Abstract
Triple‐negative breast cancer (TNBC) is a type of breast cancer that has a higher risk of distant recurrence and metastasis, leading to a relatively aggressive biological behaviour and poor outcome. So far, the clinical management of TNBC is challenging because of its heterogeneity and paucity of specific targeted therapy. Recently, various studies have identified a lot of differently expressed long non‐coding RNAs (lncRNAs) in TNBC. Those lncRNAs have been reported to play important roles in the multistep process of TNBC tumorigenesis. Here, we review the biological characteristics of lncRNAs, and present the current state of knowledge concerning the expression, function and regulation of lncRNAs in TNBC. Accumulating studies explored the potential lncRNAs‐based therapeutics in TNBC, including the techniques of genetic modification using antisense oligonucleotides, locked nucleic acid and RNA nanotechnology. In current review, we also discuss the future prospects of studies about lncRNAs in TNBC and development of lncRNA‐based strategies for clinical TNBC patients.
Keywords: biomarkers, LncRNA, LncRNA‐based therapeutics, prognosis, triple‐negative breast cancer
In current review, we accumulated literature to the understanding of lncRNAs biogenesis and function, as well as the latest findings of novel lncRNAs‐based therapeutics in TNBC. We also present the current state of knowledge concerning the expression and regulation of lncRNAs in TNBC, and discuss the future development of lncRNA‐based strategies for clinical TNBC patients.
1. INTRODUCTION
Breast cancer (BC) is the most frequently diagnosed malignancy and the leading cause of cancer death in females worldwide. 1 Triple‐negative breast cancer (TNBC) is a subgroup of breast cancers that lack the expression of oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER‐2). 2 The risk of distant recurrence and metastasis in TNBC patients is substantially higher than in non‐TNBC patients. 3 The clinical management of TNBC is challenging because of the relatively aggressive biologic behaviour and paucity of specific targeted therapy. 4 Thus, a better understanding of the regulations and mechanisms of tumorigenesis in TNBC cells and the identification of effective biomarkers for diagnosis and prognosis of TNBC patients are consequently keenly awaited.
LncRNAs, with a length exceeding 200 nucleotides, are non‐protein‐coding transcripts. 5 Accumulating studies report that lncRNAs expression is dysregulated in various types of cancer including breast cancer, ovarian cancer, hepatocellular carcinoma and many others. 6 , 7 , 8 , 9 , 10 Moreover, several lncRNAs have been reported to play crucial roles in various biological processes, including cell proliferation, apoptosis, invasion, differentiation and development. 11 , 12 , 13 , 14 In TNBC, various studies have identified a lot of dysregulated lncRNAs that play important roles in the process of tumorigenesis through diverse mechanisms. For instance, lncRNAs can act as miRNA ‘sponges’ and compete miRNA‐targeted mRNAs, thereby affecting the miRNA‐mediated gene regulation. 15 , 16 This competing endogenous RNAs (ceRNA) mechanisms and network construction, by sequestering miRNAs and sparing their protein‐coding counterparts from post‐translational regulation, have been mainly studied to act as the main molecular mechanism of lncRNA biological function. 15 Some lncRNAs were reported to assemble with mRNAs to protect them from miRNA action and increase their stability. Some lncRNAs are named scaffold lncRNAs, which could serve as a central platform to assemble with different molecular components such as proteins and RNAs and promote their intermolecular interactions. Moreover, signal lncRNAs have also been reported to interact with transcription factors (TFs) or histone‐modifying enzymes to cis‐regulate or trans‐regulate the expression of their target genes. 8 Thus, lncRNAs promise potential diagnostic and prognostic biomarkers, therapeutic targets and improve the clinical benefits for TNBC patients.
Accumulating studies have explored the potential lncRNAs‐based therapeutics in TNBC, including the techniques of genetic modification using antisense oligonucleotides (ASOs), locked nucleic acid (LNA) and RNA nanotechnology. Such as, Jin et al designed eight ASOs targeting LncRNA TROJAN and transfected TNBC cells with ASOs without using any transfection reagents to simulate in vivo conditions. They observed that lung metastasis nodules were significantly reduced in anti‐TROJAN ASO‐treated group than the control group, and the ASO toxicity was limited after detecting the murine blood biochemical indexes. 17 Hu et al reported that treatment with LINK‐A LNAs could repress cell proliferation in TNBC cells and increase the sensitivity of mammary gland tumours to immunotherapy. 18 In current review, we accumulated literature to the understanding of lncRNAs biogenesis and function, as well as the latest findings of novel lncRNAs‐based therapeutics in TNBC. We also present the current state of knowledge concerning the expression and regulation of lncRNAs in TNBC, and discuss the future development of lncRNA‐based strategies for clinical TNBC patients.
2. BIOLOGICAL CHARACTERISTICS OF LNCRNAS
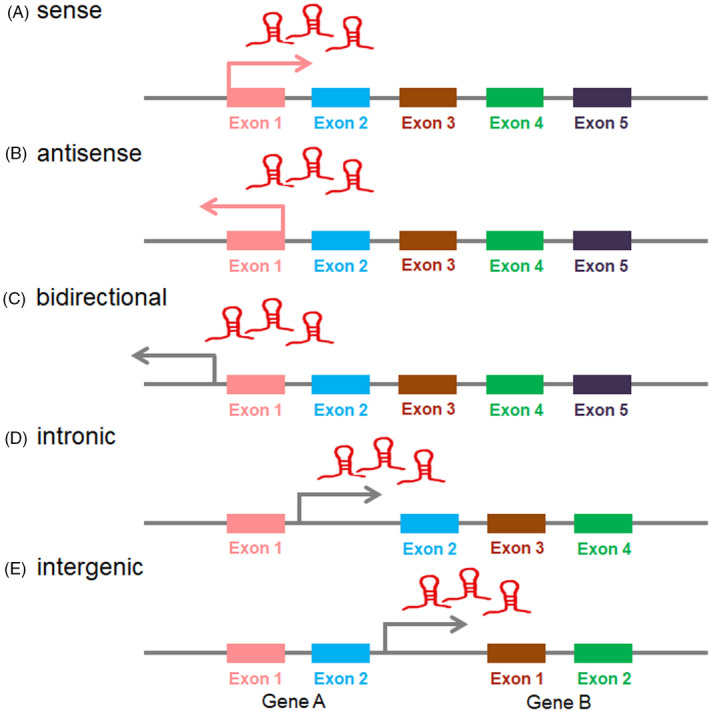
LncRNAs are functionally defined as transcripts >200 nt in length with no protein‐coding potential, many of which are uniquely expressed in differentiated tissues or specific cancer types. 19 Distinguishing lncRNAs from other protein‐coding mRNAs is not a trivial process. H19, the first lncRNA reported by Brannan et al in 1990, was just defined as not a classical mRNA, and the product of H19 gene was described to be an RNA molecule. 20 In fact, lncRNAs were first described during the large‐scale sequencing of full‐length cDNA libraries in the mouse. 21 The number of lncRNAs was reported to outnumber protein‐coding genes, and their sequences cover a larger fraction of the human genome. 22 LncRNAs may be located within nuclear or cytosolic fractions, and are overlapping with, or interspersed between, multiple coding and non‐coding transcripts. 23 , 24 Based on their genomic proximity to neighbouring transcripts, they are classified five categories (Figure 1): (a) sense, overlapping one or more exons of a protein‐coding gene on the same strand; (b) antisense, overlapping one or more exons of a protein‐coding gene on the opposite strand; (c) bidirectional, initiating its expression in close genomic proximity at <1000 base pairs away to a neighbouring coding transcript on the opposite strand; (d) intronic, deriving from an intron of a second transcript; or (e) intergenic, acting as an independent unit within the genomic interval between two genes. 25 , 26 LncRNAs were initially thought to be the products of an inconsequential transcription resulting from low RNA polymerase fidelity. 27 It is now widely recognized that lncRNAs could identify cellular pathologies such as cancer, provide prognostic value, or even inform therapeutic options for cancer patients, by serving as signals of specific cellular states or readouts of active cellular programmes. 28 Recent studies have shown that lncRNAs can regulate gene expression at different levels, including chromatin modification, transcription and post‐transcriptional regulation. 29 LncRNAs were reported to regulate several biological processes such as cell proliferation, apoptosis, cell cycle, cell invasion and metastasis, cellular differentiation, chromatin modification and nuclear‐cytoplasmic trafficking. 30 It has been suggested that the involvement of lncRNAs in human diseases could be far more prevalent than previously known. 31 Recently, lncRNAs‐related studies in cancer increased dramatically and have become one of the hottest topics in RNA biology.
FIGURE 1.
Classification of lncRNAs based on their genomic proximity to neighbouring transcripts
3. PROFILES OF LNCRNAS EXPRESSION IN TNBC
Recently, abnormal expression of many lncRNAs has been found in almost all tumours in humans, including TNBC. However, our understanding of lncRNAs remains significantly less mature than mRNAs, or even miRNAs. Next generation sequencing (NGS) is a DNA sequencing technology, which could perform sequencing of millions of small fragments of DNA in parallel. These fragments are then pieced together by mapping the individual reads to the human reference genome. 32 NGS is now used to sequence entire genomes or constrained to specific areas of interest to get the population genomic and gene expression differences in a large array of organisms. 32 Thus, NGS technologies may help researchers to accelerate the identification and characterization of important, yet‐to‐be‐annotated functional lncRNAs in TNBC.
In recent years, researchers got a lot of abnormally expressed lncRNAs in TNBC patients or cells using public databases based on the NGS technologies. Tian et al found a total of 1034 dysregulated lncRNAs in the two TNBC microarrays from the Gene Expression Omnibus (GEO) database. Among them, 537 lncRNAs were significantly correlated with 451 protein‐coding genes, which were mainly enriched in terms including cell division, cell cycle, and involved in PI3K‐Akt, MAPK, ErbB family and p53 signalling pathways. 33 In addition, further literatures related to the lncRNA expressions profiles also detected a series of dysregulated lncRNAs in TNBC. 34 , 35 , 36 , 37 , 38 , 39 , 40
Previous studies have shown that lncRNAs can act as miRNA ‘sponges’ and compete miRNA‐targeted mRNAs, thereby affecting the miRNA‐mediated gene regulation. 15 , 16 This crosstalk forms a complex post‐transcriptional regulatory network including mRNAs, lncRNAs, called competing endogenous RNAs (ceRNA) network. 15 , 16 ceRNA‐mediated regulatory mechanisms are reported to be an important pathway for lncRNAs‐modulated post‐transcriptional regulation in TNBC. Such as, Le et al developed a complex ceRNA network in TNBC using microarray mRNA and lncRNA expression data obtained from The Cancer Genome Atlas (TCGA) database and two GEO databases. 41 As a result, they identified differentially expressed 4565 mRNAs, 427 miRNAs and 4852 lncRNAs, and constructed ceRNA network using 37 lncRNAs, 28 miRNAs and 16 mRNAs. On the basis of establishing the ceRNA network, they found that two mRNAs expression are correlated with prognosis of TNBC patients. 41 Similarly, Liu et al also constructed a ceRNA network based on analysis of differentially expressed RNAs between 150 TNBC tissues and 823 non‐TNBC tissues downloaded from TCGA database. 42 They identified 190 differentially expressed lncRNAs, 48 differentially expressed mRNAs and 13 differentially expressed miRNAs in this ceRNA network. They concluded that eight lncRNAs and one mRNA could act as prognostic factors in TNBC, using survival analysis and receiver operating characteristic (ROC) curve creation in the network. 42 Additionally, they found that lncRNA OSTN‐AS1 was primarily related to immunologic function, including immune cell infiltration and immune‐related markers co‐expression. 42 Song et al also constructed a ceRNA network of TNBC using TCGA database and revealed 686 mRNAs, 26 miRNAs and 50 lncRNAs as key molecules for high risk of TNBC. 43 At the same time, the ceRNA crosstalk network of TNBC constructed by Yuan et al contains 22 hub mRNAs, and 14 key differentially expressed lncRNAs. 44 Jiang et al developed an integrated ceRNA network signature based on three mRNA (FCGR1A, RSAD2, CHRDL1) and two lncRNA (HIF1A‐AS2 and AK124454), using transcriptome microarrays for 33 paired TNBC and adjacent normal breast tissue. 45 They also found that the prognostic and predictive accuracy of this ceRNA signature was better than clinicopathological parameters to predict tumour recurrence and the benefit of taxane chemotherapy in TNBC. 45 Taken together, the ceRNA co‐regulatory network could help us understand the potential characteristics of biological function and pathological roles of lncRNAs in the development and progression of TNBC.
4. ROLES OF LNCRNAS IN TNBC
To date, numerous lncRNAs have been identified to dysregulated express and play an important role in the biological function of TNBC, including cellular proliferation, apoptosis, cell cycle, migration, invasion, angiogenesis and drug resistance (Table 1). In this chapter, we will provide an overview of lncRNA biological function in TNBC (Figure 2).
TABLE 1.
Identified lncRNAs in TNBC
| lncRNAs | Expression | Biological function | Potential Targets | References |
|---|---|---|---|---|
| GAS5 | Down | Inhibit cell proliferation and invasion; Promote cell apoptosis; Inhibit paclitaxel, cisplatin, adriamycin and PI3K/mTOR inhibitor resistance | miR‐378a‐5p/SUFU, miR‐196a‐5p | 47, 48, 49, 108 |
| LINC02095 | Up | Promote cell proliferation | SOX9 | 55 |
| HOTAIR | Up | Promote cell migration and invasion; imatinib and lapatinib resistance | miR‐148a; LEF1/TCF4 | 92, 93, 109 |
| WT1‐AS | Down | Inhibit cell migration and invasion | TGF‐β1 | 96 |
| LINC00096 | Up | Promote cell proliferation and invasion | miR‐383‐5p/RBM3 | 50 |
| DRHC | Down | Inhibit cell proliferation | HOTAIR | 60 |
| HEIH | Up |
Promote cell proliferation Inhibit cell apoptosis |
miR‐4458/SOCS1 | 51 |
| LUCAT1 | Up | Promote cell proliferation, cell cycle progression and metastasis; Inhibit cell apoptosis | miR‐5702 | 64 |
| CCAT1 | Up | Promote cell proliferation, migration, and invasion | miR‐218/ZFX | 77 |
| ASRPS | Down | Inhibit angiogenesis | STAT3 | 125 |
| HAND2‐AS1 | Down | Inhibit cell proliferation | RUNX2 | 65 |
| LINC01133 | Up | Promote cell stem cell (CSC)‐like phenotypic traits | KLF4 | 119 |
| LINC01096 | Up | Promote cell proliferation, migration, and invasion; Inhibit cell apoptosis | miR‐3130‐3p | 97 |
| PAPAS | Up | Promote cell migration and invasion | miR‐34a | 83 |
| HCP5 | Up | Promote cell proliferation; Inhibit cell apoptosis | miR‐219a‐5p/BIRC3 | 52 |
| NRAD1 | Up | Promote cell proliferation and CSC‐like phenotypic traits | ‐ | 122 |
| LINK‐A | Up | Promote immunotherapy resistance; AKT inhibitors resistance; glycolysis reprogramming | PI3K/GPCR; Akt; HIF1α | 18, 110, 127 |
| MIR503HG | Down | Inhibit cell migration and invasion | miR‐103/OLFM4 | 78 |
| AWPPH | Up | Promote cell proliferation; Promote carboplatin resistance | miR‐21; FZD7 | 63, 112 |
| PTCSC3 | Down | Inhibit cell proliferation | H19 | 61 |
| NRON | Down | Inhibit cell proliferation | snaR | 62 |
| sONE | Down | Inhibit cell proliferation, migration, and invasion | miR‐34a/15a/16, let‐7a, TP53/c‐Myc; NOS3 | 84, 85 |
| NAMPT‐AS | Up | Promote cell metastasis | miR‐548b‐3p/ NAMPT | 79 |
| DANCR | Up | Promote cell proliferation and invasion, and CSC‐like phenotypic traits | miR‐216a‐5p; RXRA; EZH2, CD44, ABCG2; Nanog, SOX2, and OCT4 | 56, 98, 120 |
| NEAT1 | Up | Inhibit cell apoptosis; Promote cell cycle progression; Promote cisplatin and paclitaxel resistance and cancer stemness | ‐ | 75 |
| TROJAN | Up | Promote cell proliferation and invasion | ZMYND8 | 17 |
| POU3F3 | Up | Promote cell proliferation; Inhibit cell apoptosis | Caspase‐9 | 66 |
| NEF | Down | Inhibit cell migration and invasion | miR‐155 | 99 |
| ZEB2‐AS1 | Up | Promote cell proliferation, metastasis and EMT | ZEB2 | 88 |
| LncKLHDC7B | Down | Inhibit cell migration and invasion; Promote cell apoptosis | KLHDC7B | 100 |
| HIF1A‐AS2 | Up | Promote cell migration and invasion | ‐ | 101 |
| LINC00339 | Up | Promote cell proliferation; Inhibit cell cycle arrest, apoptosis | miR‐377‐3p/HOXC6 | 53 |
| LINC00152 | Up | Promote cell proliferation and invasion; Inhibit cell apoptosis | PTEN, BRCA1 | 57, 58 |
| AFAP1‐AS1 | Up | Promote EMT | Wnt/β‐catenin | 81 |
| PDCD4‐AS1 | Down | Inhibit cell proliferation and migration | PDCD4 | 59 |
| HOST2 | Down | Inhibit cell proliferation | let‐7b/CDK6 | 54 |
| BORG | Up | Promote doxorubicin resistance | RPA1 | 115 |
| PVT1 | Up | Promote cell proliferation and migration, EMT | p21, KLF5/β‐catenin | 89, 90 |
| H19 | Up | Promote paclitaxel resistance and CSC‐like phenotypic traits | Akt | 114, 123 |
| TP73‐AS1 | Up | Promote cell vasculogenic mimicry | miR‐490‐3p/TWIST1 | 126 |
| TUG1 | Down | Enhance cisplatin sensitivity | miR‐197/NLK | 113 |
| MIR100HG | Up | Promote cell proliferation, Inhibit cell cycle arrest | p27 | 72 |
| LINC01638 | Up | Promote cell proliferation, metastasis and CSC‐like phenotypic traits | c‐Myc | 121 |
| ARNILA | Down | Promote EMT, invasion and metastasis | miR‐204/SOX4 | 91 |
| LINC‐ZNF469‐3 | Up | Promote cell invasion, stemness properties and lung metastasis | miR‐574‐5p/ZEB1 | 87 |
| ROR | Up | Promote cell invasion and metastasis | miR‐145/MUC1; miR‐145/ARF6 | 102, 103 |
| AIRN | Down | Inhibit cell migration and invasion | Wnt/β‐catenin/mTOR/PI3K | 104 |
| MANCR | Up | Promote cell proliferation, Inhibit DNA damage | ‐ | 128 |
| RMST | Down | Inhibit cell proliferation, invasion and migration; Promote cell apoptosis, and regulate cell cycle. | ‐ | 76 |
| SKAI1BC | Up | Promote cell migration and invasion | KAI1 | 105 |
| ANRIL | Up | Promote cell proliferation, Inhibit cell apoptosis | miR‐199a | 67 |
| MALAT1 | Up | Promote cell proliferation, cell cycle arrest, and invasion; Inhibit cell apoptosis | miR‐129‐5p; miR‐1/Slug; miR‐448/KDM5B | 68, 106, 149 |
| SNHG12 | Up | Promote cell proliferation and migration, Inhibit cell apoptosis | MMP13 | 94 |
| HULC | Up | Promote cell metastasis | MMP‐2, MMP‐9 | 107 |
| SNAR | Up | Promote cell proliferation, migration and invasion | ‐ | 95 |
| LINP1 | Up | Promote DNA DSB repair, and radiotherapy resistance | Ku80 | 117 |
| PCAT6 | Up | radiotherapy resistance | miR‐185‐5p/TPD52 | 118 |
FIGURE 2.
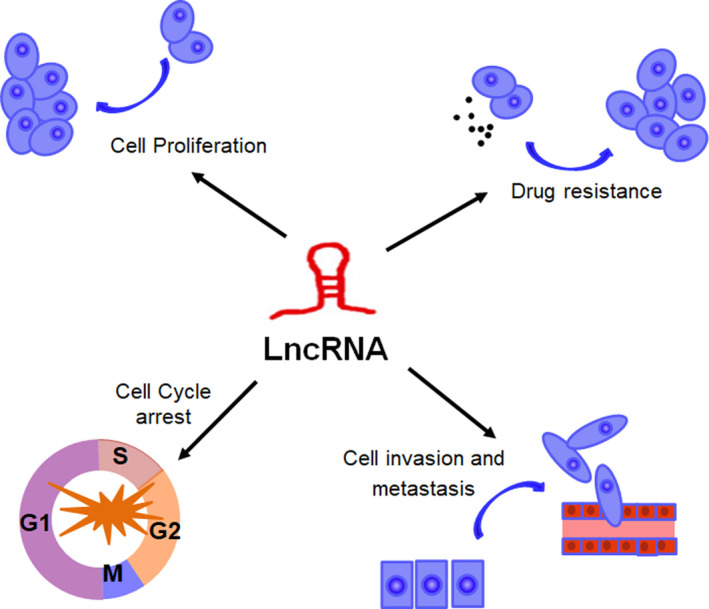
The biological function of lncRNAs in TNBC
4.1. lncRNAs involved in the regulation of cell proliferation and apoptosis
Cancer has been considered to be the result of accumulated gene mutations, which led to uncontrolled cell proliferations. And deregulated cell proliferation and inhibition of cell apoptosis lie at the heart of tumour development. 46 The role of lncRNAs in the regulation of TNBC cell proliferation and apoptosis has also been widely investigated. ceRNA mechanisms and network construction, by sequestering miRNAs and sparing their protein‐coding counterparts from post‐translational regulation, have been mainly studied to act as the main molecular mechanism of lncRNA biological function. 15 For example, lncRNA GAS5 was reported to promote apoptosis and inhibit proliferation of TNBC cells by targeting miR‐196a‐5p and miR‐378a‐5p/SUFU signalling. 47 , 48 , 49 LINC00096 promoted cell proliferation by sponging miR‐383‐5p and regulating RBM3 expression in TNBC. 50 LncRNA HEIH was shown to regulate cell proliferation and apoptosis through miR‐4458/SOCS1 axis in TNBC. 51 LncRNA HCP5 could also promote cell proliferation and inhibit cell apoptosis as a ceRNA to regulate BIRC3 by sponging miR‐219a‐5p. 52 LINC00339 promoted cell proliferation and inhibited cell apoptosis through miR‐377‐3p/HOXC6 signalling pathway. 53 Knockdown of lncRNA HOST2 could inhibit the proliferation of TNBC cells via regulation of the let‐7b/CDK6 axis. 54
There are also other molecular mechanisms of LncRNAs. Some lncRNAs assemble with mRNAs to protect them from miRNA action and increase their stability. Some lncRNAs are named scaffold lncRNAs, which could serve as a central platform to assemble with different molecular components such as proteins and RNAs and promote their intermolecular interactions. Signal lncRNAs have also been reported to interact with transcription factors (TFs) or histone‐modifying enzymes to cis‐regulate or trans‐regulate the expression of their target genes. 8 For instance, in TNBC, Tariq et al revealed that LINC02095 promotes breast cancer proliferation by facilitating the expression of oncogenic transcription factor, SOX9. 55 LncRNA DANCR was reported to bind with RXRA and increase its serine 49/78 phosphorylation, leading to activating PI3K/Akt signalling and TNBC cell proliferation. 56 Shen et al demonstrated that LINC00152 obviously enhanced NEDD4‐1‐mediated ubiquitination and degradation of PTEN protein in TNBC. 57 Meanwhile, Wu et al also revealed that LINC00152 could enhance TNBC tumorigenesis by inactivation of the BRCA1/PTEN through DNA methyltransferases. 58 Besides, LncRNA PDCD4‐AS1 was reported to stabilize PDCD4 RNA by forming RNA duplex and regulate the interaction between PDCD4 RNA and RNA decay promoting factors such as HuR. 59
Several studies have indicated that LncRNAs could also play an important role in the TNBC cell proliferation and apoptosis process by regulating other LncRNAs. LncRNA DRHC was shown to inhibit TNBC cells proliferation by down‐regulating the expression of lncRNA HOTAIR, while HOTAIR did not affect the expression level of DRHC. 60 Similarly, LncRNA PTCSC3 overexpression led to down‐regulated lncRNA H19 in TNBC cells, while H19 overexpression did not affect PTCSC3 expression. 61 LncRNA NRON overexpression inhibited cancer cell proliferation and down‐regulated lncRNA snaR in TNBC, while snaR overexpression did not significantly affect NRON expression. 62 There are other lncRNAs involved in the process of TNBC cell proliferation and apoptosis, including AWPPH, LUCAT1, HAND2‐AS1, POU3F3, MALAT1, ANRIL. 63 , 64 , 65 , 66 , 67 , 68 These lncRNAs could be potential targets for further mechanistic studies to establish their functional role in TNBC cell proliferation and apoptosis.
Cell cycle progression is regulated by cyclin‐dependent kinases (CDKs), which are activated by cyclin binding and inhibited by CDK inhibitors. 69 p27, an inhibitor of CDK, binds not only to the cyclin E/CDK2 complex, but also to the cyclin D/CDK4,6 complexes, involving in the regulation of the cell cycle. 70 , 71 LncRNA MIR100HG was reported to inhibit cell arrest in the G1 phase, through binding to p27 to form RNA‐DNA triplex structures at 275‐352 nt, 462‐557 nt and 2635‐2688 nt. 72 It was showed that lncRNA LUCAT1 plays a key role in cell cycle G1 arrest by regulating the expression of cyclin D1, CDK4 in clear cell renal cell carcinoma (ccRCC) 73 and the expression of p21, p57 in non‐small‐cell lung cancer (NSCLC). 74 In TNBC, LUCAT1 was also shown to contribute to accelerate cell cycle progression through modulating miR‐5702. 64 Besides, Wang et al reported that LINC00339 inhibited cell cycle arrest at G0/G1 phase by sponging to miR‐377‐3p and activating miR‐377‐3p/HOXC6 signalling pathway in TNBC. 53 Shin et al revealed that LncRNA NEAT1 conferred oncogenic role by regulating cell cycle progression in TNBC cells. 75 LncRNA RMST was also shown to induce the block of G0/G1 phase in TNBC. 76 Taken together, researches about lncRNAs in the regulation of cell cycle in TNBC are preliminary (Figure 3). Maybe, lncRNAs profiles to identify the abnormally expressed lncRNAs and further mechanistic studies to investigate the role of lncRNAs in the regulation of cell cycle progression in TNBC are needed.
FIGURE 3.
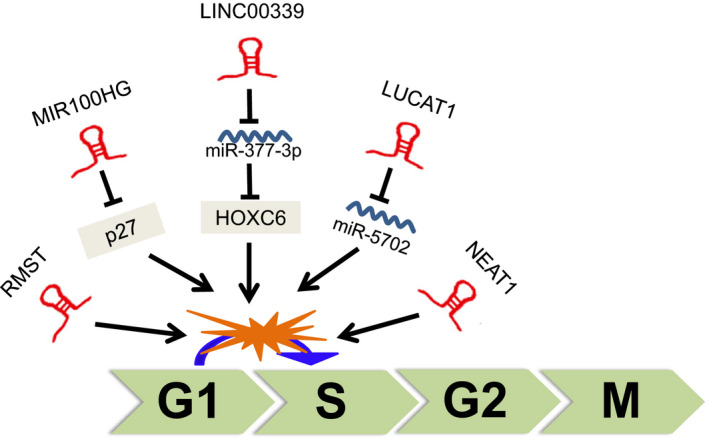
lncRNAs involved in the regulation of TNBC cell cycle
4.2. lncRNAs involved in the regulation of cell invasion and metastasis
Metastasis is the major cause of cancer‐related deaths. It has been increasingly recognized that lncRNAs play important roles in tumour invasiveness and metastasis. Overexpression of GAS5 was shown to undermine the tumour promotion effect induced by ectopic expression of miR‐196a‐5p, including cell invasion and FOXO1/PI3K/Akt signal pathway activation. 49 TROJAN can bind to metastasis‐repressing factor, ZMYND8 and increase its degradation through the ubiquitin‐proteasome pathway. 17 Han et al reported that lncRNA CCAT1 could promote TNBC cells migration and invasion by suppressing miR‐218/ZFX signalling. 77 In addition, lncRNA MIR503HG was reported to inhibit cell migration and invasion via miR‐103/OLFM4 axis in TNBC. 78 LncRNA NAMPT‐AS promoted TNBC cell metastasis and regulated autophagy, through epigenetically regulating NAMPT expression. NAMPT‐AS could recruit POU2F2 to activate the transcription of NAMPT, or serve as a ceRNA to rescue NAMPT degradation from miR‐548b‐3p in TNBC. 79
Epithelial‐mesenchymal transition (EMT) has been involved in carcinogenesis and confers metastatic properties upon cancer cells by enhancing cell mobility, invasion and resistance to apoptotic stimuli. 80 Zhang et al revealed that AFAP1‐AS1 could activate Wnt/β‐catenin pathway to promote tumorigenesis and cell invasion by inducing the expression of c‐myc and EMT‐related molecules in TNBC. 81 MiR‐34a was reported to implicate in certain EMT‐associated signal pathways to repress tumorigenesis, cancer progression and metastasis. 82 LncRNA PAPAS was shown to promote migration and invasion of TNBC cells by down‐regulating miR‐34a. 83 LncRNA sONE was also reported to repress endothelial nitric oxide synthase (eNOS)‐induced nitric oxide (NO) production, regulating TP53 and c‐Myc proteins levels and finally altering the levels of a panel of tumour‐suppressor miRNAs, including miR‐34a, miR‐15, miR‐16 and let‐7a. 84 Besides, sONE was also shown to inhibit H2S‐induced TNBC cell migration and invasion through activating sONE/NOS3/NO signalling axis. 85 ZEB1 and ZEB2 belong to the ZEB family transcription factors, which play a pivotal role in the process of EMT. 86 LncRNA LINC‐ZNF469‐3 was reported to enhance invasion ability and stemness properties, and promote lung metastasis through miR‐574‐5p/ZEB1 axis in TNBC. 87 LncRNA ZEB2‐AS1 was shown to promote TNBC cell metastasis by positively regulating ZEB2 expression and activating the EMT via the PI3K/Akt/GSK3β/ZEB2 signalling pathway. 88 Another lncRNA, PVT1, was reported to promote EMT and cell migration via regulating p21 and KLF5/β‐catenin signalling in TNBC. 89 , 90
The results of our studies also showed that lncRNA androgen receptor (AR) negatively induced long non‐coding RNA (ARNILA) could promote EMT, invasion and metastasis of TNBC, by functioning as a ceRNA for miR‐204 to facilitate expression of its target gene Sox4. 91 There are other lncRNAs involved in the process of TNBC invasion and metastasis, including HOTAIR, SNHG12, SNAR, WT1‐AS, LINC01096, DANCR, NEF, HIF1A‐AS2, LncKLHDC7B, ROR, AIRN, RMST, MALAT1, SKAI1BC, HULC. 76 , 107 Taken together, these studies revealed that lncRNAs play an important role in the regulation of cell invasion and metastasis in TNBC (Figure 4).
FIGURE 4.
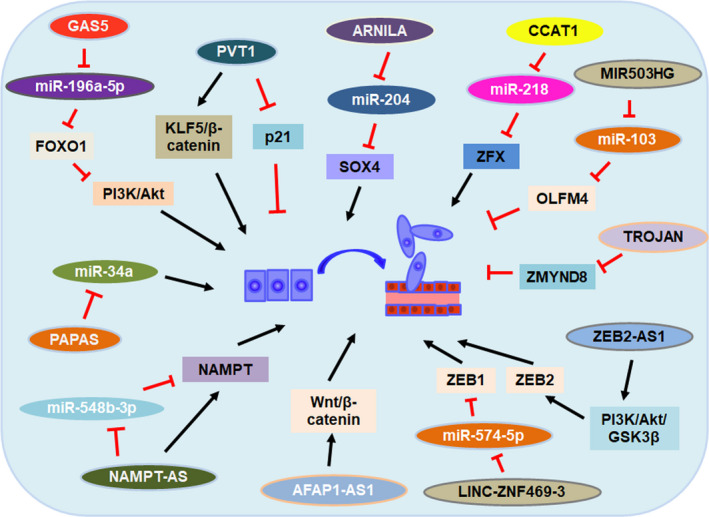
lncRNAs involved in the regulation of TNBC cell invasion and metastasis
4.3. lncRNAs involved in the regulation of drug resistance
Emerging evidences suggest that lncRNAs could implicate in regulation of drug resistance by targeting different genes in TNBC (Figure 5). LncRNA growth‐stasis‐specific transcript 5 (GAS5) is the most widely studied lncRNA involved in the regulation of various drug resistance. The expression level of GAS5 in TNBC patients was reported to associate with tumour resistance to several chemotherapeutic drugs, including adriamycin, paclitaxel and cisplatin. 47 , 48 In addition, GAS5 expression could reduce the sensitivity to not only mTORC1 inhibitor rapalogues, but also dual mTORC1/mTORC2 inhibitor AZD8055. 108 Nevertheless, they displayed a significant increase in response to the dual PI3K/mTOR inhibitor, BEZ235. 108 Considering the important role of GAS5 in the sensitivity of multiple drugs, GAS5 may be a potential biomarker for monitoring prognosis of TNBC patients.
FIGURE 5.
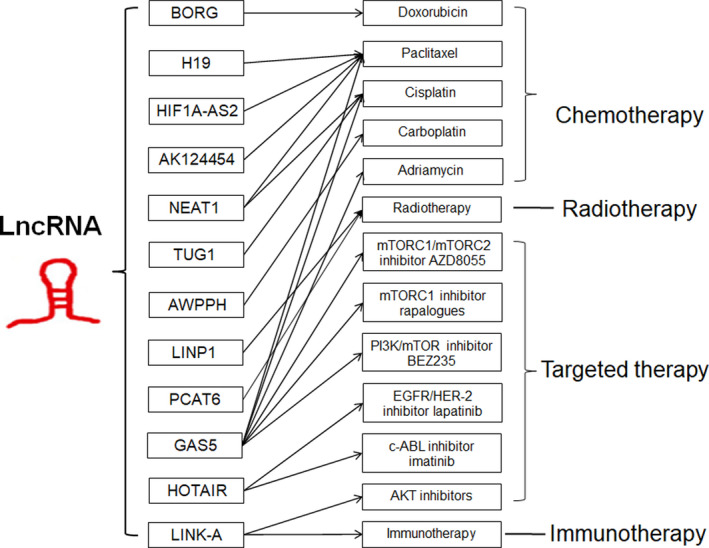
lncRNAs involved in the regulation of drug resistance in TNBC
Besides, HOTAIR expression was shown to be transcriptionally repressed by the combined treatment of EGFR/HER‐2 inhibitor lapatinib plus c‐ABL inhibitor imatinib. Enforced expression of HOTAIR conferred increased resistance to the dual treatment by recruitment of β‐catenin to the HOTAIR promoter at the LEF1/TCF4‐binding site. 109 Another lncRNA, LINK‐A (long intergenic non‐coding RNA for kinase activation, also called LINC01139), could directly interact with the AKT pleckstrin homology (PH) domain and PIP3, facilitating AKT‐PIP3 interaction and consequent enzymatic activation. 110 LINK‐A‐dependent AKT hyperactivation led to resistance to AKT inhibitors, while genomic deletions of the LINK‐A PIP3‐binding motif dramatically sensitized TNBC cells to AKT inhibitors. 110 Immunotherapy, including programmed cell death protein‐1 and programmed death ligand‐1 (PD‐1/PD‐L1) blockade, has been demonstrated to inhibit cancer progression and validated with the clinical success for the treatment of a variety of human cancers. 111 Hu and colleagues demonstrated that LINK‐A could also regulate the immunosurveillance in TNBC via LINK‐A‐PKA‐TRIM71 signalling axis. 18 Patients with PD‐1 blockade‐resistant TNBC exhibited elevated LINK‐A levels, and LINK‐A locked nucleic acids treatment sensitized mammary gland tumours to immune checkpoint blockers. 18
There are other lncRNAs involved in the regulation of drug resistance, including chemotherapy drugs paclitaxel, doxorubicin, cisplatin, carboplatin and radiotherapy resistance. For example, LncRNA AWPPH could improve cancer cell viability under carboplatin treatment, while lncRNA AWPPH small interfering RNA (siRNA) silencing led to increased chemosensitivity. 112 HIF1A‐AS2 and AK124454 were showed to contribute paclitaxel resistance in TNBC cells. 45 LncRNA TUG1 was shown to sponge miR‐197, induce expression of NLK and inactivate WNT signalling pathway, thus increasing cisplatin sensitivity of TNBC cells. 113 LncRNA H19 was also reported to confer paclitaxel resistance, while knockdown of H19 might restore the chemosensitivity in paclitaxel‐resistant TNBC by mediating the AKT signalling pathway. 114 LncRNA NEAT1 was shown to mediate paclitaxel and cisplatin resistance in TNBC. 75 Besides, lncRNA BORG led to doxorubicin resistance via binding to RPA1 and activating the NF‐κB signalling axis. 115 DNA repair is a series of processes by which damaged DNA is identified and corrected in cells. This process is essential to genomic integrity and is involved in tumorigenesis. 116 LncRNA LINP1 was reported to enhance repair of DNA double‐strand breaks (DSB) by acting as a scaffold linking Ku80 and DNA‐dependent protein kinase catalytic subunit (DNA‐PKcs), thereby coordinating the NHEJ pathway, a key determinant of ionizing radiation (IR) resistance. 117 Importantly, blocking LINP1 could increase the sensitivity of the tumour‐cell response to radiotherapy in TNBC. 117 Additionally, knockdown of lncRNA PCAT6 promoted the radiosensitivity of TNBC cells through regulating miR‐185‐5p/TPD52 axis. 118 Taken together, these studies evoke the potential of altering lncRNAs expression in future to represent a novel therapeutic approach to reverse drug resistance or radiotherapy resistance in TNBC patients. However, further studies and mechanistic investigations of the regulation mechanism of lncRNAs‐mediated drug resistance in TNBC are needed in the future.
4.4. Others
Several recent studies also demonstrated that lncRNAs could implicate in other malignant processes, including angiogenesis and cancer stemness. For example, it was reported that mesenchymal stem/stromal cells (MSCs) strongly induced the lncRNA LINC01133 in neighbouring TNBC cells. 119 LINC01133 promoted phenotypic and growth characteristics of cancer stem cell‐like cells, and that it was a direct mediator of the MSC‐triggered miR‐199a/FOXP2 pathway and pluripotency‐determining gene Kruppel‐Like Factor 4 (KLF4) in TNBC models. 119 LncRNA DANCR was shown to promote the expression of TNBC cancer stem cell markers through repressing the binding of EZH2 on the promoters of CD44 and ABCG2. 120 LINC01638 was reported to maintain the mesenchymal traits of TNBC cells, including an enriched EMT signature and cancer stem cell‐like state, through interacting with c‐Myc to prevent SPOP‐mediated c‐Myc degradation, and activate MTDH/Twist1 signalling. 121 Furthermore, there are other lncRNAs reported to involve in the regulation of TNBC stemness, including NEAT1, LINC‐ZNF469‐3, NRAD1, H19. 75 , 87 , 122 , 123 TNBC patients demonstrate enhanced angiogenesis when compared with non‐TNBC patients. 124 Wang et al recently reported that lncRNA ASRPS could directly bind to STAT3 in the coiled coil domain (CCD) and inhibit STAT3 phosphorylation, leading to reduced expression of VEGF and reduced angiogenesis. 125 Vasculogenic mimicry (VM), a malignant tumour‐specific non‐endothelial vascular network, provides oxygen and nutrients to tumour cells and facilitate tumour progression. Tao et al showed that lncRNA TP73‐AS1 was upregulated in VM positive TNBC tissues and involved in TNBC VM formation, by binding to miR‐490‐3p and activating the miR‐490‐3p/TWIST1 axis. 126 Besides, lncRNAs are also reported to implicate in other process of TNBC cells. Such as, LINK‐A was identified to promote TNBC glycolysis reprogramming by mediating HIF1α phosphorylation at Tyr 565 and Ser 797. 127 Tracy et al revealed that lncRNA MANCR significantly inhibited DNA damage and regulated genomic stability of TNBC. 128
5. LNCRNA ACTS AS BIOMARKER FOR DIAGNOSIS AND PROGNOSIS IN TNBC
Since various lncRNAs have been found to be differentially expressed in TNBC, there is increasing evidence to show lncRNAs have diagnostic or prognostic potential for clinical TNBC patients. To study the role of lncRNAs in the diagnosis and prognosis of TNBC patients, numerous researchers analysed the lncRNAs expression levels (even epigenetic level) in TNBC versus non‐TNBC patients or healthy controls from tissues specimens, plasma (circulating lncRNA), exosome lncRNA, or micropeptide, and investigated the association with the prognosis of TNBC patients, including overall survival, disease‐free survival, lymph node metastasis, and distant metastasis. For instance, Fan et al implemented a comprehensive analysis of lncRNA expression profiles and clinical data of 1097 breast cancer samples from TCGA database. They detected 1510 differentially expressed lncRNAs in normal and TNBC samples, and 672 differentially expressed lncRNAs between non‐TNBC and TNBC samples. 129 They identified three lncRNAs (AC091043.1, AP000924.1 and FOXCUT) maybe have strong diagnostic value for TNBC diagnosis. They also found that other three lncRNAs (AC010343.3, AL354793.1 and FGF10‐AS1) expression levels were associated with the clinical prognosis of TNBC patients. 129 Liu et al compared the differential lncRNAs expression in the plasma of TNBC patients (n = 25), non‐TNBC patients (n = 35) and healthy controls. At last, they found that the expression levels of three lncRNAs, ANRIL, HIF1A‐AS2 and UCA1 were significantly increased in the plasma of TNBC patients, suggesting that those three lncRNAs expression may serve as TNBC‐specific diagnostic biomarkers. 130 A recent meta‐analysis summarized the prognostic value of 24 lncRNAs from a total of 2803 TNBC patients and demonstrated that expression of nine lncRNAs (SNHG12, MALAT1, HOTAIR, HIF1A‐AS2, HULC, LINC00096, ZEB2‐AS1, LUCAT1 and LINC000173) showed a marked correlation with positive lymph node metastasis, while lncRNA MIR503HG, GAS5, TCONS_l2_00002973 showed the opposite effect. 131 The authors also found high expression level of another seven lncRNAs (MALAT1, HIF1A‐AS2, HULC, LINC00096, ADPGK‐AS1, ZEB2‐AS1, LUCAT1) was positively correlated with distant metastasis, while patients with a high lncRNA MIR503HG expression level have lower rate of distant metastasis. 131
DNA methylation is the best‐studied mechanism of epigenetic gene regulation. 132 The aberrant DNA methylation statuses play an essential role in the pathological process of many cancers. It was demonstrated that TNBC tumours are genome‐wide hypomethylation compared with other subtypes and normal breast control tissues and the hypomethylation is associated with worse overall survival (OS). 133 , 134 , 135 Plenty of evidence have revealed that cancer cells utilize DNA methylation as a strategy to abnormally silence a variety of genes including lncRNAs. Bermejo et al conducted an epigenome‐wide association study (EWAS) and identified that LINC00299 is high methylated in TNBC patients’ peripheral blood, making hypermethylation of LINC00299 a useful circulating biomarker for TNBC patients. 136
LncRNAs were also be reported to predict responses to therapy, including chemotherapy, radiotherapy and immunotherapy. One study has showed that circulating lncRNA H19 was high expressed and could predict the response to neoadjuvant chemotherapy (NAC) in TNBC patients. 137 They found patients with a pathological complete response (pCR) had lower pre‐therapeutic levels of lncRNA H19 compared with the non‐complete responders. Meanwhile, patients with higher degree of downstaging of initial tumours had lower baseline levels of lncRNA H19 among non‐complete responders. 137 Those data suggested that circulating lncRNA H19 may be a useful marker for predicting the response to neoadjuvant chemotherapy. Another study determined that lncRNA LINK‐A could predict immunosuppression and immunotherapy resistance. 18 TNBC patients who responded to pembrolizumab (anti‐PD‐1 immunotherapy) exhibited relatively lower expression of LINK‐A and higher CD8+ T‐cell infiltration compared with non‐responders. CD8+ T‐cell infiltration in this cohort of patients with TNBC negatively correlated with LINK‐A expression. 18 These results implicated the potential for lncRNA LINK‐A to serve as biomarker for predicting the outcome of TNBC patients treated with immune checkpoint inhibitors. Recently, Bi et al reported that higher lncAFAP1‐AS1 expression was detected in the patients with local recurrence, using the surgically resected tumour tissues of TNBC patients receiving postoperative radiotherapy. 138 They also found higher lncAFAP1‐AS1 expression was correlated with poor disease‐free survival and overall survival of TNBC patients. 138 These results demonstrated that high lncAFAP1‐AS1 expression is associated with radio‐resistance of TNBC patients, and the expression level of lncAFAP1‐AS1 in tumour tissues could be used to predict the outcome of TNBC radiotherapy.
In conclude, it was noticeable that lncRNAs might be more reliable diagnostic and prognostic biomarkers for TNBC patients as a result of its aberrant expression in tumorigenesis (Figure 6). However, in the future, lncRNA diagnosis and prognosis biomarker studies will need to specify more focus on the serum circulating lncRNA and predicting the response to therapy, including chemotherapy, radiotherapy, targeted therapy and immunotherapy. Additionally, further investigation of a larger patient population is necessary to confirm the diagnostic and prognostic evaluation of lncRNAs in TNBC patients.
FIGURE 6.
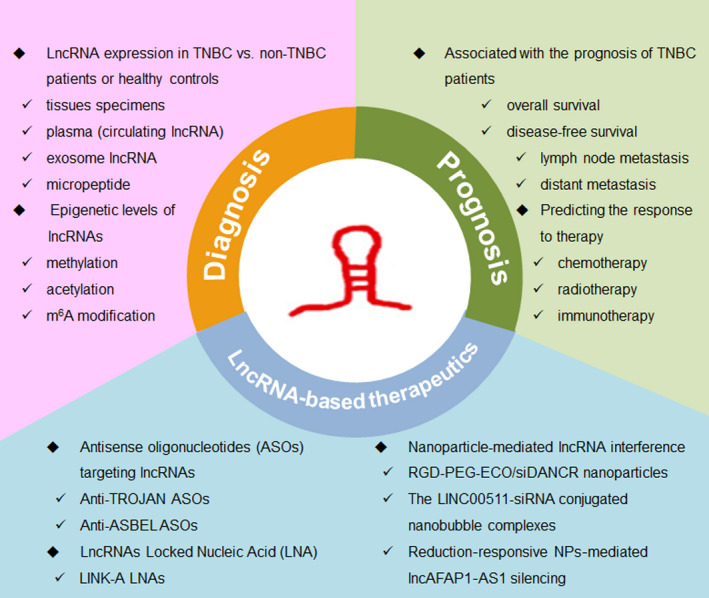
lncRNA act as biomarker for diagnosis and prognosis in TNBC
6. LNCRNA‐BASED THERAPEUTICS IN TNBC
Since lncRNAs play crucial roles in the biological processes and tumorigenesis and abnormal expression of lncRNAs occur in multiple human cancers, this presents with lncRNA‐based therapeutics possibility to correct this dysregulation. Recently, accumulating studies indicating the significance of lncRNAs in the regulation of TNBC development and drug resistance accelerated the investigation to explore the potential lncRNAs‐based therapeutics in TNBC (Figure 6).
Antisense oligonucleotides (ASOs) were able to modulate RNA processing and protein expression through different mechanisms, making them able to serve as a variety of molecular targets. 139 Recently, advancements of ASO structure and chemical modifications greatly improved the advantage and effectiveness of ASOs to act as precious tools to understand disease mechanisms and as valuable therapeutics for disease intervention. 140 Moreover, many ASOs are undergoing clinical trials, taking advantage of the various mechanisms and synthetic structures now available for the design of ASOs‐based therapies. 140 ASOs also were used to inhibit the expression of lncRNAs for lncRNA‐based therapeutics in TNBC.
LncRNA TROJAN was shown to promote TNBC proliferation and metastasis, and correlated with poor patient survival. 17 Jin et al designed eight ASOs targeting TROJAN and transfected TNBC cells with ASOs without using any transfection reagents to simulate in vivo conditions. After using anti‐TROJAN targeted therapy in an intravenous xenograft mouse model, they observed that lung metastasis nodules were significantly reduced in ASO‐treated group than the control group. 17 Meanwhile, they also found that the ASO toxicity was limited after detecting the murine blood biochemical indexes. 17 Taken together, these results demonstrated that modification of lncRNA TROJAN by ASO treatment maybe a novel therapeutic approach for TNBC clinical patients.
LncRNA ASBEL has been identified as an antisense transcript of BTG3 gene, which encodes an anti‐proliferation protein and is remarkable down‐regulated in TNBC. A number of single‐stranded modified ASOs were designed, synthesized and screened for specific lncRNA ASBEL knockdown. And anti‐ASBEL ASOs were reported to play a significant tumour suppressive role in TNBC by effective down‐regulating lncRNA ASBEL. 141 Besides, Vidovic et al revealed that ASO targeting lncRNA NRAD1 could reduce tumour growth and inhibit tumour cells to acquire and maintain stem cell characteristics in TNBC. 122
Locked Nucleic Acid (LNA) is a novel, third generation DNA analogue that has the potential to impact strongly on the future development of a diversity of nucleic acid‐based technologies. 142 LncRNA LINK‐A has been characterized as an oncogenic lncRNA in TNBC by activating HIF1α. 127 Recently, Hu et al reported that treatment with LINK‐A LNAs could repress cell proliferation in TNBC cells, but not non‐TNBC cells. 18 They also found that LINK‐A LNAs‐treated MMTV‐Tg mice exhibited inhibited tumour growth and reduced lung metastasis compared with scramble LNAs‐treated mice. 18 Besides, treatment with LINK‐A LNAs could improve the protein stability of the antigen peptide‐loading complex (PLC) components and major histocompatibility complex (MHC) class I complex, resulting in sensitization of mammary gland tumours to immunotherapy. And LINK‐A LNAs treatment could improve CD8+/CD3 + T‐cell infiltration and cytotoxicity, while tumour growth was synergistically suppressed by a combinatorial treatment of LINK‐A LNAs and immune checkpoint blockers (ICBs). 18 Therefore, LINK‐A may act as a powerful biomarker for predicting the prognosis of TNBC patients who received immunotherapy, and targeting LINK‐A could further sensitize TNBC to immune checkpoint inhibitors.
RNA nanotechnology is a rapidly evolving field that has emerged as a novel vector system for targeted therapy in various human diseases. 143 , 144 , 145 RNA nanoparticle‐based targeted therapy through inhibition of non‐coding RNA has also been reported in the treatment for human cancer. 146 LncRNA DANCR was reported to be significantly overexpressed and promote cell proliferation, invasion, and CSC‐like phenotypic traits in TNBC. 56 , 98 , 120 Vaidya et al formulated tumour‐targeting RGD‐PEG‐ECO/siDANCR nanoparticles via self‐assembly of multifunctional amino lipid ECO, cyclic RGD peptide‐PEG and siDANCR for systemic delivery. 147 The nanoparticle‐mediated RNA interference (RNAi) of the oncogenic lncRNA DANCR demonstrated effective TNBC therapy. They found that DANCR expression was 80%‐90% knockdown after treatment with the therapeutic RGD‐PEG‐ECO/siDANCR nanoparticles in TNBC cells, indicating efficient intracellular siRNA delivery and sustained target silencing. Moreover, the RGD‐PEG‐ECO/siDANCR nanoparticles mediated significant reduction in TNBC cell proliferation, invasion, migration, survival and tumour spheroid formation, suggesting excellent in vitro therapeutic efficacy. Furthermore, the RGD‐PEG‐ECO/siDANCR nanoparticles TNBC xenografts in nude mouse model also led to suppression of TNBC progression with no overt toxic side‐effects, which demonstrated the efficacy and safety of the nanoparticle therapy. Similarly, Wu et al structured a novel theranostic agent loaded with LINC00511‐siRNA to deliver siRNA, and detected the responses of drug sensitivity in TNBC. 148 They demonstrated that the combination of low‐frequency ultrasound (LFUS) irradiation and nanobubble complexes was regarded as an efficient and safe method for siRNA transfection. 148 Recently, another study engineered a reduction‐responsive nanoparticle (NP) platform for effective lncAFAP1‐AS1 siRNA (siAFAP1‐AS1) delivery, and reported that systemic delivery of siAFAP1‐AS1 with the reduction‐responsive NPs can synergistically reverse radio‐resistance by scavenging intracellular glutathione, leading to a dramatically enhanced radiotherapy effect in both xenograft and metastatic TNBC tumour models. 138 Overall, these results demonstrate that this RNA nanoparticle‐based targeted therapy by nanoparticle‐mediated modulation of onco‐lncRNAs is a promising approach that utilizes chemically modified RNPs for tumour‐specific targeting and lncRNA inhibition that will be beneficial in TNBC and other cancers setting where lncRNA knockdown is desired for a better clinical output. 138 , 147 , 148
7. CONCLUSIONS AND FUTURE PROSPECTS
Overall, recent evidences suggest that many lncRNAs were abnormal expressed and characterized as biomarkers for diagnosis and prognosis in TNBC. LncRNAs have been identified to involve in the regulation of pathological and physiological processes of TNBC cells, including cell proliferation, apoptosis, EMT, metastasis and therapy resistance. The functional lncRNAs and their regulators hold the potential for development of novel lncRNA‐based therapeutics in clinical TNBC treatment, using ASOs, LNA or RNA nanotechnology targeting lncRNA.
In the future, (a) lncRNA diagnosis and prognosis biomarker studies will need to specify more focus on the serum circulating lncRNA and predicting the response to therapy, including chemotherapy, radiotherapy, targeted therapy and immunotherapy. (b) high throughput next generation sequencing (NGS) used for lncRNA profiling has identified a lot of differential lncRNAs in TNBC versus non‐TNBC tissues. However, further comprehensive functional studies of the identified TNBC‐related lncRNAs are needed. (c) Since lncRNAs play important roles in the multiple process of TNBC development, the mechanism of the regulation of abnormally expressed lncRNAs should also need to be investigated in the future, (d) almost all of the lncRNAs‐related studies in TNBC are focused on cell lines. Future studies can play more attention in the clinical TNBC patients or animal models. (e) lncRNA‐based therapeutics in TNBC have been developed by numerous researchers. However, the technology used for current lncRNA‐based therapeutics is focus on antisense oligonucleotides to inhibit the expression of onco‐lncRNAs. More technology, such as RNA nanotechnology, may hold the potential for development of novel therapeutics in clinical TNBC treatment. Furthermore, other emerging technology through lncRNA replacement therapy to restore levels of tumour‐suppressor lncRNAs could also be developed in the future.
As lncRNAs play significant roles in the TNBC tumorigenesis, further characterization of this category of molecules to uncover their potential roles as therapeutic targets, diagnosis and prognosis biomarkers for TNBC are an important priority for the clinical TNBC treatment.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS' CONTRIBUTIONS
WZ, XG and JT conceived the study and wrote the manuscript.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (No. 81773102, 81802667), Natural Science Foundation of Jiangsu Province (BK20180133) and Key International Cooperation of the National Natural Science Foundation of China (No. 81920108029).
Zhang W, Guan X, Tang J. The long non‐coding RNA landscape in triple‐negative breast cancer. Cell Prolif.2021;54:e12966 10.1111/cpr.12966
Contributor Information
Wenwen Zhang, Email: wwzhang1022@hotmail.com.
Jinhai Tang, Email: jhtang@njmu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406. [DOI] [PubMed] [Google Scholar]
- 3. Rakha EA, El‐Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple‐negative breast cancer. Cancer. 2007;109(1):25‐32. [DOI] [PubMed] [Google Scholar]
- 4. Tan AR, Swain SM. Therapeutic strategies for triple‐negative breast cancer. Cancer J. 2008;14(6):343‐351. [DOI] [PubMed] [Google Scholar]
- 5. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansji H, Leung EY, Baguley BC, Finlay GJ, Askarian‐Amiri ME. Keeping abreast with long non‐coding RNAs in mammary gland development and breast cancer. Front Genet. 2014;5:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tripathi MK, Doxtater K, Keramatnia F, et al. Role of lncRNAs in ovarian cancer: defining new biomarkers for therapeutic purposes. Drug Discovery Today. 2018;23(9):1635‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong CM, Tsang FH, Ng IO. Non‐coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol. 2018;15(3):137‐151. [DOI] [PubMed] [Google Scholar]
- 9. Alvarez‐Dominguez JR, Lodish HF. Emerging mechanisms of long noncoding RNA function during normal and malignant hematopoiesis. Blood. 2017;130(18):1965‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhan A, Soleimani M, Mandal SS. Long Noncoding RNA and Cancer: A New Paradigm. Can Res. 2017;77(15):3965‐3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Investig. 2016;126(8):2775‐2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiong XD, Ren X, Cai MY, Yang JW, Liu X, Yang JM. Long non‐coding RNAs: an emerging powerhouse in the battle between life and death of tumor cells. Drug Resist Updat. 2016;26:28‐42. [DOI] [PubMed] [Google Scholar]
- 13. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253‐1261. [DOI] [PubMed] [Google Scholar]
- 15. Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding‐independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin X, Xu XE, Jiang YZ, et al. The endogenous retrovirus‐derived long noncoding RNA TROJAN promotes triple‐negative breast cancer progression via ZMYND8 degradation. Sci Adv. 2019;5(3):eaat9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu Q, Ye Y, Chan LC, et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat Immunol. 2019;20(7):835‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10(1):28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okazaki Y, Furuno M, Kasukawa T, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full‐length cDNAs. Nature. 2002;420(6915):563‐573. [DOI] [PubMed] [Google Scholar]
- 22. Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Consortium EP , Birney E, Stamatoyannopoulos JA, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447(7146):799‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559‐1563. [DOI] [PubMed] [Google Scholar]
- 25. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629‐641. [DOI] [PubMed] [Google Scholar]
- 26. Ma L, Bajic VB, Zhang Z. On the classification of long non‐coding RNAs. RNA Biol. 2013;10(6):925‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Louro R, Smirnova AS, Verjovski‐Almeida S. Long intronic noncoding RNA transcription: expression noise or expression choice? Genomics. 2009;93(4):291‐298. [DOI] [PubMed] [Google Scholar]
- 28. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mercer TR, Dinger ME, Mattick JS. Long non‐coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155‐159. [DOI] [PubMed] [Google Scholar]
- 30. Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354‐361.21550244 [Google Scholar]
- 32. Hardwick SA, Deveson IW, Mercer TR. Reference standards for next‐generation sequencing. Nat Rev Genet. 2017;18(8):473‐484. [DOI] [PubMed] [Google Scholar]
- 33. Tian T, Gong Z, Wang M, et al. Identification of long non‐coding RNA signatures in triple‐negative breast cancer. Cancer Cell Int. 2018;18:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koduru SV, Tiwari AK, Leberfinger A, et al. A comprehensive NGS data analysis of differentially regulated miRNAs, piRNAs, lncRNAs and sn/snoRNAs in triple negative breast cancer. J Cancer. 2017;8(4):578‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang F, Liu YH, Dong SY, et al. Co‐expression networks revealed potential core lncRNAs in the triple‐negative breast cancer. Gene. 2016;591(2):471‐477. [DOI] [PubMed] [Google Scholar]
- 36. Liu YR, Jiang YZ, Xu XE, et al. Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype‐specific RNAs of triple‐negative breast cancer. Breast Cancer Res. 2016;18(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lv M, Xu P, Wu Y, et al. LncRNAs as new biomarkers to differentiate triple negative breast cancer from non‐triple negative breast cancer. Oncotarget. 2016;7(11):13047‐13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu YR, Jiang YZ, Xu XE, Hu X, Yu KD, Shao ZM. Comprehensive transcriptome profiling reveals multigene signatures in triple‐negative breast cancer. Clin Cancer Res. 2016;22(7):1653‐1662. [DOI] [PubMed] [Google Scholar]
- 39. Shen X, Xie B, Ma Z, et al. Identification of novel long non‐coding RNAs in triple‐negative breast cancer. Oncotarget. 2015;6(25):21730‐21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen C, Li Z, Yang Y, Xiang T, Song W, Liu S. Microarray expression profiling of dysregulated long non‐coding RNAs in triple‐negative breast cancer. Cancer Biol Ther. 2015;16(6):856‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le K, Guo H, Zhang Q, et al. Gene and lncRNA co‐expression network analysis reveals novel ceRNA network for triple‐negative breast cancer. Sci Rep. 2019;9(1):15122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Z, Mi M, Li X, Zheng X, Wu G, Zhang L. lncRNA OSTN‐AS1 May Represent a Novel Immune‐Related Prognostic Marker for Triple‐Negative Breast Cancer Based on Integrated Analysis of a ceRNA Network. Front Genet. 2019;10:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song X, Zhang C, Liu Z, Liu Q, He K, Yu Z. Characterization of ceRNA network to reveal potential prognostic biomarkers in triple‐negative breast cancer. PeerJ. 2019;7:e7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yuan N, Zhang G, Bie F, et al. Integrative analysis of lncRNAs and miRNAs with coding RNAs associated with ceRNA crosstalk network in triple negative breast cancer. OncoTargets Ther. 2017;10:5883‐5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang YZ, Liu YR, Xu XE, et al. Transcriptome analysis of triple‐negative breast cancer reveals an integrated mRNA‐lncRNA signature with predictive and prognostic value. Can Res. 2016;76(8):2105‐2114. [DOI] [PubMed] [Google Scholar]
- 46. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342‐348. [DOI] [PubMed] [Google Scholar]
- 47. Zheng S, Li M, Miao K, Xu H. lncRNA GAS5‐promoted apoptosis in triple‐negative breast cancer by targeting miR‐378a‐5p/SUFU signaling. J Cell Biochem. 2019;121(3):2225‐2235. [DOI] [PubMed] [Google Scholar]
- 48. Li J, Li L, Yuan H, Huang XW, Xiang T, Dai S. Up‐regulated lncRNA GAS5 promotes chemosensitivity and apoptosis of triple‐negative breast cancer cells. Cell Cycle. 2019;18(16):1965‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49. Li S, Zhou J, Wang Z, Wang P, Gao X, Wang Y. Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR‐196a‐5p. Biomed Pharmacother. 2018;104:451‐457. [DOI] [PubMed] [Google Scholar]
- 50. Tian Y, Xia S, Ma M, Zuo Y. LINC00096 promotes the proliferation and invasion by sponging miR‐383‐5p and regulating RBM3 expression in triple‐negative breast cancer. OncoTargets Ther. 2019;12:10569‐10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li P, Zhou B, Lv Y, Qian Q. LncRNA HEIH regulates cell proliferation and apoptosis through miR‐4458/SOCS1 axis in triple‐negative breast cancer. Hum Cell. 2019;32(4):522‐528. [DOI] [PubMed] [Google Scholar]
- 52. Wang L, Luan T, Zhou S, et al. LncRNA HCP5 promotes triple negative breast cancer progression as a ceRNA to regulate BIRC3 by sponging miR‐219a‐5p. Cancer Med. 2019;8(9):4389‐4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang X, Chen T, Zhang Y, et al. Long noncoding RNA Linc00339 promotes triple‐negative breast cancer progression through miR‐377‐3p/HOXC6 signaling pathway. J Cell Physiol. 2019;234(8):13303‐13317. [DOI] [PubMed] [Google Scholar]
- 54. Zhang Y, Zhang H, Kang H, Huo W, Zhou Y, Zhang Y. Knockdown of long non‐coding RNA HOST2 inhibits the proliferation of triple negative breast cancer via regulation of the let‐7b/CDK6 axis. Int J Mol Med. 2019;43(2):1049‐1057. [DOI] [PubMed] [Google Scholar]
- 55. Tariq A, Hao Q, Sun Q, et al. LncRNA‐mediated regulation of SOX9 expression in basal sub‐type breast cancer cells. RNA. 2019;26:175‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang J, Zhong G, Zhang H, et al. LncRNA DANCR upregulates PI3K/AKT signaling through activating serine phosphorylation of RXRA. Cell Death Dis. 2018;9(12):1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shen X, Zhong J, Yu P, Zhao Q, Huang T. YY1‐regulated LINC00152 promotes triple negative breast cancer progression by affecting on stability of PTEN protein. Biochem Biophys Res Commun. 2019;509(2):448‐454. [DOI] [PubMed] [Google Scholar]
- 58. Wu J, Shuang Z, Zhao J, et al. Linc00152 promotes tumorigenesis by regulating DNMTs in triple‐negative breast cancer. Biomed Pharmacother. 2018;97:1275‐1281. [DOI] [PubMed] [Google Scholar]
- 59. Jadaliha M, Gholamalamdari O, Tang W, et al. A natural antisense lncRNA controls breast cancer progression by promoting tumor suppressor gene mRNA stability. PLoS Genet. 2018;14(11):e1007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yu F, Wang L, Zhang B. Long non‐coding RNA DRHC inhibits the proliferation of cancer cells in triple negative breast cancer by downregulating long non‐coding RNA HOTAIR. Oncol Lett. 2019;18(4):3817‐3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang N, Hou M, Zhan Y, Sheng X. LncRNA PTCSC3 inhibits triple‐negative breast cancer cell proliferation by downregulating lncRNA H19. J Cell Biochem. 2019;120(9):15083‐15088. [DOI] [PubMed] [Google Scholar]
- 62. Niu L, Fan Q, Yan M, Wang L. LncRNA NRON down‐regulates lncRNA snaR and inhibits cancer cell proliferation in TNBC. Biosci Rep. 2019;39(5):BSR20190468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang K, Li X, Song C, Li M. LncRNA AWPPH promotes the growth of triple‐negative breast cancer by up‐regulating frizzled homolog 7 (FZD7). Biosci Rep. 2018;38(6):BSR20181223. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64. Mou E, Wang H. LncRNA LUCAT1 facilitates tumorigenesis and metastasis of triple‐negative breast cancer through modulating miR‐5702. Biosci Rep. 2019;39(9):BSR20190489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wei M, Liu L, Wang Z. Long non‐coding RNA heart and neural crest derivatives expressed 2‐antisense RNA 1 overexpression inhibits the proliferation of cancer cells by reducing RUNX2 expression in triple‐negative breast cancer. Oncol Letters. 2019;18(6):6775‐6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang J, Meng X, Yu Y, Pan L, Zheng Q, Lin W. LncRNA POU3F3 promotes proliferation and inhibits apoptosis of cancer cells in triple‐negative breast cancer by inactivating caspase 9. Biosci Biotechnol Biochem. 2019;83(6):1117‐1123. [DOI] [PubMed] [Google Scholar]
- 67. Xu ST, Xu JH, Zheng ZR, et al. Long non‐coding RNA ANRIL promotes carcinogenesis via sponging miR‐199a in triple‐negative breast cancer. Biomed Pharmacother. 2017;96:14‐21. [DOI] [PubMed] [Google Scholar]
- 68. Zuo Y, Li Y, Zhou Z, Ma M, Fu K. Long non‐coding RNA MALAT1 promotes proliferation and invasion via targeting miR‐129‐5p in triple‐negative breast cancer. Biomed Pharmacother. 2017;95:922‐928. [DOI] [PubMed] [Google Scholar]
- 69. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1‐phase progression. Genes Dev. 1999;13(12):1501‐1512. [DOI] [PubMed] [Google Scholar]
- 70. Lee J, Kim SS. The function of p27 KIP1 during tumor development. Exp Mol Med. 2009;41(11):765‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. He W, Wang X, Chen L, Guan X. A crosstalk imbalance between p27(Kip1) and its interacting molecules enhances breast carcinogenesis. Cancer Biother Radiopharm. 2012;27(7):399‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang S, Ke H, Zhang H, et al. LncRNA MIR100HG promotes cell proliferation in triple‐negative breast cancer through triplex formation with p27 loci. Cell Death Dis. 2018;9(8):805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zheng Z, Zhao F, Zhu D, et al. Long non‐coding RNA LUCAT1 promotes proliferation and invasion in clear cell renal cell carcinoma through AKT/GSK‐3beta signaling pathway. Cell Physiol Biochem. 2018;48(3):891‐904. [DOI] [PubMed] [Google Scholar]
- 74. Sun Y, Jin SD, Zhu Q, et al. Long non‐coding RNA LUCAT1 is associated with poor prognosis in human non‐small lung cancer and regulates cell proliferation via epigenetically repressing p21 and p57 expression. Oncotarget. 2017;8(17):28297‐28311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shin VY, Chen J, Cheuk IW, et al. Long non‐coding RNA NEAT1 confers oncogenic role in triple‐negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019;10(4):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang L, Liu D, Wu X, et al. Long non‐coding RNA (LncRNA) RMST in triple‐negative breast cancer (TNBC): expression analysis and biological roles research. J Cell Physiol. 2018;233(10):6603‐6612. [DOI] [PubMed] [Google Scholar]
- 77. Han C, Li X, Fan Q, Liu G, Yin J. CCAT1 promotes triple‐negative breast cancer progression by suppressing miR‐218/ZFX signaling. Aging. 2019;11(14):4858‐4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fu J, Dong G, Shi H, et al. LncRNA MIR503HG inhibits cell migration and invasion via miR‐103/OLFM4 axis in triple negative breast cancer. J Cell Mol Med. 2019;23(7):4738‐4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang H, Zhang N, Liu Y, et al. Epigenetic regulation of NAMPT by NAMPT‐AS drives metastatic progression in triple‐negative breast cancer. Can Res. 2019;79(13):3347‐3359. [DOI] [PubMed] [Google Scholar]
- 80. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395‐412. [DOI] [PubMed] [Google Scholar]
- 81. Zhang K, Liu P, Tang H, et al. AFAP1‐AS1 promotes epithelial‐mesenchymal transition and tumorigenesis through wnt/beta‐catenin signaling pathway in triple‐negative breast cancer. Front Pharmacol. 2018;9:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nie D, Fu J, Chen H, Cheng J, Fu J. Roles of microRNA‐34a in epithelial to mesenchymal transition, competing endogenous RNA sponging and its therapeutic potential. Int J Mol Sci. 2019;20(4):861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kong Y, Geng C, Dong Q. LncRNA PAPAS may promote triple‐negative breast cancer by downregulating miR‐34a. J Int Med Res. 2019;47(8):3709‐3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Youness RA, Hafez HM, Khallaf E, Assal RA, Abdel Motaal A, Gad MZ. The long noncoding RNA sONE represses triple‐negative breast cancer aggressiveness through inducing the expression of miR‐34a, miR‐15a, miR‐16, and let‐7a. J Cell Physiol. 2019;234(11):20286‐20297. [DOI] [PubMed] [Google Scholar]
- 85. Youness RA, Assal RA, Abdel Motaal A, Gad MZ. A novel role of sONE/NOS3/NO signaling cascade in mediating hydrogen sulphide bilateral effects on triple negative breast cancer progression. Nitric Oxide. 2018;80:12‐23. [DOI] [PubMed] [Google Scholar]
- 86. Fardi M, Alivand M, Baradaran B, Farshdousti Hagh M, Solali S. The crucial role of ZEB2: from development to epithelial‐to‐mesenchymal transition and cancer complexity. J Cell Physiol. 2019;234(9):14783–14799. [DOI] [PubMed] [Google Scholar]
- 87. Wang PS, Chou CH, Lin CH, et al. A novel long non‐coding RNA linc‐ZNF469‐3 promotes lung metastasis through miR‐574‐5p‐ZEB1 axis in triple negative breast cancer. Oncogene. 2018;37(34):4662‐4678. [DOI] [PubMed] [Google Scholar]
- 88. Zhang G, Li H, Sun R, et al. Long non‐coding RNA ZEB2‐AS1 promotes the proliferation, metastasis and epithelial mesenchymal transition in triple‐negative breast cancer by epigenetically activating ZEB2. J Cell Mol Med. 2019;23(5):3271‐3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tang J, Li Y, Sang Y, et al. LncRNA PVT1 regulates triple‐negative breast cancer through KLF5/beta‐catenin signaling. Oncogene. 2018;37(34):4723‐4734. [DOI] [PubMed] [Google Scholar]
- 90. Wang L, Wang R, Ye Z, et al. PVT1 affects EMT and cell proliferation and migration via regulating p21 in triple‐negative breast cancer cells cultured with mature adipogenic medium. Acta Biochim Biophys Sin (Shanghai). 2018;50(12):1211‐1218. [DOI] [PubMed] [Google Scholar]
- 91. Yang F, Shen Y, Zhang W, et al. An androgen receptor negatively induced long non‐coding RNA ARNILA binding to miR‐204 promotes the invasion and metastasis of triple‐negative breast cancer. Cell Death Differ. 2018;25(12):2209‐2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liang H, Huang W, Wang Y, Ding L, Zeng L. Overexpression of MiR‐146a‐5p upregulates lncRNA HOTAIR in triple‐negative breast cancer cells and predicts poor prognosis. Technol Cancer Res Treat. 2019;18:1533033819882949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tao S, He H, Chen Q. Estradiol induces HOTAIR levels via GPER‐mediated miR‐148a inhibition in breast cancer. J Transl Med. 2015;13:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang O, Yang F, Liu Y, et al. C‐MYC‐induced upregulation of lncRNA SNHG12 regulates cell proliferation, apoptosis and migration in triple‐negative breast cancer. Am J Transl Res. 2017;9(2):533‐545. [PMC free article] [PubMed] [Google Scholar]
- 95. Lee J, Jung JH, Chae YS, et al. Long noncoding RNA snaR regulates proliferation, migration and invasion of triple‐negative breast cancer cells. Anticancer Res. 2016;36(12):6289‐6295. [DOI] [PubMed] [Google Scholar]
- 96. Wang J, Xi C, Yang X, et al. LncRNA WT1‐AS inhibits triple‐negative breast cancer cell migration and invasion by downregulating transforming growth factor beta1. Cancer Biother Radiopharm. 2019;34(10):671‐675. [DOI] [PubMed] [Google Scholar]
- 97. Wang GP, Mou ZL, Xu YY, Liu GX, Wang DM, Zhang HP. LINC01096 knockdown inhibits progression of triple‐negative breast cancer by increasing miR‐3130‐3p. Eur Rev Med Pharmacol Sci. 2019;23(17):7445‐7456. [DOI] [PubMed] [Google Scholar]
- 98. Tao W, Wang C, Zhu B, Zhang G, Pang D. LncRNA DANCR contributes to tumor progression via targetting miR‐216a‐5p in breast cancer: lncRNA DANCR contributes to tumor progression. Biosci Rep. 2019;39(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Song X, Liu Z, Yu Z. LncRNA NEF is downregulated in triple negative breast cancer and correlated with poor prognosis. Acta Biochim Biophys Sin (Shanghai). 2019;51(4):386‐392. [DOI] [PubMed] [Google Scholar]
- 100. Beltran‐Anaya FO, Romero‐Cordoba S, Rebollar‐Vega R, et al. Expression of long non‐coding RNA ENSG00000226738 (LncKLHDC7B) is enriched in the immunomodulatory triple‐negative breast cancer subtype and its alteration promotes cell migration, invasion, and resistance to cell death. Mol Oncol. 2019;13(4):909‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang Y, Zhang G, Han J. HIF1A‐AS2 predicts poor prognosis and regulates cell migration and invasion in triple‐negative breast cancer. J Cell Biochem. 2019;120(6):10513‐10518. [DOI] [PubMed] [Google Scholar]
- 102. Ma J, Yang Y, Huo D, et al. LincRNA‐RoR/miR‐145 promote invasion and metastasis in triple‐negative breast cancer via targeting MUC1. Biochem Biophys Res Commun. 2018;500(3):614‐620. [DOI] [PubMed] [Google Scholar]
- 103. Eades G, Wolfson B, Zhang Y, Li Q, Yao Y, Zhou Q. lincRNA‐RoR and miR‐145 regulate invasion in triple‐negative breast cancer via targeting ARF6. Mol Cancer Res. 2015;13(2):330‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu L, Yu D, Shi H, Li J, Meng L. Reduced lncRNA Aim enhances the malignant invasion of triple‐negative breast cancer cells mainly by activating Wnt/beta‐catenin/mTOR/PI3K signaling. Pharmazie. 2017;72(10):599‐603. [DOI] [PubMed] [Google Scholar]
- 105. Aram R, Dotan I, Hotz‐Wagenblatt A, Canaani D. Identification of a novel metastasis inducing lncRNA which suppresses the KAI1/CD82 metastasis suppressor gene and is upregulated in triple‐negative breast cancer. Oncotarget. 2017;8(40):67538‐67552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jin C, Yan B, Lu Q, Lin Y, Ma L. Reciprocal regulation of Hsa‐miR‐1 and long noncoding RNA MALAT1 promotes triple‐negative breast cancer development. Tumour Biol. 2016;37(6):7383‐7394. [DOI] [PubMed] [Google Scholar]
- 107. Shi F, Xiao F, Ding P, Qin H, Huang R. Long noncoding RNA highly up‐regulated in liver cancer predicts unfavorable outcome and regulates metastasis by MMPs in triple‐negative breast cancer. Arch Med Res. 2016;47(6):446‐453. [DOI] [PubMed] [Google Scholar]
- 108. Pickard MR, Williams GT. Regulation of apoptosis by long non‐coding RNA GAS5 in breast cancer cells: implications for chemotherapy. Breast Cancer Res Treat. 2014;145(2):359‐370. [DOI] [PubMed] [Google Scholar]
- 109. Wang YL, Overstreet AM, Chen MS, et al. Combined inhibition of EGFR and c‐ABL suppresses the growth of triple‐negative breast cancer growth through inhibition of HOTAIR. Oncotarget. 2015;6(13):11150‐11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lin A, Hu Q, Li C, et al. The LINK‐A lncRNA interacts with PtdIns(3,4,5)P3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat Cell Biol. 2017;19(3):238‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chen L, Han X. Anti‐PD‐1/PD‐L1 therapy of human cancer: past, present, and future. J Clin Investig. 2015;125(9):3384‐3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liu AN, Qu HJ, Gong WJ, Xiang JY, Yang MM, Zhang W. LncRNA AWPPH and miRNA‐21 regulates cancer cell proliferation and chemosensitivity in triple‐negative breast cancer by interacting with each other. J Cell Biochem. 2019;120(9):14860‐14866. [DOI] [PubMed] [Google Scholar]
- 113. Tang T, Cheng Y, She Q, et al. Long non‐coding RNA TUG1 sponges miR‐197 to enhance cisplatin sensitivity in triple negative breast cancer. Biomed Pharmacother. 2018;107:338‐346. [DOI] [PubMed] [Google Scholar]
- 114. Han J, Han B, Wu X, et al. Knockdown of lncRNA H19 restores chemo‐sensitivity in paclitaxel‐resistant triple‐negative breast cancer through triggering apoptosis and regulating Akt signaling pathway. Toxicol Appl Pharmacol. 2018;359:55‐61. [DOI] [PubMed] [Google Scholar]
- 115. Gooding AJ, Zhang B, Gunawardane L, Beard A, Valadkhan S, Schiemann WP. The lncRNA BORG facilitates the survival and chemoresistance of triple‐negative breast cancers. Oncogene. 2019;38(12):2020‐2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481(7381):287‐294. [DOI] [PubMed] [Google Scholar]
- 117. Zhang Y, He Q, Hu Z, et al. Long noncoding RNA LINP1 regulates repair of DNA double‐strand breaks in triple‐negative breast cancer. Nat Struct Mol Biol. 2016;23(6):522‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shi R, Wu P, Liu M, Chen B, Cong L. Knockdown of lncRNA PCAT6 enhances radiosensitivity in triple‐negative breast cancer cells by regulating miR‐185‐5p/TPD52 axis. OncoTargets Ther. 2020;13:3025‐3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tu Z, Schmollerl J, Cuiffo BG, Karnoub AE. Microenvironmental regulation of long noncoding RNA LINC01133 promotes cancer stem cell‐like phenotypic traits in triple‐negative breast cancers. Stem Cells. 2019;37(10):1281‐1292. [DOI] [PubMed] [Google Scholar]
- 120. Sha S, Yuan D, Liu Y, Han B, Zhong N. Targeting long non‐coding RNA DANCR inhibits triple negative breast cancer progression. Biol Open. 2017;6(9):1310‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Luo L, Tang H, Ling L, et al. LINC01638 lncRNA activates MTDH‐Twist1 signaling by preventing SPOP‐mediated c‐Myc degradation in triple‐negative breast cancer. Oncogene. 2018;37(47):6166‐6179. [DOI] [PubMed] [Google Scholar]
- 122. Vidovic D, Huynh TT, Konda P, et al. ALDH1A3‐regulated long non‐coding RNA NRAD1 is a potential novel target for triple‐negative breast tumors and cancer stem cells. Cell Death Differ. 2020;27(1):363‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shima H, Kida K, Adachi S, et al. Lnc RNA H19 is associated with poor prognosis in breast cancer patients and promotes cancer stemness. Breast Cancer Res Treat. 2018;170(3):507‐516. [DOI] [PubMed] [Google Scholar]
- 124. Ribatti D, Nico B, Ruggieri S, Tamma R, Simone G, Mangia A. Angiogenesis and antiangiogenesis in triple‐negative breast cancer. Transl Oncol. 2016;9(5):453‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang Y, Wu S, Zhu X, et al. LncRNA‐encoded polypeptide ASRPS inhibits triple‐negative breast cancer angiogenesis. J Exp Med. 2020;217(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tao W, Sun W, Zhu H, Zhang J. Knockdown of long non‐coding RNA TP73‐AS1 suppresses triple negative breast cancer cell vasculogenic mimicry by targeting miR‐490‐3p/TWIST1 axis. Biochem Biophys Res Commun. 2018;504(4):629‐634. [DOI] [PubMed] [Google Scholar]
- 127. Lin A, Li C, Xing Z, et al. The LINK‐A lncRNA activates normoxic HIF1alpha signalling in triple‐negative breast cancer. Nat Cell Biol. 2016;18(2):213‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tracy KM, Tye CE, Ghule PN, et al. Mitotically‐associated lncRNA (MANCR) affects genomic stability and cell division in aggressive breast cancer. Mol Cancer Res. 2018;16(4):587‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fan CN, Ma L, Liu N. Comprehensive analysis of novel three‐long noncoding RNA signatures as a diagnostic and prognostic biomarkers of human triple‐negative breast cancer. J Cell Biochem. 2019;120(3):3185‐3196. [DOI] [PubMed] [Google Scholar]
- 130. Liu M, Xing LQ, Liu YJ. A three‐long noncoding RNA signature as a diagnostic biomarker for differentiating between triple‐negative and non‐triple‐negative breast cancers. Medicine. 2017;96(9):e6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang S, Ma F, Xie X, Shen Y. Prognostic value of long non‐coding RNAs in triple negative breast cancer: a PRISMA‐compliant meta‐analysis. Medicine. 2020;99(37):e21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484‐492. [DOI] [PubMed] [Google Scholar]
- 133. Yu J, Zayas J, Qin B, Wang L. Targeting DNA methylation for treating triple‐negative breast cancer. Pharmacogenomics. 2019;20(16):1151‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cancer Genome Atlas N . Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Fang F, Turcan S, Rimner A, et al. Breast cancer methylomes establish an epigenomic foundation for metastasis. Sci Transl Med. 2011;3(75):75ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bermejo JL, Huang G, Manoochehri M, et al. Long intergenic noncoding RNA 299 methylation in peripheral blood is a biomarker for triple‐negative breast cancer. Epigenomics. 2019;11(1):81‐93. [DOI] [PubMed] [Google Scholar]
- 137. Ozgur E, Ferhatoglu F, Sen F, Saip P, Gezer U. Circulating lncRNA H19 may be a useful marker of response to neoadjuvant chemotherapy in breast cancer. Cancer Biomark. 2019;27:11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bi Z, Li Q, Dinglin X, et al. Nanoparticles (NPs)‐meditated LncRNA AFAP1‐AS1 silencing to block Wnt/beta‐catenin signaling pathway for synergistic reversal of radioresistance and effective cancer radiotherapy. Adv Sci (Weinh). 2020;7(18):2000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rinaldi C, Wood MJA. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol. 2018;14(1):9‐21. [DOI] [PubMed] [Google Scholar]
- 140. Schoch KM, Miller TM. Antisense oligonucleotides: translation from mouse models to human neurodegenerative diseases. Neuron. 2017;94(6):1056‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xia Y, Xiao X, Deng X, et al. Targeting long non‐coding RNA ASBEL with oligonucleotide antagonist for breast cancer therapy. Biochem Biophys Res Commun. 2017;489(4):386‐392. [DOI] [PubMed] [Google Scholar]
- 142. Grunweller A, Hartmann RK. Locked nucleic acid oligonucleotides: the next generation of antisense agents? BioDrugs. 2007;21(4):235‐243. [DOI] [PubMed] [Google Scholar]
- 143. Shu Y, Pi F, Sharma A, et al. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv Drug Deliv Rev. 2014;66:74‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Guo P. The emerging field of RNA nanotechnology. Nat Nanotechnol. 2010;5(12):833‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Guo P, Haque F, Hallahan B, Reif R, Li H. Uniqueness, advantages, challenges, solutions, and perspectives in therapeutics applying RNA nanotechnology. Nucleic Acid Ther. 2012;22(4):226‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lee TJ, Yoo JY, Shu D, et al. RNA nanoparticle‐based targeted therapy for glioblastoma through inhibition of oncogenic miR‐21. Mol Ther. 2017;25(7):1544‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Vaidya AM, Sun Z, Ayat N, et al. Systemic delivery of tumor‐targeting siRNA nanoparticles against an oncogenic LncRNA facilitates effective triple‐negative breast cancer therapy. Bioconjug Chem. 2019;30(3):907‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wu B, Yuan Y, Han X, et al. Structure of LINC00511‐siRNA‐conjugated nanobubbles and improvement of cisplatin sensitivity on triple negative breast cancer. FASEB J. 2020;34(7):9713‐9726. [DOI] [PubMed] [Google Scholar]
- 149. Bamodu OA, Huang WC, Lee WH, et al. Aberrant KDM5B expression promotes aggressive breast cancer through MALAT1 overexpression and downregulation of hsa‐miR‐448. BMC Cancer. 2016;16:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


