Staphylococcus epidermidis is a major cause of periprosthetic joint infection (PJI); its intracellular persistence within osteoblasts may compromise therapy if that therapy is not intracellularly active. The intracellular activity of rifampin, rifapentine, and rifabutin was assessed against five rifampin-susceptible and two rifampin-resistant S. epidermidis isolates.
KEYWORDS: Staphylococcus epidermidis, rifamycin, intracellular, periprosthetic joint infection
ABSTRACT
Staphylococcus epidermidis is a major cause of periprosthetic joint infection (PJI); its intracellular persistence within osteoblasts may compromise therapy if that therapy is not intracellularly active. The intracellular activity of rifampin, rifapentine, and rifabutin was assessed against five rifampin-susceptible and two rifampin-resistant S. epidermidis isolates. Compared to no treatment, treatment resulted in a ≥2-fold log10 reduction of intracellular rifampin-susceptible, but not rifampin-resistant, S. epidermidis. These findings show activity of rifampin, rifapentine, and rifabutin against intraosteoblast PJI-associated S. epidermidis.
INTRODUCTION
Coagulase-negative staphylococci are leading causes of periprosthetic joint infection (PJI), with Staphylococcus epidermidis accounting for the largest portion (1–3). In addition to robust biofilm production, we and others have shown that S. epidermidis can persist in the intracellular compartment of osteoblasts, though at lower concentrations than Staphylococcus aureus (4, 5). Intracellular persistence may provide bacteria a safe haven from certain antimicrobial treatments, allowing for their release and reestablishment of infection after treatment is discontinued.
Rifampin is routinely used for the treatment of staphylococcal PJI managed with implant debridement and component resection (IDCR) (1, 6, 7). Rifapentine and rifabutin, two other rifamycins, are being explored as potential rifampin alternatives for staphylococcal PJI due to their more favorable side effect profiles (8). We showed that rifampin, rifapentine, and rifabutin have activity against extracellular PJI-associated S. epidermidis and S. aureus in the planktonic and biofilm states (9). We recently showed that rifampin, rifapentine, or rifabutin combined with vancomycin were similarly active against methicillin-resistant S. aureus in a rat foreign body osteomyelitis model (10). The activity of rifampin, rifapentine, and rifabutin has also been shown against S. aureus PJI isolates infecting osteoblasts and fibroblasts (11–13). To our knowledge, activities of rifamycins against intracellular S. epidermidis have not been reported.
The purpose of this study was to determine the intraosteoblast antimicrobial activity of rifampin, rifapentine, and rifabutin against seven S. epidermidis isolates. The isolates included S. epidermidis 1457 (SE1457), a commonly studied rifampin-susceptible S. epidermidis strain (14), and IDRL-8864 and IDRL-8933, two rifampin-susceptible PJI-associated S. epidermidis isolates. Additional isolates included IDRL-9950 and IDRL-6515, two PJI-associated S. epidermidis isolates harboring Asp471Glu and Ser486Phe rpoB gene mutations, respectively (9). RP62A (ATCC 35984), a rifampin-susceptible S. epidermidis isolate, and RP62A-ΔrpoB, a rifampin-resistant S. epidermidis isolate with a His482Tyr mutation in the rpoB gene, were also analyzed. RP62A-ΔrpoB (referred to as RP62A-3Br in reference 15) was selected in vivo through rifampin treatment of rat chronic foreign body osteomyelitis in animals infected with wild-type RP62A (15). Isolates tested and their rifamycin susceptibility phenotypes are shown in Table 1.
TABLE 1.
Staphylococcus epidermidis PJI and clinical isolates used in this study with associated rifamycin MICa
| Isolate name | Source (reference no.) | Rifamycin MIC (μg/ml) with: |
||
|---|---|---|---|---|
| Rifampin | Rifapentine | Rifabutin | ||
| SE1457 | Venous catheter (14) | ≤0.015 | ≤0.015 | ≤0.015 |
| IDRL-8864 | Shoulder PJI | ≤0.015 | ≤0.015 | ≤0.015 |
| IDRL-8933 | Knee PJI | ≤0.015 | ≤0.015 | ≤0.015 |
| IDRL-9950 | Elbow PJI | 1 | 4 | 0.25 |
| IDRL-6515 | Knee PJI | >128 | >128 | >128 |
| RP62A | Catheter sepsis | ≤0.015 | ≤0.015 | ≤0.015 |
| RP62A-ΔrpoB | Rat exptl osteomyelitis (15) | >128 | >128 | >128 |
PJI, periprosthetic joint infection; exptl, experimental.
Rifamycin MICs were determined following CLSI guidelines for water-insoluble drugs (16, 17), with S. aureus ATCC 29213 used as a quality control strain (9). As no CLSI breakpoints have been defined for rifapentine or rifabutin, only breakpoints for rifampin (susceptible, ≤1 μg/ml; resistant, ≥4 μg/ml) were applied (16). SE1457, IDRL-8864, IDRL-8933, and RP62A had MICs of ≤0.015 μg/ml. IDRL-9950 had rifampin, rifapentine, and rifabutin MICs of 1, 4, and 0.25 μg/ml, respectively. IDRL-6515 and RP62A-ΔrpoB had MICs of >128 μg/ml for all three rifamycins (Table 1).
Cytotoxicity to murine osteoblasts (MC-3T3-E1) was assessed by exposing cells to rifampin, rifapentine, or rifabutin at 16 μg/ml, or no treatment, for 24 h. After exposure, osteoblasts were harvested, stained with Ghost Red 780 viability dye (Tonbo Biosciences, San Diego, CA) for 30 min, and analyzed on an Attune NxT acoustic flow cytometer (Life Technologies, Carlsbad, CA). In this assay, nonviable cells become irreversibly fluorescently labeled, while viable cells remain unstained. No difference in osteoblast viability was observed with any of the rifamycin treatments compared to untreated controls (Fig. S1 in the supplemental material).
Osteoblasts were then infected with S. epidermidis using a modified version of a previously described protocol (4), and intracellular rifamycin activity was assessed. MC-3T3-E1 cells were grown to confluence in 6-well, cell culture-treated plates (Celltreat, Pepperell, MA) with minimal essential media alpha (Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (MEM-α+FBS) (Life Technologies). Authentication of the MC-3T3-E1 cell line was performed via IDEXX BioResearch using short tandem repeat (STR) DNA profiling (4). Confluent MC-3TC-E1 cells were infected with S. epidermidis isolates at a multiplicity of infection (MOI) of 75. Cell culture plates were kept at room temperature for 30 min to allow for settling of bacterial cells, followed by incubation at 37°C in a 5% CO2 atmosphere for an additional 3 h.
After incubation, cells were washed thrice with sterile phosphate-buffered saline (PBS) and a daptomycin protection assay (DPA) performed, where MEM-α+FBS supplemented with 100 μg/ml daptomycin (MedChemExpress LLC, Monmouth Junction, NJ) was added to each well and incubated at 37°C in a 5% CO2 atmosphere for 1 h to kill extracellular bacteria. All study isolates were susceptible to daptomycin, with MIC values ≤1 μg/ml. Flow cytometric cellular viability analysis was performed on osteoblasts after daptomycin exposure, as described above. Sterility of the extracellular medium was confirmed by plating on 5% sheep blood agar (SBA). After the DPA, cells were washed with PBS; then, MEM-α+FBS supplemented with 16 μg/ml rifampin, rifapentine, or rifabutin (Sigma-Aldrich, Saint Louis, MO) was added to respective wells in triplicate. Plates were incubated at 37°C in a 5% CO2 atmosphere for 24 h, after which cells were washed thrice with PBS and another DPA was performed as described above. Cells were washed thrice with PBS and lysed in water for at least 15 min. Wells were cell scraped and contents collected, vortexed, sonicated for 10 min, and vortexed again. Serially diluted cell lysates were quantitatively cultured on SBA to determine intracellular bacterial concentrations.
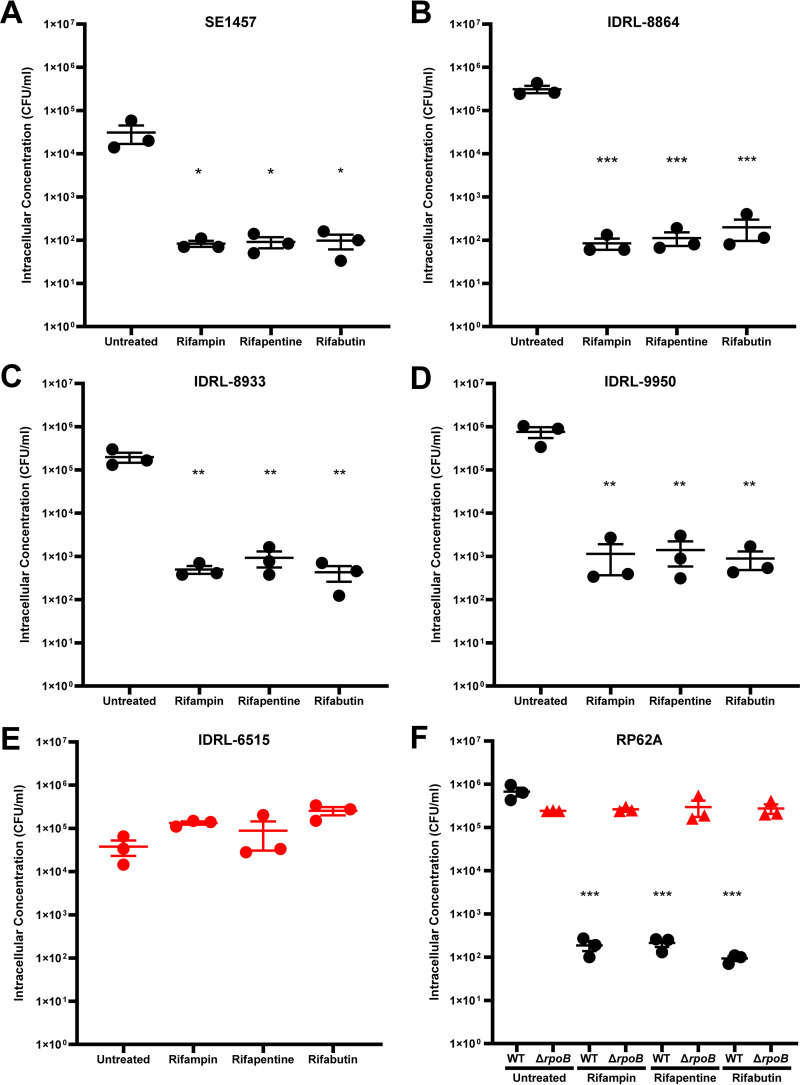
Each rifamycin treatment resulted in at least a 2-log10 reduction of SE1457 (Fig. 1A), IDRL-8864 (Fig. 1B), IDRL-8933 (Fig. 1C), and IDRL-9950 (Fig. 1D) intracellular bacterial concentrations compared to untreated controls. Mean intracellular SE1457 concentrations were reduced from 5.8 × 104 CFU/ml to 110, 140, or 160 CFU/ml with rifampin, rifapentine, or rifabutin treatment, respectively (Fig. 1A). Mean intracellular IDRL-8864 concentrations were reduced from 3.1 × 105 CFU/ml to 85, 110, or 200 CFU/ml with rifampin, rifapentine, or rifabutin treatment, respectively (Fig. 1B). Mean intracellular IDRL-8933 concentrations were reduced from 2.0 × 105 CFU/ml to 500, 930, or 430 CFU/ml with rifampin, rifapentine, or rifabutin treatment, respectively (Fig. 1C). IDRL-9950 concentrations were reduced from 7.6 × 105 CFU/ml to 1,140, 2,200, or 890 CFU/ml with rifampin, rifapentine, or rifabutin treatment, respectively (Fig. 1D). That rifamycin concentrations used during intracellular activity experiments (16 μg/ml) were higher than IDRL-9950 rifamycin MICs could explain why intracellular rifamycin activity was not abrogated, even though IDRL-9950 contains an Asp471Glu rpoB gene mutation and elevated rifamycin MICs compared to wild-type isolates SE1457, IDRL-8864, and IDRL-8933. Conversely, no decrease in IDRL-6515 intracellular bacterial concentrations was observed with any of the three rifamycins (Fig. 1E). Apparent strain-dependent differences in untreated intracellular bacteria concentrations were observed; the reason for this is unknown.
FIG 1.
Intracellular Staphylococcus epidermidis amounts in MC-3T3-E1 osteoblasts after 24 h of rifamycin exposure. (A) SE1457, (B) IDRL-8864, (C) IDRL-8933, (D) IDRL-9950, (E) IDRL-6515, and (F) RP62A and RP62A-ΔrpoB intracellular concentration (CFU/ml) after 3.5 h infection of MC-3T3-E1 cells and subsequent 24 h treatment with no treatment (untreated) or 16 μg/ml rifampin, rifapentine, or rifabutin shown. Rifampin-susceptible isolates are shown in black, with rifampin-resistant isolates shown in red. Data are depicted as the mean of experimental triplicates plus standard error of the mean for n = 3 experiments. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (one-way analysis of variance [ANOVA]).
We also compared rifampin-susceptible and -resistant versions of the same strain, RP62A, with the rifampin-resistant version having been selected in rat experimental osteomyelitis under rifampin therapy and having a His482Tyr mutation in rpoB (15). When treating infected MC-3T3-E1 cells, mean intracellular RP62A concentrations were reduced from 6.8 × 105 CFU/ml to 190, 210, or 90 CFU/ml with rifampin, rifapentine, or rifabutin, respectively (Fig. 1F), whereas no decrease in RP62A-ΔrpoB intracellular bacterial concentrations was observed with any of the three rifamycins (Fig. 1F). For all study isolates, there was no significant difference in individual isolate intracellular bacteria concentrations when comparing between rifampin, rifapentine, or rifabutin exposures.
There are several limitations to this study. The osteoblasts used here were murine; it is not known whether these findings can be extrapolated to human osteoblasts. We did not test various rifamycin concentrations or durations of exposure. Also, although each of the rifamycins showed reduced intracellular concentrations of rifampin-susceptible S. epidermidis, none eliminated all bacteria. Failure to eliminate all bacteria could be due to a number of reasons, including emergence of rifamycin-resistant bacteria. While we did not perform formal emergence of resistance studies, MICs for the rifamycins used remained ≤0.015 μg/ml for four IDRL-8864 and IDRL8933 intracellularly derived colonies isolated after each of rifampin, rifapentine, and rifabutin exposure. Although these results do not prove the absence of emergence of resistance, they are in agreement with those of Abad et al., who reported no emergence of rifamycin resistance when treating osteoblasts intracellularly infected with S. aureus (11).
Overall, results of this study show that rifampin, rifapentine, and rifabutin have intracellular antimicrobial activity during treatment of osteoblasts infected with rifampin-susceptible S. epidermidis isolates (SE1457, IDRL-8864, IDRL-8933, IDRL-9950, or RP62A). As expected, none of the study rifamycins had activity against intracellular rifampin-resistant S. epidermidis (IDRL-6515 or RP62A-ΔrpoB). Overall, the results presented here indicate that rifampin, rifapentine, and rifabutin are active against intracellular S. epidermidis.
Supplementary Material
ACKNOWLEDGMENTS
MC-3T3-E1 osteoblasts were kindly provided by Jennifer Westendorf (Mayo Clinic Department of Orthopedic Surgery, Rochester, MN), and S. epidermidis 1457 (SE1457) was generously provided by Alexander Horswill (University of Colorado, Department of Immunology and Microbiology, Denver, CO). We also thank Virginia Smith Shapiro and her team (Mayo Clinic Department of Immunology, Rochester, MN) for flow cytometer usage and for lending expertise.
C.F. was supported by the Mayo Clinic Initiative for Maximizing Student Development (IMSD) Dean’s Fellowship (NIH R25 GM055252 24) and the Ph.D. Training Grant in Basic Immunology (NIH T32 AI07425-25). R.P. was supported by R01 AR056647 and R01 AI091594.
R.P. and C.F. designed the experiments. C.F. performed and analyzed the experiments. R.P. supervised C.F. and helped edit and revise the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamad M, Deabate L, Belaieff W, Bouvet C, Zingg M, Kuczma P, Suva D, Uckay I. 2015. Prosthetic joint infections due to coagulase-negative staphylococci. Int J Infect 3:e32883. doi: 10.17795/iji-32883. [DOI] [Google Scholar]
- 3.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 4.Perez K, Patel R. 2018. Survival of Staphylococcus epidermidis in fibroblasts and osteoblasts. Infect Immun 86:e00237-18. doi: 10.1128/IAI.00237-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valour F, Trouillet-Assant S, Rasigade JP, Lustig S, Chanard E, Meugnier H, Tigaud S, Vandenesch F, Etienne J, Ferry T, Laurent F, Lyon B, Lyon BJI Study Group. 2013. Staphylococcus epidermidis in orthopedic device infections: the role of bacterial internalization in human osteoblasts and biofilm formation. PLoS One 8:e67240. doi: 10.1371/journal.pone.0067240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker A, Kreitmann L, Triffaut-Fillit C, Valour F, Mabrut E, Forestier E, Lesens O, Cazorla C, Descamps S, Boyer B, Chidiac C, Lustig S, Montbarbon E, Batailler C, Ferry T. 2020. Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France. J Bone Joint Infect 5:28–34. doi: 10.7150/jbji.40333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerli W 2006. Infection and musculoskeletal conditions: prosthetic-joint-associated infections. Best Pract Res Clin Rheumatol 20:1045–1063. doi: 10.1016/j.berh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Aristoff PA, Garcia GA, Kirchhoff PD, Showalter HD. 2010. Rifamycins – obstacles and opportunities. Tuberculosis (Edinb) 90:94–118. doi: 10.1016/j.tube.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Albano M, Karau MJ, Greenwood-Quaintance KE, Osmon DR, Oravec CP, Berry DJ, Abdel MP, Patel R. 2019. In vitro activity of rifampin, rifabutin, rifapentine, and rifaximin against planktonic and biofilm states of staphylococci isolated from periprosthetic joint infection. Antimicrob Agents Chemother 63:e00959-19. doi: 10.1128/AAC.00959-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karau MJ, Schmidt-Malan SM, Albano M, Mandrekar JN, Rivera CG, Osmon DR, Oravec CP, Berry DJ, Abdel MP, Patel R. 2020. Novel use of rifabutin and rifapentine to treat methicillin-resistant Staphylococcus aureus in a rat model of foreign body osteomyelitis. J Infect Dis 222:1498–1504. doi: 10.1093/infdis/jiaa401. [DOI] [PubMed] [Google Scholar]
- 11.Abad L, Josse J, Tasse J, Lustig S, Ferry T, Diot A, Laurent F, Valour F. 2020. Antibiofilm and intraosteoblastic activities of rifamycins against Staphylococcus aureus: promising in vitro profile of rifabutin. J Antimicrob Chemother 75:1466–1473. doi: 10.1093/jac/dkaa061. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed W, Sommer U, Sethi S, Domann E, Thormann U, Schutz I, Lips KS, Chakraborty T, Schnettler R, Alt V. 2014. Intracellular proliferation of S. aureus in osteoblasts and effects of rifampicin and gentamicin on S. aureus intracellular proliferation and survival. Eur Cell Mater 28:258–268. doi: 10.22203/ecm.v028a18. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez CJ, Jr., Shiels SM, Tennent DJ, Hardy SK, Murray CK, Wenke JC. 2015. Rifamycin derivatives are effective against staphylococcal biofilms in vitro and elutable from PMMA. Clin Orthop Relat Res 473:2874–2884. doi: 10.1007/s11999-015-4300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack D, Siemssen N, Laufs R. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun 60:2048–2057. doi: 10.1128/IAI.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wi YM, Greenwood-Quaintance KE, Brinkman CL, Lee JYH, Howden BP, Patel R. 2018. Rifampicin resistance in Staphylococcus epidermidis: molecular characterisation and fitness cost of rpoB mutations. Int J Antimicrobial Agents 51:670–677. doi: 10.1016/j.ijantimicag.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. CLSI supplement M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 11th ed CLSI standard M07 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.