Abstract
Silk fibroin (SF) is derived from Bombyx mori silkworm cocoons and has been used in textiles and as a suture material for decades. More recently, SF has been used for various new biomedical applications, including as a wound dressing, owing to its excellent biological and mechanical properties. Specifically, the mechanical stiffness, versatility, biocompatibility, biodegradability, water vapor permeability and slight bactericidal properties make SF an excellent candidate biomaterial for wound dressing applications. The effectiveness of SF as a wound dressing has been tested and well documented invitro as well as invivo, as described here. Dressings based on SF are currently used for treating a wide variety of chronic and acute (eg. burn) wounds. SF and its derivatives prepared as biomaterials are available as sponges, hydrogels, nanofibrous matrices, scaffolds, micro/nanoparticles, and films. The present review discusses the potential role of SF in wound dressing and its modulation for wound dressing applications. The comparison of SF-based dressings with other natural polymers understands the readers, the scope and limitation of the subject in-depth.
Keywords: Biomaterials, Silk Fibroin Protein, Sponges, Hydrogels, Nanofibrous matrices, Scaffolds, Micro-nanoparticles, Films, Wound dressing
1. Introduction
As our first, major barrier against viruses, fungi and bacteria, skin is one of the most important immune organs of the body and when it becomes compromised, major issues including risk of infection and death, arise. Every year, several millions of peoples are affected by skin injuries of either an acute or a chronic nature [1, 2]. Worldwide 300,000 people die every year in lower middle-income countries due to chronic wounds and burn injuries [2]. These injuries and subsequent bacterial infections are some of the most painful forms of trauma, and they require careful and effective wound management. Bacterial wound infection delays wound healing and may lead to life-threatening complications [3]. Initially, gram-positive Staphylococcus aureus (S. aureus) and Streptococcus pyogenes (S. pyogenes) microorganisms are typically found in the initial stage of the infectious process, while gram-negative bacteria like Escherichia coli (E. coli) and Pseudomonas aeruginosa (P. aeruginosa) are involved in the later stages of the infectious process, when chronic wounds develop [4]. The treatment of wounds has evolved substantially from ancient times, especially since the discovery of bacteria and anti-bacterial chemicals, dressings, and best practices. Wound dressing materials are not restricted to stop blood loss, but also to protect the wound from bacterial infection and accelerating the wound healing process.
Biologics, synthetic and combination of both are three broad categories of wound dressing [5]. Due to some inherent drawbacks like high antigenicity, poor adhesion and risk of cross-contamination, biological dressings are not so popular. [6]. The key advantages of synthetic dressings are they possess long durability, Induces minimum inflammatory response and minimum risk of bacterial contamination, but the issues related to cell compatibility remains a big challenge. The combinations of biologic and synthetic dressings are bilayered and have higher polymer content and biological materials [5]. The design of ideal wound dressings should include considerations for maintain the wound interfaces in a moist environment, provide softness, flexibility, antibacterial activity, non-cytotoxicity, and water and vapor permeability. They should also be easily removable and promote faster and complete wound healing [7, 8]. A systemic and careful approach is required to develop the material for wound dressings. The present review is an attempt to brief readers about the engineering of wound dressing materials from natural and synthetic sources upfront. Herein, we will discuss different materials for wound dressing either based on a synthetic source or derived directly from nature [9]. The review article focuses on the silk fibroin (SF) and its derivatives, which can speed up the treatment processes of wounds.
2. The process of wound healing
A wound involves damage to tissues and disruption in anatomical structure and function due to accidents, burns, surgery etc. [10]. Wounds are classified into categories according to the wound depth, tissue loss or no tissue loss, type of injury, location, and clinical appearance. Depending upon the tissue loss; the wounds can be categorized as second and third-degree burns, diabetic foot ulcers etc., and without tissue loss include first degree burns, lacerations etc. [11]. Wounds are also classified into acute wounds (which heal faster than 12 weeks) and chronic wounds (which heal very slowly, (longer than 12 weeks) or never [12]. Superficial wounds only involve thee pidermis, partial thickness wounds also involve deeper dermal layers and blood vessels, and full thickness wounds affect the epidermis, dermis and subcutaneous (fat and muscle) tissues (13).
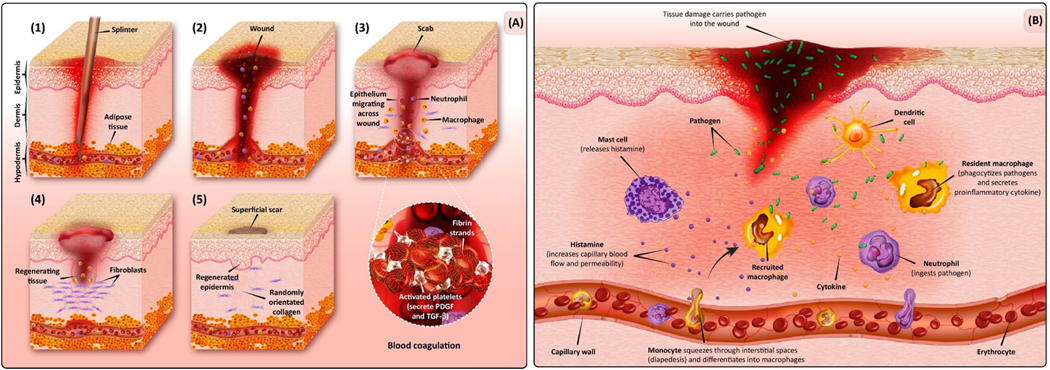
Wound healing is multistep biological process in the body, which involves tissue growth and regeneration (figure 1).Regeneration of the tissue is accomplished by four stages, including coagulation or hemostasis, inflammation, proliferation, and remodeling or maturing.
Figure 1:
Schematic of Wound Formation and Healing. (A) Wound-healing phases: hemostasis (blood clotting), inflammation, proliferation (tissue growth), and maturation (tissue remodeling). (B) Large wound defect invaded by microbial contamination that implies the need for an appropriate antibiotic-loaded wound dressing. Inflammatory cells migrate to the site of injury by increasing the permeability of the capillaries. By increasing blood flow, important factors and cells cross from the intravascular space into the extra vascular space. Macrophages and neutrophils are also shown that have a crucial role in removing pathogens at the defect site. Abbreviation: PDGF, platelet-derived growth factor. The reproduced with permission from [13].
The first phase of hemostasis occurs within the first few minutes of injury by forming a fibrin clot at the injured site and vascular constriction [14, 15].The tissue and blood clot release different cytokines [(Interlukin-1β and tumor necrosis factor (TNF)-α,)] and growth factors [(transforming growth factor (TGF) β, platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF)]. The growth factors recruit neutrophiles (after 6 hrs), and initiate formation of an initial matrix for early wound healing with fibrins, lymphocytes and histocytes (after 12 hrs.) [16].
During the inflammation phase, dead and damaged cells, along with microorganisms and debris are cleared from the wound. In this stage, neutrophils appear in the wound area, followed by lymphocytes and monocytes, which differentiate into macrophages, which phagocytose bacteria, foreign particles and damaged tissue. Proteases and reactive oxygen species (ROS) produced by neutrophils, defend against infection when cause some bystanders damage [17]. The cytokines released by macrophages in the early wound mobilize the inflammatory response by activating and recruiting additional leukocytes. Macrophages also lead to apoptosis in cells (including neutrophils) which paves the way for inflammation resolution [18].
The proliferative phase begins on the third day after the injury and lasts approximately 2 weeks. During the proliferative phase, fibroblast migration is followed by collagen synthesis and angiogenesis formation. Finally, tissue granulation takes place within the wound bed. The migrated fibroblasts produces collagen and glycan’s (glycosaminoglycans and proteoglycans), which are the key player of extracellular matrix (ECM). In the final remodeling step, many of the newly formed capillaries are regressed to a level that returns to normal vascular density. Scar maturation and collagen remodeling continues for up to two years, demonstrating a long healing period in adults, although this progresses much faster in infants [19, 21].
3. Wound dressings
3.1. Necessity of wound dressings
There are multiple factors affect wound healing that can be generally classified as local/intrinsic or systematic. Local factors include insufficient blood supply, foreign bodies, infection, and topical steroids or antiseptics. Systematic factors include the overall health and age of the patient [22]. Any wound that fails to heal within a few weeks should be assessed by a healthcare professional, because this may be a chronic wound caused by bacterial infection or an underlying disease such as diabetes, fibrosis, jaundice etc. [23].
Wound infections can occur when the natural cutaneous barrier is broken and exposing underlying tissue (figure 2)[24]. Pathogen class and concentration, the presence of vascular disease, edema, malnutrition, diabetes, obesity or poor nutrition, age corticosteroids, sex hormones, alcohol consumption, smoking, and deep, wide or necrotic wounds [22] increase the risk of infection [25]. The main characteristics of infection include increased wound fluid, increased swelling around the wound site, increased erythema and discomfort, odor and increased wound temperature [26].
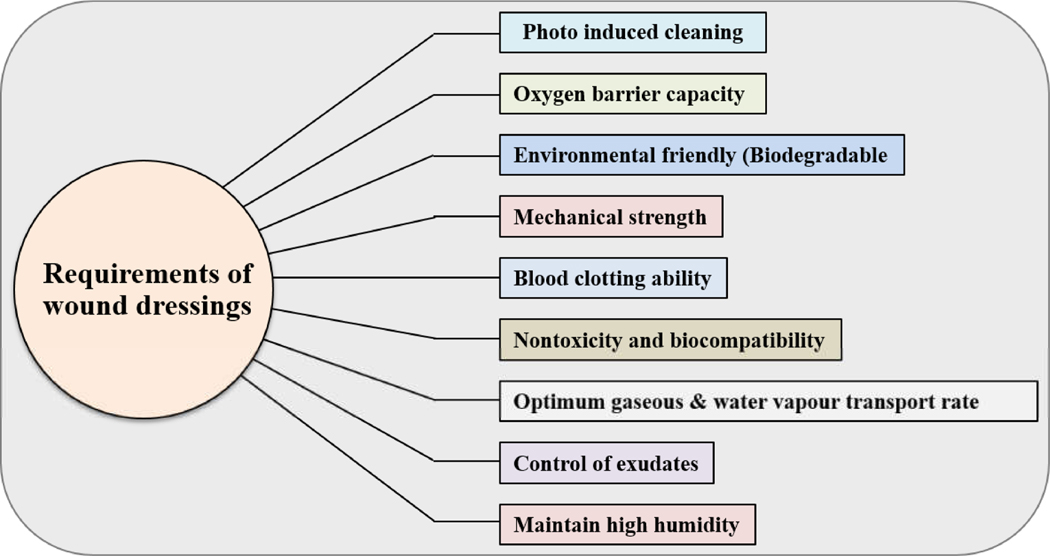
Figure 2:
The perfect wound dressing material is biocompatible, humidifier, gas permeable, suitable for a dust and microorganism barrier and non-adhesive, which can be removed without damage easily requirements of an ideal wound dressing materials.
The major wound infection-causing bacteria are Staphylococcus aureus (S. aureus), Staphylococcus epidermis (S. epidermis), Pseudomonas aeruginosa (P. aeruginosa) and Escherichia coli (E. coli). If these bacteria enter into the body, they can lead to subcutaneous infections or infections of deeper tissues; Insufficient care for infections can lead to slow healing, loss of soft tissue, limb amputation, and even death. [27]. Thus, the ideal wound dressing is the major need in wound care management.
3.2. Wound dressings: Ideal requirements
The ideal wound dressing material is biocompatible, moisture-retaining, gas permeable, adequate as a barrier against dust and microorganisms, and non-adhesive, so it can be removed easily with no trauma [5].
4. Biomaterials for wound dressings
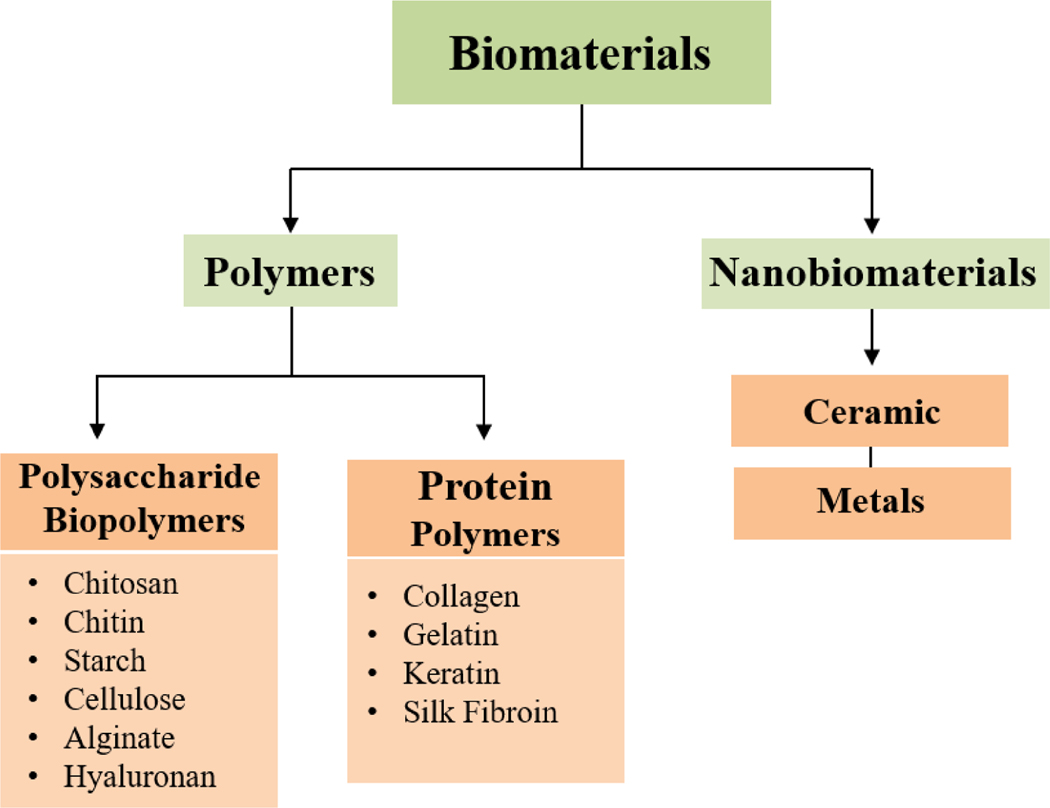
Natural biomaterials for wound dressing purposes are diverse, as shown in figure 3. The natural biomaterials are classified as polymers and nanobiomaterials. The polymers are again classified into natural (biopolymers) or synthetic polymers. Natural polymers are synthesized from renewable sources (animals and plants) and synthetic polymers are produced from nonrenewable petroleum sources [28]. Biopolymers can be polysaccharides or proteins and offer many advantages over synthetic polymers such as excellent biocompatibility, biodegradability, and fast manufacturing which can be used to manufacture bio-composite material [29].
Figure 3:
Schematic representation of different types of biomaterials used for Ideal wound dressings. This can be categorized very quickly into polymers and nanobiomaterials. Polysaccharides and proteins are two main players that represent the polymer class. Metals and metal oxide are the only class, which has included under nanobiomaterials. Chitosan, cellulose, starch and alginate are the most commonly used and formed polysaccharide biopolymers. Proteins show unique functionalities and have many applications in both biomedical and material sciences. Proteins can form films and some proteins are employed as wound and burn dressings such as collagen, gelatin, keratins and silk fibroin.
4.1. Polysaccharide biopolymers
Chitosan, cellulose, starch and alginate are the most commonly used and formed polysaccharide biopolymers [30]. Chitosan is created by deacetylation of chitin and composed of D-glucosamine and N-acetyl-D-glucosamine linked by glycosidic bonds [31, 32]. Due to its inherent compatibility and antibacterial activity, it has vast biomedical applications [32]. Due to the ability to form stable materials, generally used as antibacterial in wound dressing applications [29].
Starch is natural polysaccharide which is derived from different plants sources. The compositional ration of amylase and amylopectin in starch depends on the source [33]. A starch’s mechanical and biodegradability properties are due to the amount of amylase and amylopectin it contains [34]. The most attractive property is formation of gelatinization while heating.
Cellulose is an organic polysaccharide consisting of linear and very long macromolecular chains of one repeating unit of cellulobiose by plants. Cellulose is much more crystalline compared to starch and is insoluble in organic solvents, hence they are mostly used for wound management. Various sources of cellulose, such as plant wood or microbial, have been extensively explored for wound dressing applications. Recently, there has been a trend towards nanofibrillar cellulose synthesis for wound dressing applications that has shown to support the growth of HMSCs without cell adhesion coating [35].
Alginate is a polysaccharide, which is isolated from cell walls of brown algae. The structure of alginate is a monomer linked to α-L-guluronic acid via 1, 4-glycoside linkage in different sequences [15, 36]. Alginate is capable of forming gels in the presence of cations. Alginate-based hydrogels, composites, and films are employed as wound dressings due to alginate’s cooling sensation, biocompatibility, biodegradability, non-immunogenicity and muco-adhesive properties under normal physiological conditions [36].
Hyaluronan is an important polysaccharide present in several animal species. Hyaluronan is a polymer of disaccharides composed of D-glucoronic acid and D-N-acetylglucosamine, linked via alternating β−1, 4 and β−1, 3 glycosidic bonds. It has properties of high higroscopicity, viscoelasticity and shock-absorbing properties. Hyaluronan has the capacity to retain water, which is one reason it is distributed in many tissues, including skin, semen and umbilical cord [37].
4.2. Protein Biopolymers
Proteins show unique functionalities and have many applications in both biomedical and material sciences. Proteins can form films, following a casting method of solution display, which can be as strong as films produced by synthetic polymers [12]. Some proteins are employed as wound and burn dressings such as collagen, gelatin, keratins and silk fibroin [38].
Collagen is the main structural protein of extracellular matrix. It is the abundant protein in the human body and is produced mainly by fibroblasts cells. It stimulates the wound healing process [15]. Until date 30 different type of collagen has been identified, but 90 % of collagen resembles to Type I collagen. The animal sources for collagen type I includes bovine skin and tendons, porcine skin and rat tail. The hydrolysis of collagen yields a product which is gelatin [39].
Gelatin is prepared from animal by products and is soluble in water. Due to its biocompatibility and biodegradability, gelatin is used in biomedical fields for drug delivery and wound dressing applications [40]. Gelatin is used in various formulated products including cross-linked gelatin-alginate and gelatin-hyaluronate sponges, films and hydrogels for wound healing, which creates a novel biomaterial that has intermediate physical, mechanical and water vapor barrier properties [39, 40].
Keratins are fibrous proteins found in vertebrate epithelia and corneous tissues like horns, claws, and hooves, where their strong mechanical properties are needed. They also have thermal stability and a low sensitivity to break down by common proteolytic enzymes. Keratin proteins are classified as soft and hard based on their amino acid composition, secondary structures and functions. Keratins protect a wound dressing from breakdown in the proteolytic wound environment to facilitate the healing process.
Silk fibroin (SF) is a natural protein biopolymer synthesized by Bombyx mori silk worms, other insects, and spiders. In last few decades, SF has been shown to have many advantages such as biocompatibility, biodegradability, mechanical property in biomedical applications, including tissue engineering, tissue regeneration, and drug delivery [41]. Silk Bombyx mori cocoons consist of two types of proteins, SF and sericine, and both type of protein is used in wound dressing applications, although SF is the predominant type of silk biomaterial used [5].
4.3. Nanomaterials
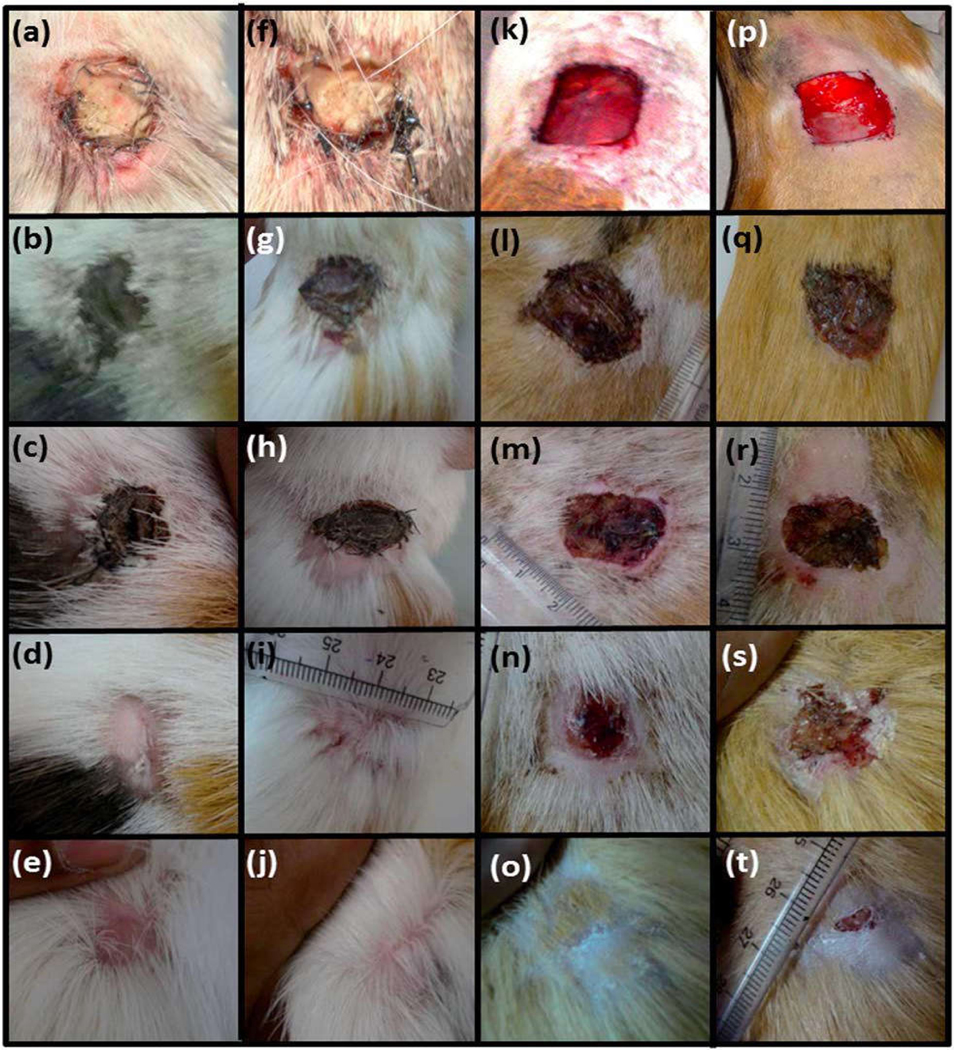
Nano biomaterials include metals and metal oxide. [42]. The properties of metals such as excellent electrical and thermal conductivity, mechanical strength, corrosion resistance (depending on the type of metal) and reasonable cost, which promotes their usage in the biomedical field including components of wound dressings [43]. Zinc is an essential trace element functions as co-factors. There has been much interest shifted on the wound healing activity of Zinc. Recently Zinc oxide (ZnO) micro particles loaded chitosan and polyvinyl alcohol acacia gum nanospheres based thin film was formulated for accelerated wound healing. Fig.4. Shows the use of polycaprolactone scaffolds to integrate the ZnO NPs and their ability to heal the wound was assessed [44]
Fig.4.
Wound healing activity of the polycaprolactone (PCL) membrane embedded with ZnO NPs. First day (a, f, k and p), on 5th day (b, g, l and q), on 10th day (c, h, m and r), on 20th day (d, I, n and s) and on 30th day (e, j, o and t) of implantation. The first column (a–e) indicates neat PCL membranes, the second column (f–j) indicates PCL membrane incorporated with 1 wt% ZnO nanoparticles, the third column (k–o) indicates povidone-iodine treated wounds (positive controls) and the fourth column (p–t) indicates negative controls. Reproduced with permission from [44]
5. Wound dressings based on silk fibroin derivatives
5.1. SF as a protein biopolymer
5.1.1. Sources of silk fibroin
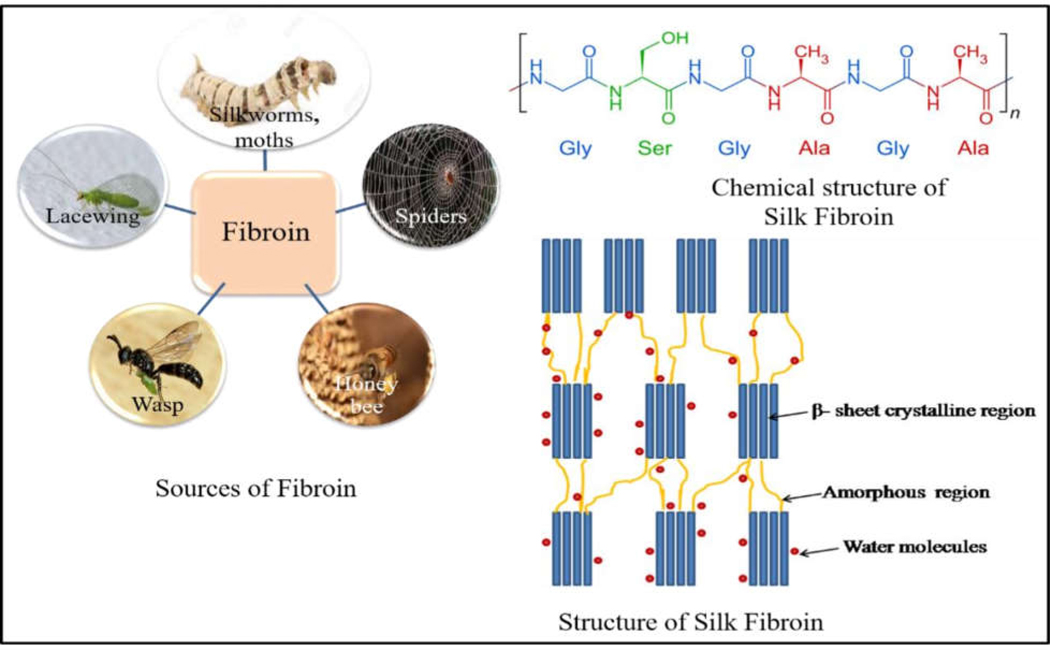
Naturally derived biomaterials such as SF are produced by spiders, silkworm, wasps and lacewings [45] (figure 5). A large amount of silk can be obtained from Bombyx mori silkworms and spiders. However, spider silk can be extracted from cannibalistic spiders, which is typically more costly than obtaining silk from silkworm cocoons [46]. Silk from silkworm and spider silks bear great resemblance in their molecular structures and mechanical properties [46]. Silk has been used for thousands of years as a textile for luxury clothing and surgical structure because of its texture, mechanical strength and biocompatibility [45]. In recent years, Bombyx mori SF has been proven to be interesting biomaterials for wound dressing applications [47].
Figure 5:
Different sources of SF proteins.In nature the protein can be made available from silkworm moths, honey bees, spiders, wasp and laccwings. The fibroin obtained from silk mainly consits of light chains and heavy chains linked by disulphide bonds. The heavy chain is comprised of crystalline and amorphous regions. The crystalline regions consist of glycine-alanine repeats interconnected with serine and tyrosine amino acids while the amorphous regions consist of amino acids such as aspartic acid.
5.1.2. Properties of SF
SF is a fibrous protein with unique properties such as slow biodegradation, mechanical stability, transparency (depending on formulation) and easy surface patterning [45, 46 47]. Different sources of SF can be explored but widely used and accepted one is from Bombyx mori cocoons. To obtain the, SF must be separated from sericin, the sticky coating around the SF fiber used to hold the cocoon together, which can elicit an immune response. Due to above properties, over the last few decades SF proteins have been considered as promising candidates in wound dressing applications [47, 49].
5.1.3. Structure of SF
SF consists of light chains and heavy chains linked by disulphide bonds. The heavy chain is comprised of crystalline and amorphous regions. The crystalline regions consist of glycine-alanine repeats interconnected with serine and tyrosine amino acids while the amorphous regions consist of amino acids such as aspartic acid [50, 51]. The crystalline regions are rich in hydrophobic β-sheet-forming structures (which provide high mechanical strength and toughness) linked by small hydrophilic linker segments. The tensile strength of B. mori silk fibers is 740 MPa [47, 51]. SF contains either a high content of β-sheet or α-helices, depending on its form. SF is tough due to the β-sheets surrounded by α-helices and coils, which act as a matrix. Silks are processed easily into semi-crystalline to crystalline structures by chemical methods such as treatment with methanol and potassium phosphate [50, 51].
5.1.4. Applications of SF Proteins
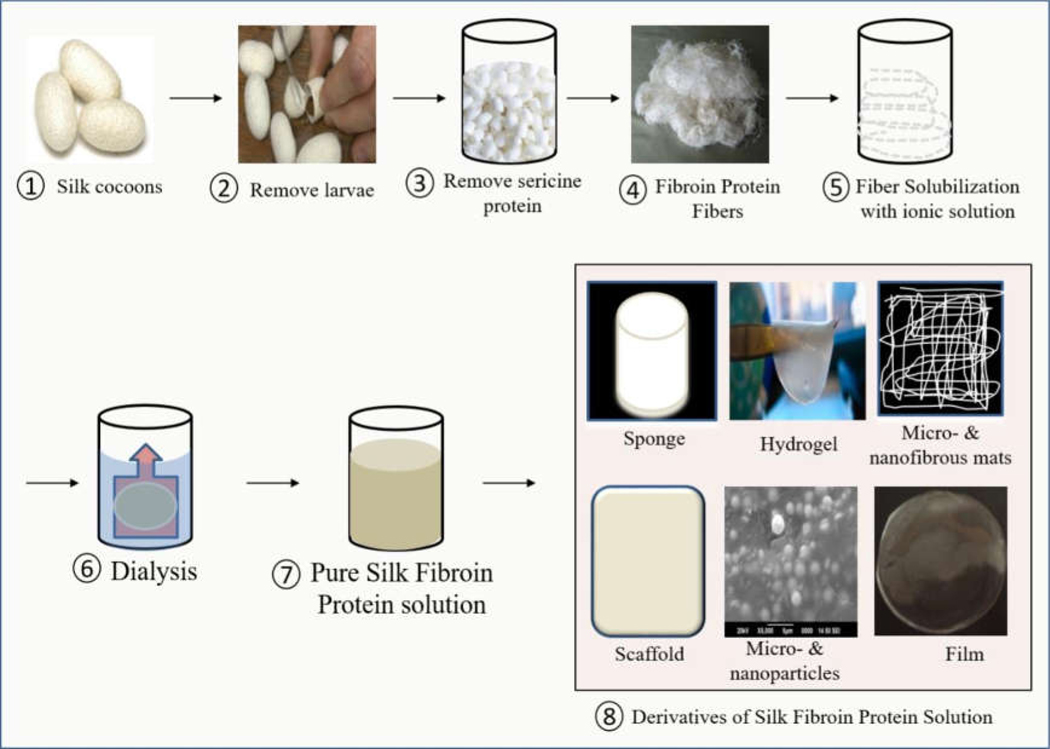
The applications of SF in wound dressings have been growing in recent years, in part thanks for the development of new SF composite biomaterials. SF fibers are used in textile industries due to their softness, lustrous appearance, and ease of dyeing due to their moisture absorption capacity [47, 49]. These are readily available biomaterials that require minimal processing, possess antibacterial activity and promote wound healing process [9]. Apart from this, SF has desirable properties of high strength, biocompatibility, biodegradability, low bacterial adherence and good handling characteristics, and therefore it is a commonly used suture material in the medical field [49]. SF can be easily fabricated into sponges, hydrogels, nanofibrous mats, scaffolds, micro-nanoparticles, and composite films for a variety of biomedical applications including tissue engineering, drug delivery, cancer therapy, skin wound healing and wound dressing applications [52–57]. The advantages of SF in wound dressing applications are its high strength, flexibility, non-cytotoxicity, oxygen permeability, and ability to support migration, proliferation, and differentiation [53].Figure 6 shows the synthesis process of SF and its different derivatives used for wound dressing applications, including sponges, hydrogels, nanofibrous matrices and scaffolds, microparticles and nanoparticles, composite films of SF-PVA films.
Figure 6:
Simplistic synthesis processes of SF solution and its different derivatives used as wound. The detail synthesis process of SF and its different derivatives used for wound dressing applications, including sponges, hydrogels, nanofibrous matrices and scaffolds, microparticles and nanoparticles, composite films, for dressing materials.
5.2. Sponges
SF sponges can be generated from SF solution by freeze-drying, gas foaming, and particle-leaching using porogens. For freeze-drying, degummed silk cocoons are dissolved in highly concentrated salt solutions such as lithium bromide and then dialyzed for 2–8 days and analyzed for wt/wt% concentrations to form a SF aqueous solution [52]. This SF solution can then freeze-dry to create scaffolds, and the pore size of these sponges can be controlled by the concentration and freezing temperature and rate. For salt leaching, the SF solution is poured into containers and salt crystals (porogens) are poured into the silk. The pore sizes ranging from 490–900 μm based on the sieves used to isolate the salt crystals scaffold formation is dependent on SF concentration as well as pore size [58]. Treatment with methanol can increase molecular crystallization into β-sheets so as to impart water stability and mechanical integrity [58]. Gas foaming processing involves the preparation of sponges with the addition of ammonium bicarbonate into SF solution and then sublimation of ammonium bicarbonate in hot water [59]. As opposed to aqueous scaffolds, non aqueous-based scaffolds can be formed using lyophilized silk dissolved in 1, 1, 1, 3, 3, 3-hexafluoro-2-propanol (HFIP), which provides smoother surfaces along the pores, greater mechanical strength, and the ability to get scaffolds of higher concentrations of silk [58, 59]. SF-based sponges can be characterized for their morphology and pore structures by Scanning Electron Microscopy (SEM), confocal imaging, or histology. Scaffold porosity can be determined by the liquid (alcohol) displacing method and sponges can be sterilized by autoclaving, ethylene oxide, γ-radiation, or 70% ethanol [59]. All of sponges provide 3–D porous scaffolds for wound dressing applications.
A SF-alginate mixture was developed and its wound treatment effects on a rat model were assessed. Results demonstrated that SF/alginate blend sponges induced better wound healing than other treatments including SF or alginate alone. Due to better re-epithelialization via a rapid proliferation of epithelial cell and enhanced collagen deposition is increased fibroblast proliferation. It may be clinically useful for skin wound treatment [60]. Xiaomeng et al (2014) developed an SF/NTCC (N- (2-hydroxy) propyl-3-trimethyl ammonium chitosan chloride)/Polyvinyl Alcohol (PVA) composite sponge for chronic wounds. This sponge was prepared by the freeze-drying method and studied using a rat chronic wound model. The results suggest that the comparative study between the composite sponges, performed better results than commercially available oil-containing non-woven fabrics [61]. Zejune et al (2015) prepared silver nanoparticles (Ag NPs) with SF and carboxyethyl chitosan into a composite sponge as an antibacterial wound dressing. The synthesized composite sponge demonstrated higher antibacterial activity against S. aureus and P. aeruginosa and P. aeruginosa activity than AQUACEL Ag, a commercial Ag+ dressing [62]. The sponges without Ag were not tested.
5.3. Hydrogels
SF-based hydrogels are produced via cross-linking of fibroin molecules in aqueous solution [53]. The gelation process can be produced by changing the physical parameters like heat/cold treatment, pH change, or adding different cross-linking agents or UV exposure. Using higher initial concentrations of SF solutions results in hydrogels are enhanced tensile strength, modulus and maximum strain. Often, a higher temperature of gelation leads to increased mechanical properties due to reduced pore sizes [63]. SF hydrogels are characterized by differential scanning calorimetry (DSC) to verify the melting point (~206 °C for hydrogels) [63]. Rheology studies determine hydrogel stiffness and dynamic mechanical analyzers using stainless steel fixtures assess their adhesive properties [64]. Due to the high water content, biocompatibility, easy application, and effective cell attachment and proliferation in SF-based hydrogels, they are easily applied in biological applications such as wound dressings [65]. Burn injury cure is one of modern surgery’s most important challenges. SF hydrogels composed of calcium alginate and carboxyethyl chitosan were formulated and studied for burn-induced wounds. The adhesive strength, water content, and cytotoxicity of fabricated hydrogels were investigated. Together, the results suggested that SF-based hydrogels are an excellent material for the healing of second-degree burns, and in vivo, wounds treated with SF-based hydrogels had the fastest healing results [66]. In another study, chitosan-based hydrogels had improved cell attachment and cell proliferation when they were loaded with SF and L-Proline. The prepared hydrogels showed better results than other polymers and have potential as an alternative wound dressing material for chronic diabetic or burn wounds [67]. Huang et al prepared successfully composite hydrogels based on SF and polyurethane, which had excellent swelling and deswelling behaviors, good mechanical properties, and potential as a novel material for biomedical applications [68]. The advantages of hydrogels for wound dressings include the ability to absorb and retain the wound exudates, a cooling sensation around the wound, promotion of fibroblast proliferation, and keratinocytes migration; these processes are necessary for complete epithelialization and healing [69, 70]. Hydrogel structures also protect wounds from infection and microorganism infiltration, and they can be loaded with antibacterial agents and antibiotics [66, 68, 71]. For example, curcumin-loaded SF gel system scaffolds, formed a weak electric fields, showed improved wound healing activity compared to absence of curcumin in silk fibroin system. They proposed that these could be new wound dressing materials [72].
5.4. Nanofibrous matrices and Electrospun mats
Naturally, silks are present in the form of fibrous architectures. Microfibers (diameters in micrometer) and nanofibers (diameters less than 1 μm) showed tremendous promise for skin tissue engineering [73, 74]. Fibers can mimic native tissue while providing topographical and biological cues for healing. Such micronano-fibers are advantages of good porosity, variable pores, high oxygen penetration, high volume to surface ratios and good mechanical characteristics. [75].
The fibers have been inserted into 2-D mats with a high surface to mass ratio, which provides excellent mechanical property. Such fibers can be synthesized by different techniques such as phase separation, self-assembly and electrospinning technique [76, 77]. Phase separation method of forming fibers can be performed by thermodynamically mixing a homogeneous solution into a polymer-rich phase and a polymer-poor phase [78]. These phases can be formed by exposure of the SF solution to another immiscible solvent. Another method for formation of SF fibers is self-assembly. It occurs by the spontaneous hierarchical organization to higher order structures [76, 78]. Self-assembly method produced very fine fibers, which can be often act as precursors to microscopic silk fibers [79]. Electrospinning is one of the advanced techniques which can be used to fabricate nanofibrous scaffolds with unique properties due to its simplicity, rapid, efficient and inexpensive method for production by applying high voltage to electrically charged liquid [80, 81]. Electrospinning is a highly scalable method for development of nano and microfibers of pure silks as well as silk composites [80]. Electrospun SF based fibers can be sterilized by using 70 % ethanol. The morphology and diameter of synthesized nanofibrous mats are examined by using SEM study. This morphology and secondary structure depends upon the electrospinning voltages, the SF solution concentration, the flow rate and the distance between them [81]. If the concentration of SF is less, therefore the prepared SF mats observed clustered or beaded fibers [47, 82]. During the process of electrospinning technique, the different spinning solvents have been used such as Polyethylene Oxide (PEO), HFIP and formic acid [59]. Recently, biocompatible Manuka honey/SF fibrous matrices were fabricated by electrospinning of aqueous PEO solution as wound dressing materials. In this study, in-vivo wound healing assay indicates that the prepared Manuka honey/SF fibrous matrices demonstrated excellent biocompatibility and enhanced wound healing process [83]. Guldmet et al (2016) prepared nanofiber webs based on olive leaf extract loaded SF/hyaluronic acid for wound dressing applications. The cytotoxicity of prepared nanofiber webs was evaluated in-vitro against the human epidermal keratinocytes cells. These results suggested that the developed electrospun nanofibers were nontoxic and good candidates for wound dressing materials [84]. Shadai et al developed starch nanoparticles as a vitamin E-TPGS carrier loaded in SF/PVA/Aloe Vera nanofibrous by electrospinning technique. These results indicated that the prepared nanofibrous dressing of SF/PVA/Aloevera containing 5% Vitamin-E-loaded starch NPs can be a potential dressing for treating skin wounds [85]. Similarly, the addition of Ag and TiO2 NPs was found to improve the antibacterial property of SF mats [86, 87]. Minsung et al. prepared Ag NPs incorporated SF for the fabrication of antibacterial wound dressings [86]. Win et al prepared electrospun SF/TiO2 nanofibrous mats for wound dressings. In this study, the antibacterial activity of prepared mats was demonstrated against gram-negative bacteria E. coli was under UV-A light exposure using the antibacterial drop-test method. Results of this study suggested that the addition of TiO2 NPs into SF nanofibrous mats showed bactericidal ability against E.coli and the ability of photocatalytic degradation of methylene blue under UV irradiation [87]. Xingwo et al. prepared polyethyleneimine/SF multilayers nanofibrous for cell culture [88]. Cai et al. demonstrated chitosan and SF composite nanofibrous membrane were fabricated by electrospinning. The antibacterial activity of composite nanofibrous membrane was studied by the turbidity measurement method against gram-positive (S. aureus) and gram-negative (E. coli) bacteria. The results concluded that the antibacterial activity of composite nanofibers varied on the type of bacteria. The biocompatibility study of prepared nanofibers was investigated by hematoxylin, eosin staining and MTT assay. The synthesized membranes were found to promote cell attachment and proliferation. The results suggested that the SF based composite nanofibrous matrix could be promising candidates for wound dressing applications [89]. For a review of electrospun silk for regenerative medicine applications, we refer the reader to the following review [90].
3D structure biomaterials are the ideal material for tissue engineering, wound healing and wound dressing applications because 3D structures mimic the in-vivo physiological environment more closely than 2D structures. Scaffolds are three-dimensional porous structures. Scaffolds are made of ceramic, polymer and metals or a combination of all these materials. SF based scaffolds must be biocompatible, excellent mechanical property and high porosity. Different types of scaffolds have been constructed using SF based materials [91, 92]. SF/amniotic 3D bi-layered artificial skins were developed as scaffolds for the reconstruction of skin in-vivo. This artificial skin promoted the remodeling of an extracellular matrix. In-vivo results indicated that the prepared artificial skin may be considered as a clinically translational product with stem cells to guide scarless healing of 3rd degree burn injuries [93]. Mojtaba et al prepared biocompatible and biodegradable SF/Chitin/Ag NPs 3 D material for wound dressing applications. The scaffolds fabricated by the freeze-drying method. The antibacterial activity, mechanical property, biodegradability, water uptake, blood clotting study was investigated. Furthermore, in this study cytocompatibility, proliferation and cell attachment with normal fibroblast (nHFFF2) cell were studied. The results of this study concluded that the prepared scaffolds proved the cytocompatibility nature and are good candidates for wound dressings and used for further in-vivo study [94]. A novel anti-adhesion nonwoven mat prepared by SFP by electrospinning technique for wound management in robotic surgery. SFP based electrospun mats prepared by addition of synthetic polymers such as SFP/PVA, SFP/polyethylene glycol (PEG), and SFP/polyethylene oxide (PEO). The results showed that the all non-woven mats were composed of submicron-fibers and showed no cytotoxicity towards skin cells such as fibroblast cells. Additionally, the prepared mats improved the anti-adhesion ability, good flexibility, high mechanical properties, promoting the biocompatibility and anti-inflammatory effects. Furthermore, in-vivo studies of SFP and SFP/PVA treated mats both have superior collagen regeneration and wound closure ability [95].
5.5. Micro particles and Nanoparticles
SF microparticles and NPs can be prepared by using diverse methods such as self-assembly [96], freezing [97], desolvation [98], spray drying [99], laminar jet break up [100], milling [101], capillary microdots [102] and electrospray techniques [97]. SF micro and NPs can be prepared by using the phenomenon of self-assembly. The prepared formulations are with highly controlled diameters. SF molecules can be arranged in hydrophilic (Tyr, Ser) and hydrophobic (Gly, Ala) segments. The addition of a certain amount of ethanol to SF solution permits SF molecules to form particles by self-assembly [96]. Microparticles sizes range from 0.2 to 1.5 μm and can be controlled by the SF solution concentration and amount of ethanol added. Microparticles have unique properties such as subcellular size, stability, high surface to volume ratio, and high carrier capacity [96]. Wang et al reported that the Silk micro- and nanospheres with controllable sizes and changeable shapes were prepared using polyvinyl alcohol (PVA). Due to PVA there were two different phases of silk solution and PVA solution. The PVA played a role of continuous phase, which makes separation of silk solution into micro- and nanospheres. The author claims that the developed method of preparation is simple, safe and accessible to many different drugs. [103]. Lammel et al studied that the addition of potassium phosphate to SF molecules to obtained SF micro and NPs ranging from size two μm to 500 nm. They proposed the addition of potassium phosphate would help to achieve tuned particle size and morphology [104]. Milling is another physical method for production of SF micro and NPs. It does not use any chemicals, particles obtained by grinding, miller and the aperture of the vibratory sieve shaker. SF NPs have been tested in wound healing and dressing applications [101]. Lee et al developed SF NPs incorporated hydrocolloid dressings (SFNHD). In this study, results suggested that the SFNHD showed structural stability, improved water uptake and swelling ratio and increase cell growth rate compared to commercially available dressings. Additionally, SFNHD reduced the burn size of rats and accelerated the growth of collagen fibers compared to Neoderm® dressing. Li et al prepared insulin- functionalized SF dressing for chronic wound healing. The results from the in-vivo test using diabetic rat model suggested that the insulin-functionalized SF dressing provides a new treatment for chronic wound due to the prepared dressings demonstrated healing of wounds, deposition of collagen and vascularization [105].
5.6. SF and its composite films
SF based composite films are prepared by casting and air-drying the aqueous SF solution [57],spin coating [106], layer-by-layer assembly [107] and vertical deposition [108]. There might be other techniques reported which can be used to develop the film [106]. Casting technique is a widely used technique for preparation of SF based films, which can be obtained by casting SF solution on a glass petri-dish with subsequent natural evaporation or drying under certain temperature [57]. Transparent SF film can be obtained by the vertical deposition method. In this method, a glass cover-slip was inserted vertically in the SF solution in an oven at 50 °C. The obtained films showed a nonhomogenous texture, because of the inherent feature of the vertical deposition method [108]. Ultrathin SF film (45± 5 nm) can be prepared by layer-by-layer assembly method, the resulting films observed higher mechanical property due to its self-reinforcing structure [107]. Water-insoluble SF films were fabricated by using methanol and ethanol with increased crystalline content [109]. SF films can be sterilized by 70 % alcohol overnight and then rinsed in sterile PBS or water. The surface morphology and thickness of SF films can be assessed by using Scanning electron microscopy (SEM). The mechanical property of synthesized SF films can be studied using Universal Testing Machine (UTM) [59]. Blended SF and chitosan showed the films had good oxygen and water vapor permeability, making the blended film useful as biomaterials. A novel flexible SF based wound dressing was prepared by addition of glucose to modify SF films. The results noticed that the addition of glucose provided good flexibility, absorption capacity, and biocompatibility provided a great effect in promoting the wound closure [110]. Gue et al (2013) prepared chitosan/SF/alginate dialdehyde (ADA) blending membrane for wound dressing applications. These results demonstrated that the addition of ADA into SF/chitosan membrane is enhanced the stability and promote cell proliferation [111]. SF has been tested for lung injury caused by burn rat model, results of this study showed that the SF promote tissue healing and complete regeneration as compared to control and saline-treated burn group. SF can be a safe, effective and helpful recovery of patients with burn-induced lung injury [111]. The chronic wound is a serious problem in wound management. Bienert et al studied the growth factor functionalized SF membranes for in-vitro wound healing. Current study observed the Fibroblast Growth Factor (FGF), Epidermal Growth Factor (EGF), Keratinocytes Growth Factor (KGF), Platelet Derived Growth Factor (PDGF) or Vascular Endothelial Growth Factor (VEGF) functionalized SF membrane, which enhanced macrophage adhesion than without growth factor. Invitro wound healing assay demonstrated that a higher wound healing capacity when functionalizing SF with EGF, FGF or VEGF compared to native SF, KGF or PDGF functionalized SF membrane [112]. Liu et al concluded that the SF film showed good biocompatibility, does not have an adverse influence on the growth and biofunction of fibroblasts and vascular endothelial cells. Additionally, SF film does not interfere with the secretion of angiogenesis growth factors such as VEGF, Ang-1, FGF2 and PDGF [113]. SF film has used in dermal wound dressing material [57]. The mechanical properties of SF films increased with increase in dextrose content in films and surface roughness of SF film. The blended films observed ability to support cell growth and proliferation as compared to SF film. SF film was prepared and studied application potential for skin repair and regeneration in the clinic. The in-vivo study by using rabbit full-thickness skin defect showed that the SF film effectively reduced the time of healing process with better skin regeneration than the commercial wound dressings. The developed film has good biocompatibility, maintained moist environment, gaseous permeability, provided waterproof surface, bacteria free, easily applied, removed, and reduced the time of healing process [57].
5.6.1. SF and PVA composite films
PVA is a synthetic polymer, soluble in water, nontoxic and semicrystalline polymer have been applied in several advanced biomedical applications e.g. wound dressings [114], drug delivery system [97], artificial organs and contact lenses [114]. It has possessed various useful properties, such as biocompatibility, gas barrier properties, high strength, flexibility and excellent membrane-forming properties together with high thermal stability making it an effective polymer to be used in wound dressing applications [115]. Blends of SF and PVA have been extensively used in dressing application since these blends can be prepared with good mechanical, biocompatible and biodegradable properties [116]. Sheik et al (2017) developed biodegradable composite film based on PVA reinforced SF fibers. The results demonstrated that the degradation rate of composite films was improved with the addition of SF fibers. Additionally, degree of crystallinity, mechanical property and thermal property was improved with the incorporation of SF fibers as compared to pure PVA [117]. Starch and PVA reinforced with SF blended films were prepared by casting method and studied for their mechanical property, antibacterial activity and biodegradability. The blended films were improved mechanical property, good antibacterial activity against Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria and showed biodegradability by addition of SF particles [118]. Electrospinning has become a popular technology for the fabrication of tissue engineering matrices and PVA is selected as the polymer additive to produce electrospun nanofibrous matrix due to its good fiber forming properties [119]. Zhao et al synthesized membrane based on recombinant spider SFP/PVA by electrospinning technique. These membranes applied as a wound dressing for Sprague Dawley rat model. The results showed that the prepared membrane has porous and promote wound healing activity [120].
An enormous population suffers from chronic diabetic foot ulcers worldwide. There is a need for bioactive dressings which can trigger the healing process for complete wound repaired. Chouhan et al fabricated functionalized PVA-SF nanofibrous mats for diabetic wound healing by electrospinning method. In this study, the prepared mats functionalized by using growth factors and LL-37 antimicrobial peptide for sustained delivery and investigated the wound healing efficacy of prepared mats in Alloxan-induced diabetic rabbit model. The results of this study observed that functionalized PVA-SF fibroin nanofibrous mats supported faster granulation tissue development, angiogenesis, and re-epithelialization of wounds [119]. Zhou et al developed nanofibrous membrane of carboxyethyl Chitosan/PVA/SF nanoparticles by electrospinning studied indirect cytotoxicity assessments of the nanofibrous. The results obtained the membranes have good biocompatibility would be used as potential material for wound dressing for skin regeneration [121]. Chouhan et al reported that the non-mulberry SF based electrospun mats functionalized with epidermal growth factor (EGF) and Ciprofloxacin (HCI) as wound dressing applications. The prepared dressings showed biocompatibility, high water vapor transmission rate, high elasticity, and good antibacterial activity. The in-vivo study observed that the prepared functionalized mats enhanced the proliferation of human dermal fibroblasts and HaCaT cells compared to non-functionalized mats. Biocompatible mats of SF and PVA promote a moist environment and wound healing process [119].
5.6.2. Composite films based on SF and Silver (Ag) NPs
Over the last few decades, NPs based wound dressing materials have become an important area in research. Ag is a well-known metal, which has long been used as an antimicrobial agent. This comes in the form of silver metallic and silver sulfadiazine onions. It possesses fast, broad-spectrum antibacterial activity [122, 123]. In recent years, researchers have focused on the Ag loaded SF composite materials. The incorporation of Ag NPs into SF film is either by insitu synthesis of NPs from it precursor or by direct addition of NPs into composite films by vigorous stirring [124]. The bacterial wound infection is the serious problem of wound management. Recently Ag oxide NPs embedded SF spun were prepared by microwave mediated and studied their synergistic wound healing and antibacterial activity. The in-vitro wound healing (scratch assay) demonstrated that fast migration of the T3T fibroblast cells through the scratch area, treated with the Ag2O-SF spuns and the area was completely cover within 24 h. The prepared spuns also showed biocompatible nature and used an ideal material for wound dressing and antibacterial applications [125]. In another study developed asymmetric wettable chitosan-SF composite dressing with embedded Ag NPs for infected wound repair. The scaffolds of chitosan-SF-Ag NPs were prepared by lyophilization and embedded particles were observed using transmission electron microscopy TEM and SEM analysis. The asymmetric wettability of prepared scaffolds was measured using a contact angle meter. The results suggested that the prepared scaffolds showed high porosity, moisture retention capability, appropriate mechanical stiffness, good antibacterial activity and highly biocompatible dressing. The in-vivo healing measured in mice infected wound models. The prepared dressing offers the potential for infected wound tissue repaired and regeneration. Ag NPs also employed in functionalization of Silk biomaterials for wound healing and dressing applications [126]. Gil et al formed silver sulfadiazine and epidermal growth factor (EGF) functionalized SFP biomaterial formats included silk films, lamellar porous silk films, and electrospun silk nanofibers and studied with a cutaneous excisional mouse wound model. The prepared functionalized silk biomaterial wound dressings increased wound healing rate, re-epithelialization, cell proliferation, collagen synthesis and reduce scar formation as compared to Tegaderm tape (3M) (− control), a commercial wound dressing and Tegaderm Hydrocolloid dressing (3M) (+ control) [127]. Recently, 3D scaffolds as an antimicrobial wound dressing bandage were prepared by using SF/chitin and Ag NPs. The bandage was prepared by the freeze-drying method and observed for successful wound dressing application. The results demonstrated that the prepared bandage has high antibacterial activity against E. coli, S. aureus and C. albicans, cell proliferation, cell viability and cell attachment with MTT assay and DAPI staining on nHFFF2 cell have proved the cytocompatibility nature of prepared SF/chitin and Ag NPs based bandages [128]. Yu et al reported in situ assemblies of Ag NPs on porous silkworm cocoon based wound film. Antibacterial activity of silkworm cocoons-based films has improved by incorporating the ability of silk sericin to act as a reducing agent for the conversion of Ag+ to Ag, yielding Ag NPs linked together by peptide bonds of silkworm cocoons wound film. The prepared film showed excellent antibacterial performance, biocompatibility and good extensibility. The invivo study was evaluated by using infected white rabbits and results observed the formation of intact and thickened epidermis during 14 days of healing of impaired wound tissue [129]. Coatings of Ag NPs on SF biomaterials have advanced technology for designing the newest wound dressing materials due to improving its antibacterial activity. Uttayarat et al reported SF mats coated with Ag NPs for wound dressing application. The results indicated that the Ag NPs coated SF mats inhibit the growth of S. aureus and P. aeurogenosa and prepared mats can be used as a prototypic wound dressing application with antibacterial properties [130]. In previous years several reports have been published describing the use of Ag NPs in a large variety of antibacterial applications. They have been used as antibacterial filler in several wound dressing materials. Calmak et al developed bio-nanotextiles using Ag NPs and SF. Ag NPs containing SF bio-nanotextiles showed strong antibacterial activity against S. aureus and P. aeruginosa bacteria [131]. Several excellent reviews on Ag NPs based biomaterials for antibacterial wound healing applications have been published in recent years [132]. All these studies have demonstrated that the novel fabrication of SF-based composites is considerable in wound dressings and that Ag NPs present in the composite aid in the antibacterial activity.
5.6.3. Innovative composite films based on SF-PVA and ZnO NPs
Metal oxide NPs such as ZnO NPs are increasingly important and considered as reinforcing filler for polymer matrix. ZnO NPs have been unique properties such as biocompatibility, bactericidal properties, self-cleaning ability, compatible with skin, sunscreens and lots of biomedical specialist [133]. Due to imparting antibacterial activity, ZnO NPs have been integrated with wound dressing materials [134]. It did not show any adverse effect on normal cells when used it in appropriated concentration. ZnO NPs released Zn+ ions and it can enhance the keratinocytes migration towards the wound site and improve the healing process. Polymer Blended ZnO NPs showed higher mechanical property, increased hydrophilicity, water vapor permeability, and swelling properties [135]. Previous studies have been reported where ZnO NPs incorporated Chitosan/PVA film used as hydrophilic wound and burn dressing [136]. Zhong et al prepared chitosan-Ag/ZnO composite dressing for enhancing antibacterial and wound healing activities [137]. Khorasani et al prepared ZnO NPs incorporated heparinised PVA/chitosan hydrogels for wound dressing materials. The results suggested that the prepared hydrogel possesses good mechanical stiffness, improve swelling capacity, enhanced antibacterial activity and prepared hydrogels did not have toxicity [138]. Liu et al reported novel nanocomposite based on genipin cross linked chitosan/poly (ethylene glycol)/ZnO/Ag nanocomposite, tested towards the bacterial species E.coli, P. aeruginosa, and S. aureus and B. subtilis. The prepared nanocomposite film showed higher antibacterial activity and potential application as burn dressings [139]. In another study reported that hydrogel based on keratin-chitosan/ZnO NPs for antibacterial treatment of burn wound healing. The antibacterial, mechanical, swelling and biocompatibility studies of the prepared nanocomposite hydrogels were evaluated. The prepared hydrogels found to be increased mechanical, swelling and bactericidal activity. The in-vivo studies using SD rats showed that increased the wound curing with assisted for quicker skin cell construction along with collagen development [140]. Shalumon et al prepared composite nanofibers using sodium alginate/PVA/ZnO NPs for antibacterial wound dressings. They have reported the antibacterial potential of the prepared mats in vitro [141]. Kumar et al formulated ZnO NPs integrated chitin hydrogel as a material used for antibacterial wound dressing. In this work, the freeze-drying method was used to prepare microporous chitin-ZnO NPs hydrogel. The versatility of prepared nanocomposite hydrogels to biocompatibility, antibacterial activity, blood clotting, swelling, and cell attachment was evaluated. The hydrogel nanocomposite was effective in swelling and blood coagulation compared to controls, while also showing antibacterial activity. Cytotoxicity study showed that this nanocomposite bandage was non-toxic, and that the composite bandages had cells attached and proliferated well [142].
A number of studies have been reported the mechanism of antibacterial effect for ZnO NPs to be cell lysis through the disruption of the cell membrane [143]. Meshram et al (2016) synthesized composite films based on deposition of ZnO NPs on PVA-Gelatin composite film. Bacterial infection generally exposed on the surfaces of wound, this issue controlled the growth of microorganisms by using antibacterial surfaces of composite films [144]. In this study antimicrobial agent, ZnO NPs first capped by using Polyethylene Glycol (PEG) and coated on the surface of PVA and gelatin membrane by screen printing method. The results suggested that the prepared composite films enhanced mechanical strength, thermal stability, and antibacterial activity. This work indicated that the composite films could be used as a wound dressing materials [144].
Recently Patil et al (2018) synthesized ZnO NPs embedded SF/PVA composite film for possible application of wound dressing [146]. In this work, a modified casting method was employed to synthesis the ZnO NPs embedded in SF/PVA composite film at room temperature. The antibacterial, swelling, blood clotting, hemocompatibility and cell viability properties of the prepared composite films were evaluated. The composite film should be enhanced swelling, blood clotting, antibacterial and cell viability study. The cytotoxicity study using fibroblasts L929 cells showed that this composite film was nontoxic. The findings suggest that the ZnO NPs embedded composite films could be used as antimicrobial wound dressing applications [145].
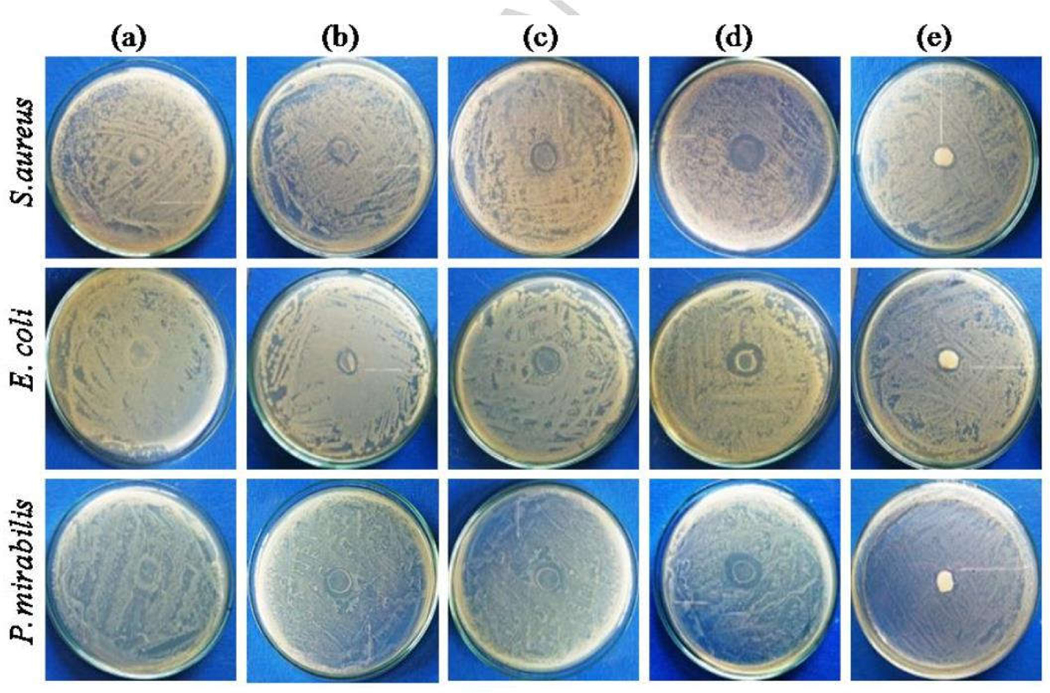
Patil and Bohara et al have reported a novel approach in which a hybrid chitosan-ZnO NPs was coated on the SF-PVA composite. The coating was done by sonochemical technique. This technique successfully ensured, the uniform distribution of the ~ 13 nm sized hybrid chitosan ZnO NPs on the surface of film [146]. The film has shown enhanced antibacterial activity against Gram-positive as well as Gram-negative bacteria with respect to commercial controls [Fig.7]. The prepared film has also shown elegant biocompatibility against L929 fibroblast cells line [146]. Table 1 shows the different SF based dressing materials in variety of forms along with their highlights.
Fig.7.
Antibacterial activity of (a) SF-PVA, (b) 0.2 wt%, (c) 0.5 wt%, (d) 1 wt% C@ZnO NPs coated SF-PVA composite films and (e) Sterizone dressing [146] Reproduced after copy right permission
Table 1:
The different SF based dressing materials in variety of forms along with their highlights.
| No. | Matrix | Name of materials | Highlights | References |
|---|---|---|---|---|
| 1. | Sponges | SF/ Alginate | Re-epithelialization via rapid proliferation of epithelial cell and collagen deposition via fibroblast proliferation. | Dae-Hyun et al 2006 |
| SF/ HTCC/PVA | Studied for chronic wound, composite sponge performed better results than commercially available oil containing non woven fabrics. | Xiaomeng et.al 2014 | ||
| SF/Carboxyethyl chitosan/ AG nanoparticles | Higher antimicrobial activity against S. aureus and P. aeruginosa then AQUACEL Ag. | Pei et.al 2015 | ||
| 2. | Hydrogels | SF/Calcium alginate/Carboxyethyl Chitosan |
Excellent cell adhesion and biocompatibility for the treatment of second-degree burns, faster cure. | Woo et al. 2014 |
| SF/Chitosan/L-Proline | Improved cell attachment, proliferation when applied in chronic wound like diabetic, burn wounds. | Thangavel et al 2017 | ||
| SF/Polyurethane | Excellent swelling, deswelling behavior and good mechanical property. | Huang et al 2013 | ||
| Curcumin loaded SF gel | Improved the wound healing activity. | Karahaliloglu et al 2018 | ||
| 3. | Nanofibrous matrices and Scaffolds | SF/Manuka honey/PEO | In vivo wound healing assay observed excellent biocompatibility and enhanced wound healing process. | Yang et al. 2017 |
| SF/Olive leaf extract/ hyluronic acid | Nontoxic and good candidates for wound dressings | Guldmet et al 2016. | ||
| SF/Vitamin E/PVA/Alovera | Antibacterial and potential dressing for treating skin wounds | Shadai et al 2018 | ||
| SF/Ag NPs | Antibacterial | Minsung et al 2007 | ||
| SF/TiO2 | Antibacterial activity demonstrated against E coli bacteria under UV light | Win et al 2012 | ||
| SF/Chitosan | Antibacterial, biocompatible | Cai et al 2010 | ||
| SF/Amniotic 3 D bilayered artificial skin scaffold | Promoted the remodeling of an extracellular matrix healing of 3 rd degree burn injuries. | Mazaher et al 2018 | ||
| SF/Chitin/Ag NPs Scaffold |
Observed good antibacterial activity, biodegradability, mechanical property, cytocompatibility, proliferation, and cell attachment with nHFFF2 cells. | Mojtaba et al 2018 | ||
| 4. | Microparticle s and NPs | SF NPs based hydrocolloid dressing | Structural stability, improved water uptake and swelling ratio and increased cell growth rate compared to commercially available dressing, reduce burn size and accelerate growth of collagen fibers compared to Neoderm dressing | Lee et al 2016 |
| Insulin functionalized SF | Chronic wound showed wound closure, collage deposition and vascularization | Lee et al 2015 | ||
| 5. | Composite films | SF/Chitosan | Good oxygen and water vapour permeability, haemostatic activity, biodegradability. | Kweon et al 2001 |
| SF/glucose | High absorption capacity, good flexibility and biocompatibility. | Haeyong et al 2001 | ||
| SF/Chitosan/alginate dialdehyde | Enhanced the stability and promote cell proliferation | Gue et al 2013 | ||
| Growth factor functionalized SF | Enhanced macrophage adhesion and higher wound healing capacity | Bienert et al 2017 | ||
| PVA reinforced SF | Biodegradability, degree of crystallinity, mechanical property, thermal property was improved | Sheik et al 2014 | ||
| Recombinant spider SF/PVA | Porosity, promoting wound healing when it applied for Sprague Dawley rat model | Zhuo et al 2017 | ||
| Asymmetric wettable chitosan SF/Ag NPs composite film | High porosity, moisture retention capacity, appropriate mechanical stiffness, antibacterial activity and highly biocompatible | Liu et al 2017 | ||
| Epidermal growth factor functionalized SF film | Increased wound healing rate, re-epithelialization, cell proliferation, collagen synthesis and reduce scar formation compared to Tegaderm tape (3M) | Gil et al 2013 | ||
| SF/PVA/ZnO NPs | Enhanced antibacterial activity, swelling, blood clotting, cell viability study than control SF/PVA composite film | Patil et al 2018 | ||
| Hybrid chitosan ZnO NPs coated SF/PVA | Improved mechanical property, swelling ability, porosity, antibacterial activity, biocompatibility the Sterizone market wound dressings | Patil et al 2018 |
6. Comparison of Silk Fibroin-based dressings with other natural polymer materials
Various dressing materials are available on the market based on natural biopolymers such as collagen, elastin and SF, etc. Most of these collagen dressings are used to support cell activity, differentiation and migration. Due to various inherent problems associated with collagen, such as the ability to activate immune responses, transfer diseases to the host tissue, complicated synthesis process, poor mechanical properties restrict the use of wound dressings. Elastin is natural biopolymers. Due to its elasticity property it is used in commercial dressings. The highly cross-linked structure of elastin hinders its process ability and decreases its solubility. Therefore, its limited supplies because of it originate from biotechnology derived sources, whereas SF has high solubility in aqueous salt solutions and easily processed into various structures. Additionally, its beneficial characteristics such as abundant supplies, high water absorption capacity, coast effectiveness and high flexibility. Due to excellent biocompatibility, mechanical properties SF based dressings is used appropriate support for complete of healing of wound. In addition, the SF wound dressing materials have been modified with nanotechnology for their added advantages like Photoinduced cleaning, enhancing antibacterial activity, strengthening mechanical property and extending durability etc. This makes nanotechnology enabled SF-based film to be more promising in the future [147].
7. Conclusions
SF has amazing natural materials for fabrication of different kinds of materials and with a great potential for various biomedical applications. Wound healing is an intricate process involving skin repairs itself after injury. SF is excellent dressing materials for wound healing purpose are well studied in the literature. In this review, we focus the recent advances of SF based materials such as sponges, hydrogels, SF micro and NPs, nanofibrous matrices, scaffolds and composite films in the wound dressings. The sponges based on SF have the properties of higher porosity, greater mechanical strength, the rapid proliferation of epithelial cell and collagen deposition via fibroblast proliferation. Hydrogels of SF may be considered as an important material for burn dressing due to its adhesive strength and water content. Moreover, SF based hydrogels provide the moist environment in wound interfaces. Nanofibrous matrix and scaffolds of SF improved porosity, oxygen permeability, and mechanical property. Electrospinning is the advanced technique for fabrication of nanofibrous mats and scaffolds. SF microparticles and NPs have shown unique properties such as subcellular size, stability, high carrier capacity, improved swelling ratio and increased cell growth of collagen fibers. To improve the properties of wound healing SF based composite films have been developed by using different types of polymers such as PEG, PVA, alginate, and hyaluronic acid. SF and PVA based composite films showed useful properties like biocompatibility, higher strength and flexibility, gas barrier property and higher swelling capacity. It is strongly proved that the nanotechnology enabled SF composite films are excellent antibacterial activity, mechanical property, non-cytotoxicity, cell attachment property and permeability to water and vapor and it is considered as nanoparticles enabled SF materials could be an excellent material for wound dressing application.
Acknowledgment
RB is supported through the Irish Research Council under the Government of Ireland Postdoctoral fellowship Grant GOIPD/2017/1283. The funding agencies are gratefully acknowledged. Funding for MRR was supplied by the NIH ( P20GM121301 (L. Liaw, PI), U54GM115516 (C. Rosen, PI), and P30GM106391 (R. Friesel, PI)), the Kane Foundation and the American Cancer Society (ACS) (#133077-RSG-19-037-01-LIB) (M.Reagan, PI).
References
- 1.Chen Fa-Ming, and Liu Xiaohua. Progress in polymer science 53 (2016): 86–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abhyankar Suhas Vidyadhar, and Vartak Arvind Madhusudan. Journal of Burn Care & Research 39, no. 1 (2017): 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Yiwei, Beekman Joanneke, Hew Jonathan, Jackson Stuart, Issler-Fisher Andrea C., Parungao Roxanne, Lajevardi Sepher S., Li Zhe, and Maitz Peter Km. Advanced drug delivery reviews 123 (2018): 3–17. [DOI] [PubMed] [Google Scholar]
- 4.Tsalik Ephraim L., Bonomo Robert A., and Fowler Vance G. Jr. Annual review of medicine 69, no. 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mir Mariam, Murtaza Najabat Ali Afifa Barakullah, Gulzar Ayesha, Arshad Munam, Fatima Shizza, and Asad Maliha. Progress in biomaterials (2018): 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrotra Shruti, and Misir Amita. Current pediatric reviews (2018). [Google Scholar]
- 7.Hu Shihao, Bi Shichao, Yan Dong, Zhou Zhongzheng, Sun Guohui, Cheng Xiaojie, and Chen Xiguang. Carbohydrate Polymers 184 (2018): 154–163. [DOI] [PubMed] [Google Scholar]
- 8.Nakod Pinaki Sachin. “The Development of a Textile Wound Dressing for Vacuum Assisted Wound Therapy.” (2017). [Google Scholar]
- 9.Singh Ajay Vikram, Gemmate Donato, Kanase Anurag, Pandey Ishan, Misra Vatsala, Kishore Vimal, Jahnke Timotheus, and Bill Joachim. Veins and Lymphatics 7, no. 1 (2018). [Google Scholar]
- 10.Eming SA, Martin P, and Tomic-Canic M, 2014. Science translational medicine, 6(265), pp.265sr6–265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A Lipsky B, Silverman MH, and Joseph WS, 2017, January In Open forum infectious diseases (Vol. 4, No. 1). Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantis JC, and Paredes JA, 2018. In The Diabetic Foot (pp. 281–304). Humana Press, Cham. [Google Scholar]
- 13.Farokhi M, Mottaghitalab F, Fatahi Y, Khademhosseini A, Kaplan DL. Trends in biotechnology. 2018. September 1;36(9):907–2 [DOI] [PubMed] [Google Scholar]
- 14.Schrementi M, Chen L, and DiPietro LA, 2018. In Skin Tissue Models (pp. 255–275). [Google Scholar]
- 15.Singh S, Young A, and McNaught CE, 2017. The physiology of wound healing. Surgery (Oxford), 35(9), pp.473–477. [Google Scholar]
- 16.Aloe C, 2017. Enhancing wound repair with marine natural products and nanoparticles. [Google Scholar]
- 17.Martin P, and Leibovich SJ, 2005. Trends in cell biology, 15(11), pp.599–607. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara N, and Kobayashi K, 2005, Current Drug Targets-Inflammation & Allergy, 4(3), pp.281–286. [DOI] [PubMed] [Google Scholar]
- 19.Velnar T, Bailey T, and Smrkolj V, 2009. Journal of International Medical Research, 37(5), pp.1528–1542. [DOI] [PubMed] [Google Scholar]
- 20.Stadelmann WK, Digenis AG, and Tobin GR, 1998. The American Journal of Surgery, 176(2), pp.26S–38S. [DOI] [PubMed] [Google Scholar]
- 21.schugrat R, Friedrnan A, Zao R, Sen CK, PNAs, wound healing is a function of tissue oxygen tension mathematical model. [Google Scholar]
- 22.Guo SA, and DiPietro LA, 2010. Journal of dental research, 89(3), pp.219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannelli V, Di Gregorio V, Iebba V, Giusto M, Schippa S, Merli M, and Thalheimer U, 2014. World journal of gastroenterology: WJG, 20(45), p.16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, and Mamelak AJ, 2008. Journal of the American Academy of Dermatology, 58(2), pp.185–206. [DOI] [PubMed] [Google Scholar]
- 25.Mayor JM, and Mills JL, 2018. Indian Journal of Vascular and Endovascular Surgery, 5(2), p.83. [Google Scholar]
- 26.Martín-Aspas A, Guerrero-Sánchez FM, García-Colchero F, Rodríguez-Roca S, and Girón-González JA, 2018. Infection and Drug Resistance, 11, p.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerry T, Queen AT, Tersagh I, and Esther E, , 2018. J Clin Case Rep, 8(1083), p.2. [Google Scholar]
- 28.Wróblewska-Krepsztul Jolanta, Rydzkowski Tomasz, Borowski Gabriel, Szczypiński Mieczysław, Klepka Tomasz, and Thakur Vijay Kumar. International Journal of Polymer Analysis and Characterization just-accepted (2018). [Google Scholar]
- 29.Shit Subhas C., and Shah Pathik M. Journal of Polymers 2014 (2014). [Google Scholar]
- 30.Mogoşanu George Dan, and Grumezescu Alexandru Mihai. Handbook of Polymers for Pharmaceutical Technologies: Structure and Chemistry, Volume 1 (2015): 477–519. [Google Scholar]
- 31.El-Sherbiny Ibrahim M., and El-Baz Nancy M. In Eco-friendly Polymer Nanocomposites, pp. 173–208. Springer, New Delhi, 2015. [Google Scholar]
- 32.LogithKumar R, KeshavNarayan A, Dhivya S, Chawla A, Saravanan S, and Selvamurugan N. Carbohydrate polymers 151 (2016): 172–188. [DOI] [PubMed] [Google Scholar]
- 33.Eliasson Ann-Charlotte. In Carbohydrates in Food, Third Edition, pp. 501–600. CRC Press, 2017. [Google Scholar]
- 34.Madhumitha G, Fowsiya J, Mohana Roopan S, and Thakur Vijay Kumar. International Journal of Polymer Analysis and Characterization (2018): 1–15. [Google Scholar]
- 35.Kiiskinen J, Merivaara A, Hakkarainen T. et al. Stem Cell Res Ther 10, 292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kizilel Seda, Alipour Mohammad, and Aydın Derya. In Functional Hydrogels in Drug Delivery, pp. 9–31. CRC Press, 2017. [Google Scholar]
- 37.Anastassiades Tassos. U.S. Patent 9,644,040, issued May 9, 2017.
- 38.Francesko Antonio, Fernandes Margarida M., Rocasalbas Guillem, Gautier Sandrine, and Tzanov Tzanko. In Advanced Polymers in Medicine, pp. 401–431. Springer, Cham, 2015. [Google Scholar]
- 39.Chattopadhyay Sayani, and Raines Ronald T. Biopolymers 101, no. 8 (2014): 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mogoşanu George Dan, and Mihai Alexandru GrumezescuInternational journal of pharmaceutics 463, no. 2 (2014): 127–136. [DOI] [PubMed] [Google Scholar]
- 41.Wang Bin. University of California, San Diego, 2016. [Google Scholar]
- 42.Khorasani Amir Mahyar, Gibson Ian, Goldberg Moshe, Nomani Junior, and Littlefair Guy. Science of Advanced Materials 8, no. 8 (2016): 1491–1511. [Google Scholar]
- 43.Santos Dos, Venina Rosmary Nichele Brandalise, and Savaris Michele. Engineering of Biomaterials. Springer, 2017. [Google Scholar]
- 44.Tiwari Atul, Bajpai AK, Bajpai Jaya, Saini Rajesh Kumar, and Agrawal Priyanka. Smart Biomaterial Devices: Polymers in Biomedical Sciences. CRC Press, 2016. [Google Scholar]
- 45.Humenik Martin, Lang Gregor, and Scheibel Thomas. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology (2018): e1509. [DOI] [PubMed] [Google Scholar]
- 46.Govorushko Sergey. Human-Insect Interactions. CRC Press, 2018. [Google Scholar]
- 47.Qi Yu, Wang Hui, Wei Kai, Yang Ya, Zheng Ru-Yue, Kim Ick Soo, and Zhang Ke-Qin. International journal of molecular sciences 18, no. 3 (2017): 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Craig Catherine L., and Riekel Christian. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 133, no. 4 (2002): 493–507. [DOI] [PubMed] [Google Scholar]
- 49.Altman Gregory H., Diaz Frank, Jakuba Caroline, Calabro Tara, Horan Rebecca L., Chen Jingsong, Lu Helen, Richmond John, and Kaplan David L. Biomaterials 24, no. 3 (2003): 401–416. [DOI] [PubMed] [Google Scholar]
- 50.Drucker B, and Smith SG Nature 165, no. 4188 (1950): 196. [Google Scholar]
- 51.Marsh Richard E., Corey Robert B., and Pauling Linus. Biochimica et Biophysica acta 16 (1955): 1–34. [DOI] [PubMed] [Google Scholar]
- 52.Roh Dae-Hyun, Kang Seuk-Yun, Kim Jeom-Yong, Kwon Young-Bae, Hae Young Kweon Kwang-Gill Lee, Park Young-Hwan et al. Journal of Materials Science: Materials in Medicine 17, no. 6 (2006): 547–552. [DOI] [PubMed] [Google Scholar]
- 53.Thangavel Ponrasu, Ramachandran Balaji, Kannan Ramya, and Muthuvijayan Vignesh. Journal of Biomedical Materials Research Part B: Applied Biomaterials 105, no. 6 (2017): 1401–1408. [DOI] [PubMed] [Google Scholar]
- 54.Kheradvar Shadi Alsadat, Nourmohammadi Jhamak, Tabesh Hadi, and Bagheri Behnam. Colloids and Surfaces B: Biointerfaces 166 (2018): 9–16. [DOI] [PubMed] [Google Scholar]
- 55.Li Chunmei, Vepari Charu, Jin Hyoung-Joon, Kim Hyeon Joo, and Kaplan David L. Biomaterials 27, no. 16 (2006): 3115–3124. [DOI] [PubMed] [Google Scholar]
- 56.Gupta Vishal, Aseh Abraham, Ríos Carmen N., Aggarwal Bharat B., and Anshu B. Mathur. International journal of nanomedicine 4 (2009): 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srivastava Chandra Mohan, Purwar Roli, Kannaujia Rekha, and Sharma Deepak. Fibers and Polymers 16, no. 5 (2015): 1020–1030. [Google Scholar]
- 58.Vepari Charu, and Kaplan David L. Progress in polymer science 32, no. 8–9 (2007): 991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rockwood Danielle N., Preda Rucsanda C., Tuna Yücel Xiaoqin Wang, Lovett Michael L., and Kaplan David L. Nature protocols 6, no. 10 (2011): 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roh Dae-Hyun, Kang Seuk-Yun, Kim Jeom-Yong, Kwon Young-Bae, Hae Young Kweon Kwang-Gill Lee, Park Young-Hwan et al. Journal of Materials Science: Materials in Medicine 17, no. 6 (2006): 547–552. [DOI] [PubMed] [Google Scholar]
- 61.Li Xiaomeng, Li Binghui, Ma Jun, Wang Xiaoyu, and Zhang Shengming. Journal of Bioactive and Compatible Polymers 29, no. 4 (2014): 398–411. [Google Scholar]
- 62.Pei Zejun, Sun Qing, Sun Xin, Wang Yaping, and Zhao Peng. Bio-medical materials and engineering 26, no. s1 (2015): S111–S118. [DOI] [PubMed] [Google Scholar]
- 63.Hoare Todd R., and Kohane Daniel S. Polymer 49, no. 8 (2008): 1993–2007. [Google Scholar]
- 64.Peppas NA, Bures P, Leobandung Ws, and Ichikawa H. European journal of pharmaceutics and biopharmaceutics 50, no. 1 (2000): 27–46. [DOI] [PubMed] [Google Scholar]
- 65.Caló Enrica, and Khutoryanskiy Vitaliy V. European Polymer Journal 65 (2015): 252–267. [Google Scholar]
- 66.Ju Hyung Woo, Lee Ok Joo, Moon Bo Mi, Sheikh Faheem A., Lee Jung Min, Kim Jung-Ho, Park Hyun Jung et al. Tissue Engineering and Regenerative Medicine 11, no. 3 (2014): 203–210. [Google Scholar]
- 67.Thangavel Ponrasu, Ramachandran Balaji, Kannan Ramya, and Muthuvijayan Vignesh. Journal of Biomedical Materials Research Part B: Applied Biomaterials 105, no. 6 (2017): 1401–1408. [DOI] [PubMed] [Google Scholar]
- 68.Huang Yiping, Zhang Baoping, Xu Gewen, and Hao Wentao. Composites Science and Technology 84 (2013): 15–22. [Google Scholar]
- 69.Boateng Joshua S., Matthews Kerr H., Stevens Howard NE, and Eccleston Gillian M. Journal of pharmaceutical sciences 97, no. 8 (2008): 2892–2923. [DOI] [PubMed] [Google Scholar]
- 70.Corkhill Philip H., Hamilton Colin J., and Tighe Brian J. Biomaterials 10, no. 1 (1989): 3–10. [DOI] [PubMed] [Google Scholar]
- 71.Kapoor Sonia, and Kundu Subhas C. Acta biomaterialia 31 (2016): 17–32. [DOI] [PubMed] [Google Scholar]
- 72.Karahaliloğlu Zeynep. Materials Technology 33, no. 4 (2018): 276–287. [Google Scholar]
- 73.Pham Quynh P., Sharma Upma, and Mikos Antonios G. Biomacromolecules 7, no. 10 (2006): 2796–2805. [DOI] [PubMed] [Google Scholar]
- 74.Daniele Michael A., Boyd Darryl A., Adams André A., and Ligler Frances S. Advanced healthcare materials 4, no. 1 (2015): 11–28. [DOI] [PubMed] [Google Scholar]
- 75.Kim Jeong In, and Kim Cheol Sang. “Nanoscale resolution 3D printing with pin-modified electrified inkjets for tailorable nano/macro hybrid constructs for tissue engineering.” ACS applied materials & interfaces (2018). [DOI] [PubMed] [Google Scholar]
- 76.Smith LA, and Ma PX Colloids and surfaces B: biointerfaces 39, no. 3 (2004): 125–131. [DOI] [PubMed] [Google Scholar]
- 77.Bhardwaj Nandana, and Kundu Subhas C. Biotechnology advances 28, no. 3 (2010): 325–347. [DOI] [PubMed] [Google Scholar]
- 78.Cheng Liao‐Ping, Lin Dar‐Jong, Shih Chien‐Hsieh, Dwan An‐Hwa, and Gryte Carl C. Journal of Polymer Science Part B: Polymer Physics 37, no. 16 (1999): 2079–2092. [Google Scholar]
- 79.Lu Qiang, Zhu Hesun, Zhang Cencen, Zhang Feng, Zhang Bing, and Kaplan David L. Biomacromolecules 13, no. 3 (2012): 826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Xiaohui, Reagan Michaela R., and Kaplan David L., Advanced drug delivery reviews 61, no. 12 (2009): 988–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martins A, Reis RL, and Neves NM International Materials Reviews 53, no. 5 (2008): 257–274. [Google Scholar]
- 82.Amiraliyan Nasim, Nouri Mahdi, and Kish Mohammad Haghighat. Fibers and Polymers 10, no. 2 (2009): 167–176. [Google Scholar]
- 83.Yang Xingxing, Fan Linpeng, Ma Linlin, Wang Yunyi, Lin Si, Yu Fan, Pan Xiaohan, Luo Gejie, Zhang Dongdong, and Wang Hongsheng. Materials & Design 119 (2017): 76–84. [Google Scholar]
- 84.Basal G, Tetik GD, Kurkcu G, Bayraktar O, Gurhan ID, and Atabey A. Digest Journal of Nanomaterials & Biostructures (DJNB) 11, no. 4 (2016). [Google Scholar]
- 85.Kheradvar Shadi Alsadat, Nourmohammadi Jhamak, Tabesh Hadi, and Bagheri Behnam. Colloids and Surfaces B: Biointerfaces 166 (2018): 9–16. [DOI] [PubMed] [Google Scholar]
- 86.Kang Minsung, Jung Rira, Kim Hun-Sik, Youk Ji Ho, and Jin Hyoung-Joon. Journal of nanoscience and nanotechnology 7, no. 11 (2007): 3888–3891. [DOI] [PubMed] [Google Scholar]
- 87.Jao Win‐Chun, Yang Ming‐Chien, Lin Chien‐Hong, and Hsu Chi‐Chuan. Polymers for Advanced Technologies 23, no. 7 (2012): 1066–1076. [Google Scholar]
- 88.Ye Xinguo, Li Sheng, Chen Xuanxuan, Zhan Yingfei, and Li Xiaonan. International journal of biological macromolecules 94 (2017): 492–499. [DOI] [PubMed] [Google Scholar]
- 89.Cai Zeng-xiao, Mo Xiu-mei, Zhang Kui-hua, Fan Lin-peng, Yin An-lin, He Chuang-long, and Wang Hong-sheng. International journal of molecular sciences 11, no. 9 (2010): 3529–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang X, Reagan MR, Kaplan DL. Advanced drug delivery reviews. 5;61(12):2009: 988–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Yun, Bella Erika, Lee Christopher SD, Migliaresi Claudio, Pelcastre Linda, Schwartz Zvi, Boyan Barbara D., and Motta Antonella. Biomaterials 31, no. 17 (2010): 4672–4681. [DOI] [PubMed] [Google Scholar]
- 92.Kim Ung-Jin, Park Jaehyung, Kim Hyeon Joo, Wada Masahisa, and Kaplan David L. Biomaterials 26, no. 15 (2005): 2775–2785. [DOI] [PubMed] [Google Scholar]
- 93.Gholipourmalekabadi Mazaher, Samadikuchaksaraei Ali, Seifalian Alexander M., Urbanska Aleksandra M., Ghanbarian Hossein, Hardy John G., Omrani Mir Davood, Mozafari Masoud, Reis Rui L., and Kundu Subhas C. Biomedical Materials 13, no. 3 (2018): 035003. [DOI] [PubMed] [Google Scholar]
- 94.Mehrabani Mojtaba Ghanbari, Karimian Ramin, Mehramouz Bahareh, Rahimi Mahdi, and Kafil Hossein Samadi. International journal of biological macromolecules 114 (2018): 961–971. [DOI] [PubMed] [Google Scholar]
- 95.Wang Yu‐Chi, Bai Meng‐Yi, Hsu Wan‐Yuan, and Yu Mu‐Hsien. Journal of Biomedical Materials Research Part A 106, no. 1 (2018): 221–230. [DOI] [PubMed] [Google Scholar]
- 96.Cao Zhengbing, Chen Xin, Yao Jinrong, Huang Lei, and Shao Zhengzhong. Soft Matter 3, no. 7 (2007): 910–915. [DOI] [PubMed] [Google Scholar]
- 97.Wang Lu, Zhen Ran Xia Lin Lin Lv, Tang Qiang, and Li Ming Zhong. In Advanced Materials Research, vol. 236, pp. 1902–1905. Trans Tech Publications, 2011. [Google Scholar]
- 98.Kundu Joydip, Chung Yong-Il, Young Ha Kim Giyoong Tae, and Kundu SC International journal of pharmaceutics 388, no. 1–2 (2010): 242–250. [DOI] [PubMed] [Google Scholar]
- 99.Hino Tomoaki, Tanimoto Masao, and Shimabayashi Saburo. Journal of colloid and interface science 266, no. 1 (2003): 68–73. [DOI] [PubMed] [Google Scholar]
- 100.Wenk Esther, Wandrey Anne J., Merkle Hans P., and Meinel Lorenz. Journal of Controlled Release 132, no. 1 (2008): 26–34. [DOI] [PubMed] [Google Scholar]
- 101.Rajkhowa Rangam, Wang Lijing, and Wang Xungai. Powder Technology 185, no. 1 (2008): 87–95. [Google Scholar]
- 102.Zhao Zheng, Li Yi, and Xie Mao-Bin. International journal of molecular sciences 16, no. 3 (2015): 4880–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Xiaoqin, Yucel Tuna, Lu Qiang, Hu Xiao, and Kaplan David L. Biomaterials 31, no. 6 (2010): 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lammel Andreas S., Hu Xiao, Park Sang-Hyug, Kaplan David L., and Scheibel Thomas R. Biomaterials 31, no. 16 (2010): 4583–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee Ok Joo, Kim Jung-Ho, Bo Mi Moon Janet Ren Chao, Yoon Jaeho, Ju Hyung Woo, Lee Jung Min et al. Tissue Engineering and Regenerative Medicine 13, no. 3 (2016): 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaviyarasu K, Maria Magdalane C, Kanimozhi K, Kennedy J, Siddhardha B, Subba Reddy E, Rotte Naresh Kumar et al. Journal of Photochemistry and Photobiology B: Biology 173 (2017): 466–475. [DOI] [PubMed] [Google Scholar]
- 107.Hong Xiaodong, Zhang Binbin, Murphy Elizabeth, Zou Jianli, and Kim Franklin. Journal of Power Sources 343 (2017): 60–66. [Google Scholar]
- 108.Russell Jennifer L., Noel Grace H., Warren Joseph M., Tran Ngoc-Lan L., and Mallouk Thomas E. Langmuir 33, no. 39 (2017): 10366–10373. [DOI] [PubMed] [Google Scholar]
- 109.Yoshioka Taiyo, Hata Tamako, Kojima Katsura, Nakazawa Yasumoto, and Kameda Tsunenori. ACS Biomaterials Science & Engineering 3, no. 12 (2017): 3207–3214. [DOI] [PubMed] [Google Scholar]
- 110.Kweon Haeyong, Ha Hyun Chul, Um In Chul, and Park Young Hwan. Journal of applied polymer science 80, no. 7 (2001): 928–934. [Google Scholar]
- 111.Gu Zhipeng, Xie HuiXu, Huang Chengcheng, Li Li, and Yu Xixun. International journal of biological macromolecules 58 (2013): 121–126. [DOI] [PubMed] [Google Scholar]
- 112.Bienert M, Hoss M, Bartneck M, Weinandy S, Böbel M, Jockenhövel S, Knüchel R. et al. Biomedical Materials 12, no. 4 (2017): 045023. [DOI] [PubMed] [Google Scholar]
- 113.Liu Tie-lian, Miao Jing-cheng, Sheng Wei-hua, Xie Yu-feng, Huang Quan, Shan Yun-bo, and Yang Ji-cheng. Journal of Zhejiang University SCIENCE B 11, no. 1 (2010): 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kamoun Elbadawy A., Kenawy El-Refaie S., and Chen Xin. Journal of advanced research 8, no. 3 (2017): 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kamoun Elbadawy A., Chen Xin, Mohy Eldin Mohamed S., and Kenawy El-Refaie S. Arabian Journal of chemistry 8, no. 1 (2015): 1–14. [Google Scholar]
- 116.Chouhan Dimple, Chakraborty Bijayshree, Nandi Samit K., and Mandal Biman B. Acta biomaterialia 48 (2017): 157–174. [DOI] [PubMed] [Google Scholar]
- 117.Sheik Sareen, Nagaraja GK, Naik Jagadish, and Bhajanthri RF International Journal of Plastics Technology 21, no. 1 (2017): 108–122. [Google Scholar]
- 118.Singha AS, and Kapoor Himanshu. International Journal of Polymer Analysis and Characterization 19, no. 3 (2014): 212–221. [Google Scholar]
- 119.Chouhan Dimple, Janani G, Chakraborty Bijayashree, Nandi Samit K., and Mandal Biman B. Journal of tissue engineering and regenerative medicine 12, no. 3 (2018): e1559–e1570. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Liang, Chen Denglong, Yao Qinghua, and LiInternational Min journal of nanomedicine 12 (2017): 8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Yingshan, Yang Hongjun, Liu Xin, Mao Jun, Gu Shaojin, and Xu Weilin. International journal of biological macromolecules 53 (2013): 88–92. [DOI] [PubMed] [Google Scholar]
- 122.Rai Mahendra, Yadav Alka, and Gade Aniket Biotechnology advances 27, no. 1 (2009): 76–83. [DOI] [PubMed] [Google Scholar]
- 123.Khampieng Thitikan, Wongkittithavorn Supisara, Chaiarwut Sonthaya, Ekabutr Pongpol, Pavasant Prasit, and Supaphol Pitt. Journal of Drug Delivery Science and Technology 44 (2018): 91–100. [Google Scholar]
- 124.Lu Zhisong, Xiao Jing, Wang Ying, and Meng Mei. Journal of colloid and interface science 452 (2015): 8–14. [DOI] [PubMed] [Google Scholar]
- 125.Babu Punuri Jayasekhar, Doble Mukesh, and Raichur Ashok M. Journal of colloid and interface science 513 (2018): 62–71. [DOI] [PubMed] [Google Scholar]
- 126.Liu Jinglong, Qian Zhiyong, Shi Quan, Yang Shuo, Wang Qianxin, Liu Bo, Xu Juan, Guo Ximin, and Liu Haifeng RSC Advances 7, no. 69 (2017): 43909–43920. [Google Scholar]
- 127.Gil Eun Seok, Panilaitis Bruce, Bellas Evangelia, and Kaplan David L. Advanced healthcare materials 2, no. 1 (2013): 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mehrabani Mojtaba Ghanbari, Karimian Ramin, Mehramouz Bahareh, Rahimi Mahdi, and Kafil Hossein Samadi. International journal of biological macromolecules 114 (2018): 961–971. [DOI] [PubMed] [Google Scholar]
- 129.Yu Kun, Lu Fei, Li Qing, Chen Honglei, Lu Bitao, Liu Jiawei, Li Zhiquan, Dai Fangying, Wu Dayang, and Lan Guangqian, Scientific Reports 7, no. 1 (2017): 2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Uttayarat Pimpon, Jetawattana Suwimol, Suwanmala Phiriyatorn, Eamsiri Jarurattana, Tangthong Theeranan, and Pongpat Suchada. Fibers and Polymers 13, no. 8 (2012): 999–1006. [Google Scholar]
- 131.Calamak Semih, Eda Ayse Aksoy Ceren Erdogdu, Sagıroglu Meral, and Ulubayram Kezban. Journal of Nanoparticle Research 17, no. 2 (2015): 87. [Google Scholar]
- 132.Kumar Sathish Sundar Dhilip, Naresh Kumar Rajendran Nicolette Nadene Houreld, and Abrahamse Heidi. International journal of biological macromolecules (2018). [DOI] [PubMed] [Google Scholar]
- 133.Salahuddin Nehal, El‐Kemary Maged, and Ibrahim Ebtisam. International Journal of Applied Ceramic Technology 15, no. 2 (2018): 448–459. [Google Scholar]
- 134.Shalumon KT, Anulekha KH, Nair Sreeja V., Nair SV, Chennazhi KP, and Jayakumar R. International journal of biological macromolecules 49, no. 3 (2011): 247–254. [DOI] [PubMed] [Google Scholar]
- 135.Sudheesh Kumar PT, Vinoth-Kumar Lakshmanan TV Anilkumar C. Ramya, Reshmi P, Unnikrishnan AG, Nair Shantikumar V., and Jayakumar R. ACS applied materials & interfaces 4, no. 5 (2012): 2618–2629. [DOI] [PubMed] [Google Scholar]
- 136.Liu He, Wang Chenyu, Li Chen, Qin Yanguo, Wang Zhonghan, Yang Fan, Li Zuhao, and Wang Jincheng. RSC Advances 8, no. 14 (2018): 7533–7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Xiaocheng, Chang Jiang, and Wu Chengtie. Applied Materials Today 11 (2018): 308–319. [Google Scholar]
- 138.Khorasani Mohammad Taghi, Joorabloo Alireza, Moghaddam Armaghan, Shamsi Hamidreza, and MansooriMoghadam Zohreh. International journal of biological macromolecules (2018). [DOI] [PubMed] [Google Scholar]
- 139.Liu Yangshuo, and Kim Hyung-Il. Carbohydrate polymers 89, no. 1 (2012): 111–116. [DOI] [PubMed] [Google Scholar]
- 140.Zhai Mingcui, Xu Yichen, Zhou Biao, and Jing Weibin. Journal of Photochemistry and Photobiology B: Biology 180 (2018): 253–258. [DOI] [PubMed] [Google Scholar]
- 141.Shalumon KT, Anulekha KH, Sreeja V. Nair SV Nair KP Chennazhi, and Jayakumar R. International journal of biological macromolecules 49, no. 3 (2011): 247–254. [DOI] [PubMed] [Google Scholar]
- 142.Kumar PT, Lakshmanan Vinoth-Kumar, Biswas Raja, Nair Shantikumar V., and Jayakumar R. Journal of biomedical nanotechnology 8, no. 6 (2012): 891–900. [DOI] [PubMed] [Google Scholar]
- 143.Singh Ajey, Singh NB, Afzal Shadma, Singh Tanu, and Hussain Imtiyaz. Journal of Materials Science 53, no. 1 (2018): 185–201. [Google Scholar]
- 144.Meshram JV, Koli VB, Phadatare MR, and Pawar SH Materials Science and Engineering: C 73 (2017): 257–266. [DOI] [PubMed] [Google Scholar]
- 145.Patil PP, Meshram JV, Bohara RA, Nanaware SG and Pawar SH, NJC, RSC 2018 [DOI] [PubMed] [Google Scholar]
- 146.Patil PP, Bohara RA, Meshram JV, Nanaware SG and Pawar SH, Inter, Jour. Of Bio. Macro. 2018. [DOI] [PubMed] [Google Scholar]
- 147.Faokhi M, Mottaghitalab F, Fatahi Y, Khademhosseini A, and Kaplan DL, Trends in biotechnology, (2018), 1642. [DOI] [PubMed] [Google Scholar]