Abstract
Study Objectives:
There is evidence that sleep deprivation or diseases such as obstructive sleep apnea (OSA) that lead to sleep disruption may adversely impact immune system functioning. We hypothesized that individuals who have OSA who did not use continuous positive airway pressure (CPAP) would have higher rates of hospitalization and complications from influenza infection than patients with OSA who were adherent to treatment.
Methods:
Medical records of patients at Dartmouth-Hitchcock Medical Center in Lebanon, New Hampshire who had both OSA and a new, laboratory-confirmed influenza infection between 2016 and 2018 were reviewed for results of polysomnography, CPAP usage, influenza vaccination records, confirmation of influenza infection, and influenza-related hospitalizations and complications.
Results:
Compared to the patients who were adherent to CPAP, patients who were either conservatively treated without CPAP or who were nonadherent to CPAP therapy had higher odds of hospitalization from influenza infections (odds ratio = 4.7, 95% confidence interval 1.3 to 19.5, P = .01) but no higher odds of complications from influenza. The patients who had untreated sleep apnea had a higher percentage of influenza vaccination for their season of illness (75% of patients) compared to patients who were adherent to CPAP (56% of patients), although the difference in vaccination was not statistically significant.
Conclusions:
Patients with OSA who did not use CPAP appear to have greater rates of hospitalization from acute influenza infection, despite having a higher trend of influenza vaccination compared to patients who were adherent to CPAP.
Citation:
Mok EM, Greenough G, Pollack CC. Untreated obstructive sleep apnea is associated with increased hospitalization from influenza infection. J Clin Sleep Med. 2020;16(12):2003–2007.
Keywords: obstructive sleep apnea, CPAP adherence, influenza, vaccine
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is evidence that sleep disruption can lead to impaired immune function. Little is known about how diseases such as obstructive sleep apnea that disrupt sleep affect clinical outcomes in respiratory infections such as influenza.
Study Impact: The results of this study suggest that nonadherence to continuous positive airway pressure for treatment of obstructive sleep apnea may increase hospitalizations from influenza infection.
INTRODUCTION
Sleep can influence many organ systems, including the immune system.1 Shared hormones, cytokines, and neurotransmitters regulate both sleep and immunity.2 There is emerging evidence that sleep deprivation or sleep disruption may adversely impact the functioning of the immune system.3 Studies have shown that poor sleep efficiency and sleep deprivation have been linked to susceptibility to respiratory viral infections.4,5 There is also evidence that decreased sleep duration and quality can negatively influence one’s response to various vaccines.6–9
Obstructive sleep apnea (OSA) is a common disorder10,11 that leads to sleep disruption and fragmentation due to arousals from sleep.12 Continuous positive airway pressure (CPAP) can effectively treat OSA and is the primary mode of treatment.13 However, adherence to CPAP remains a challenge, and a significant portion of patients remain inadequately treated and continue to experience the adverse effects of OSA.14
Influenza is a viral respiratory infection that is a major public health concern.15 Influenza infection can lead to complications such as secondary bacterial pneumonia or an exacerbation of underlying pulmonary conditions such as chronic obstructive pulmonary disease, myositis, and neurologic consequences.16 Recently, influenza infection was found to be associated with an increased incidence of acute myocardial infarction.17 Developing strategies to mitigate and control influenza are of the utmost importance.18
The objective of this retrospective cohort study was to determine whether individuals who have OSA but did not use CPAP (ie, perpetuating the effects of sleep disruption) may have an increased susceptibility to complications from influenza infection compared to individuals who were adherent to CPAP treatment.
METHODS
Study group
This is a retrospective review of adult patients over the age of 18 years within the Dartmouth-Hitchcock medical system who had OSA and a new diagnosis of acute influenza infection for the seasons of 2016–2017 or 2017–2018. Study participants were identified by using diagnosis codes for obstructive sleep apnea (G47.33) and influenza (J10.1). Patients were then excluded from analysis if there was either an incomplete or nonexistent diagnostic record of attended polysomnographic or out-of-center sleep testing, no follow-up CPAP download data within 2 years prior to influenza diagnosis, no laboratory confirmed influenza infection, or a predominance of central sleep apnea on diagnostic polysomnographic data.
Data collection
Medical records were reviewed to extract patient demographics, OSA severity through the apnea-hypopnea index (AHI), adherence to the use of CPAP, laboratory confirmation of influenza, influenza type, influenza complications, and whether the patient was hospitalized.
Adherence to CPAP was defined as at least 70% nights of usage with minimum 4 hours per night on CPAP data download. Nonadherence was defined as usage less than the aforementioned criteria or having never been prescribed CPAP. Complications were considered to be sepsis, pneumonia, acute respiratory failure, chronic obstructive pulmonary disease or asthma exacerbation, development of unstable arrythmia (eg, rapid atrial fibrillation), and development of myocardial infarction during hospitalization. The hypopnea definition varied according to the center and type of study that was conducted, a polysomnogram, or unattended home test.
The available vaccination records of the patients were also reviewed. If there was a documented influenza vaccine administered between the months of September and January immediately prior to the date of influenza diagnosis, the patient was considered to be immunized. If records showed a patient had a vaccine in a previous year but not in the season in which the patient was diagnosed with influenza, the patient was recorded as not having received an influenza vaccine. Patients that had no documentation of any influenza vaccine for any year was recorded as unknown.
The study was approved by Dartmouth-Hitchcock Committee for the Protection of Human Subjects.
Statistical analysis
Demographic and baseline characteristics were summarized using means and standard deviation for quantitative variables and percentages for categorical variables. Student t tests and Fisher exact tests were used for comparison of continuous and categorical variable, respectively. Hospitalizations were compared between the patients who were nonadherent and the patients who were adherent to CPAP using a Fisher exact test. Odds ratios and corresponding 95% confidence intervals were calculated to evaluated possible associations between nonadherence to CPAP and hospitalizations. Additional Fisher exact tests were used to compare the presence of complications from influenza infection between these groups. A priori, a P value of < .05 was considered significant. R version 3.6.1 was used for statistical analysis.
RESULTS
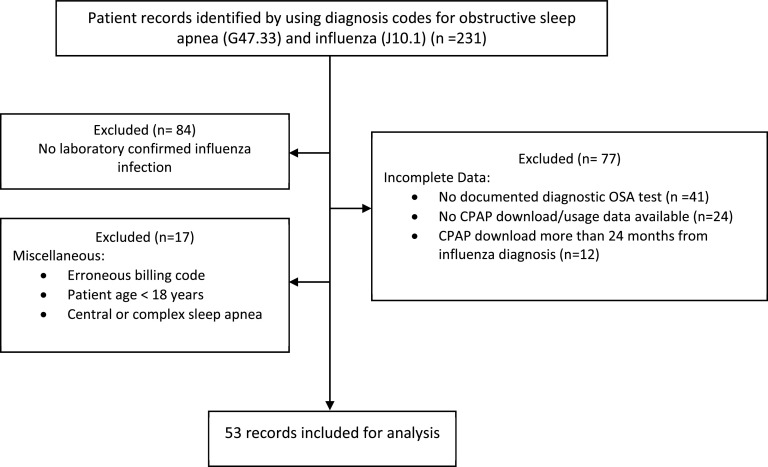
A total of 231 medical records was reviewed. Out of these, 147 had confirmed laboratory testing of acute influenza infection, either with polymerase chain reaction or through rapid antigen testing. Of these, patient charts were eliminated due to incomplete diagnostic testing of obstructive sleep apnea, no availability of CPAP download records, presence of central or complex sleep apnea, age less than 18 years, and for other miscellaneous reasons. A summary is provided in Figure 1. A total of 53 charts was used for analysis.
Figure 1. Flow diagram illustrating exclusions.
CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea.
Of these 53 charts, 28 patients were categorized as nonadherent to CPAP treatment and 25 patients were categorized as adherent to CPAP treatment. The average time between CPAP download and influenza diagnosis was 5.5 months. Patient characteristics, including sex, age, body mass index, were similar (Table 1). Patients who were not adherent to CPAP treatment had higher odds of hospitalization with influenza (odds ratio 4.7, 95% confidence interval 1.3–19.5, P = .01). However, there was no significant higher occurrences of complications from influenza (P = .10). Descriptive statistics from hospitalizations and complications from influenza are listed in Table 2.
Table 1.
Patient characteristics.
| Baseline Characteristics | Nonadherent to CPAP (n = 28) | Adherent to CPAP (n = 25) | P Value |
|---|---|---|---|
| Age, years, mean (SD) | 62.7 (11.5) | 59.5 (12.8) | .35 |
| Sex, n (%) | 1.0 | ||
| Female | 13 (46%) | 12 (48%) | |
| Male | 15 (54%) | 13 (52%) | |
| BMI, mean (SD) | 34.8 (8.3) | 35.3 (8.0) | .81 |
| AHI, mean (SD) | 30.8 (23.5) | 44.9 (34.0) | .09 |
| Influenza type, n (%) | .44 | ||
| A | 20 (71%) | 21 (84%) | |
| B | 4 (14%) | 4 (16%) | |
| A&B | 2 (7%) | 0 | |
| Unknown | 2 (7%) | 0 | |
| Tamiflu as treatment, n (%) | .49 | ||
| Did not receive | 4 (14%) | 6 (24%) | |
| Received | 24 (86%) | 19 (76%) | |
| Influenza vaccine (administered prior to the season), n (%) | .28 | ||
| No recorded vaccine administration | 4 (14%) | 8 (32%) | |
| Received | 21 (75%) | 14 (56%) | |
| Unknown | 3 (11%) | 3 (12%) |
AHI = apnea-hypopnea index, BMI = body mass index, SD = standard deviation.
Table 2.
Hospitalizations and complications.
| Nonadherent to CPAP (n = 28) | Adherent to CPAP (n = 25) | P Value | |
|---|---|---|---|
| Hospitalized, n (%) | 17 (61%) | 6 (24%) | .01 |
| Length of hospital stay in days, mean (SD) | 4.7 (5.5) | 4.7 (2.8) | .99 |
| Total number of patients with complications | 14 | 7* | .10 |
| Complication characteristics | |||
| Asthma exacerbation | 3 | - | |
| COPD exacerbation/acute bronchiolitis | 4 | 3^^ | |
| Acute respiratory failure/hypoxemia | 2 | - | |
| Pneumonia | 2 | 1 | |
| Sepsis/septic shock | 2^ | 1^^ | |
| Rapid atrial fibrillation | - | 1 | |
| NSTEMI | 1^ | 2 | |
| STEMI | 1 | - |
One patient with NSTEMI was not hospitalized. ^One patient noted to have both sepsis and demand ischemia. ^^One patient noted to have both sepsis and COPD exacerbation. COPD = chronic obstructive pulmonary disease, NSTEMI = non-ST segment elevated myocardial infarction, SD = standard deviation, STEMI = ST segment elevated myocardial infarction.
Of the patients who were nonadherent to CPAP, patients were categorized into mild (AHI 5–14 events/h), moderate (AHI 15–29 events/h), and severe (AHI above 30 events/h) OSA categories. There were no significant differences in hospitalizations among these groups (P = .13).
Documented vaccination rates were higher for patients that were nonadherent to CPAP (21 of 28 patients, 75%) compared to those that were adherent to CPAP (14 of 25 patients, 56%), although this difference did not reach statistical significance (P = .28).
Five (21%) of the 24 records excluded because of lack of CPAP download records had documented admission for influenza infection.
DISCUSSION
In this study, we found that patients with OSA who did not use CPAP had nearly 5 times higher odds of hospitalization due to influenza compared to patients that were adherent to CPAP therapy, despite having a trend of higher percentages of influenza vaccination. Although there was no significance found between the complication rates between the 2 groups, there was also a trend of higher complications in patients who were not adherent to CPAP. The findings of this study reiterate the importance of CPAP adherence for treatment of OSA for prevention of adverse consequences of untreated OSA. In addition, the findings also suggest support for the concept that sleep is essential for optimal immune function in the context of viral respiratory infection.
To our knowledge, this is the first study to examine the significance of untreated OSA and its association with clinical influenza infection. Other studies have examined the effect of OSA or sleep deprivation on the immune response to influenza vaccination. Dopp et al19 showed that patients with moderate to severe untreated OSA failed to demonstrate differences in the antibody response to influenza vaccine compared to normal controls without OSA. The study, however, was small, as it involved only 14 patients. Other studies have examined the effect of sleep loss on the development of an antibody response to vaccines in healthy human volunteers. Speigel et al8 demonstrated that sleep-restricted patients (11 patients) after influenza vaccination produced less than half of the antibody titers of control non-sleep-deprived patients (14 patients). A similar finding of sleep deprivation leading to a reduced response to a hepatitis A vaccine was found by Lange et al.6 In a community-based sample, short sleep duration was associated with decreased protection from hepatitis B after immunization.7 In a mouse study by Brown and colleagues,20 mice were orally vaccinated against influenza and then rechallenged by live virus intranasally before sleep deprivation. While the normal sleep mice achieved virus clearance, the sleep-deprived mice behaved as though they had not been immunized.
Given that most of these aforementioned studies would suggest that sleep deprivation blunts the immune response to vaccination, it is possible that the increased rate of hospitalization seen after influenza infection in patients with untreated OSA in our study group was related to this phenomenon. While most of our nonadherent CPAP group patients had received an influenza vaccine, it is possible that they did not mount an adequate antibody response to the vaccine because of their poor sleep.
The issues of how sleep loss, sleep disruption, and diseases, such as OSA that cause sleep disruption may affect immune response and vaccine efficacy, are complex.21 Additionally, vaccine efficacy is highly variable between individuals in clinical practice,22 complicating these questions. Additional research will be needed to elucidate these topics.
In addition to possibly adversely affecting immune function, it is possible that untreated OSA may also increase the severity of influenza infection due to its effects on supraglottic and upper airway edema,23 as the influenza virus also causes inflammation of the upper respiratory tree.24 This may account for the finding of higher odds of hospitalization between the nonadherent and adherent groups in this study.
Whatever the etiology of the increased rate of hospitalization among patients with untreated OSA, our study would suggest treatment of OSA with CPAP may help reduce the chance of hospitalization after influenza infection among patients with OSA. Given the significant morbidity and mortality associated with influenza infection worldwide,18 improving adherence to CPAP and thereby avoiding hospitalization could result in significant cost savings to global health systems.
There are several limitations of our study. First, this is a retrospective study with a small number of patients. Second, as the data were collected based on medical records, data on confounding variables (eg, sleep duration) were limited. A large percentage of the records reviewed were excluded from the study due to incomplete availability of data. This included 24 records in which a patient with confirmed influenza underwent a diagnostic study but had no available records of clinical follow up or CPAP usage data. The frequency of hospitalization among these patients for influenza was 21% (n = 5), which is statistically equivalent to the rate of hospitalization for the CPAP-adherent group. If these patients were assumed to be nonadherent to CPAP, this would suggest that the demonstrated risk of hospitalization in this study is an overestimation of the true odds ratio.
Additionally, whether or not a patient received an influenza vaccine for the season of their infection was also presumed based on available documentation. It is possible that patients who were recorded as not having received the vaccine may have been immunized at another unaffiliated facility or from another community source (such as a local pharmacy), in which records may not have been readily available.
Additional investigation and larger prospective studies on OSA and influenza infection are necessary to validate our findings.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continue positive airway pressure
- OSA
obstructive sleep apnea
REFERENCES
- 1.Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4(6):457–467. 10.1038/nri1369 [DOI] [PubMed] [Google Scholar]
- 2.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463(1):121–137. 10.1007/s00424-011-1044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilder-Smith A, Mustafa FB, Earnest A, Gen L, Macary PA. Impact of partial sleep deprivation on immune markers. Sleep Med. 2013;14(10):1031–1034. 10.1016/j.sleep.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169(1):62–67. 10.1001/archinternmed.2008.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prather AA, Janicki-Deverts D, Hall MH, Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38(9):1353–1359. 10.5665/sleep.4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65(5):831–835. 10.1097/01.PSY.0000091382.61178.F1 [DOI] [PubMed] [Google Scholar]
- 7.Prather AA, Hall M, Fury JM, et al. Sleep and antibody response to hepatitis B vaccination. Sleep (Basel). 2012;35(8):1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288(12):1471–1472. 10.1001/jama.288.12.1469 [DOI] [PubMed] [Google Scholar]
- 9.Benedict C, Brytting M, Markström A, Broman JE, Schiöth HB. Acute sleep deprivation has no lasting effects on the human antibody titer response following a novel influenza A H1N1 virus vaccination. BMC Immunol. 2012;13(1):1. 10.1186/1471-2172-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289(17):2230–2237. 10.1001/jama.289.17.2230 [DOI] [PubMed] [Google Scholar]
- 11.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. 10.1152/physrev.00043.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman N. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1125–1137. 10.1016/B978-0-323-24288-2.00115-X [DOI] [Google Scholar]
- 14.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1):43. 10.1186/s40463-016-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uyeki TM. Influenza. Ann Intern Med. 2017;167(5):ITC33–ITC48. 10.7326/AITC201709050 [DOI] [PubMed] [Google Scholar]
- 16.Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med. 2010;38(4, Suppl):e91–e97. 10.1097/CCM.0b013e3181c92eeb [DOI] [PubMed] [Google Scholar]
- 17.Kwong JC, Schwartz KL, Campitelli MA, et al. Acute Myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345–353. 10.1056/NEJMoa1702090 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . Global Influenza Strategy 2019-2030. https://www.who.int/influenza/global_influenza_strategy_2019_2030/en/. Accessed November 16, 2019.
- 19.Dopp JM, Wiegert NA, Moran JJ, Muller D, Weber S, Hayney MS. Humoral immune responses to influenza vaccination in patients with obstructive sleep apnea. Pharmacotherapy. 2007;27(11):1483–1489. 10.1592/phco.27.11.1483 [DOI] [PubMed] [Google Scholar]
- 20.Brown R, Pang G, Husband AJ, King MG. Suppression of immunity to influenza virus infection in the respiratory tract following sleep disturbance. Reg Immunol. 1989;2(5):321–325. [PubMed] [Google Scholar]
- 21.Opp M, Krueger J. Sleep and host defense. In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed., Philadelphia, PA: Elsevier; 2017: 193–201. 10.1016/B978-0-323-24288-2.00019-2 [DOI] [Google Scholar]
- 22.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2):e00084-e18. 10.1128/CMR.00084-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fogel RB, Malhotra A, White DP. Sleep. 2: pathophysiology of obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(2):159–163. 10.1136/thorax.2003.015859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3(1):499–522. 10.1146/annurev.pathmechdis.3.121806.154316 [DOI] [PMC free article] [PubMed] [Google Scholar]