Abstract
Pancreatic cancer (PC) is difficult to defeat due to mechanism (s) driving metastasis and drug resistance. Cancer stemness is a major challenging phenomenon associated with PC metastasis and limiting therapy efficacy. In this study, we evaluated the pre-clinical and clinical significance of eradicating pancreatic cancer stem cells (PCSC) and its components using a pan-EGFR inhibitor afatinib in combination with gemcitabine. Afatinib in combination with gemcitabine, significantly reduced KrasG12D/+; Pdx-1 Cre (KC) (P<0.01) and KrasG12D/+; p53R172H/+; Pdx-1 Cre (KPC) (P<0.05) derived mouse tumoroids and KPC-derived murine syngeneic cell line growth compared to gemcitabine/afatinib alone treatment. The drug combination also reduced PC xenograft tumor burden (P<0.05) and the incidence of metastasis by affecting key stemness markers, as confirmed by co-localization studies. Moreover, the drug combination significantly decreases the growth of various PC patient-derived organoids (P<0.001). We found that SOX9 is significantly overexpressed in high-grade PC tumors (P<0.05) and in chemotherapy-treated patients compared to chemo-naïve patients (P<0.05). These results were further validated using publicly available datasets. Moreover, afatinib alone or in combination with gemcitabine decreased stemness and tumorspheres by reducing phosphorylation of EGFR family proteins, ERK, FAK, and CSC markers. Mechanistically, afatinib treatment decreased CSC markers by downregulating SOX9 via FOXA2. Indeed, EGFR and FOXA2 depletion reduced SOX9 expression in PCSCs. Taken together, pan EGFR inhibition by afatinib impedes PCSCs growth and metastasis via the EGFR/ERK/FOXA2/SOX9 axis. This novel mechanism of panEGFR inhibitor and its ability to eradicate CSC may serve as a tailor-made approach to enhance chemotherapeutic benefits in other cancer types.
Keywords: Afatinib, pancreatic cancer metastasis, pancreatic cancer stem cells, EGFR, therapy
Introduction
Improving disease-free survival and a patient’s quality of life is the overall goal of research against pancreatic cancer (PC). Despite the availability of several therapeutic options, patient outcomes have not significantly improved. The present therapy for advanced metastatic PC primarily includes gemcitabine and its combination with erlotinib or nab-paclitaxel or FOLFIRINOX [1]. Despite all the available therapies and surgical interventions, the average five-year survival rate for pancreatic ductal adenocarcinoma (PDAC) patients is merely 10% [2]. Approximately 53% of patients upon diagnosis present with an advanced metastatic stage of the disease [1]. In addition, emerging evidence suggests that PC is metastatic even at its conception, which makes targeting this aggressive metastatic cancer challenging [3]. One of the significant factors contributing to metastasis is the mobilization of cancer stem cells (CSCs) [4, 5], a small subpopulation of cells in the tumor, also referred to as side population (SP), which is vital for PC metastasis and drug-resistance [6, 7]. The success of new PC therapies in achieving durable remissions will depend on targeting pancreatic CSCs (PCSCs); therefore, understanding mechanisms for targeting PCSCs is critical.
The EGFR family of proteins is involved in PC’s initiation and progression, with EGFR overexpression being essential for the initiation process [8]. Many ongoing clinical trials are using inhibitors against the EGFR family of proteins for therapy against cancers. The USFDA recently approved one such inhibitor, erlotinib, in combination with gemcitabine for the treatment of advanced PC. However, later this treatment caused dose-limiting toxicity and failed to control the compensatory changes in the phosphorylation of HER3, resulting in resistance against erlotinib [9, 10]. Afatinib is a third-generation pan-EGFR inhibitor (targeting all EGFR family proteins) and is superior in inhibiting PC cell lines compared to erlotinib [9]. It is also an FDA-approved drug for non-small cell lung carcinoma. It is presently being investigated as a single agent and in combination with other chemotherapeutic drugs in 49 clinical trials [11, 12]. Moreover, targeting EGFR alone is not sufficient to eradicate the CSC population [13, 14]. We have previously shown that afatinib can be used to inhibit EGFR proteins in vitro and reduce colony formation and invasion of PDAC cell lines [15]. Here, we tested the potential of EGFR family inhibition via afatinib to specifically target the PCSCs. The use of murine, PDAC patient-derived organoids, novel activated KRAS-dependent murine syngeneic cell lines, and metastatic orthotopic mouse models to directly study the effects of afatinib, gemcitabine, or the drug combination on tumor progression and in vivo growth would be much more relevant to clinical practice.
Results
Afatinib and gemcitabine inhibit the growth of mouse PDAC tumoroids and xenograft tumors
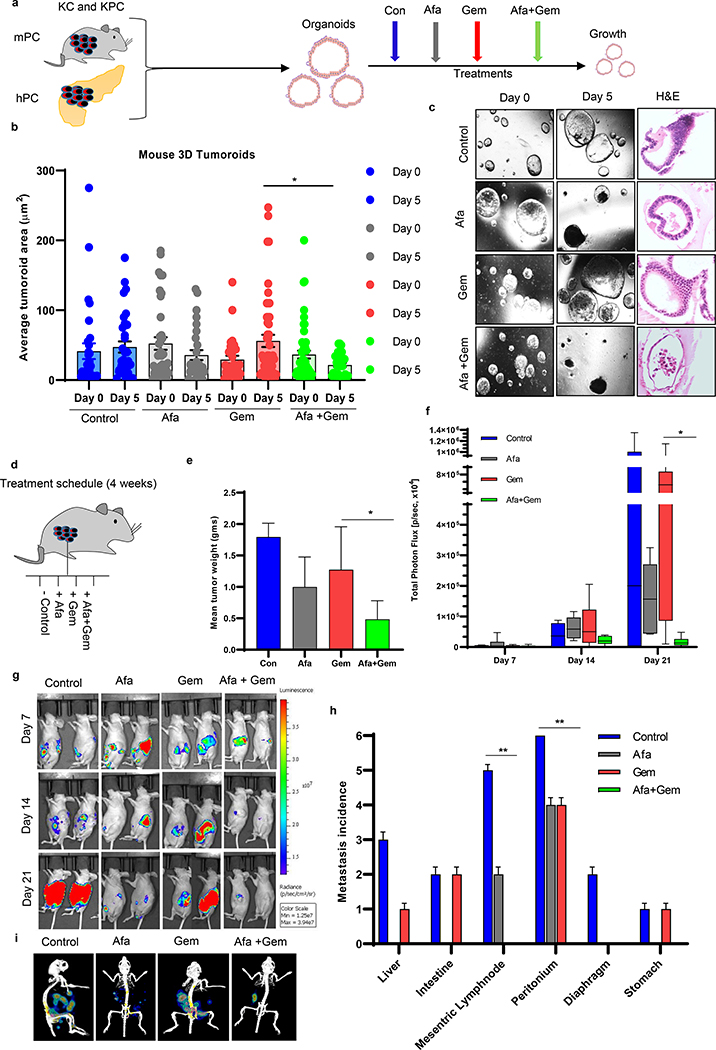
As shown in the schematic overview of the experimental approach (Fig. 1a), mouse autochthonous (KC and KPC) tumoroids and orthotopic xenograft PDAC tumors were treated with afatinib and gemcitabine alone and in combination. During early tumoroid development (both human and murine tumoroids), they grew as single-cell-layered ductal structures; however, as the days progress, they developed more complex structures (Supplementary Fig. 1a, b). Upon treatment with gemcitabine and/or afatinib, a significant (P<0.05) decrease in size (measured in μm2) and significant increase in the percentage of dead KC (P<0.001) and KPC (P<0.001) organoids were observed relative to their respective controls, supporting an enhanced effect of the combination in inhibiting PDAC (Fig 1b, c, and Supplementary Fig. 2a–d). Pairwise comparison between different groups for each day revealed more organoid growth inhibition in afatinib and gemcitabine combination treatment than gemcitabine alone treated groups (Adjusted P<0.05). Time-dependent and different genetic backgrounds (KC versus KPC) based treatment of afatinib and/or gemcitabine in murine organoids exhibited profound and significant drug effects in controlling the growth of KC (P<0.001) organoids compared to KPC (P<0.01) organoids (Supplementary Fig. 2a–d). Few drug-treated organoids remained distorted with no structural complexity, but the majority of organoids were eradicated, as revealed by H&E staining (Fig. 1c, Supplementary Fig. 2e and f).
Fig.1. Effects of afatinib and gemcitabine on mouse pancreatic tumoroids and xenograft models.
a An experimental scheme shows murine (KC and KPC) and human tumoroids development and the treatment strategies. b Box and whisker plots were demonstrating quantitative analysis of the average change in the size of KPC tumoroids treated with afatinib and gemcitabine alone and in combination over 5 days of treatment (N=25/group). c Light microscopic and hematoxylin and eosin (H&E)-stained images of KPC mouse PC tumoroids at days 0 and 5. d Study design and treatment strategies for in vivo PDAC orthotopic mouse experiments. e Bar graph showing mean tumor weights of xenografts (N=6/group) exposed to afatinib and /or gemcitabine for 3 weeks (*P<0.05). f Mice in various treatment conditions were injected (intraperitoneal) with D-luciferin (150 mg/kg body weight) and imaged after 10–15 minutes of administration. All the luminescence images were normalized to the same scale for each time point, and region of interest (ROI) were selected, and photons intensities in the ROIs were measured and quantified in the units of photons/second/cm2/steradian. Bar graph showing the quantification of total photon flux measured for each mouse during bioluminescence imaging on days 7, 14, and 21 day (N=6/group, *P<0.05). g Representative images of mice bearing orthotopic PC tumor and their response towards afatinib and gemcitabine as a single agent and in drug combination at days 7, 14, and 21 after the start of drug treatment. h After 3 weeks of treatment, mice were sacrificed, and the incidence of metastasis to internal organs of the mice was counted; the number of metastatic spots were noted for each organ/group (N=6/group). i Representative Bioluminescence images of mouse with metastasis incidence after treatment with afatinib and /or gemcitabine.
Furthermore, we tested the combination therapy in the PDAC xenograft mouse model. After the orthotopic implantation of luciferase-tagged Capan-1 cells, mice were randomized into four groups as control (PBS), afatinib (orally), gemcitabine (intraperitoneal), and the combination of both (Fig. 1d). Afatinib and gemcitabine treatment significantly reduced primary tumor burden (Fig. 1e–g). When we made pairwise comparisons between groups following an ANOVA method, we observed that the combination of afatinib and gemcitabine significantly reduced tumor weight compared to gemcitabine alone administered mice tumors (Fig. 1e, P<0.05). Further, we used ANOVA to compare the efflux of photon flux between treatment groups for days 7, 14, and 21. We didn’t observe any statistically significant difference among treatment groups on day 7 and 14, but the overall difference among the groups (ANOVA, P<0.05) and pairwise comparison between total photon flux of gemcitabine and its combination with afatinib treated mice (Adjusted P<0.05) at day 21 is significant from gemcitabine alone (Fig. 1f and Supplementary Fig. 3a). Specifically, treatment with afatinib alone and its combination with gemcitabine decreased the metastatic incidence to the liver, mesenteric lymph nodes, intestine and diaphragm, and stomach compared to Afatinib/Gemcitabine alone group (Fig. 1h, i and Supplementary Fig. 3b, c). Fisher’s exact test was used to compare the incidence of metastases between treatment groups. A statistically significant difference in mesenteric lymph node (P<0.01) and peritoneum metastasis (P<0.01) was found among the groups. Taken together, these results suggest that afatinib, in combination with gemcitabine, can inhibit PC growth and metastasis.
Co-treatment of afatinib and gemcitabine inhibit 3D human PDAC tumoroid growth
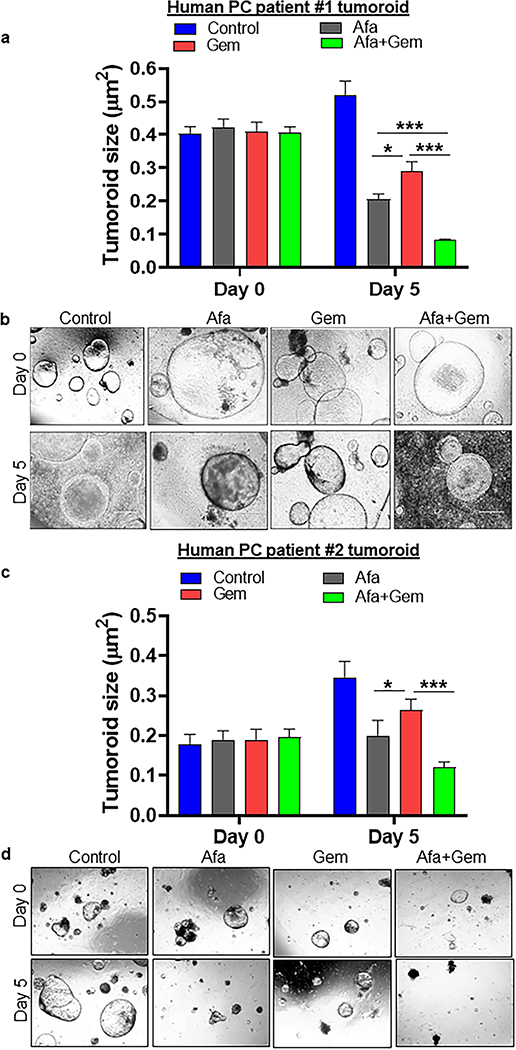
Treatment with afatinib significantly reduced the size of patient-derived PDAC organoids compared to control, whereas the gemcitabine-treated organoids did not show any significant variation in size. However, the combination treatment significantly reduced pancreatic tumor organoids size (Fig. 2a–d and Supplementary Fig. 2g, h). To better understand whether the drugs combination treatment is more effective than a single agent, we performed a two-way ANOVA model. We found a statistical significance between gemcitabine versus afatinib+gemcitabine treatment in human patient-derived organoid # 1, 2, and 3 (P<0.001) (Fig. 2). Comparing gemcitabine versus afatinib as a single agent treatment also showed a statistically significant difference in reducing the growth of organoid #1 (P<0.05), 2 (P<0.05), and 3 (P<0.001). However, treatment with afatinib alone and its combination with gemcitabine significantly reduced organoid growth only in organoid #1 (P<0.001). The difference between these groups in organoid # 2 and #3 is not statistically significant. The variation of statistical differences may be due to different phenotypes of the tumoroids.
Fig. 2. Afatinib and gemcitabine combination treatment inhibited human pancreatic cancer patients-derived tumoroids.
Human pancreatic tumoroids were established and propagated from two different PDAC patients who participated in our in house (UNMC) rapid autopsy program. Microscopic images were taken using Motic digital microscope camera, and tumoroid areas were measured using Motic software. (a and c) Bar graph demonstrating the average tumoroid size before (Day 0) and after treatment (Day 5) with afatinib and /or gemcitabine treatment (patient #1 and 2) (N=25/group, ***P<0.001). (b and d) Representative bright-field microscopic images were depicting the changes in the tumoroid size as a result of afatinib and/or gemcitabine treatments at day 0 and 5, respectively (patient #1 and 2).
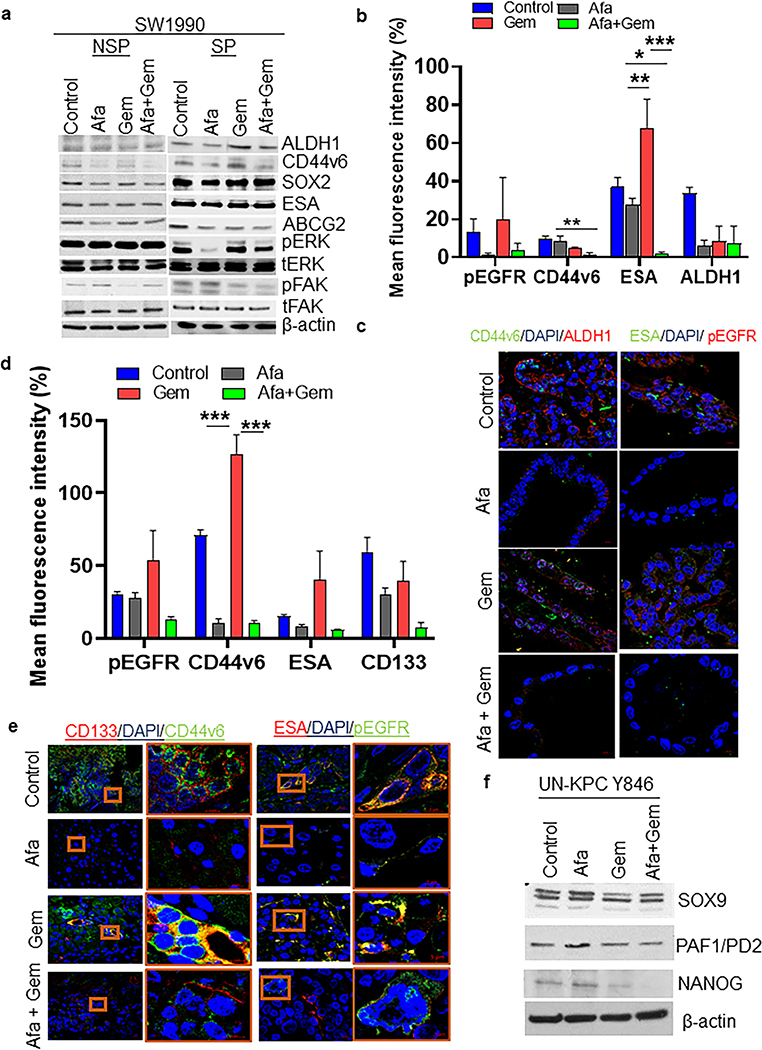
Afatinib specifically targets SP/CSC populations by abrogating CSC markers and oncogenic signaling in human and murine PDAC cells
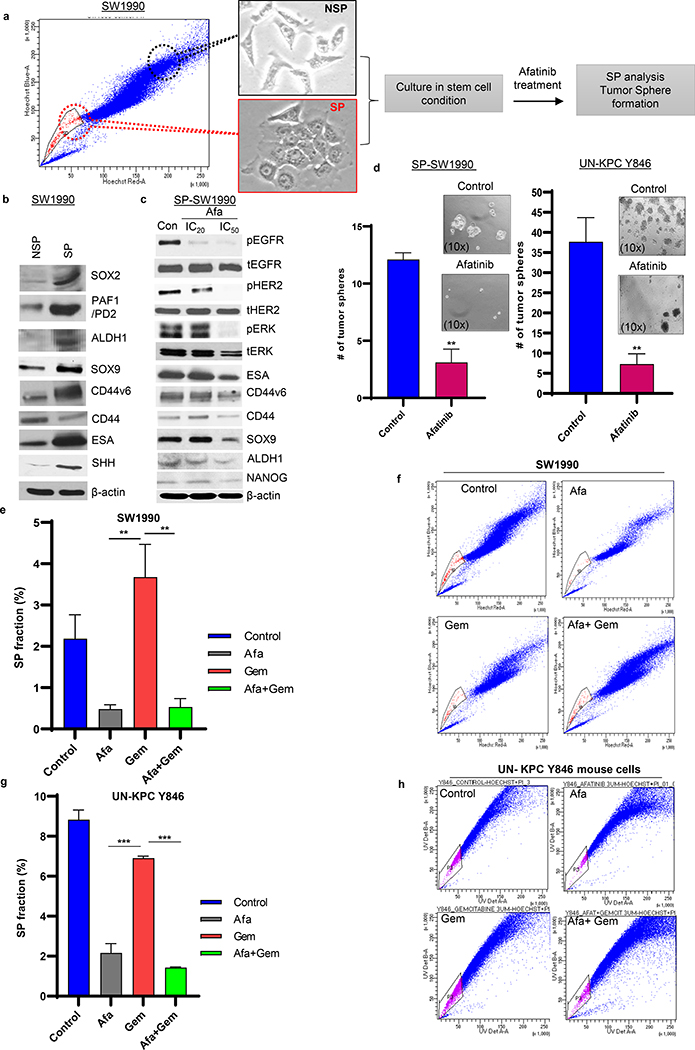
To examine the response of the PCSC population to proposed therapy, we performed Hoechst-based FACS analysis for isolation of CSC (SP)/Non-SP (NSP) from SW1990 PC cells (Fig. 3a) [21]. As shown in Fig. 3b, SP-SW1990 expressed a higher level of SOX2, PAF1/PD2, ESA, ALDH1, SOX9, CD44v6, and SHH CSC markers compared to NSP, which substantiated their identity as CSC. The SP-SW1990 cells were subjected to IC20 and IC50 concentrations of afatinib to elucidate their effects on CSCs (Supplementary Table 1). Consequently, decreased expression of CD44v6, CD44, ESA, SOX9, NANOG, and ALDH1, along with pEGFR, pHER2, and pERK, was observed in SP PDAC cells upon afatinib treatment (Fig. 3c). Additionally, immunofluorescence analysis on the SP cells (isolated from SW1990 and Capan1) also revealed the downregulation of ESA, ALDH1, OCT4, and SOX9 upon afatinib treatment (Supplementary Fig. 4a–d). Tumorsphere assay was performed in human SP-SW1990 (student’s t. test, P<0.01), SP- Capan-1 PDAC cells and murine derived KPC Y846 (student’s t. test, P<0.01) cells treated with afatinib significantly reduced the number of PDAC tumorspheres compared to untreated controls (Fig. 3d and Supplementary Fig. 5a, b). These results suggest that afatinib inhibits PCSC by down-regulating molecules vital for CSC maintenance and self-renewal.
Fig.3. Afatinib inhibited human and mouse pancreatic cancer stem cells and stemness.
a Experimental design for side population (SP)/CSC isolation and functional characterization upon afatinib treatment. b Western blot analysis demonstrating CSC marker expression in the isolated SP/non-SP SW1990 PC cells. c Western blot analysis showing dose-dependent (IC20 and IC50) effects of afatinib and its response on CSC markers in PCSC cells. d Isolated SP/CSC population was seeded in low attachment plates and treated with IC50 concentrations of Afatinib. Bar graph depicting the average number of tumorspheres counts following afatinib treatment in SP cells isolated from SW1990 (left, N=3/group, **P<0.01) and mouse UN-KPC Y846 (right, N=3/group, ***P<0.001) PC cells. Representative light microscopic images showing tumor spheroids affected in response to no-drug and afatinib treatment (insert). e-h Human and mouse PC cells were exposed to afatinib, gemcitabine, and a combination of both for 48 hours. Following treatment, both SW1990 (e and f) and UN-KPC Y846 (g and h) PDAC cells were subjected to SP analysis (FACS) using the Hoechst staining method. The Bar graph represents the percentage of SP cells (using FACS analysis) affected upon drug treatment in SW1990 (N=3/group) and UN-KPC Y846 (N=3/group) PC cells (e and g). FACS plots showing affected SP fraction in treatment groups (Afatinib, Gemcitabine and Afatinib and Gemcitabine combination treatments) compared to unaffected controls PC cells (SW1990 and UN-KPC Y846) (f and h).
Afatinib reduces CSC population/markers, thereby, enhances gemcitabine sensitivity in human and mouse PDAC cells
To confirm the inhibitory effect of afatinib on the EGFR family of proteins, we treated SW1990, Capan1, and UN-KPC Y846 cells with afatinib and gemcitabine alone and in combination for 48 h using respective IC50 values (Supplementary Table 1). We found that treatment with afatinib alone and in combination with gemcitabine effectively downregulated the phosphorylation of EGFR, HER2, and HER3 with no significant change to their total protein expression (Supplementary Fig. 6). Further, to evaluate the impact of afatinib on CSC/SP populations, PC cells subjected to drug combinations were analyzed for the effects on CSC/SP populations [18, 22]. When we compare gemcitabine versus afatinib and gemcitabine combination-treated cells, we found a significantly decreased CSC (ANOVA pairwise adjusted with Tukey’s method (SW1990 (P<0.01), UN-KPC Y846 (P<0.001)) in both human and murine cells (Fig. 3e–h and Supplementary Fig. 5c–f). Our data suggest that afatinib treatment inhibited the CSC population, which is enriched by gemcitabine exposure.
Self-renewal markers are enriched in high-grade PDAC and chemotherapy-treated PDAC patients
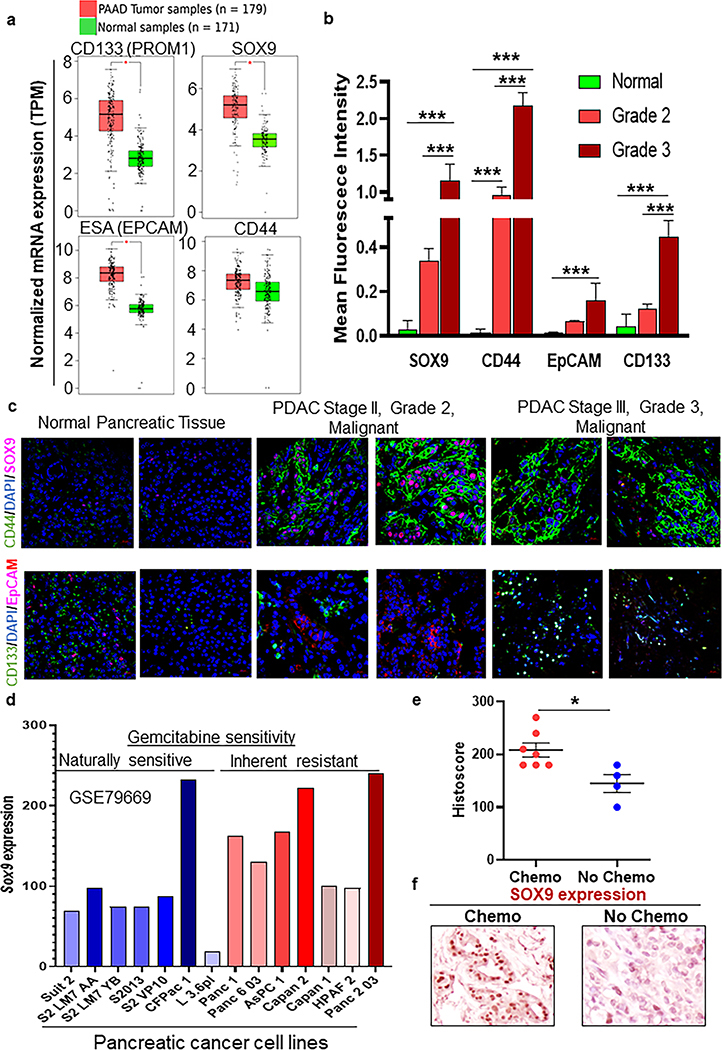
To examine the presence of the CSC population in PDAC patients, we screened cancer databases for expression profiles of PCSC markers. We found SOX9, CD44, CD133 (PROM1), and ESA (EpCAM) CSC markers, and maintenance genes were enhanced in PDAC compared to normal tissues (Fig. 4a and Supplementary Fig. 7a). We also performed immunofluorescence analysis for PCSC markers such as SOX9, CD44, ESA, and CD133 in PDAC tissues consisting of normal (n= 3) and various grades (Grade 2 (n= 6), Grade 3 (n=6) of PDAC progression (Fig. 4b, c). Interestingly, upon segregating PDAC tissues according to patient grades, we observed that SOX9 (adjusted p<0.001), CD44 (adjusted p<0.001), and CD133 (adjusted p<0.001) expression was increased in Grade 3 PDAC tissues than Grade 2 tissues, whereas the difference of ESA (EpCAM) expression was not statistically significant (Fig. 4b, c). Among our CSC marker analysis, SOX9 is known to be a master regulator for several stem cell genes in the pancreas and a valuable marker for the ductal lineage of PC cells [23, 24]. In addition, we analyzed SOX9 expression among gemcitabine sensitive and resistant PDAC cell lines in the GEO database (GSE79669). We found higher SOX9 transcript was associated with inherently gemcitabine resistant PC cell lines compared to gemcitabine sensitive cell lines (Fig. 4d). Consistently, another dataset containing the differential expression of several genes in response to chronic treatment (2 months) of gemcitabine in Panc-1 (gemcitabine resistant) cells showed higher enrichment of SOX9 as compared to untreated parental Panc-1 cells (GSE 80617) (Supplementary Fig. 7b). Further, to identify the clinical significance of chemotherapeutic treatments and its impact on PCSCs, we immunohistochemically stained human PDAC tissue microarray consisting of known history of patients in response to therapy for SOX9 expression. A subset of PDAC tumors with a history of chemotherapeutic treatment was found to exhibit a significantly higher Histoscore of SOX9 expression (Wilcoxon rank-sum test, P<0.05) compared to tissues with no history of chemotherapy (Fig. 4e, f). To substantiate the positive correlation between SOX9 expression in tissues with increased tumor stage or aggressiveness, migration assay was performed in SP PC cells. Supplementary figure 8a–c shows that genetic knockdown (siRNA) of SOX9 in SP cells isolated from SW1990 and Capan-1 PC cells resulted in significantly decreased invasive potential than scramble siRNA transfected cells (P<0.05), suggesting that SOX9 expression is associated with invasive potential and migration (Supplementary Fig. 8a–c).
Fig.4. Cancer stem cell-associated self-renewal markers SOX9, CD44, EpCAM and CD133 expression in normal pancreas, grade 2 and grade 3 PDAC, chemo-treated and untreated pancreatic cancer tissues.
a Box plot representing the expression of vital cancer stem cell markers (PROM1, SOX9, EPCAM, and CD44) and self-renewal genes on PDAC patients (N=179) versus normal controls (N=171, *P<0.05). b Bar graph showing quantitative analysis of CSC markers (SOX9, CD44, EpCAM, and CD133) expression in a stained tissue microarray (Normal =3, Grade 2= 6, and Grade 3 =6). Mean fluorescent intensities for Red, Green, and Blue (DAPI) stains were noted for each image. Red and green staining was then normalized by blue stain to get normalized mean fluorescent intensities (*P<0.05, **P<0.01, ***P<0.001). c Representative immunofluorescence images of human tissue microarray with pancreatic cancer and normal pancreatic cores stained with SOX9, CD44, EpCAM, and CD133. CSC and self-renewal markers expression in normal pancreatic and PDAC pathological stage II, tumor grade 2 malignant, PDAC pathological stage III, tumor grade 3 malignant, tissues. d Bar graph depicting relative expression of SOX9 between gemcitabine sensitive and resistant PDAC cell lines. e Dot plot demonstrating IHC quantification of Sox 9 expression in a subset of patients treated with chemotherapy (N=7) and no chemotherapy (N=4, *P<0.05). f Representative images were showing SOX9 protein expression in chemotherapy-treated and un-treated human PDAC tissues.
Treatment of afatinib significantly decreases the CSC markers in PDAC CSC/SP cells, tumor organoids, pancreatic xenograft tumors, and treatment-resistant PDAC cells
Afatinib alone and in combination with gemcitabine decreased the enrichment of self-renewal and CSC markers ALDH1, CD44v6, SOX2, SOX9, ESA, CD44, ABCG2, and inhibited activation of oncogenic pathways such as ERK and FAK in SP-SW1990 compared and NSP-SW1990 cells (Fig. 5a). As shown in supplementary figure 5g, afatinib alone and its combination with gemcitabine treatment modestly reduced stemness markers CD44 and SOX9 in Capan-1 PC cells, suggesting a pronounced mode of afatinib action on PC stemness reduction. Additionally, afatinib treatment alone and in combination with gemcitabine decreased CSC markers such as CD44v6, ALDH1, CD133, and ESA, along with pEGFR in KPC organoids and primary xenograft tumors, suggesting that afatinib inhibits PCSC stemness by downregulating CSC markers (Fig. 5b–e). In addition, murine PDAC cell lines (UN-KPC Y846) treated with afatinib and/or gemcitabine showed decreased CSC markers expression (SOX9, PAF1/PD2, and NANOG) (Fig. 5f). The expression of CSC markers has been shown to be critical drivers of PC metastasis [25–28], and ESA is a well-known marker for PCSCs [29]. In addition, we tested whether gemcitabine treatment-induced enrichment of CSC markers is reduced upon afatinib treatment using gemcitabine resistant cell lines. As shown in supplementary fig. 7c, afatinib treatment in SW1990 GemR cells demonstrates a reduction in CSC markers protein expression (ESA, ALDH1, OCT3/4, β-Catenin, SOX-9) compared to the untreated isogeneic cells. Hence, a reduction in these markers further demonstrates the efficacy of afatinib against PCSC. Interestingly, we also observed an increase in the same markers with gemcitabine treatment, which corroborated our previous data suggesting gemcitabine enriches CSCs (Fig. 4a–f). These data and our earlier results of primary xenograft tumor weight reduction suggest that the addition of afatinib in concurrent with gemcitabine treatment might reduce the drug resistance phenotype by affecting CSC markers expression.
Fig. 5. Afatinib inhibited CSC stemness by downregulating multiple CSC markers in vitro and in vivo xenograft PC models.
a NSP and SP cells were isolated from SW1990 PC cells and subjected to drug treatment for 24 h. Immunoblot analysis of CSC (ALDH1, CD44v6, SOX2, ESA, and ABCG2), oncogenic/proliferative (total and activated ERK) and migratory (total and activated FAK) effectors upon afatinib treatment. (b - e) Co-immunolocalization analysis of CSC proteins in KPC tumoroids and PC xenograft tissues. Bar graph demonstrating quantification of mean fluorescent intensities of CSC proteins (pEGFR, CD44v6, ESA, ALDH1, and CD133) in KPC organoids (b) and PC xenograft tissues (N=5/group, *P<0.05, **P<0.01, ***P<0.001) (d). Representative confocal microscopic images are showing a response of CSC markers upon afatinib/gemcitabine treatment in KPC tumoroids (c) and xenograft tissues (e), scale bars=20 μM. (f) Western blot analysis of CSC markers (SOX9, PAF1/PD2, and NANOG) in mouse UN-KPC Y846 PC cells treated with/without afatinib and gemcitabine and combination. Beta-actin served as loading control in NSP-SW1990, SP-SW1990, and UN-KPC Y846 PC cells.
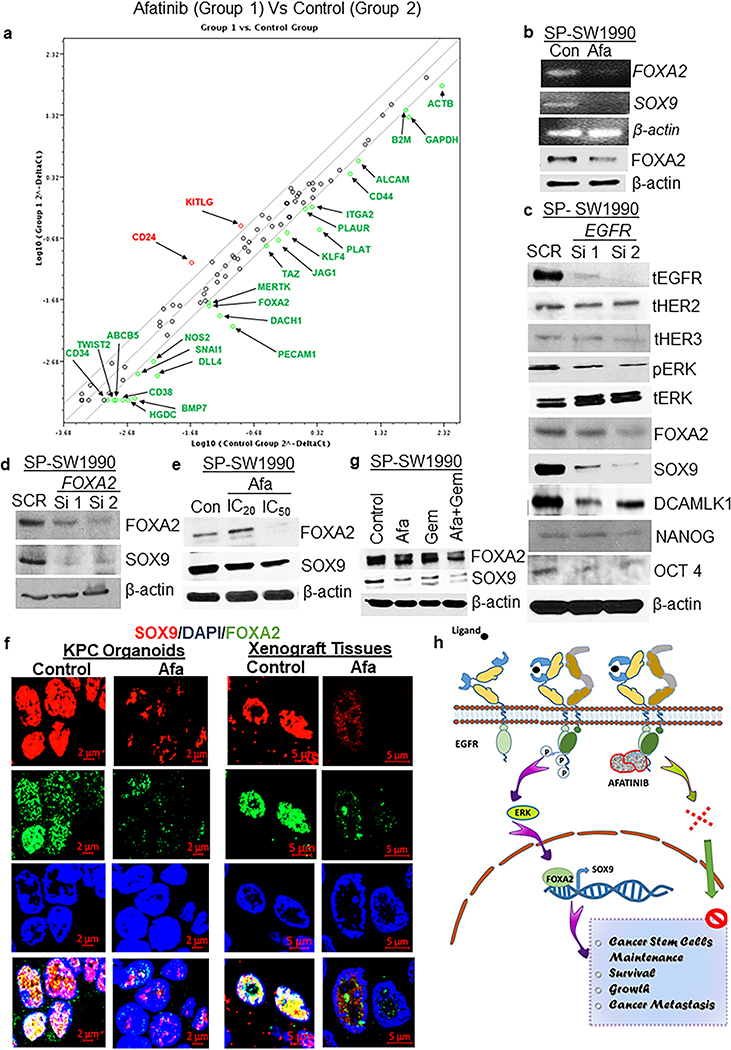
Afatinib decreases CSC populations via ERK/FOXA2/SOX9 signaling in PCSCs
To delineate the mechanism of action of afatinib, SW1990 cells were treated with afatinib (IC50) for 4 days, followed by RNA isolation and PCR array analysis. Afatinib treatment led to a greater than 2-fold decrease in 23 transcription factors (ABCB5, ALCAM, BMP7, CD38, CD44, DACH1, DLL4, FOXA2, ITGA2, JAG1, KLF4, MERTK, NOS2, PECAM1, PLAT, PLAUR, PTPRC, SNAI1, TAZ, TWIST2, ACTB, B2M, and HGDC) important for CSCs (Fig. 6a). FOXA2 is an important player in promoting the self-renewal of pancreatic progenitor cells [30, 31]. It is involved in the regulation of SOX9 expression, which is another self-renewal marker for PCSC [31], both of which were downregulated upon afatinib treatment. Further, qRT-PCR and western blot analysis for FOXA2 and SOX9 expression validated a decrease in mRNA and protein levels upon afatinib treatment on SP-SW1990 (Fig. 6b), suggesting a probable mechanism of action.
Fig.6. Afatinib decreased the CSC population via EGFR/ERK/FOXA2/SOX9 signaling in PCSCs.
a PCR array for CSC and self-renewal markers in SP/CSC cells (SP-SW1990) after 4 days of treatment with afatinib. b RT-PCR analysis showing downregulation of FOXA2 and SOX9 mRNA (Top 3 images) and immunoblot analysis of FOXA2 and β-actin (loading control) proteins (bottom 2 blots) in PCSC in response to afatinib treatment. c Transient suppression of EGFR in SP-SW1990 cells and its effects on CSC markers (FOXA2, SOX9, DCAMLK1, NANOG, and OCT4) and oncogenic signaling molecules (pERK) along with a reduction of total EGFR protein. d Immunoblot analysis of PCSC cells transiently knockdown for FOXA2 and its impact on SOX9 protein. e Immunoblot analysis showing a reduction in FOXA2 and SOX9 protein expression upon dose-dependent (IC20 and IC50 concentrations) treatments with afatinib alone in SPPC cells. f Immunofluorescence analysis of FOXA2 and SOX9 protein co-expression in afatinib-treated KPC tumoroids and mouse primary xenograft tumors. g Immunoblot analysis of FOXA2 and SOX9 protein expression in PC cells treated with afatinib/gemcitabine alone and combination therapies. h Schematic model of the hypothesized signaling axis by which afatinib inhibits the PCSC, scale bars= 5 μM and 2 μM.
To further corroborate the possibility of FOXA2 regulating SOX9, the SOX9 promoter region was scanned for FOXA2 binding motifs revealing eight possible binding sites (Supplementary Fig. 9a). It has been reported previously that the phosphorylated form of ERK regulates FOXA2 transcription [32], and hence we examined pERK levels upon EGFR gene knockdown studies and pharmacological treatment with afatinib. As expected, afatinib and knockdown of EGFR decreased pERK levels in the PC cell line and the isolated SP/CSC population, suggesting that afatinib inhibits PCSCs by inhibiting EGFR/ERK/FOXA2/SOX9 signaling along with multiple other CSC markers such as OCT4, NANOG, and DCAMLK1 (Fig. 3c, Fig. 6b and c).
Further, two independent siRNAs and dose-dependent treatment of afatinib downregulate FOXA2 mRNA, which led to a decrease in SOX9 expression (Fig. 6d and e). To determine if afatinib engages the same mechanism in organoids and xenograft tissues, KPC organoids and xenograft sections were stained with FOXA2 and SOX9. The results revealed that afatinib decreased expression of these markers (Fig. 6f). Next, our logical step is to investigate the robust response of afatinib beyond the primary tumor. Hence, we evaluated the differential expression pattern of SOX9 in the different metastatic tumors collected from the same mice of the Capan-1 xenograft model. Immunofluorescence analysis revealed that oral administration of afatinib for 5 days a week sequentially for 3 weeks resulted in a decrease in mean fluorescent intensity of SOX9 protein expression in liver (P<0.01) and diaphragmatic (P<0.05) metastatic tumor cells compared with untreated control metastatic tumors (Supplementary Fig. 10a, b). We also observed that SOX9 expression is high in primary xenograft tissues compared with metastatic tumors, which may be due to bulk tumor mass in the primary site compared with metastatic organs. Our results suggest that panEGFR inhibition by afatinib reduces not only primary xenograft growth by reducing SOX9 expression but also suppresses SOX9 expression in metastatic PC tumor, substantiating the role of afatinib in mitigating tumor escape. The NSP/SP-PDAC cells treated with afatinib alone and in combination with gemcitabine were also examined for FOXA2 and SOX9 protein expression.
Interestingly, PC SP lysates show a decrease in FOXA2 and SOX9 levels (Fig. 6g) whereas, NSP cells treated with the same drug combination exhibited a marginal reduction in FOXA2 and SOX9 expression (Supplementary Fig. 9b). Further, to evaluate whether the mode of action of afatinib is specific to SP or a general phenomenon that could happen in NSP cells, we performed western blot analysis to detect common cell death markers activation such as PARP and caspase in single-agent and drug combination-treated SP and NSP PC cells. We found that afatinib alone and in combination with gemcitabine induces cell death/apoptosis by activating PARP and Caspase 3 cleavage in both SP and NSP cells relative to respective untreated controls (Supplementary Fig. 9c). In parallel, KPC tumoroids and PC xenografts treated with afatinib alone and in combination with gemcitabine also showed a drastic and significant decrease in the mean fluorescent intensity of CSC markers (SOX9, and DCAMLK1) along with pERK and FOXA2 in the drug combination (Supplementary Fig. 9d–g). Altogether, our data provide a novel mechanism of action by which afatinib inhibits the PCSCs in PC (Fig. 6h).
Discussion
PC is a devastating disease with an abysmal prognosis [1, 33]. Current treatment options offer an average overall survival benefit of 5.6 months to 11 months [34]. Aggressiveness and early metastasis is the primary cause of mortalities in PDAC [3, 13, 29, 35]. The cells that likely initiate early metastasis are CSCs, which have the potential to seed tumors, promote metastasis and provide drug resistance [13, 29, 35]. Afatinib is a multipotent panEGFR family inhibitor that provides a broad spectrum of the molecular targeted therapeutic protocol. Pre-clinical studies on afatinib in glioblastoma and head and neck cancer specifically targeted the CSC population by inhibiting CSC markers expression. In particular, afatinib treatment specifically affected temozolomide and radiotherapy-induced CSC population in these two cancers [36, 37]. Interestingly, a recent Phase IIIB clinical study demonstrated an encouraging safety and efficacy profile of afatinib in tyrosine kinase inhibitor naïve and chemotherapy pre-treated locally advanced/metastatic NSCLC patients [38]. Afatinib demonstrates a complete response to EGFR mutated metastatic lung cancer of primary pancreatic tumors [39]. Currently, early phases (I and II) of clinical trials recruit patients with advanced solid tumors, including PC, with objectives of (i) evaluating safety and efficacy of afatinib in combination with capecitabine (NCT02451553), (ii) utilizing afatinib for personalized medicine approach based on inclusion and exclusion criteria specific for each cancer (NCT03878524) and (iii) targeted approach coupled with genetic abnormality testing (mutation, amplification) (NCT02465060). Thus, pre-clinical and clinical studies support the combination approach of using afatinib with traditional cytotoxic agents. In addition, targeting CSCs using afatinib may provide an opportunity to impede tumor growth, metastasis and to overcome treatment failures in PDAC; however, currently used chemotherapies are not designed to target these cells [1, 13, 40].
To begin targeting CSC, we showed the inhibitory effects of afatinib and gemcitabine combination and monotherapies on mouse organoids and xenograft tumors. We identified that pan–EGFR inhibitor afatinib targeted SOX9 and thereby reduced the CSC population in 2D, 3D, and in vivo models. Secondly, we analyzed the expression of PC specific CSC markers in PC patient specimens in varying tumor grades and observed a high SOX9, CD133, and CD44 expression was found to be associated with disease progression. Additionally, we showed that traditional chemotherapy in patients was associated with enrichment of SOX9 expression. Finally, we elucidated that signaling through EGFR/SOX9 axis vital for cancer stem cells can be targeted via afatinib. Our results provide a rationale for targeting CSC in PDAC to improve chemotherapeutic efficacy.
As a strategy to target pancreatic CSCs, we tested combination therapy of pan-EGFR inhibitor afatinib with first-line therapeutic drug gemcitabine. PDAC is known to overexpress EGFR family members HER1 (40–70%) and HER2 (22%) [15], which are vital for the self-renewal and maintenance of CSCs in PC [40]. Inhibition of EGFR family proteins has been reported to downregulate tumor growth and metastasis in multiple cancers, including PDAC [41]. In PC, the presence of EGFR in serum is correlated with poor prognosis [42], and inhibition of EGFR via erlotinib has shown limited survival benefits for PC patients when combined with gemcitabine [43]. Afatinib inhibits all EGFR family members by binding to their ATP binding domain and inactivating downstream signaling [44]. Although afatinib is an FDA-approved drug for non-small cell lung carcinoma, emerging evidence suggests its role as a potent inhibitor of multiple tumor types [9, 11, 44]. In this study, we illustrated that inhibition of the EGFR family of proteins using afatinib significantly reduced the stemness properties of pancreatic CSCs and provided antitumor activity against PC that was enhanced with gemcitabine treatment. Similarly, several other studies have reported that EGFR family inhibition suppresses CSCs in brain, lung, and breast cancers, amongst others [14, 45, 46].
To test the effect of afatinib in PDAC models, we developed 3-D tumor organoids from KC, KPC autochthonous mouse tumors, and human PDAC tumors. Tumor organoids recapitulate tumor architecture and biology, as observed in the patient population [47], making them suitable for therapy-related studies. Treatment with afatinib alone or in combination with gemcitabine effectively inhibited the tumor size and architecture in mouse and human tumor organoids compared to gemcitabine alone, providing evidence for its efficacy as a combination therapy. Our 3D PDAC organoid results are promising to move forward towards precision medicine. Our organoids model results are in concordance with the reduction in tumor burden and metastatic incidence compared to gemcitabine alone, as observed in our xenograft mouse model experiments. These results also align with our previous study depicting a reduction in tumor burden upon inhibition of EGFR family proteins by canertinib [15]. The observed increase in tumor organoid size and structural complexity upon gemcitabine treatment, although not significant, may stem from the enrichment of pancreatic CSCs, since CSCs are resistant to gemcitabine [35].
Interestingly, afatinib alone and in combination with gemcitabine decreased distant metastatic spread, suggesting afatinib could be inhibiting the metastasis in PDAC patients. This observation is supported by other reports of metastasis being linked to the overexpression and amplification of EGFR and its other family members HER2, HER3, and HER4 in primary cancers like colorectal, ovarian, non-small cell lung carcinoma, and PC [48–51]. We decided to analyze the effect of afatinib on the CSC population since CSC is vital for metastasis. Our data revealed that afatinib significantly reduced the SP/CSC population in PDAC cell lines and inhibited their self-renewal and tumorigenic potential. Correspondingly, our immunofluorescence staining results showed that afatinib treatment downregulated the expression of pEGFR and CSC markers in PC organoids and xenograft tumor tissues.
In our study, we delineated a novel mechanism by which afatinib selectively inhibits CSCs in PC. Afatinib treatment reduced several CSC markers along with oncogenic signaling molecules like ERK and FAK. Additionally, we found afatinib downregulated 22 vital transcription factors and CSC markers by more than two-fold compared to control. Amongst the downregulated genes, FOXA2 caught our attention because of its role in stem cell self-renewal [52]. It is vital for lineage specification in the pancreatic organogenesis, and its expression is essential for pancreatic progenitor cells. FOXA2 is also known to regulate SOX9 [31], both SOX9 and FOXA2 were downregulated by afatinib treatment, and availability of FOXA2 binding motifs in the SOX9 proximal region suggested a probable mode of action. SOX9 is known to be vital for CSC self-renewal and is a master regulator for several stem cell markers, is also regulated by pERK [53], which is a downstream molecule of EGFR. This led us to hypothesize that afatinib targets the EGFR/ERK/FOXA2/SOX9 axis to inhibit PCSCs and their stemness properties. Knockdown of EGFR decreased expression of FOXA2 and SOX9, and silencing of FOXA2 decreased expression of SOX9, confirming our hypothesis. To further verify the mechanism of action, we stained afatinib-treated tissue and organoid sections with fluorescent antibodies against pERK, SOX9, and FOXA2 and observed decreased expression of these stemness molecules. Overall, these results affirm that afatinib inhibits CSC maintenance and proliferation via EGFR/ERK/FOXA2/SOX9 inhibition in PC, thereby decreasing PC growth and metastasis.
Conclusion
In the current study, afatinib treatment alone decreased the CSC/SP fraction in PC models by altering their self-renewal/tumorigenic potential. Afatinib, unlike gemcitabine, reduced tumor organoid size and inhibited CSC markers. Furthermore, combination therapy with afatinib and gemcitabine effectively reduced tumor burden and metastatic incidence in the xenograft mouse model of PC. Our mechanistic studies indicate that afatinib acts on pancreatic CSC by inhibiting EGFR/ERK/FOXA2 to target SOX9 (Fig. 6h). Overall, Afatinib and gemcitabine combination therapy may improve clinical outcomes for advanced metastatic PDAC patients by targeting PCSC.
Materials and methods
Immunohistochemistry and confocal immunofluorescence microscopy
PDAC specimens (TMA), tumoroids, and orthotopic tumors were processed for immunohistochemistry (IHC) and confocal studies and scored as described before (16).
PC organoid and syngeneic cell line development from LSL-KrasG12D/+; Pdx-1-Cre (KC), and LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx-1-Cre (KPC) mouse and human PDAC patient tumors
3D pancreatic tumoroids were established from KC (50 weeks old) and KPC (25 weeks old) autochthonous mouse models and human pancreatic tissues using methods described previously [16] with minor modifications as given in supplementary materials and methods.
In vivo xenograft mouse model and treatment strategy
Details about ethical statements, study animals, and experimental strategies adapted for in vivo xenograft studies are given in supplementary materials and methods.
Cell culture, reagents, and transfection
SW1990, COLO 357, and Capan-1 human PC cell lines were obtained from ATCC and were cultured in high glucose DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin. Novel mouse syngeneic cell line UN-KPC Y846 was developed using our previously-established protocol [17]. We also developed gemcitabine resistant SW1990 cell lines initiated with IC20 value of gemcitabine (Supplementary Table 1). Afatinib was purchased from Selleck Chemicals, TX, USA, while gemcitabine was obtained from Sigma Aldrich. SOX9 human siRNA oligo duplex (cat#: SR304532) was purchased from Origene Technologies. Additional details about isogenic cell line generation and transient transfection methods are described in supplementary materials and methods.
Isolation of CSC/side population
Side population/CSC population was sorted using BD FACS Aria (BD Biosciences), as shown previously [18] and briefly described in supplementary materials and methods.
Tumorsphere assay and drug treatments
An in vitro tumorsphere assay was performed using CSC and non-CSC populations isolated from SW1990 and UN-KPC Y846 cells, as shown previously [19]. After seven days, spheres were viewed under the microscope, counted and photographed.
Immunoblot analysis
Western blot analysis was performed as described previously [20]. The blots were incubated with the following primary antibodies: pEGFR, EGFR, pHER2, HER2, pHER3, HER3, pERK, ERK, ESA, FOXA2, SOX2, SOX9, CD44V6, CD44, EpCAM, ALDH1, ABCG2, pFAK, FAK, NANOG, SHH, PD2/PAF1, OCT4, DCAMLK1, β- Catenin, Cleaved PARP and Cleaved Caspase-3. All primary antibodies were purchased from Cell Signaling Technology (CST). Secondary antibodies used were: Rabbit, 1:1000; CST and β-actin (mouse, 1:5000; Sigma Aldrich, A1978).
RNA isolation and quantitative real-time PCR (qRT-PCR)
RNA isolation, quantification, cDNA synthesis, and qRT-PCR analysis were briefly described in supplementary materials and methods and primers used in this study as supplementary table 2.
Statistical analysis
Detailed statistical analysis were briefly described in supplementary materials and methods.
Supplementary Material
Acknowledgments
Funding
This research manuscript is supported, in parts, by grants from the National Institutes of Health (This work was supported, in parts, by the National Institutes of Health (P01 CA217798, R01 CA183459, R01 CA195586, R01 CA201444, R01 CA210637, R01 CA228524, R01 CA247471, U01 CA200466, and U01 CA210240).
Footnotes
Conflict of interest
SKB is one of the co-founders of Sanguine Diagnostics and Therapeutics, Inc. The other authors disclosed no potential conflicts of interest.
References
- 1.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015; 12: 319–34. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012; 148: 349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clevers H The cancer stem cell: premises, promises and challenges. Nat Med. 2011; 17: 313–9. [DOI] [PubMed] [Google Scholar]
- 5.Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell. 2007; 1: 241–2. [DOI] [PubMed] [Google Scholar]
- 6.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007; 1: 313–23. [DOI] [PubMed] [Google Scholar]
- 7.Tanase CP, Neagu AI, Necula LG, Mambet C, Enciu AM, Calenic B et al. Cancer stem cells: involvement in pancreatic cancer pathogenesis and perspectives on cancer therapeutics. World J Gastroenterol. 2014; 20: 10790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012; 22: 304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioannou N, Dalgleish AG, Seddon AM, Mackintosh D, Guertler U, Solca F et al. Anti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cells. Br J Cancer. 2011; 105: 1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007; 445: 437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engle JA, Kolesar JM. Afatinib: A first-line treatment for selected patients with metastatic non-small-cell lung cancer. Am J Health Syst Pharm. 2014; 71: 1933–8. [DOI] [PubMed] [Google Scholar]
- 12.Modjtahedi H, Cho BC, Michel MC, Solca F. A comprehensive review of the preclinical efficacy profile of the ErbB family blocker afatinib in cancer. Naunyn Schmiedebergs Arch Pharmacol. 2014; 387: 505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Pancreatic cancer stem cells: emerging target for designing novel therapy. Cancer Lett. 2013; 338: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark PA, Iida M, Treisman DM, Kalluri H, Ezhilan S, Zorniak M et al. Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition. Neoplasia. 2012; 14: 420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seshacharyulu P, Ponnusamy MP, Rachagani S, Lakshmanan I, Haridas D, Yan Y et al. Targeting EGF-receptor(s) - STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget. 2015; 6: 5164–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011; 141: 1762–72. [DOI] [PubMed] [Google Scholar]
- 17.Torres MP, Rachagani S, Souchek JJ, Mallya K, Johansson SL, Batra SK. Novel pancreatic cancer cell lines derived from genetically engineered mouse models of spontaneous pancreatic adenocarcinoma: applications in diagnosis and therapy. PLoS One. 2013; 8: e80580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karmakar S, Seshacharyulu P, Lakshmanan I, Vaz AP, Chugh S, Sheinin YM et al. hPaf1/PD2 interacts with OCT3/4 to promote self-renewal of ovarian cancer stem cells. Oncotarget. 2017; 8: 14806–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimmakayala RK, Seshacharyulu P, Lakshmanan I, Rachagani S, Chugh S, Karmakar S et al. Cigarette Smoke Induces Stem Cell Features of Pancreatic Cancer Cells via PAF1. Gastroenterology. 2018; 155: 892–908.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majhi PD, Lakshmanan I, Ponnusamy MP, Jain M, Das S, Kaur S et al. Pathobiological implications of MUC4 in non-small-cell lung cancer. Journal of thoracic oncology. 2013; 8: 398–407. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar FH, Li Y, Wang Z, Kong D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir. 2009; 64: 489–500. [PMC free article] [PubMed] [Google Scholar]
- 22.Vaz AP, Ponnusamy MP, Rachagani S, Dey P, Ganti AK, Batra SK. Novel role of pancreatic differentiation 2 in facilitating self-renewal and drug resistance of pancreatic cancer stem cells. Br J Cancer. 2014; 111: 486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seymour PA. Sox9: a master regulator of the pancreatic program. Rev Diabet Stud. 2014; 11: 51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shroff S, Rashid A, Wang H, Katz MH, Abbruzzese JL, Fleming JB et al. SOX9: a useful marker for pancreatic ductal lineage of pancreatic neoplasms. Hum Pathol. 2014; 45: 456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K, Li Z, Jiang P, Zhang X, Zhang Y, Jiang Y et al. Co-expression of CD133, CD44v6 and human tissue factor is associated with metastasis and poor prognosis in pancreatic carcinoma. Oncol Rep. 2014; 32: 755–63. [DOI] [PubMed] [Google Scholar]
- 26.Ding Q, Miyazaki Y, Tsukasa K, Matsubara S, Yoshimitsu M, Takao S. CD133 facilitates epithelial-mesenchymal transition through interaction with the ERK pathway in pancreatic cancer metastasis. Mol Cancer. 2014; 13: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou G, Chiu D, Qin D, Niu L, Cai J, He L et al. Detection and clinical significance of CD44v6 and integrin-beta1 in pancreatic cancer patients using a triplex real-time RT-PCR assay. Appl Biochem Biotechnol. 2012; 167: 2257–68. [DOI] [PubMed] [Google Scholar]
- 28.Nomura A, Banerjee S, Chugh R, Dudeja V, Yamamoto M, Vickers SM et al. CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget. 2015; 6: 8313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald TL, McCubrey JA. Pancreatic cancer stem cells: association with cell surface markers, prognosis, resistance, metastasis and treatment. Adv Biol Regul. 2014; 56: 45–50. [DOI] [PubMed] [Google Scholar]
- 30.Herreros-Villanueva M, Zhang JS, Koenig A, Abel EV, Smyrk TC, Bamlet WR et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013; 2: e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci U S A. 2007; 104: 10500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010; 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hennig R, Grippo P, Ding XZ, Rao SM, Buchler MW, Friess H et al. 5-Lipoxygenase, a marker for early pancreatic intraepithelial neoplastic lesions. Cancer Res. 2005; 65: 6011–6. [DOI] [PubMed] [Google Scholar]
- 34.Han H, Von Hoff DD. SnapShot: pancreatic cancer. Cancer Cell. 2013; 23: 424–24 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Broeck A, Gremeaux L, Topal B, Vankelecom H. Human pancreatic adenocarcinoma contains a side population resistant to gemcitabine. BMC Cancer. 2012; 12: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macha MA, Rachagani S, Qazi AK, Jahan R, Gupta S, Patel A et al. Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget. 2017; 8: 20961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vengoji R, Macha MA, Nimmakayala RK, Rachagani S, Siddiqui JA, Mallya K et al. Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J Exp Clin Cancer Res. 2019; 38: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passaro A, Laktionov KK, Poltoratskiy A, Egorova I, Hochmair M, Migliorino MR et al. Afatinib in EGFR TKI-naive patients (pts) with locally advanced/metastatic NSCLC harbouring EGFR mutations: An interim analysis of a phase IIIB trial. Ann Oncol. 2019; 30 Suppl 2: ii48–ii49. [Google Scholar]
- 39.Furuya T, Shimada J, Okada S, Tsunezuka H, Kato D, Inoue M. Successful treatment with afatinib for pancreatic metastasis of lung adenocarcinoma: a case report. J Thorac Dis. 2017; 9: E890–E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzgerald TL, Lertpiriyapong K, Cocco L, Martelli AM, Libra M, Candido S et al. Roles of EGFR and KRAS and their downstream signaling pathways in pancreatic cancer and pancreatic cancer stem cells. Adv Biol Regul. 2015; 59: 65–81. [DOI] [PubMed] [Google Scholar]
- 41.Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer. 2013; 13: 663–73. [DOI] [PubMed] [Google Scholar]
- 42.Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004; 29: e1–8. [DOI] [PubMed] [Google Scholar]
- 43.Kelley RK, Ko AH. Erlotinib in the treatment of advanced pancreatic cancer. Biologics. 2008; 2: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirsh V Next-Generation Covalent Irreversible Kinase Inhibitors in NSCLC: Focus on Afatinib. BioDrugs. 2015; 29: 167–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abhold EL, Kiang A, Rahimy E, Kuo SZ, Wang-Rodriguez J, Lopez JP et al. EGFR kinase promotes acquisition of stem cell-like properties: a potential therapeutic target in head and neck squamous cell carcinoma stem cells. PLoS One. 2012; 7: e32459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steelman LS, Fitzgerald T, Lertpiriyapong K, Cocco L, Follo MY, Martelli AM et al. Critical Roles of EGFR Family Members in Breast Cancer and Breast Cancer Stem Cells: Targets for Therapy. Curr Pharm Des. 2016; 22: 2358–88. [DOI] [PubMed] [Google Scholar]
- 47.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015; 160: 324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang H, Wu L, Chen J, Mishra M, Chawsheen HA, Zhu H et al. Sulfiredoxin Promotes Colorectal Cancer Cell Invasion and Metastasis through a Novel Mechanism of Enhancing EGFR Signaling. Mol Cancer Res. 2015; 13: 1554–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukhopadhyay P, Lakshmanan I, Ponnusamy MP, Chakraborty S, Jain M, Pai P et al. MUC4 overexpression augments cell migration and metastasis through EGFR family proteins in triple negative breast cancer cells. PLoS One. 2013; 8: e54455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Zhao W, Xu QW, Wang XS, Zhang Y, Zhang J. IQGAP3 promotes EGFR-ERK signaling and the growth and metastasis of lung cancer cells. PLoS One. 2014; 9: e97578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou X, Hu Y, Dai L, Wang Y, Zhou J, Wang W et al. MicroRNA-7 inhibits tumor metastasis and reverses epithelial-mesenchymal transition through AKT/ERK1/2 inactivation by targeting EGFR in epithelial ovarian cancer. PLoS One. 2014; 9: e96718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavon N, Yanuka O, Benvenisty N. The effect of overexpression of Pdx1 and Foxa2 on the differentiation of human embryonic stem cells into pancreatic cells. Stem Cells. 2006; 24: 1923–30. [DOI] [PubMed] [Google Scholar]
- 53.Ling S, Chang X, Schultz L, Lee TK, Chaux A, Marchionni L et al. An EGFR-ERK-SOX9 signaling cascade links urothelial development and regeneration to cancer. Cancer Res. 2011; 71: 3812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.