Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a respiratory disorder caused by the obstruction of the upper airway during sleep. The most common cause of pediatric OSA is adenotonsillar hypertrophy. Adenotonsillectomy is the first-line treatment for pediatric OSA; however, OSA persists in a significant number of patients due, in part, to the method of evaluating enlarged adenoids and tonsil tissue. The reason for these effects on OSA severity is not clear. This study aimed to establish a method to diagnose the need for adenoidectomy or tonsillectomy.
Methods:
Twenty-seven Japanese children (mean age 6.6 years) participated in this study, undergoing polysomnography and computed tomography examination. Pharyngeal airway morphology (adenoids and tonsil tissue size, volume, and cross-sectional area [CSA]) and pressure on the upper airway were evaluated at each site using computational fluid dynamic analysis.
Results:
Apnea-hypopnea index (AHI) showed a strong linear association with maximum negative pressure (Pmax) (AHI = −0.055* events/h Pmax −1.326, R2 = .805). The relationship between minimum CSA (CSAmin) and Pmax was represented by an inversely proportional fitted curve (Pmax = −4797/CSAmin −5.1, R2 = .507). The relationship between CSAmin and AHI was also represented by an inversely proportional fitted curve (AHI = 301.6 events/h/CSAmin1.22, R2 = .680). Pmax greatly increased if CSAmin became ≤ 30 mm2. The negative pressure of each site increased when CSA measured ≤ 50 mm2.
Conclusions:
In children, when the CSA for each site is ≤ 50 mm2, AHI is likely to be elevated, and the patient may require tonsillectomy or adenoidectomy.
Citation:
Iwasaki T, Sugiyama T, Yanagisawa-Minami A, Oku Y, Yokura A, Yamasaki Y. Effect of adenoids and tonsil tissue on pediatric obstructive sleep apnea severity determined by computational fluid dynamics. J Clin Sleep Med. 2020;16(12):2021–2028.
Keywords: adenoid, tonsil, airway negative pressure, computed fluid dynamics, obstructive sleep apnea, pediatrics
BRIEF SUMMARY
Current Knowledge/Study Rationale: Indication of adenoidectomy and tonsillectomy for obstructive sleep apnea (OSA) in children is important to determine treatment strategies. However, the adaptive decision method of the current method is insufficient.
Study Impact: The purpose of this study was to determine a new adaptive evaluation method to determine the usefulness of adenoidectomy or tonsillectomy in pediatric OSA. We determined that adenoidectomy and tonsillectomy can improve treatment results for pediatric OSA.
INTRODUCTION
Obstructive sleep apnea (OSA) is a respiratory disorder caused by the obstruction of the upper airway during sleep.1 The prevalence of OSA in children is approximately 1.2–5.7%.2,3 Hypertrophy of the adenoidal and tonsillar tissue (AT) is thought to be one of the primary anatomical causes of OSA in the pediatric population.4 Therefore, adenotonsillectomy is considered the first-line treatment for pediatric patients with OSA.4,5 AT size assessment during the presurgical physical examination is often used as a key component of clinical decision-making, specifically to estimate the potential success or failure of adenotonsillectomy.
The success rate of adenotonsillectomy for pediatric OSA is variable, ranging from 27.2 to 82.9%.6 Nolan et al7 reviewed 20 studies that determined if tonsillar size was related to OSA. Eleven of the 20 studies concluded there was an association between tonsil size and OSA, and showed that diagnosis of OSA was possible using varying statistical techniques, either liner or logistic regression analysis, and correlations. However, the other 9 studies did not find an association. The review concluded that there was a weak association between tonsillar size and diagnosable OSA in pediatric patients. Therefore, it is not clear to what extent AT enlargement causes OSA. Bally et al8 reported that these evaluation methods not only require further evaluation themselves, but they also must ascertain the effect that enlarged adenoids and tonsils have on the airway.
In children without OSA, pharyngeal airway volume is 29,718.6 mm3, and in children with OSA, the volume is 17,387.4 mm3, as determined by Van Holsbeke et al.9 This study also determined that a pharyngeal airway of small volume is characteristic in children with OSA and that the pharyngeal airway of children with OSA greatly narrows upon inhalation.1 This decrease in size is regarded as a consequence of major negative pressure occurring during inhalation.10 Therefore, not only the degree of enlargement of AT but also the airway size at the AT site and the negative AT pressure at inhalation are thought to influence airway obstruction.11
OSA severity reflects ventilation condition of the whole upper airway. Therefore, it is necessary to investigate OSA that involves nasal obstruction. Increased obstruction of the pharyngeal airway affects OSA severity.
Recently, we have been able to evaluate the ventilation condition of each upper airway site by using computational fluid dynamics (CFD).10 CFD can evaluate the flow of air in a manner similar to that during actual breathing, even in cases of upper airways with complicated morphologies.12 As we used CFD that was only able to evaluate the ventilation condition for the nasal airway, only children without nasal obstruction were selected for the study.
The purpose of this study was to identify the factors influencing OSA disease severity by investigating the relationships between pharyngeal airway morphology (eg, AT size, volume, and cross-sectional area [CSA]), negative pressure of the pharyngeal airway by CFD, and OSA disease severity. Factors identified in this study may help to predict the need for adenoidectomy or tonsillectomy.
METHODS
Patients
Thirty-three patients (25 boys, 8 girls) treated at a national university hospital (Yamanashi, Japan) for OSA were included in this retrospective study. The inclusion criteria for the study included children aged 3–13 years who had undergone polysomnography and computed tomography (CT) scan for diagnosis. Polysomnography was used to measure the apnea-hypopnea index (AHI). Apnea was defined as the complete cessation of airflow for 2 respiratory cycles, and hypopnea was defined as a 50% reduction in oronasal airflow for 10 seconds with at least 3% desaturation. The AHI was calculated as the number of apnea and hypopnea events per hour of sleep.13 Severe OSA was defined as an AHI of 15 events/h or more. CT scanning was nonroutine; it was performed only for cases (eg, adenoidectomy or tonsillectomy candidate, severe maxillofacial form abnormality) in which detailed examination of the morphology of the upper airway was needed. A CT scan was only performed when CT scan data required for OSA treatment outweighed the risk of the radiation. A craniocervical inclination of 95° to 105° was used as seen in previous work,14 because the volume of the airway is influenced by head posture. The exclusion criteria for the study included nasal obstruction with a nasal airway resistance greater than 0.5 Pa/ml/sec. Because nasal obstruction affected OSA severity, it was unclear in these cases whether OSA severity was due to adenoid or/and tonsil hypertrophy.15,16 Other exclusions were craniofacial or growth abnormalities, a history of tonsillectomy or adenoidectomy, or treatment for systematic disease. The final patients included in this study were 27 children (21 boys, 6 girls; mean age 6.6 ± 2.2 years). On the basis of a previous study,10 the correlation coefficient was set to .60. With the significance and power set to .05 and .80, respectively, the correlation coefficient showed that the required sample size was 20. This study was approved by the institutional review board of Kagoshima University, Japan (180073 (657) Epi-ver. 5) and Yamanashi University, Japan (1594). Due to the study’s retrospective nature, the need for informed consent was waived.
Method
Polysomnography and the CT scan method were previously reported.10 Adenoid and tonsil hypertrophy was evaluated. AT growth was evaluated in reference to past studies as AT grade.15,16,17,18 Morphological evaluation of the pharyngeal airway (pharyngeal airway volume [PAv] and cross-sectional area [CSA]) had been previously reported.10 Minimum CSA in the pharyngeal airway (CSAmin), smallest CSA in the nasopharyngeal airway (NA) (CSANA), smallest CSA in the retropalatal airway (RA) (CSARA), and smallest CSA in the oropharyngeal airway (OA) (CSAOA) were all measured.
Adenoids
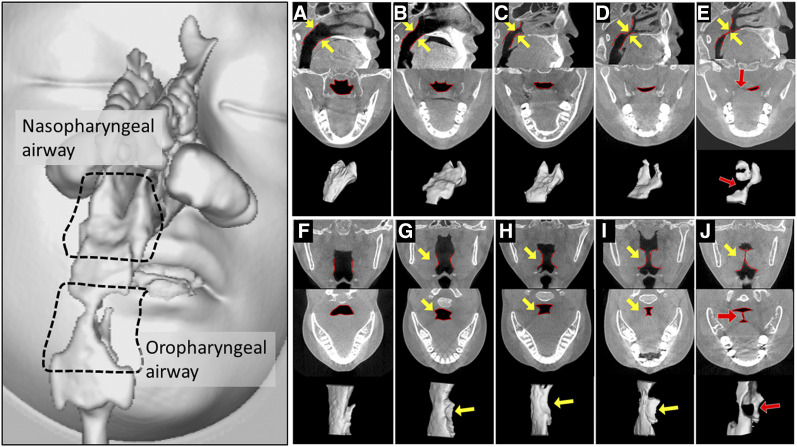
The midsagittal plane between the posterior outline of the soft palate and the closest point on the adenoid tissue from CT images was measured to classify the relative size of the adenoids into 5 grades ( Figure 1A, Figure 1B, Figure 1C, Figure 1D, and Figure 1E). In addition, the nasopharyngeal airway was visualized by reversing the size of adenoids and modeling three-dimensionally.15
Figure 1. Measurement of adenoid and tonsil size.
Measurement of relative adenoid size (A–E). Upper figure: mid sagittal plane. Middle figures: cross section of nasopharyngeal airway of closest part. Lower figure: three-dimensional nasopharyngeal airway model from the right rear. The distance in the midsagittal plane from the posterior outline of the soft palate to the closest point on the adenoid tissue from cone-beam computed tomography images was used to classify the relative size of the adenoids into five groups. (A) Grade I was not present in adenoid. (B) Grade II adenoid extended quarter of the way to the midline (yellow arrow). (C) Grade IIl adenoid extended halfway to the midline (yellow arrow). (D) Grade IV adenoid extended three quarters of the way to the midline (yellow arrow). (E) Grade V adenoid partially occluded the airway (red arrow). Measurement of relative palatine tonsil size (F–J). Upper figure: coronal plane of oropharyngeal airway of closest part. Middle figure: horizontal plane of oropharyngeal airway of closest part. Lower figure: three-dimensional oropharyngeal airway model from the right rear. (F) Grade I was not present in hyperplasia of palatine tonsil. (G) Grade lI tonsils extended quarter of the way to the midline (yellow arrow). (H) Grade III tonsils extended halfway to the midline (yellow arrow). (I) Grade IV tonsils extended three quarters of the way to the midline (yellow arrow). (J) Grade V tonsils were completely obstructing the airway, also known as “kissing” tonsils (red arrow).
Tonsils
The narrowest distance between the tonsils in the midcoronal plane was used to classify their relative sizes into 5 grades (Figure 1F, Figure 1G, Figure 1H, Figure 1I, and Figure 1J). The oropharyngeal airway was visualized by reversing the size of adenoids and modeling three-dimensionally.18
Evaluations of ventilation conditions of the nasal airway, NA, RA, and OA were conducted. The analysis of the ventilation condition using CFD of the nasal airway and the upper airway was carried out according to a method previously reported10 (Figure 1D and Figure 1E). The nasal resistance was calculated from external naris, choanal difference in pressure, and flow quantity obtained in CFD using the nasal airway model.
The upper airway three-dimensional model obtained with CFD at inhalation was used to evaluate multiple values. First, it evaluated maximal negative pressure (Pmax) in the upper airway. Pmax is defined as the largest value measured in the upper airway. Second, it evaluated the pressure change in NA (ΔNA), the difference in the pharyngeal airway pressure between the choana and pharyngeal airway at the palatal plane. Third, it evaluated the pressure change in RA (ΔRA), where a difference in adenoid and palatine tonsil length influence pressure. This was determined by calculating the difference in the pharynx airway pressure between the palatal plane and tip of the soft palate. Finally, pressure changes in OA (ΔOA) were evaluated by calculating the difference in the pharynx airway pressure between the tip of the soft palate and the bottom of the epiglottis, equivalent to the palatine tonsil.
Statistics
All data were analyzed blindly. Depending on distribution, Pearson’s (using AHI and morphological values) and Spearman (using AHI and CFD values) correlation coefficients (Rs) were used to evaluate relationships between OSA severity (AHI), pharyngeal airway morphology (AT grade, volume, and CSA), and pharyngeal airway functional value (pressure determined with CFD). Nonlinear regression was used to describe the relationship between the measurement values. Multiple regression was used to examine all measured values that had greater influence on AHI. For all tests, P < .05 was considered statistically significant. For intra- and interexaminer reliability, a random number generator was used to select 10 patients. The measurements were repeated 1 week after the initial measurements. Both intra- and interexaminer reliability tests exhibited high correlations ranging from 0.965 to 0.981 for all measures.
RESULTS
The mean, standard deviation, minimum, and maximum of characteristics of patients in this study are listed in Table 1. AHI was 7.38 ± 7.00 events/h and Pmax was −159.30 ± 114.87 Pa. Adenoid grade was 3.41 ± 0.93 degrees and tonsil grade was 4.07 ± 0.96 degrees. Table 1 describes the correlation between AHI and morphological values and pressures. AHI was significantly negatively correlated to pressure changes in OA corresponding to tonsil part (ΔOA) pressure and Pmax (Rs = −.838, P < .001, Rs = −.838, P < .001, respectively). A significant negative correlation also existed between AHI, CSANA, CSARA, and CSAmin (Rs = −.384, P < .048, Rs = −.442, P < .021, Rs = −.485, P < .010, respectively). However, AHI was not significantly correlated with airway volumes. A significantly positive correlation existed between AHI and AT grade (Rs = −.427, P < .026, Rs = −.399, P < .039, respectively). Table 2 describes pressure and morphological values.
Table 1.
Morphologic and functional variable of the upper airway and correlation coefficient to AHI (n = 27).
| Min | Max | Mean | SD | AHI Correlation Coefficients | P | |
|---|---|---|---|---|---|---|
| Age (years) | 3.70 | 13.10 | 6.62 | 2.26 | — | — |
| Height (cm) | 91.90 | 153.40 | 116.88 | 16.35 | — | — |
| Body weight (Kg) | 12.10 | 54.00 | 24.01 | 10.74 | — | — |
| BMI (kg/mm2) | 12.60 | 23.10 | 16.80 | 2.95 | — | — |
| Percentiles BMI (%ile) | 0.10 | 98.20 | 54.23 | 34.83 | — | — |
| AHI (events/h) | 1.10 | 36.30 | 7.38 | 7.00 | — | — |
| Max pressure (Pa)† | −576.00 | −50.00 | −159.30 | 114.87 | −.838** | .000 |
| ΔNA (Pa)† | −310.00 | 2.00 | −43.49 | 81.39 | −.220 | .271 |
| ΔRA (Pa)† | −210.00 | 1.00 | −30.24 | 44.59 | −.301 | .127 |
| ΔOA (Pa)† | −170.90 | −0.70 | −51.87 | 51.90 | −.838** | .000 |
| Adenoid grade (degree)† | 1.00 | 5.00 | 3.41 | 0.93 | .427* | .026 |
| Tonsil grade (degree)† | 1.00 | 5.00 | 4.07 | 0.96 | .399* | .039 |
| NAv (cm3)†† | 0.40 | 4.50 | 1.54 | 1.05 | −.148 | .461 |
| RAv (cm3)†† | 0.70 | 4.50 | 2.05 | 0.88 | −.106 | .600 |
| OAv (cm3)†† | 0.30 | 6.70 | 2.15 | 1.39 | −.131 | .514 |
| PAv (cm3)†† | 1.70 | 10.40 | 4.21 | 1.78 | −.082 | .685 |
| CSANA (mm2)†† | 16.30 | 327.90 | 106.24 | 76.40 | −.384* | .048 |
| CSARA (mm2)†† | 19.80 | 178.90 | 74.62 | 43.31 | −.442* | .021 |
| CSAOA (mm2)†† | 15.80 | 208.00 | 61.45 | 45.03 | .054 | .788 |
| CSAmin (mm2)†† | 15.80 | 178.90 | 44.14 | 33.79 | −.485* | .010 |
†Spearman correlation coefficient; ††Pearson correlation coefficient; *P < .05; **P < .01. AHI = apnea-hypopnea index, BMI = body-mass index, CSA = cross-sectional area, CSAmin = minimum CSA, CSANA = CSA of NA, CSAOA = CSA of OA, CSARA = CSA of RA, NA = nasopharyngeal airway, ΔNA = pressure changes in NA corresponding to adenoids part, NAv = NA volume, OA = oropharyngeal airway, OAv = OA volume, ΔOA = pressure changes in OA corresponding to tonsil part, PAv = pharyngeal airway volume, RA = retropalatal airway, ΔRA = pressure changes in RA where adenoids and palatine tonsil influence, RAv = RA volume, SD = standard deviation.
Table 2.
Relationship between airway morphological values and pressure drop.
| Max Pressure (Pa) | ΔNA (Pa) | ΔRA (Pa) | ΔOA (Pa) | |
|---|---|---|---|---|
| CSA (mm2) | ||||
| CSAmin (mm2) | .849** | .306 | .544** | .550** |
| P | .000 | .121 | .003 | .003 |
| CSANA (mm2) | .503** | .853** | .275 | −.189 |
| P | .008 | .000 | .165 | .345 |
| CSARA (mm2) | .558** | .290 | .842** | .493** |
| P | .002 | .143 | .000 | .009 |
| CSAOA (mm2) | .431* | −.223 | .414* | .865** |
| P | .025 | .264 | .032 | .000 |
| Volume (cm3) | ||||
| PAv (cm3) | .225 | .509** | .134 | −.123 |
| P | .258 | .007 | .504 | .540 |
| NAv (cm3) | .234 | .743** | .046 | −.439* |
| P | .240 | .000 | .819 | .022 |
| RAv (cm3) | .183 | .435* | .099 | −.149 |
| P | .361 | .023 | .623 | .458 |
| OAv (cm3) | .140 | .200 | .029 | .056 |
| P | .486 | .318 | .885 | .780 |
*P < .05; **P < .01. CSA = cross-sectional area, CSAmin = minimum CSA, CSANA = CSA of NA, CSAOA = CSA of OA, CSARA = CSA of RA, Max = maximum value, NA = nasopharyngeal airway, ΔNA = pressure changes in NA corresponding to adenoids part, NAv = NA volume, OA = oropharyngeal airway, ΔOA = pressure changes in OA corresponding to tonsil part, OAv = OA volume, PAv = pharyngeal airway volume, RA = retropalatal airway, ΔRA = pressure changes in RA where adenoids and palatine tonsil influence, RAv = RA volume.
CSAmin was significantly correlated with Pmax (Rs = .849). Each site of CSANA, CSARA, and CSAmin was significantly correlated with pressure change of each site (Rs = .853, .842, .865). NA volume was significantly correlated with pressure change of NA (Rs = .743). However, RA and OA volumes were not significantly correlated with the pressure change at each corresponding site.
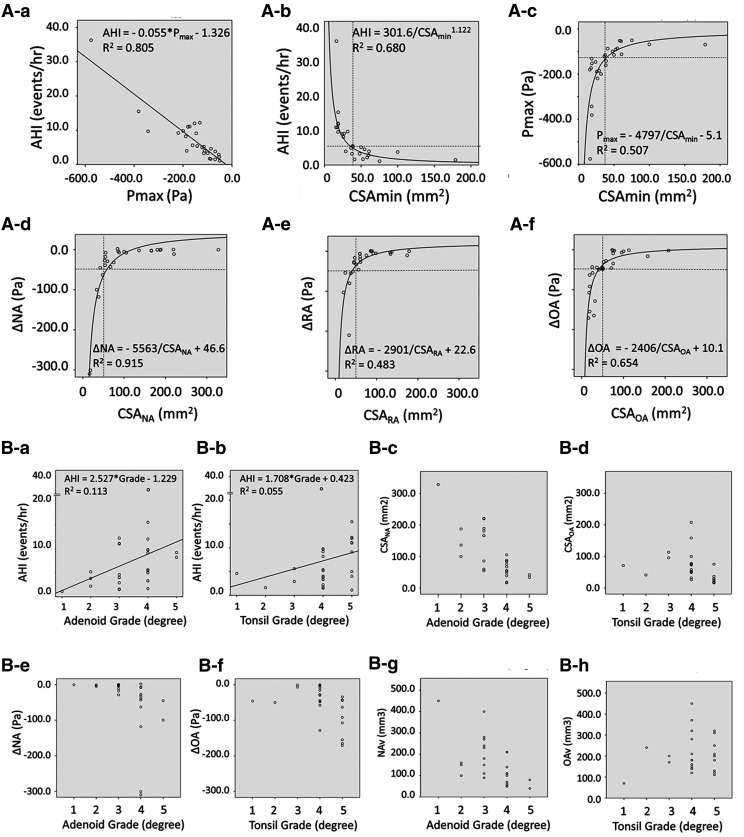
AHI showed strong linear association between Pmax (Figure 2A-a, AHI = −0.055* events/h Pmax −1.326 R2 = .805, P < .001). The relationship between AHI and CSAmin was shown in inversely proportional data and represented by a fitted curve (Figure 2A-b, AHI = 301.6 events/h/CSAmin 11.22, R2 = .680, P < .001). AHI increased suddenly if CSA became 30 mm2 or less.
Figure 2. Relationships measurement variables.
(A) Relationship of AHI and maximum negative pressure (Pmax) and cross-sectional area. Relationship of (A-a) AHI and Pmax; (A-b) AHI and minimum cross-sectional area (CSAmin); (A-c) Pmax and CSAmin; (A-d) pressure changes at NA and cross-sectional area at NA site; (A-e) pressure change at RA and cross-sectional area at RA site; and (A-f) pressure change at OA and cross-sectional area at OA site. (B) Association between enlargement of adenoids and the palatine tonsil (AT) and AHI, pressure change, airway volume of each site, and cross-sectional area (CSA) of each site. Each grade of AT did not correlate to the variables (AHI, negative pressure, airway volume, and CSA). Relationship of (B-a) AHI and Adenoid grade; (B-b) AHI and tonsil grade; (B-c) CSANA and Adenoid grade; (B-d) CSAOA and tonsil grade; (B-e) ΔNA and Adenoid grade; (B-f) ΔOA and tonsil grade; (B-g) NAv and Adenoid grade; and (B-h) relationship of OAv and tonsil grade. AHI = apnea-hypopnea index, CSA = cross-sectional area, CSAmin = minimum CSA, CSANA = CSA of NA, CSAOA = CSA of OA, CSARA = CSA of RA, NA = nasopharyngeal airway, Nav = NA volume, ΔNA = pressure changes in NA corresponding to adenoids, OA = oropharyngeal airway, OAv = OA volume, ΔOA = pressure changes in OA corresponding to tonsil, Pmax = maximum negative pressure, RA = retropalatal airway, ΔRA = pressure changes in RA where adenoids and palatine tonsil influence.
The relationship between Pmax and CSAmin was represented by an inversely proportional fitted curve (Figure 2A-c, AHI = −4797 events/h/CSAmin −5.1, R2 = .507, P < .01). Negative pressure quickly increased by more than −120 Pa if CSAmin measured 30 mm2 or less. The distributions between the CSANA, CSARA, and CSAOA, and the pressure of each site in this study, are shown in Figure 2A-d–f. The relationship between CSANA, CSARA, and CSAOA and corresponding pressures was represented by an inversely proportional fitted curve. Negative pressure increased if CSANA, CSARA, or CSAOA increased from 30 mm2 to 50 mm2 or less.
The distributions of AHI, pressure, CSA, and volume of each site compared to AT grade is shown in Figure 2B-a–h. The values of AHI for AT grade, negative pressure, airway volume, and CSA of each site were widely distributed.
Multiple regression analysis was performed to examine AT grade, pharyngeal airway volume and cross section, and negative pressure at inhalation. This determined which endpoint had the greatest influence on AHI, which our study revealed to be Pmax (β = 0.889, P < .001).
When CSAmin of the site was 30 mm2 or less, adenoidectomy or tonsillectomy was usually completed (Figure 2A-b–c). Furthermore, when the pharyngeal airway (including AT) had multiple sites with a CSA measuring 50 mm2 or less, major negative pressure increased, even if it did not occur alone. When nasal obstruction was severe, negative pressure of the pharyngeal airway increased and obstruction occurred.
DISCUSSION
This study showed that Pmax had the greatest influence on AHI. AT grade was not the greatest influencer of Pmax as expected. Instead, CSAmin had the greatest influence on Pmax. As a result, AHI was affected when CSA was 50 mm2 or less. AHI became severe when CSA measured 30 mm2 or less. From these results, we concluded that when each site’s CSANA, CSARA, and CSAOA were 50 mm2 or less, this indicated the need for adenoidectomy or tonsillectomy.
Concerning the influences that Pmax has on AHI, Arens et al1 reported that the pharyngeal airway greatly decreased in size at inhalation in children with OSA. Their hypothesis was that the pharyngeal airway shrank due to great negative pressure at inhalation. Based on this study, we have found a strong association between AHI and negative pressure and determined that negative pressure is associated with obstruction. CSA showed strong association with AHI because Pmax was heavily influenced by CSA. From previous reports1,10 and our present results, a small CSA in patients caused negative pharyngeal airway pressure at inhalation. During sleep, muscles of the pharyngeal airway relax but collapse when negative pressure is large. Therefore, we concluded that negative pressure affected AHI.
Previous studies in adults have shown the relationship between a nasal airway cross section and nasal resistance.19 Relationships between constricted pharyngeal airway cross sections and negative pharyngeal airway pressure were reported to show the inversely proportional relationship, similar to the earlier study.20,21 Previous studies reported negative pressure increases when cross sections of the pharyngeal airway were 100 mm2 or less.20,21 In our study, a change of pressure was measured at 50 mm2 or less. Because our patients were children (mean age 6.6 years old), this discrepancy may be due to the smaller size of the pharyngeal airway and a subsequent change in flow rate. This study determined that the relationship between CSA and pressure was similar to that in previous studies.20,21
When CSAmin was 30 mm2 or less, the pharyngeal airway exhibited increased negative pressure, and collapse of the pharyngeal airway was expected due to muscle relaxation. Previous studies showed that CSA in children with OSA is small.9,22 Van Holsbeke et al9 reported that CSA in Belgian children (mean AHI = 8.7 events/h) was 17.9 mm2, measured at the time the patients awoke from CT sedation, as in this study. Similar CSA results were obtained when OSA severity in children in the present study and the results of Van Holsbeke et al9 were compared. However, in previous studies,9,22 only mean CSA values were shown. The need for adenoidectomy or tonsillectomy was not considered in every case individually. However, we believe we can easily determine the need for adenoidectomy or tonsillectomy using three-dimensional morphological and CFD evaluations. Additionally, negative pressure tended to moderately increase when CSA was less than 50 mm2. This showed a relationship between CSANA, CSARA, and CSAOA and the change in the pressure of all parts. In this case, a CSA of 50 mm2 or less should be considered as indication of adenoidectomy or tonsillectomy.
In a previous drug-induced sleep endoscopy study,23 it was reported that even a small AT grade or large pharyngeal airway may be obstructed during sleep. When the upper airway is obstructed, major negative pressure occurs in the lower airway.10,24 For example, our data showed that severe nasal obstruction can cause negative pressure of the pharyngeal airway, even if CSA of the pharyngeal airway is large and AT grade is small. When the pharyngeal airway form determined AHI, which reflected the ventilation of the upper airway, cases of nasal obstruction were excluded. However, adenoids may affect nasal airway ventilation even after conventional rhinomanometry.25,26 Isolating the nasal airway to evaluate the ventilation condition was difficult. This study excluded the possibility of a nasal obstruction because we were able to evaluate CFD for ventilation conditions of the upper airway. We were also able to evaluate the ventilation condition of each pharyngeal airway site. CFD proved to be a useful method to determine airway ventilation. We were able to use CFD to analyze the pharyngeal airway and determine an association between pharyngeal airway pressure, pharyngeal airway size, and OSA severity.
Several epidemiologic studies6–8 examined AT grade and its relationship to OSA. However, these studies6–8 only examined the association statistically without discussing mechanism. Therefore, a consensus was not reached concerning the association between AT grade and OSA severity until now. In this study, the relationship between AHI and AT grade was examined, with a result that showed only a weak correlation between AT grade and AHI.
For AT grade, the values of not only AHI but also CSA, volume, and pressure changes were distributed widely (Figure 2B-c–h). As a result, we showed cases in which AT grade did not associate with each CSA. In contrast, there were cases in which AT grade strongly associated with each CSA. Therefore, because it is difficult to determine CSA that influences ventilation condition from AT grade, there was no strong association between AT grade and AHI. When the extraction was based on the AT grade, it was less clear that adenoidectomy and tonsillectomy would be indicated for an elevated AHI. From these results, we concluded that CSA evaluation was more effective than AT grade evaluation in determining a patient’s need for adenoidectomy or tonsillectomy.
This study had several limitations. The sample size was small, and the analysis of the rigid model was performed using awakening CT data. Because awakening CT data was different from sleep CT data, it was difficult to define the characteristics of OSA. However, our study was able to characterize the airflow properties of children with OSA. These data clearly showed that CFD-evaluated values were correlated with AHI. Further research comparing the patient outcomes of AT by CSA analysis is warranted to confirm the efficacy of CSA. In addition, this study was not able to examine the effect of obesity. A similar study for children with obesity is necessary.
Clinical implications
The past study showed that the association between the degree of AT enlargement and pediatric OSA was weak. In this study, however, CSA was more closely associated with OSA disease severity than with the degree of AT enlargement, and was therefore, thought to be useful as a criterion for AT. When CSA for each site equals 50 mm2 or less, adenectomy or tonsillectomy may be necessary.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest. This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (17K11965, 18K09860, and 20K10230).
ACKNOWLEDGMENTS
The authors thank Editage for English language editing.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AT
adenoidal and tonsillar tissue
- CFD
computational fluid dynamics
- CSA
cross-sectional area
- CSAmin
minimum CSA
- CSANA
CSA of NA
- CSAOA
CSA of OA
- CSARA
CSA of RA
- CT
computed tomography
- NA
nasopharyngeal airway
- ΔNA
pressure changes in NA corresponding to adenoids part
- NAv
NA volume
- OA
oropharyngeal airway
- ΔOA
pressure changes in OA corresponding to tonsil part
- OAv
OA volume
- OSA
obstructive sleep apnea
- Pmax
maximum negative pressure
- RA
retropalatal airway
- ΔRA
pressure changes in RA where adenoids and palatine tonsil influence
- RAv
RA volume
REFERENCES
- 1.Arens R, Sin S, McDonough JM, et al. Changes in upper airway size during tidal breathing in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2005;171(11):1298–1304. 10.1164/rccm.200411-1597OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736. 10.1093/sleep/32.6.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li AM, So HK, Au CT, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010;65(11):991–997. 10.1136/thx.2010.134858 [DOI] [PubMed] [Google Scholar]
- 4.Venekamp RP, Hearne BJ, Chandrasekharan D, Blackshaw H, Lim J, Schilder AG. Tonsillectomy or adenotonsillectomy versus non-surgical management for obstructive sleep-disordered breathing in children. Cochrane Database Syst Rev. 2015:CD011165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 6.Friedman M, Wilson M, Lin HC, Chang HW. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2009;140(6):800–808. 10.1016/j.otohns.2009.01.043 [DOI] [PubMed] [Google Scholar]
- 7.Nolan J, Brietzke SE. Systematic review of pediatric tonsil size and polysomnogram-measured obstructive sleep apnea severity. Otolaryngol Head Neck Surg. 2011;144(6):844–850. 10.1177/0194599811400683 [DOI] [PubMed] [Google Scholar]
- 8.Pierce B, Brietzke S. Association of Preoperative, Subjective Pediatric Tonsil Size With Tonsillectomy Outcomes: A Systematic Review. JAMA Otolaryngol Head Neck Surg. 2019;145(9):854. 10.1001/jamaoto.2019.1842 [DOI] [PubMed] [Google Scholar]
- 9.Van Holsbeke C, Vos W, Van Hoorenbeeck K, et al. Functional respiratory imaging as a tool to assess upper airway patency in children with obstructive sleep apnea. Sleep Med. 2013;14(5):433–439. 10.1016/j.sleep.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 10.Yanagisawa-Minami A, Sugiyama T, Iwasaki T, Yamasaki Y. Primary site identification in children with obstructive sleep apnea by computational fluid dynamics analysis of the upper airway. J Clin Sleep Med. 2020;16(3):431–439. 10.5664/jcsm.8224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27(5):997–1019. 10.1093/sleep/27.5.997 [DOI] [PubMed] [Google Scholar]
- 12.Suga H, Iwasaki T, Mishima K, Nakano H, Ueyama Y, Yamasaki Y. Evaluation of the effect of oral appliance treatment on upper-airway ventilation conditions in obstructive sleep apnea using computational fluid dynamics [published online ahead of print, 2019 Mar 31]. Cranio. 2019;1–9. 10.1080/08869634.2019.1596554. [DOI] [PubMed] [Google Scholar]
- 13.Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med. 1997;127(8 Pt 1):581–587. 10.7326/0003-4819-127-8_Part_1-199710150-00001 [DOI] [PubMed] [Google Scholar]
- 14.Muto T, Takeda S, Kanazawa M, Yamazaki A, Fujiwara Y, Mizoguchi I. The effect of head posture on the pharyngeal airway space (PAS). Int J Oral Maxillofac Surg. 2002;31(6):579–583. 10.1054/ijom.2002.0279 [DOI] [PubMed] [Google Scholar]
- 15.Major MP, Witmans M, El-Hakim H, Major PW, Flores-Mir C. Agreement between cone-beam computed tomography and nasoendoscopy evaluations of adenoid hypertrophy. Am J Orthod Dentofacial Orthop. 2014;146(4):451–459. 10.1016/j.ajodo.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki T, Sato H, Suga H, et al. Relationships among nasal resistance, adenoids, tonsils, and tongue posture and maxillofacial form in Class II and Class III children. Am J Orthod Dentofacial Orthop. 2017;151(5):929–940. 10.1016/j.ajodo.2016.10.027 [DOI] [PubMed] [Google Scholar]
- 17.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36(6):1551–1569. 10.1016/S0031-3955(16)36806-7 [DOI] [PubMed] [Google Scholar]
- 18.Friedman M, Tanyeri H, La Rosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109(12):1901–1907. 10.1097/00005537-199912000-00002 [DOI] [PubMed] [Google Scholar]
- 19.Warren DW, Hairfield WM, Seaton DL, Hinton VA. The relationship between nasal airway cross-sectional area and nasal resistance. Am J Orthod Dentofacial Orthop. 1987;92(5):390–395. 10.1016/0889-5406(87)90259-9 [DOI] [PubMed] [Google Scholar]
- 20.Yajima Y, Oshima M, Iwai T, Kitajima H, Omura S, Tohnai I. Computational fluid dynamics study of the pharyngeal airway space before and after mandibular setback surgery in patients with mandibular prognathism. Int J Oral Maxillofac Surg. 2017;46(7):839–844. 10.1016/j.ijom.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 21.Shirazawa Y, Iwasaki T, Ooi K, et al. Relationship between pharyngeal airway depth and ventilation condition in mandibular setback surgery: A computational fluid dynamics study. Orthod Craniofac Res. 2020;23(3):313–322. 10.1111/ocr.12371 [DOI] [PubMed] [Google Scholar]
- 22.Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164(4):698–703. 10.1164/ajrccm.164.4.2101127 [DOI] [PubMed] [Google Scholar]
- 23.Chen J, He S. Drug-induced sleep endoscopy-directed adenotonsillectomy in pediatric obstructive sleep apnea with small tonsils. PLoS One. 2019;14(2):e0212317. 10.1371/journal.pone.0212317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki T, Takemoto Y, Inada E, et al. The effect of rapid maxillary expansion on pharyngeal airway pressure during inspiration evaluated using computational fluid dynamics. Int J Pediatr Otorhinolaryngol. 2014;78(8):1258–1264. 10.1016/j.ijporl.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 25.Jones AS, Lancer JM. Rhinomanometry. Clin Otolaryngol Allied Sci. 1987;12(3):233–236. 10.1111/j.1365-2273.1987.tb00193.x [DOI] [PubMed] [Google Scholar]
- 26.Clarke RW, Jones AS. The limitations of peak nasal flow measurement. Clin Otolaryngol Allied Sci. 1994;19(6):502–504. 10.1111/j.1365-2273.1994.tb01277.x [DOI] [PubMed] [Google Scholar]