at room temperature (19). The saturated salt solutions of LiCl, CH3COOK, MgCl2, K2CO3, Mg(NO3)2, NaNO2, NaCl, and KCl could generate a consistent relative humidity of 11.3%, 22.5%, 32.8%, 43.2%, 52.9%, 65.8%, 75.3%, and 84.3%, respectively, at room temperature, which corresponded to the equivalent target aw values of 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.8, respectively, at room temperature. In measuring the moisture sorption isotherms by high-temperature cells, freeze-dried S. Enteritidis PT 30 was conditioned at all the above relative humidity levels. For the thermal treatments, freeze-dried S. Enteritidis PT 30 was conditioned at four selected relative humidity levels (22.5%, 32.8%, 43.2%, and 65.8%). The equilibration of the freeze-dried S. Enteritidis PT 30 samples was verified by measuring the aw value using the water activity meter. Samples were used for all experiments after the target aw ± 0.02 was reached.
Thermal treatments of freeze-dried of S. Enteritidis PT 30.
Thermal treatments of freeze-dried S. Enteritidis PT 30 were performed at 80°C using the improved aluminum thermal-death-time test cells (TDT II) in triplicate. TDT II cells are thinner (height, 1.39 mm) and have a larger cavity (diameter, 31.18 mm) than traditional TDT cells (51). During the heating process, the XW of freeze-dried S. Enteritidis PT 30 should remain constant in well-sealed TDT II cells, while the aw of freeze-dried S. Enteritidis PT 30 could change with increasing temperatures.
Come-up time (CUT, in minutes) is the time required for samples to reach the target temperature within 0.5°C. It was measured using a specially designed TDT II cell installed with a 0.5-mm-diameter thermocouple (type T; Omega Engineering, Inc., Stamford, CT, USA) secured on the top center of the lid. The cell was filled with 100 mg of freeze-dried S. Enteritidis PT 30 and sealed. The core temperature of the freeze-dried S. Enteritidis PT 30 in the test cell was measured by a thermometer (Digi-Sense DuaLogR, model 99100-50; Cole-Parmer Instruments Co., Vernon Hills, IL, USA) when it was subjected to thermal treatment at 80°C. The CUT was determined in triplicate.
Freeze-dried S. Enteritidis PT 30 samples were conditioned for 2 to 3 days to reach the target aw (0.2, 0.3, 0.4, and 0.6). A portion (0.100 ± 0.010 g) of the equilibrated freeze-dried S. Enteritidis PT 30 was loaded into a TDT II cell and sealed. Thermal treatment at 80°C was carried out by immersing test cells in a preheated ethylene glycol bath circulator (Fisherbrand Isotemp 5150 H24; Thermo Fisher Scientific, PA, USA). Duplicate TDT II cells were removed from the circulator at five predetermined sampling points (come-up time was regarded as 0 min) and immediately cooled in an ice water bath for 1 min. Thermal inactivation treatments were independently repeated in triplicate.
Thermally treated freeze-dried S. Enteritidis PT 30 was transferred from the test cell to a 2-ml sterile snap-lock microtube (MCT-200-C; Axygen, Union City, CA, USA), and 0.9 ml of sterile BPW was added to achieve a 10-fold dilution. Next, the bacterial suspension was homogenized using a vortex and was further serially diluted 10-fold. The appropriate dilutions were spread on modified TSAYE plates in two technical replicates and were incubated at 37°C for 48 h. The average number of viable colonies at each time point was converted to CFU per gram based on two technical replicates.
Statistical analysis.
The first-order kinetic model (log-linear model; equation 1) and the Weibull model (equation 2) were applied to analyze thermal inactivation (52). The thermal decimal time (D-value) in minutes was estimated by these models, and the goodness of fit was evaluated by the R2 coefficient. Statistical analysis of the standard deviation (SD) was performed using Microsoft Excel (version 16.35; Microsoft)
Determination of aw changes of freeze-dried S. Enteritidis PT 30 with increasing temperatures.
The aw changes of freeze-dried S. Enteritidis PT 30 at different temperatures were determined using aluminum high-temperature cells (Meter Group, Inc., Pullman, WA, USA) designed by Tadapaneni et al. (19). The aw was measured by a capacitance-based relative humidity and temperature sensor (HumidIcon; Honeywell, Morristown, NJ, USA) located on the center of the inner side lid. The sample cup with pre-equilibrated freeze-dried S. Enteritidis PT 30 (300 mg) was placed in a high-temperature cell and sealed tightly to prevent any leakage. High-temperature cells were kept at room temperature (∼21°C) and then heated in 20°C increments from 40°C to 80°C by immersion in a heated glycol bath (Fisherbrand Isotemp 5150 H24; Fisher Scientific, PA, USA). The relative humidity and temperature of the headspace were read every minute, and the equilibrium state at the respective temperature was achieved when constant relative humidity values were obtained for at least 10 readings. Then the temperature and relative humidity values were recorded, and the relative humidity value was considered to be the corresponding aw value of the equilibrated freeze-dried S. Enteritidis PT 30 in this closed system at a certain temperature (19). After the above series of measurements was recorded, and the high-temperature cells were cooled to room temperature, the XW of freeze-dried S. Enteritidis PT 30 was determined. All experiments were performed in triplicate.
Clausius-Clapeyron equation and moisture sorption isotherms.
The experimental aw data were fitted by a modified Clausius-Clapeyron equation (CCE) (equation 3) according to Tadapaneni et al. (19). Data measured by high-temperature cells were further fitted according to the Guggenheim-Anderson-de Boer (GAB) model (equation 4) to generate moisture sorption isotherms, providing the relationship between aw and XW (28).
ACKNOWLEDGMENTS
This study was funded by the USDA Agricultural and Food Research Initiative (AFRI) CAP grant (2015-68003-2341) and was partially funded by the Washington State University Agriculture Research Center. This work was also supported in part by the USDA National Institute of Food and Agriculture, Hatch project (accession no. 1016366). Yucen Xie appreciates the grant from the China Scholarship Council (CSC) for supporting her Ph.D. study.
REFERENCES
- 1.Enache E, Podolak R, Kataoka A, Harris LJ. 2017. Persistence of Salmonella and other bacterial pathogens in low-moisture foods, p 67–86. In Podolak R, Black DG (ed), Control of Salmonella and other bacterial pathogens in low-moisture foods. John Wiley & Sons, Ltd, Chichester, United Kingdom. [Google Scholar]
- 2.Labuza TP. 1980. The effect of water activity on reaction kinetics of food deterioration. Food Technol 34:36–41. [Google Scholar]
- 3.Syamaladevi RM, Tang J, Villa-Rojas R, Sablani S, Carter B, Campbell G. 2016. Influence of water activity on thermal resistance of microorganisms in low-moisture foods: a review. Compr Rev Food Sci Food Saf 15:353–370. doi: 10.1111/1541-4337.12190. [DOI] [PubMed] [Google Scholar]
- 4.Podolak R, Black DG. 2017. Introduction and overview, p 1–27. In Podolak R, Black DG (ed), Control of Salmonella and other bacterial pathogens in low-moisture foods. John Wiley & Sons, Ltd, Chichester, United Kingdom. [Google Scholar]
- 5.Li H, Wang H, D’Aoust J-Y, Maurer J. 2013. Salmonella species, p 225–261. In Doyle MP, Buchanan RL (ed), Food microbiology: fundamentals and frontiers, 4th ed. American Society for Microbiology, Washington, DC. [Google Scholar]
- 6.Syamaladevi RM, Tadapaneni RK, Xu J, Villa-Rojas R, Tang J, Carter B, Sablani S, Marks B. 2016. Water activity change at elevated temperatures and thermal resistance of Salmonella in all purpose wheat flour and peanut butter. Food Res Int 81:163–170. doi: 10.1016/j.foodres.2016.01.008. [DOI] [Google Scholar]
- 7.CDC. 2020. Salmonella and food. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/foodsafety/communication/salmonella-food.html.
- 8.EFSA. 2019. Salmonella the most common cause of foodborne outbreaks in the European Union. European Food Safety Authority, Parma, Italy: https://www.efsa.europa.eu/en/news/salmonella-most-common-cause-foodborne-outbreaks-european-union. [Google Scholar]
- 9.Podolak R, Enache E, Stone W, Black DG, Elliott PH. 2010. Sources and risk factors for contamination, survival, persistence, and heat resistance of Salmonella in low-moisture foods. J Food Prot 73:1919–1936. doi: 10.4315/0362-028X-73.10.1919. [DOI] [PubMed] [Google Scholar]
- 10.Villa-Rojas R, Tang J, Wang S, Gao M, Kang DH, Mah JH, Gray P, Sosa-Morales ME, Pez-Malo AL. 2013. Thermal inactivation of Salmonella Enteritidis PT 30 in almond kernels as influenced by water activity. J Food Prot 76:26–32. doi: 10.4315/0362-028X.JFP-11-509. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Tang J, Jin Y, Song J, Yang R, Sablani SS, Zhu MJ. 2019. High temperature water activity as a key factor influencing survival of Salmonella Enteritidis PT30 in thermal processing. Food Control 98:520–528. doi: 10.1016/j.foodcont.2018.11.054. [DOI] [Google Scholar]
- 12.Tadapaneni RK, Xu J, Yang R, Tang J. 2018. Improving design of thermal water activity cell to study thermal resistance of Salmonella in low-moisture foods. LWT 92:371–379. doi: 10.1016/j.lwt.2018.02.046. [DOI] [Google Scholar]
- 13.Jin Y, Tang J, Zhu M-J. 2020. Water activity influence on the thermal resistance of Salmonella in soy protein powder at elevated temperatures. Food Control 113:107160. doi: 10.1016/j.foodcont.2020.107160. [DOI] [Google Scholar]
- 14.Liu S, Tang J, Tadapaneni RK, Yang R, Zhu MJ. 2018. Exponentially increased thermal resistance of Salmonella spp. and Enterococcus faecium at reduced water activity. Appl Environ Microbiol 84:e02742-17. doi: 10.1128/AEM.02742-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang R, Guan J, Sun S, Sablani SS, Tang J. 2020. Understanding water activity change in oil with temperature. Curr Res Food Sci 3:158–165. doi: 10.1016/j.crfs.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang R, Xie Y, Lombardo SP, Tang J. 2020. Oil protects bacteria from humid heat in thermal processing. Food Control 2020:107690. doi: 10.1016/j.foodcont.2020.107690. [DOI] [Google Scholar]
- 17.Syamaladevi RM, Tang J, Zhong QP. 2016. Water diffusion from a bacterial cell in low-moisture foods. J Food Sci 81:R2129–R2134. doi: 10.1111/1750-3841.13412. [DOI] [PubMed] [Google Scholar]
- 18.Jin Y, Tang J, Sablani SS. 2019. Food component influence on water activity of low-moisture powders at elevated temperatures in connection with pathogen control. LWT 112:108257. doi: 10.1016/j.lwt.2019.108257. [DOI] [Google Scholar]
- 19.Tadapaneni RK, Yang R, Carter B, Tang J. 2017. A new method to determine the water activity and the net isosteric heats of sorption for low moisture foods at elevated temperatures. Food Res Int 102:203–212. doi: 10.1016/j.foodres.2017.09.070. [DOI] [PubMed] [Google Scholar]
- 20.Horton RA, Moran LA, Scrimgeour G, Perry M, Rawn D. 2006. Principles of biochemistry, 4th ed Pearson Prentice Hall, Upper Saddle River, NJ. [Google Scholar]
- 21.Finn S, Condell O, McClure P, Amézquita A, Fanning S. 2013. Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Front Microbiol 4:331. doi: 10.3389/fmicb.2013.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Liu S, Song J, Tang J, Zhu MJ, Gray P, Villa-Rojas R. 2018. Dry-inoculation method for thermal inactivation studies in wheat flour using freeze-dried Enterococcus faecium NRRL B-2354. LWT 89:10–17. doi: 10.1016/j.lwt.2017.10.006. [DOI] [Google Scholar]
- 23.Alshammari J, Xu J, Tang J, Sablani S, Zhu M-J. 2020. Thermal resistance of Salmonella in low-moisture high-sugar products. Food Control 114:107255. doi: 10.1016/j.foodcont.2020.107255. [DOI] [Google Scholar]
- 24.Jeong S, Marks BP, Orta-Ramirez A. 2009. Thermal inactivation kinetics for Salmonella Enteritidis PT30 on almonds subjected to moist-air convection heating. J Food Prot 72:1602–1609. doi: 10.4315/0362-028X-72.8.1602. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Rojas RV, Gray P, Zhu MJ, Tang J. 2018. Enterococcus faecium as a Salmonella surrogate in the thermal processing of wheat flour: influence of water activity at high temperatures. Food Microbiol 74:92–99. doi: 10.1016/j.fm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y, Pickens SR, Hildebrandt IM, Burbick SJ, Grasso-Kelley EM, Keller SE, Anderson NM. 2018. Thermal inactivation of Salmonella Agona in low-water activity foods: predictive models for the combined effect of temperature, water activity, and food component. J Food Prot 81:1411–1417. doi: 10.4315/0362-028X.JFP-18-041. [DOI] [PubMed] [Google Scholar]
- 27.Santillana Farakos SM, Frank JF, Schaffner DW. 2013. Modeling the influence of temperature, water activity and water mobility on the persistence of Salmonella in low-moisture foods. Int J Food Microbiol 166:280–293. doi: 10.1016/j.ijfoodmicro.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Labuza TP, Altunakar L. 2007. Water activity prediction and moisture sorption isotherms, p 109–154. In Barbosa-Cnovas GV, Fontana AJ, Schmidt SJ, Labuza TP (ed), Water activity in foods. Blackwell Publishing Ltd, Oxford, United Kingdom. [Google Scholar]
- 29.Jay JM. 2000. Modern food microbiology, 6th ed Aspen Publishers, Gaithersburg, MD. [Google Scholar]
- 30.Labuza TP, Kaanane A, Chen JY. 2006. Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods. J Food Sci 50:385–392. doi: 10.1111/j.1365-2621.1985.tb13409.x. [DOI] [Google Scholar]
- 31.Corrêa PC, Goneli ALD, Jaren C, Ribeiro DM, Resende O. 2007. Sorption isotherms and isosteric heat of peanut pods, kernels and hulls. Food Sci Technol Int 13:231–238. doi: 10.1177/1082013207079601. [DOI] [Google Scholar]
- 32.Al-Muhtaseb AH, McMinn WAM, Magee TRA. 2002. Moisture sorption isotherm characteristics of food products: a review. Food Bioprod Process 80:118–128. doi: 10.1205/09603080252938753. [DOI] [Google Scholar]
- 33.Labuza TP. 1977. The properties of water in relationship to water binding in foods: a review. J Food Process Preserv 1:167–190. doi: 10.1111/j.1745-4549.1977.tb00321.x. [DOI] [Google Scholar]
- 34.Mathlouthi M. 2001. Water content, water activity, water structure and the stability of foodstuffs. Food Control 12:409–417. doi: 10.1016/S0956-7135(01)00032-9. [DOI] [Google Scholar]
- 35.Guo MQ, Hu X, Wang C, Ai L. 2017. Polysaccharides: structure and solubility, p 7–21. In Xu Z. (ed), Solubility of polysaccharides. InTech, London, United Kingdom. [Google Scholar]
- 36.McMurry J. 2010. Fundamentals of general, organic and biological chemistry, 6th ed. Pearson Prentice Hall, Upper Saddle River, NJ. [Google Scholar]
- 37.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. 2015. Molecular biology of the cell, 6th ed. Garland Science, Taylor and Francis Group, New York, NY. [Google Scholar]
- 38.Reference deleted.
- 39.Yang R, Xu J, Lombardo SP, Ganjyal GM, Tang J. 2020. Desiccation in oil protects bacteria in thermal processing. Food Res Int 137:109519. doi: 10.1016/j.foodres.2020.109519. [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Shah DH, Song J, Tang J. 2020. Changes in cellular structure of heat‐treated Salmonella in low‐moisture environments. J Appl Microbiol 129:e14614. doi: 10.1111/jam.14614. [DOI] [PubMed] [Google Scholar]
- 41.Mackey BM, Miles CA, Parsons SE, Seymour DA. 1991. Thermal denaturation of whole cells and cell components of Escherichia coli examined by differential scanning calorimetry. J Gen Microbiol 137:2361–2374. doi: 10.1099/00221287-137-10-2361. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Kaletunç G. 2002. Evaluation of the heat inactivation of Escherichia coli and Lactobacillus plantarum by differential scanning calorimetry. Appl Environ Microbiol 68:5379–5386. doi: 10.1128/AEM.68.11.5379-5386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cebrián G, Condón S, Mañas P. 2017. Physiology of the inactivation of vegetative bacteria by thermal treatments: mode of action, influence of environmental factors and inactivation kinetics. Foods 6:107. doi: 10.3390/foods6120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Killian MS, Taylor AJ, Castner DG. 2018. Stabilization of dry protein coatings with compatible solutes. Biointerphases 13:e06E401. doi: 10.1116/1.5031189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roychoudhury A, Bieker A, Häussinger D, Oesterhelt F. 2013. Membrane protein stability depends on the concentration of compatible solutes – a single molecule force spectroscopic study. Biol Chem 394:1465–1474. doi: 10.1515/hsz-2013-0173. [DOI] [PubMed] [Google Scholar]
- 46.Yoneda JS, Miles AJ, Araujo APU, Wallace BA. 2017. Differential dehydration effects on globular proteins and intrinsically disordered proteins during film formation. Protein Sci 26:718–726. doi: 10.1002/pro.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ball P. 2008. Water as an active constituent in cell biology. Chem Rev 108:74–108. doi: 10.1021/cr068037a. [DOI] [PubMed] [Google Scholar]
- 48.Potts M. 1994. Desiccation tolerance of prokaryotes. Microbiol Rev 58:755–805. doi: 10.1128/MMBR.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLaughlin MR, Balaa MF. 2006. Enhanced contrast of bacteriophage plaques in Salmonella with ferric ammonium citrate and sodium thiosulfate (FACST) and tetrazolium red (TZR). J Microbiol Methods 65:318–323. doi: 10.1016/j.mimet.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Helrich K. 1990. Solids (total) and moisture in flour, method 925.10 In Official methods of analysis of the AOAC, 15th ed Association of Official Analytical Chemists, Washington, DC. [Google Scholar]
- 51.Jin Y, Tang J. 2019. Improved design of aluminum test cell to study the thermal resistance of Salmonella enterica and Enterococcus faecium in low-water activity foods. Food Control 104:343–348. doi: 10.1016/j.foodcont.2019.05.008. [DOI] [Google Scholar]
- 52.Peleg M. 2006. Advanced quantitative microbiology for foods and biosystems: models for predicting growth and inactivation. CRC Press, Boca Raton, FL. [Google Scholar]