Abstract
Study Objectives:
Adults with obesity and obstructive sleep apnea (OSA) are at risk for cardiometabolic disease, and this risk likely extends to children with both conditions. Noninvasive ventilation (NIV; including continuous and bilevel positive airway pressure) is often used to treat OSA in children with obesity. The aim of this study was to examine the impact of NIV treatment on heart rate variability (HRV), as a marker of cardiovascular risk, in children with obesity and newly diagnosed OSA.
Methods:
A prospective multicenter cohort study was conducted in children with obesity prescribed NIV therapy for newly diagnosed moderate-severe OSA. Measurements of HRV were derived from polysomnography recordings at baseline and after 12 months of treatment. HRV parameters were examined by sleep stage, before and after arousal and oxygen desaturation events. HRV parameters were compared between time points using pair t tests as well as mixed model analysis.
Results:
Twelve children had appropriate data for analysis at baseline and 12 months. Heart rate decreased by 4.5 beats/min after NIV treatment, with no change in HRV parameters. HRV parameters differed by sleep stage and showed an increase in arousal-related sympathetic-parasympathetic balance after 12 months of NIV treatment. HRV parameters did not differ before and after oxygen desaturation events.
Conclusions:
NIV for the treatment in children with obesity and OSA resulted in a small decrease in heart rate and an increase in arousal-related sympathetic-parasympathetic balance. These findings suggest small, potentially positive impacts of NIV on cardiovascular risk in children with concurrent obesity and OSA.
Citation:
Kirk VG, Edgell H, Joshi H, et al. Cardiovascular changes in children with obstructive sleep apnea and obesity after treatment with noninvasive ventilation. J Clin Sleep Med. 2020;16(12):2063–2071.
Keywords: adolescent, youth, continuous positive airway pressure, bilevel positive airway pressure
BRIEF SUMMARY
Current Knowledge/Study Rationale: Cardiovascular disease risk is increased in children with obesity and compounded by obstructive sleep apnea. Heart rate variability, an established marker of cardiovascular risk in adults, differs between children with obstructive sleep apnea and control children, and improves with adenotonsillectomy. It is not known whether treatment with noninvasive ventilation (including continuous and positive airway pressure) has the same effect.
Study Impact: Our results demonstrate a small reduction in heart rate and improvement in heart rate variability to arousal events in children with obesity after 12 months of treatment of obstructive sleep apnea. These results support a potentially positive impact of noninvasive ventilation treatment on cardiovascular risk in a largely adherent group of children and youth.
INTRODUCTION
High rates of childhood obesity have contributed to an increasing number of pediatric cases of obesity-related sleep-related breathing disorders, including obstructive sleep apnea (OSA). With 1 in 3 North American children being overweight or obese, obesity is an important risk factor for childhood OSA.1,2 OSA prevalence is significantly higher among children with obesity, with estimates of 13–71% in cohorts who have undergone polysomnography3–7 compared with 1–5% in otherwise healthy children.8 Residual OSA post-adenotonsillectomy is present in 22–59% of children with obesity,9–11 making noninvasive ventilation (NIV), including continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BPAP), common secondary treatments.8
Obesity-related complications are increasingly recognized in children, including metabolic disturbances with insulin resistance and hypertension, and are particularly prevalent in those with concurrent OSA. Our group previously reported a high prevalence of metabolic dysfunction and hypertension at the time of OSA diagnosis in a cohort of children with obesity and OSA who were started on NIV.12 Forty percent of our population had elevated homeostatic model assessment of insulin resistance (HOMA-IR) relative to a reference population at baseline and 40% were hypertensive, with 70% of the sample having a loss of nocturnal blood pressure dip. Increased inflammatory markers were also present in 63% at the time of OSA diagnosis, independent of OSA severity.12 These findings highlight the need to evaluate markers of early cardiovascular disease in this high-risk population of children with OSA and obesity, many of whom will already have subclinical cardiometabolic disease at the time of OSA diagnosis.
Heart rate variability (HRV) is an established marker of cardiovascular risk in adults. HRV measures the fluctuation in the time from one R wave peak, or heartbeat, to the next.13 Analysis of HRV can be described in the time domain, quantifying the amount of variability in the interbeat interval, and in the frequency domain, which separates the HRV waveform into its component rhythms to describe how variance and amplitude is distributed as a function of the time period of a given rhythm (eg, low frequency, LF, 0.04–0.15 Hz; high frequency, HF, 0.15–0.4 Hz).13 The LF band captures rhythms repeating 7–25 s and is affected by breathing rates between 3 and 9 breaths/min. The HF, or respiratory band, captures rhythms repeating 2.5–7 s and is influenced by breathing rates between 9 and 24 breaths/min. The standard deviation of the NN interval measures the difference between R-R intervals and largely reflects parasympathetic activity. HF changes reflect the activity of the parasympathetic system as well. The etiology of LF changes are less clear and may be under both sympathetic and parasympathetic influence, while the LF/HF is an index of the balance of sympathetic and parasympathetic tone or vagal activity.14
Changes in both heart rate and HRV reflect the combined activity of the internal control system, primarily via the autonomic nervous system, interaction with other systems, including the respiratory system, and stressors, which include specific disease states.14,15 In the context of cardiovascular risk for at-risk adults, lower HRV is associated with greater risk of cardiovascular events and mortality.16 Reduced HRV is also implicated in sudden infant death syndrome, while factors shown to be protective for sudden infant death syndrome improve HRV.17–20 Compared to children acting as controls, children with OSA demonstrate evidence of altered HRV with a reduction in HRV that is more pronounced in more severe OSA.21 While age is the strongest determinant of HRV in children, central obesity adds to the reduction in HRV seen with OSA.22 In otherwise healthy preschool aged children, resolution of OSA is associated with a reduction in both parasympathetic and sympathetic activity as measured by a reduction in LF and HF power HRV parameters.23 A study of a novel method of heart rate pattern analysis demonstrated that school-aged children who underwent adenotonsillectomy for OSA showed a reduction in cardiac autonomic modulation during sleep regardless of whether OSA had been cured as measures by apnea-hypopnea index (AHI).24 The impact of treatment of OSA with NIV on HRV, as a marker of cardiovascular risk, has not been studied.
Given the mounting evidence that the cardiometabolic disease associated with obesity and OSA in adults is also present in children and youth, we hypothesize that NIV treatment for children with obesity and OSA will improve HRV and lower cardiovascular disease risk. The primary aim of this study was, therefore, to compare HRV at the time of sleep-related breathing disorders diagnosis and after 1 year of NIV treatment in children with obesity and sleep-related breathing disorders .
METHODS
Study design
This study is a substudy of a larger prospective, multicenter cohort study of children and youth with obesity and OSA where participants were recruited from 4 tertiary care pediatric centers across Canada. Twenty-seven participants were followed for 1 year after enrolment, with study evaluations performed at baseline (within 3 months of starting NIV), 6 months, and 12 months. The primary aim of the overall study was to determine the effect of NIV treatment on metabolic and cardiovascular parameters in children and youth with newly diagnosed OSA. Ethics approval was obtained from the Research Ethics Board at each of the participating study sites. Informed consent/assent was obtained from participants and their parents/guardians. The current study included a subset of the data collected with the main study protocol and additional results published elsewhere.12,25
Study population
The study population included children and youth 8–16 years old with a body mass index ≥ 95th percentile for sex and age26 who had newly diagnosed moderate-severe OSA and/or hypoventilation and were prescribed NIV and followed by their treating physician as per standard clinical protocols. Participants and their parent/guardian were fluent in either English or French. Exclusion criteria included craniofacial anomalies; central nervous system lesions; neuromuscular, neurological, or genetic syndromes; congenital heart disease and/or diagnosed ventricular dysfunction; chronic respiratory disease (with the exception of asthma); use of systemic corticosteroids within the past 3 months; current use of pharmacological sleep aids or medication for the treatment of type 1 or 2 diabetes.
The presence of OSA and hypoventilation were identified by overnight in-laboratory diagnostic polysomnography conducted and scored by sleep technologists, according to the American Academy of Sleep Medicine recommendations.27,28 Moderate to severe OSA was defined as an obstructive apnea-hypopnea index ≥ 5 events/h. Hypoventilation was defined as CO2 > 50 mm Hg for > 25% of total sleep time.28
Participants were excluded from this substudy if they did not have polysomnography recordings at baseline and 12 months with sufficient data for HRV analysis.
Measurements
Data included in the analysis included demographics, anthropometric measures, NIV adherence, and polysomnography. Height, weight, and waist measurements taken at baseline and the 12-month visit were used to calculate height, weight, waist, waist:height ratio, and body mass index z-scores.26 Sleep duration was obtained from a self-report diary and activity from a self-report questionnaire. Adherence to NIV was assessed by 1-month machine downloads as well as participant usage diaries and self/parent-report at clinic visits with adherence to therapy defined as use for an average of ≥ 4 h/night for > 50% of nights. If machine downloads were unavailable, a summary judgement of adherence to therapy was made by the treating physician based on the review of available information and was used where machine download data were unavailable.
Polysomnography was completed as a diagnostic study at baseline and with a treatment study (NIV titration) 12 months after starting NIV. AHI was calculated as the total number of obstructive, mixed and central apneas and hypopneas per hour of sleep. The obstructive-mixed AHI included obstructive and mixed apneas and hypopneas per hour of sleep, while the central AHI included central apneas and hypopneas per hour of sleep.
Short-term HRV and heart rate were derived from electrocardiogram tracings acquired during polysomnography testing for baseline and follow-up studies. Sampling rates for electrocardiogram measurements during polysomnography were > 200 Hz. Heart rate was measured from the R-R interval. HRV was analyzed in time domains via the standard deviation of the NN interval and the frequency domain via the LF and HF powers, including the LF/HF ratio.29 HRV was determined from the electrocardiogram recordings using a Hann (cosine-bell) data window (window overlap of 50%) and a fast-Fourier transform size of 1,024. A range of 0.04–0.15 Hz and 0.15–0.45 Hz was used for the LF and HF spectrums, respectively (LabChart Pro software; Version 8.1.9, ADInstruments, Colorado Springs, CO). Two-minute sections of recording, uninterrupted by apneas/hypopneas, arousals, or oxygen desaturation events, were analyzed with data from 2 to 5 sections across the night averaged together. HRV was analyzed for wake and each sleep stage, including non-rapid eye movement (NREM) stage 2 (NREM2), NREM3 (also known as slow-wave sleep), and rapid eye-movement sleep. This process was repeated for periods uninterrupted by apneas/hypopneas before and after arousal and oxygen desaturation events independent of sleep stage.
Statistical analysis
Descriptive statistics were used to summarize the sample characteristics expressed as median ± interquartile range. HRV parameters are expressed as means ± standard error. Student’s t test and Wilcoxon signed rank test were used to compare parametric and nonparametric measures. Paired t tests were used to compare HRV parameters between baseline and follow-up time points with mixed model used for multivariable analysis.
RESULTS
Of the 57 potential participants, 27 participants enrolled in the main study, and 12 had sufficient polysomnography data for HRV analysis at baseline and 12-month follow-up to be included in this analysis; 2 of these 12 (17%) were female. The majority of participants self-identified as White (60%). Of the 12 participants, 10 had OSA (83%) and 3 had hypoventilation (17%). Most were initiated on CPAP (9/12; 75%), with the remainder on bilevel positive airway pressure (3/12, 25%). There was a mean time between baseline and follow-up of 1.18 ± 0.51 years, and 75% of participants were considered adherent with therapy (9/12; adherence data unavailable for 2 participants, 1 nonadherent). No participants were on medication to control blood pressure, 1 was prescribed a beta-agonist, and one was prescribed a stimulant medication. Average reported sleep duration did not differ between baseline and follow-up (8:55 ± 0:45 vs 9:08 ± 0:55, mean difference −0:12 ± 0:21, P = not significant). Average reported weekday active time did not differ between baseline and follow-up (3:06 ± 3:00 vs 1:57 ± 1:48, mean difference 1:12 ± 1:49, P = not significant).
Anthropometric measures between baseline and 12-month follow-up remained stable (Table 1). NIV was effective in improving measures of sleep and breathing at 12 months compared to baseline diagnostic testing, with improvements in arousal index, AHI, obstructive-mixed AHI, lowest oxygen saturation, and mean as well as highest CO2. Sleep efficiency and mean oxygen saturation were unchanged from baseline on NIV at follow-up. AHI was normalized (< 2 events/h) for 6 of 12 participants (50%), 8 of 12 (67%) had an OMAHI < 2 events/h, and 9 of 12 (75%) had AHI <5 events/h. For those with AHI ≥ 2 events/h at the follow-up titration, the residual AHI was 4.3 events/h (interquartile range 4.47, range 2.1–8.9 events/h).
Table 1.
Characteristics of the cohort at baseline and follow-up (n = 12).
| Baseline Median (IQR) | Follow-up Median (IQR) | |
|---|---|---|
| Age (years) | 12.8 (6.1) | 14.3 (6.2) |
| Height z-score | 1.4 (3.3) | 1.6 (3.3) |
| Weight z-score | 3.3 (1.56) | 3.1 (1.7) |
| BMI z-score | 2.6 (0.6) | 2.7 (0.5) |
| Sleep efficiency (%) | 87.6 (15.7) | 87.5 (10.2) |
| Arousal index (events/h)** | 19.2 (22.0) | 7.9 (5.0) |
| Apnea-hypopnea index (events/h)** | 16.0 (13.6) | 1.9 (4.1) |
| Obstructive mixed apnea-hypopnea index (events/h)** | 13.8 (17.3) | 0.90 (2.6) |
| Central apnea-hypopnea index (events/h) | 0.35 (6.1) | 0.80 (1.6) |
| Mean oxygen saturation (%) | 95.8 (4.0) | 97.3 (2.7) |
| Mean carbon dioxide (mmHg)** | 44.2 (4.4) | 40.5 (5.4) |
| Lowest oxygen saturation (%)** | 84.7 (9.2) | 89.9 (3.9) |
| Highest carbon dioxide (mmHg)** | 50.0 (5.8) | 42.7 (9.4) |
Follow-up polysomnography data are from treatment studies where NIV was used during the study. **P < .001. BMI = body mass index, IQR = interquartile ratio, NIV = noninvasive ventilation.
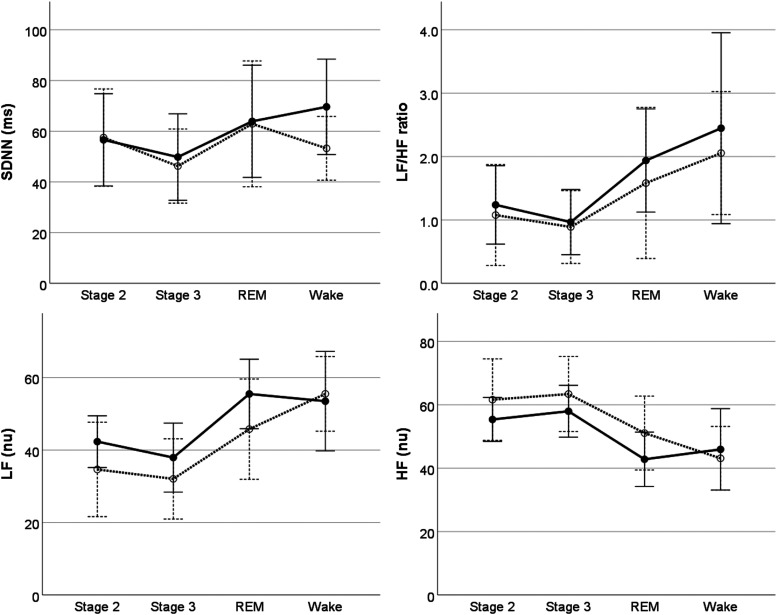
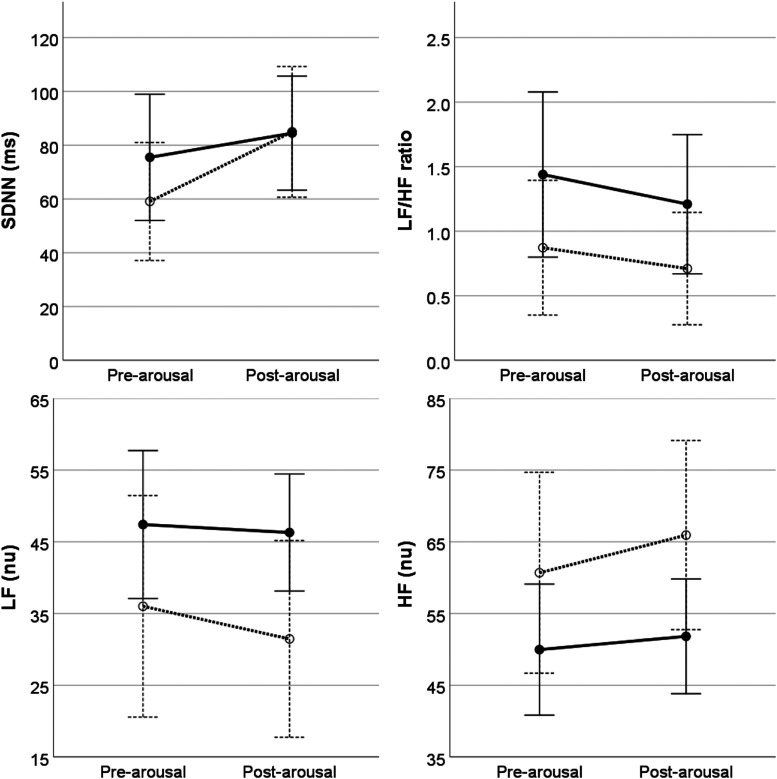
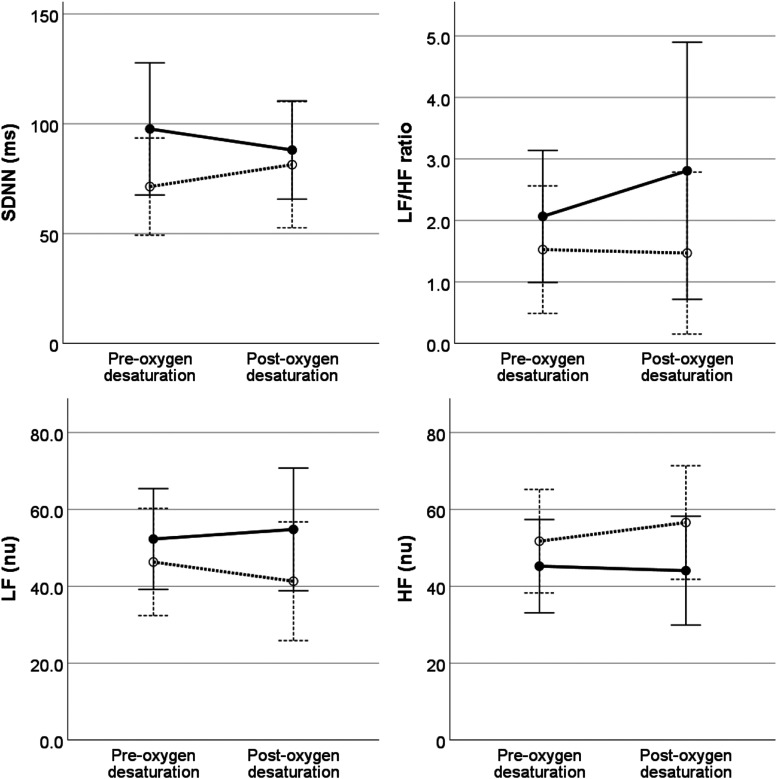
On univariable analysis, heart rate during sleep decreased an average of 4.5 beats/minute after 12 months of NIV treatment, with no change in overall HRV parameters (Table 2). For HRV parameters, multivariable analysis showed that LF, HF, and LF/HF ratio differed by sleep stage but not before and after treatment; LF power was lower and HF power higher in NREM2 and NREM3 compared to rapid-eye movement, with no differences by individual sleep stages for LH/HF ratio (Figure 1). While HRV parameters did not differ before and after arousal, HRV parameters around arousal events did differ before and after NIV treatment, with an increase in LF and decreased in HF after treatment compared to baseline (Figure 2). HRV parameters did not differ before and after oxygen desaturation events nor was this response altered by NIV treatment (Figure 3).
Table 2.
Univariate comparison of heart rate and heart rate variability during sleep at baseline and after 12 months of treatment.
| Baseline (mean ± SE) | Posttreatment (mean ± SE) | Mean Difference (mean ± SE) | |
|---|---|---|---|
| Heart rate (beats/min)* | 82.0 ± 2.9 | 77.6 ± 3.5 | −4.5 ± 2.0 |
| SDNN (ms) | 54.1 ± 7.1 | 56.8 ± 8.6 | 6.0 ± 7.6 |
| LF/HF ratio | 1.40 ± 0.34 | 1.68 ± 0.32 | 0.28 ± 0.25 |
| LF (nu) | 41.2 ± 5.0 | 48.0 ± 4.2 | 6.2 ± 3.9 |
| HF (nu) | 55.0 ± 5.0 | 49.9 ± 3.8 | −5.0 ± 4.0 |
*P < .05. nu = normal units.
Figure 1. Heart rate variability measures by sleep stage at baseline (dashed lines) and after 12 months of treatment (solid lines).
LF/HF ratio (P < 0.05), LF and HF (P < 0.01) differ between sleep stages, with no effect of treatment. Individual sleep stage estimates did not differ for LF/HF ratio. Compared to REM sleep, LF power was lower in NREM2 (−12.4 ± 5.6, 95%CI −23.6 to −1.1, P < 0.05) and NREM3 (−15.9 ± 5.7, 95%CI −27.3 to −4.53, P < 0.05), with no difference from wake (3.64, 95% CI −7.60 to 14.89). For HF, there was an increase in HF for NREM2 (11.8 ± 5.4, 95%CI 1.0 to 22.5, P < 0.05) and NREM3 (14.0 ± 5.4, 95% CI 3.2 to 24.8, P < 0.05) compared to REM, with no difference from wake (−2.1, 95%CI −12.91 to 8.50). Error bars indicate ± 2 standard errors. CI = confidence interval, HF = high frequency, LF = low frequency, nu = normal units, NREM = non-rapid eye movement, REM = rapid eye movement, SDNN = standard deviation of NN intervals.
Figure 2. Heart rate variability measured before and after arousal events at baseline (dashed lines) and after 12 months of treatment (solid lines).
There was a main effect of treatment for LF and HF, with no effect of pre-arousal/post-arousal differences. After 12 months of treatment, LF increased (13.19 ± 5.9, 95% CI 1.29 to 24.9, P < 0.05) and HF decreased (−12.41 ± 5.4, 95% CI 1.43 to 23.4) relative to baseline. Error bars indicate ± 2 standard errors. CI = confidence interval, HF = high frequency, LF = low frequency, nu = normal units, SDNN = standard deviation of NN intervals.
Figure 3. Heart rate variability measured before and after oxygen desaturation events at baseline (dashed lines) and after 12 months of treatment (solid lines).
Heart rate variability parameters did not differ pre-oxygen/post-oxygen desaturation events or after 12 months of treatment. Error bars indicate ± 2 standard errors. HF = high frequency, LF = low frequency, nu = normal units, SDNN = standard deviation of NN intervals.
DISCUSSION
In this study, we examined heart rate and HRV in a group of children with obesity and OSA before and after 12 months of treatment with NIV. Our results demonstrate an overall small reduction in heart rate, which may be consistent with an increase in parasympathetic activation, but no overall change in HRV parameters after 12 months of NIV treatment. While HRV parameters did not differ before and after arousal events, an increase in LF power and decrease in HF power around arousal events after 12 months of NIV treatment may be indicative of a relative impairment in arousal response at baseline that is restored after treatment. HRV parameters did vary by sleep stage as expected, so the relative parasympathetic/sympathetic balance across sleep stages was preserved both before and after treatment. Overall, our results support minimal changes in heart rate and HRV after 12 months of treatment with NIV in children with obesity and OSA.
There has been considerable interest in the impact of OSA treatment in adults on HRV as a marker of cardiovascular disease risk. One of the proposed mechanisms for an increased risk of cardiovascular disease associated with OSA is an increase in sympathetic activation secondary to intermittent hypoxia and arousal from sleep.30 Treatment of OSA with CPAP, by extension, would decrease cardiovascular disease risk by decreasing sympathetic activation. Despite smaller studies demonstrating a change in HRV with CPAP use,31–33 a recent meta-analysis investigating the association between CPAP and HRV in adults with OSA found little change in HRV parameters after at least 1 month of treatment.34 The results showed that CPAP use was associated with a small reduction in LF power, little impact on HF power, and a small reduction in LF/HF ratio. In a meta-analysis of the cardiovascular effects of oral appliance use for the treatment of OSA, 2 studies reported on HRV parameters35; 1 showed an increase in HF power after 3 months of use, but only during 1 of 4 breathing techniques used to test autonomic function,36 and the other showed no change in HRV parameters after 1 month of treatment.37 Similar results of minimal, although statistically significant, decreases in LF and increase in HF power were seen after successful upper airway surgery for OSA.38,39 Our results showed no overall treatment effect of 12 months of NIV use on HRV parameters despite a small decrease in heart rate and changes in HRV parameters around arousal events. Taken together, these results suggest little impact of different OSA treatments on HRV parameters and support a suggestion that, in the context of OSA, analysis of HRV may not provide additional information over the analysis of heart rate across the night.40,41
Arousal from sleep is a potential protective factor for the occurrence of OSA as well as having an important role in the pathophysiology of OSA and cardiovascular risk. Arousal or awakening from sleep is an important protective response to respiratory events as it restores muscle tone to the airway, reducing airway collapsibility and enabling movement to improve airway patency.42,43 By contrast, a low arousal threshold, where a smaller stimulus is needed to precipitate arousal, along with a collapsible airway, results in OSA associated with fragmented sleep, an OSA phenotype that is associated with increased risk for cardiovascular events or death in adults.44 While intermittent hypoxia is clearly an important stimulus for cardiac sympathetic activity, arousal also modulates sympathetic overactivity. Arousal from sleep enhances the increase in autonomic nervous system and sympathetic nerve activity associated with respiratory events.45 Both arousal and hypoxia accompanying an apnea/hypopnea event increases heart rate and LF/HF ratio.46 Infants with apparent life-threatening events and OSA demonstrate altered arousal responses that normalize after treatment with CPAP.47,48 Although our study results suggest that HRV arousal response may be blunted before treatment of OSA and potentially restored after treatment with NIV, additional work is needed to examine the role of arousal responses and their relationship to the underlying risk of OSA and subsequent cardiovascular risk in children and youth with obesity and OSA.
It is important to highlight the significance of the context of HRV measurement, as a number of factors may impact measurements and likely explain some of the variability between results from different studies.13 Because respiratory rate may alter heart rate, both the time and frequency domain measurements of HRV parameters may be altered by a change in breathing frequency related to NIV use rather than a direct impact on cardiovascular function. This may explain why studies where HRV was measured on NIV show a moderate reduction in LF power, a moderate reduction in HF, and no change in LF/HF ratio, whereas studies where HRV was measured off CPAP show increased HF power with a decline in LF/HF ratio.34 HRV parameters also change with age, so differences in age and time between measurements may also account for some differences.49 Sympathetic-parasympathetic balance changes from wake to sleep, so measures taken during wake vs sleep will differ. Position is also important, with higher reproducibility of measurements in the supine position.50 In the present study, HRV was measured during polysomnography off NIV at baseline and on NIV at 12-month follow-up with the data for both acquired during sleep in a recumbent position. These factors may impact our results and the comparability of our results with other forms of OSA treatment as well as other HRV measurement paradigms.
This study has some limitations that may impact interpretation of the results. This sample of children and youth with obesity were a select group, as all were referred to tertiary care centers for evaluation of breathing during sleep. In addition, all met our strict inclusion criteria, returned for follow-up evaluations, and had complete data. As a result, the sample size is small. The children and youth included in this analysis represent 44% of the original cohort enrolled in this study and 63% of those who completed follow-up,12 so the results may not generalize to other children and youth with obesity using NIV. In addition, 50% of participants demonstrated a residual AHI ≥ 2 events/h and, therefore, may be considered to have residual OSA despite significant improvements from baseline, and 25% of participants were considered nonadherent to therapy; our results may underestimate the benefits of NIV on heart rate and HRV. We were unable to look at the impact of apnea-hypopnea events on HRV because apnea events were uncommon and hypopnea events were associated with arousal or oxygen desaturation so we could not isolate the impact of the hypopnea event alone.
CONCLUSIONS
In our cohort of children and youth with obesity and OSA, there was a small reduction in heart rate during sleep after 1 year of prescribed NIV therapy, although no overall changes were observed in HRV parameters. We did see expected sleep stage-related differences in HRV parameters, as well as alterations in HRV arousal responses after treatment. These findings support potentially small positive impacts of NIV treatment on cardiovascular risk in a group of children and youth with obesity. Further work is needed to understand the longer term implications of OSA treatment in children and youth with obesity on cardiovascular health.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Children’s Hospital of Eastern Ontario, Ottawa, Ontario; Montreal Children’s Hospital, Montreal, Quebec; and Stollery Children’s Hospital, Edmonton, Alberta. This study was funded by the Canadian Institutes of Health Research and facilitated by the Women and Children’s Health Research Institute through the generous support of the Stollery Children's Hospital Foundation. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to the children and youth who participated in the study and their families who supported them.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- HF
high frequency
- HR
heart rate
- HRV
heart rate variability
- LF
low frequency
- NIV
non-invasive ventilation
- NREM
non-rapid eye-movement sleep, stage
- OSA
obstructive sleep apnea
REFERENCES
- 1.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics. 2018;141(3):e20173459. 10.1542/peds.2017-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao DP, Kropac E, Do MT, Roberts KC, Jayaraman GC. Childhood overweight and obesity trends in Canada. HPCDP. 2016;36:194–198. 10.24095/hpcdp.36.9.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohler M, Lushington K, Couper R, Martin J, van den Heuvel C, Pamula Y, Kennedy D. Obesity and risk of sleep related upper airway obstruction in Caucasian children. J Clin Sleep Med. 2008;4(2):129–136. 10.5664/jcsm.27129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso-Álvarez ML, Cordero-Guevara JA, Terán-Santos J, et al. Obstructive sleep apnea in obese community-dwelling children: the NANOS study. Sleep. 2014;37(5):943–949. 10.5665/sleep.3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhulst SL, Van Gaal L, De Backer W, Desager K. The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents. Sleep Med Rev. 2008;12(5):339–346. 10.1016/j.smrv.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Van Eyck A, Van Hoorenbeeck K, De Winter BY, Van Gaal L, De Backer W, Verhulst SL. Sleep-disordered breathing and pulmonary function in obese children and adolescents. Sleep Med. 2014;15(8):929–933. 10.1016/j.sleep.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 7.Bhushan B, Maddalozzo J, Sheldon SH, Haymond S, Rychlik K, Lales GC, Billings KR. Metabolic alterations in children with obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2014;78(5):854–859. 10.1016/j.ijporl.2014.02.028 [DOI] [PubMed] [Google Scholar]
- 8.Marcus CL, Brooks LJ, Draper KA, et al.; American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. 10.1542/peds.2012-1671 [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL, Moore RH, Rosen CL, et al.; Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Com G, Carroll JL, Tang X, Melguizo MS, Bower C, Jambhekar S. Characteristics and surgical and clinical outcomes of severely obese children with obstructive sleep apnea. J Clin Sleep Med. 2015;11(4):467–474. 10.5664/jcsm.4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–683. 10.1164/rccm.200912-1930OC [DOI] [PubMed] [Google Scholar]
- 12.Katz SL, MacLean JE, Hoey L, et al. Insulin resistance and hypertension in obese youth with sleep-disordered breathing treated with positive airway pressure: a prospective multicenter study. J Clin Sleep Med. 2017;13(9):1039–1047. 10.5664/jcsm.6718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh N, Moneghetti KJ, Christle JW, Hadley D, Plews D, Froelicher V. Heart rate variability: an old metric with new meaning in the era of using mHealth technologies for health and exercise training guidance. part one: physiology and methods. Arrhythm Electrophysiol Rev. 2018;7(3):193–198. 10.15420/aer.2018.27.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draghici AE, Taylor JA. The physiological basis and measurement of heart rate variability in humans. J Physiol Anthropol. 2016;35(1):22. 10.1186/s40101-016-0113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh N, Moneghetti KJ, Christle JW, Hadley D, Froelicher V, Plews D. Heart rate variability: an old metric with new meaning in the era of using mhealth technologies for health and exercise training guidance. part two: prognosis and training. Arrhythm Electrophysiol Rev. 2018;7(4):247–255. 10.15420/aer.2018.30.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Heart rate variation in normal infants and victims of the sudden infant death syndrome. Early Hum Dev. 1989;19(3):167–181. 10.1016/0378-3782(89)90077-7 [DOI] [PubMed] [Google Scholar]
- 18.Horne RSC, Fyfe KL, Odoi A, Athukoralage A, Yiallourou SR, Wong FY. Dummy/pacifier use in preterm infants increases blood pressure and improves heart rate control. Pediatr Res. 2016;79(2):325–332. 10.1038/pr.2015.212 [DOI] [PubMed] [Google Scholar]
- 19.Ariagno RL, Mirmiran M, Adams MM, Saporito AG, Dubin AM, Baldwin RB. Effect of position on sleep, heart rate variability, and QT interval in preterm infants at 1 and 3 months’ corrected age. Pediatrics. 2003;111(3):622–625. 10.1542/peds.111.3.622 [DOI] [PubMed] [Google Scholar]
- 20.Stéphan-Blanchard E, Chardon K, Léké A, Delanaud S, Bach V, Telliez F. Heart rate variability in sleeping preterm neonates exposed to cool and warm thermal conditions. PLoS One. 2013;8(7):e68211. 10.1371/journal.pone.0068211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nisbet LC, Yiallourou SR, Walter LM, Horne RS. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Med Rev. 2014;18(2):179–189. 10.1016/j.smrv.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 22.Walter LM, Tamanyan K, Nisbet LC, Davey MJ, Nixon GM, Horne RSC. Obesity and anthropometric determinants of autonomic control in children with sleep-disordered breathing-which measurements matter? Int J Obes Lond. 2018;42(6):1195–1201. 10.1038/s41366-018-0130-1 [DOI] [PubMed] [Google Scholar]
- 23.Walter LM, Biggs SN, Nisbet LC, et al. Improved long-term autonomic function following resolution of sleep-disordered breathing in preschool-aged children. Sleep Breath. 2016;20(1):309–319. 10.1007/s11325-015-1268-x [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Immanuel S, Kennedy D, Martin J, Pamula Y, Baumert M. Effect of adenotonsillectomy for childhood obstructive sleep apnea on nocturnal heart rate patterns. Sleep. 2018;41(11):01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz SL, MacLean JE, Barrowman N, et al. Long-term impact of sleep-disordered breathing on quality of life in children with obesity. J Clin Sleep Med. 2018;14(3):451–458. 10.5664/jcsm.6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC National Centre for Health Statistics : National Health and Nutrition Examination Survey. CDC growth charts: body Mass index for age. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm. Accessed February 26, 2019.
- 27.Kushida CADA, Chediak A, Berry RB, et al.; Positive Airway Pressure Titration Task Force ; American Academy of Sleep Medicine . Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. 10.5664/jcsm.27133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 29.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354–381. 10.1093/oxfordjournals.eurheartj.a014868 [DOI] [PubMed] [Google Scholar]
- 30.Khoo MC, Blasi A. Sleep-related changes in autonomic control in obstructive sleep apnea: a model-based perspective. Respir Physiol Neurobiol. 2013;188(3):267–276. 10.1016/j.resp.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belozeroff V, Berry RB, Sassoon CSH, Khoo MC. Effects of CPAP therapy on cardiovascular variability in obstructive sleep apnea: a closed-loop analysis. Am J Physiol Heart Circ Physiol. 2002;282(1):H110–H121. 10.1152/ajpheart.2002.282.1.H110 [DOI] [PubMed] [Google Scholar]
- 32.Quadri F, Boni E, Pini L, Bottone D, Venturoli N, Corda L, Tantucci C. Exercise tolerance in obstructive sleep apnea-hypopnea (OSAH), before and after CPAP treatment: Effects of autonomic dysfunction improvement. Respir Physiol Neurobiol. 2017;236:51–56. 10.1016/j.resp.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 33.Palma JA, Iriarte J, Fernandez S, Alegre M, Valencia M, Artieda J, Urrestarazu E. Long-term continuous positive airway pressure therapy improves cardiac autonomic tone during sleep in patients with obstructive sleep apnea. Clin Auton Res. 2015;25(4):225–232. 10.1007/s10286-015-0297-7 [DOI] [PubMed] [Google Scholar]
- 34.Guo W, Lv T, She F, et al. The impact of continuous positive airway pressure on heart rate variability in obstructive sleep apnea patients during sleep: A meta-analysis. Heart Lung. 2018;47(5):516–524. 10.1016/j.hrtlng.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 35.de Vries GE, Wijkstra PJ, Houwerzijl EJ, Kerstjens HAM, Hoekema A. Cardiovascular effects of oral appliance therapy in obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2018;40:55–68. 10.1016/j.smrv.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 36.Glos M, Penzel T, Schoebel C, et al. Comparison of effects of OSA treatment by MAD and by CPAP on cardiac autonomic function during daytime. Sleep Breath. 2016;20(2):635–646. 10.1007/s11325-015-1265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dal-Fabbro C, Garbuio S, D’Almeida V, Cintra FD, Tufik S, Bittencourt L. Mandibular advancement device and CPAP upon cardiovascular parameters in OSA. Sleep Breath. 2014;18(4):749–759. 10.1007/s11325-014-0937-5 [DOI] [PubMed] [Google Scholar]
- 38.Choi JH, Yi JS, Lee SH, et al. Effect of upper airway surgery on heart rate variability in patients with obstructive sleep apnoea syndrome. J Sleep Res. 2012;21(3):316–321. 10.1111/j.1365-2869.2011.00978.x [DOI] [PubMed] [Google Scholar]
- 39.Süslü AE, Pamuk G, Pamuk AE, Özer S, Jafarov S, Önerci TM. Effects of expansion sphincter pharyngoplasty on the apnea-hypopnea index and heart rate variability. J Oral Maxillofac Surg. 2017;75(12):2650–2657. 10.1016/j.joms.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 40.Aeschbacher S, Bossard M, Schoen T, et al. heart rate variability and sleep-related breathing disorders in the general population. Am J Cardiol. 2016;118(6):912–917. 10.1016/j.amjcard.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 41.Alvarez-Estevez D, Moret-Bonillo V. Spectral heart rate variability analysis using the heart timing signal for the screening of the sleep apnea-hypopnea syndrome. Comput Bio. Med. 2016;71:14–23. 10.1016/j.compbiomed.2016.01.023 [DOI] [PubMed] [Google Scholar]
- 42.Phillipson EA, Sullivan CE. Arousal: the forgotten response to respiratory stimuli. Am Rev Respir Dis. 1978;118(5):807–809. [DOI] [PubMed] [Google Scholar]
- 43.Deacon N, Malhotra A. Potential protective mechanism of arousal in obstructive sleep apnea. J Thorac Dis. 20168, S7, Suppl 7:S545–S546. 10.21037/jtd.2016.07.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zinchuk AV, Jeon S, Koo BB, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73(5):472–480. 10.1136/thoraxjnl-2017-210431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang J, Zhang X, He X, Ling L, Zeng C, Luo Y. The independent and combined effects of respiratory events and cortical arousals on the autonomic nervous system across sleep stages. Sleep Breath. 2018;22(4):1161–1168. 10.1007/s11325-018-1669-8 [DOI] [PubMed] [Google Scholar]
- 46.Chouchou F, Pichot V, Barthélémy JC, Bastuji H, Roche F. Cardiac sympathetic modulation in response to apneas/hypopneas through heart rate variability analysis. PLoS One. 2014;9(1):e86434. 10.1371/journal.pone.0086434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington C, Kirjavainen T, Teng A, Sullivan CE. Altered autonomic function and reduced arousability in apparent life-threatening event infants with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(8):1048–1054. 10.1164/ajrccm.165.8.2102059 [DOI] [PubMed] [Google Scholar]
- 48.Harrington C, Kirjavainen T, Teng A, Sullivan CE. nCPAP improves abnormal autonomic function in at-risk-for-SIDS infants with OSA. J Appl Physiol. 2003;95(4):1591–1597. 10.1152/japplphysiol.00354.2002 [DOI] [PubMed] [Google Scholar]
- 49.Gozal D, Hakim F, Kheirandish-Gozal L. Chemoreceptors, baroreceptors, and autonomic deregulation in children with obstructive sleep apnea. Respir Physiol Neurobiol. 2013;185(1):177–185. 10.1016/j.resp.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva CC, Bertollo M, Reichert FF, Boullosa DA, Nakamura FY. Reliability of heart rate variability in children: influence of sex and body position during data collection. Pediatr Exerc Sci. 2017;29(2):228–236. 10.1123/pes.2016-0085 [DOI] [PubMed] [Google Scholar]