The aim of this study was to develop a population pharmacokinetics (PK) model for vancomycin and to evaluate its pharmacodynamic target attainment (TA) in adults on extracorporeal membrane oxygenation (ECMO). After a single 1,000-mg dose of vancomycin, samples were collected 9 times per patient prospectively.
KEYWORDS: vancomycin, extracorporeal membrane oxygenation, MIC, area under the curve, population pharmacokinetics
ABSTRACT
The aim of this study was to develop a population pharmacokinetics (PK) model for vancomycin and to evaluate its pharmacodynamic target attainment in adults on extracorporeal membrane oxygenation (ECMO). After a single 1,000-mg dose of vancomycin, samples were collected 9 times per patient prospectively. A population PK model was developed using a nonlinear mixed-effect model. The probability of target attainment (PTA) of vancomycin was evaluated for various dosing strategies using Monte Carlo simulation. The ratio of the area under the vancomycin concentration-time curve at steady state over 24 h to the MIC (AUC/MIC ratio) was investigated by applying the vancomycin breakpoint distribution of MICs for methicillin-resistant Staphylococcus aureus. A total of 22 adult patients with 194 concentration measurements were included. The population PK was best described by a three-compartment model with a proportional residual error model. Vancomycin clearance and steady-state volume of distribution were 4.01 liters/h (0.0542 liters/h/kg) and 29.6 liters (0.400 liters/kg), respectively. If the treatment target AUC/MIC value was only ≥400, a total daily dose of 3 to 4 g would be optimal (PTA of ≥90%) for patients with normal renal function (estimated glomerular filtration rate [eGFR] = 60 to 120 ml/min/1.73 m2) when the MIC was presumed to be 1 mg/liter. However, AUC/MIC values of 400 to 600 were difficult to attain with any dosing strategy regardless of MIC and eGFR. Thus, it is hard to achieve efficacy and safety targets in patients on ECMO using the population dosing approach with Monte Carlo simulations, and therapeutic drug monitoring should be implemented in these patients.
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) in critically ill patients has markedly increased in usage over the past 2 decades (1). The use of ECMO in these patients can result in complex pharmacokinetics (PK) alterations, making it difficult to maintain optimal pharmacotherapy and achieve favorable clinical outcomes (2, 3). Augmented cardiac function, hemodilution with a priming solution, and drug sequestration in the pump circuit resulting from ECMO may affect drug concentrations. Therefore, a lack of understanding of the effects of ECMO on clearance (CL) and volume of distribution (Vd) may result in an increased likelihood of therapeutic failure due to suboptimal drug concentrations or in drug toxicity due to excessive concentrations in these critically ill patients.
Despite ECMO’s life-saving impact on critically ill patients, its use increases the risk of infections, which may adversely affect outcomes regardless of preexisting indications for ECMO (4). Therefore, antibiotics are frequently used in these patients as a therapeutic and/or prophylactic strategy. Vancomycin is commonly prescribed for procedural prophylaxis or treatment of infections caused by Gram-positive organism such as methicillin-resistant Staphylococcus aureus (MRSA). With the increasing use of ECMO, changes of PK have been reported for many drugs, mainly in pediatric studies, but studies on the PK of vancomycin are limited and their results are conflicting (5, 6). A population PK study of vancomycin that included pediatric and adult subjects found that ECMO use resulted in a decrease in the CL and an increase in the Vd of vancomycin (7), and several pediatric studies have reported similar PK alterations (8–11). None of the relatively few clinical studies have reported any significant PK changes in adult patients with ECMO (12–14), but failure to achieve target concentrations was more frequent in adult patients with ECMO than in those without ECMO (15, 16). In contrast, a recent study showed that ECMO did not impact vancomycin serum concentrations in lung transplant patients (17). The results of these studies thus indicate that there is a lack of understanding of the appropriateness of dosing strategy for vancomycin in adult patients on ECMO, and there are few studies investigating the pharmacodynamic (PD) attainment of vancomycin using the ratio of the area under the concentration-time curve at steady state over 24 h to the MIC (AUC/MIC ratio) (17, 18).
The aim of this study was to develop a population PK model for vancomycin and to evaluate pharmacodynamic attainment in adults on ECMO by means of Monte Carlo simulations.
RESULTS
Patients.
During the study period, a total of 22 patients were enrolled. The demographic characteristics of these patients are described in Table 1. Eighteen patients received venoarterial (VA) ECMO, 3 venovenous (VV) ECMO, and 1 venovenoarterial (VVA) ECMO. Since VVA ECMO is a variant of VA ECMO, the one patient on VVA EMCO was included in the VA ECMO group for the covariate analysis.
TABLE 1.
Characteristics of the study patientsa
| Parameter | Values |
|---|---|
| Sex | Males, n = 17; females, n = 5 |
| ECMO type | VA, n = 19; VV, n = 3 |
| Age (yrs) | 56 (43.3–64.5) |
| Ht (cm) | 168 (165–174) |
| Wt (kg) | 74.0 (55.5–82.3) |
| Body surface area (m2) | 1.85 (1.60–1.96) |
| Protein (g/dl) | 6.40 (5.20–6.80) |
| Albumin (g/dl) | 3.65 (2.95–3.90) |
| BUN (mg/dl) | 20.9 (17.2–33.7) |
| Scr (mg/dl) | 1.33 (0.965–1.77) |
| CLCR, Cockcroft-Gault (ml/min) | 62.1 (48.9–76.6) |
| GFR, MDRD (ml/min/1.73 m2) | 53.0 (38.3–85.1) |
| GFR, modified MDRD (ml/min)b | 56.4 (43.9–77.4) |
| GFR, CKD-EPI (ml/min/1.73 m2) | 56.8 (40.0–85.2) |
| GFR, modified CKD-EPI (ml/min)b | 59.0 (45.8–85.6) |
Values represent median (interquartile range [IQR]) except where otherwise indicated. Abbreviations: IQR, interquartile range; ECMO, extracorporeal membrane oxygenation; VA, venoarterial; VV, venovenous; BSA, body surface area; Scr, serum creatinine level; BUN, serum blood urea nitrogen level; CLCR, creatinine clearance; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
The modified MDRD and CKD-EPI equations adjusted to individual BSA are as follows: GFR (ml/min) = GFR (MDRD or CKD-EPI) × (BSA/1.73 m2).
Population PK analysis.
A total of 194 plasma samples were used for this analysis. The time course of vancomycin concentrations was best described by a three-compartment model. The objective function values (OFVs) for one-, two-, and three-compartment models were 363.89, −37.69, and −68.64, respectively. The PK structure was parameterized in terms of total clearance (CL), central volume of distribution (V1), volume of distribution for the first peripheral compartment (V2), intercompartmental clearance between V1 and V2 (Q2), volume of distribution for the second peripheral compartment (V3), and intercompartmental clearance between V1 and V3 (Q3). The interindividual variability (IIV) was estimated for CL, V1, V2, and V3 (Table 2). In the final PK model (OFV = −89.33), the glomerular filtration rate (GFR) estimated by the CKD-EPI equation was identified as a significant covariate for CL, while the OFV of a reduced model without this covariate increased to −81.90. The interindividual variability for CL was reduced from 40.1% to 33.9% after adding the covariate. ECMO type was also a significant covariate for Q2, while the OFV of a reduced model without ECMO type on Q2 increased to −73.18. Residual error was well described by a proportional error model.
TABLE 2.
Population PK parameter estimates for vancomycin in patients on ECMOa
| Parameter | Estimate | RSE (%) | Bootstrap median (95% CI) |
|---|---|---|---|
| Structural model | |||
| CL = θ1 × [1 + θ2 × (CE − 56.75)] | |||
| Θ1 (liters/h) | 4.01 | 7.51 | 4.02 (3.42–4.68) |
| Θ2 | 0.00752 | 30.0 | 0.00754 (0.00187–0.0133) |
| V1 (liters) | 8.01 | 8.43 | 8.02 (6.79–9.54) |
| Q2 = θ3 [VA] orθ3 + θ4 [VV] | |||
| Θ3 (liters/h) | 4.95 | 5.27 | 4.94 (4.36–6.03) |
| Θ4 (liters/h) | 1.28 | 17.1 | 1.30 (0.702–2.07) |
| V2 (liters) | 15.4 | 8.53 | 15.4 (12.7–18.1) |
| Q3 (liters/h) | 9.09 | 10.0 | 8.96 (6.50–10.5) |
| V3 (liters) | 6.21 | 14.0 | 6.17 (4.03–8.17) |
| Interindividual variability | |||
| ωCL (%) | 33.9 | 13.7 | 31.9 (22.3–40.1) |
| ωV1 (%) | 27.9 | 18.2 | 26.9 (13.1–36.6) |
| ωV2 (%) | 34.3 | 17.5 | 32.8 (17.2–44.5) |
| ωV3 (%) | 56.9 | 23.3 | 56.4 (30.4–92.2) |
| Residual variability | |||
| σProportional (%) | 6.64 | 15.5 | 6.53 (4.39–8.51) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; RSE, relative standard error; CL, total clearance; V1, central volume of distribution; V2, volume of distribution for the first peripheral compartment; Q2, intercompartmental clearance between V1 and V2; V3, volume of distribution for the second peripheral compartment; Q3, intercompartmental clearance between V1 and V3; CE, glomerular filtration rate estimated by CKD-EPI equation; VA, venoarterial ECMO; VV, venovenous ECMO.
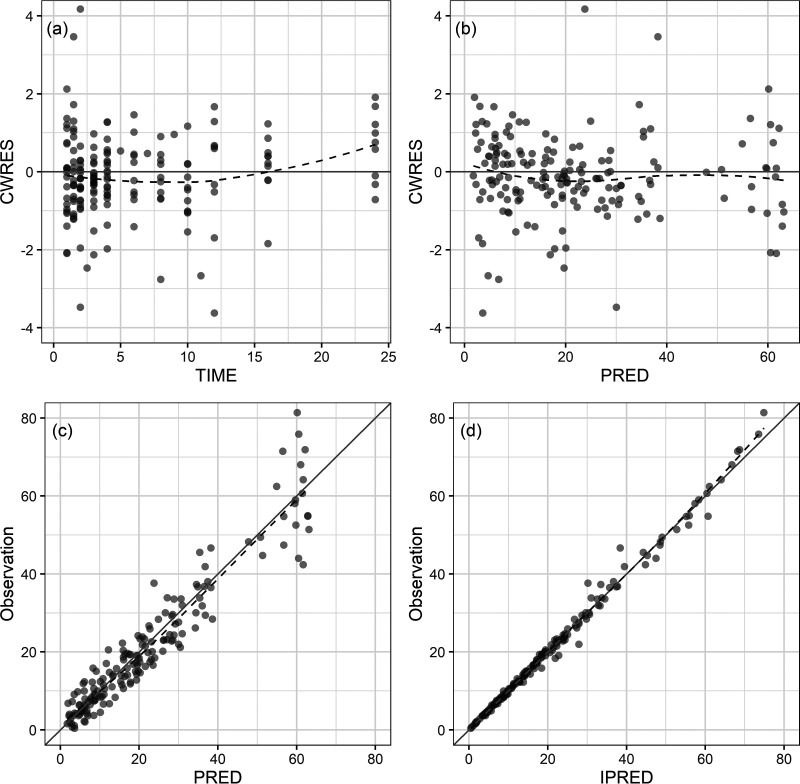
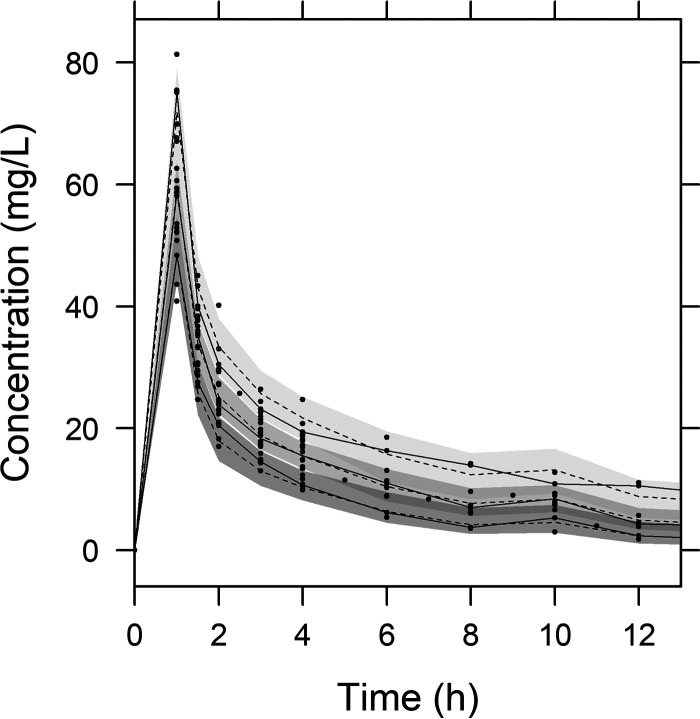
Diagnostic goodness-of-fit plots for the final PK model are shown in Fig. 1. The conditional weighted residual values (CWRES) are evenly distributed about the zero line, indicating the absence of major bias in the structural model (Fig. 1a) or in the residual error model (Fig. 1b). The observed concentrations are evenly distributed about the line of identity, indicating that there is no evidence of misspecification of the structural model, interindividual variability model, or residual error model (Fig. 1c and d). Results of a prediction-corrected visual predictive check (VPC) are shown in Fig. 2. This plot shows that 159 of the 194 observed concentrations (82.0%) fall within the 80% prediction intervals and that the observed 10th, 50th, and 90th percentiles fall within the 95% confidence intervals (CIs) of the simulated 10th, 50th, and 90th percentiles. These findings suggest that the final PK model correctly describes the data and has adequate predictive performance.
FIG 1.
Goodness-of-fit plots: (a) conditional weighted residuals versus time, (b) conditional weighted residuals versus population predicted concentration (PRED), (c) observed concentration versus population predicted concentration, and (d) observed concentration versus individual predicted concentration (IPRED). The dashed lines are smooth curves.
FIG 2.
Visual predictive check from simulated concentrations of 1,000 virtual data sets. Closed circles, observed concentrations; solid lines, 90th, 50th, and 10th percentiles of observations; dashed lines, 90th, 50th, and 10th percentiles of simulated concentrations; shaded areas, 95% confidence intervals for the 90th, 50th, and 10th percentiles of simulated concentrations.
Pharmacodynamic target attainment.
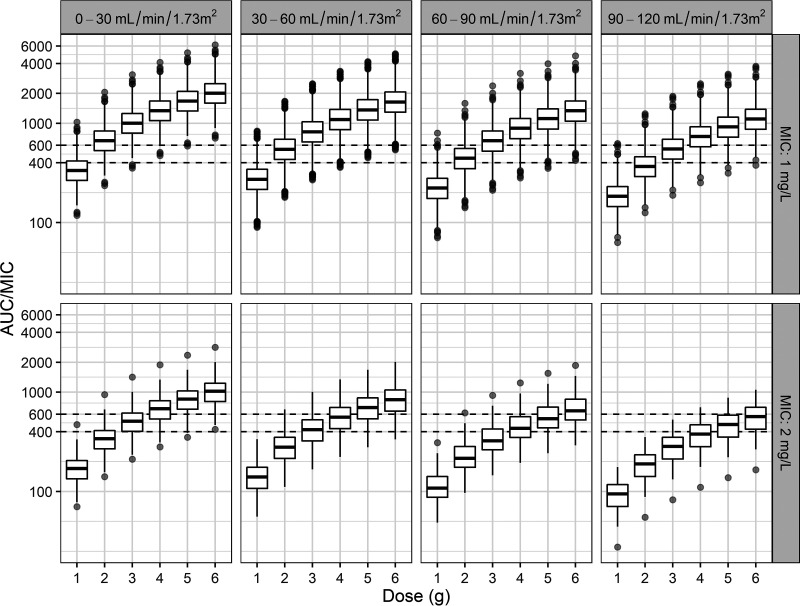
A Monte Carlo simulation was implemented using the final PK parameter estimates for CL, which incorporated the estimated GFR (eGFR) obtained by the CKD-EPI formula. eGFR was simulated at between 1 and 120 ml/min/1.73 m2. Figure 3 gives the distribution of AUC/MIC values that were generated using six total daily doses (1 to 6 g in increments of 1 g) and four renal function groups (0 to 120 ml/min/1.73 m2 in increments of 30 ml/min/1.73 m2) and the MIC distribution for MRSA. For patients with eGFR values of 30 to 90 ml/min/1.73 m2, a total daily dose of 3 g was optimal (probability of target attainment [PTA], >90%) when the MIC was ≤1 mg/liter. For patients with eGFR values of 90 to 120 ml/min/1.73 m2, a total daily dose of 4 g was optimal (PTA of >90%) when the MIC was ≤1 mg/liter (see Table S1 in the supplemental material).
FIG 3.
AUC/MIC distributions with various total daily doses in patients on ECMO with four renal function groups and two MICs (horizontal line inside the box, median; ends of each box, upper and lower quartiles; whiskers, 1.5× quartiles; gray dots, outliers). A MIC of less than 1 mg/liter was presumed to be a MIC of 1 mg/liter.
DISCUSSION
In this prospective study, we analyzed the concentration-time profiles of vancomycin by dense sampling to evaluate the vancomycin population PK properties in adults on ECMO. To the best of our knowledge, this was the largest prospective study to date investigating vancomycin PK and evaluating the PTA in adults with ECMO. Until now, about 100 adult patients with ECMO had been included in studies of vancomycin PK (7, 12–18). We could have assumed a distribution of PK parameters similar to that in the literature. However, the existing models are based on small numbers of ECMO patients and/or small numbers of samples per individual. The typical value of vancomycin PK can be obtained from the models, but the data make it difficult to predict any individual concentration because they contain little information about individual patients. Therefore, we considered that it would be difficult to predict AUCs using Bayesian dose optimization with those models and decided to develop a new PK model based on prospectively enrolled patients and dense sampling. In that way, we were able to evaluate the optimal dosing regimen to achieve the PK/PD target by Monte Carlo simulation.
In this study, we found that the PK profile of vancomycin in adult patients on ECMO was best described by a three-compartment model, whereas vancomycin PK is usually described by a one-compartment model in a sparse sampling design (13) or by a two-compartment model in a dense sampling design (7, 12, 14). The number and timing of blood collections can affect the number of compartments of a PK model. The number of blood samples in our study was 9, and the number of samples in the study on which the 2-compartment model was constructed was 6 (14). The rapid infusion rate (16.7 mg/min = 1,000 mg/hour) also seemed to play a role in the development of the three-compartment model by allowing us to identify the first distributional compartment, even though the rate did not exceed the recommended value.
Typical values of weight-normalized CL (± standard deviation [SD]) and V1 (± SD) for vancomycin in our study were 0.0542 liters/h/kg of body weight (± 0.0184) and 0.108 liters/kg (± 0.0366), respectively. The CL data obtained in this study were consistent with those reported in 10 previous population PK studies in adults (including one ECMO study), where the median (range) CL was 0.051 (0.031 to 0.086) liters/h/kg in adults (19). V1 in our study was lower than in the studies cited above (0.864 [0.388 to 2.040] liters/kg). However, in terms of the steady-state volume of distribution (VSS = V1 + V2 + V3 = 29.6 liters, 0.400 liters/kg), one vancomycin PK study in patients without ECMO that developed a three-compartment model found values similar to ours: the mean value of VSS for patients of normal weight (65.9 to 89.1 kg) was 0.391 liters/kg and for obese patients (111.4 to 225.4 kg) was 0.263 liters/kg (20). As for previous ECMO studies, the value of CL (4.01 liters/h, 0.0542 liters/h/kg) determined in the present study is within the range of values reported in the literature (0.03 to 0.071 liters/h/kg), while our estimate of VSS (29.6 liters, 0.400 liters/kg) is lower than previous estimates, which ranged from 0.65 to 1.41 liters/kg (6). However, a recent study of patients with ECMO in China reported Vss values similar to ours (29.03 liters, 0.46 liters/kg) (17). Since the differences in pharmacokinetics of vancomycin between patients or study cohorts are known to be large (21), and since there have been few studies of vancomycin PK in adults with ECMO, our value of Vss, although lower than those reported from previous studies, does not seem to be extreme. A physiologically based PK (PBPK) model, which would provide a more sophisticated tool, might be more appropriate for exploring changes in PK caused by ECMO (22); however, such a model has not yet been used in studies on vancomycin PK in adults with ECMO.
An early study that included both children and adults with ECMO found significant changes in vancomycin PK (7), but recent adult studies with matched controls reported no significant alterations of vancomycin PK (12, 13). The conflicting results could be due to population differences; children are more likely to be influenced by the addition of priming fluid owing to their low body weight, so that PK changes could be more prominent. In addition, they could also be the result of differences between pumps. Roller pumps have large circuits and surface areas, making drug sequestration more likely and altering the PK of many drugs. Wu et al. also reported that vancomycin CL was significantly lower in adult patients in studies using roller pumps whereas it was not changed in those using centrifugal pumps (13). Centrifugal pumps are widely used and have potential advantages over roller pumps, and recent studies of patients using centrifugal pumps did not reveal vancomycin PK changes (12, 13, 15). Furthermore, the physicochemical properties of vancomycin (high molecular weight, hydrophilic nature, and moderate protein binding activity) would make it unlikely to bind to the ECMO apparatus (23). Patients with ECMO may have failed to achieve target concentrations more frequently despite the absence of significant PK changes (15, 16) because of the critical nature of the illness rather than because of the ECMO therapy itself (18). Given the complex circumstances of these populations, aggressive dosing with frequent therapeutic drug monitoring is needed to reach the therapeutic PK/PD target.
The covariate effect of the eGFR obtained from the CKD-EPI formula on CL was included in the final PK model. However, it is difficult to conclude that the CKD-EPI formula is the best formula to use, as the medians and ranges of the four renal functions in the demographic factors are similar and the degrees of OFV reduction are similar. ECMO type was also a significant covariate affecting Q2 (intercompartmental clearance between V1 and V2). The typical Q2 values were 4.95 liters for VA ECMO and 6.23 liters for VV ECMO. In patients on VV ECMO, the circulation is maintained by native cardiac function and pulsatile blood flow is maintained. In patients on VA ECMO, blood flow bypasses both heart and lungs, and nonpulsatile flow circulates at high flow rates via the ECMO system (24). Since pulseless flow can reduce capillary circulation and alter tissue perfusion (25, 26), this could be the reason why Q2 was lower in VA ECMO than in VV ECMO.
In the present study, we were able to identify an optimal dosage regimen for an AUC/MIC ratio of ≥400 using Monte Carlo simulations (27). If MIC is presumed to be 1 mg/liter (i.e., when MIC is 1 mg/liter or less than 1 mg/liter), our simulations showed that in patients with normal renal function (eGFR = 60 to 120 ml/min/1.73 m2), 3 to 4 g of vancomycin daily was optimal (PTA of >90%), which is not beyond the range of recommended dosages (15 to 20 mg/kg/dose every 8 to 12 h, not exceeding 2 g per dose) (28). In patients with moderately decreased renal function (eGFR of <30 ml/min/1.73 m2) and patients with mildly decreased renal function (eGFR = 30 to 60 ml/min/1.73 m2), daily doses of 2 g and 2.5 g, respectively, are needed to achieve a PTA of ≥90%. ECMO patients with decreased renal function seemed to need higher dosages than non-ECMO patients with the same degree of renal dysfunction to achieve the PK/PD goal in our study. Mahmoud et al. also reported that higher doses (1.5 g and 2 g twice daily) were needed to produce a PTA of >90% when MIC was 1 mg/liter (median creatinine clearance was 45 ml/min) (18). When the upper limit of the PK/PD target (AUC/MIC = 600) was applied to prevent acute kidney injury (27), no dosing regimen used in these simulations reached a PTA of >50%, regardless of renal functions, when MIC was ≤1 mg/liter. In view of these findings, therapeutic drug monitoring taking AUC into account should be performed in these populations to achieve the PK/PD goal and to avoid renal toxicity.
This study had several limitations. First, the sample size of 22 was too small to detect various meaningful covariates such as sex, age, weight, and ECMO flow rate, although eGFR and ECMO type were identified as covariates. Second, the study was conducted in a single center, and the results were based on the use of a single dose and a single simulation tool, so the results may not be generalizable to other patients and should be interpreted cautiously in the clinical field, even though ours was the largest prospective population PK study to have evaluated target attainment for vancomycin in adult patients on ECMO.
In conclusion, this report describes the vancomycin PK profile of ECMO patients appropriately with a three-compartment PK model. The simulation results show that if patients are administered appropriate doses, they can attain the therapeutic PK/PD target (AUC/MIC ratio of ≥400) when MIC is ≤1 mg/liter. However, if one includes the upper limit of PK/PD (AUC/MIC ratios of ≥400 and ≤600), our simulations show that it is difficult to achieve the optimal pharmacodynamic range with a one-size-fits-all dosing method. Therefore, therapeutic drug monitoring is needed in patients with ECMO to achieve efficacy and safety targets.
MATERIALS AND METHODS
Patients.
This was a prospective study performed in Hallym University Sacred Heart Hospital (830-bed academic center), Anyang, South Korea. From November 2018 to August 2019, adult patients (aged ≥19 years) undergoing ECMO for respiratory and/or cardiac dysfunction and receiving vancomycin for prophylaxis or treatment were eligible for the study. Patients receiving renal replacement therapy due to acute or chronic kidney injury were excluded. Written informed consent forms were signed by legally authorized representatives of each subject before enrollment. The study was reviewed and approved by the Institutional Review Board of the Hallym University Sacred Heart Hospital (IRB no. 2018-05-037) and conducted in accordance with the Declaration of Helsinki and good clinical practice.
ECMO machine.
The ECMO system was a permanent life support system (Maquet, Rastatt, Germany) consisting of a PLS-i oxygenator with Bioline coating and a Rotaflow RF-32 centrifugal pump. The permanent life support (PLS) circuit was primed with 1 liter of normal saline or plasma solution, and the total circuit volume was 500 to 600 ml.
Study design.
A single 1,000-mg dose of vancomycin diluted in 200 ml of 5% dextrose fluid was infused intravenously over the course of 1 h. Serial blood samples of 3 ml were obtained through the use of an arterial catheter at 0, 1, 1.5, 2, 3, 4, 6, 8, and 12 h in patients with normal renal function (defined as estimated glomerular filtration rate [eGFR] of ≥50 ml/min/1.73 m3) and at 0, 1, 1.5, 2, 3, 4, 10, 16, and 24 h in those with decreased renal function (defined as eGFR of <50 ml/min/1.73 m3).
Vancomycin assay.
Vancomycin drug concentrations were analyzed with an Architect iVancomycin assay (Abbott Laboratories, Germany) by the Department of Laboratory Medicine using chemiluminescent microparticle immunoassay technology. This assay is based on a homogenous enzyme immunoassay technique. The measuring range was 3.0 to 100 μg/ml. Assay precision was determined using human serum/plasma samples and controls in a modified CLSI (formerly NCCLS) EP17-A protocol. No significant cross-reactivity or interference with additional drugs was noted.
Population PK analysis.
Population PK analyses were implemented using NONMEM 7.4 (Icon Development Solutions). First-order conditional estimation with interaction method was used for the PK models; this method accounts for the interaction between interindividual variability (IIV) in PK parameters and residual unexplained variability (RUV), arising from intraindividual variability, assay error, errors in independent variables, model misspecification, etc. (29). One-, two-, and three-compartment models with first-order kinetics were tested. IIV was modeled using an exponential function. Additive, proportional, and combined additive and proportional error models were tested for RUV.
Model selection was based on NONMEM objective function value (OFV), relative standard errors for parameter estimates, and diagnostic goodness-of-fit plots. In a log-likelihood ratio test, a decrease in the OFV between two nested models of >3.84 with 1 degree of freedom or of >5.99 with 2 degrees of freedom was considered a significant model improvement. Stepwise forward inclusion and backward exclusion were performed with Perl-speaks-NONMEM software (version 4.8.1 [https://uupharmacometrics.github.io/PsN]) to search significant covariates for PK parameters. The statistical significance criteria were a P value of <0.05 (ΔOFV < −3.84 with 1 degree of freedom) for inclusion and a P value of <0.01 (ΔOFV > 6.64 with 1 degree of freedom) for exclusion. The tested covariates for all PK parameters were age, height, weight, body surface area (BSA), serum protein level, serum albumin level, sex, ECMO type (venoarterial or venovenous), serum creatinine level, serum blood urea nitrogen level, ECMO flow rate, and renal function; renal functions were estimated by applying the Cockcroft-Gault (CG) (30), modification of diet in renal disease (MDRD) (31), and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (32) equations; modified MDRD and modified CKD-EPI equations were applied for estimation of total clearance (CL) only. The modified MDRD and CKD-EPI equations, in which BSA is estimated by the Du Bois formula, were adjusted to individual BSA values (33). A prediction-corrected VPC was obtained by comparing the observed plasma concentrations with the 80% prediction intervals from 1,000 simulated data sets, using the final PK parameters and significant covariates (34). The nonparametric bootstrap method was used to evaluate the stability of the final model. Median and 2.5% to 97.5% intervals for the PK parameter estimates of bootstrap samples (n = 2,000) were generated for comparison with the final PK parameter estimates (35).
Pharmacodynamic target attainment.
The primary pharmacokinetic/pharmacodynamic (PK/PD) index for vancomycin is the AUC/MIC ratio (27). AUC/MIC values of ≥400 and ≤600 are recommended for vancomycin in serious MRSA infections in the new consensus guidelines (27). To evaluate the vancomycin AUC/MIC values for the study population, a 10,000-subject Monte Carlo simulation for CL was conducted using the final PK parameter estimates, including the typical value and its IIV. Continuous covariates were generated as normally distributed random variables within a range twice the standard deviation of the demographic data. In this simulation, six doses (1 to 6 g in increments of 1 g) were used to calculate the AUC values for 10,000 subjects. The AUC/MIC ratio was derived by applying the vancomycin breakpoint distribution of MICs for MRSA set by the European Committee on Antimicrobial Susceptibility Testing (36). The revised guidelines do not recommend decreasing the dose of vancomycin to achieve the AUC/MIC target when the MIC is reported to be <1 mg/liter (27). Therefore, MIC values of <1 mg/liter were included as a group of MICs of 1 mg/liter when the Monte Carlo simulation was conducted. The AUC/MIC distributions were compared in patients with four renal function groups (0 to 30, 30 to 60, 60 to 90, and 90 to 120 ml/min/1.73 m2 of eGFR) using box and whisker plots.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Hallym University Research Fund, 2018 (HURF-2018-36).
We confirm that we do not have any conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Squiers JJ, Lima B, DiMaio JM. 2016. Contemporary extracorporeal membrane oxygenation therapy in adults: fundamental principles and systematic review of the evidence. J Thorac Cardiovasc Surg 152:20–32. doi: 10.1016/j.jtcvs.2016.02.067. [DOI] [PubMed] [Google Scholar]
- 2.Shekar K, Fraser JF, Smith MT, Roberts JA. 2012. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 27:741.e9–741.e18. doi: 10.1016/j.jcrc.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Sherwin J, Heath T, Watt K. 2016. Pharmacokinetics and dosing of anti-infective drugs in patients on extracorporeal membrane oxygenation: a review of the current literature. Clin Ther 38:1976–1994. doi: 10.1016/j.clinthera.2016.07.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun HY, Ko WJ, Tsai PR, Sun CC, Chang YY, Lee CW, Chen YC. 2010. Infections occurring during extracorporeal membrane oxygenation use in adult patients. J Thorac Cardiovasc Surg 140:1125–1132.e2. doi: 10.1016/j.jtcvs.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Hahn J, Choi JH, Chang MJ. 2017. Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients. J Clin Pharm Ther 42:661–671. doi: 10.1111/jcpt.12636. [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Aziz MH, Roberts JA. 2020. Antibiotic dosing during extracorporeal membrane oxygenation: does the system matter? Curr Opin Anaesthesiol 33:71–82. doi: 10.1097/ACO.0000000000000810. [DOI] [PubMed] [Google Scholar]
- 7.Mulla H, Pooboni S. 2005. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol 60:265–275. doi: 10.1111/j.1365-2125.2005.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer DM, Jessen ME, Eberhart RC; Extracorporeal Life Support Organization. 1995. Neonatal extracorporeal membrane oxygenation complicated by sepsis. Ann Thorac Surg 59:975–980. doi: 10.1016/0003-4975(95)00044-L. [DOI] [PubMed] [Google Scholar]
- 9.Hoie EB, Swigart SA, Leuschen MP, Willett LD, Bolam DL, Goodrich PD, Bussey ME, Nelson RM, Jr. 1990. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm 9:711–715. [PubMed] [Google Scholar]
- 10.Buck ML 1998. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy 18:1082–1086. [PubMed] [Google Scholar]
- 11.An SH, Lee EM, Kim JY, Gwak HS. 2020. Vancomycin pharmacokinetics in critically ill neonates receiving extracorporeal membrane oxygenation. Eur J Hosp Pharm 27:e25–e29. doi: 10.1136/ejhpharm-2018-001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donadello K, Roberts JA, Cristallini S, Beumier M, Shekar K, Jacobs F, Belhaj A, Vincent JL, de Backer D, Taccone FS. 2014. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study. Crit Care 18:632. doi: 10.1186/s13054-014-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CC, Shen LJ, Hsu LF, Ko WJ, Wu FL. 2016. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc 115:560–570. doi: 10.1016/j.jfma.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Moore JN, Healy JR, Thoma BN, Peahota MM, Ahamadi M, Schmidt L, Cavarocchi NC, Kraft WK. 2016. A population pharmacokinetic model for vancomycin in adult patients receiving extracorporeal membrane oxygenation therapy. CPT Pharmacometrics Syst Pharmacol 5:495–502. doi: 10.1002/psp4.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SJ, Yang JH, Park HJ, In YW, Lee YM, Cho YH, Chung CR, Park CM, Jeon K, Suh GY. 2015. Trough concentrations of vancomycin in patients undergoing extracorporeal membrane oxygenation. PLoS One 10:e0141016. doi: 10.1371/journal.pone.0141016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang CJ, Wu CW, Wu CC. 2018. Effect of extracorporeal membrane oxygenation on the new vancomycin dosing regimen in critically ill patients receiving continuous venovenous hemofiltration. Ther Drug Monit 40:310–314. doi: 10.1097/FTD.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Chen W, Wang Q, Li M, Zhang Z, Cui G, Li P, Zhang X, Ma Y, Zhan Q, Wang C. 2020. Influence of venovenous extracorporeal membrane oxygenation on pharmacokinetics of vancomycin in lung transplant recipients. J Clin Pharm Ther 45:1066–1075. doi: 10.1111/jcpt.13163. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud AA, Avedissian SN, Al-Qamari A, Bohling T, Pham M, Scheetz MH. 28 May 2020, posting date Pharmacokinetic assessment of pre- and post-oxygenator vancomycin concentrations in extracorporeal membrane oxygenation: a prospective observational study. Clin Pharmacokinet doi: 10.1007/s40262-020-00902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsot A, Boulamery A, Bruguerolle B, Simon N. 2012. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet 51:1–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Blouin RA, Bauer LA, Miller DD, Record KE, Griffen WO, Jr. 1982. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother 21:575–580. doi: 10.1128/aac.21.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aljutayli A, Marsot A, Nekka F. 2020. An update on population pharmacokinetic analyses of vancomycin, part I: in adults. Clin Pharmacokinet 59:671–698. doi: 10.1007/s40262-020-00866-2. [DOI] [PubMed] [Google Scholar]
- 22.Watt KM, Cohen-Wolkowiez M, Barrett JS, Sevestre M, Zhao P, Brouwer KLR, Edginton AN. 2018. Physiologically based pharmacokinetic approach to determine dosing on extracorporeal life support: fluconazole in children on ECMO. CPT Pharmacometrics Syst Pharmacol 7:629–637. doi: 10.1002/psp4.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dzierba AL, Abrams D, Brodie D. 2017. Medicating patients during extracorporeal membrane oxygenation: the evidence is building. Crit Care 21:66. doi: 10.1186/s13054-017-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mousavi S, Levcovich B, Mojtahedzadeh M. 2011. A systematic review on pharmacokinetic changes in critically ill patients: role of extracorporeal membrane oxygenation. Daru 19:312–321. [PMC free article] [PubMed] [Google Scholar]
- 25.Shevde K, DeBois WJ. 1987. Pro: pulsatile flow is preferable to nonpulsatile flow during cardiopulmonary bypass. J Cardiothorac Anesth 1:165–168. doi: 10.1016/0888-6296(87)90012-3. [DOI] [PubMed] [Google Scholar]
- 26.O'Neil MP, Fleming JC, Badhwar A, Guo LR. 2012. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: microcirculatory and systemic effects. Ann Thorac Surg 94:2046–2053. doi: 10.1016/j.athoracsur.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 27.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. 2020. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 77:835–864. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF, Infectious Diseases Society of A. 2011. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 29.Dosne AG, Bergstrand M, Karlsson MO. 2016. A strategy for residual error modeling incorporating scedasticity of variance and distribution shape. J Pharmacokinet Pharmacodyn 43:137–151. doi: 10.1007/s10928-015-9460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration. 2006. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glatard A, Bourguignon L, Jelliffe RW, Maire P, Neely MN, Goutelle S. 2015. Influence of renal function estimation on pharmacokinetic modeling of vancomycin in elderly patients. Antimicrob Agents Chemother 59:2986–2994. doi: 10.1128/AAC.04132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baverel PG, Savic RM, Karlsson MO. 2011. Two bootstrapping routines for obtaining imprecision estimates for nonparametric parameter distributions in nonlinear mixed effects models. J Pharmacokinet Pharmacodyn 38:63–82. doi: 10.1007/s10928-010-9177-x. [DOI] [PubMed] [Google Scholar]
- 36.EUCAST. 2007. Antimicrobial wild type distributions of microorganisms. https://mic.eucast.org/Eucast2/. Accessed 5 March 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.