Abstract
Study Objectives:
The purpose of this study was to evaluate the feasibility and acceptability of a novel cognitive behavioral therapy for hypersomnia (CBT-H) in people with central disorders of hypersomnolence and co-occurring depressive symptoms using a telehealth model for delivery and assessment.
Methods:
Thirty-five adults with narcolepsy or idiopathic hypersomnia received a 6-session CBT-H delivered individually or in small groups using videoconferencing. The clinical impact of CBT-H was evaluated using the Patient Health Questionnaire, Patient-Reported Outcomes Measurement Information System measures, Epworth Sleepiness Scale, and other patient-reported outcomes collected online at baseline and posttreatment. Feasibility and acceptability of the intervention and telehealth model was also evaluated using qualitative data collected from exit interviews conducted through videoconferencing.
Results:
Forty percent of the sample achieved a clinically significant baseline to posttreatment change in depressive symptoms (decrease in Patient Health Questionnaire ≥ 5), which is below the prespecified efficacy benchmark (50% of the sample). The prespecified benchmark for a minimal clinically important difference (Cohen’s d > 0.5) on other psychosocial measures was met only on the Patient-Reported Outcomes Measurement Information System global self-efficacy (d = 0.62) in the total sample. Qualitative data revealed enthusiasm for the accessibility of telehealth delivery and the usefulness of several cognitive and behavioral modules but also revealed opportunities to refine the CBT-H program.
Conclusions:
These findings indicate that this new CBT-H program can potentially reduce depressive symptoms and improve self-efficacy in people with central disorders of hypersomnolence. Furthermore, telehealth is a promising model for remote delivery and data collection to enhance participant accessibility and engagement.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Psychosocial Adjunctive Treatment for Hypersomnia (PATH); URL: https://clinicaltrials.gov/ct2/show/NCT03904238; Identifier: NCT03904238.
Citation:
Ong JC, Dawson SC, Mundt JM, Moore C. Developing a cognitive behavioral therapy for hypersomnia using telehealth: a feasibility study. J Clin Sleep Med. 2020;16(12):2047–2062.
Keywords: narcolepsy, idiopathic hypersomnia, cognitive-behavior therapy, depression, psychosocial, health-related quality of life
BRIEF SUMMARY
Current Knowledge/Study Rationale: Observational studies have consistently found that people with central disorders of hypersomnolence experience poor psychosocial functioning, including symptoms of depression. It is not known if an adjunctive therapy using cognitive and behavioral strategies can effectively reduce depressive symptoms and improve health-related quality of life.
Study Impact: This phase 1b study indicates that a cognitive-behavior therapy designed to improve psychosocial functioning in people with central disorders of hypersomnolence and co-occurring depressive symptoms is feasible and acceptable. In addition, the findings support the use of a telehealth model for delivery and assessment.
INTRODUCTION
Central disorders of hypersomnolence (CDH), including narcolepsy type 1 (NT1), narcolepsy type 2 (NT2), and idiopathic hypersomnia (IH), are serious and debilitating conditions that account for 5–10% of patients who present to sleep clinics1 and occur in about 0.025–0.16% of the general population.2 Health-related quality of life (HRQoL) is significantly diminished in people with CDH, with elevations in depressive symptoms and poor psychosocial functioning.3 One study found that 56.9% of patients with narcolepsy reported at least mild symptoms of depression,4 whereas another study found that 25% of patients with narcolepsy reported stable levels of moderate to severe symptoms of depression over a 5-year period.5 In a large cohort study of people with CDH in France, 28.8% reported moderate to severe depression despite taking medications for CDH, with similar levels reported for narcolepsy and IH.6 A recent study found that 36% of patients with narcolepsy also take antidepressant medications to treat mood or anxiety symptoms,7 and another study found that patients with narcolepsy use twice the amount of nonnarcolepsy drugs (medical and psychiatric) compared with matched controls.8 With regard to social impact, 72% of patients with narcolepsy reported interpersonal distress (marital and family conflicts), with 20% identifying narcolepsy as the reason for divorce or separation.9 In studies across different countries around the world,4,5,10–12 people with CDH consistently reported a profound impact on HRQoL.
Despite the consistent findings of poor psychosocial functioning, there are currently no empirically based behavioral treatments that can effectively improve these aspects of HRQoL in people with CDH. Previous studies in narcolepsy have found some support for reducing self-reported and objectively measured sleepiness and improving performance using scheduled naps or a combination of scheduled naps with regulating nighttime sleep.13–15 However, these studies could not determine the optimal frequency, timing, or duration of naps, and limitations included small sample sizes and a short study duration (1–2 weeks). Furthermore, none of these studies evaluated mood or HRQoL so it is unclear if behavioral treatments can improve other patient-reported outcomes. The treatment guidelines from the American Academy of Sleep Medicine,16 European Federation of Neurological Sciences,17 and the Brazilian Sleep Society18 all suggest the use of behavioral strategies (eg, scheduled naps) and/or psychosocial support (eg, general counseling) as potentially beneficial adjunctive therapies for narcolepsy and IH. However, these guidelines also acknowledge the lack of sufficient evidence to recommend any of these nonpharmacologic strategies as part of standard practice. Specifically, there are no published randomized controlled trials that have tested a psychological or behavioral treatment aimed at improving psychosocial functioning in people with CDH.
To address this research gap, recent work in our laboratory has been aimed at systematically developing an adjunctive psychosocial treatment program to improve HRQoL in people with CDH. First, we conducted a survey study to determine the need for and interest in a psychosocial intervention among people with CDH.19 Between 61% and 91% endorsed at least 1 symptom of depression and anxiety and 73.9% reported being somewhat or extremely interested in learning cognitive and behavioral strategies for improving psychosocial functioning and managing symptoms of CDH. Next, we conducted a mixed-methods study in people with narcolepsy to gather more specific information on potential treatment targets, preferred delivery format, and assessment instruments for patient-reported outcome measures.20 Findings from this study revealed the importance of addressing self-esteem and self-efficacy to help manage the stigma of being diagnosed with narcolepsy and emotion-regulation strategies to manage anxiety and depressive symptoms related to the unpredictable nature of the narcolepsy symptoms. There was also a strong preference for using a telehealth model of delivery to enhance accessibility to treatment and reduce the potential for narcolepsy symptoms to interfere with attending treatment.
The purpose of this proof-of-concept study was to determine the feasibility and acceptability of a novel cognitive behavioral therapy for people with CDH and depressive symptoms. Consistent with phase 1b of the staged development of behavioral therapies,19,21 the aims for this study were to (1) gather feasibility data on preliminary efficacy on depressive symptoms and treatment effects on related psychosocial domains and (2) evaluate methodologic considerations using telehealth for remote delivery and data collection (ie, recruitment, retention, assessment protocol, treatment delivery).
METHODS
Participants and procedures
Participants were adults at least 18 years of age with an established diagnosis of NT1, NT2, or IH who were receiving standard care for these conditions. Additionally, participants had to report at least mild symptoms of depression based on a cutoff score ≥5 on the Patient Health Questionnaire (PHQ-8) at baseline. Exclusion criteria included unstable medical condition, severe or unstable psychiatric condition (psychotic disorder, substance abuse, active suicidal ideation), women who were pregnant at the time of assessment, and untreated sleep-related breathing disorder. For feasibility purposes, individuals who did not reside in North America, did not have reliable internet connection, or had scheduling conflicts with the timing of the study intervention were also excluded. Participants were recruited between December 2018 and July 2019 using several strategies including (1) social media postings by nonprofit patient organizations (eg, Wake up Narcolepsy, Hypersomnia Foundation, Narcolepsy Network), (2) presentations and word of mouth at conferences hosted by these patient organizations, (3) invitations sent to participants from a prior study who agreed to be contacted for future studies,20 and (4) referrals from local sleep disorders clinics.
All screening procedures were conducted remotely. Participants were first screened using an online survey to obtain general information about eligibility (eg, age, diagnosis, depressive symptoms). Those who were eligible to continue then completed a screening interview using a live videoconferencing platform with the study coordinator. During this interview, the coordinator reviewed and obtained informed consent, completed a review of medical information (ie, medications, medical history, current treatments), and completed a brief structured interview of current psychiatric symptoms. Participants who qualified for the study were then asked to provide documentation of their CDH diagnosis in the form of a diagnostic polysomnography/multiple sleep latency test report, progress note from their physician with diagnosis/International Classification of Diseases code, or a letter from their physician documenting diagnosis and standard care. Finally, participants who met all eligibility criteria completed a set of baseline measures and were eligible to be assigned to a treatment condition. This study was approved by the Institutional Review Board at Northwestern University, and all participants provided informed consent as described above.
Eligible participants were assigned sequentially to receive either individual or group delivery of the cognitive behavioral therapy. Assignment was made in clusters (n = 7–10) based on matching availability between participants and study therapists. Study assessments were conducted at posttreatment, approximately 6 weeks after the start of treatment and within 1 month of completing the last session.
Treatment
Cognitive behavioral therapy for hypersomnia: design and therapeutic targets
The cognitive behavioral therapy for hypersomnia (CBT-H) program was developed as an adjunctive therapy for people with narcolepsy or IH who were currently receiving standard care for their condition. The program was grounded in a cognitive behavioral framework tailored to address the psychosocial needs of people with CDH. The contents and delivery methods were informed by stakeholder input,20 previous work in the broader literature on CDH, evidence-based psychosocial interventions for managing other chronic conditions, such as cancer,22–24 chronic pain,25–27 and chronic worry,28 and anecdotal clinical experience with patients with CDH. To optimize efficiency and test feasibility, CBT-H was designed as a modular treatment to address issues related to HRQoL for both narcolepsy and IH. Each module consisted of a CDH-related theme with specific psychological and/or behavioral activities and homework assignments that were customized to provide flexibility in addressing disease-specific symptoms of people with NT1, NT2, and IH (see Table 1 for summary). For example, modules addressing anxiety with cataplexy were delivered to individuals with NT1 (as appropriate) but were not delivered to those with NT2 or IH. Specific behavioral strategies included scheduled naps (if beneficial for the individual) and regulating sleep at night with specific timing and duration tailored to each participant. In addition, a structured daytime schedule was developed for each participant using alarms and reminders to take planned naps and/or breaks throughout the day to manage sleepiness, energy, and performance. Customization of these behavioral strategies were based on disease-specific considerations (eg, people with IH are less likely to benefit from naps) patient-reported benefits, and other lifestyle factors. Cognitive strategies were aimed at processing the changes in self-identity or functional limitations that have emerged because of the symptoms of CDH and the challenges encountered in getting properly diagnosed and treated for CDH. Topics included dealing with the stigma of being diagnosed with CDH, implications of CDH symptoms on self-perception, occupational goals and interpersonal relationships, and specific coping skills to manage mood and anxiety associated with the unpredictability of CDH symptoms. Specific strategies included developing a structured worry time,28 evaluating the energy transactions of daily activities (nurturing/depleting activities),29 and findings ways to maintain value-congruent living.
Table 1.
Summary of CBT modules and activities.
| Module | Key Concepts/Activities | Suggested Session |
|---|---|---|
| Education about CDH | Provide education about the prevalence and etiology of narcolepsy and/or IH (as appropriate for the patient). Discuss the patient’s experience with the emergence of symptoms, the journey to getting diagnosed, and the perception of others about having narcolepsy. Provide resources for learning more about narcolepsy or IH (as appropriate). | Session 1 |
| Self-identity and self-image | Discuss self-identity and changes that have developed as the result of CDH symptoms. This includes strategies for active acceptance and value-congruent living. | Session 2 |
| Structured daytime activities | Use of sleep/wake diaries to develop a personalized structure for scheduled naps (as appropriate) and waking activities in small segments throughout the day (Pomodoro technique). Explain the nurturing/depleting activity to evaluate energy transactions throughout the day. | Session 3 |
| Structured nighttime activities | Use of sleep/wake diaries to develop a structure for regulating bedtime and waketime and to practice good sleep hygiene. | Session 4 |
| Coping skills and emotion-regulation | Discuss problem-focused and emotion-focused coping strategies to manage the unpredictability and/or constancy of CDH symptoms. This include cognitive flexibility for dealing with limitations or setting a structured worry time to manage anxiety. | Session 5 |
| Social support | Explain the importance of support from family and friends and connecting with others through patient organizations for people with CDH. | Session 5 |
| Medical, legal, and occupational issues | Discuss disability accommodations at work/school (if applicable), disclosing CDH diagnosis at work/school, and preparing for doctor’s visits. | Session 6 |
| Other topics | Discuss topics as appropriate: (1) managing the unpredictability of cataplexy; (2) medication adherence; (3) impact of CDH symptoms on family relationships; (4) using service or emotional support animals | Optional |
This table provides a summary of the CBT modules and key concepts and activities within each module. CDH including narcolepsy type 1, narcolepsy type 2, and IH. CBT = cognitive behavioral therapy, CDH = central disorders of hypersomnolence, IH = idiopathic hypersomnia.
Delivery format
CBT-H was delivered in 6 weekly sessions approximately 1 hour in duration. All sessions were conducted remotely using a live videoconferencing platform. Participants assigned to individual CBT-H received the modules described above during individual sessions with their assigned study therapist. The putative benefit of individual CBT-H was to enhance rapport between the participant and therapist and to provide more opportunities to customize the treatment toward personal issues and challenges in living with CDH. Participants assigned to group CBT-H received the same modules in small group sessions (n = 3–5). The study therapist led the group through the modules and activities and moderated group discussions. The putative benefit of group CBT-H was to include social interactions that allowed participants to share their experiences with others who also have CDH. In this study, therapists were encouraged to deliver all modules but were given flexibility to modify the timing or order of the modules based on clinical judgment. In addition, make-up sessions were provided to compensate for absences as needed.
Study therapists
The program was delivered by 4 study therapists. Two therapists were licensed clinical psychologists (JO, JM), 1 therapist was a postdoctoral fellow (SD), and 1 therapist was a doctoral student in clinical psychology (EA). Before the study, training was conducted with all therapists to provide instructions on the treatment manual and study protocol. During the study, the therapists held regular meetings to review the treatment program, share their clinical experience, and discuss issues. All 4 therapists delivered individual CBT-H and 2 therapists (JO, SD) delivered group CBT-H.
Measures
All questionnaire data were collected remotely using Research Electronic Data Capture, a secure, web-based application designed to support data capture for research studies.30
Clinical measures
The Patient Health Questionnaire (PHQ-8) is a measure of depressive symptoms that has been validated to assess the severity of depression in clinical practice.31 In this study, we used the 8-item version (without item 9, suicidal ideation) as a screening tool to determine eligibility for the study and to measure change in depressive symptoms from pretreatment to posttreatment.
The Patient-Reported Outcomes Measurement Information Systems (PROMIS) measures were used to assess the impact of the intervention from baseline to posttreatment across several domains of interest related to psychosocial functioning. The measures used in this study include the following: depression, anxiety, sleep disturbance, sleep-related impairment, fatigue, general self-efficacy, self-efficacy for managing emotions, self-efficacy for managing social interactions, self-efficacy for managing symptoms, ability to participate in social roles and activities, social isolation, cognitive function, physical function, and global health (mental and physical). Computer adaptive test forms were used for all measures except global health, which was assessed with fixed-length short forms. Computer adaptive test uses item response theory to deliver questions tailored to the participant based on prior responses.32 The T-scores from each scale were used to determine the level of severity in each domain, with a mean of 50 corresponding to the average level relative to the normative sample and standard deviation of 10.
The Epworth Sleepiness Scale (ESS) is a measure of excessive daytime sleepiness.33 The ESS was administered at baseline, at weekly intervals during treatment, and at posttreatment. Although the CBT-H program was not designed to specifically reduce sleepiness, we used the ESS in this study to allow comparisons to other behavioral and pharmacologic studies because it is commonly used as an outcome measure in CDH treatment studies.
In addition to these main clinical measures, we collected baseline and posttreatment data on secondary measures of daytime functioning and sleep to explore the impact of the intervention and to evaluate the feasibility of using these measures in this population. The Functional Outcomes of Sleep Questionnaire (FOSQ) was used to assess the impact of sleepiness on daily behaviors and quality of life.34 The Sleep Inertia Questionnaire (SIQ) was used to measure low arousal and impaired ability to perform on waking, which can be symptomatic of people with IH.35 The Restorative Sleep Questionnaire (RSQ) was used to measure self-reported sleep quality related to feeling restored.36
Treatment acceptability and credibility
Data on treatment acceptability were collected to evaluate the delivery and contents of CBT-H and to inform potential strategies to refine and optimize the intervention. A treatment credibility and expectancy questionnaire37 was administered between the first and second sessions of treatment. This scale consisted of 4 items: the first 3 items assess how logical the treatment appeared, how successful participants believed it would be in reducing their symptoms, and how confident they would feel about recommending the treatment to someone else using a 1–9 scale. The fourth item assesses the degree of improvement (0–100%) expected because of the treatment. After completing CBT-H, participants were invited to complete an exit interview conducted remotely by the study coordinator using a videoconferencing platform to gather qualitative data on the acceptability of the format, contents, and delivery of the intervention and telehealth model.
Data analysis
Based on recommendations for pilot studies,38 we focused on evaluating the feasibility and acceptability of the intervention and telehealth model for delivery and data collection to inform the design of a subsequent efficacy study. Rather than conducting formal hypothesis testing, we report the patterns of recruitment and retention along with results based on prespecified benchmarks, indications of treatment effects, and emerging themes from qualitative data. For preliminary efficacy on depressive symptoms, the prespecified benchmark was that ≥50% of the sample would achieve a clinically significant change in symptoms of depression, as indicated by a decrease of ≥5 points on the PHQ-8 from baseline to posttreatment. For indications of treatment effects on HRQoL, daytime functioning, and other secondary measures, the prespecified benchmark for a minimal clinically important difference (MCID) was an effect size of Cohen’s d > 0.5. To complement these benchmarks, we report P values using paired samples t tests on baseline-to-posttreatment changes for the entire sample. Subsequently, exploratory post hoc analyses were conducted to compare baseline-to-posttreatment changes for treatment format (individual vs group) and diagnosis (NT1, NT2, IH). For qualitative data, we examined the responses from the exit interview for emerging patterns and themes across participants and collated the responses into common themes. Data analyses were conducted using an intent-to-treat approach on all participants who were allocated to treatment (n = 35) with the last observation carried forward for the 3 participants who did not provide posttreatment data.
RESULTS
Recruitment and retention
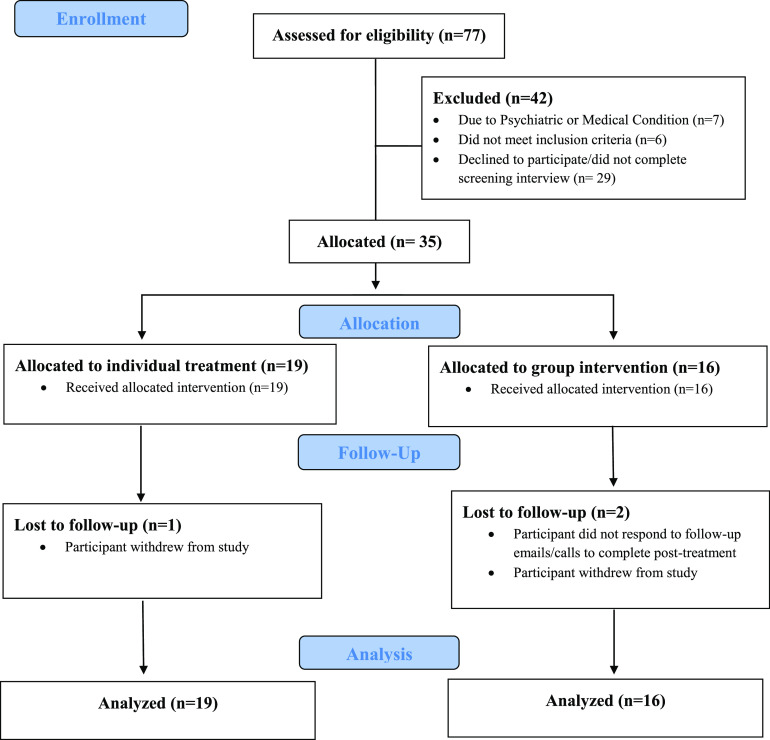
A total of 77 individuals contacted the study and were assessed for eligibility (Figure 1). Over the 7-month period of recruitment, this yielded an average pace of 11 participants screened per month and 5 participants allocated to treatment per month. Of the 77 individuals screened, 35 were allocated to treatment (45.5%), with 32 participants (91.4%) who completed the treatment program and provided baseline and posttreatment data. There were 19 participants assigned to individual treatment with 18 participants who attended all 6 sessions of the treatment and 1 participant who dropped out and did not complete the posttreatment assessment. There were 16 participants assigned to group treatment with 7 participants who attended 6 sessions, 5 participants who attended 5 sessions, and 1 participant who attended 4 sessions. One participant attended 3 group sessions and 3 make-up individual sessions with the study therapist. Two participants were dropouts and did not complete group CBT-H or posttreatment assessment.
Figure 1. CONSORT flow diagram.
Demographics
The final sample for intent-to-treat analyses consisted of 35 participants (32 female, 3 male) who were allocated to treatment. The average age was 32.0 years (standard deviation [SD] = 12.9 years) with a range from 19 to 79 years. Thirty-one participants (88.6%) identified as white, 1 participant (2.8%) identified as Asian, and 3 participants (8.6%) as more than one race. Three participants (8.6%) identified as Hispanic, and the remaining 32 participants identified as non-Hispanic. Participants had an average of 16.8 years of education (SD = 2.8). With regard to diagnosis, 12 participants (34.3%) were diagnosed with NT1, 11 participants (31.4%) with NT2, and 12 participants (34.3%) with IH. The mean time since CDH diagnosis was 4.5 years (SD = 4.7). Table 2 provides the demographic information.
Table 2.
Demographics.
| Characteristic | Individual | Group | Total |
|---|---|---|---|
| Sex (n, %) | |||
| Female | 17 (89.5%) | 15 (93.8%) | 32 (91.4%) |
| Male | 2 (10.5%) | 1 (6.3%) | 3 (8.6%) |
| Race (n, %) | |||
| White | 31 (88.6%) | ||
| Asian | 18 (94.7%) | 13 (81.3%) | 1 (2.8%) |
| More than one race | 1 (5.3%) | 3 (18.8%) | 3 (8.6%) |
| Ethnicity (n, %) | |||
| Hispanic | 3 (15.8%) | 3 (8.6%) | |
| Non-Hispanic | 16 (84.2%) | 16 (100%) | 32 (91.4%) |
| Age (mean, SD) | 32.9 (14.4) | 30.9 (11.3) | 32.0 (12.9) |
| Years of education (mean, SD) | 17.3 (3.1) | 16.2 (2.4) | 16.8 (2.8) |
| CDH diagnosis (n, %) | |||
| NT1 | 6 (31.6%) | 6 (37.5%) | 12 (34.3%) |
| NT2 | 8 (42.1%) | 3 (18.8%) | 11 (31.4%) |
| IH | 5 (26.3%) | 7 (43.8%) | 12 (34.3%) |
| Time since diagnosis (mean, SD) | 4.3 (5.2) | 4.6 (4.2) | 4.5 (4.7) |
Age and time since diagnosis are in years. SD = standard deviation, CDH = central disorders of hypersomnolence, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, IH = idiopathic hypersomnia.
Clinical measures
Clinical measures for the total sample and each treatment format are presented in Table 3 and Figure 2. For treatment effects on depressive symptoms, 40% of the total sample (14 of 35) achieved a clinically significant change in depressive symptoms (PHQ decrease ≥ 5), which is below the prespecified benchmark of 50%. Paired samples t tests revealed a reduction for the overall sample on the PHQ score with a large effect size (P < .0001, d = 0.80). When examining by treatment format, the benchmark was achieved among those receiving group CBT-H (50%, 8 of 16) but not among those receiving individual CBT-H (31.5%, 6 of 19). Paired samples t tests revealed a reduction for both group CBT-H (P = .0011, d = 1.04) and individual CBT-H (P = .0300, d = 0.54).
Table 3.
Clinical measures.
| Measure | N | Baseline | Posttreatment | t | P | d |
|---|---|---|---|---|---|---|
| PHQ | ||||||
| Individual | 19 | 12.68 (4.03) | 10.42 (4.89) | −2.36 | .0300 | −0.54 |
| Group | 16 | 15.75 (3.87) | 11.31 (5.85) | −4.04 | .0011 | −1.01 |
| Total | 35 | 14.09 (4.20) | 10.83 (5.29) | −4.42 | <.0001 | −0.75 |
| PROMIS | ||||||
| Dep | ||||||
| Individual | 19 | 59.02 (6.97) | 57.58 (9.08) | −0.86 | .4034 | −0.20 |
| Group | 16 | 61.12 (5.97) | 57.65 (9.32) | −2.21 | .0431 | −0.55 |
| Total | 35 | 59.98 (6.52) | 57.61 (9.05) | −2.05 | .0486 | −0.35 |
| Anx | ||||||
| Individual | 19 | 61.68 (9.27) | 58.85 (8.15) | −1.84 | .0822 | −0.42 |
| Group | 16 | 62.73 (5.59) | 61.72 (7.59) | −0.65 | .5244 | −0.16 |
| Total | 35 | 62.16 (7.71) | 60.16 (7.92) | −1.83 | .0755 | −0.31 |
| Sleep Dist | ||||||
| Individual | 19 | 57.15 (8.04) | 55.21 (7.44) | −1.41 | .1759 | −0.32 |
| Group | 16 | 51.78 (5.43) | 51.59 (8.14) | −0.20 | .8473 | −0.05 |
| Total | 35 | 54.69 (7.39) | 53.55 (7.87) | −1.31 | .2005 | −0.22 |
| Sleep Imp | ||||||
| Individual | 19 | 63.83 (7.30) | 62.46 (5.63) | −0.90 | .3780 | −0.21 |
| Group | 16 | 65.58 (6.02) | 64.94 (6.72) | −0.36 | .7239 | −0.09 |
| Total | 35 | 64.63 (6.71) | 63.59 (6.19) | −0.91 | .3708 | −0.15 |
| Fatigue | ||||||
| Individual | 19 | 65.56 (7.18) | 64.68 (6.85) | −0.75 | .4650 | 0.17 |
| Group | 16 | 69.13 (4.54) | 68.13 (6.98) | −0.64 | .5290 | −0.16 |
| Total | 35 | 67.19 (6.30) | 66.26 (7.02) | −0.99 | .3274 | −0.17 |
| GSE | ||||||
| Individual | 19 | 40.23 (8.67) | 44.62 (9.35) | 2.51 | .0218 | 0.58 |
| Group | 16 | 40.05 (8.66) | 44.38 (5.96) | 2.61 | .0197 | 0.65 |
| Total | 35 | 40.15 (8.54) | 44.51 (7.87) | 3.64 | .0009 | 0.62 |
| SEMEM | ||||||
| Individual | 19 | 42.28 (5.68) | 44.18 (7.07) | 1.53 | .1425 | 0.35 |
| Group | 16 | 39.66 (4.99) | 41.84 (7.18) | 1.45 | .1677 | 0.36 |
| Total | 35 | 41.08 (5.46) | 43.11 (7.11) | 2.14 | .0396 | 0.36 |
| SEMSS | ||||||
| Individual | 19 | 42.93 (6.93) | 43.97 (5.85) | 0.95 | .3544 | 0.22 |
| Group | 16 | 40.03 (6.48) | 41.94 (5.60) | 2.10 | .0530 | 0.53 |
| Total | 35 | 41.60 (6.79) | 43.04 (5.75) | 2.00 | .0533 | 0.34 |
| SEMSX | ||||||
| Individual | 19 | 39.56 (6.21) | 41.39 (5.35) | 1.11 | .2828 | 0.25 |
| Group | 16 | 38.15 (4.61) | 39.24 (4.49) | 1.38 | .1882 | 0.34 |
| Total | 35 | 38.92 (5.51) | 40.41 (5.02) | 1.56 | .1282 | 0.26 |
| Participate | ||||||
| Individual | 19 | 38.74 (6.90) | 40.47 (4.34) | 1.57 | .1340 | 0.36 |
| Group | 16 | 37.93 (6.04) | 38.00 (6.03) | 0.11 | .9157 | 0.03 |
| Total | 35 | 38.37 (6.44) | 39.34 (5.25) | 1.44 | .1578 | 0.24 |
| Soc Isolation | ||||||
| Individual | 19 | 58.70 (6.71) | 56.36 (7.07) | −1.48 | .1550 | −0.34 |
| Group | 16 | 58.31 (5.15) | 56.82 (6.58) | −1.18 | .2573 | −0.29 |
| Total | 35 | 58.52 (5.96) | 56.57 (6.76) | −1.91 | .0644 | −0.32 |
| Cog Funct | ||||||
| Individual | 19 | 37.28 (6.51) | 37.73 (4.57) | 0.33 | .7464 | 0.08 |
| Group | 16 | 36.14 (4.29) | 37.10 (3.79) | 0.99 | .3389 | 0.25 |
| Total | 35 | 36.76 (5.56) | 37.44 (4.19) | 0.80 | .4299 | 0.14 |
| Phys Funct | ||||||
| Individual | 19 | 44.18 (6.12) | 44.62 (6.84) | 0.44 | .6634 | 0.10 |
| Group | 16 | 42.84 (6.21) | 42.46 (7.20) | −0.37 | .7192 | −0.09 |
| Total | 35 | 43.57 (6.11) | 43.63 (6.99) | 0.09 | .9262 | 0.02 |
| Global MH | ||||||
| Individual | 19 | 37.38 (7.15) | 38.82 (7.34) | 0.70 | .4920 | 0.16 |
| Group | 16 | 35.18 (6.59) | 36.16 (7.40) | 0.95 | .3564 | 0.24 |
| Total | 35 | 36.37 (6.89) | 37.60 (7.38) | 1.03 | .3110 | 0.17 |
| Global PH | ||||||
| Individual | 19 | 43.90 (8.37) | 42.71 (6.96) | −1.08 | .2950 | −0.25 |
| Group | 16 | 40.84 (6.28) | 41.75 (5.07) | 0.71 | .4902 | 0.18 |
| Total | 35 | 42.50 (7.54) | 42.27 (6.10) | 0.27 | .7911 | 0.05 |
| ESS | ||||||
| Individual | 19 | 12.68 (4.06) | 11.95 (3.89) | −0.89 | .3865 | −0.20 |
| Group | 16 | 13.81 (3.25) | 12.44 (3.46) | −2.91 | .0109 | −0.73 |
| Total | 35 | 13.20 (3.70) | 12.17 (3.66) | −2.07 | .0458 | −0.35 |
| FOSQ | ||||||
| Activity | ||||||
| Individual | 19 | 1.98 (0.43) | 2.11 (0.62) | 1.04 | .3130 | 0.24 |
| Group | 16 | 1.94 (0.46) | 1.99 (0.58) | 0.45 | .6585 | 0.11 |
| Total | 35 | 1.96 (0.44) | 2.05 (0.60) | 1.12 | .2703 | 0.19 |
| Vigilance | ||||||
| Individual | 19 | 2.45 (0.68) | 2.70 (0.59) | 1.67 | .1126 | 0.38 |
| Group | 16 | 2.36 (0.58) | 2.39 (0.70) | 0.24 | .8161 | 0.06 |
| Total | 35 | 2.41 (0.63) | 2.56 (0.65) | 1.52 | .1373 | 0.26 |
| Intimacy | ||||||
| Individual | 17 | 2.54 (0.84) | 2.60 (0.92) | 0.44 | .6684 | 0.11 |
| Group | 15 | 2.27 (0.95) | 2.51 (0.92) | 1.19 | .2544 | 0.31 |
| Total | 32 | 2.41 (0.89) | 2.56 (0.91) | 1.22 | .2319 | 0.22 |
| Productivity | ||||||
| Individual | 19 | 2.51 (0.60) | 2.63 (0.46) | 0.81 | .4265 | 0.19 |
| Group | 16 | 2.46 (0.62) | 2.45 (0.62) | −0.14 | .8938 | −0.03 |
| Total | 35 | 2.49 (0.60) | 2.55 (0.54) | 0.70 | .4891 | 0.12 |
| Social | ||||||
| Individual | 19 | 2.61 (0.81) | 2.97 (0.56) | 2.35 | .0305 | 0.54 |
| Group | 16 | 2.34 (0.72) | 2.63 (0.74) | 1.38 | .1881 | 0.34 |
| Total | 35 | 2.49 (0.77) | 2.81 (0.67) | 2.64 | .0125 | 0.45 |
| Total FOSQ | ||||||
| Individual | 19 | 2.34 (0.38) | 2.49 (0.38) | 1.50 | .1503 | 0.34 |
| Group | 16 | 2.24 (0.49) | 2.29 (0.61) | 0.75 | .4675 | 0.19 |
| Total | 35 | 2.30 (0.43) | 2.40 (0.50) | 1.68 | .1024 | 0.28 |
| SIQ | ||||||
| Individual | 19 | 77.00 (13.16) | 74.21 (14.86) | −1.06 | .3034 | −0.24 |
| Group | 16 | 81.94 (11.73) | 77.75 (16.26) | −1.81 | .0899 | −0.45 |
| Total | 35 | 79.26 (12.59) | 75.83 (15.39) | −1.95 | .0591 | −0.33 |
| RSQ | ||||||
| Individual | 19 | 22.37 (14.78) | 28.95 (11.82) | 2.09 | .0513 | 0.48 |
| Group | 16 | 20.70 (12.02) | 16.80 (17.19) | −1.62 | .1262 | −0.40 |
| Total | 35 | 21.61 (13.42) | 23.39 (15.56) | 0.81 | .4221 | 0.14 |
PHQ = Patient Health Questionnaire, PROMIS = Patient-Reported Outcomes Measurement Information Systems, Dep = Depression, Anx = Anxiety, Sleep Dist = Sleep Disturbance, Sleep Imp = Sleep-Related Impairment, GSE = General Self-Efficacy, SEMEM = Self-Efficacy for Managing Emotions, SEMSS = Self-Efficacy for Managing Social Interactions, SEMSX = Self-Efficacy for Managing Symptoms, Participate = Ability to Participate in Social Roles and Activities, Soc Isolation = Social Isolation, Cog Funct = Cognitive Functioning, Phys Funct = Physical Functioning, Global MH = Global Mental Health, Global PH = Global Physical Health, ESS = Epworth Sleepiness Scale, FOSQ = Functional Outcomes of Sleep Questionnaire, SIQ = Sleep Inertia Questionnaire, RSQ = Restorative Sleep Questionnaire.
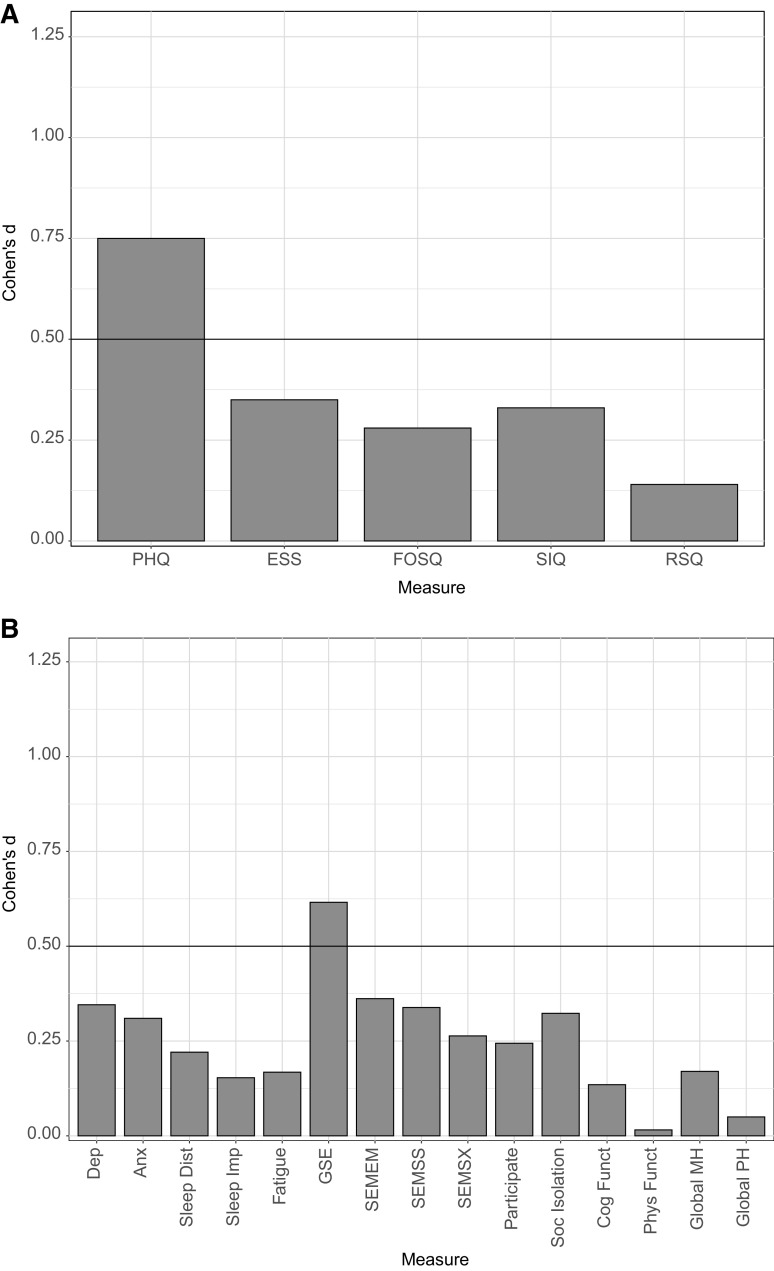
Figure 2. Within-subject effect sizes by clinical measures for total sample.
(A) The within-subject effect size (Cohen’s d) from baseline to posttreatment for each of the following clinical measures: Patient Health Questionnaire (PHQ), Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire (FOSQ), Sleep Inertia Questionnaire (SIQ), and Restorative Sleep Questionnaire (RSQ). (B) The within-subject effect size (Cohen’s d) from baseline to posttreatment for each of the following Patient-Reported Outcomes Measurement Information Systems measures: Depression (Dep), Anxiety (Anx), Sleep Disturbance (Sleep Dist), Sleep-Related Impairment (Sleep Imp), Fatigue, General Self-Efficacy (GSE), Self-Efficacy for Managing Emotions (SEMEM), Self-Efficacy for Managing Social Interactions (SEMSS), Self-Efficacy for Managing Symptoms (SEMSX), Ability to Participate in Social Roles and Activities (Participate), Social Isolation (Soc Isolation), Cognitive Functioning (Cog Funct), Physical Functioning (Phys Funct), Global Mental Health (Global MH), and Global Physical Health (Global PH). An effect size of d > 0.5 was the prespecified benchmark for a minimal clinically important difference.
For changes from baseline to posttreatment on other measures using the prespecified MCID benchmark (d > 0.05), only the PROMIS global self-efficacy (P = .0009, d = 0.62) met this benchmark in the total sample. When examining by allocation to treatment format, both individual (P = .0218, d = 0.58) and group CBT-H (P = .0197, d = 0.65) met the benchmark on PROMIS global self-efficacy. In addition, group CBT-H met this benchmark on the PROMIS depression (P = .0431, d = 0.55), PROMIS self-efficacy for managing social interactions (P = .0530, d = 0.53), and ESS (P = .0109, d = 0.73). Individual CBT-H met the benchmark on the FOSQ social subscale (P = .0305, d = 0.54). No other measures met the MCID benchmark.
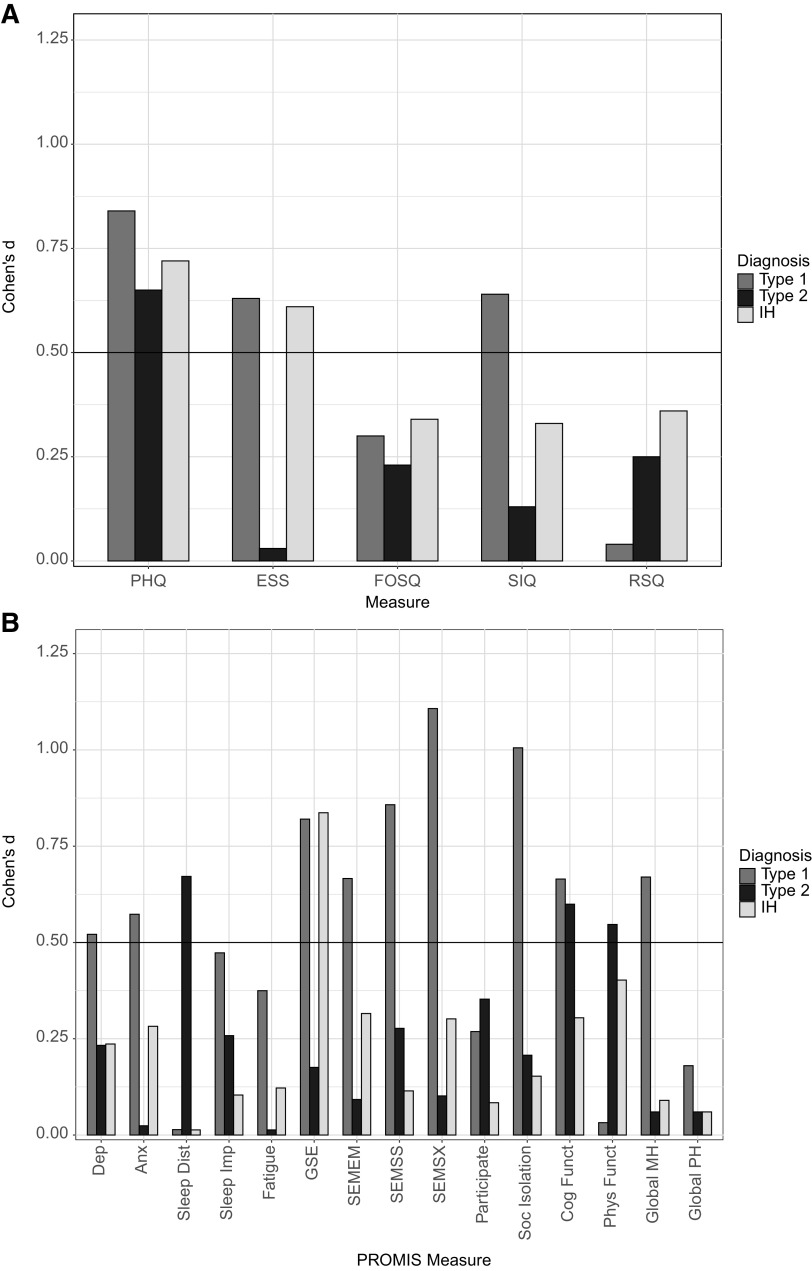
Exploratory analyses conducted across diagnostic groups revealed that all 3 groups met the MCID benchmark on the PHQ (NT1: P = .0140, d = 0.84; NT2: P = .0576, d = 0.65; IH: P = .0297, d = 0.72). On the PROMIS global self-efficacy, NT1 (P = .0160, d = 0.82) and IH (P = .0145, d = 0.84) met the benchmark but not NT2 (P = .5731, d = 0.18). A similar pattern was found on ESS, with NT1 (P = .0528, d = 0.63) and IH (P = .0598, d = 0.61) meeting the benchmark but not NT2 (P = .9309, d = 0.03). Only NT1 met the benchmark on several other PROMIS scales including depression (P = .0984, d = 0.52), anxiety (P = .0726, d = 0.57), self-efficacy to manage emotions (P = .0415, d = 0.67), self-efficacy to manage social interactions (P = .0127, d = 0.86), self-efficacy to manage symptoms (P = .0028, d = 1.11), social isolation (P = .0051, d = 1.01), cognitive functioning (P = .0419, d = 0.66), and global mental health (P = .0390, d = 0.67), and the SIQ (P = .0487, d = 0.64). Only NT2 met the MCID benchmark on sleep disturbance (P = .0501, d = 0.67) and physical functioning (P = .0998, d = 0.55). Only IH met the MCID benchmark on the FOSQ social subscale (P = .0468, d = 0.65), whereas NT1 and NT2 did not (Table 4 and Figure 3).
Table 4.
Clinical measures by diagnosis.
| Measure | n | Baseline | Posttreatment | t | P | d |
|---|---|---|---|---|---|---|
| PHQ | ||||||
| NT1 | 12 | 14.33 (4.98) | 10.58 (6.50) | −2.92 | .0140 | −0.84 |
| NT2 | 11 | 13.45 (4.08) | 10.09 (4.87) | −2.14 | .0576 | −0.65 |
| IH | 12 | 14.42 (3.73) | 11.75 (4.59) | −2.50 | .0297 | −0.72 |
| PROMIS | ||||||
| Dep | ||||||
| NT1 | 12 | 59.91 (7.51) | 56.00 (10.09) | −1.81 | .0984 | −0.52 |
| NT2 | 11 | 60.50 (7.40) | 59.01 (10.00) | −0.77 | .4576 | −0.23 |
| IH | 12 | 59.57 (4.99) | 57.94 (7.46) | −0.82 | .4302 | −0.24 |
| Anx | ||||||
| NT1 | 12 | 64.40 (5.83) | 60.28 (8.69) | −1.99 | .0726 | −0.57 |
| NT2 | 11 | 62.45 (10.73) | 62.29 (9.54) | −0.08 | .9381 | −0.02 |
| IH | 12 | 59.67 (5.74) | 58.09 (5.13) | −0.98 | .3409 | −0.28 |
| Sleep Dist | ||||||
| NT1 | 12 | 57.30 (7.68) | 57.23 (9.11) | −0.05 | .9618 | −0.01 |
| NT2 | 11 | 57.09 (7.06) | 53.46 (6.28) | −2.23 | .0501 | −0.67 |
| IH | 12 | 49.89 (5.12) | 49.96 (6.61) | 0.05 | .9638 | 0.01 |
| Sleep Imp | ||||||
| NT1 | 12 | 68.66 (4.99) | 65.85 (8.46) | −1.64 | .1295 | −0.47 |
| NT2 | 11 | 61.59 (5.80) | 60.35 (3.15) | −0.86 | .4119 | −0.26 |
| IH | 12 | 63.38 (7.41) | 64.30 (4.60) | 0.36 | .7256 | 0.10 |
| Fatigue | ||||||
| NT1 | 12 | 69.37 (4.08) | 67.32 (7.26) | −1.30 | .2206 | −0.37 |
| NT2 | 11 | 63.16 (7.95) | 63.24 (7.91) | 0.04 | .9652 | 0.01 |
| IH | 12 | 68.71 (4.96) | 67.97 (5.43) | −0.42 | .6801 | −0.12 |
| GSE | ||||||
| NT1 | 12 | 39.29 (10.80) | 45.09 (8.37) | 2.84 | .0160 | 0.82 |
| NT2 | 11 | 41.14 (7.20) | 42.33 (8.49) | 0.58 | .5731 | 0.18 |
| IH | 12 | 40.09 (7.74) | 45.92 (6.96) | 2.90 | .0145 | 0.84 |
| SEMEM | ||||||
| NT1 | 12 | 41.77 (7.19) | 45.80 (7.88) | 2.31 | .0415 | 0.67 |
| NT2 | 11 | 40.39 (4.82) | 40.96 (6.53) | 0.31 | .7655 | 0.09 |
| IH | 12 | 41.03 (4.28) | 42.38 (6.52) | 1.09 | .2977 | 0.32 |
| SEMSS | ||||||
| NT1 | 12 | 41.18 (7.31) | 44.27 (6.36) | 2.97 | .0127 | 0.86 |
| NT2 | 11 | 41.01 (8.37) | 42.58 (7.15) | 0.92 | .3797 | 0.28 |
| IH | 12 | 42.56 (4.90) | 42.24 (3.54) | −0.40 | .6990 | −0.11 |
| SEMSX | ||||||
| NT1 | 12 | 38.52 (5.98) | 42.23 (6.31) | 3.84 | .0028 | 1.11 |
| NT2 | 11 | 40.16 (6.20) | 39.36 (4.03) | −0.34 | .7428 | −0.10 |
| IH | 12 | 38.18 (4.55) | 39.54 (4.24) | 1.05 | .3183 | 0.30 |
| Participate | ||||||
| NT1 | 12 | 37.21 (6.61) | 38.03 (6.54) | 0.93 | .3721 | 0.27 |
| NT2 | 11 | 38.81 (8.38) | 40.68 (5.25) | 1.17 | .2689 | 0.35 |
| IH | 12 | 39.13 (4.28) | 39.43 (3.70) | 0.29 | .7765 | 0.08 |
| Soc Isolation | ||||||
| NT1 | 12 | 59.58 (5.84) | 54.48 (5.90) | −3.48 | .0051 | −1.01 |
| NT2 | 11 | 58.85 (6.90) | 57.41 (5.76) | −0.69 | .5074 | −0.21 |
| IH | 12 | 57.16 (5.41) | 57.90 (8.29) | 0.53 | .6068 | 0.15 |
| Cog Funct | ||||||
| NT1 | 12 | 34.72 (5.09) | 37.24 (4.25) | 2.30 | .0419 | 0.66 |
| NT2 | 11 | 40.12 (5.67) | 37.62 (3.75) | −1.99 | .0749 | −0.60 |
| IH | 12 | 35.73 (4.83) | 37.48 (4.81) | 1.05 | .3143 | 0.30 |
| Phys Funct | ||||||
| NT1 | 12 | 43.08 (5.29) | 43.26 (6.94) | 0.11 | .9135 | 0.03 |
| NT2 | 11 | 42.75 (6.02) | 44.19 (8.08) | 1.81 | .0998 | 0.55 |
| IH | 12 | 44.81 (7.19) | 43.49 (6.54) | −1.39 | .1908 | −0.40 |
| Global MH | ||||||
| NT1 | 12 | 33.71 (7.35) | 37.30 (7.90) | 2.34 | .0390 | 0.67 |
| NT2 | 11 | 37.78 (7.88) | 38.37 (7.22) | 0.21 | .8383 | 0.06 |
| IH | 12 | 37.74 (4.96) | 37.19 (7.61) | −0.32 | .7584 | −0.09 |
| Global PH | ||||||
| NT1 | 12 | 42.18 (6.31) | 41.63 (7.04) | −0.63 | .5421 | −0.18 |
| NT2 | 11 | 42.80 (8.96) | 43.12 (7.09) | 0.19 | .8522 | 0.06 |
| IH | 12 | 42.53 (7.92) | 42.13 (4.30) | −0.22 | .8310 | −0.06 |
| ESS | ||||||
| NT1 | 12 | 15.00 (3.25) | 13.92 (3.48) | −2.17 | .0528 | −0.63 |
| NT2 | 11 | 10.55 (2.70) | 10.64 (3.35) | 0.09 | .9309 | 0.03 |
| IH | 12 | 13.83 (3.76) | 11.83 (3.64) | −2.10 | .0598 | −0.61 |
| FOSQ | ||||||
| Activity | ||||||
| NT1 | 12 | 1.93 (0.43) | 1.97 (0.62) | 0.48 | .6430 | 0.14 |
| NT2 | 11 | 2.19 (0.43) | 2.21 (0.71) | 0.10 | .9212 | 0.03 |
| IH | 12 | 1.78 (0.38) | 1.99 (0.46) | 1.48 | .1665 | 0.43 |
| Vigilance | ||||||
| NT1 | 12 | 2.27 (0.63) | 2.38 (0.69) | 0.63 | .5431 | 0.18 |
| NT2 | 11 | 2.61 (0.58) | 2.89 (0.52) | 1.51 | .1619 | 0.46 |
| IH | 12 | 2.36 (0.68) | 2.43 (0.66) | 0.44 | .6667 | 0.13 |
| Intimacy | ||||||
| NT1 | 12 | 2.23 (0.94) | 2.40 (0.91) | 1.23 | .2437 | 0.36 |
| NT2 | 9 | 2.50 (0.83) | 2.58 (1.03) | 0.35 | .7328 | 0.12 |
| IH | 11 | 2.55 (0.93) | 2.71 (0.87) | 0.65 | .5301 | 0.20 |
| Productivity | ||||||
| NT1 | 12 | 2.33 (0.59) | 2.39 (0.65) | 0.74 | .4774 | 0.21 |
| NT2 | 11 | 2.75 (0.70) | 2.80 (0.43) | 0.30 | .7733 | 0.09 |
| IH | 12 | 2.41 (0.47) | 2.47 (0.46) | 0.36 | .7278 | 0.10 |
| Social | ||||||
| NT1 | 12 | 2.54 (0.92) | 2.67 (0.78) | 0.67 | .5152 | 0.19 |
| NT2 | 11 | 2.64 (0.67) | 2.95 (0.52) | 1.47 | .1717 | 0.44 |
| IH | 12 | 2.29 (0.72) | 2.83 (0.69) | 2.24 | .0468 | 0.65 |
| Total FOSQ | ||||||
| NT1 | 12 | 2.19 (0.41) | 2.27 (0.57) | 1.05 | .3147 | 0.30 |
| NT2 | 11 | 2.50 (0.45) | 2.62 (0.43) | 0.77 | .4581 | 0.23 |
| IH | 12 | 2.21 (0.39) | 2.33 (0.46) | 1.16 | .2688 | 0.34 |
| SIQ | ||||||
| NT1 | 12 | 81.08 (10.77) | 76.25 (13.88) | −2.22 | .0487 | −0.64 |
| NT2 | 11 | 71.64 (12.48) | 70.00 (17.45) | −0.43 | .6789 | −0.13 |
| IH | 12 | 84.42 (11.91) | 80.75 (14.21) | −1.14 | .2777 | −0.33 |
| RSQ | ||||||
| NT1 | 12 | 26.56 (12.82) | 27.08 (16.50) | 0.15 | .8808 | 0.04 |
| NT2 | 11 | 27.27 (13.48) | 25.57 (12.95) | −0.82 | .4316 | −0.25 |
| IH | 12 | 11.46 (7.46) | 17.71 (16.39) | 1.24 | .2412 | 0.36 |
PHQ = Patient Health Questionnaire, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, IH = idiopathic hypersomnia, PROMIS = Patient-Reported Outcomes Measurement Information Systems, Dep = Depression, Anx = Anxiety, Sleep Dist = Sleep Disturbance, Sleep Imp = Sleep-Related Impairment, GSE = General Self-Efficacy, SEMEM = Self-Efficacy for Managing Emotions, SEMSS = Self-Efficacy for Managing Social Interactions, SEMSX = Self-Efficacy for Managing Symptoms, Participate = Ability to Participate in Social Roles and Activities, Soc Isolation = Social Isolation, Cog Funct = Cognitive Functioning, Phys Funct = Physical Functioning, Global MH = Global Mental Health, Global PH = Global Physical Health, ESS = Epworth Sleepiness Scale, FOSQ = Functional Outcomes of Sleep Questionnaire, SIQ = Sleep Inertia Questionnaire, RSQ = Restorative Sleep Questionnaire.
Figure 3. Within-subject effect sizes by diagnosis.
(A) The within-subject effect size (Cohen’s d) from baseline to posttreatment for narcolepsy type 1 (NT1), narcolepsy type 2 (NT2), and idiopathic hypersomnia (IH) across the following clinical measures: Patient Health Questionnaire (PHQ), Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire (FOSQ), Sleep Inertia Questionnaire (SIQ), and Restorative Sleep Questionnaire (RSQ). (B) The within-subject effect size (Cohen’s d) from baseline to posttreatment for each diagnostic group across the following Patient-Reported Outcomes Measurement Information Systems measures: Depression (Dep), Anxiety (Anx), Sleep Disturbance (Sleep Dist), Sleep-Related Impairment (Sleep Imp), Fatigue, General Self-Efficacy (GSE), Self-Efficacy for Managing Emotions (SEMEM), Self-Efficacy for Managing Social Interactions (SEMSS), Self-Efficacy for Managing Symptoms (SEMSX), Ability to Participate in Social Roles and Activities (Participate), Social Isolation (Soc Isolation), Cognitive Functioning (Cog Funct), Physical Functioning (Phys Funct), Global Mental Health (Global MH), and Global Physical Health (Global PH). An effect size of d > 0.5 was the prespecified benchmark for a minimal clinically important difference.
Treatment acceptability and credibility
The treatment expectancy scale was completed by 29 participants. The scores on how logical (mean = 7.24, SD = 1.79) and how confident (mean = 6.07, SD = 1.87) were relatively high, with relatively moderate scores on being successful with treatment (mean = 4.34, SD = 1.88) and likelihood of seeing improvement (mean = 40.70%, SD = 22.82%). There were no differences (P > .05) between individual and group CBT-H on any of these subscales. There were no adverse events reported in either individual or group CBT-H.
All 32 of the completers participated in the exit interview. With regard to treatment modules, participants generally found that cognitive strategies on emotion regulation (eg, active acceptance) and coping skills for the constancy and unpredictability of CDH symptoms were beneficial. Behaviorally, participants reported improved energy and productivity by implementing structure during the day, taking planned naps, using the nurturing/depleting activity, and following proper sleep hygiene. Some participants noted that they had not yet experienced change but felt that the skills acquired from the intervention would lead to benefits beyond treatment termination. Many participants disliked completing the sleep/wake diary every week, stating it was helpful in setting up a daytime structure and routine but that it became burdensome to continue completing on a daily basis.
There was consensus support for using a telehealth delivery model, including fewer barriers to access the program and improved ability to engage during the treatment sessions (eg, more comfortable to participate from home). Participants were generally satisfied with their assigned treatment format. Most participants who received individual CBT-H appreciated the individualized attention and felt more comfortable discussing emotional issues in this format. However, others felt that they would have benefitted from having a social connection with others who also had CDH. Participants who received group CBT-H enjoyed meeting others with CDH and sharing experiences but some felt that the group setting limited their opportunity to share, and others felt reluctant to discuss topics that might not be relevant to other group members. The majority of participants felt that 6 weekly sessions was acceptable, although some participants in group CBT-H suggested additional or longer sessions to facilitate participation. In addition, there were some suggestions for a hybrid approach using both group and individual sessions to optimize social interactions and personal attention. Finally, some participants suggested greater emphasis or additional modules devoted to interpersonal relationships, raising families, and disclosing their CDH diagnosis to others.
DISCUSSION
This phase 1b study of a novel adjunctive CBT-H program for people with CDH and depressive symptoms found indications of feasibility and acceptability in some areas along with the need for further refinement in other areas. A significant baseline to posttreatment reduction in depressive symptoms (≥5-point change on the PHQ) was achieved by 40% of the total sample, falling short of the benchmark set at ≥50% for preliminary efficacy. It is possible that our prespecified benchmark was overly ambitious. One study comparing different criteria for treatment response on the PHQ found that between 36.5% to 40.6% achieved clinically significant changes in depressive symptoms using different criteria following a community-level intervention for depression,39 which is within the range of the proportion of responders in this study. Notably, 50% of the participants who received the group CBT-H achieved a significant reduction and baseline to posttreatment effect sizes also indicated larger effects for group CBT-H (d = 1.01) compared with individual CBT-H (d = 0.54). These findings suggest that the group format might be more potent for reducing depressive symptoms. Qualitative data collected on the treatment modules revealed that participants found the coping skills and emotion-regulation exercises to be useful, indicating that these could be important modules in CBT-H for managing depressive symptoms.
On other psychosocial domains, the CBT-H program met the MCID benchmark on the PROMIS global self-efficacy scale. This benchmark was achieved in the overall sample and both individual and group formats, indicating that both formats are capable of increasing self-efficacy, which is the belief in an individual’s ability to carry out a behavior necessary to reach a desired goal, even when the situation is unpredictable or stressful.40,41 Self-efficacy has been found to be a predictor in managing other chronic conditions such as heart disease42 and type 2 diabetes43 and has also been shown to be an important mediator between self-management skills and patient-reported outcomes such as depression and pain.41 Findings in this study suggest that self-efficacy could be an important psychological mechanism for improving psychosocial functioning in CDH.
The effect size on the ESS in the overall sample was below the MCID benchmark (d = 0.35), but group CBT-H met the MCID benchmark (d = 0.73), and the change from baseline to posttreatment was below P < .05 for group CBT-H and the overall sample. This indicates that CBT-H is capable of reducing hypersomnolence. The behavioral modules in CBT-H included sleep hygiene and scheduled naps, strategies that have previously received some support for reducing hypersomnolence in people with narcolepsy.13–15 In addition, this CBT-H program introduced a new module for structured waking activities, which involved organizing each day into small segments of planned activities in between major (ie, nighttime sleep) or minor (ie, scheduled naps) sleep periods. A structured morning routine has also been used as a treatment component to reduce sleep inertia in people with insomnia and bipolar disorder.44 Together, these findings indicate that structured behavioral routines might be beneficial for managing symptoms of hypersomnolence in certain sleep disorders. Another new behavioral strategy aimed at daytime activities was the nurturing/depleting activity, which was used to evaluate the energy valence of each activity during the day and to examine ways to rebalance these energy transactions. This activity has previously been used as part of mindfulness-based cognitive therapy29 to help prevent the relapse of depression and thus could also account for the effects on depressive symptoms observed in this study. During the exit interviews, participants also expressed satisfaction for using these cognitive and behavioral strategies for managing energy.
Post hoc analyses by treatment format revealed a pattern that somewhat favored the group format. Group CBT-H met the MCID benchmark on the PROMIS depression (d = 0.55) and PROMIS self-efficacy for managing social interactions (d = 0.53), whereas individual CBT-H met the benchmark on the FOSQ social subscale (P = .0305, d = 0.54). Post hoc analysis did not reveal a consistent pattern across CDH diagnostic types. All 3 diagnostic conditions met the MCID benchmark on PHQ, indicating that CBT-H can be beneficial for people with either narcolepsy or IH. Compared with NT2 and IH, people with NT1 showed a more favorable pattern of responses using the MCID benchmark in several psychosocial domains. However, these analyses were not direct comparisons between the diagnostic groups, and the study was not sufficiently powered to test these between-group comparisons. Therefore, these results should be interpreted with caution. The current findings demonstrate that the modular approach used in CBT-H is feasible and potentially beneficial for people with narcolepsy or IH, although the clinical impact might be somewhat different with each diagnostic group.
The findings generally supported the acceptability of the CBT-H program and the telehealth model. Of the 35 participants assigned to treatment, 91.4% completed the study, and no adverse events were reported. The CBT-H program was found to be credible with moderate expectations for achieving success. Qualitative data revealed enthusiasm for the telehealth delivery model. The 6-week timeframe was acceptable, although some participants desired having more time or more individual attention. Participants generally had positive feedback regarding their assigned delivery format, but there were mixed opinions regarding preferences for individual or group formats. The findings also provide indications for specific areas to refine and optimize the CBT-H program. Considerations should be given to modify or reduce the patient burden for sleep/wake diaries, because participants did not find it beneficial to keep regular diaries throughout the program. Consideration might also be given to alternative formats, such as a hybrid of individual and group sessions or providing dedicated time for each participant to share during the group sessions. Finally, additional emphasis on managing interpersonal relationships and communication skills in disclosing CDH diagnosis could enhance the clinical impact of CBT-H.
Several limitations should be considered when interpreting the findings from this study. First, this was an uncontrolled study, and the changes observed could have been because of factors such as passage of time, regression to the mean, or treatment expectations. Second, the sample size was relatively small, and the data on treatment effects could be unstable and should be interpreted with caution. Third, treatment format was assigned rather than randomized, which could allow for selection bias. Fourth, there were differences in clinical experience among the study therapists, and there was no formal monitoring of treatment fidelity, which could have impacted the patient’s experience and clinical outcomes. Fifth, the study only included self-report measures, which are subject to bias. However, the priority for this phase 1b study was to focus on patient-reported outcomes and remote delivery in preparation for a future pragmatic trial. Finally, the demographics of this sample were disproportionately high for women and whites, and the inclusion criteria for access to stable internet could have led to selection bias that might not generalize to all people with narcolepsy and IH.
The present findings provide preliminary support for the feasibility and acceptability of using a novel CBT-H program as an adjunctive therapy to reduce depressive symptoms and improve psychosocial functioning in CDH with depressive symptoms. Further refinement and optimization of the treatment program should be considered before moving into a phase 2 study. Additionally, findings supported the use of telehealth for remote treatment delivery and data collection. Overall, these findings provide important guidance for future studies to build on and demonstrate the potential for improving HRQoL in people with CDH.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was funded by a research grant from the American Academy of Sleep Medicine Foundation (185-SR-17). Dr. Dawson also received grant support from the National Institutes of Health T32HL007909. Dr. Ong has received grant support from Harmony Biosciences and is on the medical advisory board for Wake up Narcolepsy, Narcolepsy Network, and Hypersomnia Foundation. These activities are unrelated to the current study. Dr. Mundt is a consultant for Loona, Inc., which is unrelated to the current study. All other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Elizabeth Adkins for serving as a study therapist and Wake up Narcolepsy, Narcolepsy Network, and the Hypersomnia Foundation for assistance with recruitment and distribution of the study information to their members.
ABBREVIATIONS
- Anx
anxiety
- CBT-H
cognitive behavioral therapy for hypersomnia
- CDH
central disorders of hypersomnolence
- Cog Funct
cognitive functioning
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- Global MH
global mental health
- Global PH
global physical health
- GSE
general self-efficacy
- HRQoL
health-related quality of life
- IH
idiopathic hypersomnia
- MCID
minimal clinically important difference
- NT1
narcolepsy type 1
- NT2
narcolepsy type 2
- Participate
ability to participate in social roles and activities
- PHQ
Patient Health Questionnaire
- Phys Funct
physical functioning
- PROMIS
Patient-Reported Outcomes Measurement Information Systems
- RSQ
Restorative Sleep Questionnaire
- SEMEM
self-efficacy for managing emotions
- SEMSS
self-efficacy for managing social interactions
- SEMSX
self-efficacy for managing symptoms
- SIQ
Sleep Inertia Questionnaire
- Sleep Dist
sleep disturbance
- Sleep Imp
sleep-related impairment
- Soc Isolation
social isolation
REFERENCES
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Khan Z, Trotti LM. Central disorders of hypersomnolence: focus on the narcolepsies and idiopathic hypersomnia. Chest. 2015;148(1):262–273. 10.1378/chest.14-1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozaki A, Inoue Y, Nakajima T, Hayashida K, Honda M, Komada Y, Takahashi K. Health-related quality of life among drug-naïve patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time. J Clin Sleep Med. 2008;4(6):572–578. 10.5664/jcsm.27352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10(1):75–81. 10.1046/j.1365-2869.2001.00234.x [DOI] [PubMed] [Google Scholar]
- 5.Vignatelli L, Plazzi G, Peschechera F, Delaj L, D’Alessandro R. A 5-year prospective cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2011;12(1):19–23. 10.1016/j.sleep.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 6.Dauvilliers Y, Paquereau J, Bastuji H, Drouot X, Weil JS, Viot-Blanc V. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry. 2009;80(6):636–641. 10.1136/jnnp.2008.161588 [DOI] [PubMed] [Google Scholar]
- 7.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14(6):488–492. 10.1016/j.sleep.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Black J, Reaven NL, Funk SE, et al. The burden of narcolepsy disease (BOND) study: health-care utilization and cost findings. Sleep Med. 2014;15(5):522–529. 10.1016/j.sleep.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 9.Kales A, Soldatos CR, Bixler EO, Caldwell A, Cadieux RJ, Verrechio JM, Kales JD. Narcolepsy-cataplexy. II. Psychosocial consequences and associated psychopathology. Arch Neurol. 1982;39(3):169–171. 10.1001/archneur.1982.00510150039009 [DOI] [PubMed] [Google Scholar]
- 10.David A, Constantino F, dos Santos JM, Paiva T. Health-related quality of life in Portuguese patients with narcolepsy. Sleep Med. 2012;13(3):273–277. 10.1016/j.sleep.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 11.Vignatelli L, D’Alessandro R, Mosconi P, Ferini-Strambi L, Guidolin L, De Vincentiis A, Plazzi G; GINSEN (Gruppo Italiano Narcolessia-Studio Epidemiologico Nazionale) . Health-related quality of life in Italian patients with narcolepsy: the SF-36 health survey. Sleep Med. 2004;5(5):467–475. 10.1016/j.sleep.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 12.Dodel R, Peter H, Spottke A, et al. Health-related quality of life in patients with narcolepsy. Sleep Med. 2007;8(7-8):733–741. 10.1016/j.sleep.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 13.Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep. 2001;24(4):385–391. 10.1093/sleep/24.4.385 [DOI] [PubMed] [Google Scholar]
- 14.Mullington J, Broughton R. Scheduled naps in the management of daytime sleepiness in narcolepsy-cataplexy. Sleep. 1993;16(5):444–456. 10.1093/sleep/16.5.444 [DOI] [PubMed] [Google Scholar]
- 15.Helmus T, Rosenthal L, Bishop C, Roehrs T, Syron ML, Roth T. The alerting effects of short and long naps in narcoleptic, sleep deprived, and alert individuals. Sleep. 1997;20(4):251–257. 10.1093/sleep/20.4.251 [DOI] [PubMed] [Google Scholar]
- 16.Morgenthaler TI, Kapur VK, Brown T, et al.; Standards of Practice Committee of the American Academy of Sleep Medicine . Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705–1711. 10.1093/sleep/30.12.1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billiard M, Bassetti C, Dauvilliers Y, et al.; EFNS Task Force . EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13(10):1035–1048. 10.1111/j.1468-1331.2006.01473.x [DOI] [PubMed] [Google Scholar]
- 18.Aloe F, Alves RC, Araujo JF, et al. Diretrizes brasileiras para o tratamento da narcolepsia. Br J Psychiatry. 2010;32(3):305–314. 10.1590/S1516-44462010000300016 [DOI] [PubMed] [Google Scholar]
- 19.Neikrug AB, Crawford MR, Ong JC. Behavioral sleep medicine services for hypersomnia: a survey study. Behav Sleep Med. 2016;15(2):158–171. [DOI] [PubMed] [Google Scholar]
- 20.Ong JC, Fox RS, Brower RF, Mazurek S, Moore C. How does narcolepsy impact health-related quality of life? A mixed-methods study. Behav Sleep Med. 2020:1–14. 10.1080/15402002.2020.1715411 [DOI] [PubMed] [Google Scholar]
- 21.Czajkowski SM, Powell LH, Adler N, et al. From ideas to efficacy: the ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015;34(10):971–982. 10.1037/hea0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psychooncology. 2013;22(8):1688–1704. 10.1002/pon.3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. Int J Psychiatry Med. 2006;36(1):13–34. 10.2190/EUFN-RV1K-Y3TR-FK0L [DOI] [PubMed] [Google Scholar]
- 24.Rehse B, Pukrop R. Effects of psychosocial interventions on quality of life in adult cancer patients: meta analysis of 37 published controlled outcome studies. Patient Educ Couns. 2003;50(2):179–186. 10.1016/S0738-3991(02)00149-0 [DOI] [PubMed] [Google Scholar]
- 25.Trompetter HR, Bohlmeijer ET, Veehof MM, Schreurs KMG. Internet-based guided self-help intervention for chronic pain based on Acceptance and Commitment Therapy: a randomized controlled trial. J Behav Med. 2015;38(1):66–80. 10.1007/s10865-014-9579-0 [DOI] [PubMed] [Google Scholar]
- 26.Eccleston C, Morley SJ, Williams AC. Psychological approaches to chronic pain management: evidence and challenges. Br J Anaesth. 2013;111(1):59–63. 10.1093/bja/aet207 [DOI] [PubMed] [Google Scholar]
- 27.Keefe FJ, Porter L, Somers T, Shelby R, Wren AV. Psychosocial interventions for managing pain in older adults: outcomes and clinical implications. Br J Anaesth. 2013;111(1):89–94. 10.1093/bja/aet129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borkovec TD, Wilkinson L, Folensbee R, Lerman C. Stimulus control applications to the treatment of worry. Behav Res Ther. 1983;21(3):247–251. 10.1016/0005-7967(83)90206-1 [DOI] [PubMed] [Google Scholar]
- 29.Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: The Guilford Press; 2002. [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai JS, Cella D, Choi S, Junghaenel DU, Christodoulou C, Gershon R, Stone A. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil. 201192(10 suppl):S20–S27. 10.1016/j.apmr.2010.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 34.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 35.Kanady JC, Harvey AG. Development and validation of the sleep inertia questionnaire (SIQ) and assessment of sleep inertia in analogue and clinical depression. Cognit Ther Res. 2015;39(5):601–612. 10.1007/s10608-015-9686-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drake CL, Hays RD, Morlock R, et al. Development and evaluation of a measure to assess restorative sleep. J Clin Sleep Med. 2014;10(7):733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73–86. 10.1016/S0005-7916(00)00012-4 [DOI] [PubMed] [Google Scholar]
- 38.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–629. 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMillan D, Gilbody S, Richards D. Defining successful treatment outcome in depression using the PHQ-9: a comparison of methods. J Affect Disord. 2010;127(1-3):122–129. 10.1016/j.jad.2010.04.030 [DOI] [PubMed] [Google Scholar]
- 40.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44(9):1175–1184. 10.1037/0003-066X.44.9.1175 [DOI] [PubMed] [Google Scholar]
- 41.Gruber-Baldini AL, Velozo C, Romero S, Shulman LM. Validation of the PROMIS® measures of self-efficacy for managing chronic conditions. Qual Life Res. 2017;26(7):1915–1924. 10.1007/s11136-017-1527-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark NM, Dodge JA. Exploring self-efficacy as a predictor of disease management. Health Educ Behav. 1999;26(1):72–89. 10.1177/109019819902600107 [DOI] [PubMed] [Google Scholar]
- 43.Aljasem LI, Peyrot M, Wissow L, Rubin RR. The impact of barriers and self-efficacy on self-care behaviors in type 2 diabetes. Diabetes Educ. 2001;27(3):393–404. 10.1177/014572170102700309 [DOI] [PubMed] [Google Scholar]
- 44.Kaplan KA, Talavera DC, Harvey AG. Rise and shine: A treatment experiment testing a morning routine to decrease subjective sleep inertia in insomnia and bipolar disorder. Behav Res Ther. 2018;111:106–112. 10.1016/j.brat.2018.10.009 [DOI] [PubMed] [Google Scholar]