This study investigates the optimal meropenem (MEM) dosing regimen for critically ill pediatric patients, for which there is a lack of pharmacokinetic (PK) studies. We conducted a retrospective single-center PK and pharmacodynamic (PD) analysis of 34 pediatric intensive care unit patients who received MEM.
KEYWORDS: extracorporeal membrane oxygenation, continuous renal replacement therapy, therapeutic drug monitoring, systemic inflammatory response syndrome, meropenem
ABSTRACT
This study investigates the optimal meropenem (MEM) dosing regimen for critically ill pediatric patients, for which there is a lack of pharmacokinetic (PK) studies. We conducted a retrospective single-center PK and pharmacodynamic (PD) analysis of 34 pediatric intensive care unit patients who received MEM. Individual PK parameters were determined by a two-compartment analysis. The median (range) age and body weight were 1.4 (0.03 to 14.6) years and 8.9 (2.7 to 40.9) kg, respectively, and eight (23.5%) patients received continuous renal replacement therapy (CRRT), three of whom received extracorporeal membrane oxygenation. Renal function, the systemic inflammatory response syndrome (SIRS) score for the clearance (CL), and the use of CRRT for the central volume of distribution (Vc) were identified as significant covariates. The mean CL, Vc, and peripheral volume of distribution (Vp) were 0.45 liters/kg/h, 0.49 liters/kg, and 0.34 liters/kg, respectively. The mean population CL of MEM increased by 35% in patients with SIRS and Vc increased by 66% in patients on CRRT in the final model. Dosing simulations suggested that the standard dosing regimen provided insufficient PD exposures of a 100% free time above the MIC, and higher doses (40 to 80 mg/kg of body weight/dose every 8 h) with a prolonged 3-h infusion were required to ensure the appropriate PD exposures for patients with SIRS. Our PK model indicated that critically ill pediatric patients are at risk of subtherapeutic exposure under the standard dosing regimen of MEM. A larger, prospective investigation confirming the safety and efficacy of higher concentrations and prolonged infusion of MEM is necessary.
TEXT
Meropenem (MEM) is a broad-spectrum carbapenem with high levels of activity against Gram-positive and Gram-negative pathogens, including Pseudomonas aeruginosa, Acinetobacter spp., and anaerobes (1), and it is one of the most commonly prescribed antibiotics for the empirical treatment of severe infections (2). Because MEM is a small hydrophilic molecule with a low volume of distribution (V) (0.3 liters/kg) and an extremely low degree of protein binding (<2%), its clearance (CL) is mainly influenced by renal function (3). The V is also affected by increased capillary leakage into adjacent tissues, aggressive fluid resuscitation, and ascites in critically ill patients (4). These conditions may result in subtherapeutic serum and tissue concentrations under standard dosing. Pharmacokinetic (PK) parameters can also be significantly altered in cases receiving extracorporeal circulation, such as continuous renal replacement therapy (CRRT) and extracorporeal membrane oxygenation (ECMO) (5–7). These conditions and patients’ characteristics are likely to be inversely related to age because the circuit volume and increased fluid volume in pediatric patients are often proportionally higher than those in adult patients (8).
PK and pharmacodynamic (PD) concepts have shown that β-lactam antimicrobials kill bacteria in a time-dependent manner. Optimal killing activity occurs when the plasma concentration of the unbound fraction (fu) of the drug is maintained above the MIC of the bacteria for a certain percentage of the dosing interval (T) (%fuT>MIC), which has been reported to be approximately 40% in animal studies in vitro and in vivo (9). Some clinical data have shown that critically ill patients may require a higher %fuT>MIC, even as much as 100% (10, 11). Several reports have suggested that an increased %fuT>MIC was associated with improved outcomes (12), while subtherapeutic antimicrobial therapy was associated with increased mortality (13).
Considering these facts, an appropriate dosing modification according to patients’ severity, extracorporeal circulation settings, the presence of ascites, and residual renal function is necessary. Although various studies have separately described MEM PKs in pediatric patients with sepsis, CRRT, and ECMO (5, 14–19), a large proportion of the patients in pediatric intensive care units (PICUs) have multiple factors that affect the MEM PKs.
This study describes the MEM PKs in critically ill pediatric patients who required CRRT and ECMO, including cases with sepsis, to identify the sources of PK variability in these patients. Different dosing simulations were performed to assess their probability of target attainment (PTA) by MIC, which provides more appropriate dosing recommendations based on clinical characteristics.
RESULTS
Clinical characteristics of subjects.
We evaluated 34 patients admitted to the PICU. The overall median (range) age, body weight (BW), serum creatinine (SCr) level, SCr-based estimated glomerular filtration rate (eGFR), and MEM dose were 1.4 (0.03 to 14.6) years, 8.9 (2.7 to 40.9) kg, 0.2 (0.1 to 5.5) mg/dl, 38.2 (1.4 to 183.8) ml/min, and 36.4 (14.7 to 97.8) mg/kg of body weight/dose, respectively (Table 1). Twenty-one patients (61.8%) were female. Eight patients (23.5%) received CRRT (six patients were prescribed continuous venovenous hemodiafiltration, and two were prescribed continuous venovenous hemodialysis). Of them, three patients on continuous venovenous hemodiafiltration also received ECMO. Four patients (11.8%) had massive ascites (10 ml/kg/day or more) (20). Fourteen patients (41.1%) had undergone liver transplantation. Among these 14 patients, 6 patients had been administered tacrolimus monotherapy, 5 had been administered tacrolimus and prednisolone, 2 had been administered mycophenolate monotherapy, and 1 had been administered tacrolimus and mycophenolate as immunosuppressive medications during MEM medication.
TABLE 1.
Demographics and clinical characteristics of subjectsc
| Variable | Value for patient population (n = 34) |
|---|---|
| Median age (yrs) (range)a | 1.4 (0.03–14.6) |
| No. (%) of female subjects | 21 (61.8) |
| Median body wt (kg) (range)a | 8.9 (2.7–40.9) |
| Median ht (cm) (range)a | 142.5 (74.5–93.5) |
| Median serum creatinine concn (mg/dl) (range)a | 0.2 (0.1–5.5) |
| Median eGFR (ml/min) (range)a | 38.2 (1.4–183.8) |
| Median vol of diuresis (ml/kg/24 h) (range)a | 53.9 (0–323.9) |
| No. (%) of patients with ascitesa | 17 (50.0) |
| Median vol of ascites (ml/24 h) (range)a | 105 (0–9,000) |
| No. (%) of patients by SIRS scoreb | |
| 0 | 10 (29.4) |
| 1 | 7 (20.6) |
| 2 | 9 (26.5) |
| 3 | 7 (20.6) |
| 4 | 1 (2.9) |
| No. (%) of patients who received liver transplantation | 14 (41.1) |
| No. (%) of patients receiving: | |
| CRRT | 8 (23.5) |
| ECMO | 3 (8.8) |
| Vasopressors | 19 (55.9) |
| Median dialysate flow rate (ml/h) (range) | 1,600 (600–4,100) |
| Median filtrate flow rate (ml/h) (range) | 1,600 (600–8,400) |
| Median blood flow (ml/min) (range) | 40 (15–80) |
| Median time of MEM administration (days) (range) | 5 (1–16) |
| Median MEM dose (mg/kg/day) (range) | 105.2 (40.0–293.4) |
| Median MEM dose (mg/kg/dose) (range) | 36.4 (14.7–97.8) |
| No. (%) of patients receiving intravenous MEM by: | |
| Standard 1-h infusion | 27 (79.4) |
| Prolonged 3-h infusion | 9 (26.5) |
| No. (%) of patients with proven bacterial infection | 20 (58.8) |
| Median time to negative conversion of blood culture (h) (range) | 48 (24–120) |
| Median time to antipyretic response (days) (range) | 4 (0–23) |
| 30-day survival [no. (%) of patients] | 33 (97.1) |
| Serum samples analyzed for MEM concn (n = 290) | |
| No. (%) of scavenged samples | 256 (88.3) |
| No. (%) of prospective samples | 34 (11.7) |
| Median no. of samples per patient (range) | 9 (1–22) |
On the day of the study.
SIRS scores for each patient are indicated as the number of variables that met the SIRS criteria. The variables were provided as age-specific vital signs and laboratory variables (lower values for heart rate, leukocyte count, and systolic blood pressure). SIRS and CRRT were treated as categorical covariates. If the patient met ≥2 SIRS criteria for pediatric patients, the patient was categorized as SIRS+. If the patient was on continuous hemodialysis, the patient was categorized as CRRT+.
eGFR, estimated glomerular filtration rate; SIRS, systemic inflammatory response syndrome; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation.
Regarding the CRRT settings, the median (range) dialysate flow rate on the day of the study was 1,600 (600 to 4,100) ml/h, and the median blood flow rate was 40 (15 to 80) ml/min. On the first day of MEM therapy, 6 (17.6%) patients were anuric (<0.3 ml/kg/h for 24 h or anuria for 12 h), 10 (29.4%) were oliguric (<0.5 ml/kg/h for 24 h), and 18 (52.9%) had preserved diuresis (>0.5 ml/kg/h for 24 h). The median urine output was 53.9 (0 to 323.9) ml/kg/24 h. The suspected foci of infection were catheter-related septicemia (n = 6), catheter-related peritonitis or pleurisy (n = 5), cholangitis (n = 3), liver abscess (n = 1), urinary tract infection (n = 2), endocarditis (n = 1), or unknown (n = 2). Microbiological analysis revealed clinically significant positive cultures from 20 patients (58.8%). Of them, 17 (85.0%) cases were positive by blood culture results, 4 (20.0%) were positive by ascites culture, 2 (10.0%) were positive by urine culture, and 1 (5.0%) was positive by operative wound culture. The most frequently isolated microorganisms were Escherichia coli (n = 7; 20.6%) (MEM MIC ≤ 1 μg/ml in 6 cases; MIC = 2 μg/ml in 1 case) and Pseudomonas aeruginosa (n = 3; 8.8%) (MEM MIC ≤ 1 μg/ml in 2 cases; MIC = 2 μg/ml in 1 case). The number of patients who met ≥2 of the pediatric systemic inflammatory response syndrome (SIRS) criteria was 17 (50%). The 30-day survival rate after MEM therapy initiation was 97.1%. One patient with recurrent neuroblastoma died of pneumonia that was probably caused by Stenotrophomonas maltophilia 6 days after MEM initiation.
PK specimens.
We collected 380 serum samples and analyzed 290 drug concentrations. The median time of PK sampling (range) was 5.4 (0.06 to 19.7) h after the dose, and the median concentration was 9.1 (0.3 to 75.2) μg/ml. An average of 9 (1 to 22) samples per patient was collected, and the overwhelming majority of measurable PK samples were the leftover samples from the clinical laboratory (256/290; 88.3%). Ninety samples (23.7%) had MEM concentrations below the limit of quantitation, and these measurements were excluded from the PK analysis.
Population PK model building.
The preliminary analysis for the base model showed that the two-compartment model resulted in a better fit for describing MEM concentrations, while the multiplicative model best described the residual variability. The −2 log likelihoods of the objective function value (OFV) for exploration of the structure of the one- and two-compartment models were 1,745 and 1,609, respectively, suggesting that a two-compartment model with a multiplicative residual error was the appropriate basic structural model for our population. The shrinkage factors of the V for the peripheral compartment (Vp) (liters) and the intercompartmental CL (Q) (liters per hour) were all >0.3, indicating minor interindividual variability of the parameters that could be eliminated without significantly altering the OFVs. Thus, each was excluded in the model-building process. The η shrinkage for V of the central compartment (Vc) and CL in the base model was small (<0.3), confirming that our estimates were not overparameterized. CL, Vc, Vp, and Q were validated by a hypothesis test using forward inclusion/backward elimination in the modeling process. CL and Vc were scaled by BW for the base model. The SCr-based eGFR and the SIRS score for the covariate analysis significantly influenced the MEM CL, reducing the OFVs by 75.34 and 53.53, respectively, whereas daily urine excretion, daily ascites excretion, patients on CRRT, and patients undergoing liver transplantation did not.
Although the type of immunosuppressant was evaluated as a categorical covariate (tacrolimus monotherapy or concomitant therapy), it was not included as a significant factor. CRRT use was a significant covariate that statistically improved the base model when added to MEM Vc, reducing the OFV by 47.09. The final model included the effects of the eGFR and SIRS score on CL and of CRRT on Vc (see Table S1 in the supplemental material). For Vp and Q, only BW was included as a covariate. The final PK model (Table 2) was as follows: CLi (liters per hour) = CLpop × (eGFR [milliliters per minute]/38.2)θeGFR × (1 + θSIRS)SIRS × BW × exp(ηCL), where SIRS = 1 if the patient met ≥2 SIRS criteria and SIRS = 0 if the patient met ≤1 SIRS criterion; Qi (liters per hour) = Qpop × BW; Vci (liters) = Vcpop × (1 + θCRRT)CRRT × BW × exp(ηV), where CRRT = 1 if the patients received CRRT; and Vp (liters) = Vppop × BW. The ηi values are normally distributed, with a mean of 0 and a variance of ω2. The magnitude of ε shrinkage was 5.91%. The model parameters had moderate levels of η shrinkage for CL (22.3%) and Vc (27.9%).
TABLE 2.
Population pharmacokinetic estimates for the final model and bootstrap resultse
| Parameter | Base model estimate (RSE%) | Final model estimate (RSE%) | Median bootstrap value of the final model (95% CI) |
|---|---|---|---|
| Population parameters | |||
| CL (liters/kg/h)a | |||
| θCL | 0.46 (32.01) | 0.45 (12.63) | 0.45 (0.36–0.52) |
| θeGFR | 0.19 (20.60) | 0.20 (0.11–0.27) | |
| θSIRS | 0.35 (40.70) | 0.36 (0.10–0.63) | |
| Vc (liters/kg)b | |||
| θV | 0.49 (34.59) | 0.49 (24.22) | 0.48 (0.32–0.70) |
| θCRRT | 0.66 (20.58) | 0.67 (0.57–0.98) | |
| Vp (liters/kg)c | |||
| θV2 | 0.34 (24.22) | 0.34 (23.60) | 0.33 (0.21–0.50) |
| Q (liters/kg/h)d | |||
| θQ | 0.29 (33.98) | 0.28 (34.90) | 0.28 (0.09–0.47) |
| Between-subject variability | |||
| % ωCL | 48.54 (20.51) | 27.10 (19.01) | 26.28 (17.09–36.81) |
| % ωVc | 56.42 (20.13) | 31.50 (14.30) | 30.47 (20.04–40.17) |
| Residual variability | |||
| Proportional (%) | 59.72 (12.15) | 33.23 (4.28) | 0.33 (0.31–0.35) |
CL (liters per kilogram per hour) = θCL × (eGFR [milliliters per minute]/38.2)θeGFR × (1 + θSIRS)SIRS × BW × exp(ηCL).
Vc (liters per kilogram) = θV × (1 + θCRRT)CRRT × BW × exp(ηCL).
Vp (liters per kilogram) = θV2 × BW.
Q (liters per kilogram per hour) = θQ × BW.
RSE%, percent relative standard error; CL, total body clearance; eGFR, estimated glomerular filtration rate; SIRS, systemic inflammatory response syndrome (0 or 1); Vc, central volume of distribution; CRRT, receiving continuous renal replacement therapy (0 or 1); Vp, peripheral volume of distribution; Q, intercompartmental CL; ω, between-subject variability; BW, body weight.
Model validation.
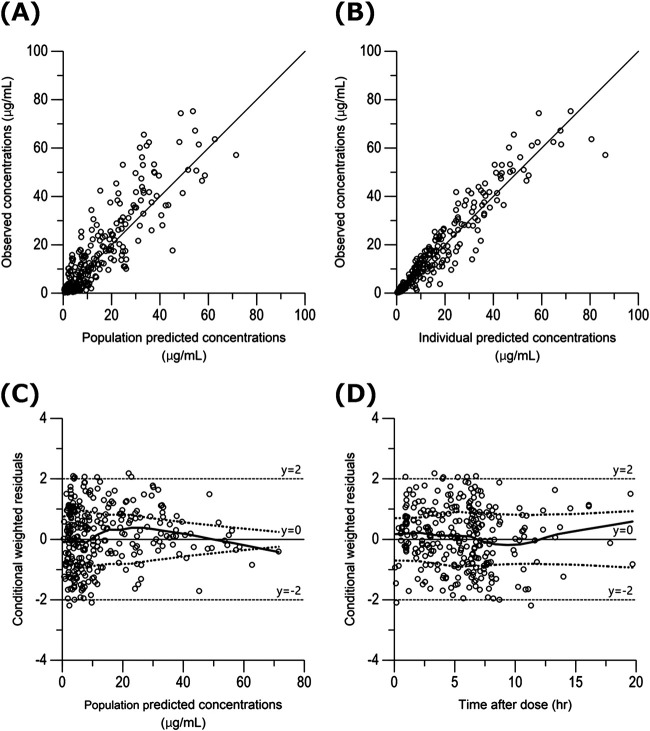
Table 2 shows the statistical distributions of the parameter estimates obtained from the bootstrap analyses. The final model converged in 1,000 bootstrap samples represents a convergence rate of 100%. The median values of the parameters estimated from the bootstrap analyses were in good agreement, and the 95% confidence intervals (CIs) were narrow, demonstrating satisfactory precision. The basic goodness-of-fit plots of the final model showed that the scatterplots of observed concentrations versus population predicted concentrations (PRED) and observed concentrations versus individual predicted concentrations (IPRED) were uniformly distributed around the line of identity (Fig. 1A and B). Additionally, the values of conditional weighted residuals (CWRES) were distributed symmetrically around zero across the entire PRED and time after dose range (Fig. 1C and D). The predictive performance of the model observed by a visual predictive check is shown, where the 5th, 50th, and 95th percentiles of the observed concentrations are close to the respective percentiles of the simulated concentrations (Fig. 2).
FIG 1.
Goodness-of-fit plots for the final population pharmacokinetic model. (A) Plot of observed MEM concentrations (micrograms per milliliter) versus PRED (micrograms per milliliter). (B) Plot of observed MEM concentrations (micrograms per milliliter) versus IPRED (micrograms per milliliter). (C) Plot of CWRES versus PRED. (D) Plot of CWRES versus time after the last dose. In panels A and B, the solid line is the line of unity (y = x). In panels C and D, the top dotted curve is locally weighted scatterplot smoothing fitted to the absolute values of the residuals, and the bottom red curve is a reflection of the top dotted curve about the x axis. The solid middle line is locally weighted scatterplot smoothing fitted to the raw residuals.
FIG 2.
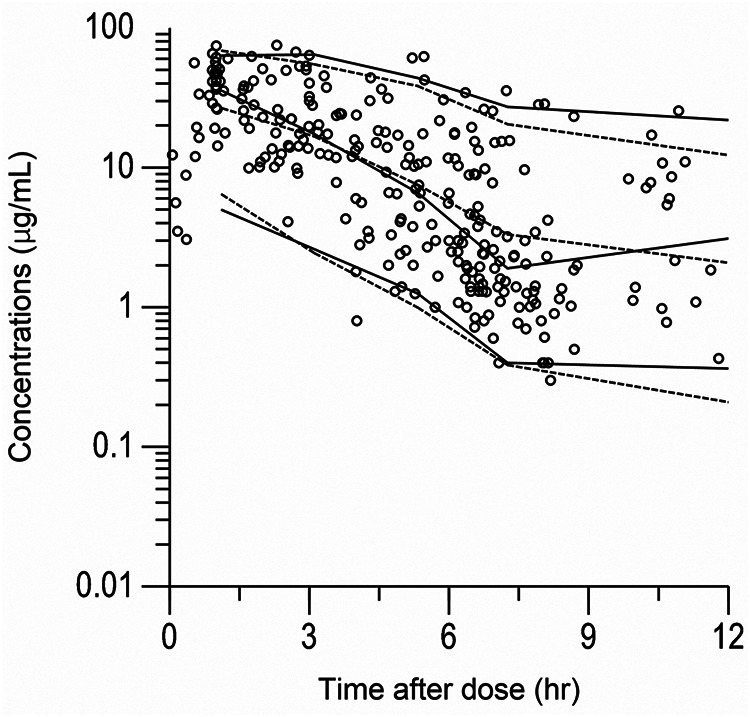
Visual predictive check of observed and simulated concentrations. Shown are visual predictive checks (n = 1,000) of observed MEM concentrations (micrograms per milliliter) along with 5th, 50th, and 95th percentiles overlaid on the median and 90% prediction intervals of simulated concentrations generated from the final model. The solid lines are the 5th, 50th, and 95th percentiles of the observed concentrations. The dotted lines are the median and the 90% prediction intervals of the simulated concentrations.
Dosing simulation.
Monte Carlo simulations were performed using the final model to determine the appropriate dosing regimens corresponding to the patients with SIRS and the patients on CRRT. The results of the Monte Carlo simulation after a standard dosing regimen adjusted by the renal function are shown in Table 3. The simulation revealed that it was difficult to achieve a 90% PTA of 100% fuT>MIC using the standard dosing regimens, even by prolonged infusion in many cases.
TABLE 3.
PTAs for MEM at various fuT>MIC targets after the standard dosing regimen for each renal functiona
| Infusion duration (h) | fuT>MIC (%) | SIRS status | CRRT status | PTA (%) for SCr-based eGFR (ml/min) (standard dosing regimen) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC = 1 μg/ml |
MIC = 2 μg/ml |
MIC = 4 μg/ml |
MIC = 8 μg/ml |
||||||||||||||||
| <10 (20 mg/kg q24h) | 10–25 (20 mg/kg q12h) | 26–50 (40 mg/kg q12h) | >50 (40 mg/kg q8h) | <10 (20 mg/kg q24h) | 10–25 (20 mg/kg q12h) | 26–50 (40 mg/kg q12h) | >50 (40 mg/kg q8h) | <10 (20 mg/kg q24h) | 10–25 (20 mg/kg q12h) | 26–50 (40 mg/kg q12h) | >50 (40 mg/kg q8h) | <10 (20 mg/kg q24h) | 10–25 (20 mg/kg q12h) | 26–50 (40 mg/kg q12h) | >50 (40 mg/kg q8h) | ||||
| 0.5 | 40 | − | − | 73.2 | 100.0 | 100.0 | 100.0 | 12.3 | 100.0 | 100.0 | 100.0 | 0.1 | 36.5 | 75.0 | 100.0 | 0.0 | 0.1 | 9.0 | 26.3 |
| − | + | 100.0 | 100.0 | 100.0 | 100.0 | 73.7 | 100.0 | 100.0 | 100.0 | 2.6 | 93.3 | 100.0 | 100.0 | 0.0 | 1.3 | 48.2 | 100 | ||
| + | − | 5.8 | 89.2 | 100.0 | 100.0 | 0.1 | 30.2 | 51.9 | 100.0 | 0.0 | 1.1 | 6.7 | 19.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| + | + | 63.7 | 100.0 | 100.0 | 100.0 | 6.1 | 100.0 | 100.0 | 100.0 | 0.0 | 12.7 | 66.4 | 100.0 | 0.0 | 0.0 | 1.9 | 0.6 | ||
| 100 | − | − | 0.1 | 10.5 | 21.3 | 79.8 | 0.0 | 0.4 | 1.5 | 24.8 | 0.0 | 0.0 | 0.0 | 1.7 | 0.0 | 0.0 | 0.0 | 0.0 | |
| − | + | 1.3 | 64.9 | 82.6 | 100.0 | 0.0 | 9.9 | 24.8 | 89.8 | 0.0 | 0.1 | 1.2 | 30.4 | 0.0 | 0.0 | 0.0 | 0.7 | ||
| + | − | 0.0 | 0.1 | 0.7 | 11.6 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| + | + | 0.0 | 4.8 | 9.8 | 71.4 | 0.0 | 0.1 | 0.5 | 17.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| 3 | 40 | − | − | 100.0 | 100.0 | 100.0 | 100.0 | 30.8 | 100.0 | 100.0 | 100.0 | 0.6 | 97.5 | 100.0 | 100.0 | 0.0 | 1.7 | 52.5 | 100.0 |
| − | + | 100.0 | 100.0 | 100.0 | 100.0 | 98.9 | 100.0 | 100.0 | 100.0 | 6.3 | 100.0 | 100.0 | 100.0 | 0.0 | 2.2 | 89.4 | 100.0 | ||
| + | − | 39.6 | 100.0 | 100.0 | 100.0 | 5.4 | 20.7 | 100.0 | 100.0 | 0.0 | 16.2 | 68.4 | 100.0 | 0.0 | 0.0 | 2.3 | 100.0 | ||
| + | + | 96.5 | 100.0 | 100.0 | 100.0 | 17.1 | 100.0 | 100.0 | 100.0 | 0.0 | 42.0 | 100.0 | 100.0 | 0.0 | 0.0 | 10.7 | 100.0 | ||
| 100 | − | − | 0.1 | 21.0 | 36.0 | 100.0 | 0.0 | 1.1 | 4.6 | 59.9 | 0.0 | 0.0 | 0.1 | 9.1 | 0.0 | 0.0 | 0.0 | 0.1 | |
| − | + | 2.5 | 90.5 | 100.0 | 100.0 | 0.0 | 23.1 | 45.7 | 100.0 | 0.0 | 0.3 | 4.5 | 70.3 | 0.0 | 0.0 | 0.0 | 5.8 | ||
| + | − | 0.0 | 0.4 | 1.6 | 33.2 | 0.0 | 0.0 | 0.0 | 3.5 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| + | + | 0.0 | 13.2 | 23.6 | 89.1 | 0.0 | 0.4 | 2.0 | 52.7 | 0.0 | 0.0 | 0.0 | 5.3 | 0.0 | 0.0 | 0.0 | 0.0 | ||
Shaded values indicate a probability of target attainment of >90%. SCr, serum creatinine; eGFR, estimated glomerular filtration rate; SIRS, systemic inflammatory response syndrome; CRRT, continuous renal replacement therapy.
A simulation for exploring the optimal dosing regimen was performed. The PTAs of 40% or 100% fuT>MIC by four dosing regimens at two infusion rates and according to the target MIC are presented in Table S2. When the target was set at 40% fuT>MIC and the MIC was 1 mg/dl, a dosing regimen of 10 to 20 mg/kg every 8 h (q8h) in a 0.5-h infusion nearly achieved a 90% PTA regardless of the presence or absence of SIRS or CRRT. However, when the target %fuT>MIC was set at 100%, the conventional 0.5-h infusion was sometimes unable to achieve a sufficient PTA, and a 3-h prolonged infusion, with or without higher doses, was necessary for such situations.
PD assessment regarding treatment response in each patient.
The median time to negative conversion of positive bacterial culture was 48 h and the median time to antipyretic response was 4 days in the 20 patients with proven bacterial infections. The estimated individual %fuT>MIC (MIC = 1 μg/ml) values were compared among the patients whose bacterial culture was negative within and for more than 48 h and the patients who showed an antipyretic response within and for more than 4 days. The median %fuT>MIC values in patients with bacterial cultures showing a sustained positive result over 48 h (n = 10) and within 48 h (n = 10) were 57.5% and 82.6%, respectively. Temperature charts were available for 13 patients for assessment of the antipyretic response. The median %fuT>MIC values in patients whose times to antipyretic response were more than 4 days (n = 7) and within 4 days (n = 6) were 60.7% and 96.6%, respectively. Thus, patients who achieved a higher %fuT>MIC tended to have a better treatment response.
DISCUSSION
The selection of an optimal dosage regimen in critically ill pediatric patients with multiple factors affecting the MEM PK parameters is complicated by the lack of PK studies in this population. This study presents the PKs for MEM in critically ill pediatric patients and suggests dosage regimens based on a population PK/PD approach.
The model-building process for MEM PKs revealed that BW, renal function, and the pediatric SIRS score were covariates of the CL. Likewise, we included BW and the use of CRRT as covariates of the Vc. We used Schwartz’s formula to estimate individual renal function. The formula of Uemura et al. (21, 22), a new SCr-based eGFR formula for Japanese pediatric patients, showed better estimations of interindividual differences for the MEM CL in this study (the OFVs for the final model were 1,488.38 and 1,512.41 using the formula of Uemura et al. and Schwartz’s formula, respectively [data not shown]). Although we selected Schwarz’s formula for our final model because it is the standard formula for estimating pediatric renal function worldwide, this result suggests that the formula of Uemura et al., which was developed with Japanese patients’ data, could be a better fit for our patient population. This fact may indicate the usefulness of developing an original formula for renal function estimation for patients of different ethnicities.
The final model showed that the MEM CL increased by 35% in patients who met ≥2 SIRS criteria compared with patients who met ≤1 SIRS criterion, and the Vc increased by 66% in patients on CRRT compared with those not on CRRT. A previous study showed the increasing recognition of augmented renal clearance (ARC) of solutes in the adult intensive care population (23). Several studies have illustrated the risk of therapeutic failure due to an enhanced CL of renally eliminated drugs such as β-lactams, MEM, and vancomycin leading to the development of adjusted dosing recommendations in adult patients with ARC (24–26). ARC is also highly prevalent in the pediatric intensive care population (27). Shimamoto et al. reported that patients with SIRS had a significantly higher vancomycin CL that was positively correlated with the SIRS score (28). To the best of our knowledge, this is the first report indicating that an increased MEM dosage is necessary for patients with a higher pediatric SIRS score. Critically ill pediatric patients meeting ≥2 pediatric SIRS criteria appear to be at risk for subtherapeutic drug exposure.
Theoretically, CRRT settings, such as the dialysate flow rate and blood flow rate, in each patient may affect CL and V, but these CRRT settings were not retained as continuous variables in our study, probably due to the small sample size. ECMO use was also excluded as a significant contributing factor of MEM PKs, most likely due to the small number of pediatric patients on ECMO. Although MEM is not a lipophilic agent and has low levels of protein binding, the loss of MEM in the ECMO circuit may be due to drug sequestration (or “sticking”) as reported in previous studies (29). In contrast, other studies found no significant loss of MEM in ECMO and CRRT circuits (30, 31). Whether the PK of MEM is influenced by a loss of MEM in ECMO and CRRT is controversial. Furthermore, previous reports have shown that the effect of CRRT and ECMO on V is inversely related to patient age (8, 32, 33). Considering these facts, a large-scale study is necessary to determine the effect of CRRT settings and ECMO on MEM PKs in pediatric patients.
The estimated V and CL for MEM were reported to be 0.4 liters/kg and 0.3 liters/kg/h, respectively (34, 35), in pediatric patients with clinically stable conditions and 0.2 liters/kg and 0.3 liters/kg/h, respectively, in pediatric patients with critically ill conditions (median age, 6.0 years) (16). Another study of critically ill pediatric patients (median age, 2.0 years) showed a V of 0.6 to 0.8 liters/kg and a CL of 0.3 to 0.4 liters/kg/h (15). Our study results showed mean estimated V and CL for patients without SIRS or CRRT of 0.83 liters/kg and 0.45 liters/kg/h, respectively, and these parameters increased to 1.15 liters/kg and 0.54 liters/kg/h, respectively, in patients with SIRS or CRRT. It was reported that the MEM V in pediatric patients on extracorporeal circulation, such as CRRT and ECMO, was higher than the population PK estimates derived from healthy volunteers (19). Furthermore, a higher CL was observed in critically ill patients, and it was established in adults that sepsis causes enhanced blood flow to the kidney and increased glomerular filtration, known as ARC, which we discussed previously (36). The age of the study population also affects the MEM PK parameters. Parker et al. (37) suggested that V and CL were markedly different in patients <2 years old and weighing <10 kg. However, the median age of our study subjects was 1.4 years, which was younger than that of the pediatric patients reported by Parker et al. Considering these facts, critically ill conditions and age might contribute to changes in the V and CL.
The results of the Monte Carlo simulations suggested that the currently recommended MEM dose is insufficient in some situations. Although the optimal PD target of MEM for critically ill pediatric patients remains undetermined, several studies have recommended a higher fuT>MIC, such as 100% fuT>MIC, for critically ill patients (11, 38).
When the target was set at 100% fuT>MIC, only the highest MEM dosage regimen of 40 mg/kg/dose q8h given as a 3-h prolonged infusion provided appropriate PD exposure at a susceptible MIC against Gram-negative bacilli such as Enterobacterales (MIC ≤ 1 μg/ml) and Pseudomonas aeruginosa (MIC ≤ 2 μg/ml) (39). Thus, 40 to 80 mg/kg/dose q8h might be required to obtain 100% fuT>MIC. In particular, patients who met ≥2 SIRS criteria (SIRS+) without CRRT (CRRT−) were at a high risk of achieving an insufficient PTA of 100% fuT>MIC. If the eGFR was ≥50 ml/min, dosing escalation to 80 mg/kg q8h with 3-h prolonged dosing was required, even for a target MIC of 1 μg/ml, except for patients who met ≥2 SIRS criteria without CRRT (SIRS+ and CRRT−). It is difficult to achieve a sufficient enough PTA of 100% fuT>MIC in the majority of situations for a target MIC of ≥2 μg/ml, even under prolonged infusion and with higher dosing, if the patient’s eGFR is ≥50 ml/min. In our PICU, the MIC50 and MIC90 (defined as the MICs at which the growth of 50% and 90% of the organisms was inhibited, respectively) of MEM for 5 recent years (2015 to 2019) were ≤2 and 8 μg/ml for P. aeruginosa and ≤1 and ≤1 μg/ml for E. coli, respectively. In addition, based on a nationwide surveillance study conducted in Japan in 2012, the MIC50 and MIC90 of MEM were 1 and 16 μg/ml for P. aeruginosa, respectively, and ≤0.06 and 0.12 μg/ml for extended-spectrum β-lactamase (ESBL)-producing E. coli, respectively (40). The MIC distribution of MEM varies among institutions and countries; therefore, an understanding of local factors that may affect the MIC distribution of MEM for Gram-negative organisms is important for clinicians in determining an empirical antibiotic dosing strategy.
Many studies recommend high doses and prolonged infusion regimens for severe bacterial infection in adult patients or critically ill patients where subtherapeutic drug exposure is expected (11, 38, 41–43) based on PD concepts. It has been reported that a continuous infusion or a higher dose of MEM was associated with a higher microbiological cure rate, shorter intensive care unit admission, and improved clinical outcomes in critically ill adult patients (36, 44, 45). Our study demonstrated that a higher % fuT>MIC was associated with a shortened time of sustained positive bacterial culture and a rapid antipyretic response. These findings support a higher %fuT>MIC, such as 100% fuT>MIC, being beneficial, even in critically ill pediatric patients. Although MEM is well tolerated in pediatric patients (46), the safety of a high-dose regimen remains unclear. Thus, it is essential to evaluate new dosage regimens when they are applied to clinical practice.
There are several limitations to our study. First, this was a single-center investigation that included patients with various underlying diseases. Second, our study population consisted of a wide age range, and the results were not stratified by age. Younger patients are known to clear renally excreted drugs more rapidly than older patients and typically have a larger volume of distribution normalized to their BW. Further analysis of the developmental PK changes in critically ill children is necessary. Third, only 3/34 patients included in this PK analysis were on ECMO, and we were unable to conduct an adequate evaluation of the impact of ECMO on MEM PKs. Thus, the results of the Monte Carlo simulations in our study are not applicable to pediatric patients on ECMO. Finally, this study was conducted using a scavenged sampling strategy. The sampling times and the sparse number of samples collected for each child may not have been ideal to fully characterize the distribution phase of MEM in each patient. However, this method allowed us to analyze critical pediatric samples without putting the patients at additional risk. Although MEM levels in a scavenged sample may change from the true value due to MEM instability and inaccuracies in sampling time, the sampling method (scavenged sampling or interventional sampling) was not included as a significant categorical covariate (OFV = 1,512.35 for the included model, and OFV = 1,512.41 for the final model).
We demonstrated the MEM PKs in critically ill pediatric patients, including cases on CRRT and ECMO, and we developed a population PK model considering various factors affecting subtherapeutic MEM exposure. The data suggested that the standard dosing regimens for MEM did not attain an appropriate PD target in critically ill pediatric patients in some situations. Patients with SIRS required a higher MEM dose and prolonged infusion to achieve an optimal target for Gram-negative bacilli. A larger, prospective investigation is necessary to confirm the safety and efficacy of a higher dose and prolonged infusion of MEM for critically ill pediatric patients with various conditions.
MATERIALS AND METHODS
Study design.
We performed a single-center retrospective PK study in the PICU at the National Center for Child Health and Development (NCCHD), a tertiary children’s hospital in Tokyo, Japan. The NCCHD has a large PICU with approximately 1,100 annual admissions. It is the largest pediatric liver transplant center in Japan, performing approximately 60 pediatric liver transplantations annually. The patients were enrolled between September 2016 and June 2019. The inclusion criteria were as follows: age of <18 years, critically ill patients treated with MEM for suspected or proven bacterial infection, and an unstable condition, including sepsis, renal failure, massive ascites, or receipt of CRRT or ECMO. The MEM dose and infusion time were determined at the discretion of the PICU physician and the pediatric infectious diseases physician.
Data collection.
The following data were retrieved from the medical records of all study subjects: gender, age, BW, height, body surface area, SCr, SCr-based eGFR (milliliters per minute per 1.73 m2) (eGFR = 0.413 × height [centimeters]/SCr [milligrams per deciliter]) (47), blood urea nitrogen, alanine aminotransferase, albumin, and concomitant drug therapy. The eGFR determined using body surface area was used to calculate the GFR (milliliters per minute) of individuals. Data on MEM dose, time of administration, time of blood sampling, and serum MEM concentration were also collected. Schwartz’s formula was chosen to calculate the eGFR because it is currently the most commonly used method in pediatric practice worldwide (56). The formula of Uemura et al., a new SCr-based eGFR formula for Japanese pediatric patients, was also used to estimate renal function (21, 22). Additionally, the CRRT settings, ECMO settings, pediatric SIRS criterion score (48), daily urine excretion volume, daily ascites excretion volume, and culture results for pathogenic bacterial cultures were recorded. The SIRS score was calculated using the heart rate, respiratory rate, leukocyte count, and systolic blood pressure and was described as the number of inspection items (0 to 4) meeting the age-specific criteria for pediatric patients from neonates to young adults. Patients meeting ≥2 pediatric SIRS criteria (2 to 4 criteria) were categorized as 1, and the others were categorized as 0, in the PK modeling analysis.
PK sample collection.
This study followed a sparse-sampling approach. The samples were divided into two types: scavenged samples and interventional blood samples. Scavenged samples were collected from leftover discarded blood obtained for routine clinical tests. Interventional blood sampling was defined as samples obtained by collecting extra blood from the enrolled pediatric patients. Each blood draw was approximately 0.4 ml and was collected in a plastic tube (Venoject II; Terumo Corporation, Tokyo, Japan). PK sample collection was planned at the following time points: immediately before MEM infusion (0 h), immediately after infusion completion (1 h for a standard infusion or 3 h for an extended infusion), and approximately 4 h after infusion completion.
For scavenged samples, the sampling times that were recorded electronically in routine clinical practice were extracted from the medical chart to ensure the accuracy of the data. The MEM infusion time was clinically determined. The samples were centrifuged immediately after collection at 1,500 × g at 4°C for 5 min. Next, the sera from the interventional blood samples were stored at −80°C. The sera from the scavenged blood samples were also stored at −80°C immediately after routine biochemical tests. The samples were stored for a maximum of 2 weeks before analysis.
LC-MS analysis.
The total serum MEM concentration was measured using liquid chromatography (LC) coupled with tandem mass spectrometry (MS/MS) (TSQ Vantage LC-MS system with the Dionex UltiMate 3000 rapid-separation liquid chromatography [RSLC] system; Thermo Fisher Scientific KK, Tokyo, Japan) in the hospital’s internal laboratory using a validated method (49). The instrument parameters were optimized for MEM transition (m/z 384.1→68.1). The ion spray voltage and turbo heater temperature were maintained at 3,000 V and 270°C, respectively; the argon collision gas pressure was 1.5 mTorr; and the collision energy was −41 V. MEM and MEM-d6 (internal standard) were purchased from Toronto Research Chemicals Inc. (Ontario, Canada). LC separation was achieved using a reverse-phase C18 column (Imtakt Corporation, Kyoto, Japan) with a flow rate of 0.4 ml/min using a gradient mobile phase. Mobile phase A consisted of 0.1% formic acid in H2O, and mobile phase B consisted of 0.1% formic acid in acetonitrile. The total run time was 4 min. Quan Browser software (version 2.1.0; Thermo Fisher Scientific KK) was used to acquire the analytical data. The lower limit of quantitation of serum MEM was 0.2 μg/ml. Intraday and interday coefficients of variation of <5% at concentrations of 0.5 to 1,000 μg/ml were considered adequate results according to the guidelines of the Food and Drug Administration (50).
PK analysis.
The dosing, sampling, and demographic information were merged with the bioanalytical information to create the PK data set. The population PK analysis was performed using Phoenix NLME 8.2 software (Certara USA Inc., Princeton, NJ). One- and two-compartmental models with first-order elimination and multiplicative, additive, proportional, and additive residual-error models were evaluated according to a three-step strategy: (i) basic population model selection, (ii) covariate selection, and (iii) validation (51). Interindividual random effects (ω2) were evaluated according to CL (liters per hour) and Vc (liters). The goodness of fit for a model was assessed by (i) significant decreases in the −2 log likelihood of the OFV, (ii) plots of PRED and IPRED versus observed concentrations and of CWRES versus observed concentrations and time (52), and (iii) changes in the standard errors of parameter estimates (precision). Shrinkage was calculated for all model parameters. A shrinkage value of <20% was considered acceptable (53). Demographic and laboratory characteristics, including SCr-based eGFR, daily urine excretion volume, and daily ascites excretion volume, were evaluated as continuous covariates, and CRRT use, SIRS score, proven bacterial infection, history of liver transplantation, and kind of immunosuppressant medications for each liver transplant patient were evaluated as categorical covariates for potential model covariates. Continuous covariates were implemented using an allometric model with the equation Pi = Ppop × (Covi/Covmedian)PWR, where Pi represents the individual parameter estimate of the ith patient, Ppop represents the population parameter estimates, Cov is the covariate, and PWR is the exponent. Categorical covariates were included in the model as , where Pj is the PK parameter for the jth patient, Covi is a numeric index value, Ppop is the typical value of a PK parameter for the reference covariate values, and θCOV is the multiplicative factor for the influence of the covariate on the PK parameter.
Each covariate investigated was retained if it led to an improved fit, as evaluated by biological plausibility, graphical displays based on the agreement between the observed and predicted drug concentrations, the uniformity of the distribution of the CWRES, improvement of the precision in parameter estimates, and reduction of the OFV by >3.84 (P < 0.05). A forward-addition/backward-elimination approach to covariate selection was planned if >1 covariate was found to be significant. A reduction of 7.88 (P = 0.005) was required for covariate retention in the final model.
Model evaluation.
A bootstrapping approach (n = 1,000) and a visual predictive check (n = 1,000) using Phoenix NLME 8.2 software were used for final model qualifications. The reliability of the final PK parameter estimates and their 95% CIs was assessed using the bootstrap approach. Eta (η) and epsilon (ε) shrinkages were used to assess the reliability of individual estimations and the power to detect model misspecifications in goodness-of-fit diagnostics (54).
Dosing simulations.
Monte Carlo simulations (1,000 replicates) were employed to determine the PTA of achieving a PK/PD target of %fuT>MIC values of 40% and 100% during a 0.5-h standard infusion or a 3-h prolonged infusion. The PTAs after the standard dosing regimen corresponding to varying renal function (eGFR = 10, 25, 50, and 100 ml/min) were evaluated before the appropriate dosage exploration, as follows: 40 mg/kg q8h (optimal dose for a patient with an eGFR of >50 ml/min), 40 mg/kg q12h (eGFR, 26 to 50 ml/min), 20 mg/kg q12h (eGFR, 10 to 25 ml/min), and 20 mg/kg q24h (eGFR, <10 ml/min). Intravenous infusions of 10, 20, 40, and 80 mg/kg/dose were simulated at 8-h intervals to explore the dosing regimen in pediatric patients with SIRS or on CRRT. Different renal functions of eGFRs of 10, 25, 50, and 100 ml/min were included in each simulation. Because an MIC breakpoint of >8 μg/ml is considered to indicate an isolate resistant to MEM monotherapy (55), the simulated MICs ranged from 1 to 8 μg/ml. A fixed 2% fu of MEM was used for simulations, according to previously reported data (9). If the PTA was >90%, the dosing regimen was considered successful.
Evaluation of PD profiles and outcomes.
The efficacy of MEM treatment was evaluated by determining the associations between the calculated %fuT>MIC in individual patients estimated using the individual post hoc estimates and the time to negative conversion of positive bacterial cultures and the time to the antipyretic response. The MIC was set at 1 μg/ml, which is the CLSI breakpoint of MEM for Enterobacterales (39).
Statistical analysis.
Patient demographic characteristics and their MEM PK parameter estimates were summarized and are reported as means ± standard deviations and as medians and ranges. A P value of ≤0.05 was considered statistically significant.
Ethical approval.
Ethical approval was obtained from the Ethics Committee at our institution (NCCHD-1328). The study was conducted according to the guidelines of the Declaration of Helsinki. Written consent to participate was obtained from the guardians of the pediatric patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Enago for the English language review.
This study was supported by grants from the Japanese Society for Pediatric Infectious Diseases and the NCCHD (2020C-8 and 30-31) awarded to K.S.
J.S. and K.S. contributed to conceptualizing and designing the study, analyzed the data, and drafted the manuscript. Y.O., H.K., S.M., and S.A. contributed to data collection and helped revise the manuscript. H.N., A.Y., and I.M. supervised the study and helped revise the manuscript. T.O. and M.H. contributed to the data evaluation and helped revise the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
We have no conflict of interest to declare related to the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Wiseman LR, Wagstaff AJ, Brogden RN, Bryson HM. 1995. Meropenem. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs 50:73–101. doi: 10.2165/00003495-199550010-00007. [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Ulldemolins M, Lisboa T, Koulenti D, Manez R, Martin-Loeches I, De Waele JJ, Putensen C, Guven M, Deja M, Diaz E, EU-VAP/CAP Study Group . 2011. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur Respir J 37:1332–1339. doi: 10.1183/09031936.00093010. [DOI] [PubMed] [Google Scholar]
- 3.Moon YS, Chung KC, Gill MA. 1997. Pharmacokinetics of meropenem in animals, healthy volunteers, and patients. Clin Infect Dis 24(Suppl 2):S249–S255. doi: 10.1093/clinids/24.Supplement_2.S249. [DOI] [PubMed] [Google Scholar]
- 4.Honore PM, Jacobs R, Hendrickx I, De Waele E, Van Gorp V, Spapen HD. 2015. Meropenem therapy in extracorporeal membrane oxygenation patients: an ongoing pharmacokinetic challenge. Crit Care 19:263. doi: 10.1186/s13054-015-0953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulldemolins M, Vaquer S, Llaurado-Serra M, Pontes C, Calvo G, Soy D, Martin-Loeches I. 2014. Beta-lactam dosing in critically ill patients with septic shock and continuous renal replacement therapy. Crit Care 18:227. doi: 10.1186/cc13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cies JJ, Moore WS, II, Dickerman MJ, Small C, Carella D, Chopra A, Parker J. 2014. Pharmacokinetics of continuous-infusion meropenem in a pediatric patient receiving extracorporeal life support. Pharmacotherapy 34:e175–e179. doi: 10.1002/phar.1476. [DOI] [PubMed] [Google Scholar]
- 7.Cies JJ, Moore WS, II, Calaman S, Brown M, Narayan P, Parker J, Chopra A. 2015. Pharmacokinetics of continuous-infusion meropenem for the treatment of Serratia marcescens ventriculitis in a pediatric patient. Pharmacotherapy 35:e32–e36. doi: 10.1002/phar.1567. [DOI] [PubMed] [Google Scholar]
- 8.Sherwin J, Heath T, Watt K. 2016. Pharmacokinetics and dosing of anti-infective drugs in patients on extracorporeal membrane oxygenation: a review of the current literature. Clin Ther 38:1976–1994. doi: 10.1016/j.clinthera.2016.07.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig WA 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen K-M, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, DALI Study . 2014. DALI: defining antibiotic levels in intensive care unit patients. Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 51:1725–1730. doi: 10.1128/AAC.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courter JD, Kuti JL, Girotto JE, Nicolau DP. 2009. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr Blood Cancer 53:379–385. doi: 10.1002/pbc.22051. [DOI] [PubMed] [Google Scholar]
- 13.Weiss SL, Fitzgerald JC, Balamuth F, Alpern ER, Lavelle J, Chilutti M, Grundmeier R, Nadkarni VM, Thomas NJ. 2014. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 42:2409–2417. doi: 10.1097/CCM.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cies JJ, Moore WS, II, Conley SB, Dickerman MJ, Small C, Carella D, Shea P, Parker J, Chopra A. 2016. Pharmacokinetics of continuous infusion meropenem with concurrent extracorporeal life support and continuous renal replacement therapy: a case report. J Pediatr Pharmacol Ther 21:92–97. doi: 10.5863/1551-6776-21.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cies JJ, Moore WS, II, Enache A, Chopra A. 2017. Population pharmacokinetics and pharmacodynamic target attainment of meropenem in critically ill young children. J Pediatr Pharmacol Ther 22:276–285. doi: 10.5863/1551-6776-22.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kongthavonsakul K, Lucksiri A, Eakanunkul S, Roongjang S, Issaranggoon Na Ayuthaya S, Oberdorfer P. 2016. Pharmacokinetics and pharmacodynamics of meropenem in children with severe infection. Int J Antimicrob Agents 48:151–157. doi: 10.1016/j.ijantimicag.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Cojutti P, Maximova N, Pea F. 2015. Pharmacokinetics and pharmacodynamics of continuous-infusion meropenem in pediatric hematopoietic stem cell transplant patients. Antimicrob Agents Chemother 59:5535–5541. doi: 10.1128/AAC.00787-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoji K, Saito J, Oho Y, Matsumoto S, Aoki S, Fukuda A, Sakamoto S, Kasahara M, Capparelli E, Miyairi I. 2019. Meropenem pharmacokinetics during relapsing peritonitis due to ESBL-producing Enterobacteriaciae [sic] in a liver transplant recipient. Clin Case Rep 7:2169–2173. doi: 10.1002/ccr3.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito J, Shoji K, Oho Y, Aoki S, Matsumoto S, Yoshida M, Nakamura H, Kaneko Y, Hayashi T, Yamatani A, Capparelli E, Miyairi I. 2020. Meropenem pharmacokinetics during extracorporeal membrane oxygenation and continuous hemodialysis: a case report. J Glob Antimicrob Resist 22:651–655. doi: 10.1016/j.jgar.2020.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Sanada Y, Mizuta K, Urahashi T, Umehara M, Wakiya T, Okada N, Hayashida M, Egami S, Hishikawa S, Kawano Y, Ushijima K, Otomo S, Sakamoto K, Fujiwara T, Sakuma Y, Hyodo M, Yasuda Y, Kawarasaki H. 2010. Management of intra-abdominal drain after living donor liver transplantation. Transplant Proc 42:4555–4559. doi: 10.1016/j.transproceed.2010.09.159. [DOI] [PubMed] [Google Scholar]
- 21.Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, Fujita N, Akioka Y, Kaneko T, Honda M. 2014. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol 18:626–633. doi: 10.1007/s10157-013-0856-y. [DOI] [PubMed] [Google Scholar]
- 22.Uemura O, Ishikura K, Gotoh Y, Honda M. 2018. Creatinine-based estimated glomerular filtration rate for children younger than 2 years. Clin Exp Nephrol 22:483–484. doi: 10.1007/s10157-017-1460-3. [DOI] [PubMed] [Google Scholar]
- 23.Bilbao-Meseguer I, Rodriguez-Gascon A, Barrasa H, Isla A, Solinis MA. 2018. Augmented renal clearance in critically ill patients: a systematic review. Clin Pharmacokinet 57:1107–1121. doi: 10.1007/s40262-018-0636-7. [DOI] [PubMed] [Google Scholar]
- 24.Baptista JP, Sousa E, Martins PJ, Pimentel JM. 2012. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents 39:420–423. doi: 10.1016/j.ijantimicag.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Troger U, Drust A, Martens-Lobenhoffer J, Tanev I, Braun-Dullaeus RC, Bode-Boger SM. 2012. Decreased meropenem levels in intensive care unit patients with augmented renal clearance: benefit of therapeutic drug monitoring. Int J Antimicrob Agents 40:370–372. doi: 10.1016/j.ijantimicag.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Carrié C, Petit L, d’Houdain N, Sauvage N, Cottenceau V, Lafitte M, Foumenteze C, Hisz Q, Menu D, Legeron R, Breilh D, Sztark F. 2018. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion. A prospective observational study. Int J Antimicrob Agents 51:443–449. doi: 10.1016/j.ijantimicag.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA. 2020. Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol 35:25–39. doi: 10.1007/s00467-018-4120-2. [DOI] [PubMed] [Google Scholar]
- 28.Shimamoto Y, Fukuda T, Tanaka K, Komori K, Sadamitsu D. 2013. Systemic inflammatory response syndrome criteria and vancomycin dose requirement in patients with sepsis. Intensive Care Med 39:1247–1252. doi: 10.1007/s00134-013-2909-9. [DOI] [PubMed] [Google Scholar]
- 29.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. 2010. Determinants of drug absorption in different ECMO circuits. Intensive Care Med 36:2109–2116. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J, Shin DA, Lee S, Cho Y-J, Jheon S, Lee JC, Kim HC. 2017. Investigation of key circuit constituents affecting drug sequestration during extracorporeal membrane oxygenation treatment. ASAIO J 63:293–298. doi: 10.1097/MAT.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 31.Sime FB, Pandey S, Karamujic N, Parker S, Alexander E, Loutit J, Durso S, Griffith D, Lipman J, Wallis SC, Roberts JA. 2018. Ex vivo characterization of effects of renal replacement therapy modalities and settings on pharmacokinetics of meropenem and vaborbactam. Antimicrob Agents Chemother 62:e01306-18. doi: 10.1128/AAC.01306-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buck ML 2003. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet 42:403–417. doi: 10.2165/00003088-200342050-00001. [DOI] [PubMed] [Google Scholar]
- 33.Zuppa AF, Zane NR, Moorthy G, Dalton HJ, Abraham A, Reeder RW, Carcillo JA, Yates AR, Meert KL, Berg RA, Sapru A, Mourani P, Notterman DA, Dean JM, Gastonguay MR, Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network . 2019. A population pharmacokinetic analysis to study the effect of extracorporeal membrane oxygenation on cefepime disposition in children. Pediatr Crit Care Med 20:62–70. doi: 10.1097/PCC.0000000000001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blumer JL, Reed MD, Kearns GL, Jacobs RF, Gooch WM, III, Yogev R, Willims K, Ewing BJ. 1995. Sequential, single-dose pharmacokinetic evaluation of meropenem in hospitalized infants and children. Antimicrob Agents Chemother 39:1721–1725. doi: 10.1128/aac.39.8.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du X, Li C, Kuti JL, Nightingale CH, Nicolau DP. 2006. Population pharmacokinetics and pharmacodynamics of meropenem in pediatric patients. J Clin Pharmacol 46:69–75. doi: 10.1177/0091270005283283. [DOI] [PubMed] [Google Scholar]
- 36.Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. 2010. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet 49:1–16. doi: 10.2165/11318140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Parker EM, Hutchison M, Blumer JL. 1995. The pharmacokinetics of meropenem in infants and children: a population analysis. J Antimicrob Chemother 36:63–71. doi: 10.1093/jac/36.suppl_A.63. [DOI] [PubMed] [Google Scholar]
- 38.Chytra I, Stepan M, Benes J, Pelnar P, Zidkova A, Bergerova T, Pradl R, Kasal E. 2012. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trial. Crit Care 16:R113. doi: 10.1186/cc11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstein MP 2020. CLSI M100-ED30: 2020 performance standards for antimicrobial susceptibility testing, 30th ed CLSI, Wayne, PA: https://clsi.org/standards/products/microbiology/documents/m100/. Accessed 10 August 2020. [Google Scholar]
- 40.Yamaguchi K, Ishii Y, Tateda K, Iwata M, Watanabe N, Shinagawa M, Kayaba H, Kimura M, Suwabe A, Kaku M, Abe Y, Kanemitsu K, Taniguchi N, Murakami M, Maesaki S, Kawamura T, Nomura F, Watanabe M, Kanno H, Horiuchi H, Tazawa Y, Kondo S, Misawa S, Takemura H, Nakashima H, Matsuto T, Fujimoto Y, Ishigo S, Gotoh H, Watanabe O, Yagi T, Shimaoka N, Mikamo H, Yamagishi Y, Fujita N, Komori T, Ichiyama S, Kawano S, Nakayama A, Nakamura F, Kohno H, Fukuda S, Kusano N, Nose M, Yokozaki M, Onodera M, Murao K, Negayama K, Nishimiya T, Miyamoto H, et al. 2014. Nationwide surveillance of parenteral antibiotics containing meropenem activities against clinically isolated strains in 2012. Jpn J Antibiot 67:73–107. [PubMed] [Google Scholar]
- 41.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. 2004. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 39:1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 42.Liebchen U, Paal M, Jung J, Schroeder I, Frey L, Zoller M, Scharf C. 2020. Therapeutic drug monitoring-guided high dose meropenem therapy of a multidrug resistant Acinetobacter baumannii—a case report. Respir Med Case Rep 29:100966. doi: 10.1016/j.rmcr.2019.100966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL, International Society of Anti-Infective Pharmacology, Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases . 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pea F, Della Siega P, Cojutti P, Sartor A, Crapis M, Scarparo C, Bassetti M. 2017. Might real-time pharmacokinetic/pharmacodynamic optimisation of high-dose continuous-infusion meropenem improve clinical cure in infections caused by KPC-producing Klebsiella pneumoniae? Int J Antimicrob Agents 49:255–258. doi: 10.1016/j.ijantimicag.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Lertwattanachai T, Montakantikul P, Tangsujaritvijit V, Sanguanwit P, Sueajai J, Auparakkitanon S, Dilokpattanamongkol P. 2020. Clinical outcomes of empirical high-dose meropenem in critically ill patients with sepsis and septic shock: a randomized controlled trial. J Intensive Care 8:26. doi: 10.1186/s40560-020-00442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hornik CP, Herring AH, Benjamin DK, Jr, Capparelli EV, Kearns GL, van den Anker J, Cohen-Wolkowiez M, Clark RH, Smith PB, Best Pharmaceuticals for Children Act-Pediatric Trials Network . 2013. Adverse events associated with meropenem versus imipenem/cilastatin therapy in a large retrospective cohort of hospitalized infants. Pediatr Infect Dis J 32:748–753. doi: 10.1097/INF.0b013e31828be70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. 2009. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis . 2005. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 49.Barco S, Bandettini R, Maffia A, Tripodi G, Castagnola E, Cangemi G. 2015. Quantification of piperacillin, tazobactam, meropenem, ceftazidime, and linezolid in human plasma by liquid chromatography/tandem mass spectrometry. J Chemother 27:343–347. doi: 10.1179/1973947814Y.0000000209. [DOI] [PubMed] [Google Scholar]
- 50.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine. 2013. Guidance for industry: bioanalytical method validation. US Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 51.Sheiner LB, Steimer JL. 2000. Pharmacokinetic/pharmacodynamic modeling in drug development. Annu Rev Pharmacol Toxicol 40:67–95. doi: 10.1146/annurev.pharmtox.40.1.67. [DOI] [PubMed] [Google Scholar]
- 52.Hooker AC, Staatz CE, Karlsson MO. 2007. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24:2187–2197. doi: 10.1007/s11095-007-9361-x. [DOI] [PubMed] [Google Scholar]
- 53.Karlsson MO, Savic RM. 2007. Diagnosing model diagnostics. Clin Pharmacol Ther 82:17–20. doi: 10.1038/sj.clpt.6100241. [DOI] [PubMed] [Google Scholar]
- 54.Savic RM, Karlsson MO. 2009. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J 11:558–569. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mian AN, Schwartz GJ. 2017. Measurement and estimation of glomerular filtration rate in children. Adv Chronic Kidney Dis 24:348–356. doi: 10.1053/j.ackd.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.