A2059G mutation in the 23S rRNA gene is the only reported mechanism conferring high-level azithromycin resistance (HL-AZMR) in Neisseria gonorrhoeae. Through U.S. gonococcal antimicrobial resistance surveillance projects, we identified four HL-AZMR gonococcal isolates lacking this mutational genotype. Genetic analysis revealed an A2058G mutation of 23S rRNA alleles in all four isolates. In vitro selected gonococcal strains with homozygous A2058G recapitulated the HL-AZMR phenotype.
KEYWORDS: 23s rRNA, A2058G, HL-AZMR, Neisseria gonorrhoeae, antibiotic resistance, azithromycin resistant, gonorrhea
ABSTRACT
A2059G mutation in the 23S rRNA gene is the only reported mechanism conferring high-level azithromycin resistance (HL-AZMR) in Neisseria gonorrhoeae. Through U.S. gonococcal antimicrobial resistance surveillance projects, we identified four HL-AZMR gonococcal isolates lacking this mutational genotype. Genetic analysis revealed an A2058G mutation of 23S rRNA alleles in all four isolates. In vitro selected gonococcal strains with homozygous A2058G recapitulated the HL-AZMR phenotype. Taken together, we postulate that the A2058G mutation confers HL-AZMR in N. gonorrhoeae.
INTRODUCTION
Antibiotics play an essential role in the management of gonorrhea, a sexually transmitted disease (STD) caused by Neisseria gonorrhoeae. The dwindling number of effective antibiotics to treat this pervasive disease and an increase in the number of antimicrobial-resistant cases globally are of major public health concern. Currently, the U.S. Centers for Disease Control and Prevention (CDC) as well as several other countries are recommending a combination regimen that includes ceftriaxone (CRO) and azithromycin (AZM) for uncomplicated gonococcal infection (1, 2). In the United States, CRO remains fully effective against N. gonorrhoeae, while the percentage of isolates displaying reduced susceptibility to AZM has steadily increased since 2012 (3). Approximately 4.6% of gonococcal isolates collected through the U.S. Gonococcal Isolate Surveillance Project (GISP) in 2018 are considered nonsusceptible to AZM (3).
AZM is a widely used anti-infective macrolide (4). It binds to the bacterial 50S ribosomal subunit at the peptidyl transferase moiety (formed by 23S rRNA and ribosomal proteins) and abolishes protein synthesis (5–8). Genetic aberrations in the 23S rRNA gene such as single nucleotide polymorphism at positions 2058 and 2059 (Escherichia coli nomenclature) are known to significantly reduce the efficacy of AZM in various bacteria (9–12). Site-directed mutagenesis experiments substituting the adenine with a guanine at either of these positions (hereafter A2058G or A2059G) increased Mycobacterium smegmatis MIC more than 64-fold for AZM (11). In N. gonorrhoeae, in vitro studies have shown that the A2059G mutation conferred high-level azithromycin resistance (HL-AZMR; MIC ≥ 256 μg/ml) (12). Moreover, HL-AZMR gonococcal strains have been cultured from clinical samples worldwide (2, 13–22). CDC’s GISP and the Strengthening the U.S. Response to Resistant Gonorrhea (SURRG) project each reported a cluster of HL-AZMR isolates in 2016 and 2018, respectively (20, 22). In the United Kingdom, Fifer et al. identified a sustained outbreak of HL-AZMR isolates from 2014 to 2017 (21). Last, Wan et al. reported 40 isolates collected in China between 2013 and 2014 that were HL-AZMR (9). All previously reported HL-AZMR gonococcal isolates harbored the A2059G genotype. Here, we report four HL-AZMR gonococcal isolates lacking the A2059G genotype identified through CDC’s GISP and SURRG projects.
GISP monitors resistance patterns based on urethral isolates collected from men presenting at STD clinics with symptomatic gonococcal urethritis (23). In recent years, CDC has expanded antibiotic resistance response efforts, and a subset of projects participating in GISP also participate in the SURRG project. SURRG collects urogenital and extragenital (i.e., pharyngeal and rectal) isolates from men and women attending STD and community health clinics. Between 2016 and 2019, four different patients attending clinics participating in GISP and SURRG yielded four gonococcal isolates with atypical HL-AZMR genotype: two isolates were cultured from urethral samples in 2016 (AZMR-16) and 2018 (AZMR-18), while one isolate each was cultured from pharyngeal (AZMR-19A) and rectal (AZMR-19B) samples in 2019. Initial Etest (bioMérieux, Durham, NC, USA) (24) antimicrobial susceptibility testing (AST) performed at local SURRG laboratories suggested that AZMR-16, AZMR-19A, and AZMR-19B were HL-AZMR. An agar dilution AST method was also used to assess the susceptibility levels of the isolates (Table 1) against AZM, cefixime (CFM), ciprofloxacin (CIP), gentamicin (GEN), penicillin (PEN), tetracycline (TET), and CRO at the Antibiotic Resistance Laboratory Network regional laboratories using antibiotic powders purchased from Sigma-Aldrich (St. Louis, MO, USA) (25). Agar dilution was performed and antibiotic susceptibility was interpreted as described by the Clinical and Laboratory Standards Institute (CLSI) (26, 27). All four isolates displayed an MIC of >16 μg/ml (highest concentration tested with agar dilution) to AZM. The HL-AZMR phenotype was confirmed at CDC for all four isolates using Etest. Resistance to TET alone or to TET and CIP was also observed in AZMR-16 and AZMR-18, respectively.
TABLE 1.
Antibiogram of the novel HL-AZMR isolatesa
| N. gonorrhoeae isolate | MIC (μg/ml)b
|
β-Lactamase | Sample type | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AZM | CFM | CRO | CIP | GEN | PEN | TET | |||
| AZMR-16 | 4 & ≥256 | 0.06 | 0.03 | 0.015 | 8 | 1 | 1 | Negative | Urethral |
| AZMR-16-MCc | ≥256 | 0.06 | 0.03 | ND | ND | ND | ND | Negative | |
| AZMR-18 | ≥256 | 0.06 | 0.06 | 16 | 4 | 1 | 4 | Negative | Urethral |
| AZMR-19A | ≥256 | 0.03 | 0.015 | 0.015 | 8 | 0.25 | 1 | Negative | Pharyngeal |
| AZMR-19B | 4 & ≥256 | 0.03 | 0.015 | 0.03 | 8 | 0.25 | 2 | Negative | Rectal |
| AZMR-19B-MCd | ≥256 | 0.03 | 0.015 | ND | ND | ND | ND | Negative | |
Antimicrobial susceptibility profile of N. gonorrhoeae isolates harboring the A2058G mutation in the 23S rRNA. Isolates with the A2058G mutation were HL-AZMR. These isolates displayed varying levels of susceptibility to other antibiotics.
ND, not determined.
Laboratory N. gonorrhoeae strain derived from clinical strain AZMR-16.
Laboratory N. gonorrhoeae strain derived from clinical strain AZMR-19B.
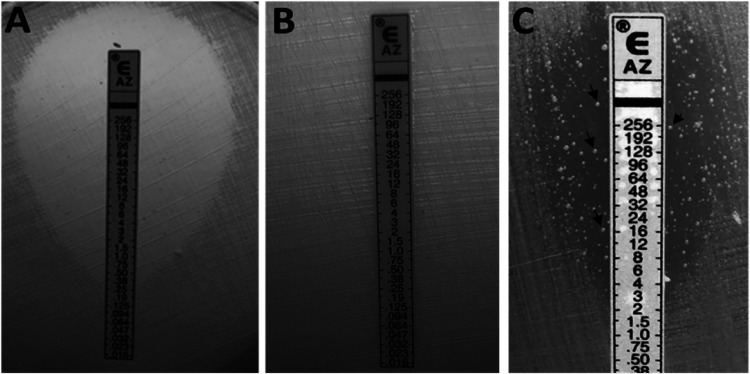
Of the four isolates, two showed a homogenous HL-AZMR phenotype, while two displayed heterogenous resistance phenotypes. Both AZMR-18 and AZMR-19A displayed homogenous and confluent growth (MIC ≥256 μg/ml) throughout the AZM Etest strip (Fig. 1B). In contrast, AZMR-16 and AZMR-19B ellipses intersected the AZM Etest strip between the 4 and 8 μg/ml marks, and macrocolonies were visible inside the ellipses exceeding ≥256 μg/ml (Fig. 1C). Such phenotypic display is referred to as heteroresistance hereafter. Unlike AZMR-16 and AZMR-19B, susceptible and nonheteroresistant isolates failed to produce a macrocolony inside the ellipse (Fig. 1A). Colonies derived from AZMR-16 and AZMR-19B after five serial passages of a single colony on antibiotic-free, nutrient-enriched medium all displayed heteroresistance phenotype (data not shown). In contrast, macrocolonies (MC) isolated from within the ellipses of AZMR-16 (AZMR-16-MC) or AZMR-19B (AZMR-19B-MC) all displayed HL-AZMR.
FIG 1.
HL-AZMR phenotype displayed by N. gonorrhoeae harboring the A2058G mutation in the 23S rRNA. These are representative images of N. gonorrhoeae Etest assays depicting isolates with differing levels of susceptibility to AZM caused by the A2058G mutation; wild type (A), homozygous A2058G (B), and heterozygous A2058G (C). The heterozygous A2058G strains also displayed heteroresistance phenotype to AZM with macrocolonies growing inside the ellipse and along the Etest strip (arrows in panel C).
Molecular analysis of HL-AZMR and heteroresistant gonococcal isolates revealed differing mutation profiles of the 23S rRNA gene. These isolates lacked the A2059G mutation typically associated with HL-AZMR in N. gonorrhoeae (Table 2). Whole-genome sequencing (WGS) (22) showed that these isolates also lacked mutations that confer AZM resistance, e.g., C2611T mutation in the 23S rRNA, mosaic mtr, and sequence aberrations in RplD and RplV ribosomal proteins (Table 2). Only AZMR-18 was found to have an adenine deletion in the mtr promoter (ΔAmtrR-p) and an amino acid substitution at position 105 (H105Y) in MtrR. However, these aberrations are unlikely the cause of HL-AZMR in this isolate because such mutations were associated with an AZM MIC of ≤8 μg/ml (28).
TABLE 2.
Genetic profiles of the novel HL-AZMR isolatesa
|
N. gonorrhoeae strain |
23S rRNA |
mtr locus |
Ribosomal protein |
Molecular sequence type |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate ID | WGS ID | 2058 A/G | 2059 A/G | 2611 C/T | Mosaic mtr | ΔAmtrR-p | mtr 120 | G45D | H105Y | RpID | RpIV | MLST | NG-STAR | NG-MAST |
| AZMR-16 | GCWGS_1720 | 1/3 | 4/0 | 4/0 | No | No | C | No | No | WT | WT | 9363 | NA | 298 |
| AZMR-16-MC | LRRBGS_0776 | 0/4 | 4/0 | 4/0 | No | No | C | No | No | WT | WT | 9363 | NA | 298 |
| AZMR-18 | GCWGS_2473 | 0/4 | 4/0 | 4/0 | No | Yes | C | No | Yes | WT | WT | 7363 | NA | 16982 |
| AZMR-19A | GCWGS_6721 | 0/4 | 4/0 | 4/0 | No | No | C | No | No | WT | WT | 11982 | NA | NA |
| AZMR-19B | LRRBGS_0777 | 1/3 | 4/0 | 4/0 | No | No | C | No | No | WT | WT | 11982 | NA | NA |
| AZMR-19B-MC | LRRBGS_0778 | 0/4 | 4/0 | 4/0 | No | No | C | No | No | WT | WT | 11982 | NA | NA |
Molecular profiles of AZM mutation-mediated resistance markers in N. gonorrhoeae isolates harboring the A2058G mutations in the 23S rRNA. The 23S rRNA sequences were analyzed for mutations at nucleotide positions 2058, 2059, and 2611. The ratio of wild-type/mutant nucleotides at the three positions is shown. This table also lists the molecular profiles for ribosomal proteins RplD and RplV and for a limited number of mutations in the mtrR locus. The MLST, NG-MAST, and NG-STAR (N. gonorrhoeae sequence typing for antimicrobial resistance) profiles were included when available. Whole-genome sequencing (WGS) data are available in the Sequence Read Archive (SRA) NCBI under BioProject numbers PRJNA317462 and PRJNA329501. Abbreviations: ID, identifier; WT, wild type; NA, not assigned.
Interestingly, all HL-AZMR isolates in this study harbored an A2058G mutation in the 23S rRNA gene. AZMR-18 and AZMR-19 were A2058G homozygous (mutation occurs in all four 23S rRNA alleles) while the heteroresistant strains, AZMR-16 and AZMR-19B, were A2058G heterozygous based on Sanger sequencing (29). A2058G mutation occurred in only three of the four alleles with allele 1 and allele 3 being wild type in AZMR-19B and AZMR-16, respectively. In vitro conversion of the wild-type allele to A2058G in the heteroresistant strains led to the HL-AZMR phenotype. All 11 macrocolonies (AZMR-16-MC and AZMR-19B-MC) isolated from the Etest ellipses have an A2058G mutation in all four 23S rRNA alleles and displayed HL-AZMR phenotype. AZMR-16-MC and AZMR-19B-MC shared identical MLST (multilocus sequence type), NG-MAST (N. gonorrhoeae multiantigen sequence type), and AZMR markers as their respective clinical parent strains (Table 2).
A2058G-mediated HL-AZMR has also been documented in clinical isolates of Legionella pneumophila, Moraxella catarrhalis, Mycoplasma genitalium, and Treponema pallidum (11, 30–32). An L. pneumophila isolate with the A2058G mutation in all three copies of its 23S rRNA alleles displayed an AZM MIC of ≥1,024 μg/ml (10). Together, the data imply that the A2058G resistance determinant confers HL-AZMR across diverse types of bacteria, in this case N. gonorrhoeae. However, additional studies (e.g., site-directed mutagenesis and transformation) are necessary to definitively establish the cause and effect of the A2058G mutation and HL-AZMR phenotype in N. gonorrhoeae.
In conclusion, genetic mutations continue to develop in N. gonorrhoeae, and in this case, it allowed this pathogen to become resistant to AZM. Therefore, robust and vigilant gonococcal surveillance programs such as GISP and SURRG are integral in the detection of novel resistance mechanisms. These programs successfully detected a novel mutation that confers HL-AZMR in N. gonorrhoeae. The identification of A2058G alteration in the 23S rRNA of N. gonorrhoeae will help inform and enhance antimicrobial resistance molecular surveillance and detection activities against this pervasive pathogen.
ACKNOWLEDGMENTS
The AR Lab Network Workgroup members are Robert D. Kirkcaldy, Alesia Harvey, and Elizabeth A. Torrone. These members are affiliated with the Centers for Disease Control and Prevention, Atlanta, GA (Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB).
We also thank the CDC Antibiotic Resistance Coordination and Strategy Unit (ARX), the AR Lab Network regional labs for GC, prior GISP regional laboratories, GISP, SURRG, and the grantees of these projects for their contributions to N. gonorrhoeae antimicrobial resistance surveillance.
This study was funded by CDC, partially with funds from the Combating Antibiotic-Resistant Bacteria (CARB) initiative.
The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137. https://www.cdc.gov/std/tg2015/default.htm. [PMC free article] [PubMed] [Google Scholar]
- 2.Martin I, Sawatzky P, Allen V, Lefebvre B, Hoang LMN, Naidu P, Minion J, Van Caeseele P, Haldane D, Gad RR, Zahariadis G, Corriveau A, German G, Tomas K, Mulvey MR. 2019. Multidrug-resistant and extensively drug-resistant Neisseria gonorrhoeae in Canada, 2012–2016. Can Commun Dis Rep 45:45–53. doi: 10.14745/ccdr.v45i23a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2019. Gonococcal Isolate Surveillance Project (GISP) profiles, 2018. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/std/stats18/Gonorrhea.htm.
- 4.Centers for Disease Control and Prevention. 2016. Outpatient antibiotic prescriptions — United States, 2016. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-local-activities/outpatient-antibiotic-prescriptions-US-2016.html.
- 5.Schlunzen F, Zarivach R, Harms J, Bashan A, Tocilj A, Albrecht R, Yonath A, Franceschi F. 2001. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 413:814–821. doi: 10.1038/35101544. [DOI] [PubMed] [Google Scholar]
- 6.Bakheit AHH, Al-Hadiya BMH, Abd-Elgalil AA. 2014. Chapter one: azithromycin, p 1–40. In Profiles of drug substances, excipients and related methodology, volume 39. Academic Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 7.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. 2014. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Gomes C, Ruiz-Roldán L, Mateu J, Ochoa TJ, Ruiz J. 2019. Azithromycin resistance levels and mechanisms in Escherichia coli. Sci Rep 9:6089. doi: 10.1038/s41598-019-42423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan C, Li Y, Le WJ, Liu YR, Li S, Wang BX, Rice PA, Su XH. 2018. Increasing resistance to azithromycin in Neisseria gonorrhoeae in eastern Chinese cities: resistance mechanisms and genetic diversity among isolates from Nanjing. Antimicrob Agents Chemother 62:e02499-17. doi: 10.1128/AAC.02499-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descours G, Ginevra C, Jacotin N, Forey F, Chastang J, Kay E, Etienne J, Lina G, Doublet P, Jarraud S. 2017. Ribosomal mutations conferring macrolide resistance in Legionella pneumophila. Antimicrob Agents Chemother 61:e02188-16. doi: 10.1128/AAC.02188-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfister P, Jenni S, Poehlsgaard J, Thomas A, Douthwaite S, Ban N, Böttger EC. 2004. The structural basis of macrolide-ribosome binding assessed using mutagenesis of 23S rRNA positions 2058 and 2059. J Mol Biol 342:1569–1581. doi: 10.1016/j.jmb.2004.07.095. [DOI] [PubMed] [Google Scholar]
- 12.Chisholm SA, Dave J, Ison CA. 2010. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob Agents Chemother 54:3812–3816. doi: 10.1128/AAC.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsson S, Golparian D, Cole M, Spiteri G, Martin I, Bergheim T, Borrego MJ, Crowley B, Crucitti T, Van Dam AP, Hoffmann S, Jeverica S, Kohl P, Mlynarczyk-Bonikowska B, Pakarna G, Stary A, Stefanelli P, Pavlik P, Tzelepi E, Abad R, Harris SR, Unemo M. 2016. WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014. J Antimicrob Chemother 71:3109–3116. doi: 10.1093/jac/dkw279. [DOI] [PubMed] [Google Scholar]
- 14.Galarza PG, Alcalá B, Salcedo C, Canigia LF, Buscemi L, Pagano I, Oviedo C, Vázquez JA. 2009. Emergence of high-level azithromycin-resistant Neisseria gonorrhoeae strain isolated in Argentina. Sex Transm Dis 36:787–788. doi: 10.1097/OLQ.0b013e3181b61bb1. [DOI] [PubMed] [Google Scholar]
- 15.Katz AR, Komeya AY, Soge OO, Kiaha MI, Lee MV, Wasserman G, Maningas EV, Whelen AC, Kirkcaldy RD, Shapiro SJ, Bolan GA, Holmes KK. 2012. Neisseria gonorrhoeae with high-level resistance to azithromycin; case report of the first isolate identified in the United States. Clin Infect Dis 54:841–843. doi: 10.1093/cid/cir929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unemo M, Golparian D, Hellmark B. 2014. First three Neisseria gonorrhoeae isolates with high-level resistance to azithromycin in Sweden: a threat to currently available dual-antimicrobial regimens for treatment of gonorrhea? Antimicrob Agents Chemother 58:624–625. doi: 10.1128/AAC.02093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen VG, Seah C, Martin I, Melano RG. 2014. Azithromycin resistance is coevolving with reduced susceptibility to cephalosporins in Neisseria gonorrhoeae in Ontario, Canada. Antimicrob Agents Chemother 58:2528–2534. doi: 10.1128/AAC.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens K, Zaia A, Tawil S, Bates J, Hicks V, Whiley D, Limnios A, Lahra MM, Howden BP. 2015. Neisseria gonorrhoeae isolates with high-level resistance to azithromycin in Australia. J Antimicrob Chemother 70:1267–1268. doi: 10.1093/jac/dku490. [DOI] [PubMed] [Google Scholar]
- 19.Lynagh Y, Mac Aogáin M, Walsh A, Rogers TR, Unemo M, Crowley B. 2015. Detailed characterization of the first high-level azithromycin-resistant Neisseria gonorrhoeae cases in Ireland. J Antimicrob Chemother 70:2411–2413. doi: 10.1093/jac/dkv106. [DOI] [PubMed] [Google Scholar]
- 20.Katz AR, Komeya AY, Kirkcaldy RD, Whelen AC, Soge OO, Papp JR, Kersh EN, Wasserman GM, O’Connor NP, O’Brien PS, Sato DT, Maningas EV, Kunimoto GY, Tomas JE. 2017. Cluster of Neisseria gonorrhoeae isolates with high-level azithromycin resistance and decreased ceftriaxone susceptibility, Hawaii, 2016. Clin Infect Dis 65:918–923. doi: 10.1093/cid/cix485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fifer H, Cole M, Hughes G, Padfield S, Smolarchuk C, Woodford N, Wensley A, Mustafa N, Schaefer U, Myers R, Templeton K, Shepherd J, Underwood A. 2018. Sustained transmission of high-level azithromycin-resistant Neisseria gonorrhoeae in England: an observational study. Lancet Infect Dis 18:573–581. doi: 10.1016/S1473-3099(18)30122-1. [DOI] [PubMed] [Google Scholar]
- 22.Pham CD, Sharpe S, Schlanger K, St Cyr S, Holderman J, Steece R, Soge OO, Masinde G, Arno J, Schmerer M, Kersh EN, SURRG Working Group. 2019. Emergence of Neisseria gonorrhoeae strains harboring a novel combination of azithromycin-attenuating mutations. Antimicrob Agents Chemother 63:e02313-18. doi: 10.1128/AAC.02313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. 2020. Gonococcal Isolate Surveillance Project (GISP). Division of Sexually Transmitted Diseases Prevention, Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/std/gisp/.
- 24.Liu H, Taylor TH, Jr, Pettus K, Trees D. 2014. Assessment of Etest as an alternative to agar dilution for antimicrobial susceptibility testing of Neisseria gonorrhoeae. J Clin Microbiol 52:1435–1440. doi: 10.1128/JCM.02131-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kersh EN, Pham CD, Papp JR, Myers R, Steece R, Kubin G, Gautom R, Nash EE, Sharpe S, Gernert KM, Schmerer M, Raphael BH, Henning T, Gaynor AM, Soge O, Schlanger K, Kirkcaldy RD, St Cyr SB, Torrone EA, Bernstein K, Weinstock H. 2020. Expanding U.S. laboratory capacity for Neisseria gonorrhoeae antimicrobial susceptibility testing and whole-genome sequencing through the CDC’s Antibiotic Resistance Laboratory Network. J Clin Microbiol 58:e01461-19. doi: 10.1128/JCM.01461-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically - eleventh edition: CLSI standard M07 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. 30th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Ohneck EA, Zalucki YM, Johnson PJ, Dhulipala V, Golparian D, Unemo M, Jerse AE, Shafer WM. 2011. A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio 2:e00187-11. doi: 10.1128/mBio.00187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson SR, Grad Y, Abrams AJ, Pettus K, Trees DL. 2017. Use of whole-genome sequencing data to analyze 23S rRNA-mediated azithromycin resistance. Int J Antimicrob Agents 49:252–254. doi: 10.1016/j.ijantimicag.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasai A, Ogihara S, Yamada K, Tanimichi Y, Nishiyama H, Saito R. 2015. Prevalence and molecular analysis of macrolide-resistant Moraxella catarrhalis clinical isolates in Japan, following emergence of the highly macrolide-resistant strain NSH1 in 2011. J Med Microbiol 64:708–713. doi: 10.1099/jmm.0.000076. [DOI] [PubMed] [Google Scholar]
- 31.Nijhuis RH, Severs TT, Van der Vegt DS, Van Zwet AA, Kusters JG. 2015. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother 70:2515–2518. doi: 10.1093/jac/dkv136. [DOI] [PubMed] [Google Scholar]
- 32.Noda AA, Matos N, Blanco O, Rodríguez I, Stamm LV. 2016. First report of the 23S rRNA gene A2058G point mutation associated with macrolide resistance in Treponema pallidum from syphilis patients in Cuba. Sex Transm Dis 43:332–334. doi: 10.1097/OLQ.0000000000000440. [DOI] [PubMed] [Google Scholar]