Abstract
OBJECTIVE
Cardiovascular disease (CVD) mortality has declined substantially in the U.S. The aims of this study were to examine trends and demographic disparities in mortality due to CVD and CVD subtypes among adults with and without self-reported diabetes.
RESEARCH DESIGN AND METHODS
We used the National Health Interview Survey (NHIS) (1985–2014) with mortality follow-up data through the end of 2015 to estimate nationally representative trends and disparities in major CVD, ischemic heart disease (IHD), stroke, heart failure, and arrhythmia mortality among adults ≥20 years of age by diabetes status.
RESULTS
Over a mean follow-up period of 11.8 years from 1988 to 2015 of 677,051 adults, there were significant decreases in major CVD death (all P values <0.05) in adults with and without diabetes except adults 20–54 years of age. Among adults with diabetes, 10-year relative changes in mortality were significant for major CVD (−32.7%[95%CI −37.2, −27.9]), IHD(−40.3%[−44.7, −35.6]), and stroke (−29.2% [−40.0, −16.5]), but not heart failure (−0.5% [−20.7, 24.7]), and arrhythmia (−12.0% [−29.4, 77.5]); the absolute decrease of major CVD among adults with diabetes was higher than among adults without diabetes (P < 0.001). Men with diabetes had larger decreases in CVD death than women with diabetes (P < 0.001).
CONCLUSIONS
Major CVD mortality in adults with diabetes has declined, especially in men. Large reductions were observed for IHD and stroke mortality, although heart failure and arrhythmia deaths did not change. All race and education groups benefitted to a similar degree, but significant gaps remained across groups.
Cardiovascular disease (CVD) is the leading cause of death and morbidity in U.S. adults; compared with the population without diabetes, persons with diabetes have about twice the risk of CVD mortality (1). In recent decades, however, CVD mortality decreased nationally among persons both with and without diabetes in Sweden (2), Australia (3), and the U.S. (4). However, CVD is a diverse set of conditions and the pathophysiology of CVD has been shown to differ according to diabetes status (5-7). Additionally, there are disparities in diabetes and CVD outcomes across sociodemographic groups in the U.S. (8), and declines in death rates from coronary heart disease from 1979 to 2011 were most pronounced in adults ≥65 years of age (9). It is unclear whether recent improvements in CVD have accentuated or diminished these sociodemographic disparities, and whether similar patterns are also present in people with diabetes.
Although many U.S. national mortality surveillance reports have been based on the registration of deaths, it is impossible to calculate national mortality among persons with or without diabetes using vital registration records without a decedent’s diabetes status information. The nationally representative National Health Interview Survey (NHIS) mortality follow-up data provide a unique opportunity to concurrently examine nationally representative trends in annual mortality rates between people with and without diabetes (10). In this study, we used data from NHIS participants over the period from 1985 to 2014 with mortality linkage up to 2015 to address the following questions among U.S. adults by diabetes status: 1) whether trends in mortality from major CVD and CVD subtypes have decreased and 2) whether sociodemographic disparities in mortality from CVD and CVD subtypes among different age, sex, race/ethnicity, and education groups have narrowed.
RESEARCH DESIGN AND METHODS
Data Source
The NHIS is an annual, in-person, interviewer-administered household survey of the health status and behaviors of the U.S. noninstitutionalized population. The survey uses a multistage, probability-sampling approach to select >30,000 U.S. adults each year. The annual response rate of NHIS is ~80% of the eligible households in the sample (11).
This study included all sampled adults who participated in the diabetes survey module in the NHIS from 1985 to 2014 who were ≥18 years of age with mortality follow-up. In addition, demographic data (age, sex, and race/ethnicity) and diabetes status of the NHIS 2015 without mortality follow-up were used to provide the population structure and sampling weights for poststratification reweighting for those sampled adults with mortality follow-up beyond 2014 (10). To describe the demographic characteristics of the samples, the surveys were aggregated into the following six periods: 1985–1989, 1990–1994, 1995–1999, 2000–2004, 2005–2009, and 2010–2014.
NHIS 1985–2014 baseline surveys were linked with the restricted-use version of the NHIS linked to the National Death Index File. Participants were followed from the date of interview up to the date of death or 31 December 2015, whichever came first. An estimated 96% of baseline participants had the eligible mortality follow-up information. The National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention used poststratification reweighting based on the U.S. population to account for ineligible follow-up after year 1997.
To minimize potential selection biases at baseline caused by other factors such as immobility, aging, poor health, or pre-existing conditions at baseline, all follow-up times of participants were excluded up to the end of the second year of follow-up as a washout period (10). Therefore, trends and disparities in CVD mortality were estimated only for 1988–2015 and adults ≥20 years of age at follow-up. Since diabetes status was assessed only at baseline, adults without diabetes at baseline could become incident case patients during the follow-up. To minimize the bias with sufficient statistical power, the maximum follow-up duration was 10 years.
Diabetes Status and Duration
The content of the survey has been updated about every 10 years. From 1980 to 1996, only a subsample of participants (one of every six) was asked whether in the past 12 months they or any family member had diabetes. Adults were classified as having prevalent self-reported diagnosed diabetes (hereafter termed “diabetes”) if they answered “yes” to the question, “During the past 12 months, did you have diabetes?” Starting from 1997, all sampled adults were asked whether a health professional had ever told them they had diabetes. Participants were classified as having prevalent diabetes if they answered “yes” to the question, “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” for women, or “Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” for men. Participants ≥18 years of age with diagnosed diabetes were asked “How old were you when a doctor first told you that you had diabetes or sugar diabetes?” (i.e., age at diagnosis of diabetes) (12). Therefore, the duration of diabetes was calculated from their age at diagnosis and their age at the time of the interview.
Mortality Outcomes
The coding of underlying causes of death prior to 1999 used the ICD-9 guidelines, whereas the coding of causes during 1999 and onward used the ICD-10 guidelines. We used the grouped codes for causes of death in major CVD death (ICD-9 codes 390–448 or ICD-10 codes I00–I78), and four subtypes of CVD death including deaths due to ischemic heart disease (IHD) (ICD-9 codes 410–414 or ICD-10 codes I20-I25): cerebrovascular diseases (stroke, ICD-9 codes 430–434, 436–438; or ICD-10 codes I60-I69); heart failure (ICD-9 code 428 or ICD-10 code I50); and arrhythmia (ICD-9 code 426 or 427 [excluding 427.5]; or ICD-10 codes I44-I45 or I48-I49) (13).
Data collection for NHIS was approved by the NCHS Research Ethics Review Board. Analysis of deidentified data from the survey is exempt from the federal regulations for the protection of human research participants. Analysis of restricted data through the NCHS Research Data Center was also approved by the NCHS Ethics Review Board.
Other Covariates
To describe baseline characteristics, we used sex, age at baseline (in years) grouped as 18–54, 55–64, and ≥65, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanics, and others). Education was used as an indicator of social position and presented as the following three categories: less than high school, high school, and more than high school. In trend and disparity analysis, to increase the sample sizes of lower education in the later years with short follow-up time, education level was limited to the following two categories: high school completed or less and more than high school.
To calculate yearly and period-specific death rates, we divided the continuous time-to-event survival data into discrete survival years from the date at interview to the date of death or censoring on 31 December 2015. We used year at follow-up, age at follow-up, and status of event (alive or dead) at follow-up as time-varying variables. For modeling age-adjusted estimates of death rates, age at follow-up was treated as a continuous variable. For age stratification, age at follow-up was divided into the following three strata: 20–54, 55–64, and ≥65 years of age. Depending on the purposes of the analyses, discrete years during the follow-up period were treated as either categorical (1988–1994, 1995–1999, 2000–2004, 2005–2009, and 2010–2015) for tables or continuous for modeling annual change.
Statistical Analysis
To describe baseline characteristics from 1985 to 2014, we used means (with SEs) for continuous variables and proportions (with SEs) for categorical variables. For categorical variables, we used Pearson’s χ2 tests to compare baseline characteristics across survey periods. Linear regression was used to estimate means and test differences between means.
We used discrete Poisson regression to model mortality. We modeled the trend of rates with quadratic terms for calendar year and age. Average marginal prediction was used to estimate crude and adjusted mortality. To adjust for the fact that cohorts from the baseline surveys are older by calendar year, we poststratified the overlapped cohort sample weights at each follow-up calendar year using the U.S. population structure of that specific year (10). Both absolute (10-year absolute change) and relative (10-year percentage change) terms were used to examine trends in mortality. We used a 10-year derivative and derivative as an elasticity approach to estimate average absolute change and mortality rate ratio (RR). The 10-year percentage change is calculated using (RR − 1) × 100.
Quadratic and cubic terms of year at follow-up were evaluated but were not statistically significant, and were excluded from the final models. We used the interaction term of diabetes status and periods in models to examine potential differences in mortality decline by diabetes status. The delta method was used to compute the SE and CI for difference or the ratio of independent estimates. In a sensitivity analysis, to examine whether mortality patterns changed because of a potentially earlier diagnosis of diabetes in later years, we reclassified persons with diabetes in the first 2, 3, 4, and 5 years of follow-up as not having diabetes over the four baseline survey periods (1997–1999, 2000–2004, 2005–2009, 2010–2014), respectively.
We used Stata version 15.1 (StataCorp LP, College Station, TX) to manage and analyze data accounting for the complex multistage sampling design and to produce weighted estimates and 95% CIs and total case estimates. For comparisons of estimates, we used a two-sided t test with significance defined as P < 0.05. We used R (ggplot2 package: http://ggplot2.org/) for data visualization.
RESULTS
In the baseline surveys from 1985 to 2014, the final analytical unweighted sample sizes (representing annual average U.S. adult population in millions [M]) during each period were 59,869 (172 M), 69,185 (181 M), 113,323 (193 M), 148,418 (208 M), 123,430 (223 M), and 162,835 (235 M), respectively, in 1985–1989, 1990–1994, 1995–1999, 2000–2004, 2005–2009, and 2010–2014. Table 1 describes the baseline characteristics and the prevalence and duration of diabetes by time period from 1985 to 2014 at baseline survey. Across the time periods, the U.S. adult population became older and more racially diverse, improved in education level, and had higher diabetes prevalence.
Table 1—
Characteristics at baseline of the population aged 18 years or older by time period, NHIS 1985–2014
| 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 | |
|---|---|---|---|---|---|---|
| Total sample, n | 59,869 | 69,185 | 113,323 | 148,418 | 123,430 | 162,835 |
| Represented population, N | 171.7 M | 181.4 M | 193.2 M | 207.8 M | 222.6 M | 234.5 M |
| Adults with diabetes, n | 2,293 | 2,850 | 6,524 | 10,665 | 11,122 | 16,745 |
| Represented diabetes, N | 6.2 M | 7.1 M | 9.5 M | 13.7 M | 18.1 M | 21.5 M |
| Prevalent diabetes, % (SE) | 3.6 (0.1) | 3.9 (0.1) | 4.9 (0.1) | 6.6 (0.1) | 8.1 (0.1) | 9.2 (0.1) |
| Diabetes duration (years) among adults with diabetes, % (SE) | ||||||
| ≤1.0 | 10.4 (0.7) | 9.7 (0.6) | 13.6 (0.5) | 16.5 (0.4) | 15.8 (0.5) | 12.9 (0.3) |
| 1.1–5.0 | 27.6 (1.1) | 28.0 (0.9) | 27.2 (0.7) | 27.7 (0.5) | 26.7 (0.5) | 23.5 (0.4) |
| >5.0 | 62.0 (1.2) | 62.2 (1.0) | 59.2 (0.8) | 55.8 (0.5) | 57.5 (0.6) | 63.6 (0.5) |
| Age (years), mean (SE) | 43.5 (0.2) | 44.2 (0.1) | 44.7 (0.1) | 45.3 (0.1) | 45.9 (0.1) | 46.7 (0.1) |
| 20–54 | 71.5 (0.4) | 72.0 (0.3) | 72.5 (0.3) | 71.4 (0.2) | 69.1 (0.2) | 66.0 (0.2) |
| 55–64 | 12.4 (0.2) | 11.4 (0.2) | 11.1 (0.1) | 12.5 (0.1) | 14.6 (0.1) | 16.2 (0.1) |
| 65 or older | 16.1 (0.3) | 16.6 (0.3) | 16.4 (0.2) | 16.1 (0.2) | 16.3 (0.2) | 17.8 (0.2) |
| Women, % (SE) | 52.6 (0.1) | 52.3 (0.2) | 52.1 (0.2) | 52.0 (0.2) | 51.7 (0.2) | 51.7 (0.2) |
| Race/ethnicity, % (SE) | ||||||
| Non-Hispanic white | 79.4 (0.6) | 76.1 (0.6) | 75.0 (0.3) | 73.1 (0.3) | 69.7 (0.3) | 67.4 (0.3) |
| Non-Hispanic black | 10.8 (0.4) | 11.0 (0.5) | 11.1 (0.2) | 11.3 (0.2) | 11.7 (0.2) | 11.9 (0.2) |
| Hispanic | 7.2 (0.4) | 8.8 (0.4) | 9.9 (0.2) | 11.4 (0.2) | 13.3 (0.2) | 14.7 (0.3) |
| Others | 2.6 (0.3) | 4.1 (0.3) | 4.0 (0.2) | 4.1 (0.2) | 5.3 (0.1) | 6.0 (0.1) |
| Education, % (SE) | ||||||
| Less than high school | 23.8 (0.4) | 21.3 (0.3) | 19.4 (0.2) | 17.8 (0.2) | 16.5 (0.2) | 14.4 (0.2) |
| High school | 38.6 (0.3) | 37.8 (0.3) | 32.4 (0.2) | 29.6 (0.2) | 28.4 (0.2) | 26.3 (0.2) |
| More than high school | 37.6 (0.5) | 40.9 (0.4) | 48.2 (0.3) | 52.6 (0.3) | 55.1 (0.3) | 59.3 (0.3) |
Over the period, among 677,051 adults with a mean follow-up period of 11.8 years, the average crude death rates (per 1,000 person-years) for major CVD, IHD, stroke, heart failure, and arrhythmia were 4.3 (95% CI 4.2, 4.4), 2.3 (2.2, 2.4), 0.7 (0.6, 0.7), 0.3 (0.2, 0.3), and 0.1 (0.1, 0.2), respectively (not shown in the table). These estimates approximate those from the National Vital Statistics System queried from the Centers for Disease Control and Prevention’s WONDER system (14). From 1988 to 2015, there were 3,830 deaths among sampled adults with diabetes and 14,993 deaths among adults without diabetes in our analytical sample (Table 2). As expected, major CVD mortality among adults with diabetes (8.2 per 1,000 person-years) was −110% higher than major CVD mortality among adults without diabetes (3.9 per 1,000 person-years), and the mortality of each type of CVD was ~70% to ~130% higher among adults with diabetes compared with those without diabetes.
Table 2—
Age-, sex-, and race/ethnicity-adjusted average annual mortality (per 1,000 person-years) among adults ≥20 years of age, NHIS mortality follow-up 1988–2015
| All years | 1988–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2015 | 10-Year absolute change |
10-Year percentage change |
|
|---|---|---|---|---|---|---|---|---|
| Sample CVD death, n | ||||||||
| With diabetes | 3,830 | 315 | 421 | 781 | 1,039 | 1,274 | ||
| Without diabetes | 14,993 | 1,711 | 2,200 | 3,263 | 3,861 | 3,958 | ||
| Major CVD | ||||||||
| With diabetes | 8.2 (7.7, 8.6)* | 11.2 (9.3, 13.0) | 11.2 (10.0, 12.4) | 8.7 (7.9, 9.6) | 6.7 (6.2, 7.2) | 5.2 (4.8, 5.6) | −3.2 (−3.9, −2.6) | −32.7 (−37.2, −27.9) |
| Without diabetes | 3.9 (3.8, 4.0) | 5.2 (4.9, 5.6) | 4.8 (4.6, 5.1) | 4.1 (4.0, 4.3) | 3.2 (3.1, 3.3) | 2.8 (2.7, 3.0) | −1.2 (−1.3, −1.0) | −26.2 (−28.6, −23.7) |
| RR (95% CI)† | 2.1 (2.0, 2.2) | 2.1 (1.8, 2.5) | 2.3 (2.0, 2.6) | 2.1 (1.9, 2.3) | 2.1 (1.9, 2.3) | 1.8 (1.7, 2.0) | P < 0.001‡ | P = 0.011‡ |
| IHD | ||||||||
| With diabetes | 4.7 (4.4, 5.0) | 7.3 (6.1, 8.5) | 6.8 (5.9, 7.8) | 5.2 (4.5, 5.8) | 3.5 (3.1, 3.8) | 2.6 (2.3, 2.9) | −2.5 (−2.9, −2.0) | −40.3 (−44.7, −35.6) |
| Without diabetes | 2.1 (2.0, 2.1) | 3.2 (2.9, 3.4) | 2.8 (2.6, 3.0) | 2.2 (2.0, 2.3) | 1.6 (1.5, 1.7) | 1.3 (1.2, 1.4) | −0.9 (−1.0, −0.8) | −35.3 (−38.0, −32.4) |
| RR (95% CI) | 2.3 (2.1, 2.5) | 2.3 (1.9, 2.8) | 2.5 (2.1, 2.9) | 2.4 (2.1, 2.8) | 2.1 (1.9, 2.4) | 2.0 (1.8, 2.3) | P < 0.001 | P = 0.067 |
| Stroke | ||||||||
| With diabetes | 1.2 (1.0, 1.3) | 1.4 (0.8, 2.1) | 1.6 (1.2, 2.1) | 1.2 (0.9, 1.5) | 1.1 (0.9, 1.3) | 0.8 (0.6, 0.9) | −0.4 (−0.6, −0.2) | −29.2 (−40.0, −16.5) |
| Without diabetes | 0.7 (0.6, 0.7) | 0.8 (0.7, 0.9) | 0.9 (0.8, 0.9) | 0.8 (0.7, 0.9) | 0.6 (0.5, 0.6) | 0.5 (0.4, 0.5) | −0.2 (−0.2, −0.1) | −25.5 (−30.2, −20.4) |
| RR (95% CI) | 1.7 (1.5, 2.0) | 1.7 (1.1, 2.7) | 1.9 (1.4, 2.6) | 1.5 (1.2, 2.0) | 1.9 (1.6, 2.4) | 1.6 (1.3, 2.0) | P = 0.078 | P = 0.564 |
| Heart failure | ||||||||
| With diabetes | 0.6 (0.5, 0.7) | 0.5 (0.1, 0.8) | 0.6 (0.3, 0.9) | 0.6 (0.4, 0.8) | 0.6 (0.5, 0.8) | 0.5 (0.4, 0.6) | −0.0 (−0.1, 0.1) | −0.5 (−20.7, 24.7) |
| Without diabetes | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.3 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.0 (−0.0, 0.0) | 2.6 (−9.4, 16.2) |
| RR (95% CI) | 2.4 (2.0, 2.9) | 2.0 (0.9, 4.2) | 2.8 (1.6, 4.9) | 2.4 (1.7, 3.5) | 2.7 (2.0, 3.7) | 2.3 (1.7, 2.9) | P = 0.893 | P = 0.809 |
| Arrhythmia | ||||||||
| With diabetes | 0.2 (0.1, 0.2) | 0.2 (0.0, 0.3) | 0.2 (0.0, 0.3) | 0.2 (0.0, 0.3) | 0.2 (0.1, 0.2) | 0.2 (0.2, 0.3) | −0.0 (−0.1, 0.1) | 12.0 (−29.4, 77.5) |
| Without diabetes | 0.1 (0.1, 0.1) | 0.1 (0.0, 0.1) | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.2) | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.2) | 0.0 (0.0, 0.0) | 40.4 (19.6, 64.7) |
| RR (95% CI) | 1.7 (1.3, 2.4) | 4.2 (1.2, 14.0) | 1.8 (0.7, 4.5) | 1.3 (0.6, 2.8) | 1.5 (0.9, 2.5) | 1.6 (1.1, 2.3) | P = 0.714 | P = 0.325 |
Numbers in parentheses are 95% CIs.
Values are RR (95% CI): hazard ratio (with diabetes vs. without diabetes) and 95% CI.
P value for difference of absolute or percentage change between adults with and without diabetes.
For adults both with and without diabetes, death rates from major CVD, IHD, and stroke declined over time. However, there was no significant change in heart failure and arrhythmia deaths for adults with and without diabetes, except for the increase of arrhythmia among adults without diabetes. The 10-year relative changes of major CVD, IHD, stroke, heart failure, and arrhythmia were −32.7% (95% CI −37.2, −27.9), −40.3% (−44.7, −35.6), −29.2% (−40.0, −16.5), −0.5% (−20.7, 24.7), and 12.0% (−29.4, 77.5) for adults with diabetes and −26.2% (−28.6, −23.7), −35.3% (−38.0, −32.4), −25.5% (−30.2, −20.4), 2.6% (−9.4, 16.2), and 40.4% (19.6, 64.7) for adults without diabetes. Compared with adults without diabetes, adults with diabetes experienced a 25% [ = 100 × (−32.7 − (−26.2))/−26.2] greater 10-year percentage reduction of major CVD, and the 10-year absolute changes (per 1,000 person-years) in major CVD and IHD death rates among adults with diabetes were −2.0 [ = −3.2 − (−1.2)] and −1.6 [= −2.5 − (−0.9)] greater, respectively (all P < 0.001), than among adults without diabetes. The P values for interaction of diabetes and survey periods on mortality secular changes were 0.023, 0.221, 0.523, 0.847, and 0.612, respectively, for major CVD, IHD, stroke, heart failure, and arrhythmia. Although both the absolute and relative reduction in death rates for stroke, heart failure, and arrhythmia tended to be higher for adults with diabetes compared with those without diabetes, differences in changes in rates over time by diabetes status were NS.
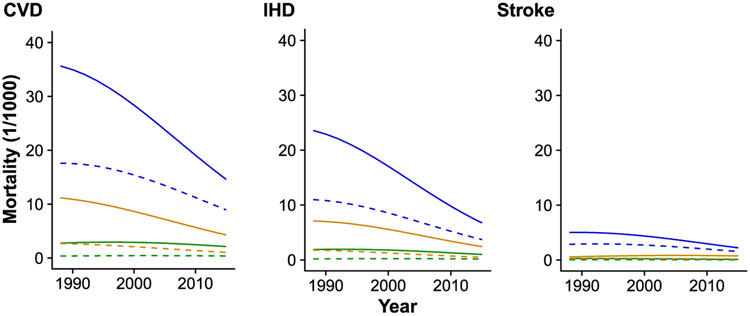
Figure 1 displays sex- and race/ethnicity-adjusted trends in major CVD, IHD, and stroke mortality by three age-groups (20–54, 55–64, and ≥65 years of age) for adults with diabetes. For both major CVD and IHD, declines in rates were larger among older adults than younger adults (P < 0.001), resulting in a narrowing of the gaps in death rates. Supplementary Figs. 1-3 display adjusted trends in major CVD, IHD, and stroke mortality by sex, race/ethnicity, or education, respectively. We did not plot the similar figures for heart failure and arrhythmia because of an insufficient number of events.
Figure 1—
Trends in mortality by age-groups and select CVDs among adults with diabetes. Among U.S. adults both with and without diabetes by three age-groups (20–54, 55–65, and ≥65 years of age), the sex- and race/ethnicity-adjusted death rates from major CVD including IHD and stroke have decreased steadily from 1988 to 2015, especially among adults ≥65 years of age with diabetes. The solid lines represent the mortality of adults with diabetes, and the dashed lines represent the mortality of adults without diabetes. The green lines represent the mortality of adults 20–54 years of age, the orange lines represent the mortality of adults 55–65 years of age, and the blue lines represent the mortality of adults ≥65 years of age.
Table 3 presents average mortality rates for 1988–2015 in terms of absolute and relative changes in rates over that time period for adults with and without diabetes. The results show a slight increase in adjusted trends in heart failure mortality relatively (P < 0.001 for adults 20–54 years of age only) (Table 3). There was no evidence of a secular change in arrhythmia deaths among adults with diabetes by age-group. In general, there were statistically significant disparities in major CVD mortality across age, sex, race/ethnicity, and education subgroups among persons with and without diabetes (all P values of RRs <0.05); the only reductions of absolute gaps over time were seen by age and sex subgroups. Disparities by educational level and race/ethnicity for major CVD and IHD remained with no significant differences in 10-year absolute and relative changes. For stroke, race/ethnicity disparities narrowed with higher declines in rates among non-Hispanic black individuals than among other groups in absolute terms (P < 0.001) and relative terms (P < 0.001).
Table 3—
Age-, sex-, and race/ethnicity-adjusted trends and disparity in mortality (per 1,000 person-years) among adults ≥20 years of age, NHIS mortality follow-up 1988–2015
| Adults with diabetes | Adults without diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| Average annual mortality |
Hazard ratio | 10-Year absolute change |
10-Year percentage change |
Average annual mortality |
Hazard ratio | 10-Year absolute change |
10-Year percent change |
|
| Major CVD | ||||||||
| Age-group, years | ||||||||
| 20–54 | 2.7 (2.2, 3.2)* | 1.0 (Reference) | −0.3 (−0.9, 0.4) | −1.1 (−3.6, 1.4) | 0.4 (0.4, 0.5) | 1.0 (Reference) | 0.0 (−0.0, 0.0) | 0.0 (−1.1, 1.1) |
| 55–64 | 7.9 (6.6, 9.1) | 2.9 (2.3, 3.7) | −2.6 (−4.3, −0.8) | −3.6 (−5.6, −1.7) | 1.9 (1.7, 2.1) | 4.5 (4.0, 5.1) | −0.6 (−0.8, −0.4) | −3.6 (−4.7, −2.5) |
| 65 or older | 25.8 (24.3, 27.3) | 9.5 (7.8, 11.8) | −7.9 (−9.9, −5.8) | −3.4 (−4.1, −2.7) | 14.1 (13.7, 14.4) | 33.4 (30.9, 36.2) | −3.3 (−3.9, −2.7) | −2.6 (−3.0, −2.3) |
| P value† | <0.001 | <0.001 | 0.210 | <0.001 | <0.001 | <0.001 | ||
| Sex | ||||||||
| Men | 10.5 (9.7, 11.3) | 1.6 (1.4, 1.7) | −4.6 (−5.8, −3.3) | −4.5 (−5.4, −3.5) | 5.0 (4.8, 5.2) | 1.6 (1.5, 1.7) | −1.6 (−1.9, −1.4) | −3.3 (−3.8, −2.9) |
| Women | 6.7 (6.2, 7.1) | 1.0 (Reference) | −2.0 (−2.7, −1.3) | −3.1 (−3.9, −2.2) | 3.1 (3.0, 3.2) | 1.0 (Reference) | −0.8 (−1.0, −0.7) | −2.7 (−3.2, −2.3) |
| P value | <0.001 | <0.001 | 0.015 | <0.001 | <0.001 | 0.075 | ||
| Race/ethnicity | ||||||||
| Non-Hispanic white | 8.4 (7.9, 8.9) | 1.0 (Reference) | −3.1 (−3.9, −2.4) | −3.8 (−4.6, −3.1) | 3.8 (3.7, 4.0) | 1.0 (Reference) | −1.2 (−1.3, −1.0) | −3.2 (−3.5, −2.8) |
| Non-Hispanic black | 8.4 (7.1, 9.7) | 1.0 (0.8, 1.2) | −3.1 (−5.1, −1.1) | −3.8 (−5.8, −1.8) | 5.2 (4.9, 5.5) | 1.4 (1.3, 1.4) | −1.5 (−1.9, −1.1) | −2.9 (−3.7, −2.1) |
| Other | 6.9 (5.7, 8.2) | 0.8 (0.7, 1.0) | −2.7 (−4.4, −1.0) | −3.9 (−5.9, −2.0) | 3.0 (2.7, 3.3) | 0.8 (0.7, 0.9) | −0.5 (−1.0, −0.0) | −1.6 (−3.1, −0.1) |
| P value | 0.070 | 0.863 | 0.990 | <0.001 | 0.006 | 0.145 | ||
| Education | ||||||||
| High school or less | 8.6 (8.0, 9.1) | 1.2 (1.1, 1.4) | −3.1 (−3.9, −2.3) | −3.7 (−4.5, −2.9) | 4.4 (4.2, 4.5) | 1.5 (1.4, 1.5) | −1.1 (−1.3, −1.0) | −2.7 (−3.0, −2.3) |
| More than high school | 7.1 (6.3, 7.9) | 1.0 (Reference) | −2.4 (−3.5, −1.3) | −3.5 (−4.8, −2.2) | 3.0 (2.8, 3.1) | 1.0 (Reference) | −0.7 (−1.0, −0.5) | −2.5 (−3.2, −1.8) |
| P value | 0.003 | 0.316 | 0.768 | <0.001 | 0.002 | 0.654 | ||
| IHDs | ||||||||
| Age-group, years | ||||||||
| 20–54 | 1.6 (1.2, 2.1) | 1.0 (Reference) | −0.3 (−0.9, 0.2) | −2.5 (−6.1, 1.0) | 0.2 (0.2, 0.2) | 1.0 (Reference) | −0.0 (−0.0, 0.0) | −0.3 (−1.9, 1.2) |
| 55–64 | 5.0 (4.1, 5.8) | 3.1 (2.2, 4.3) | −1.7 (−2.8, −0.7) | −4.1 (−6.0, −2.2) | 1.1 (1.0, 1.3) | 5.5 (4.7, 6.5) | −0.5 (−0.7, −0.3) | −4.9 (−6.4, −3.4) |
| 65 or older | 15.2 (14.1, 16.4) | 9.5 (7.1, 12.7) | −6.2 (−7.7, −4.8) | −4.7 (−5.5, −3.9) | 7.6 (7.3, 7.9) | 36.8 (33.0, 41.2) | −2.7 (−3.2, −2.3) | −4.1 (−4.6, −3.7) |
| P value | <0.001 | <0.001 | 0.429 | <0.001 | <0.001 | <0.001 | ||
| Sex | ||||||||
| Men | 3.0 (2.8, 3.1) | 1.8 (1.6, 2.0) | −3.3 (−4.1, −2.4) | −5.4 (−6.4, −4.3) | 6.4 (5.9, 7.0) | 2.0 (1.9, 2.1) | −1.3 (−1.5, −1.0) | −4.5 (−5.1, −3.9) |
| Women | 1.5 (1.4, 1.6) | 1.0 (Reference) | −1.5 (−2.0, −1.1) | −4.4 (−5.5, −3.4) | 3.6 (3.3, 4.0) | 1.0 (Reference) | −0.6 (−0.7, −0.5) | −4.4 (−5.0, −3.7) |
| P value | <0.001 | <0.001 | 0.176 | <0.001 | <0.001 | 0.788 | ||
| Race/ethnicity | ||||||||
| Non-Hispanic white | 4.9 (4.5, 5.2) | 1.0 (Reference) | −2.3 (−2.8, −1.8) | −5.0 (−5.9, −4.2) | 2.1 (2.0, 2.2) | 1.0 (Reference) | −0.9 (−1.0, −0.8) | −4.6 (−5.0, −4.1) |
| Non-Hispanic black | 4.6 (3.8, 5.4) | 0.9 (0.8, 1.1) | −2.1 (−3.3, −0.9) | −4.8 (−7.0, −2.7) | 2.5 (2.3, 2.8) | 1.2 (1.1, 1.3) | −0.9 (−1.2, −0.6) | −3.8 (−5.0, −2.7) |
| Other | 4.2 (3.2, 5.2) | 0.9 (0.7, 1.1) | −2.1 (−3.4, −0.7) | −5.2 (−7.5, −2.8) | 1.6 (1.4, 1.9) | 0.8 (0.7, 0.9) | −0.6 (−1.0, −0.2) | −3.7 (−5.8, −1.5) |
| P value | 0.415 | 0.896 | 0.977 | <0.001 | 0.277 | 0.429 | ||
| Education | ||||||||
| High school or less | 5.1 (4.6, 5.5) | 1.3 (1.1, 1.5) | −2.3 (−2.9, −1.8) | −4.9 (−5.8, −4.0) | 2.3 (2.3, 2.4) | 1.5 (1.4, 1.6) | −0.9 (−1.1, −0.8) | −4.2 (−4.7, −3.7) |
| More than high school | 3.9 (3.4, 4.5) | 1.0 (Reference) | −1.6 (−2.4, −0.8) | −4.4 (−6.0, −2.7) | 1.6 (1.4, 1.7) | 1.0 (Reference) | −0.5 (−0.7, −0.3) | −3.5 (−4.4, −2.6) |
| P value | 0.004 | 0.166 | 0.582 | <0.001 | <0.001 | 0.162 | ||
| Stroke | ||||||||
| Age-group, years | ||||||||
| 20–54 | 0.2 (0.1, 0.4) | 1.0 (Reference) | −0.1 (−0.2, 0.1) | −3.6 (−10.8, 3.6) | 0.1 (0.0, 0.1) | 1.0 (Reference) | −0.0 (−0.0, 0.0) | −1.8 (−5.0, 1.4) |
| 55–64 | 0.8 (0.5, 1.0) | 3.5 (1.7, 7.0) | 0.0 (−0.2, 0.3) | 0.8 (−3.8, 5.3) | 0.3 (0.2, 0.3) | 4.2 (3.2, 5.6) | −0.0 (−0.1, 0.0) | −2.2 (−5.3, 0.9) |
| 65 or older | 3.9 (3.3, 4.5) | 17.6 (9.4, 32.6) | −1.1 (−1.9, −0.3) | −3.2 (−5.0, −1.3) | 2.5 (2.3, 2.6) | 40.3 (33.2, 49.0) | −0.5 (−0.7, −0.3) | −2.4 (−3.1, −1.7) |
| P value | <0.001 | 0.033 | 0.261 | <0.001 | <0.001 | 0.929 | ||
| Sex | ||||||||
| Men | 1.3 (1.0, 1.6) | 1.2 (0.9, 1.6) | −0.6 (−1.1, −0.2) | −4.9 (−7.6, −2.2) | s0.7 (0.7, 0.8) | 1.1 (1.0, 1.3) | −0.2 (−0.3, −0.2) | −3.5 (−4.6, −2.4) |
| Women | 1.1 (0.9, 1.2) | 1.0 (Reference) | −0.2 (−0.4, 0.0) | −2.0 (−4.1, 0.0) | 0.6 (0.6, 0.7) | 1.0 (Reference) | −0.2 (−0.2, −0.1) | −2.5 (−3.5, −1.6) |
| P value | 0.151 | 0.104 | 0.082 | 0.016 | 0.121 | 0.224 | ||
| Race/ethnicity | ||||||||
| Non-Hispanic white | 1.2 (1.0, 1.4) | 1.0 (Reference) | −0.5 (−0.8, −0.2) | −4.1 (−6.2, −2.1) | 0.6 (0.6, 0.7) | 1.0 (Reference) | −0.2 (−0.2, −0.1) | −3.2 (−4.0, −2.4) |
| Non-Hispanic black | 1.0 (0.8, 1.3) | 0.8 (0.6, 1.1) | −0.1 (−0.3, 0.2) | −0.4 (−3.5, 2.7) | 0.9 (0.7, 1.0) | 1.3 (1.1, 1.6) | −0.4 (−0.6, −0.2) | −4.5 (−6.6, −2.4) |
| Other | 0.9 (0.6, 1.2) | 0.7 (0.5, 1.0) | −0.0 (−0.3, 0.3) | 0.1 (−3.7, 3.9) | 0.6 (0.5, 0.7) | 1.0 (0.8, 1.2) | 0.0 (−0.1, 0.2) | 0.9 (−1.3, 3.2) |
| P value | 0.081 | 0.044 | 0.036 | 0.006 | <0.001 | <0.001 | ||
| Education | ||||||||
| High school or less | 1.1 (1.0, 1.3) | 0.9 (0.6, 1.2) | −0.3 (−0.5, −0.1) | −2.6 (−4.4, −0.7) | 0.7 (0.7, 0.8) | 1.4 (1.2, 1.6) | −0.2 (−0.2, −0.1) | −2.6 (−3.4, −1.8) |
| More than high school | 1.3 (0.9, 1.7) | 1.0 (Reference) | −0.7 (−1.4, 0.0) | −5.5 (−9.3, −1.8) | 0.5 (0.5, 0.6) | 1.0 (Reference) | −0.1 (−0.2, −0.0) | −2.6 (−4.2, −1.0) |
| P value | 0.479 | 0.254 | 0.145 | <0.001 | 0.378 | 0.967 | ||
| Heart failure | ||||||||
| Age-group, years | ||||||||
| 20–54 | 0.1 (0.0, 0.1) | 1.0 (Reference) | 0.1 (−0.0, 0.2) | 20.2 (2.0, 38.4) | 0.0 (0.0, 0.0) | 1.0 (Reference) | 0.0 (0.0, 0.0) | 11.8 (3.5, 20.0) |
| 55–64 | 0.3 (0.1, 0.4) | 5.0 (2.0, 12.8) | 0.1 (−0.1, 0.2) | 4.2 (−3.6, 12.0) | 0.1 (0.0, 0.1) | 19.1 (9.8, 37.2) | −0.0 (−0.0, 0.0) | −0.4 (−5.5, 4.6) |
| 65 or older | 1.8 (1.5, 2.2) | 36.2 (15.9, 82.7) | 0.1 (−0.3, 0.5) | 0.8 (−1.9, 3.4) | 0.9 (0.8, 1.0) | 281.0 (160.7, 491.2) | 0.1 (−0.0, 0.2) | 1.2 (−0.2, 2.6) |
| P value | <0.001 | 0.979 | 0.099 | <0.001 | 0.194 | 0.040 | ||
| Sex | ||||||||
| Men | 0.6 (0.5, 0.8) | 1.2 (0.9, 1.7) | 0.1 (−0.1, 0.2) | 1.2 (−2.1, 4.6) | 0.3 (0.2, 0.3) | 1.4 (1.2, 1.7) | 0.0 (−0.1, 0.1) | 0.3 (−2.2, 2.7) |
| Women | 0.5 (0.4, 0.6) | 1.0 (Reference) | −0.0 (−0.2, 0.1) | −0.5 (−3.9, 2.9) | 0.2 (0.2, 0.2) | 1.0 (Reference) | 0.0 (−0.0, 0.0) | 0.3 (−1.1, 1.7) |
| P value | 0.219 | 0.465 | 0.471 | <0.001 | 0.985 | 0.970 | ||
| Race/ethnicity | ||||||||
| Non-Hispanic white | 0.6 (0.5, 0.7) | 1.0 (Reference) | 0.0 (−0.1, 0.1) | 0.4 (−2.3, 3.1) | 0.2 (0.2, 0.3) | 1.0 (Reference) | −0.0 (−0.0, 0.0) | −0.0 (−1.5, 1.5) |
| Non-Hispanic black | 0.4 (0.2, 0.7) | 0.7 (0.4, 1.3) | −0.1 (−0.5, 0.2) | −2.4 (−10.1, 5.2) | 0.3 (0.2, 0.4) | 1.2 (0.9, 1.5) | 0.0 (−0.1, 0.1) | 0.7 (−2.5, 3.8) |
| Other | 0.3 (0.1, 0.4) | 0.5 (0.3, 0.8) | 0.1 (−0.1, 0.3) | 3.4 (−4.7, 11.4) | 0.1 (0.0, 0.1) | 0.4 (0.2, 0.6) | 0.1 (−0.0, 0.2) | 10.2 (−1.2, 21.6) |
| P value | 0.002 | 0.637 | 0.583 | <0.001 | 0.266 | 0.206 | ||
| Education | ||||||||
| High school or less | 0.6 (0.5, 0.7) | 1.7 (1.2, 2.4) | −0.0 (−0.2, 0.1) | −0.5 (−3.2, 2.2) | 0.3 (0.2, 0.3) | 1.3 (1.0, 1.7) | 0.0 (−0.0, 0.1) | 1.2 (−0.4, 2.8) |
| More than high school | 0.4 (0.3, 0.5) | 1.0 (Reference) | 0.2 (0.0, 0.4) | 6.2 (0.2, 12.1) | 0.2 (0.2, 0.2) | 1.0 (Reference) | −0.0 (−0.1, 0.0) | −1.2 (−4.4, 2.1) |
| P value | 0.002 | 0.038 | 0.040 | 0.015 | 0.214 | 0.199 | ||
| Arrhythmia | ||||||||
| Age-group, years | ||||||||
| 20–54 | 0.1 (0.0, 0.2) | 1.0 (Reference) | 0.0 (−0.1, 0.2) | 5.4 (−12.2, 23.0) | 0.0 (0.0, 0.0) | 1.0 (Reference) | 0.0 (−0.0, 0.0) | 2.4 (−2.4, 7.2) |
| 55–64 | 0.1 (0.0, 0.2) | 1.2 (0.4, 3.4) | 0.1 (−0.0, 0.2) | 9.4 (−3.3, 22.2) | 0.0 (0.0, 0.0) | 2.2 (1.2, 4.1) | −0.0 (−0.0, 0.0) | −1.2 (−6.4, 4.0) |
| 65 or older | 0.6 (0.3, 0.8) | 6.3 (2.5, 15.7) | 0.1 (−0.2, 0.3) | 1.2 (−4.6, 7.1) | 0.4 (0.3, 0.4) | 27.4 (18.4, 40.9) | 0.2 (0.1, 0.2) | 5.2 (3.2, 7.2) |
| P value | <0.001 | 0.889 | 0.483 | <0.001 | <0.001 | 0.044 | ||
| Sex | ||||||||
| Men | 0.2 (0.1, 0.3) | 1.6 (0.8, 3.0) | 0.0 (−0.1, 0.2) | 1.7 (−5.5, 8.8) | 0.1 (0.1, 0.1) | 1.3 (1.0, 1.6) | 0.0 (0.0, 0.1) | 3.6 (1.0, 6.2) |
| Women | 0.1 (0.1, 0.2) | 1.0 (Reference) | 0.0 (−0.1, 0.1) | 0.6 (−7.1, 8.3) | 0.1 (0.1, 0.1) | 1.0 (Reference) | 0.0 (0.0, 0.1) | 4.1 (1.6, 6.5) |
| P value | 0.171 | 0.778 | 0.845 | 0.042 | 0.820 | 0.799 | ||
| Race/ethnicity | ||||||||
| Non-Hispanic white | 0.2 (0.1, 0.2) | 1.0 (Reference) | 0.0 (−0.0, 0.1) | 3.4 (−3.3, 10.0) | 0.1 (0.1, 0.1) | 1.0 (Reference) | 0.0 (0.0, 0.1) | 4.2 (2.1, 6.3) |
| Non-Hispanic black | 0.1 (0.0, 0.2) | 0.7 (0.3, 1.5) | 0.0 (−0.1, 0.2) | 3.2 (−12.1, 18.6) | 0.1 (0.1, 0.2) | 1.2 (0.8, 1.9) | 0.0 (−0.0, 0.1) | 1.8 (−3.3, 6.9) |
| Other | 0.4 (0.0, 0.8) | 2.3 (0.8, 6.6) | −0.3 (−0.8, 0.3) | −7.4 (-15.9, 1.1) | 0.1 (0.0, 0.1) | 0.6 (0.3, 1.0) | 0.0 (−0.0, 0.0) | 3.9 (−1.9, 9.7) |
| P value | 0.291 | 0.555 | 0.139 | 0.015 | 0.522 | 0.694 | ||
| Education | ||||||||
| High school or less | 0.2 (0.1, 0.3) | 1.3 (0.7, 2.7) | −0.0 (−0.1, 0.1) | 0.1 (−5.8, 6.1) | 0.1 (0.1, 0.1) | 1.7 (1.3, 2.1) | 0.0 (0.0, 0.1) | 4.3 (2.3, 6.4) |
| More than high school | 0.1 (0.1, 0.2) | 1.0 (Reference) | 0.1 (−0.0, 0.2) | 6.8 (−4.1, 17.6) | 0.1 (0.1, 0.1) | 1.0 (Reference) | 0.0 (0.0, 0.0) | 4.8 (1.3, 8.3) |
| P value | 0.387 | 0.267 | 0.283 | <0.001 | 0.366 | 0.827 | ||
Numbers in parentheses are 95% CIs.
P value for difference of an estimate among subgroups.
Mortality from heart failure was lower than for IHD or stroke (Table 3). As expected, older adults with diabetes had a significantly higher risk of death from heart failure than those without diabetes. Interestingly, the 10-year percentage change in heart failure deaths was significantly increased in the youngest age-group (20–54 years of age) and did not change significantly in the other two age-groups. Finally, arrhythmia deaths did not statistically significantly differ by sex, race/ethnicity, or education among adults with diabetes. Nevertheless, there was a small increase of arrhythmia deaths in older, non-Hispanic white adults and lower educated adults.
To examine the potential impact of earlier diagnosis related to diabetes diagnostic criteria changes and increasing diabetes awareness, we conducted the sensitivity analysis as described in RESEARCH DESIGN AND METHODS. As shown in Supplementary Table 1A and B, the sensitivity analyses yielded no appreciable changes in death rates compared with the primary analyses.
As adults with major CVD death (n = 16,118) who died before 2015 but after 10th year of follow-up were right censored at 10 years, we conducted a sensitivity analysis that included each individual’s whole follow-up period and the results did not change the inference.
CONCLUSIONS
This analysis of national trends in CVD subtype mortality revealed four main findings. First, the large CVD mortality rate reductions among persons with diabetes significantly narrowed CVD mortality gap between persons with and without diabetes over the past 30 years. Second, subgroup analyses revealed a concerning lack of significant improvement in mortality rates among young adults. Third, disparities in CVD mortality among adults with diabetes persisted by age, sex, race/ethnicity, and education subgroups, but narrowed for age and sex due to larger decreases in the elderly and in men. Finally, mortality changes were not homogenous across CVD subtypes, because heart failure death rates improved the least and even increased in younger adults (10-year percentage change 11.8 [95% CI 3.5, 20.0]) in Table 3].
Factors explaining these improvements remain an area of debate. The assessment of risk factors in NHIS is limited to baseline and self-report, making it difficult to assess the factors explaining reductions in mortality. However, previous studies (15) have suggested that improvements in overall CVD mortality are due to multiple factors, ranging from advances in medical and lifesaving treatments for acute CVD events, improvements in medication accessibility, and reduction in the risk of complications of diabetes. Tobacco use, an unhealthy diet and physical inactivity, obesity, elevated blood pressure, abnormal blood lipids, elevated blood glucose levels, and family history, in addition to diabetes, are major risk factors for CVD (15). Our previous studies (16) have shown that the proportions of smoking, high cholesterol, and high blood pressure, and medication for hyperlipidemia, high blood pressure, and hyperglycemia have improved among the U.S. population and especially among people with diabetes. The improvements in the control of risk factors might lead to reduced complications; a recent study found large reductions over the past 2 decades in the incidence of a wide range of diabetes-related complications (including acute myocardial infarction and stroke) that often lead to death. Thus, adults with diabetes may have benefited more than those without diabetes from risk factor control (16).
We found that CVD mortality decreased among both men and women, and the CVD mortality gap between men and women narrowed for adults both with and without diabetes. Part of this attenuated sex gap of CVD mortality is likely a function of men having started from a much higher rate of exposure of risk factors and thus are more apt to benefit from the improvements in risk factor management that occurred. However, greater improvements in men could also be due to lower perceptions of CVD in women (17) and less improvement of traditional risk factor control (18). Women might also have a different spectrum of heart disease that is more related to inflammation, reproductive hormones, and microvascular disease, each of which is outside the scope of regular CVD risk factor management (19).
Heart failure and arrhythmia are two major cardiovascular complications among adults with diabetes (20,21). Contrasted to a major study in Sweden that showed a significant decrease of hospitalization for heart failure among persons with type 2 diabetes, our findings suggest that the risk of heart failure death increased among younger adults with diabetes (Table 3), which is consistent with the trends in the general population (15) and with another Swedish study (22) that found an increase in hospitalization for heart failure among young adults. Our analysis could not assess the pathophysiological mechanisms underlying the differences in trends in mortality that were observed; however, both heart failure and arrhythmia can be complications of macrovascular diseases such as IHD. They may also have the same risk factors and have particular associations with diabetes due to the effects of diabetes and obesity on diastolic heart dysfunction, microvascular disease, cardiomyopathy, and neuropathy (23,24). The increases in heart failure deaths, especially in younger adults, and the increases in arrhythmia deaths in older adults could also have been influenced by an increase in survival rate from macrovascular coronary disease or an increase in other risk factors. It is worth raising awareness of these increases among adults with diabetes and their physicians, and to encourage attentive management of traditional risk factors as well as consideration of non-traditional risk factors (25).
Our findings confirmed the persistence of disparities in CVD mortality across racial/ethnic and education groups (15). Major CVD mortality among adults with diabetes decreased across all subgroups without significant differences, except that non-Hispanic white individuals experienced higher reductions in stroke deaths.
There were some limitations in our study. First, our study relied on self-reported diabetes status assessed only at baseline. The persons with diabetes might also experience a small portion of remission in a short period (26). We could not distinguish between type 1 and type 2 diabetes. The differences in mortality between young and old adults could be affected by the different type of diabetes. Although self-reported diabetes is highly specific and closely correlated with medical records (27), it is known to be nonsensitive to the broader population with undiagnosed diabetes (28). If persons with undiagnosed diabetes have better health status than persons with diagnosed diabetes, the misclassification of persons with undiagnosed diabetes into the group without diabetes in our study could lead to the overestimation of CVD mortality rates in both the group with diabetes and the group without diabetes. However, the prevalence of undiagnosed diabetes and the ratio of undiagnosed diabetes to nondiabetes has been relatively stable nationally and thus unlikely to have had a major effect on the differential trends in CVD mortality between the populations with and without diabetes (29). The possible impact of changes from ICD-9 to ICD-10 on the trends were evaluated by Anderson et al. (13). Compared with ICD-9, ICD-10 classification results in a slightly lower likelihood of being coded as a heart disease death coding and a higher likelihood of stroke death coding as the underlying causes of death. An additional limitation is that the attributions of cause of death relied on death certificates, which could be misclassified. That said, the accuracy of death certificates for CVD have been shown to be acceptable and relatively stable during the study period (30). Recommended diabetes diagnostic criteria had been changed twice since 1997. Although we cannot rule out some impact of earlier diagnosis leading to a healthier denominator in later years, our sensitivity analyses simulating the effect of increased detection had little change on our main findings and the duration of diabetes changed little between the NHIS cohorts. In addition, we excluded the first 2 years of follow-up time and followed up adults for up to 10 years to minimize potential selection bias and misclassification. Although our study demonstrates trends in CVD mortality of U.S. adults with diabetes, the factors driving this trend need further exploration in the future because the available NHIS data do not permit further exploration.
Several significant factors differentiate ours from previous studies. To our knowledge, this is the first U.S. national report of long-term trends and disparities in CVD mortality and its subtypes among adults with diabetes. We found that there was no reduction of major CVD mortality but, rather, an increase in heart failure among young adults; whereas gaps in major CVD deaths among race and education narrowed in absolute terms. We report findings both in terms of relative changes and in terms of absolute change. Our study provides several design and analytic advances over previous work, including the first comprehensive examination of cause-specific CVD mortality in the U.S. population who have received diagnoses of diabetes. Instead of presenting survival data and changes by cohorts, we used weighted discrete Poisson models to permit estimation of nationally representative rates by calendar year, which are more descriptive and informative for surveillance and health policy making.
We conclude that in the U.S. from 1988 to 2015, although adults with diabetes experienced larger reductions in CVD mortality over time, they still remain at higher risk of CVD mortality than those without diabetes. The major portion of decreases in CVD mortality was observed in decreases in IHD and stroke deaths. Disparities in CVD mortality among adults with diabetes have substantially narrowed, especially between women and men, which suggests that mortality and disparities can be reduced by primary prevention of risk factor exposure, and improvements in detection and treatment at individual, community, health care system, and environmental levels (31). However, disparities in mortality still continue and need to be closely monitored and improved.
Supplementary Material
Acknowledgments.
The authors thank the women and men who participated in the study as well as all of the staff involved at the U.S. NHIS for the study design, data collection, and data dissemination.
Funding. This work was funded by the U.S. Department of Health and Human Services and the U.S. Centers for Disease Control and Prevention (CDC).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0831/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Sarwar N, Gao P, Seshasai SR, et al. ; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies [published correction appears in Lancet 2010;376:958]. Lancet 2010; 375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418 [DOI] [PubMed] [Google Scholar]
- 3.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-specific trends from 2000-2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care 2016;39:1018–1026 [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 2018;391:2430–2440 [DOI] [PubMed] [Google Scholar]
- 5.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J 2013;34:2436–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J 2013;34:2444–2452 [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary AK, Aneja GK, Shukla S, Razi SM. Study on diastolic dysfunction in newly diagnosed type 2 diabetes mellitus and its correlation with glycosylated haemoglobin (HbA1C). J Clin Diagn Res 2015;9:OC20–OC22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havranek EP, Mujahid MS, Barr DA, et al. American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2015;132:873–898 [DOI] [PubMed] [Google Scholar]
- 9.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults especially women. Circulation 2015;132:997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng YJ, Gregg EW, Rolka DB, Thompson TJ. Using multi-year national survey cohorts for period estimates: an application of weighted discrete Poisson regression for assessing annual national mortality in US adults with and without diabetes, 2000-2006. Popul Health Metr 2016;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. About the National Health Interview Survey [Internet], 2018. Available from http://www.cdc.gov/nchs/nhis/about_nhis.htm. Accessed 2 July 2018
- 12.Centers for Disease Control and Prevention. National Health Interview Survey - questionnaires [Internet], 2016. Available from ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS/. Accessed 1 July 2018
- 13.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep 2001;49:1–32 [PubMed] [Google Scholar]
- 14.Friede A, Reid JA, Ory HW. CDC WONDER: a comprehensive on-line public health information system of the Centers for Disease Control and Prevention. Am J Public Health 1993;83:1289–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–1523 [DOI] [PubMed] [Google Scholar]
- 17.Hart PL. Women’s perceptions of coronary heart disease: an integrative review. J Cardiovasc Nurs 2005;20:170–176 [DOI] [PubMed] [Google Scholar]
- 18.Towfighi A, Zheng L, Ovbiagele B. Sex-specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med 2009;169:1762–1766 [DOI] [PubMed] [Google Scholar]
- 19.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol 2009;54:1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahtiyar G, Gutterman D, Lebovitz H. Heart failure: a major cardiovascular complication of diabetes mellitus. Curr Diab Rep 2016;16:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakou ES, Mavrakis H, Vardas PE. Are diabetic patients at increased risk of arrhythmias? Hellenic J Cardiol 2012;53:335–339 [PubMed] [Google Scholar]
- 22.Barasa A, Schaufelberger M, Lappas G, Swedberg K, Dellborg M, Rosengren A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J 2014;35:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pop-Busui R Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 2010;33:434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battiprolu PK, Gillette TG, Wang ZV, Lavandero S, Hill JA. Diabetic cardiomyopathy: mechanisms and therapeutic targets. Drug Discov Today Dis Mech 2010;7:e135–e143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuire DK, Gore MO, Masoudi FA. Diabetes and heart failure in patients with coronary disease: separating markers from mediators. Diabetes Care 2010;33:2120–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregg EW, Chen H, Wagenknecht LE, et al. ; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012;308:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE. Concordance between respondent self-reports and medical records for chronic conditions: experience from the Veterans Health Study. J Ambul Care Manage 2005;28:102–110 [DOI] [PubMed] [Google Scholar]
- 28.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 29.Geiss LS, Bullard KM, Brinks R, Hoyer A, Gregg EW. Trends in type 2 diabetes detection among adults in the USA, 1999-2014. BMJ Open Diabetes Res Care 2018;6:e000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roulson J, Benbow EW, Hasleton PS. Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta-analysis and review. Histopathology 2005;47:551–559 [DOI] [PubMed] [Google Scholar]
- 31.Jousilahti P, Laatikainen T, Peltonen M, et al. Primary prevention and risk factor reduction in coronary heart disease mortality among working aged men and women in eastern Finland over 40 years: population based observational study. BMJ 2016;352:i721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.