Cefiderocol is a cephalosporin designed to treat multidrug-resistant Gram-negative infections. By forming a chelated complex with ferric iron, cefiderocol is transported into the periplasmic space via bacterial iron transport systems and primarily binds to penicillin-binding protein 3 (PBP3) to inhibit peptidoglycan synthesis. This mode of action results in cefiderocol having greater in vitro activity against many Gram-negative bacilli than currently used carbapenems, β-lactam/β-lactamase inhibitor combinations, and cephalosporins.
KEYWORDS: melioidosis, Burkholderia pseudomallei, cefiderocol, antimicrobial resistance, AMR, minimum inhibitory concentration, MIC
ABSTRACT
Cefiderocol is a cephalosporin designed to treat multidrug-resistant Gram-negative infections. By forming a chelated complex with ferric iron, cefiderocol is transported into the periplasmic space via bacterial iron transport systems and primarily binds to penicillin-binding protein 3 (PBP3) to inhibit peptidoglycan synthesis. This mode of action results in cefiderocol having greater in vitro activity against many Gram-negative bacilli than currently used carbapenems, β-lactam/β-lactamase inhibitor combinations, and cephalosporins. Thus, we investigated the in vitro activity of cefiderocol against a total of 246 clinical isolates of Burkholderia pseudomallei from Queensland, Australia. The collection was composed primarily of bloodstream (56.1%), skin and soft tissue (16.3%), and respiratory (15.9%) isolates. MICs of cefiderocol ranged from ≤0.03 to 16 mg/liter, whereas the MIC90 was 0.125 mg/liter. Based upon CLSI clinical breakpoints for cefiderocol against Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia, three isolates (1.2%) would be classified as nonsusceptible (MIC > 4 mg/liter). Using EUCAST non-species-specific (pharmacokinetic/pharmacodynamic [PK/PD]) clinical breakpoints or those set for Pseudomonas aeruginosa, four isolates (1.6%) would be resistant (MIC > 2 mg/liter). Further testing for coresistance to meropenem, ceftazidime, trimethoprim-sulfamethoxazole, amoxicillin-clavulanate, and doxycycline was performed on the four isolates with elevated cefiderocol MICs (>2 mg/liter); all isolates exhibited resistance to amoxicillin-clavulanic acid, while three isolates also displayed resistance to at least one other antimicrobial. Cefiderocol was found to be highly active in vitro against B. pseudomallei primary clinical isolates. This compound shows great potential for the treatment of melioidosis in countries of endemicity and should be explored further.
INTRODUCTION
Burkholderia pseudomallei is endemic to tropical and subtropical regions, including Southeast Asia and northern Australia, and has a presence in Africa and South America (1–3). The bacteria are known to cause melioidosis, where pneumonia, sepsis, neurological disease, and visceral abscesses are commonly described clinical presentations (1, 4). Treatment for B. pseudomallei infections requires an intensive 2- to 8-week intravenous treatment with an antimicrobial such as a carbapenem or cephalosporin, followed by an orally administered eradication treatment for 3 to 6 months with an antimicrobial such as trimethoprim-sulfamethoxazole (5). Infections frequently require intensive care admission and are associated with significant morbidity (6). The associated morbidity is due, in part, to the intrinsic antimicrobial resistance (AMR) of B. pseudomallei as well as acquired resistance to antimicrobials such as tetracyclines, β-lactam/β-lactamase inhibitors, and, rarely, carbapenems (7–9). The mortality rate for patients presenting with melioidosis ranges from 10% to 40% in Burma, Singapore, Thailand, and Vietnam (6), while in Australia, it remains approximately 10% (10). Recent research has focused on new or repurposed compounds in order to provide improved treatment options for such infections, particularly given the potential of B. pseudomallei to be genetically manipulated or used as an agent of biologic warfare (11, 12).
Cefiderocol (formerly S-649266; Shionogi & Co. Ltd., Osaka, Japan) inhibits peptidoglycan synthesis and has been described as almost ubiquitously stable against β-lactamases, resulting in greater efficacy than those of carbapenems, currently available β-lactam/β-lactamase inhibitor combinations, and cephalosporins (13–16). It has great potential for the treatment of pathogenic multidrug-resistant (MDR) and carbapenem-nonsusceptible Gram-negative bacilli (GNB), as they have increased significantly worldwide (17, 18). This has resulted in diminished appropriate antimicrobial therapy options (19, 20).
Cefiderocol recently received FDA approval for the treatment of complicated urinary tract infections (21) on the basis of noninferiority against imipenem/cilastatin (22) as well as approval for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (23, 24). Another trial against nosocomial pneumonia has also been completed (13, 25), with efficacy demonstrated against Gram-negative pathogens such as Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii (26, 27). Provisional CLSI (Clinical and Laboratory Standards Institute) in vitro breakpoints have been set at ≤4 mg/liter (susceptible [S]) and ≥16 mg/liter (resistant [R]) for these pathogens (26). Recently, EUCAST (European Committee on Antimicrobial Susceptibility Testing) set much lower in vitro breakpoints of ≤2 mg/liter (susceptible) and >2 mg/liter (resistant) for Enterobacterales and Pseudomonas aeruginosa and as a pharmacokinetic/pharmacodynamic (PK/PD; non-species specific) breakpoint (28). Furthermore, cefiderocol has shown promising efficacy against Burkholderia pseudomallei and Burkholderia mallei, resulting in MIC90s of 0.25 and 4 mg/liter, respectively (n = 30) (29). Therefore, with the need for new treatment options for B. pseudomallei or other GNB and the promising efficacy of cefiderocol as a treatment option, the aim of this study was to assess the in vitro activity of cefiderocol against a large sample size of clinical B. pseudomallei isolates from Queensland, Australia, where melioidosis is endemic.
RESULTS
Disc diffusion.
All 246 isolates were subjected to cefiderocol disc diffusion testing. Zone diameters ranged from 11 to 46 mm (Table 1). One isolate, C137 (38 mm), demonstrated inner growth on cefiderocol disc diffusion testing (12 mm), indicating a heterogeneous population or expression. The resistant subpopulation, deemed C137R, was directly subjected to broth microdilution (BMD) susceptibility testing. However, the resistant subpopulation could not be isolated via subculture from the C137 sample; therefore, the original C137 sample was used for further testing.
TABLE 1.
In vitro MICs and disc diffusion ranges for clinical B. pseudomallei isolates against cefiderocol
| Species | No. of isolates at a cefiderocol MIC (mg/liter) of: |
MIC (mg/liter) |
Disc zone (mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ≥32 | Range | 50% | 90% | ||
| B. pseudomallei (n = 246) | 112 | 89 | 37 | 1 | 2 | 1 | 0 | 1 | 1 | 2 | 0 | ≤0.03–16 | 0.06 | 0.125 | 11–46 |
Broth microdilution.
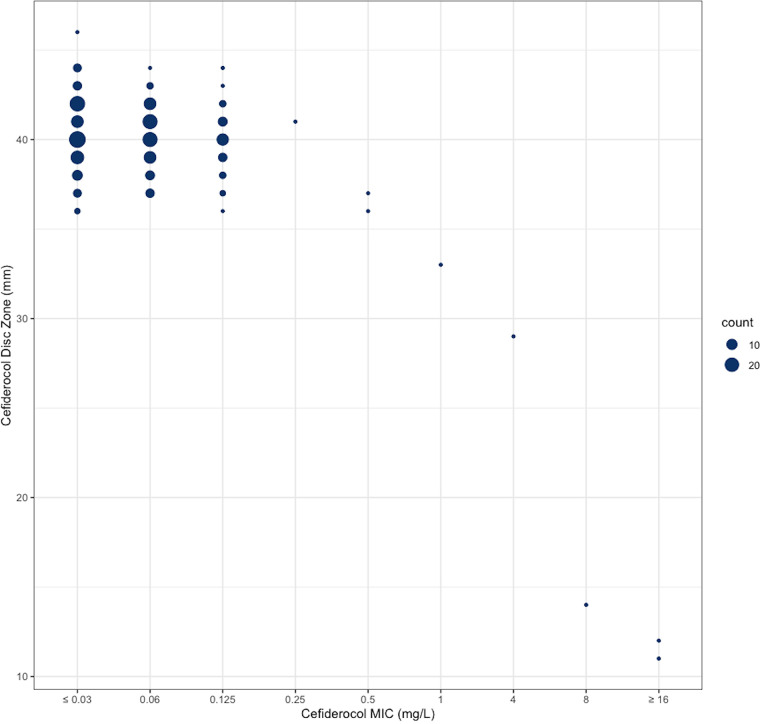
Cefiderocol BMD testing was performed on all 246 isolates. The MIC range, MIC50, and MIC90 were ≤0.03 to 16, 0.06, and 0.125 mg/liter, respectively (Table 1; Fig. 1). An association between zone diameter and MIC was only observed for MIC values ≥8 mg/liter (Fig. 2).
FIG 1.
Histogram of cefiderocol MICs (mg/liter) for 246 clinical Burkholderia pseudomallei isolates.
FIG 2.
Scatterplot of cefiderocol disc zone diameters (mm) versus cefiderocol MICs (mg/liter) for 246 clinical Burkholderia pseudomallei isolates.
Isolates expressing elevated cefiderocol MICs.
According to CLSI breakpoints for cefiderocol against other GNB, two isolates (0.8%) would be categorized as resistant (MIC, ≥16 mg/liter), while one isolate (0.4%) would be categorized as intermediate (MIC, 8 mg/liter). With respect to EUCAST breakpoints, four isolates would be deemed resistant (MIC, >2 mg/liter). These four isolates comprised two lung specimens, one from a cystic fibrosis patient and one from a persistent B. pseudomallei infection, one skin and soft tissue infection, and one bloodstream infection. All four individuals had serious underlying comorbidities. Coresistance was examined via BMD testing between cefiderocol and meropenem, ceftazidime, amoxicillin-clavulanic acid, doxycycline, and trimethoprim-sulfamethoxazole; all isolates exhibited resistance to amoxicillin-clavulanic acid, while three isolates also displayed resistance to meropenem or trimethoprim-sulfamethoxazole (Table 2).
TABLE 2.
MICs of B. pseudomallei clinical isolates with elevated cefiderocol concentrations
| Isolate IDa |
B. pseudomallei isolate metadata collection |
Clinically relevant antimicrobial (mg/liter) and categoryb
|
||||||
|---|---|---|---|---|---|---|---|---|
| Site | Yr | FDC | MEM | CAZ | AMC | SXT | DOX | |
| C137Rc | SSTI | 2016 | 16 | 1 | 2 | >32 R | 0.5/9.5 | 2 |
| C4 | Lung | 2006 | 8 | >16 R | 2 | >32 R | 0.12/2.28 | 2 |
| C50 | Lung | 2000 | 4 | 4 | ≥16 NS | >32 R | 4/76 R | 4 |
| C194 | Blood | 2018 | 16 | >16 R | 2 | >32 R | 0.12/2.28 | 2 |
ID, identifier.
FDC, cefiderocol. Clinically relevant antimicrobials included meropenem (MEM), ceftazidime (CAZ), amoxicillin-clavulanic acid (AMC), trimethoprim-sulfamethoxazole (SXT), and doxycycline (DOX). Nonsusceptible (NS) and resistant (R) categories were determined by CLSI breakpoints.
Broth microdilution testing was performed on isolate C137 as the resistant subpopulation was not able to be subcultured from this sample.
DISCUSSION
B. pseudomallei infections pose a significant mortality risk to patients. Furthermore, treatment can be especially burdensome in terms of the need for prolonged intravenous and oral antibiotic therapy. Due to the virulence of the organism and its predilection for immunocompromised hosts, patients with melioidosis frequently present with severe disease requiring intensive care admission. While uncommon, emergent resistance may also occur to compromise treatment. A compound such as cefiderocol with such low MICs could be used as an empirical therapy to improve clinical outcomes of B. pseudomallei infection in regions of endemicity, where multidrug-resistant Gram-negative bacilli are circulating. An approach such as this may result in improved clinical responses during empirical therapy and increased bacterial killing in difficult-to-treat infections, such as those in the central nervous system, bone, joint, and lymphatic system.
Disc diffusion.
The relationship between cefiderocol disc diffusion zone diameter and BMD MIC values was observed at concentrations ≥8 mg/liter of cefiderocol. As there are no zone diameters specified for cefiderocol disc diffusion against B. pseudomallei, very major errors, major errors, and minor errors were unable to be calculated. Zone diameter was able to differentiate isolates with MICs of ≥8 mg/liter consistently at 25 mm or less. Isolates with MICs between 1 and 4 mg/liter ranged between 35 to 25 mm, where the relationship may be more prominent with a larger number of isolates with MICs within this range (Fig. 2). This relationship was previously observed in more than 1,300 Gram-negative bacteria, where isolates with MICs of ≥8 mg/liter were consistently differentiated at 20 mm or less (30). These relationships were present in species within the Enterobacterales as well as P. aeruginosa and A. baumannii; however, Stenotrophomonas maltophilia isolates (≥2 mg/liter) were differentiated at 15 mm or less (30). Better discrimination among susceptible isolates may be seen with a lower disc mass, but only 30-μg discs are currently available commercially.
Broth microdilution.
In this study, isolates with MIC values of >2 mg/liter were considered to be elevated based on EUCAST clinical breakpoints set for Enterobacterales, P. aeruginosa, and S. maltophilia (31). Only a few were identified in this collection (n = 4; 1.6%), coinciding with the rates previously reported for Australian B. pseudomallei (0.0% to 4.0%) against other clinically relevant antimicrobials (32–34). This finding is expected, with previous in vitro studies identifying very few resistant isolates for several Gram-negative bacilli species. For example, only 24/753 (3.2%) isolates showed MICs of ≥8 mg/liter from a global data set (35), while 54/8,954 (0.6%) isolates in North America and Europe had MICs of ≥4 mg/liter (27), and no resistant isolates were identified among 189 isolates from Greece (36). The MIC90 of the 246 B. pseudomallei isolates in this study was 0.125 mg/liter, not significantly less than that of the previously described MIC90 of 0.25 mg/liter obtained from a study of 30 B. pseudomallei isolates (29). However, an MIC90 of 0.125 mg/liter is similar to that observed in the closely related Burkholderia cepacia complex, with MIC90 values of 0.016 to 0.12 mg/liter attained from small studies (13, 27), yet much less than that of sister species B. mallei, with an MIC90 of 4 mg/liter (29). Notably, an increase in sample size has not substantially increased the MIC90 of cefiderocol in other pathogens such as P. aeruginosa, A. baumannii, K. pneumoniae, and Escherichia coli (27, 35, 36). Therefore, it is reasonable to assume that the MIC90 of Australian B. pseudomallei will remain low against cefiderocol, even with a dramatic increase in sample size.
Isolates with elevated cefiderocol MICs.
As no individual had received cefiderocol previously, we suggest any MIC increase is likely a reflection of including isolates from clinically complex individuals with underlying comorbidities. The four isolates with MICs of ≥4 mg/liter were derived from individuals with cystic fibrosis, diabetes mellitus, and persistent B. pseudomallei infection. Our results predict elevated cefiderocol MICs will more than likely be encountered in individuals that may have previously received extensive antimicrobial exposure due to underlying comorbidities such as cystic fibrosis or in the case of persistent or relapsing B. pseudomallei infections.
Five clinically relevant antimicrobials were selected for further BMD testing against B. pseudomallei isolates with elevated cefiderocol MICs to assess the presence of coresistance. Interestingly, all four isolates exhibited resistance to amoxicillin-clavulanic acid (introduced by penA mutations in B. pseudomallei [8]), while one of these isolates also exhibited nonsusceptibility to ceftazidime and resistance to trimethoprim-sulfamethoxazole. Two isolates also exhibited meropenem resistance. Previous studies have highlighted that cefiderocol activity is not generally impacted by resistance to other antimicrobials (13–15, 27, 36). Our findings agree, with amoxicillin-clavulanic acid, meropenem, ceftazidime, or trimethoprim-sulfamethoxazole resistance not appearing to impact cefiderocol MICs. As these isolates are from individuals with known significant comorbidities and who were likely exposed to antimicrobials previously, it is suggested that an increase in MIC could be a result of exposure to multiple antimicrobials in these individuals. Characterization of the mechanism of resistance in strains with elevated MIC is under way.
Prospective role of cefiderocol against B. pseudomallei infections.
Cefiderocol has great potential as an antimicrobial therapy for multidrug-resistant B. pseudomallei infections. This is supported by the findings of this study in combination with the efficacy of cefiderocol being unaffected by resistance to other antimicrobials in vitro (36) as well as the antimicrobial being well tolerated by patients (13, 16) and found to be safe in clinical trials to date (16). However, this study also suggests that cefiderocol may lose efficacy in vitro against B. pseudomallei isolates derived from individuals with significant underlying comorbidities or with significant prior antibiotic exposure. Nevertheless, further in vitro testing, followed by in vivo trials of cefiderocol against B. pseudomallei, is warranted.
Conclusion.
Cefiderocol demonstrates a high degree of activity in vitro against 246 clinical isolates of B. pseudomallei from Queensland, Australia. The MIC50 and MIC90 were 0.06 and 0.125 mg/liter, respectively. Resistant isolates with MICs of >2 mg/liter were infrequently demonstrated in this collection (1.6%) based on EUCAST non-species-specific breakpoints and were most commonly associated with significant underlying comorbidities. Cefiderocol shows promise as an intravenous agent for the management of acute melioidosis based upon in vitro susceptibility testing, particularly in regions where carbapenem-resistant Gram-negative organisms and B. pseudomallei cocirculate. Further investigation into the role of cefiderocol as a treatment for melioidosis and likely mechanisms of resistance would be of great value.
MATERIALS AND METHODS
Isolate collection and storage.
Clinical B. pseudomallei isolates were prospectively collected from patients admitted to Queensland Health hospitals, Australia, over a period from 1999 to 2018. All isolates during this period were glycerol stocked at the time of collection and stored at −80°C until further use. A small number of isolates were referred from external laboratories. These isolates were retrieved from −80°C storage from three microbiology laboratories in Queensland (Forensic and Scientific Services, Coopers Plains, Australia; Pathology Queensland and Townsville and Central laboratories). Isolates were transferred to the University of Queensland Centre for Clinical Research (UQCCR) and stored at −80°C prior to testing. Demographic and clinical information for the isolates and patients was retrieved from the laboratory information system (Auslab; PJA Solutions). Ethical approval for this study was granted by the Forensic and Scientific Services Human Ethics Committee (HREC/17/QFSS/12). Biosafety approvals for this study were granted by the Institutional Biosafety Committee UQCCR (IBC/210B/SOM/2017). This project was performed under the study number S-649266-EF-312-N.
Isolates.
A total of 246 isolates from 246 individuals were included in this study (Table 3). All isolates were from Queensland, where B. pseudomallei was predominantly isolated from the blood, lung, skin, and soft tissues of male patients (Table 3).
TABLE 3.
Demographic and clinical data for primary B. pseudomallei isolates
| Category | Value(s) |
|---|---|
| Demographics | |
| No. of individuals | 246 |
| Age range (yrs) | 6–90 |
| Gender (%) | |
| Female | 25.6 |
| Male | 73.2 |
| Unknown | 1.2 |
| Clinical data | |
| Isolate collection site (n) | |
| Blood | 138 |
| Skin and soft tissue | 40 |
| Sputum | 21 |
| Lung | 18 |
| Urine | 12 |
| Bone/joint/fluid | 4 |
| Unknown | 3 |
| Pleural fluid | 3 |
| Liver | 1 |
| Prostate | 1 |
| Brain | 1 |
| Lymph node | 1 |
| Gastrointestinal tract | 1 |
| Gut | 1 |
| Peritoneal fluid | 1 |
| No. of isolates | 246 |
Disc diffusion.
Disc diffusion susceptibility testing was performed using 30-μg cefiderocol discs provided by Mast Group Ltd. (Bootle, UK). Discs were stored at 4°C prior to use. Isolates were subcultured from storage on 5% horse blood agar (Micromedia, Victoria, Australia) for 18 to 24 h at 37°C prior to preparation of a 0.5 McFarland solution in 0.9% sterile saline. Mueller-Hinton agar plates (Micromedia) were inoculated with test isolates using the Kirby-Bauer method and incubated for 16 to 20 h at 37°C. Control strains E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were included with each run (Table 4).
TABLE 4.
Accepted range of MICs and disc inhibition zone diameters for cefiderocol against quality control organisms E. coli and P. aeruginosa and respective non-species-specific breakpoints
| Organism | MIC (mg/liter) |
Zone diameter (mm) |
||||
|---|---|---|---|---|---|---|
| CLSI |
EUCAST |
CLSI | EUCAST | |||
| Range | Break pointa | Range | Break pointb | |||
| E. coli ATCC 25922 | 0.06–0.5 | 0.06–0.5 | 25–31 | 24–30 | ||
| P. aeruginosa ATCC 27853 | 0.06–0.5 | 0.06–0.5 | 22–31 | 22–28 | ||
| Non-species specific | ||||||
| Susceptible | ≤4 | ≤2 | ||||
| Resistant | ≥16 | >2 | ||||
CLSI breakpoints set for Enterobacterales, P. aeruginosa, A. baumannii, and S. maltophilia.
EUCAST PK/PD, Enterobacterales and P. aeruginosa breakpoints.
Broth microdilution.
Broth microdilution (BMD) testing was performed using 96-well plates provided by Shionogi & Co., Ltd. (Osaka, Japan) and prepared by International Health Management Associates (IHMA; Schaumburg, USA). Iron-depleted cation-adjusted Mueller-Hinton broth (ID-CAMHB) was used according to CLSI and the manufacturer’s recommendations (37). Plates were stored at −20°C and thawed for 1 h prior to use. Isolates were subcultured from storage on to 5% horse blood agar (Micromedia, Victoria, Australia) for 18 to 24 h at 37°C prior to preparation of a 0.5 McFarland solution in 0.85% sterile saline for a final inoculum of 5 × 105 cells, as per CLSI BMD guidelines. Plates were inoculated once for each isolate and incubated at 37°C for 16 to 20 h prior to reading. One positive-growth-control well and one negative-control well were included in each plate. Quality control of cefiderocol was performed using E. coli ATCC 25922 and P. aeruginosa ATCC 27853 (Table 4) (38). CLSI provides susceptible (≤4 mg/liter) and resistant (≥16 mg/liter) interpretative criteria for Enterobacterales and some nonfermenters such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia (but not B. pseudomallei) (26). More recently, EUCAST breakpoints of susceptible (≤2 mg/liter) and resistant (>2 mg/liter) have been described (28), and as such, EUCAST breakpoints were applied to isolates in this study (Table 4).
Further antimicrobial susceptibility testing was performed on isolates with cefiderocol MICs of >2 mg/liter (n = 4), including clinically relevant antimicrobials meropenem, ceftazidime, doxycycline, amoxicillin-clavulanic acid, and trimethoprim-sulfamethoxazole (Table 2). BMD testing was performed in-house where 96-well plates were prepared using the Tecan D300e Digital Dispenser (HP Inc., CA, USA). Standard inoculation (5 × 105 CFU), incubation in ambient air at 37°C, and endpoint reading at 24 h were performed. Quality control was performed in duplicates for each batch of plates made, including E. coli ATCC 25922, E. coli ATCC 35218, and S. aureus ATCC 29213, to ensure correct dilutions of each antimicrobial (38). The ranges of meropenem, ceftazidime, doxycycline, amoxicillin-clavulanic acid, and trimethoprim-sulfamethoxazole tested were 0.06 to 16, 0.06 to 16, 0.12 to 16, 0.12/2 to 32/2, and 0.06/1.19 to 32/608 mg/liter, respectively. MICs were interpreted according to CLSI breakpoints (39).
Longitudinal analysis.
Multiple isolates collected from 11 patients over time were also identified in the collection (n = 13). These isolates comprised specimens collected from an alternate anatomical site or isolates collected weeks, months, or years apart. The antimicrobial treatment of patients remains unknown. These isolates were subject to the same methods as described above and are presented in Tables S1 and S2 the supplemental material.
Supplementary Material
ACKNOWLEDGMENTS
This work received financial support from Shionogi & Co., LTD., under the stand-alone service agreement.
We thank Pathology Queensland collaborators Haakon Bergh and Tracey Shepherd for their efforts during this study.
D. L. Paterson reports nonfinancial support from Ecolab Pty Ltd., Whiteley Corporation, and Kimberly-Clark Professional during the conduct of the study, personal fees from Merck, Shionogi, Achaogen, AstraZeneca, Leo Pharmaceuticals, Bayer, GlaxoSmithKline, Cubist, Venatorx, Accelerate, and Pfizer, and grants from Shionogi and Merck (MSD), outside the submitted work. P. N. A. Harris reports grants from Merck (MSD), Sandoz, and Shionogi and personal fees from Pfizer and Sandoz, outside the submitted work. All other authors declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Cheng AC, Currie BJ. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McRobb E, Kaestli M, Price EP, Sarovich DS, Mayo M, Warner J, Spratt BG, Currie BJ. 2014. Distribution of Burkholderia pseudomallei in Northern Australia, a land of diversity. Appl Environ Microbiol 80:3463–3468. doi: 10.1128/AEM.00128-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, Golding N, Dance DAB, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NPJ, Peacock SJ, Hay SI. 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1:15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 4.Hemarajata P, Baghdadi JD, Hoffman R, Humphries RM. 2016. Burkholderia pseudomallei: challenges for the clinical microbiology laboratory. J Clin Microbiol 54:2866–2873. doi: 10.1128/JCM.01636-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiersinga WJ, Currie BJ, Peacock SJ. 2012. Melioidosis. N Engl J Med 367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 6.Gassiep I, Armstrong M, Norton R. 2020. Human melioidosis. Clin Microbiol Rev 33:e00006-19. doi: 10.1128/CMR.00006-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhodes KA, Schweizer HP. 2016. Antibiotic resistance in Burkholderia species. Drug Resist Updat 28:82–90. doi: 10.1016/j.drup.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweizer HP 2012. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 7:1389–1399. doi: 10.2217/fmb.12.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wuthiekanun V, Amornchai P, Saiprom N, Chantratita N, Chierakul W, Koh GCKW, Chaowagul W, Day NPJ, Limmathurotsakul D, Peacock SJ. 2011. Survey of antimicrobial resistance in clinical Burkholderia pseudomallei isolates over two decades in northeast Thailand. Antimicrob Agents Chemother 55:5388–5391. doi: 10.1128/AAC.05517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currie BJ, Dance DAB, Cheng AC. 2008. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg 102:S1–S4. doi: 10.1016/S0035-9203(08)70002-6. [DOI] [PubMed] [Google Scholar]
- 11.Ross BN, Myers JN, Muruato LA, Tapia D, Torres AG. 2018. Evaluating new compounds to treat Burkholderia pseudomallei infections. Front Cell Infect Microbiol 8:210. doi: 10.3389/fcimb.2018.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laws TR, Taylor AW, Russell P, Williamson D. 2019. The treatment of melioidosis: is there a role for repurposed drugs? A proposal and review. Expert Rev Anti Infect Ther 17:957–967. doi: 10.1080/14787210.2018.1496330. [DOI] [PubMed] [Google Scholar]
- 13.Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, Idowu T, Domalaon R, Schweizer F, Zhanel MA, Lagacé-Wiens PRS, Walkty AJ, Noreddin A, Lynch Iii JP, Karlowsky JA. 2019. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant Gram-negative bacilli. Drugs 79:271–289. doi: 10.1007/s40265-019-1055-2. [DOI] [PubMed] [Google Scholar]
- 14.Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. doi: 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito A, Sato T, Ota M, Takemura M, Nishikawa T, Toba S, Kohira N, Miyagawa S, Ishibashi N, Matsumoto S, Nakamura R, Tsuji M, Yamano Y. 2017. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 62:e01454-17. doi: 10.1128/AAC.01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saisho Y, Katsube T, White S, Fukase H, Shimada J. 2018. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for Gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother 62:e02163-17. doi: 10.1128/AAC.02163-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 2014. Antimicrobial resistance global report on surveillance. http://www.who.int/drugresistance/documents/surveillancereport/en/.
- 19.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 20.Hawkey PM, Warren RE, Livermore DM, McNulty CAM, Enoch DA, Otter JA, Wilson APR. 2018. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother 73:iii2–iii78. doi: 10.1093/jac/dky027. [DOI] [PubMed] [Google Scholar]
- 21.Shionogi & Co., Ltd. 2019. FETROJA (cefiderocol) approved by the FDA for treatment of complicated urinary tract infections (cUTI) in adult patients with limited or no alternative treatment options. Shionogi & Co., Ltd., Osaka, Japan. [Google Scholar]
- 22.Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JCA, Ariyasu M, Tenke P, Nagata TD. 2018. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis 18:1319–1328. doi: 10.1016/S1473-3099(18)30554-1. [DOI] [PubMed] [Google Scholar]
- 23.Office of the Commissioner, FDA. 2020. FDA approves antibiotic to treat hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. https://www.fda.gov/news-events/press-announcements/fda-approves-antibiotic-treat-hospital-acquired-bacterial-pneumonia-and-ventilator-associated.
- 24.Pharmacy Practice News Staff. 2020. FDA grants HABP/VABP indication for Fetroja. https://www.pharmacypracticenews.com/FDA-Approvals/Article/09-20/FDA-Grants-HABP-VABP-Indication-for-Fetroja-/60715?ses=ogst.
- 25.Tsuji M, Jakielaszek C, Marchand CL. 2016. Cefiderocol (S-649266), a novel siderophore cephalosporin: in vitro activity against biothreat pathogens, poster. IDWeek, New Orleans, LA. [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial disk susceptibility tests. CLSI standard M02. 13th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 27.Karlowsky JA, Hackel MA, Tsuji M, Yamano Y, Echols R, Sahm DF. 2019. In vitro activity of cefiderocol, a siderophore cephalosporin, against Gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015–2016: SIDERO-WT-2015. Int J Antimicrob Agents 53:456–466. doi: 10.1016/j.ijantimicag.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 28.EUCAST. 2020. Breakpoints for cefiderocol from EUCAST. Addendum (May 20202) to EUCAST breakpoint tables V.10.0. Breakpoints to be included in EUCAST breakpoint tables v 11.0, January 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/Cefiderocol_addendum_20200501.pdf.
- 29.Tsuji M, Jakielaszek C, Marchand CL. 2016. S-649266, a novel siderophore cephalosporin: in vitro activity against biothreat pathogen. Open Forum Infect Dis 3 (Suppl 1):1832. doi: 10.1093/ofid/ofw172.1380. [DOI] [Google Scholar]
- 30.Masakatsu Tsuji MH, Yoshinori Yamano NK, Daniel F, Sahm RE. 2018. Correlations between cefiderocol broth microdilution MICs and disk diffusion inhibitory zone diameters among target Gram-negative organisms, poster P0186. 28th ECCMID, Madrid, Spain. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2017. CLSI. Performance Standards for antimicrobial susceptibility testing. 27th ed, CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.Crowe A, McMahon N, Currie BJ, Baird RW. 2014. Current antimicrobial susceptibility of first-episode melioidosis Burkholderia pseudomallei isolates from the Northern Territory, Australia. Int J Antimicrob Agents 44:160–162. doi: 10.1016/j.ijantimicag.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Smith S, Hanson J, Currie BJ. 2018. Melioidosis: an Australian perspective. Trop Med Infect Dis 3:27. doi: 10.3390/tropicalmed3010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenney AWJ, Lum G, Fisher DA, Currie BJ. 2001. Antibiotic susceptibility of Burkholderia pseudomallei from tropical northern Australia and implications for therapy of melioidosis. Int J Antimicrob Agents 17:109–113. doi: 10.1016/S0924-8579(00)00334-4. [DOI] [PubMed] [Google Scholar]
- 35.Dobias J, Dénervaud-Tendon V, Poirel L, Nordmann P. 2017. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis 36:2319–2327. doi: 10.1007/s10096-017-3063-z. [DOI] [PubMed] [Google Scholar]
- 36.Falagas ME, Skalidis T, Vardakas KZ, Legakis NJ, Hellenic Cefiderocol Study Group. 2017. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J Antimicrob Chemother 72:1704–1708. doi: 10.1093/jac/dkx049. [DOI] [PubMed] [Google Scholar]
- 37.Huband MD, Ito A, Tsuji M, Sader HS, Fedler KA, Flamm RK. 2017. Cefiderocol MIC quality control ranges in iron-depleted cation-adjusted Mueller-Hinton broth using a CLSI M23-A4 multi-laboratory study design. Diagn Microbiol Infect Dis 88:198–200. doi: 10.1016/j.diagmicrobio.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Kahlmeter G, Brown DFJ, Goldstein FW, MacGowan AP, Mouton JW, Odenholt I, Rodloff A, Soussy C-J, Steinbakk M, Soriano F, Stetsiouk O. 2006. European Committee on Antimicrobial Susceptibility Testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infect 12:501–503. doi: 10.1111/j.1469-0691.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 39.Clinical and Laboratory Standards Institute. 2016. CLSI guideline M45 Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.