Abstract
Study Objectives:
To assess, in a large cohort of patients with obstructive sleep apnea, the factors that are independently associated with positional obstructive sleep apnea (POSA) and exclusive POSA (e-POSA) and determine their prevalence. The secondary objective was to evaluate the outcome of positive airway pressure (PAP) therapy for patients with POSA and e-POSA.
Methods:
This retrospective study included 6,437 patients with typical mild‐to‐severe OSA from the Pays de la Loire sleep cohort. Patients with POSA and e-POSA were compared to those with non-POSA for clinical and polysomnographic characteristics. In a subgroup of patients (n = 3,000) included in a PAP follow-up analysis, we determined whether POSA and e-POSA phenotypes were associated with treatment outcomes at 6 months.
Results:
POSA and e-POSA had a prevalence of 53.5% and 20.1%, respectively, and were independently associated with time in supine position, male sex, younger age, lower apnea-hypopnea index and lower body mass index. After adjustment for confounding factors, patients with POSA and e-POSA had a significantly lower likelihood of treatment adherence (PAP daily use ≥ 4 h) at 6 months and were at higher risk of PAP treatment withdrawal compared to those with non-POSA.
Conclusions:
The prevalence and independent predictors of POSA and e-POSA were determined in this large clinical population. Patients with POSA and e-POSA have lower PAP therapy adherence, and this choice of treatment may not be optimal. Thus, there is a need to offer these patients an alternative therapy.
Citation:
Sabil A, Blanchard M, Trzepizur W, et al. Positional obstructive sleep apnea within a large multicenter French cohort: prevalence, characteristics, and treatment outcomes. J Clin Sleep Med. 2020;16(12):2037–2046.
Keywords: positional sleep apnea, continuous positive airway pressure, prevalence, treatment adherence
BRIEF SUMMARY
Current Knowledge/Study Rationale: Clinicians need to be aware of the high prevalence of positional obstructive sleep apnea and the challenges of its treatment. A consensus is needed to determine the best definition of positional obstructive sleep apnea and to further identify its clinical characteristics.
Study Impact: Patients with positional obstructive sleep apnea patients have lower positive airway pressure therapy adherence, and this choice of treatment may not be optimal to them, and thus, the need of an alternative treatment such as positional therapy. However, 1-night recording may not be reliable enough to phenotype patients as having positional obstructive sleep apnea, and positional therapy has limited and conflicting data on long-term effectiveness and adherence.
INTRODUCTION
A recently published study1 suggests that nearly 1 billion adults aged 30–69 years worldwide could have obstructive sleep apnea (OSA), and the number of people with moderate to severe OSA, for which treatment is generally recommended, is estimated to be almost 425 million worldwide. There are variable clinical phenotypes of OSA, the most common of which is that of supine position-related OSA, where OSA is more severe in the supine compared to lateral sleeping position.2 The principal mechanisms causing frequent and severe surge of OSA in the supine position are likely to be a combination of inadequate upper airway geometry, with an increase in collapsibility, reduced lung volume, and a failure of the airway dilator muscles to properly compensate.3
The reported prevalence of positional OSA (POSA) ranges from 20 to 75% of patients with OSA, and POSA is predominantly present in those with mild and moderate OSA.2,4,5 This variability is a consequence of several factors, including the size of the studied cohorts, the monocentric design, the ethnicity of the patients, and the use of different POSA definitions.4–7 Several definitions of POSA have been used in the literature, but it is most commonly defined according to Cartwright’s definition as OSA with a ratio of respiratory events in the supine to nonsupine position greater than 2:1.8 When OSA occurs exclusively in the supine position, it is referred to as exclusive POSA (e-POSA) and is defined by a ratio of respiratory events in the supine to nonsupine position greater than 2:1 and a nonsupine apnea-hypopnea index (AHI) < 5 events/hour.5
Patients with POSA are more likely to be younger men and less obese, with fewer symptoms, less comorbidity, and a tendency to have a smaller neck circumference.9,10 Furthermore, in patients with POSA, desaturations, heart rate cyclic variations, loud snoring, and respiratory events appear almost exclusively in the supine position.11
While positional therapy (PT) devices are an option for patients with POSA, positive airway pressure (PAP) remains the most common treatment of both OSA and POSA. However, its effectiveness is limited by inconsistent adherence to therapy, and poor PAP adherence is widely recognized as a critical problem in the treatment of OSA.12–14 Moreover, PAP nonadherence of mild or asymptomatic patients could be even worse.15 Thus, many patients with OSA, including those with POSA, who should be treated are not, leading to severe health consequences.16 Positional therapy devices have been compared to PAP therapy and some have been shown to be as effective as PAP therapy in terms of AHI reduction and treatment success.17,18 However, long-term PT compliance could be low for certain devices and close follow-up of patient adherence with PT is necessary.19–22 In addition, some PT devices are found to be too uncomfortable by the patients, leading to treatment discontinuation.22,23 Finally, PAP adherence and PAP therapy success in patients with POSA and e-POSA have not been extensively studied.
The objective of this study was, therefore, to analyze in a large cohort of OSA patients both clinical and polygraphic characteristics to assess the factors that are independently associated with POSA and e-POSA and determine their prevalence in a clinical population. The secondary objective of this study was to evaluate PAP treatment adherence of patients with POSA and e-POSA and determine PAP therapy success factors for these patients.
METHODS
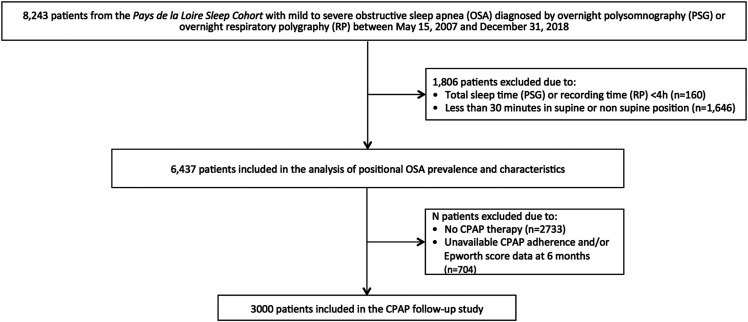
The study was conducted on the Pays de la Loire sleep cohort.24 Since May 15, 2007, consecutive patients ≥ 18 years investigated by overnight polysomnography (PSG) or overnight home sleep apnea test (HSAT) for suspected OSA in 7 centers from the Pays de la Loire were eligible for inclusion in the Pays de la Loire sleep cohort. Patients with learning difficulties, who were unable to fill in the questionnaires or read and/or speak French, and patients with neuromuscular diseases or chronic respiratory failure were excluded from the Pays de la Loire sleep cohort. All patients from the Pays de la Loire sleep cohort diagnosed with mild-to-severe OSA between May 15, 2007 and December 31, 2018 were eligible for the present study. Patients with total sleep time (PSG) or recording time (HSAT) < 4 h and/or less than 30 minutes in supine or nonsupine position were excluded from the present study.
Approval was obtained from the University of Angers ethics committee and the “Comité Consultatif sur le Traitement de l'Information en matière de Recherche dans le domaine de la Santé” (CCTIRS; 07.207bis). The database was anonymous and complied with the restrictive requirements of the “Commission Nationale Informatique et Liberté” (CNIL), the French information technology, and personal data protection authority. All patients have given their written informed consent.
Baseline evaluation and questionnaires
Each patient enrolled in the Pays de la Loire sleep cohort completed surveys, including anthropometric data, medical history, and socioprofessional status.24 Obesity was defined by a body mass index (BMI) of at least 30 kg/m2. Systemic hypertension and diabetes mellitus were defined as a physician diagnosis on data reported during a baseline standardized health interview combined with appropriate medication treatment. Comorbid cardiovascular disease was defined as history of physician-diagnosed angina, myocardial infarction, coronary revascularization procedure, heart failure, stroke, or atrial fibrillation. Excessive daytime sleepiness and depressive symptoms were assessed by the Epworth Sleepiness Score (ESS) and the QD2A depression score respectively.25 Health-related quality of life (HR-QOL) was assessed using the Outcomes Study 36-item short-form (SF-36).26 Socioprofessional status was described by marital status (married or living as a couple/living alone [never married, divorced, separated, widowed]) and the occupational status (employed full time or part time, unemployed, or retired).
Sleep studies and PAP treatment
According to French guidelines, patients with a high clinical probability of OSA were investigated by HSAT (CID102LM; CIDELEC, Sainte-Gemmes-sur-Loire, France).27 Patients with a low likelihood of OSA and/or coexisting sleep disorders underwent a PSG (CID102L8DM; CIDELEC, Sainte-Gemmes-sur-Loire, France). Recorded data included oronasal airflow (thermistor and nasal pressure cannula), tracheal sounds and suprasternal pressure, chest and abdominal wall motion (respiratory inductance plethysmograph belts), arterial oxygen saturation (pulse oximetry), body position, and electrophysiological signals for sleep evaluation (PSG).
During the installation of sensors, the CIDELEC interface software takes the user through a procedure that requires confirmation of the orientation of the body position sensor. A pop-up window showing different options of the sensor’s positioning asks the user to check the orientation of the sensor. This procedure helps minimize the inaccuracy of body position, particularly for an HSAT where video recording is unavailable. The accuracy of sleep position sensors could also depend on the technology used. Levendowski et al28 showed in a cohort of 30 patients that supine position was underdetected by > 5% in 3% of cases. Bignold et al29 tested another device and showed that it had a high posture classification agreement with Kappa = 0.95 and supine time measurements with negligible systematic bias, with limits of agreement within 5% compared to simultaneous in-laboratory video recordings. However, in a third study evaluating an older system, the MESAM, Fietze et al30 reported that 17% (6/35) of studies were deleted because of sleep position errors. In addition to the type of technology used, the mapping between sensor orientation and body position depends on where the sensor is worn and its default orientation with respect to the anatomical planes.31 Placing the CIDELEC sensor just above an anatomical landmark (the sternal notch) increases the likelihood of proper placement and accurate measurement of body position.
Respiratory events were scored manually using recommended criteria.32 Apnea was defined as an at least 90% decrease in the oronasal thermal sensor signal, and hypopnea was defined as an at least 30% decrease in the nasal pressure signal combined with either ≥ 3% arterial oxygen desaturation or an arousal (PSG), both lasting at least 10 s. As previously described,5 POSA was defined as an AHI ≥ 5 events/h and supine/nonsupine AHI ratio ≥ 2; e-POSA was defined as POSA criteria and nonsupine AHI < 5 events/h.
According to the criteria defined by the French national health insurance, PAP therapy was prescribed in patients with severe OSA and in those with moderate OSA and cardiovascular comorbidities or severe daytime sleepiness. As previously described,24 a single home respiratory care company (ALISEO, Beaucouzé, France) was involved in PAP device delivery and follow-up support program. Sleep specialists reviewed patients in consultation at 5 months, 12 months, and then at least annually. Objective daily PAP use was monitored at each follow-up visit and recorded in the database. The average daily PAP use during the follow-up period was calculated to determine treatment adherence.
Statistical analysis
The first objective of our study was to determine the prevalence and characteristics of POSA and e-POSA within the entire study population. Continuous and qualitative variables were respectively described as mean (standard deviation) and percentages. Patients with POSA and e-POSA were compared to those with non-POSA (NPOSA) for clinical and polygraphic characteristics, including events duration,33 the fraction of events that were hypopneas,34 and the hypoxic burden.35 Continuous and categorical variables were compared using Student t test and chi-square test, respectively. Then, variables for which there was a significant difference were entered in multivariate analyses. The second objective of our study was to determine whether POSA and e-POSA phenotypes were associated with specific treatment outcomes in patients in whom PAP had been prescribed for at least 6 months. Logistic regression analyses were conducted to calculate unadjusted and adjusted odds ratios (OR) for treatment success defined as daily PAP use ≥ 4 h and a decrease in ESS ≥ 2 points from baseline to 6-month follow-up,36,37 according to POSA or e-POSA phenotype.
All statistical analyses were performed with SAS 9.4 software (SAS Institute, Cary, NC). Associations were considered statistically significant for a P value < .05.
RESULTS
Out of 8,243 patients from the Pays de la Loire sleep cohort diagnosed with mild-to-severe OSA between May 15, 2007 and December 31, 2018, 1,806 were excluded due to total sleep (PSG) or recording (HSAT) time < 4 h (n = 160) or < 30 minutes in supine or nonsupine position (n = 1,646). Therefore, the final study sample size comprised 6,437 patients at the time of the diagnostic sleep study (Figure 1).
Figure 1. Flow diagram of the patients during the study.
CPAP = continuous positive airway pressure.
As shown in Table 1, the study population consisted of patients with typical mild‐to‐severe OSA (mean AHI = 29.4 [19.8] events/h and mean ESS score = 9.9 [5.1]), of average age (61.1 ± 13.3 years), predominantly male (66.2%), overweight or obese (mean BMI = 30.7 [6.5] kg/m2), and frequently presenting with systemic hypertension (35.5%), cardiovascular diseases (16.8%), and diabetes (14.8%).
Table 1.
Baseline clinical characteristics of the study population according to positional category.
| All | NPOSA | POSA | P Value vs NPOSA | e-POSA | P Value vs NPOSA | |
|---|---|---|---|---|---|---|
| n | 6,437 | 2,996 | 3,441 | 1,293 | ||
| Time in supine position, % | 46.3 (22.6) | 45.3 (22.9) | 47.2 (22.3) | .0009 | 53.9 (21.9) | < .0001 |
| Age, years | 61.1 (13.3) | 62.8 (13.7) | 59.6 (12.8) | < .0001 | 57.4 (12.9) | < .0001 |
| Men, % | 66.2 | 63.7 | 68.4 | < .0001 | 64.2 | .7671 |
| BMI, kg/m2 | 30.7 (6.5) | 32.8 (6.9) | 28.9 (5.5) | < .0001 | 27.4 (5.5) | < .0001 |
| Obesity, % | 48.0 | 62.4 | 35.4 | < .0001 | 26.2 | < .0001 |
| ESS | 9.9 (5.1) | 9.9 (5.0) | 10.0 (5.1) | .4164 | 9.9 (5.1) | .6738 |
| ESS ≥ 11, % | 44.7 | 44.4 | 45.0 | .6606 | 44.2 | .9176 |
| QD2A score | 3.6 (3.4) | 3.7 (3.4) | 3.4 (3.4) | .0022 | 3.5 (3.4) | .0839 |
| QD2A score ≥ 7, % | 19.9 | 20.8 | 19.1 | .0967 | 19.5 | .3470 |
| SF36 MCS | 46.9 (5.6) | 46.8 (5.7) | 46.9 (5.5) | .2626 | 46.6 (5.5) | .3735 |
| SF36 PCS | 50.7 (2.2) | 50.6 (2.2) | 50.8 (2.1) | .0089 | 50.9 (2.2) | < .0001 |
| AHI | 29.4 (19.8) | 36.8 (22.7) | 23.0 (14.0) | < .0001 | 13.5 (8.6) | < .0001 |
| Supine AHI | 39.6 (23.9) | 39.3 (25.3) | 39.9 (22.7) | .2746 | 24.3 (15.0) | < .0001 |
| Nonsupine AHI | 21.5 (20.9) | 34.8 (22.8) | 9.9 (8.8) | < .0001 | 2.5 (1.3) | < .0001 |
| Hypertension, % | 35.5 | 41.6 | 30.1 | < .0001 | 25.3 | < .0001 |
| Diabetes, % | 14.8 | 19.2 | 10.9 | < .0001 | 8.0 | < .0001 |
| CV disease, % | 16.8 | 19.8 | 14.1 | < .0001 | 12.3 | < .0001 |
| PSG, % | 44.6 | 35.7 | 52.3 | < .0001 | 57.0 | < .0001 |
Data are expressed as mean (standard deviation) or percentages. P values are based on t tests for continuous variables and chi-square test for categorical variables. AHI = apnea-hypopnea index, BMI = body mass index, CV = cardiovascular, e-POSA = exclusive positional obstructive sleep apnea, ESS = Epworth Sleepiness Score, MCS = mental composite score, NPOSA = nonpositional obstructive sleep apnea, PCS = physical composite score, POSA = positional obstructive sleep apnea, PSG = polysomnography.
Prevalence and characteristics of POSA and e-POSA
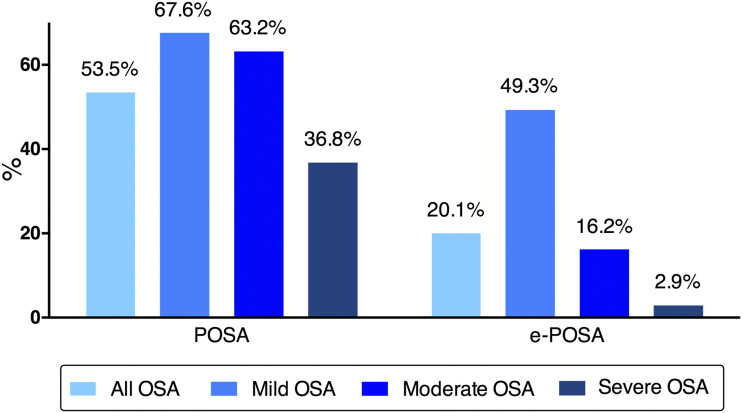
Among the included 6,437 patients, 2,996 (46.5%) had NPOSA, POSA was present in 3,441 patients (53.5%) of all, and e-POSA in 1,293 (20.1%). The prevalence of POSA and e-POSA according to OSA severity is shown in Figure 2. The prevalence of POSA decreased from 67.6% in mild OSA to 36.8% in severe OSA. The prevalence of e-POSA decreased from 49.3% in mild OSA to 2.9% in severe OSA. Baseline characteristics of the study population according to positional category are presented in Table 1. Compared to NPOSA, patients with POSA and e-POSA spent more time on their back, were younger; had lower BMI and AHI; were less likely to have hypertension, diabetes, and cardiovascular disease, and more likely to have undergone PSG recording than HSAT. Patients with POSA and e-POSA had a slightly higher SF-36 physical composite score than those with NPOSA. A higher proportion of men and lower depression scores were also observed in patients with POSA.
Figure 2. Prevalence of nonpositional obstructive sleep apnea (NPOSA), positional obstructive sleep apnea (POSA) and exclusive positional obstructive sleep apnea (e-POSA) according to obstructive sleep apnea (OSA) severity.
On multivariate analysis, POSA and e-POSA were positively associated with time in supine position and male sex and were negatively associated with age, BMI, and AHI. Being investigated by PSG rather than HSAT was also significantly associated with POSA (Table 2).
Table 2.
Multivariate analysis for positional obstructive sleep apnea and exclusive positional obstructive sleep apnea.
| POSA | e-POSA | |||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Time in supine position (1 SD) | 1.20 (1.13–1.28) | < .0001 | 1.91 (1.72–2.13) | < .0001 |
| Age (1 SD) | 0.85 (0.79–0.92) | < .0001 | 0.89 (0.79–1.00) | .0434 |
| Sex (male) | 1.45 (1.25–1.68) | < .0001 | 1.66 (1.33–2.07) | < .0001 |
| Body mass index (1 SD) | 0.61 (0.57–0.67) | < .0001 | 0.57 (0.51–0.65) | < .0001 |
| QD2A depression score (1 SD) | 0.96 (0.89–1.03) | .2406 | 0.94 (0.83–1.05) | .2768 |
| SF36 PCS (1 SD) | 1.01 (0.93–1.08) | .8708 | 1.02 (0.91–1.15) | .6939 |
| Apnea-hypopnea index (1 SD) | 0.47 (0.44–0.51) | < .0001 | 0.12 (0.09–0.14) | < .0001 |
| Hypertension | 0.97 (0.83–1.13) | .6802 | 1.06 (0.83–1.35) | .6334 |
| Diabetes | 0.90 (0.74–1.10) | .3123 | 0.80 (0.92–1.19) | .2291 |
| Cardiovascular disease | 0.84 (0.70–1.01) | .0682 | 0.80 (0.59–1.09) | .1587 |
| PSG vs HSAT | 1.28 (1.11–1.47) | .0006 | 1.24 (1.00–1.53) | .0537 |
CI = confidence interval, e-POSA = exclusive positional obstructive sleep apnea, HSAT = home sleep apnea testing, NPOSA = nonpositional obstructive sleep apnea, OR = odds ratio, PCS = physical composite score, POSA = positional obstructive sleep apnea, PSG = polysomnography, SD = standard deviation.
As shown in Table 3, POSA and e-POSA were characterized by significantly shorter apneas but a longer duration of all respiratory events, which was only significant for e-POSA. POSA and e-POSA were also significantly associated with a higher fraction of events that were hypopneas and lower oxygen desaturations as assessed by 3 and 4% oxygen desaturation index, percent nighttime with oxygen saturation < 90%, nadir oxygen saturation, and the hypoxic burden.
Table 3.
Baseline polygraphic characteristics of the study population according to positional category.
| All | NPOSA | POSA | P Value vs NPOSA | e-POSA | P Value vs NPOSA | |
|---|---|---|---|---|---|---|
| Apnea duration, s | 16.6 (6.3) | 17.0 (6.5) | 16.3 (6.0) | < .0001 | 15.4 (6.6) | < .0001 |
| Events duration, s | 19.2 (4.1) | 19.1 (4.3) | 19.3 (4.0) | .1166 | 19.4 (4.2) | .0050 |
| Fhyponea, % | 73.2 (23.9) | 70.2 (26.2) | 75.8 (21.3) | < .0001 | 79.9 (18.8) | < .0001 |
| Desaturation indices | ||||||
| 3% ODI, events/h | 13.9 (17.6) | 20.0 (21.9) | 8.5 (10.1) | < .0001 | 3.9 (5.3) | < .0001 |
| 4% ODI, events/h | 10.4 (15.8) | 15.7 (20.1) | 5.9 (8.4) | < .0001 | 2.4 (4.0) | < .0001 |
| T90, % | 9.2 (16.5) | 13.7 (19.9) | 5.2 (11.5) | < .0001 | 2.8 (9.0) | < .0001 |
| Nadir Sao2, % | 80.7 (8.7) | 78.1 (9.8) | 83.1 (6.7) | < .0001 | 85.4 (5.3) | < .0001 |
| Hypoxic burden | 67.1 (90.5) | 96.2 (117.5) | 41.7 (43.8) | < .0001 | 20.4 (21.4) | < .0001 |
Data are expressed as mean (standard deviation) or percentages. P values are based on t tests. e-POSA = exclusive positional obstructive sleep apnea, Fhypopnea = fraction of events that were hypopneas, NPOSA = nonpositional obstructive sleep apnea, ODI = oxygen desaturation index, POSA = positional obstructive sleep apnea, T90 = percentage of sleep (recording) time with oxygen saturation (Sao2) < 90%.
Treatment outcomes in POSA and e-POSA
Out of the 6,437 patients included in the analysis of positional OSA prevalence and characteristics, 2,733 were not prescribed PAP treatment and 704 had no adherence and/or ESS score data available at 6 months. Thus, 3,000 patients were included in the PAP follow-up analysis, of whom 1,398 (46.6%) had POSA and 279 (9.3%) had e-POSA. Detailed baseline clinical characteristics of PAP treated patients according to positional category are shown in Table 4. Compared to NPOSA, both patients with POSA and e-POSA were significantly less likely to be PAP adherent. Conversely, there was no statistically significant difference for both POSA and e-POSA in term of PAP treatment response. The probability of PAP treatment success was lower in patients with POSA and e-POSA compared to those with NPOSA, but the difference reached statistical significance only for POSA (P = .0116).
Table 4.
Baseline clinical characteristics of patients treated with positive airway pressure according to positional category.
| All | NPOSA | POSA | P Value vs NPOSA | e-POSA | P Value vs NPOSA | |
|---|---|---|---|---|---|---|
| n | 3,000 | 1,602 | 1,398 | 279 | ||
| Age, years | 63.1 (12.5) | 64.4 (12,7) | 61.5 (12.2) | < .0001 | 59.4 (12.3) | < .0001 |
| Men, % | 69.2 | 66.7 | 72.2 | .0011 | 68.5 | .5571 |
| BMI, kg/m2 | 31.5 (6.2) | 33.4 (6.4) | 29.5 (5.2) | < .0001 | 28.1 (5.2) | < .0001 |
| Obesity, % | 54.4 | 67.8 | 39.1 | < .0001 | 27.2 | < .0001 |
| ESS | 10.4 (5.1) | 10.2 (4.9) | 10.8 (5.1) | .0011 | 11.2 (5.1) | .0013 |
| ESS ≥ 11, % | 49.5 | 46.9 | 52.4 | .0030 | 56.9 | .0023 |
| QD2A score | 3.6 (3.4) | 3.7 (3.4) | 3.6 (3.4) | .3271 | 3.7 (3.4) | .8507 |
| QD2A score ≥ 7, % | 19.8 | 20.1 | 19.5 | .7018 | 21.0 | .7358 |
| AHI | 39.7 (19.9) | 46.8 (21.9) | 31.7 (13.2) | < .0001 | 22.8 (11.1) | < .0001 |
| Hypertension, % | 39.2 | 44.5 | 32.9 | < .0001 | 26.2 | < .0001 |
| Diabetes, % | 17.1 | 20.9 | 12.7 | < .0001 | 8.8 | < .0001 |
| CV disease, % | 17.4 | 19.7 | 14.7 | .0003 | 13.2 | .0105 |
| PAP treatment adherence*, % | 64.6 | 69.0 | 59.6 | < .0001 | 53.8 | < .0001 |
| PAP treatment response†, % | 64.9 | 63.7 | 66.3 | .1714 | 67.7 | .2493 |
| PAP treatment success‡, % | 42.6 | 44.7 | 40.1 | .0116 | 39.4 | .1011 |
Data are expressed as mean (standard deviation) or percentages. P values are based on t tests. *Treatment adherence was defined by a mean daily positive airway pressure (PAP) use ≥ 4 h; †PAP treatment response was defined by a decrease in Epworth score of at least 2 points from baseline to 6-month follow-up; ‡PAP treatment success was defined by a mean daily PAP use ≥ 4 h and a decrease in Epworth score of at least 2 points from baseline to 6-month follow-up. AHI = apnea-hypopnea index, BMI = body mass index, CV = cardiovascular, e-POSA = exclusive positional obstructive sleep apnea, ESS = Epworth sleepiness score, MCS = mental composite score, NPOSA = nonpositional obstructive sleep apnea, PCS = physical composite score, POSA = positional obstructive sleep apnea.
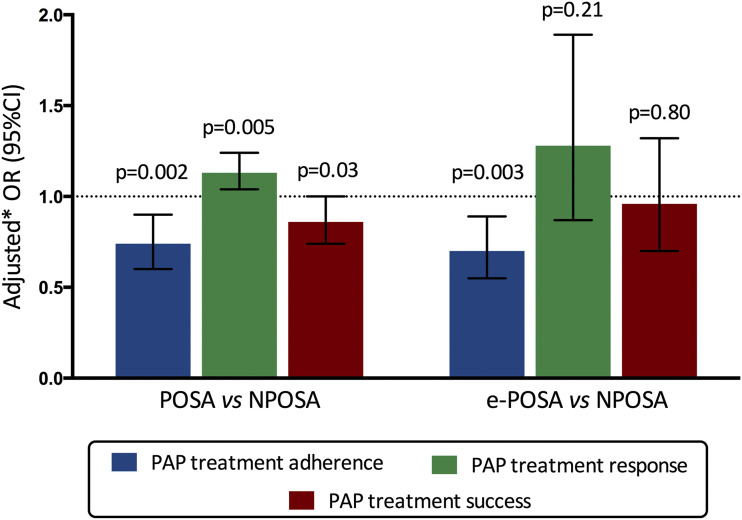
Figure 3 illustrates a multivariable analysis for PAP treatment outcomes according to positional category. After adjustment for age, sex, BMI, ESS score, AHI, cardiovascular diseases, marital and occupational status, type of sleep study, and study site, both POSA and e-POSA were associated with a significantly lower likelihood of PAP treatment adherence at 6 months compared to NPOSA (adjusted OR 0.74 [0.60–0.90] for POSA and 0.70 [0.55–0.89] for e-POSA). Despite a higher likelihood of PAP treatment response (adjusted OR 1.13 [1.04–1.24]), POSA was negatively associated with treatment success (adjusted OR 0.86 [0.74–1.00]). Conversely, e-POSA was not significantly associated with PAP treatment response and success after adjustment for confounders.
Figure 3. Multivariate analysis for positive airway pressure (PAP) treatment outcomes according to positional category.
Treatment adherence was defined by a mean daily PAP use ≥ 4 h; PAP treatment response was defined by a decrease in Epworth score of at least 2 points from baseline to 6-month follow-up; PAP treatment success was defined by a mean daily PAP use ≥ 4 h and a decrease in Epworth score of at least 2 points from baseline to 6-month follow-up. *Adjusted for age, sex, body mass index, Epworth score, apnea-hypopnea index, cardiovascular diseases, marital and occupational status, type of sleep study and study site. CI = confidence interval, e-POSA = exclusive positional sleep apnea, NPOSA = nonpositional sleep apnea, OR = odds ratio, POSA = positional sleep apnea.
Finally, PAP treatment was stopped within the first 6 months in 10.3% of patients with NPOSA vs 15.9% and 20.7% in those with POSA and e-POSA, respectively (P < .0001 compared to NPOSA). After adjustment for confounders, both patients with POSA (OR 1.27 [1.09–1.48], P = .003) and e-POSA (OR 1.45 [1.14–1.85], P < .0001) were at higher risk of PAP treatment withdrawal.
DISCUSSION
To the best of our knowledge, this is the largest cohort where both clinical and polygraphic characteristics were analyzed to provide a complete report on the prevalence as well as on PAP treatment adherence of POSA and e-POSA in a clinic population. In a population with typical mild-to-severe OSA, the prevalence of POSA and e-POSA was, respectively, 53.5% and 20.1% and decreased with OSA severity. The main independent predictors of POSA and e-POSA were male sex, younger age, BMI, and AHI. After adjustment for the main factors of PAP adherence, POSA and e-POSA were both associated with a lower likelihood of being adherent to the therapy at 6 months.
Prevalence and characteristics of POSA and e-POSA
In patients with mild-to-severe OSA, the prevalence of POSA and e-POSA ranged between 20–75% and 5.4–36%, respectively.1,4,5,8 This variability is not only a consequence of several factors, including the number and ethnicity of the patients,5–7 but also due to the use of 3 different classification systems (Cartwright, Bignold, and the new Amsterdam Positional OSA Classification [APOC]) for positional OSA.38 Studying the prevalence of POSA in a South Korean cohort of patients with OSA (n = 1,170), Mo et al7 demonstrated that, among Asians, the prevalence of POSA is almost 75% of the OSA patients. A short cranial base and retrognathia in the far east Asian population may indicate a prolate airway that easily collapses in the supine position compared with the lateral position and may be the reason of higher prevalence of POSA in this population.6,39 In a population-based European Study, Heinzer et al5 found that the prevalence of POSA and e-POSA in a middle to older age Swiss population-based sample (n = 1,719), was, respectively, 53% and 26% of all patients. In a recent study evaluating the incidence of POSA in older patients (n = 434) using different definitions of POSA, Iannella et al38 showed that the prevalence of POSA in older patients differed according to the classification system used. It was 49.3% using Cartwright’s classification system, 20.5% with the Bignold classification, and 22.6%, 38.9%, and 5.4% of APOC 1, APOC 2, and APOC3 subclasses were, respectively, identified for the APOC classification system. Compared to previous reports, our multicenter study included the largest number of typical OSA patients, with a wide range of sleep-disordered breathing severity, and used the definitions of POSA previously defined by Cartwright9 and e-POSA by Heinzer et al.5
Our findings that lower AHI, lower BMI, male sex, and younger age were associated with the presence of POSA and e-POSA are consistent with previous reports.3,5,11,12 In addition, the results of a multivariate analysis in our sample of patients, adjusted for all significant factors, showed that only sex, age, BMI, and AHI were significantly associated with POSA and e-POSA, and comorbidities were not associated factors.
In terms of OSA severity, our results showed that patients with POSA were predominantly mild to moderate and e-POSA were mainly mild (Figure 2). Older patients were less likely to have POSA and more likely to have severe OSA with associated comorbidities. This may support findings from a recent study suggesting that supine position OSA could represent an early stage of OSA natural history. In a recent study, Oksenberg and al,40 assessed the stability over time of POSA and the main factors that are involved in the conversion of POSA to NPOSA. They showed that after an average of 6.6 years, 30% of patients with POSA had significant changes in BMI and AHI and became NPOSA. On the other hand, the changes in these parameters were not significant in patients who remained POSA (70%). Further prospective studies are needed to determine if having POSA at younger age is a predictive factor for developing severe OSA later on.
Our results showed that POSA and e-POSA patients were more likely to have undergone a PSG recording. This could be explained by the fact that these patients usually are younger, leaner, and have fewer symptoms and comorbidities and therefore a lower pretest probability of having sleep apnea, which prompts the clinician to proceed directly with a PSG recording as recommended by the American Academy of Sleep Medicine and French clinical practice recommendations.41,42
In a study assessing the effect of in-lab PSG and HSAT on sleep position, Cartwright et al43 demonstrated that some patients report feeling constrained during PSG recordings due to the use of numerous sensors and cables, resulting in them spending more time in the supine position than they would have during a typical night at home. This finding was confirmed in a later study by Metersky et al44 that found that the mean percentage of supine sleep during PSG recordings was 56% greater than was seen during the HSAT night. However, in a recent study analyzing percentage of supine sleep in 445 PSG and 416 HSAT recordings, Kukwa et al45 showed that there was no difference in the percentage of supine position between in-laboratory PSG and HSAT. Women had even more supine sleep during HSAT than during PSG.
In comparison to NPOSA, both patients with POSA and e-POSA spent more time on their back, with a more significant difference for those with e-POSA. This means that the more time patients sleep in the supine position, the more likely they were to have positional OSA. The time spent sleeping in supine position is an important determining factor of the overall AHI in patients with POSA and e-POSA. Studies evaluating PT devices have shown that treated patients spent less time on their back and reduced their AHI significantly.46–48 This suggests the potential interest of PT devices as a therapeutic alternative to PAP, at least for patients with e-POSA.
Both patients with POSA and e-POSA had lower desaturation indices, which is consistent with less severe OSA with a lower AHI compared to NPOSA. These patients seem to have a higher fraction of hypopneas, as reported in other studies.49 This could be due to minor changes in the upper airway structure during supine position that is more likely to induce hypopneas. Some studies have shown that when unselected patients with OSA move from lateral to supine position, small but significant changes occur in pharyngeal cross sectional area.50,51 However, others have demonstrated no significant change.52,53
Treatment outcomes in POSA and e-POSA
Patients were selected for treatment with CPAP according to the criteria defined by the French clinical practice guidelines. PAP therapy was prescribed to all patients with severe OSA (AHI ≥ 30 events/h) and to those with moderate OSA (15 events/h ≤ AHI < 30 events/h) associated with cardiovascular comorbidities or severe daytime sleepiness. Our results are based on a large cohort of 3,000 patients with typical CPAP treatment with moderate-to-severe OSA without specific selection bias. Some patients eligible for PAP treatment declined to be treated. Among patients who were prescribed CPAP, only those who refused the therapy straightaway were excluded from the analysis. In a previously published study exploring data from the same cohort, Gagnadoux et al24 demonstrated that only 3.7% (42/1,389 patients) of patients refused PAP treatment.
About half the patients (46.6%) included in the PAP follow-up program were patients with POSA, with a minor subgroup of patients with e-POSA (9.2%). Compared to patients with NPOSA, both patients with POSA and e-POSA were significantly less adherent to their PAP treatment. While PAP adherence is known to be a challenge for all patients with OSA, it is even more so with those with POSA and e-POSA.
An adjusted multivariate analysis for confounders revealed that despite a higher likelihood of PAP treatment response when adherence was sufficient, POSA was negatively associated with treatment success. The same differences were observed in patients with e-POSA but were not significant. Furthermore, even when early adherence was adequate, patients with POSA and e-POSA were more likely to withdraw from PAP treatment than those with NPOSA. Thus, PAP treatment may not be efficient and alternative treatment should be considered for patients with POSA and particularly for those with e-POSA. However, if alternative treatment is an option, a close follow-up of adherence, effectiveness, and side effects to the proposed treatment should be considered. In addition, patient preferences and treatment costs should be taken into consideration, given that some positional treatment devices are expensive and not covered by medical insurance in most countries.
Mandibular advancement devices, hypoglossal nerve stimulation, upper airway surgery, maxillofacial surgery, and bariatric surgery are alternative options for management of OSA. Lifestyle modifications, including weight loss, avoidance of alcohol, opioids, and sedatives, are also beneficial. The most effective treatment for OSA is the one that is personalized to each patient’s need: for patients with POSA, it would be to avoid sleeping in the supine position. Avoiding the supine posture during sleep could be a better treatment alternative than suboptimal PAP use. PT treatment is effective at maintaining patients in the lateral position during sleep, and this cannot be achieved using PAP treatment.18 While PT devices are effective in reducing AHI in the short term and the new generation devices can monitor PT compliance, more studies are needed to prove long-term effectiveness, adherence, and clinical outcomes of these devices.
Furthermore, patients with POSA could continue to benefit from PT only as long as adherence for this therapy is good and their OSA remains positional. For those who switch to NPOSA, PT would not be the optimal treatment anymore. Thus, patients with mild-to-moderate POSA using PT should be frequently monitored to detect those who become less adherent as well as those whose POSA changes to a more severe OSA to assess the need to switch their treatment from PT to PAP.
However, if PT devices are to be used as a first choice treatment for patients with POSA, further prospective studies are needed to determine the best definition of POSA and to identify clinical characteristics that will predict a good response to PT and its long-term adherence.
Study strengths and limitations
The present study had a number of strengths, including the analysis of most relevant clinical and polygraphic characteristics of patients with POSA and e-POSA, the evaluation of PAP therapy adherence in these patients, and the evaluation of the prevalence for these phenotypes of OSA in the largest multicentric cohort compared to previous studies. However, we acknowledge a number of limitations. First, only 45% of patients in our cohort had a PSG recording, while the remainder had a HSAT without the possibility to use video recordings to correct the position signal if needed. This may have yielded occasional inaccuracies in the evaluation of the posture of some patients. The accuracy of position sensors depends on the technology used and on the placement of the sensor.28–31 Placing the sensor just above an anatomical landmark (the sternal notch) increases the likelihood of proper placement and accurate measurement of body position. Therefore, the CIDELEC sensor should have a high degree of accuracy. However, while errors are still possible, they likely did not have an impact on such a large cohort. Second, the diagnosis of POSA was made on a single-night recording. Investigating intraindividual variability, Fietze et al54 showed, in a case report recording the same patient with OSA for a consecutive 28 nights, that the supine body position ranged from 8.1 to 58.8% of total sleep time. Furthermore, in a study investigating how many patients with POSA will change to NPOSA in the follow-up PSG, Chou et al6 showed that one-third of the patients with POSA changed to NPOSA in the second PSG. Thus, POSA may not be a stable night-to-night phenotype. Recording of position for several consecutive nights might be necessary to confirm the patients’ POSA phenotype. Furthermore, we decided to use a definition of PAP treatment success based only on a daily PAP use ≥ 4 h and a decrease in ESS ≥ 2 points from baseline to 6-month follow-up. Although adherence and ESS may not be the only variables to assess PAP therapy success, we believe that they are most likely to identify patients that are properly responding to the therapy, as suggested in previous studies.36,37 Finally, PAP low adherence and discontinuation in patients with POSA could have been influenced by the clinician’s favorable attitude toward alternative treatments. However, patients from the cohort were seen in clinic by sleep specialists 5 months after PAP therapy onset. In other words, patients were given enough time to get accustomed to PAP treatment before the clinician could propose an alternative treatment in case of low PAP adherence (< 4 h/night). In addition, all patients benefited from the same follow-up support program managed by the home care provider.
CONCLUSIONS
In this large French population of patients with typical mild‐to‐severe OSA, we found that POSA and e-POSA were present in 53.5% and 20.1%, respectively, and were associated with male sex, younger age, lower AHI and BMI, and without the usual OSA associated comorbidities. Patients with POSA have lower PAP therapy adherence. Based on our data, CPAP may be an acceptable treatment for over 50% of patients with POSA, but PT should perhaps be considered when CPAP failed or based on patient preferences.
Clinicians need to be aware of the high prevalence of POSA and the challenges of its treatment. A consensus to determine the best definition of POSA and to further identify its clinical characteristics is needed. Our findings highlight the need to offer these patients an alternative treatment such as PT. However, one-night recording may not be reliable enough to phenotype patients as having POSA or e-POSA. Thus, future studies should evaluate the use of a simple position detection sensor for several nights to confirm the positional OSA phenotype of patients.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by grants from the Institut de Recherche en Santé Respiratoire des Pays de la Loire. At the time of the study, A. Sabil was a full-time employee of Philips Respironics and M. Blanchard worked was doing an internship at CIDELEC. All other authors declare that they have no conflict of interest on the present study. F. Gagnadoux reports personal fees from AIR LIQUIDE SANTE, CIDELEC, RESMED, SEFAM, outside the submitted work; non-financial support from AIR LIQUIDE SANTE, ASTEN SANTE, SEFAM, outside the submitted work.
ACKNOWLEDGMENTS
The authors thank the Pays de la Loire Sleep Cohort Group: Centre Hospitalier, Le Mans: Olivier Molinier; Centre Hospitalier, Pôle santé des Olonnes, Olonne sur Mer: Marie Langelot-Richard; Christelle Gosselin and Professor Jean-Louis Racineux from the Institut de Recherche en Santé Respiratoire des Pays de la Loire; and the sleep technicians from the Department of Respiratory and Sleep Medicine of Angers University Hospital: Julien Godey, Laetitia Moreno and Marion Vincent.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- APOC
Amsterdam Positional OSA Classification
- BMI
body mass index
- e-POSA
exclusive positional obstructive sleep apnea
- ESS
Epworth sleepiness scale
- HSAT
home sleep apnea test
- OR
odds ratio
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- POSA
positional obstructive sleep apnea
- PSG
polysomnography
- PT
positional therapy
REFERENCES
- 1.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joosten SA, Sands SA, Edwards BA, et al. Evaluation of the role of lung volume and airway size and shape in supine-predominant obstructive sleep apnoea patients. Respirology. 2015;20(5):819–827. 10.1111/resp.12549 [DOI] [PubMed] [Google Scholar]
- 3.Joosten SA, O’Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev. 2014;18(1):7–17. 10.1016/j.smrv.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 4.Ravesloot MJ, van Maanen JP, Dun L, de Vries N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea-a review of the literature. Sleep Breath. 2013;17(1):39–49. 10.1007/s11325-012-0683-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinzer R, Petitpierre NJ, Marti-Soler H, Haba-Rubio J. Prevalence and characteristics of positional sleep apnea in the HypnoLaus population-based cohort. Sleep Med. 2018;48:157–162. 10.1016/j.sleep.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 6.Chou Y-T, Yang T-M, Lin C-K, et al. Pay attention to treating a subgroup of positional obstructive sleep apnea patients. J Formos Med Assoc. 2017;116(5):359–365. 10.1016/j.jfma.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 7.Mo JH, Lee CH, Rhee CS, Yoon IY, Kim JW. Positional dependency in Asian patients with obstructive sleep apnea and its implication for hypertension. Arch Otolaryngol Head Neck Surg. 2011;137(8):786–790. 10.1001/archoto.2011.122 [DOI] [PubMed] [Google Scholar]
- 8.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7(2):110–114. 10.1093/sleep/7.2.110 [DOI] [PubMed] [Google Scholar]
- 9.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128(4):2130–2137. 10.1378/chest.128.4.2130 [DOI] [PubMed] [Google Scholar]
- 10.Laub RR, Mikkelsen KL, Tønnesen P. Prevalence of positional obstructive sleep apnea and patients characteristics using various definitions. Eur Respir J. 2015;46(suppl):59. [Google Scholar]
- 11.Oksenberg A, Gadoth N. Are we missing a simple treatment for most adult sleep apnea patients? The avoidance of the supine sleep position. J Sleep Res. 2014;23(2):204–210. 10.1111/jsr.12097 [DOI] [PubMed] [Google Scholar]
- 12.Haniffa M, Lasserson TJ, Smith I. Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea. Cochrane Database Syst Rev. 2004:CD003531. [DOI] [PubMed] [Google Scholar]
- 13.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS). Sleep Med Rev. 2003;7(1):81–99. 10.1053/smrv.2001.0197 [DOI] [PubMed] [Google Scholar]
- 14.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. 10.1513/pats.200708-119MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal L, Gerhardstein R, Lumley A, et al. CPAP therapy in patients with mild OSA: implementation and treatment outcome. Sleep Med. 2000;1(3):215–220. 10.1016/S1389-9457(00)00012-5 [DOI] [PubMed] [Google Scholar]
- 16.Kendzerska T, Mollayeva T, Gershon AS, Leung RS, Hawker G, Tomlinson G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev. 2014;18(1):49–59. 10.1016/j.smrv.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 17.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6(3):238–243. 10.5664/jcsm.27820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha SCN, Hirai HW, Tsoi KKF. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta-analysis of randomized trials. Sleep Med Rev. 2014;18(1):19–24. 10.1016/j.smrv.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 19.de Vries GE, Hoekema A, Doff MH, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med. 2015;11(2):131–137. 10.5664/jcsm.4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laub RR, Tønnesen P, Jennum PJ. A Sleep Position Trainer for positional sleep apnea: a randomized, controlled trial. J Sleep Res. 2017;26(5):641–650. 10.1111/jsr.12530 [DOI] [PubMed] [Google Scholar]
- 21.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115(3):771–781. 10.1378/chest.115.3.771 [DOI] [PubMed] [Google Scholar]
- 22.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5(5):428–430. 10.5664/jcsm.27597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: A 6-month follow-up study. Laryngoscope. 2006;116(11):1995–2000. 10.1097/01.mlg.0000237674.66716.a7 [DOI] [PubMed] [Google Scholar]
- 24.Gagnadoux F, Le Vaillant M, Goupil F, et al. IRSR sleep cohort group . Influence of marital status and employment status on long-term adherence with continuous positive airway pressure in sleep apnea patients. PLoS One. 2011;6(8):e22503. 10.1371/journal.pone.0022503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagnadoux F, Le Vaillant M, Goupil F, et al. IRSR Sleep Cohort Group . Depressive symptoms before and after long-term CPAP therapy in patients with sleep apnea. Chest. 2014;145(5):1025–1031. 10.1378/chest.13-2373 [DOI] [PubMed] [Google Scholar]
- 26.Gagnadoux F, Le Vaillant M, Paris A, et al. Institut de Recherche en Santé Respiratoire des Pays de la Loire Sleep Cohort Group . Relationship between OSA clinical phenotypes and CPAP treatment outcomes. Chest. 2016;149(1):288–290. 10.1016/j.chest.2015.09.032 [DOI] [PubMed] [Google Scholar]
- 27.Meurice J-C, Gagnadoux F. [Recommendations for clinical practice in the management of obstructive sleep apnea syndrome in adults. Preface]. Rev Mal Respir. 2010;27(Suppl 3):S113–S114. 10.1016/S0761-8425(10)70016-4 [DOI] [PubMed] [Google Scholar]
- 28.Levendowski DJ, Seagraves S, Popovic D, Westbrook PR. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med. 2014;10(8):863–871. 10.5664/jcsm.3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7(4):376–383. 10.5664/JCSM.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fietze I, Dingli K, Diefenbach K, et al. Night-to-night variation of the oxygen desaturation index in sleep apnoea syndrome. Eur Respir J. 2004;24(6):987–993. 10.1183/09031936.04.00100203 [DOI] [PubMed] [Google Scholar]
- 31.Roebuck A, Monasterio V, Gederi E, et al. A review of signals used in sleep analysis. Physio. Meas 2014;35(1):R1–R57. 10.1088/0967-3334/35/1/R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry RB, Budhiraja R, Gottlieb DJ, et al. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler MP, Emch JT, Rueschman M, et al. Apnea-hypopnea event duration predicts mortality in men and women in the Sleep Heart Health Study. Am J Respir Crit Care Med. 2019;199(7):903–912. 10.1164/rccm.201804-0758OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respi. Crit Care Med. 2014;190(11):1293–1300. 10.1164/rccm.201404-0718OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40(14):1149–1157. 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crook S, Sievi NA, Bloch KE, et al. Minimum important difference of the Epworth Sleepiness Scale in obstructive sleep apnoea: estimation from three randomised controlled trials. Thorax. 2019;74(4):390–396. [DOI] [PubMed] [Google Scholar]
- 37.Patel S, Kon SSC, Nolan CM, et al. The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(7):961–963. 10.1164/rccm.201704-0672LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iannella G, Magliulo G, Lo Iacono CAM, et al. Positional obstructive sleep apnea syndrome in elderly patients. Int J Environ Res Public Health. 2020;17(3):1120. 10.3390/ijerph17031120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villaneuva AT, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev. 2005;9(6):419–436. 10.1016/j.smrv.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 40.Oksenberg A, Goizman V, Eitan E, Nasser K, Gadoth N, Leppänen T. Obstructive sleep apnea: Do positional patients become nonpositional patients with time? Laryngoscope. 2019;130(9):2263–2268. [DOI] [PubMed] [Google Scholar]
- 41.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479–504. 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escourrou P, Meslier N, Raffestin B, et al. [Which clinical approach and which diagnostic procedures for obstructive sleep apnea syndrome?]. Rev Mal Respir. 201027, 27, Suppl 3:S115–S123. 10.1016/S0761-8425(10)70017-6 [DOI] [PubMed] [Google Scholar]
- 43.Cartwright RD, Lloyd S, Lilie J, Kravitz H. Sleep position training as treatment for sleep apnea syndrome: a preliminary study. Sleep. 1985;8(2):87–94. 10.1093/sleep/8.2.87 [DOI] [PubMed] [Google Scholar]
- 44.Metersky ML, Castriotta RJ. The effect of polysomnography on sleep position: possible implications on the diagnosis of positional obstructive sleep apnea. Respiration. 1996;63(5):283–287. 10.1159/000196561 [DOI] [PubMed] [Google Scholar]
- 45.Kukwa W, Migacz E, Lis T, Ishman SL. The effect of in-lab polysomnography and home sleep polygraphy on sleep position. Sleep Breath. 2020. 10.1007/s11325-020-02099-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dieltjens M, Vroegop AV, Verbruggen AE, et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath. 2015;19(2):637–644. 10.1007/s11325-014-1068-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eijsvogel MM, Ubbink R, Dekker J, et al. Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. J Clin Sleep Med. 2015;11(2):139–147. 10.5664/jcsm.4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Maanen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome. Sleep. 2014;37(7):1209–1215. 10.5665/sleep.3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leppänen T, Töyräs J, Muraja-Murro A, et al. Length of individual apnea events is increased by supine position and modulated by severity of obstructive sleep apnea. Sleep Disord. 2016;2016:9645347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isono S, Tanaka A, Nishino T. Lateral position decreases collapsibility of the passive pharynx in patients with obstructive sleep apnea. Anesthesiology. 2002;97(4):780–785. 10.1097/00000542-200210000-00006 [DOI] [PubMed] [Google Scholar]
- 51.Ono T, Otsuka R, Kuroda T, Honda E, Sasaki T. Effects of head and body position on two- and three-dimensional configurations of the upper airway. J Dent Res. 2000;79(11):1879–1884. 10.1177/00220345000790111101 [DOI] [PubMed] [Google Scholar]
- 52.Jan MA, Marshall I, Douglas NJ. Effect of posture on upper airway dimensions in normal human. Am J Respir Crit Care Med. 1994;149(1):145–148. 10.1164/ajrccm.149.1.8111573 [DOI] [PubMed] [Google Scholar]
- 53.Walsh JH, Leigh MS, Paduch A, et al. Effect of body posture on pharyngeal shape and size in adults with and without obstructive sleep apnea. Sleep. 2008;31(11):1543–1549. 10.1093/sleep/31.11.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fietze I, Glos M, Zimmermann S, Penzel T. Long-term variability of the apnea-hypopnea index in a patient with mild to moderate obstructive sleep apnea. J Clin Sleep Med. 2020;16(2):319–323. 10.5664/jcsm.8192 [DOI] [PMC free article] [PubMed] [Google Scholar]