Fungal infections are a universal problem and are routinely associated with high morbidity and mortality rates in immunocompromised patients. Existing therapies comprise five different classes of antifungal agents, four of which target the synthesis of ergosterol and cell wall glucans.
KEYWORDS: acylhydrazones, antifungal agents, arylamidine, calcineurin, drug therapy, new targets, nikkomycin, olorofilm, sphingolipids, threalose
ABSTRACT
Fungal infections are a universal problem and are routinely associated with high morbidity and mortality rates in immunocompromised patients. Existing therapies comprise five different classes of antifungal agents, four of which target the synthesis of ergosterol and cell wall glucans. However, the currently available antifungals have many limitations, including poor oral bioavailability, narrow therapeutic indices, and emerging drug resistance resulting from their use, thus making it essential to investigate the development of novel drugs which can overcome these limitations and add to the antifungal armamentarium. Advances have been made in antifungal drug discovery research and development over the past few years as evidenced by the presence of several new compounds currently in various stages of development. In the following minireview, we provide a comprehensive summary of compounds aimed at one or more novel molecular targets. We also briefly describe potential pathways relevant for fungal pathogenesis that can be considered for drug development in the near future.
INTRODUCTION
An estimated 1.7 billion individuals suffer from fungal infections worldwide (1, 2). Fungal infections that are pathologically relevant can be categorized into two main types: superficial fungal infections and invasive fungal infections (3). Superficial infections affect the skin, mucous membranes, and keratinous tissues, causing ailments such as thrush, oropharyngeal candidiasis, and dermatophyte infections. Invasive fungal infections are more life-threatening and affect sterile areas of the body such as the bloodstream, organs (lungs, liver, and kidneys), and the central nervous system (3, 4). Fungal infections can affect immunocompetent and immunocompromised individuals; however, the severity of invasive fungal infections in persons having an underlying disease, immunocompromised individuals undergoing organ transplant or chemotherapy, or patients with HIV/AIDS or autoimmune diseases is concerning (3–5) as such infections result in approximately 1.7 million deaths per year (1, 6, 7).
Of 5 million known fungal species, 300 are known to cause diseases in humans (8, 9); of these, 20 infect humans frequently (8). Examples include Candida albicans, Candida auris, Aspergillus fumigatus, Cryptococcus neoformans, Histoplasma capsulatum, Coccidioides immitis, Malassezia furfur, Blastomyces dermatitidis, Sporothrix spp., Fusarium, and Scedosporium (4, 5, 8, 10).
Globally, invasive fungal infections of aspergillosis account for 300,000 cases per year, candidiasis accounts for 750,000 cases, and cryptococcosis (in AIDS patients) accounts for 223,000 cases. Mortality rates are estimated to be 30% to 90%, 10% to 75%, and 20% to 70% for aspergillosis, candidiasis, and cryptococcosis, respectively (6, 7, 11, 12).
There are currently 5 structural classes of antifungal drugs being used to treat infections—polyenes, azoles, allylamines, pyrimidines, and echinocandins (2, 4–6). Polyenes (e.g., amphotericin B) bind ergosterol on the surface of the fungus, altering the permeability of the cell membrane (13, 14). They have potent fungicidal activity against Aspergillus spp., Cryptococcus spp., Candida spp., and other fungi. Azoles target lanosterol 14α-demethylase enzymatic activity, thus decreasing ergosterol content in fungi. Most azoles are fungistatic although they can behave as a fungicidal in certain molds, such as Aspergillus spp. Echinocandins target 1,3-β-glucan synthase activity, thus altering cell wall organization. Echinocandins are fungicidal against Candida spp. and fungistatic against Aspergillus, and they have no activity against Cryptococcus spp. Pyrimidines disrupt DNA and RNA biosynthesis by interfering with pyrimidine metabolism. This class is fungistatic against Cryptococcus spp. and against Candida spp. when used in conjunction with polyenes and with azoles, respectively (6, 7, 15, 16). Allylamines act by attenuating an enzyme (squalene epoxidase) of the ergosterol synthesis pathway (17, 18). They are fungicidal against dermatophytes and are fungistatic against C. albicans (19).
Although agents in these classes are effectively used as treatments today, there are some drawbacks to their use. Overuse, long treatment courses, and environmental exposure of azoles, polyenes, and echinocandins in the past decade have resulted in drug resistance (4). There is a high prevalence of Candida resistance to azoles and echinocandins. According to the 2019 Antibiotic Resistance Threats in the United States report generated by the CDC, there were 34,800 cases of infection and 1,700 deaths caused by drug-resistant Candida spp. Azole resistance is likely attributable to the drug being fungistatic in nature, creating a selection pressure leading to resistance, while resistance to echinocandins is relatively recent and has emerged due to the overuse of the drug in the past decade. Aspergillus and Cryptococcus also display azole resistance (7). Drug resistance arises from a reduced intracellular accumulation of the drug, decreased affinity between the drug target and drug, or a counteraction of the effect of a drug (15, 20). In addition to drug resistance, the polyenes and echinocandins have been shown to be highly toxic with a narrow therapeutic index which is confounded by poor (and variable) oral availability (5, 20). The use of extended-spectrum triazoles, posaconazole, and voriconazole is restricted by considerable drug-drug interactions, variable bioavailability, acute adverse events, and emergence of resistance (21).
With the large numbers of fungal infections, mortality rates associated with invasive fungal infections, and shortcomings of currently used antifungals, there is an ever-increasing need to discover new drugs with an improved range of properties. Only two antifungal drugs have been approved since the start of the 21st century: (i) caspofungin, the first echinocandin to be approved for use (in 2001) (echinocandins were the latest class of antifungals to be discovered in 1970 [2]); and (ii) isavuconazole, a triazole effective against dimorphic fungi, yeast, and molds (approved for use in 2015 (21, 22).
There are a variety of approaches to antifungal drug discovery. Such approaches can take the form of either a whole-cell-based or growth-based assay, where optical density as an indication of cell growth is utilized (23). Usually, protocols from the Clinical and laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) are used to determine in vitro susceptibility against a variety of fungal pathogens (24). Another approach could be by targeting specific pathways in fungi. Advantages of this approach include minimal risk of toxicity and also utility in helping to identify unique classes of compounds (23). Libraries of compounds used to screen utilizing these approaches could include synthetic, semisynthetic, or natural product libraries (24). They could also be compounds which have already been approved for other indications, an approach known as drug repurposing (24). The pursuit of natural or synthetic compounds as antifungal drugs has its own set of advantages. Natural products are highly complex, and they help us access chemical space that might be difficult to achieve with synthetic compounds (25). They also offer a good source for semisynthetic derivatives (26). Advantages of synthetic compounds include certainty of purity in terms of compounds not being mixtures of isomers, ease of large-scale synthesis, and availability of a large number of libraries for initial screening.
Antifungal drug discovery is challenging as fungal pathogens use much the same eukaryotic machinery as humans, thus reducing the number of pathogen-specific targets. Therefore, it is essential to identify biochemical mechanisms unique to fungi as drug discovery targets in order to develop the next generation(s) of antifungal therapies. An antifungal should ideally have the following properties: (i) minimal or manageable toxicities/side effects, providing a wide therapeutic index; (ii) pharmaceutical properties commensurate with multiple routes of delivery; (iii) activity corresponding to fungus-specific primary pathways and targets; (iv) effects that are preferably fungicidal; (v) a broad spectrum of activity against a range of fungi (7, 10). Development of a drug with all these properties will be arduous. In this minireview, we address the metabolic and signaling pathways that are unique to fungi and/or regulate virulence and which constitute promising targets for drug development.
AGENTS THAT TARGET FUNGAL CELL WALL SYNTHESIS
Fosmanogepix (APX001).
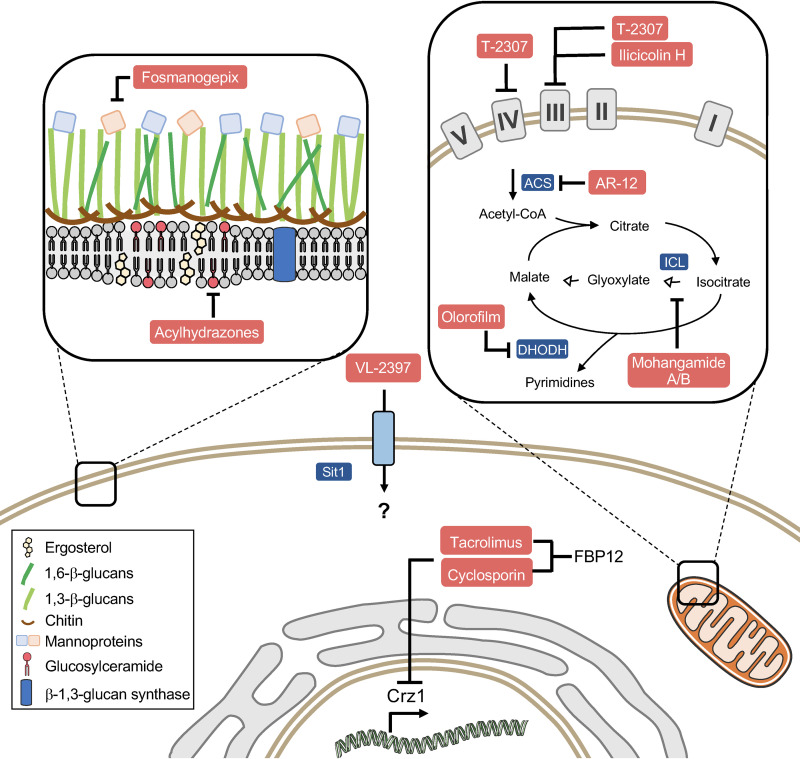
Fosmanogepix (Fig. 1) is a small-molecule antifungal developed by Amplyx Pharmaceuticals. It is an N-phosphonooxymethyl prodrug of APX001A, which targets the Gwt1 enzyme that catalyzes one of the early steps in the glycosylphosphatidylinositol (GPI)-anchored biosynthesis pathway (Fig. 2) (27, 28). Glycosylphosphatidylinositol (GPI)-anchored proteins are found in eukaryotic organisms, playing a crucial role in fungal adhesion to the host cells. Inhibition of Gwt1 prevents proper localization of mannoproteins, which are essential for cell wall integrity and fungal growth (27, 29). Fosmanogepix inhibited the growth of yeasts such as Candida spp. and C. neoformans, as well as filamentous fungi such as A. fumigatus, Fusarium solani, Scedosporium prolificans, and Pseudallescheria boydii (30). In addition, fosmanogepix prevented the inositol acylation of GPI in C. albicans and A. fumigatus, but not in human cells, suggesting that the compound is selective toward fungal cells (29). In mouse models, data from experiments performed with APX001 and APX001A displayed high rates of survival and reduced CFU levels of fungi in lung, kidney, and brain (27). Fosmanogepix was well tolerated when administered orally or intravenously in clinical phase 1 studies and was given fast-track status by the US FDA in September 2019 for seven invasive fungal infections, including candidiasis, aspergillosis, scedosporiosis, fusariosis, mucormycosis, cryptococcosis, and coccidioidomycosis (31). It is currently in phase 2 clinical trial for invasive candidiasis (31).
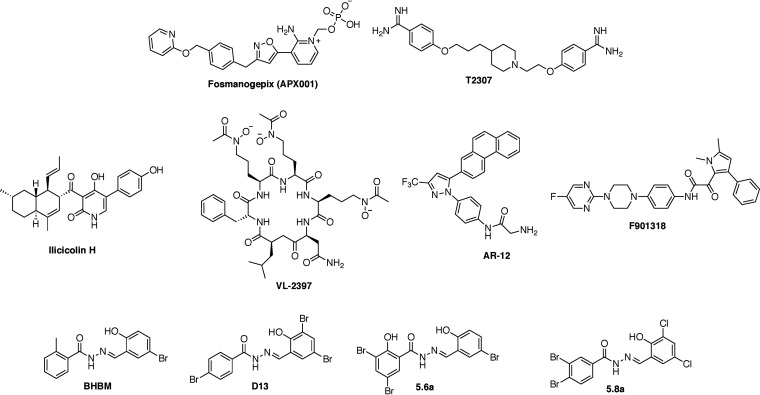
FIG 1.
Structures of the antifungal agents in development.
FIG 2.
New antifungal drugs and targets. Acylhydrazones impair the production of glucosylceramide. T-2307 and ilicicolin H act by inhibiting the mitochondrial respiratory chain complexes. AR-12, olorofilm, and mohangamides target metabolism-related enzymes. Tacrolimus and cyclosporine inhibit the fungal calcineurin (Crz1) pathway. ACS, acetyl-CoA synthetase.
Nikkomycin Z.
Nikkomycin is a pyrimidine nucleoside isolated from Streptomyces tendae (32). It inhibits the synthesis of chitin, an essential component of fungal cell wall, by competitively inhibiting chitin synthase and thus septation and causing osmotic stress to the fungal cell (32, 33). Since chitin is absent in mammalian cells, it makes an excellent antifungal target and in turn renders nikkomycin devoid of cytotoxic effects (33). Coccidiomycosis, also known as valley fever, is caused by Coccidioides posadasii or Coccidioides immitis (34). Investigators at the University of Arizona who were involved in the development of nikkomycin Z for treatment of pulmonary coccidiomycosis reported in 2014 that preparations were being made for a phase 2 clinical trial (35), although there have been no updates since.
INHIBITORS OF THE FUNCTION OF MITOCHONDRIA
Mitochondria represent the powerhouse of eukaryotic cells, producing most of the cellular ATP pool through the tricarboxylic acid (TCA) cycle and oxidative phosphorylation. The roles of mitochondria in energy metabolism include the synthesis of amino acids and phospholipids, which, in addition to respiration, govern processes such as senescence, virulence, and antifungal drug resistance (36, 37). Although the mitochondrial genomes of fungi and humans share high similarity, fungus-specific proteins (such as yeast Nuo1 and Nuo2) constitute promising targets for the development of selective antifungals (38). To our knowledge, the following two inhibitors of the fungal mitochondria have been described so far.
T-2307.
The arylamidine T-2307 exhibits fungicidal activity in vitro against Candida, Aspergillus, and Cryptococcus spp., preventing disseminated infection in mice (39, 40). This compound is efficiently internalized by C. albicans cells through polyamine transporters, which does not seem to occur in rat hepatocytes (41, 42). Once internalized into the fungal cell, T-2307 inhibits mainly complexes III and IV of the respiratory chain, disrupting the mitochondrial membrane potential (Fig. 2) (43, 44). Interestingly, only a minimal effect on rat mitochondria was observed, highlighting this compound’s potential to act as a selective inhibitor (44). Initially studied by the Fujifilm Toyama Chemical Co., T-2307 was licensed to Appili Therapeutics in November 2019, where it was renamed ATI-2307. The compound was well tolerated in human phase 1 studies and is currently under preclinical analysis prior phase 2, expected for 2021 (https://www.appilitherapeutics.com/ati-2307).
Ilicicolin H.
Ilicicolin H is a polyketide that was isolated from the fungus Cylindrocladium ilicicola and shows activity against Cryptococcus, Candida, and Aspergillus spp. (45). The mechanism of action of this compound involves the inhibition of the mitochondrial cytochrome bc1 reductase (50% inhibitory concentration [IC50], 2 to 3 ng/ml) (Fig. 2) (45–47). In the animal model, ilicicolin H reduced the fungal burden in mice infected with C. albicans and C. neoformans (45), exhibiting a low affinity toward rat mitochondria (48). Together, these observations highlight the potential of ilicicolin as an efficacious and selective antifungal.
OTHER/UNKNOWN
AR-12.
The celecoxib derivative AR-12 (Arno Therapeutics Inc.) was developed as a protein kinase inhibitor (PKI) and was initially used as an anticancer agent in phase I trial (ClinicalTrials registration no. NCT00978523). As protein kinases share structural and functional similarities across distinct organisms, PKI compounds were also screened for antibacterial and antifungal activity (49, 50). AR-12 inhibits the growth of several fungi, including yeasts such as Candida and C. neoformans and filamentous species such as A. fumigatus (51). The most interesting features of AR-12 involve its potent activity against dimorphic fungi and molds that are notoriously challenging in clinical settings, such as Scedosporium and Rhizopus oryzae (51). In addition, combination therapy using AR-12 and fluconazole reduced the fungal burden in brain in a mouse model of cryptococcosis (51). Although initially characterized as a PDK1 inhibitor, AR-12 does not inhibit the kinase ortholog in C. neoformans (52). Apparently, the antifungal activity of AR-12 involves dual mechanisms of action: (i) targeting of fungal acetyl coenzyme A (acetyl-CoA) synthetase, which catalyzes the production of acetyl-CoA from acetate and CoA (Fig. 2), regulating the histone acetylation and carbon metabolism (52), and (ii) downregulation of the host chaperones, modulating the immune response (8, 53). The future progress of AR-12 in clinical trials for antifungal therapy remains uncertain, as Arno Therapeutics declared bankruptcy in 2017 (2).
Acylhydrazones.
Aromatic acylhydrazones BHBM and D2 were identified as inhibitors of fungal sphingolipid synthesis through screening of a commercially available library (54, 55). Further analysis of BHBM and D2 derivatives led to the identification of a more potent compound, D13, that was highly active in vitro and performed better than BHBM in in vivo models of cryptococcosis, candidiasis, and pulmonary aspergillosis (56). Based on the structures of BHBM, D2, and D13, a novel library of ∼300 aromatic acylhydrazones was designed and synthesized. Further study resulted in the identification of 5 compounds which are potent, fungicidal, and highly selective toward fungi, with selectivity index values of >500 (57). Among the 5 lead compounds, SB-AF-1002 was tested in mouse models of a variety of invasive fungal infections and was found to outperform the current standard of care (58).
VL-2397.
VL-2397 (formerly termed ASP2397) is a cyclic hexapeptide isolated from the fungus Acremonium persicinum (59), potentially representing a novel class of antifungals with a unique mode of action. This compound chelates aluminum and is structurally related to ferrichrome, a low-molecular-weight siderophore (2, 59). Supplementation of the culture media with 0.03 mM iron increased the VL-2397 MIC for A. fumigatus from 1 mg/liter to 2 mg/liter (60). Similarly, the addition of bathophenanthroline disulfonate (BPS), an iron chelator, reduced the VL-2397 MIC from 1 mg/liter to 0.06 mg/liter. Together, these observations suggest that the compounds’ activity is affected by iron availability (60). In addition, the replacement of the Al in VL-2397 structure for Fe, generating the compound AS2488053, impaired antifungal activity (59). It was recently reported that iron abundance regulates expression of Sit1, the siderophore transporter that promotes VL-2397 internalization in the fungal cell (60). In fact, A. fumigatus cells lacking Sit1 were resistant to VL-2397 (63). Furthermore, expression of A. fumigatus Sit1 renders the intrinsically resistant species Saccharomyces cerevisiae susceptible to VL-2397 (63). Despite the requirement of Sit1 for VL-2397 uptake by the fungal cell, the intracellular target of the compound remains to be elucidated (Fig. 2).
VL-2397 inhibits the in vitro growth of Aspergillus spp., Fusarium solani, Candida glabrata, and C. neoformans (61). The compound also kills A. fumigatus conidia and prevents hyphal elongation in germlings (61). Potent and fungicidal activity of VL-2397, especially against Aspergillus, was also observed in vivo, as the drug increased survival rates in mouse and silkworm larva models of aspergillosis (61, 62). VL-2397 was well tolerated by healthy volunteers, successfully passing the phase I trials as a treatment for invasive aspergillosis (2, 60). Unfortunately, phase II studies using VL-2397 were discontinued for financial reasons.
Olorofilm.
Another promising class of antifungals includes the orotomide F901318 (olorofilm; F2G Ltd.), which targets the dihydroorotate dehydrogenase (DHODH) enzyme, involved in de novo pyrimidine biosynthesis (Fig. 2) (63). Pyrimidines act as structural precursors of molecules required for the synthesis of DNA/RNA, cell wall, and phospholipids (64), playing a crucial role in fungal virulence (65, 66). Although the DHODH enzyme is also found in mammals, F901318 affinity toward the human enzyme was 2,000-fold lower, suggesting that the drug inhibits specifically the fungal protein (63). F901318 showed potent activity against molds, such as Aspergillus species, and dimorphic fungi, such as H. capsulatum, B. dermatitidis, C. immitis, and Paracoccidioides brasiliensis (63, 67). Potential limitations in the clinical use of orotomides include the lack of activity against yeasts (such as Candida and Cryptococcus) (63). F901318 improved the rate of survival in a murine model of invasive aspergilosis, possibly through preventing A. fumigatus germination and hyphal extension (63, 68). Pharmacokinetic (PK) studies of F901318 showed good tissue distribution in mice, and the drug is being studied in phase IIb trials for the treatment of invasive infections caused by Aspergillus, Scedosporium, and other resistant species (ClinicalTrials registration no.03583164).
FUNGAL PATHWAYS AS PROMISING TARGETS FOR DRUG DEVELOPMENT
The glyoxylate cycle.
The glyoxylate cycle corresponds to an anaplerotic route of the tricarboxylic acid (TCA) cycle, bypassing the reactions that generate CO2 and allowing the use of two-carbon compounds as carbon sources for gluconeogenesis (69, 70). This metabolic pathway includes five enzymatic reactions; two of them, catalyzed by isocitrate lyase (ICL) and malate synthase (MS), are unique to this cycle, while the remaining three (citrate synthase, aconitase, and malate dehydrogenase) are shared with the TCA cycle (71). Interestingly, C. albicans cells lacking ICL1, the isocitrate lyase-encoding gene, were avirulent in mice (70). In addition, phagocytosis of C. albicans and P. brasiliensis induced glyoxylate cycle-related genes, suggesting that this pathway plays an important role in fungal survival inside the macrophages (72, 73). Besides its relevance to fungal pathogenesis, the glyoxylate cycle is not observed in the mammalian host and therefore constitutes a promising target for selective antifungals (74).
Mohangamides A and B were isolated from Streptomyces sp. and shown to inhibit C. albicans ICL (75). Additionally, mohangamide A impaired the growth of C. albicans in vitro when acetate but not glucose was used as a carbon source (75). The efficacy of these compounds in the treatment of a murine model of candidiasis and their pharmacokinetics properties remain to be further elucidated.
The calcineurin pathway.
Calcineurin is a Ca2+/calmodulin-activated protein phosphatase, conserved from fungi to mammals (76). The calcineurin pathway is the target of tacrolimus (FK506) and cyclosporine (CsA), which are widely used as immunosuppressive agents that prevent graft rejection. These drugs bind to the corresponding immunophilins (FK506-FKBP12 and CsA-CypA) and impair the access of phosphatase substrates to calcineurin, ultimately inhibiting T-cell proliferation. In pathogenic fungi, the calcineurin pathway plays a pivotal role in growth and virulence (77–79). Targeting of calcineurin for antifungal drug development has been restricted by the immunomodulatory effects that the drugs exert in the host. An attempt to circumvent this limitation included the synthesis of FK506 antagonists, permeative with respect to mammalian cells but not fungal cells, which likely minimize the immunosuppression while retaining their antifungal properties (80). The structural characterization of the FK506-FKBP12 complex in fungi also shed light on regions that differ from their mammalian counterparts, allowing the development of the APX879 compound (81). APX879 efficiently reduced fungal burden in a murine model of cryptococcosis, improving survival (81). In addition, this compound showed reduced immunosuppressive activity in comparison to the parental drug, FK506 (81). These new findings pave the way for the design of selective and efficacious inhibitors of the fungal calcineurin.
Hsp90.
Hsp90 is a conserved chaperone which regulates the function and stability of several client proteins, including its downstream effector calcineurin (82). In pathogenic fungi, Hsp90 mediates stress responses, virulence, and drug resistance (83–85). Hsp90 inhibitors improved fluconazole efficacy and prevented a lethal C. albicans infection (86), highlighting the therapeutic potential of molecules which target Hsp90. In fact, patients with invasive candidiasis who received an antibody against Hsp90 (efungumab [Mycograb; NeuTec Pharma/Novartis]) along with amphotericin B showed a better clinical response than those who were on amphotericin B monotherapy (87). Marketing authorization for Mycograb was denied in November 2006 due to quality concerns. A modified version of Mycograb, named Mycograb C28Y, was further developed but unfortunately was not as efficacious as the original formulation (88). Geldamycin and its derivatives, which target Hsp90, have been used for anticancer therapy; however, their use was restricted as they show host toxicity (86, 89). Novel inhibitors with reduced toxicity toward mammalian cells, enabling the development of Hsp90 inhibitors which retain antifungal activity and display increased selectivity, were previously described (90).
The trehalose pathway.
The disaccharide trehalose is an energy storage molecule and serves as a source of carbon (91). This sugar also functions as a stress protectant, preventing protein degradation and preserving the cell membrane structure under stress conditions (92, 93). Trehalose synthesis is linked to the glycolytic pathway, as the first step of trehalose production involves the conversion of glucose-6-phosphate to trehalose-6-phosphate by trehalose 6-phosphate synthase 1 (Tps1) (94). Next, trehalose 6-phosphate phosphatase (Tps2) generates trehalose from trehalose-6-phosphate. In Cryptococcus, deletion of TPS1 or TPS2 genes impaired growth at 37°C and rendered cells avirulent (95, 96). Interestingly, disruption of TPS2 was followed by the accumulation of the toxic intermediate trehalose 6-phosphate, causing fungal cell death (96). These observations suggest that targeting of the trehalose pathway, especially Tps2, might compromise fungal viability and virulence. In addition, mammalian cells lack trehalose synthesis, indicating that Tps2 inhibitors might act as selective antifungals.
Sphingolipid pathway.
Along with sterols, sphingolipids such as glucosylceramide (GlcCer), inositol phosphorylceramide (IPC), and mannosylinositol phosphorylceramide (MIPC) are major constituents of fungal lipid rafts (97). These molecules play crucial roles in fungal growth, differentiation, and virulence (98). Sphingolipid synthesis starts in the endoplasmic reticulum, with the condensation of serine and palmitoyl-CoA. This reaction produces 3-keto dihydrosphingosine and is catalyzed by the enzyme serine palmitoyltransferase (SPT), targeted by myriocin and serine (99, 100). The use of myriocin in antifungal therapy depends on the development of selective derivatives, as the compound also targets mammalian SPT (101). Another promising step in sphingolipid production to be targeted by novel compounds includes the synthesis of ceramide. Deletion of the ceramide synthase (CerS)-encoding gene renders C. neoformans cells avirulent (102), indicating that CerS is an important regulator of fungal pathogenesis. Fumonisin and australifungin were previously described to inhibit CerS (103) but showed a limited spectrum of activity and poor selectivity (104). Inhibitors of CerS might exhibit a dual mechanism of action, by depleting the pool of complex sphingolipids and leading to the accumulation of toxic intermediates. Therefore, the development of compounds which target the fungal but not the mammalian CerS might give rise to a novel class of potent antifungals.
Once produced, ceramides are transported to the Golgi apparatus, where synthesis of complex sphingolipids occurs. The transfer of a phosphoinositol from phosphatidylinositol to phytoceramide leads to the production of IPC, through the activity of IPC synthase (105). Aureobasidin and khafrefungin inhibit IPC synthase at nanomolar concentrations (106), showing activity against C. neoformans and C. albicans (107–109). Aureobasidin is also well tolerated in animals, efficiently treating invasive candidiasis (107). Aureogen Biosciences developed 58 novel derivatives from aureobasidin with improved potency against A. fumigatus, which were licensed to Merck in 2015.
Other pathways.
To efficiently colonize the host, fungal cells must sense and adapt to the challenges imposed by the physiological conditions. Stress-responsive pathways in fungi include the cyclic AMP signaling pathway, protein kinase C (PKC)/Mpk1 mitogen-activated protein kinase pathway and the high-osmolarity glycerol (HOG) pathway (110). In C. albicans and C. neoformans, the disturbance of the HOG pathway attenuated virulence in mice, highlighting the relevance of this cascade for fungal pathogenesis and its potential as an antifungal target (111–113). A variety of antifungal compounds, including ambutricins and phenylpyrroles (as fludioxonil), targeted the HOG pathway, leading to the accumulation of glycerol and fatty acids (114). The disbalance in the osmoregulation led to the leakage of intracellular content and, ultimately, cell death (115). Conversely, the antifungal cercosporamide inhibited selectively fungal Pkc1 (116), which has a central role in cell wall biosynthesis and remodeling (117). The loss of Pkc1 function was accompanied by cell lysis (118), indicating that pharmacological targeting of the Pkc1 might lead to the synthesis of fungicidal drugs.
REPURPOSING OF EXISTING DRUGS
Recently, in a continued effort to find new antifungal agents, drug repurposing was widely undertaken. This involves identifying new uses for drugs that had previously been approved by the U.S. FDA for different conditions (119). The advantages of this strategy over conventional drug discovery include lower risk of failure, especially in terms of safety, and shorter time frame for the development of the drug (120). Some of the drugs that were previously identified/used for other indications were repurposed to treat fungal infections.
Sertraline.
X. Lin and coworkers at Texas A&M University screened the Johns Hopkins clinical compound library and found that sertraline displayed modest inhibitory activity against Aspergillus nidulans (121). Further analysis of sertraline against Aspergillus and Candida species showed that its MIC against these fungi was much higher than the serum concentration of sertraline that can be achieved therapeutically (121). However, sertraline was found to be fungicidal against Cryptococcus at concentrations of <10 µg/ml and previous PK data in rats and dogs showed that its concentration in cerebrospinal fluid was 20-to-40-fold higher than its serum concentration. In an in vivo model of cryptococcosis, sertraline was found to reduce the fungal burden in the brain (121). In order to understand the antifungal mechanism of sertraline, X. Lin and coworkers screened a whole-genome deletion collection of Saccharomyces cerevisiae isolates and found that sertraline perturbs translation and, in turn, protein synthesis (121).
Tamoxifen.
Tamoxifen is a drug that is generally used to treat breast cancer and also as a protective adjuvant in women who have high risk of developing breast cancer (122). Its anticancer properties are known to be mediated by estrogen receptor antagonism and also by oxidative stress on breast cancer cells (123, 124). In 1989, Wiseman, Cannon, and Arnstein reported inhibitory effect of tamoxifen against Saccharomyces cerevisiae (125). Tamoxifen was found to be fungicidal against C. albicans at 15 to 20 µM concentration, whereas its MIC against C. neoformans and other Candida species was 8 to 64 g/ml (122). Tamoxifen was also effective in a murine model of candidiasis at 200 mg/kg of body weight. Although the exact mechanism by which tamoxifen exerts antifungal activity is not well known, inhibition of some components of the calcium-calcineurin pathway and inhibition of the calmodulin site are two methods proposed by researchers (122).
SUMMARY
Despite the increased mortality caused by fungal infections in the past decade, only minor advances in antifungal therapy have been reported during this decade. In fact, most of the drugs that were recently approved or that are currently in development consist of derivatives of azoles and echinocandins. Several compounds highlighted here inhibited fungus-specific proteins/pathways, exhibiting low toxicity toward mammalian cells and good pharmacological properties with a broader spectrum of activity than current antifungals. As only a small fraction of compounds undergoing clinical studies will be approved and released into the market, it is imperative that more antifungal compounds are identified and thoroughly explored in the coming years.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI136934, AI116420, and AI125770 and by Merit Review Grant I01BX002924 from the Veterans Affairs Program to M.D.P. M.D.P. is a Burroughs Welcome Investigator in Infectious Diseases.
M.D.P. is a cofounder of and Chief Scientific Officer (CSO) of MicroRid Technologies Inc. J.B.M. is a cofounder of and Chief Executive Office of MicroRid Technologies Inc. J.M. is a cofounder of and Chief Research and Development Officer of MicroRid Technologies Inc.
REFERENCES
- 1.Denning DW, Bromley MJ. 2015. Infectious disease. How to bolster the antifungal pipeline. Science 347:1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- 2.Rauseo AM, Coler-Reilly A, Larson L, Spec A. 2020. Hope on the horizon: novel fungal treatments in development. Open Forum Infect Dis 7:ofaa016. doi: 10.1093/ofid/ofaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roemer T, Krysan DJ. 2014. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez de Ullivarri M, Arbulu S, Garcia-Gutierrez E, Cotter PD. 2020. Antifungal peptides as therapeutic agents. Front Cell Infect Microbiol 10:105. doi: 10.3389/fcimb.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasim S, Coleman JJ. 2019. Targeting the fungal cell wall: current therapies and implications for development of alternative antifungal agents. Future Med Chem 11:869–883. doi: 10.4155/fmc-2018-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houst J, Spizek J, Havlicek V. 2020. Antifungal drugs. Metabolites 10:106. doi: 10.3390/metabo10030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pianalto KM, Alspaugh JA. 2016. New horizons in antifungal therapy. J Fungi (Basel) 2:26. doi: 10.3390/jof2040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perfect JR 2017. The antifungal pipeline: a reality check. Nat Rev Drug Discov 16:603–616. doi: 10.1038/nrd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmiedel Y, Zimmerli S. 2016. Common invasive fungal diseases: an overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med Wkly 146:w14281. doi: 10.4414/smw.2016.14281. [DOI] [PubMed] [Google Scholar]
- 10.Mazu TK, Bricker BA, Flores-Rozas H, Ablordeppey SY. 2016. The mechanistic targets of antifungal agents: an overview. Mini Rev Med Chem 16:555–578. doi: 10.2174/1389557516666160118112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bongomin F, Gago S, Oladele RO, Denning DW. 2017. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. 2012. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A 109:2234–2239. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, Nieuwkoop AJ, Comellas G, Maryum N, Wang S, Uno BE, Wildeman EL, Gonen T, Rienstra CM, Burke MD. 2014. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol 10:400–406. doi: 10.1038/nchembio.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spampinato C, Leonardi D. 2013. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed Res Int 2013:204237. doi: 10.1155/2013/204237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Daele R, Spriet I, Wauters J, Maertens J, Mercier T, Van Hecke S, Bruggemann R. 2019. Antifungal drugs: what brings the future? Med Mycol 57:S328–S343. doi: 10.1093/mmy/myz012. [DOI] [PubMed] [Google Scholar]
- 17.Birnbaum JE 1990. Pharmacology of the allylamines. J Am Acad Dermatol 23:782–785. doi: 10.1016/0190-9622(90)70288-s. [DOI] [PubMed] [Google Scholar]
- 18.Nowosielski M, Hoffmann M, Wyrwicz LS, Stepniak P, Plewczynski DM, Lazniewski M, Ginalski K, Rychlewski L. 2011. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J Chem Inf Model 51:455–462. doi: 10.1021/ci100403b. [DOI] [PubMed] [Google Scholar]
- 19.Bondaryk M, Kurzątkowski W, Staniszewska M. 2013. Antifungal agents commonly used in the superficial and mucosal candidiasis treatment: mode of action and resistance development. Postepy Dermatol Alergol 30:293–301. doi: 10.5114/pdia.2013.38358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parente-Rocha JA, Bailao AM, Amaral AC, Taborda CP, Paccez JD, Borges CL, Pereira M. 2017. Antifungal resistance, metabolic routes as drug targets, and new antifungal agents: an overview about endemic dimorphic fungi. Mediators Inflamm 2017:9870679. doi: 10.1155/2017/9870679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miceli MH, Kauffman CA. 2015. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis 61:1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 22.Pettit NN, Carver PL. 2015. Isavuconazole: a new option for the management of invasive fungal infections. Ann Pharmacother 49:825–842. doi: 10.1177/1060028015581679. [DOI] [PubMed] [Google Scholar]
- 23.Beattie SR, Krysan DJ. 2020. Antifungal drug screening: thinking outside the box to identify novel antifungal scaffolds. Curr Opin Microbiol 57:1–6. doi: 10.1016/j.mib.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderone R, Sun N, Gay-Andrieu F, Groutas W, Weerawarna P, Prasad S, Alex D, Li D. 2014. Antifungal drug discovery: the process and outcomes. Future Microbiol 9:791–805. doi: 10.2217/fmb.14.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aldholmi M, Marchand P, Ourliac-Garnier I, Le Pape P, Ganesan A. 2019. A decade of antifungal leads from natural products: 2010-2019. Pharmaceuticals (Basel) 12:182. doi: 10.3390/ph12040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katiyar C, Gupta A, Kanjilal S, Katiyar S. 2012. Drug discovery from plant sources: an integrated approach. Ayu 33:10–19. doi: 10.4103/0974-8520.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw KJ, Schell WA, Covel J, Duboc G, Giamberardino C, Kapoor M, Moloney M, Soltow QA, Tenor JL, Toffaletti DL, Trzoss M, Webb P, Perfect JR. 2018. In vitro and in vivo evaluation of APX001A/APX001 and other Gwt1 inhibitors against cryptococcus. Antimicrob Agents Chemother 62:e00523-18. doi: 10.1128/AAC.00523-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukahara K, Hata K, Nakamoto K, Sagane K, Watanabe N-a, Kuromitsu J, Kai J, Tsuchiya M, Ohba F, Jigami Y, Yoshimatsu K, Nagasu T. 2003. Medicinal genetics approach towards identifying the molecular target of a novel inhibitor of fungal cell wall assembly. Mol Microbiol 48:1029–1042. doi: 10.1046/j.1365-2958.2003.03481.x. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe N-a, Miyazaki M, Horii T, Sagane K, Tsukahara K, Hata K. 2012. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother 56:960–971. doi: 10.1128/AAC.00731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki M, Horii T, Hata K, Watanabe N-A, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M. 2011. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother 55:4652–4658. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amplyx Pharmaceuticals. 2020. Our pipeline. https://amplyx.com/our-pipeline/. Accessed 5 November 2020.
- 32.Groll AH, Piscitelli SC, Walsh TJ. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol 44:343–500. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 33.Bell AS 2007. 7.15—Major antifungal drugs, p 445–468. In Taylor JB, Triggle DJ (ed), Comprehensive medicinal chemistry II. Elsevier, Oxford, United Kingdom. doi: 10.1016/B0-08-045044-X/00216-9. [DOI] [Google Scholar]
- 34.Van Dyke MCC, Thompson GR, Galgiani JN, Barker BM. 2019. The rise of coccidioides: forces against the dust devil unleashed. Front Immunol 10:2188–2188. doi: 10.3389/fimmu.2019.02188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shubitz LF, Trinh HT, Perrill RH, Thompson CM, Hanan NJ, Galgiani JN, Nix DE. 2014. Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials. J Infect Dis 209:1949–1954. doi: 10.1093/infdis/jiu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malina C, Larsson C, Nielsen J. 2018. Yeast mitochondria: an overview of mitochondrial biology and the potential of mitochondrial systems biology. FEMS Yeast Res 18:foy040. doi: 10.1093/femsyr/foy040. [DOI] [PubMed] [Google Scholar]
- 37.Chatre L, Ricchetti M. 2014. Are mitochondria the Achilles’ heel of the Kingdom Fungi? Curr Opin Microbiol 20:49–54. doi: 10.1016/j.mib.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Calderone R. 2017. Exploiting mitochondria as targets for the development of new antifungals. Virulence 8:159–168. doi: 10.1080/21505594.2016.1188235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitsuyama J, Nomura N, Hashimoto K, Yamada E, Nishikawa H, Kaeriyama M, Kimura A, Todo Y, Narita H. 2008. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother 52:1318–1324. doi: 10.1128/AAC.01159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishikawa H, Fukuda Y, Mitsuyama J, Tashiro M, Tanaka A, Takazono T, Saijo T, Yamamoto K, Nakamura S, Imamura Y, Miyazaki T, Kakeya H, Yamamoto Y, Yanagihara K, Mukae H, Kohno S, Izumikawa K. 2017. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine, against Cryptococcus gattii: an emerging fungal pathogen. J Antimicrob Chemother 72:1709–1713. doi: 10.1093/jac/dkx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishikawa H, Yamada E, Shibata T, Uchihashi S, Fan H, Hayakawa H, Nomura N, Mitsuyama J. 2010. Uptake of T-2307, a novel arylamidine, in Candida albicans. J Antimicrob Chemother 65:1681–1687. doi: 10.1093/jac/dkq177. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa H, Sakagami T, Yamada E, Fukuda Y, Hayakawa H, Nomura N, Mitsuyama J, Miyazaki T, Mukae H, Kohno S. 2016. T-2307, a novel arylamidine, is transported into Candida albicans by a high-affinity spermine and spermidine carrier regulated by Agp2. J Antimicrob Chemother 71:1845–1855. doi: 10.1093/jac/dkw095. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita K, Miyazaki T, Fukuda Y, Mitsuyama J, Saijo T, Shimamura S, Yamamoto K, Imamura Y, Izumikawa K, Yanagihara K, Kohno S, Mukae H. 2019. The novel arylamidine T-2307 selectively disrupts yeast mitochondrial function by inhibiting respiratory chain complexes. Antimicrob Agents Chemother 63:e00374-19. doi: 10.1128/AAC.00374-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibata T, Takahashi T, Yamada E, Kimura A, Nishikawa H, Hayakawa H, Nomura N, Mitsuyama J. 2012. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother 56:5892–5897. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh SB, Liu W, Li X, Chen T, Shafiee A, Card D, Abruzzo G, Flattery A, Gill C, Thompson JR, Rosenbach M, Dreikorn S, Hornak V, Meinz M, Kurtz M, Kelly R, Onishi JC. 2012. Antifungal spectrum, in vivo efficacy, and structure–activity relationship of ilicicolin H. ACS Med Chem Lett 3:814–817. doi: 10.1021/ml300173e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Covian R, Trumpower BL. 2009. Ilicicolin inhibition and binding at center N of the dimeric cytochrome bc1 complex reveal electron transfer and regulatory interactions between monomers. J Biol Chem 284:8614–8620. doi: 10.1074/jbc.M808914200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutierrez-Cirlos EB, Merbitz-Zahradnik T, Trumpower BL. 2004. Inhibition of the yeast cytochrome bc1 complex by ilicicolin H, a novel inhibitor that acts at the Qn site of the bc1 complex. J Biol Chem 279:8708–8714. doi: 10.1074/jbc.M311805200. [DOI] [PubMed] [Google Scholar]
- 48.Singh SB, Liu W, Li X, Chen T, Shafiee A, Dreikorn S, Hornak V, Meinz M, Onishi JC. 2013. Structure–activity relationship of cytochrome bc1 reductase inhibitor broad spectrum antifungal ilicicolin H. Bioorg Med Chem Lett 23:3018–3022. doi: 10.1016/j.bmcl.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 49.Walsh CT, Fischbach MA. 2009. Repurposing libraries of eukaryotic protein kinase inhibitors for antibiotic discovery. Proc Natl Acad Sci U S A 106:1689–1690. doi: 10.1073/pnas.0813405106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baxter BK, DiDone L, Ogu D, Schor S, Krysan DJ. 2011. Identification, in vitro activity and mode of action of phosphoinositide-dependent-1 kinase inhibitors as antifungal molecules. ACS Chem Biol 6:502–510. doi: 10.1021/cb100399x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koselny K, Green J, DiDone L, Halterman JP, Fothergill AW, Wiederhold NP, Patterson TF, Cushion MT, Rappelye C, Wellington M. 2016. The celecoxib derivative AR-12 has broad-spectrum antifungal activity in vitro and improves the activity of fluconazole in a murine model of cryptococcosis. Antimicrob Agents Chemother 60:7115–7127. doi: 10.1128/AAC.01061-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koselny K, Green J, Favazzo L, Glazier VE, DiDone L, Ransford S, Krysan DJ. 2016. Antitumor/antifungal celecoxib derivative AR-12 is a non-nucleoside inhibitor of the ANL-family adenylating enzyme acetyl CoA synthetase. ACS Infect Dis 2:268–280. doi: 10.1021/acsinfecdis.5b00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Mambro T, Guerriero I, Aurisicchio L, Magnani M, Marra E. 2019. The yin and yang of current antifungal therapeutic strategies: how can we harness our natural defenses? Front Pharmacol 10:80. doi: 10.3389/fphar.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mor V, Rella A, Farnoud AM, Singh A, Munshi M, Bryan A, Naseem S, Konopka JB, Ojima I, Bullesbach E, Ashbaugh A, Linke MJ, Cushion M, Collins M, Ananthula HK, Sallans L, Desai PB, Wiederhold NP, Fothergill AW, Kirkpatrick WR, Patterson T, Wong LH, Sinha S, Giaever G, Nislow C, Flaherty P, Pan X, Cesar GV, de Melo Tavares P, Frases S, Miranda K, Rodrigues ML, Luberto C, Nimrichter L, Del Poeta M. 2015. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. mBio 6:e00647-15. doi: 10.1128/mBio.00647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mor V, Rella A, Farnoud AM, Singh A, Munshi M, Bryan A, Naseem S, Konopka JB, Ojima I, Bullesbach E, Ashbaugh A, Linke MJ, Cushion M, Collins M, Ananthula HK, Sallans L, Desai PB, Wiederhold NP, Fothergill AW, Kirkpatrick WR, Patterson T, Wong LH, Sinha S, Giaever G, Nislow C, Flaherty P, Pan X, Cesar GV, de Melo Tavares P, Frases S, Miranda K, Rodrigues ML, Luberto C, Nimrichter L, Del Poeta M. 2018. Erratum for Mor et al., “Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids”. mBio 9:e00188-18. doi: 10.1128/mBio.00188-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazzarini C, Haranahalli K, Rieger R, Ananthula HK, Desai PB, Ashbaugh A, Linke MJ, Cushion MT, Ruzsicska B, Haley J, Ojima I, Del Poeta M. 2018. Acylhydrazones as antifungal agents targeting the synthesis of fungal sphingolipids. Antimicrob Agents Chemother 62:e00156-18. doi: 10.1128/AAC.00156-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haranahalli K, Lazzarini C, Sun Y, Zambito J, Pathiranage S, McCarthy JB, Mallamo J, Del Poeta M, Ojima I. 2019. SAR Studies on aromatic acylhydrazone-based inhibitors of fungal sphingolipid synthesis as next-generation antifungal agents. J Med Chem 62:8249–8273. doi: 10.1021/acs.jmedchem.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazzarini C, Haranahalli K, McCarthy JB, Mallamo J, Ojima I, Del Poeta M. 2020. Preclinical evaluation of acylhydrazone SB-AF-1002 as a novel broad-spectrum antifungal agent. Antimicrob Agents Chemother 64:e00946-20. doi: 10.1128/AAC.00946-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura I, Yoshimura S, Masaki T, Takase S, Ohsumi K, Hashimoto M, Furukawa S, Fujie A. 2017. ASP2397: a novel antifungal agent produced by Acremonium persicinum MF-347833. J Antibiot (Tokyo) 70:45–51. doi: 10.1038/ja.2016.107. [DOI] [PubMed] [Google Scholar]
- 60.Dietl A-M, Misslinger M, Aguiar MM, Ivashov V, Teis D, Pfister J, Decristoforo C, Hermann M, Sullivan SM, Smith LR, Haas H. 2019. The siderophore transporter Sit1 determines susceptibility to the antifungal VL-2397. Antimicrob Agents Chemother 63:e00807-19. doi: 10.1128/AAC.00807-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakamura I, Ohsumi K, Takeda S, Katsumata K, Matsumoto S, Akamatsu S, Mitori H, Nakai T. 2019. ASP2397 is a novel natural compound that exhibits rapid and potent fungicidal activity against Aspergillus species through a specific transporter. Antimicrob Agents Chemother 63:e02689-18. doi: 10.1128/AAC.02689-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakamura I, Kanasaki R, Yoshikawa K, Furukawa S, Fujie A, Hamamoto H, Sekimizu K. 2017. Discovery of a new antifungal agent ASP2397 using a silkworm model of Aspergillus fumigatus infection. J Antibiot (Tokyo) 70:41–44. doi: 10.1038/ja.2016.106. [DOI] [PubMed] [Google Scholar]
- 63.Oliver JD, Sibley GEM, Beckmann N, Dobb KS, Slater MJ, McEntee L, Du Pré S, Livermore J, Bromley MJ, Wiederhold NP, Hope WW, Kennedy AJ, Law D, Birch M. 2016. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci U S A 113:12809–12814. doi: 10.1073/pnas.1608304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garavito MF, Narváez-Ortiz HY, Zimmermann BH. 2015. Pyrimidine metabolism: dynamic and versatile pathways in pathogens and cellular development. J Genet Genomics 42:195–205. doi: 10.1016/j.jgg.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 65.D'Enfert C, Diaquin M, Delit A, Wuscher N, Debeaupuis J-P, Huerre M, Latge J-P. 1996. Attenuated virulence of uridine-uracil auxotrophs of Aspergillus fumigatus. Infect Immun 64:4401–4405. doi: 10.1128/IAI.64.10.4401-4405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Gontijo FA, Pascon RC, Fernandes L, Machado J, Jr, Alspaugh JA, Vallim MA. 2014. The role of the de novo pyrimidine biosynthetic pathway in Cryptococcus neoformans high temperature growth and virulence. Fungal Genet Biol 70:12–23. doi: 10.1016/j.fgb.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buil J, Rijs A, Meis J, Birch M, Law D, Melchers W, Verweij P. 2017. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J Antimicrob Chemother 72:2548–2552. doi: 10.1093/jac/dkx177. [DOI] [PubMed] [Google Scholar]
- 68.Du Pré S, Beckmann N, Almeida MC, Sibley GE, Law D, Brand AC, Birch M, Read ND, Oliver JD. 2018. Effect of the novel antifungal drug F901318 (olorofim) on growth and viability of Aspergillus fumigatus. Antimicrob Agents Chemother 62:e00231-18. doi: 10.1128/AAC.00231-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunn M, Ramirez-Trujillo J, Hernández-Lucas I. 2009. Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology (Reading) 155:3166–3175. doi: 10.1099/mic.0.030858-0. [DOI] [PubMed] [Google Scholar]
- 70.Lorenz MC, Fink GR. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83–86. doi: 10.1038/35083594. [DOI] [PubMed] [Google Scholar]
- 71.Piekarska K, Hardy G, Mol E, van den Burg J, Strijbis K, van Roermund C, van den Berg M, Distel B. 2008. The activity of the glyoxylate cycle in peroxisomes of Candida albicans depends on a functional β-oxidation pathway: evidence for reduced metabolite transport across the peroxisomal membrane. Microbiology (Reading) 154:3061–3072. doi: 10.1099/mic.0.2008/020289-0. [DOI] [PubMed] [Google Scholar]
- 72.Lorenz MC, Bender JA, Fink GR. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Derengowski L, Tavares A, Silva S, Procópio L, Felipe M, Silva-Pereira I. 2008. Upregulation of glyoxylate cycle genes upon Paracoccidioides brasiliensis internalization by murine macrophages and in vitro nutritional stress condition. Med Mycol 46:125–134. doi: 10.1080/13693780701670509. [DOI] [PubMed] [Google Scholar]
- 74.Li X, Hou Y, Yue L, Liu S, Du J, Sun S. 2015. Potential targets for antifungal drug discovery based on growth and virulence in Candida albicans. Antimicrob Agents Chemother 59:5885–5891. doi: 10.1128/AAC.00726-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bae M, Kim H, Moon K, Nam S-J, Shin J, Oh K-B, Oh D-C. 2015. Mohangamides A and B, new dilactone-tethered pseudo-dimeric peptides inhibiting Candida albicans isocitrate lyase. Org Lett 17:712–715. doi: 10.1021/ol5037248. [DOI] [PubMed] [Google Scholar]
- 76.Juvvadi PR, Lamoth F, Steinbach WJ. 2014. Calcineurin as a multifunctional regulator: unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol Rev 28:56–69. doi: 10.1016/j.fbr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J 16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blankenship JR, Heitman J. 2005. Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect Immun 73:5767–5774. doi: 10.1128/IAI.73.9.5767-5774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steinbach WJ, Cramer RA, Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, Kirkpatrick WR, Patterson TF, Benjamin DK, Heitman J, Perfect JR. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell 5:1091–1103. doi: 10.1128/EC.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nambu M, Covel JA, Kapoor M, Li X, Moloney MK, Numa MM, Soltow QA, Trzoss M, Webb P, Webb RR, Mutz M. 2017. A calcineurin antifungal strategy with analogs of FK506. Bioorg Med Chem Lett 27:2465–2471. doi: 10.1016/j.bmcl.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Juvvadi PR, Fox D, Bobay BG, Hoy MJ, Gobeil SMC, Venters RA, Chang Z, Lin JJ, Averette AF, Cole DC, Barrington BC, Wheaton JD, Ciofani M, Trzoss M, Li X, Lee SC, Chen Y-L, Mutz M, Spicer LD, Schumacher MA, Heitman J, Steinbach WJ. 2019. Harnessing calcineurin-FK506-FKBP12 crystal structures from invasive fungal pathogens to develop antifungal agents. Nat Commun 10:4275. doi: 10.1038/s41467-019-12199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Meara TR, Robbins N, Cowen LE. 2017. The Hsp90 chaperone network modulates Candida virulence traits. Trends Microbiol 25:809–819. doi: 10.1016/j.tim.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, Heitman J, Cowen LE. 2009. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol 19:621–629. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. 2009. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog 5:e1000532. doi: 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cowen LE, Lindquist S. 2005. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 86.Cowen LE, Singh SD, Köhler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, Lindquist S. 2009. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A 106:2818–2823. doi: 10.1073/pnas.0813394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pachl J, Svoboda P, Jacobs F, Vandewoude K, van der Hoven B, Spronk P, Masterson G, Malbrain M, Aoun M, Garbino J, Takala J, Drgona L, Burnie J, Matthews R, Mycograb Invasive Candidiasis Study Group. 2006. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis 42:1404–1413. doi: 10.1086/503428. [DOI] [PubMed] [Google Scholar]
- 88.Bugli F, Cacaci M, Martini C, Torelli R, Posteraro B, Sanguinetti M, Paroni Sterbini F. 2013. Human monoclonal antibody-based therapy in the treatment of invasive candidiasis. Clin Dev Immunol 2013:403121. doi: 10.1155/2013/403121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jhaveri K, Taldone T, Modi S, Chiosis G. 2012. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta 1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whitesell L, Robbins N, Huang DS, McLellan CA, Shekhar-Guturja T, LeBlanc EV, Nation CS, Hui R, Hutchinson A, Collins C, Chatterjee S, Trilles R, Xie JL, Krysan DJ, Lindquist S, Porco JA, Tatu U, Brown LE, Pizarro J, Cowen LE. 2019. Structural basis for species-selective targeting of Hsp90 in a pathogenic fungus. Nat Commun 10:17. doi: 10.1038/s41467-018-08248-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lillie SH, Pringle JR. 1980. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol 143:1384–1394. doi: 10.1128/JB.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crowe JH, Crowe LM, Chapman D. 1984. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science 223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 93.Gadd G, Chalmers K, Reed R. 1987. The role of trehalose in dehydration resistance of Saccharomyces cerevisiae. FEMS Microbiol Lett 48:249–254. doi: 10.1111/j.1574-6968.1987.tb02551.x. [DOI] [Google Scholar]
- 94.Perfect JR, Tenor JL, Miao Y, Brennan RG. 2017. Trehalose pathway as an antifungal target. Virulence 8:143–149. doi: 10.1080/21505594.2016.1195529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ngamskulrungroj P, Himmelreich U, Breger JA, Wilson C, Chayakulkeeree M, Krockenberger MB, Malik R, Daniel H-M, Toffaletti D, Djordjevic JT, Mylonakis E, Meyer W, Perfect JR. 2009. The trehalose synthesis pathway is an integral part of the virulence composite for Cryptococcus gattii. Infect Immun 77:4584–4596. doi: 10.1128/IAI.00565-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petzold EW, Himmelreich U, Mylonakis E, Rude T, Toffaletti D, Cox GM, Miller JL, Perfect JR. 2006. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect Immun 74:5877–5887. doi: 10.1128/IAI.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farnoud AM, Toledo AM, Konopka JB, Del Poeta M, London E. 2015. Raft-like membrane domains in pathogenic microorganisms. Curr Top Membr 74:233–268. doi: 10.1016/bs.ctm.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fernandes CM, Goldman GH, Del Poeta M. 2018. Biological roles played by sphingolipids in dimorphic and filamentous fungi. mBio 9:e00642-18. doi: 10.1128/mBio.00642-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, Wright GD. 2011. Cross‐species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol 7:499. doi: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T. 1995. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem Biophys Res Commun 211:396–403. doi: 10.1006/bbrc.1995.1827. [DOI] [PubMed] [Google Scholar]
- 101.Hanada K, Nishijima M, Fujita T, Kobayashi S. 2000. Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells: a novel evaluation system using an SPT-defective mammalian cell mutant. Biochem Pharmacol 59:1211–1216. doi: 10.1016/s0006-2952(00)00251-3. [DOI] [PubMed] [Google Scholar]
- 102.Munshi MA, Gardin JM, Singh A, Luberto C, Rieger R, Bouklas T, Fries BC, Del Poeta M. 2018. The role of ceramide synthases in the pathogenicity of Cryptococcus neoformans. Cell Rep 22:1392–1400. doi: 10.1016/j.celrep.2018.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Delgado A, Casas J, Llebaria A, Abad JL, Fabrias G. 2006. Inhibitors of sphingolipid metabolism enzymes. Biochim Biophys Acta 1758:1957–1977. doi: 10.1016/j.bbamem.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 104.Voss KA, Riley RT, Norred W, Bacon CW, Meredith FI, Howard PC, Plattner RD, Collins T, Hansen DK, Porter JK. 2001. An overview of rodent toxicities: liver and kidney effects of fumonisins and Fusarium moniliforme. Environ Health Perspect 109:259–266. doi: 10.2307/3435017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heidler SA, Radding JA. 2000. Inositol phosphoryl transferases from human pathogenic fungi. Biochim Biophys Acta 1500:147–152. doi: 10.1016/S0925-4439(99)00097-6. [DOI] [PubMed] [Google Scholar]
- 106.Aeed PA, Young CL, Nagiec MM, Elhammer ÅP. 2009. Inhibition of inositol phosphorylceramide synthase by the cyclic peptide aureobasidin A. Antimicrob Agents Chemother 53:496–504. doi: 10.1128/AAC.00633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takesako K, Kuroda H, Inoue T, Haruna F, Yoshikawa Y, Kato I, Uchida K, Hiratani T, Yamaguchi H. 1993. Biological properties of aureobasidin A, a cyclic depsipeptide antifungal antibiotic. J Antibiot (Tokyo) 46:1414–1420. doi: 10.7164/antibiotics.46.1414. [DOI] [PubMed] [Google Scholar]
- 108.Nagiec MM, Nagiec EE, Baltisberger JA, Wells GB, Lester RL, Dickson RC. 1997. Sphingolipid synthesis as a target for antifungal drugs complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J Biol Chem 272:9809–9817. doi: 10.1074/jbc.272.15.9809. [DOI] [PubMed] [Google Scholar]
- 109.Mandala SM, Thornton RA, Rosenbach M, Milligan J, Garcia-Calvo M, Bull HG, Kurtz MB. 1997. Khafrefungin, a novel inhibitor of sphingolipid synthesis. J Biol Chem 272:32709–32714. doi: 10.1074/jbc.272.51.32709. [DOI] [PubMed] [Google Scholar]
- 110.Bahn Y-S 2008. Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot Cell 7:2017–2036. doi: 10.1128/EC.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alonso-Monge R, Navarro-Garcia F, Molero G, Diez-Orejas R, Gustin M, Pla J, Sanchez M, Nombela C. 1999. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol 181:3058–3068. doi: 10.1128/JB.181.10.3058-3068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bahn Y-S, Kojima K, Cox GM, Heitman J. 2005. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol Biol Cell 16:2285–2300. doi: 10.1091/mbc.e04-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bahn Y-S, Kojima K, Cox GM, Heitman J. 2006. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol Biol Cell 17:3122–3135. doi: 10.1091/mbc.e06-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vetcher L, Menzella HG, Kudo T, Motoyama T, Katz L. 2007. The antifungal polyketide ambruticin targets the HOG pathway. Antimicrob Agents Chemother 51:3734–3736. doi: 10.1128/AAC.00369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Knauth P, Reichenbach H. 2000. On the mechanism of action of the myxobacterial fungicide ambruticin. J Antibiot (Tokyo) 53:1182–1190. doi: 10.7164/antibiotics.53.1182. [DOI] [PubMed] [Google Scholar]
- 116.Sussman A, Huss K, Chio L-C, Heidler S, Shaw M, Ma D, Zhu G, Campbell RM, Park T-S, Kulanthaivel P, Scott JE, Carpenter JW, Strege MA, Belvo MD, Swartling JR, Fischl A, Yeh W-K, Shih C, Ye XS. 2004. Discovery of cercosporamide, a known antifungal natural product, as a selective Pkc1 kinase inhibitor through high-throughput screening. Eukaryot Cell 3:932–943. doi: 10.1128/EC.3.4.932-943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fuchs BB, Mylonakis E. 2009. Our paths might cross: the role of the fungal cell wall integrity pathway in stress response and cross talk with other stress response pathways. Eukaryot Cell 8:1616–1625. doi: 10.1128/EC.00193-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Levin DE, Bartlett-Heubusch E. 1992. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol 116:1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ashburn TT, Thor KB. 2004. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 120.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M. 2019. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 121.Zhai B, Wu C, Wang L, Sachs MS, Lin X. 2012. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother 56:3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Al-Janabi AAHS, Al-Mosawe HAS, Ai-Moswai K. 2019. Tamoxifen: from anti-cancer to antifungal drug. Int J Med Rev 6:88–91. doi: 10.29252/IJMR-060304. [DOI] [Google Scholar]
- 123.Bekele RT, Venkatraman G, Liu R-Z, Tang X, Mi S, Benesch MGK, Mackey JR, Godbout R, Curtis JM, McMullen TPW, Brindley DN. 2016. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: implications for tamoxifen therapy and resistance. Sci Rep 6:21164. doi: 10.1038/srep21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osborne CK 1998. Tamoxifen in the treatment of breast cancer. N Engl J Med 339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 125.Wiseman H, Cannon M, Arnstein HRV. 1989. Observation and significance of growth inhibition of Saccharomyces cerevisiae (A224A) by the antioestrogen drug tamoxifen. Biochem Soc Trans 17:1038–1039. doi: 10.1042/bst0171038. [DOI] [PubMed] [Google Scholar]