Lyme borreliosis is caused by a growing list of related, yet distinct, spirochetes with complex biology and sophisticated immune evasion mechanisms.
KEYWORDS: Borrelia, Borrelia burgdorferi, Borreliella, borreliosis, diagnosis, diagnostics, Lyme disease, molecular methods, serology
SUMMARY
Lyme borreliosis is caused by a growing list of related, yet distinct, spirochetes with complex biology and sophisticated immune evasion mechanisms. It may result in a range of clinical manifestations involving different organ systems, and can lead to persistent sequelae in a subset of cases. The pathogenesis of Lyme borreliosis is incompletely understood, and laboratory diagnosis, the focus of this review, requires considerable understanding to interpret the results correctly. Direct detection of the infectious agent is usually not possible or practical, necessitating a continued reliance on serologic testing. Still, some important advances have been made in the area of diagnostics, and there are many promising ideas for future assay development. This review summarizes the state of the art in laboratory diagnostics for Lyme borreliosis, provides guidance in test selection and interpretation, and highlights future directions.
INTRODUCTION
Lyme borreliosis, also known as Lyme disease, is a bacterial infection caused by spirochetes of the Borrelia burgdorferi sensu lato complex (also more recently grouped under a new genus taxon named Borreliella) (1). In this review, we retain the former taxonomy of Borrelia burgdorferi sensu lato complex (Bbsl), because the new taxonomy is still not widely accepted (2, 3). The causative agents of Lyme borreliosis are transmitted to humans by ixodid (hard) ticks. The infection usually begins with an expanding skin lesion, termed erythema migrans. Untreated patients may develop acute Lyme neuroborreliosis or Lyme carditis within weeks of infection, or Lyme arthritis within months of infection, with rarer manifestations also described (4). Direct detection of the infectious agent is often not possible or practical, necessitating reliance on serologic testing for support of the diagnosis. Serologic testing is insensitive during the first weeks of infection, but after that time the standard two-tiered approach of enzyme immunoassay (EIA) followed by immunoblots, or newer two-test approaches, have high sensitivity and specificity. Thus, using validated interpretative criteria, laboratory testing substantially improves overall diagnostic accuracy.
AGENTS OF INFECTION
Spirochetes of the family Borreliaceae are separated into two distinct phylogenetic groups: (i) Lyme-related borreliae and genetically similar species and (ii) relapsing fever borreliae and their relatives (1, 5, 6). The Borrelia burgdorferi sensu lato genospecies complex (Bbsl) includes the three most frequent agents of Lyme borreliosis worldwide—Borrelia burgdorferi (sensu stricto), Borrelia afzelii, and Borrelia garinii—along with closely related Borrelia species that rarely, if ever, cause human infection (7, 8). Nearly all Lyme borreliosis cases acquired in North America are caused by B. burgdorferi, with the remaining few cases caused by B. mayonii, a recently recognized species in the upper Midwest (7, 9, 10). The B. burgdorferi type strain is B31, which was the original isolate, recovered from ticks collected on Shelter Island, New York (11). In Europe, most Lyme borreliosis cases are caused by B. afzelii or B. garinii, with a lesser contribution from B. burgdorferi, B. spielmanii, B. bavariensis, and rarer species (8). In Asia, B. garinii predominates as the causative species (8).
Each of the three most important pathogenic species is associated with certain differences in clinical expression. For example, classic Lyme neuroborreliosis (Bannwarth’s syndrome) in Europe is associated with B. garinii, whereas B. afzelii more typically causes skin manifestations (12–16). B. burgdorferi in the northeastern and mid-Atlantic United States is particularly arthritogenic, accounting for the greater frequency of Lyme arthritis cases in North America compared to Europe or Asia (8, 16, 17). Regional differences in species distribution can have important implications for diagnostic testing as well, since diagnostic assays intended to detect infection by one species may perform less well in detecting infection by another species (18–22). This is less of a problem in the United States, where almost all domestically acquired infections are caused by B. burgdorferi.
Intraspecies diversity allows subclassification of clinical strains. Several phenotypic and genetic strain-typing systems have been used to separate clinical strains of a given species into subgroups (5, 16). In North America, a common strategy to differentiate among B. burgdorferi strains has been analysis of a single genetic locus, either the plasmid-located outer surface protein C gene (ospC), or the chromosomal 16S-23S rRNA intergenic spacer (IGS) region (5, 23). ospC sequence analysis divides North American B. burgdorferi strains into at least 23 ospC genotypes (24–27), whereas restriction fragment-length polymorphism analysis of the 16S–23S rRNA intergenic spacer divides B. burgdorferi strains into 3 major groups, ribosomal spacer type 1 (RST1) through RST3 (28, 29). A third system, multilocus sequence typing, which is based on sequence analysis of 8 housekeeping genes, divides B. burgdorferi strains into at least 33 sequence types (30). Notably, B. burgdorferi populations in Europe and North America constitute distinct lineages (30), and clinical isolates collected from patients in the Northeast or Upper Midwest regions of the U.S. represent distinct B. burgdorferi populations (27).
The existence of divergent genotypes within Borrelia species has clinical and diagnostic implications. First, exposure to one genotype does not necessarily confer immunity to other genotypes (31), and serial distinct infections caused by B. burgdorferi strains of different genotypes are possible in the same individual (32–35). Reinfection may occur after an episode of antibiotic-treated erythema migrans (36, 37), whereas reinfection is very rarely documented after resolution of a late Lyme borreliosis manifestation, presumably because the expanded immune response associated with the latter is more broadly protective (37).
Second, in Europe, where there is greater diversity of genospecies, optimization of serologic assays requires inclusion of antigens or epitopes derived from the prevalent genospecies (38–42). To a lesser degree, genotypic diversity among North American B. burgdorferi strains may also have the potential to affect serologic test performance. For example, antigenic differences linked to genotype might explain (at least in part) why the sensitivity of immunoblots prepared from the original isolate of B. burgdorferi (strain B31, an RST1 isolate which is used in most North American serologic assays) is higher in patients infected with RST1 strains than in patients infected with RST2 or RST3 strains (42).
Third, some B. burgdorferi genotypes are more virulent than others (27). For example, the B. burgdorferi RST1 subtype has greater inflammatory potential (43, 44), is more frequently detectable in blood (23, 45, 46), and is associated with more severe early disease and with higher rates of postinfectious, antibiotic-refractory Lyme arthritis (44, 47). In the Northeastern United States, more than half of isolates from EM skin lesions are OspC type A (part of the RST1 group) or OspC type K (part of the RST2 group), whereas in the upper Midwest, OspC type H (also in the RST2 group) appears to be most common (27). Certain OspC genotypes, especially types A, B, H, I, and K, confer a higher risk of dissemination (23, 26, 27, 46, 48). Thus, genotype-associated virulence factors, combined with certain host factors, account (at least in part) for the wide variation in clinical manifestations and outcomes among patients with Lyme borreliosis (44).
TRANSMISSION, EPIDEMIOLOGY, AND RISK FACTORS
Agents of Lyme borreliosis are transmitted between reservoir hosts and humans (incidental hosts) by hard-bodied ticks of the Ixodes ricinus complex (49). B. burgdorferi must be coated with a tick protein, Salp15, to survive initial transmission from tick to host (50). Neither direct person-to-person transmission, direct zoonotic transmission, nor transmission via blood product transfusion has ever been documented, although Lyme-related borreliae can survive in packed red blood cells at refrigeration temperatures and transfusion-acquired infection is a theoretical transfusion risk (51, 52). Vertical human transmission leading to congenital infection has also not been described (53–56). Most infections are acquired from nymphal ticks rather than adult ticks (57), in part because transmission usually requires 36 to 48 h of tick attachment, and adult, engorged ticks are more likely to be noticed and removed in that time frame compared to feeding nymphs (7, 58). Larval ticks, which are not infected, do not transmit Lyme disease.
Nymphal ixodid ticks are most active and abundant in the late spring and early summer months; this, along with greater human outdoor activity during warmer weather, accounts for the marked seasonality of erythema migrans cases, which peak during the summer months in the United States (59, 60). Certain later manifestations, especially Lyme arthritis, typically manifest after months of latent infection, and therefore the onset of arthritis does not follow a seasonal pattern (61). In the United States, Lyme borreliosis has a slight male predominance and a bimodal age distribution, with peak incidence at 5 to 15 years and at >45 years of age (7, 17). Risk factors include peridomestic, recreational, or occupational exposure to ixodid tick habitat (62–65).
The necessity of vector intermediates limits human transmission to specific geographical regions, in which the natural Bbsl enzootic cycle can be maintained. Areas of endemicity exist throughout temperate regions of the Northern Hemisphere, including large swaths of North America, Europe and Asia (8). In North America, most cases are acquired in the Northeast, Mid-Atlantic, and Upper Midwestern regions of the U.S. extending into Canada, and there are now highly affected areas in southern Canada, especially in Nova Scotia (66–68). However, the disease incidence is low in the U.S. Pacific Northwest, and there is minimal or negligible incidence elsewhere in the United States. (7, 32, 69, 70). Levels of endemicity can vary significantly at the local level, with pockets of hyperendemicity neighboring areas of low-disease incidence (71). Regions of endemicity are expanding (68, 71–73), mirroring territorial expansion of competent vectors (74), and the annual incidence of reported infection has increased sharply over the past few decades in North America, Europe, and Asia (7, 69).
CLINICAL MANIFESTATIONS
The most common presenting manifestation of Lyme borreliosis is an annular, expanding, erythematous skin rash termed erythema migrans (EM), which results from localized infection at the inoculation site after an incubation period of about 1 week (range, 3 to 32 days) after a tick bite (4, 75, 76). As the lesion expands, it may develop partial central clearing surrounded by a redder outer border, or classically, a “bull’s eye” configuration with concentric rings of erythema alternating with partial clearing (77). However, if the patient is seen within days of rash onset, less distinctive lesions are more common. Typical appearances include expanding, homogeneous erythema, or expanding pale erythema surrounding a darker red center (78, 79). In the United States, many patients with EM also have systemic symptoms, most frequently fatigue, arthralgia, myalgia or headache, and regional lymphadenopathy may be present (77, 80, 81). During the first days of infection, fever and chills may also be present, particularly in children. In some cases, multiple EM lesions occur (81), with a primary lesion at the site of the tick bite and secondary skin lesions resulting from hematogenous dissemination (82). Peripheral leukocytosis, leukopenia, anemia, or thrombocytopenia are not associated with EM, whereas lymphopenia or elevated liver function tests sometimes are (83).
Dissemination may also lead to noncutaneous organ involvement. If the patient is not treated with antimicrobial therapy, early Lyme neuroborreliosis may develop in approximately 15% of patients within weeks after initial infection, usually manifesting with cranial neuropathy (most commonly unilateral or bilateral facial nerve palsy), lymphocytic meningitis, or radiculitis (76, 84). Within the same time frame, a less frequent (but potentially fatal) manifestation of early disseminated disease is Lyme carditis, usually causing atrioventricular conduction block in about 5% of untreated patients (4, 84, 85). In the northeastern United States, Lyme arthritis is the most common late manifestation of the disease, which occurs in approximately 60% of untreated patients, usually beginning months to as long as 2 years after untreated initial infection (4, 76, 86). Lyme arthritis typically affects only one or a few large joints, especially the knee (84, 86). Affected knees usually have large joint effusions, with a neutrophilic leukocytosis in synovial fluid (87). Arthritis occurs only in a small proportion of European patients with Lyme borreliosis, often earlier in the disease course than in U.S. patients (17). In contrast, several later manifestations recognized with some frequency in Europe are rare or absent among North American cases, including acrodermatitis chronica atrophicans, borrelial lymphocytoma, and late encephalomyelitis (8, 84).
In clinical practice today, most patients present with—and are treated for—erythema migrans, preventing later manifestations of the disease. Among cases reported to the Centers for Disease Control and Prevention (CDC) in recent years, 72% had EM, 28% had arthritis, 13% had neurologic involvement, and only 1.5% had carditis (88). In addition, among participants in a Lyme disease vaccine trial in the United States, 11% had asymptomatic infection (89). In Europe, as many as half of cases may be asymptomatic (90).
DIAGNOSTIC TESTING
There are two broad categories of diagnostic tests for Lyme borreliosis: (i) direct detection methods, which detect the agent of infection in primary patient specimens, and (ii) indirect detection methods, which detect a host response to the infection. The first tests adopted for routine clinical diagnostic use were serum antibody tests (91). These indirect detection assays have evolved substantially in their methodology and chemistry (92), and they remain the most useful and widely available diagnostic aids. Despite prodigious recent advances in molecular methods for direct detection of other infectious agents, these and other direct detection strategies currently play little role in clinical diagnostics for Lyme borreliosis.
Antibody Detection
Kinetics and evolution of the antibody response in untreated patients.
A specific antibody response against B. burgdorferi is not detectable during a “window period” of several days to a few weeks after initial infection (93–95). As the antibody response develops, IgM-class antibodies directed against a relatively limited repertoire of immunogenic antigens are produced, often with rapid IgM-to-IgG isotype switching. Immunodominant early antigens include outer surface protein C (OspC; molecular weight, 21 to 25 kDa) and variable major protein (VMP)-like sequence, expressed (VlsE; molecular weight, 34 to 35 kDa), both of which are surface-exposed, outer membrane lipoproteins, and the 41-kDa flagellar protein (p41/flagellin/FlaB) (93–105). Other immunogenic antigens which may provoke an antibody response during early infection include the 37-kDa flagellar protein (FlaA), decorin binding proteins (Dbp) A and B, RevA, p66, BBK07, BBK32, BBG33, LA7, BmpA (molecular weight, 39 kDa), FliL, and several oligopeptide permeases (OppA1, -A2, and -A4) (95, 98, 102, 106–117). The sequence in which antibodies against specific early antigens become detectable, and the number of antibody specificities detectable at a given time point varies from patient to patient.
After 1 to 2 months of untreated active infection, an expanded IgG antibody response is reliably present, with reactivity against a range of immunogenic antigens, typically including most or all those specified in the IgG immunoblot interpretive criteria (Table 1), plus VlsE and potentially many others (94, 98). Lyme arthritis patients in the United States typically have an exceptionally expanded IgG antibody response to spirochetal proteins. In a microarray analysis of >1,200 spirochetal proteins, these patients had reactivity with as many as 89 antigens, particularly outer surface proteins (108). This commonly includes IgG reactivity with OspC (118), as well as spirochetal proteins that are usually expressed only in the tick, including OspA, OspD, and Borrelia iron- and copper-binding protein (BicA) (119). These tick program responses are only found in U.S. Lyme arthritis patients, presumably because of the highly inflammatory milieu in their inflamed joints (120).
TABLE 1.
Interpretive criteria for North American immunoblotsa
| Immunoblot | Criteria for a positive resultb |
|---|---|
| IgM | Two of the following three bands must be present: 23 kDa (OspC), 39 kDa (BmpA), and 41 kDa (Fla) |
| IgG | Five of the following ten bands must be present: 18 kDa, 21 kDa (OspC), 28 kDa, 30 kDa, 39 kDa (BmpA), 41 kDa (Fla), 45 kDa, 58 kDa (not GroEL), 66 kDa, and 93 kDa |
Adapted from reference 135.
The apparent molecular mass of OspC is dependent on the strain of B. burgdorferi being tested. The 23- and 21-kDa proteins referred to are the same.
While the IgM antibody response usually wanes and may become undetectable in late active disease (91, 121, 122), the IgG antibody response persists (91, 123). However, in some cases, the IgM antibody response also persists (91, 122), and therefore the presence of specific IgM antibodies does not necessarily indicate an infection of short duration or early-stage infection.
Kinetics and evolution of the antibody response after prompt antimicrobial therapy.
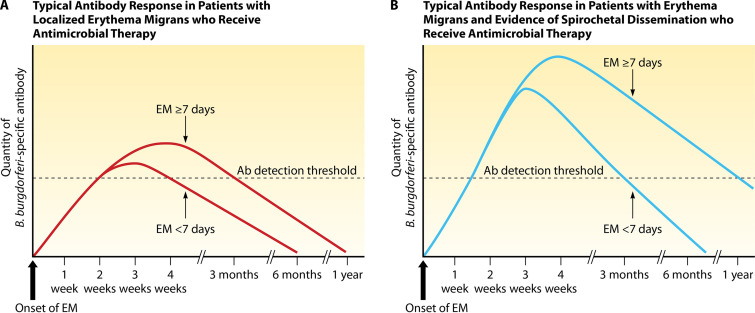
The early antibody response can be impacted by effective antimicrobial therapy (Fig. 1). In general, the eventual degree of expansion and maturation of the antibody response relates proportionately to the duration and extent of infection prior to initiation of effective therapy. When antimicrobial therapy is administered during the window period of seronegativity soon after initial infection, up to half of patients with localized cutaneous infection may remain seronegative during convalescence (78, 91, 93, 95, 124, 125). Failure to develop a detectable antibody response in convalescence strongly suggests that antimicrobial treatment, combined with the innate immune response, resulted in spirochetal elimination in the skin. More commonly, spirochetal killing during localized infection of short duration may dampen, rather than abrogate, the developing antibody response. Peak antibody titers may be lower and antibodies may develop against fewer spirochetal antigens compared with cases not treated promptly or in cases of disseminated Lyme borreliosis; isotype switching from IgM- to IgG-class antibodies may also be prevented for some or all antibody specificities (91, 95, 126).
FIG 1.
Kinetics of the antibody response in patients treated with antimicrobial therapy for localized erythema migrans (A) or erythema migrans with evidence of dissemination (B), as detected by polyvalent ELISAs. As shown, the antibody response typically reaches a higher titer and declines less rapidly when the duration of EM prior to administration of effective antimicrobial therapy is ≥7 days, compared with <7 days. (Based on data from references 93 and 95.)
Within a few weeks after successful antimicrobial treatment of early infection, or within weeks to months after antimicrobial treatment of late infection, antibody titers begin to decline, as determined by semiquantitative or quantitative EIAs. In contrast, immunoblots, which are qualitative tests, do not change much, or change very slowly, after antimicrobial therapy (Fig. 2). In patients with early Lyme borreliosis, prompt treatment may result in disappearance of B. burgdorferi-specific antibodies over time (seroreversion) (122, 125, 127, 128), or a ≥4-fold decline in IgG antibody titer (123, 129). Although antibody responses decline after effective treatment of late-stage Lyme borreliosis (122), a significant decline in IgG antibody titer is generally delayed in proportion to the duration and extent of active infection prior to therapy (93) and may require months after effective treatment of late-stage infection (129, 130). In these patients, the IgG antibody response usually remains detectable at a low level for many years, which is a sign of immune memory rather than active infection.
FIG 2.
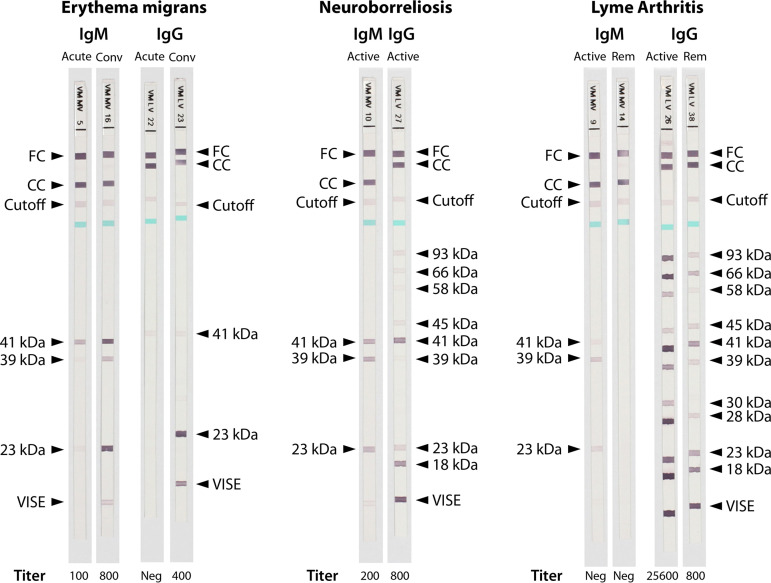
Ladder immunoblots demonstrating typical IgM and IgG antibody responses in individual patients with common manifestations of Lyme borreliosis. The correlating antibody titer, as measured using a semiquantitative whole-cell sonicate ELISA method, is shown at the bottom of each test strip. For the patient with erythema migrans, serum studies are shown using a sample collected during the acute phase of illness (at the time of initial presentation, prior to antimicrobial administration; strips 5 and 22) and using a sample collected in convalescence (28 days after initial presentation, following a standard course of antimicrobial therapy; strips 16 and 23). For the patient with neuroborreliosis, serum studies are shown using a sample collected during active infection (at the time of initial presentation, prior to antimicrobial administration; strips 10 and 27). For the patient with Lyme arthritis, serum studies are shown using a sample collected during active infection (at the time of initial presentation, prior to antimicrobial administration; strips 9 and 26) and using a sample collected 30 years after antimicrobial therapy (remote past infection; strips 14 and 38). Abbreviations: Conv, convalescent; Rem, remote; FC, functional control; CC, conjugate control; Cutoff, cutoff control; kDa, kilodalton; Neg, negative.
IgM antibodies to B. burgdorferi may also persist for many years after successful treatment (122), so the presence of specific IgM antibodies does not necessarily indicate active or recent infection, or reinfection, unless the appropriate clinical presentation is present. For example, in one study, 10% of patients with antimicrobial-treated early Lyme borreliosis and 15% of those with treated Lyme arthritis still had IgM seroreactivity using two-tiered testing 10 to 20 years after active infection (122).
Because B. burgdorferi-specific IgM and IgG antibody responses may persist qualitatively after effective antimicrobial treatment, serologic testing cannot be used to distinguish between active and past (effectively treated) infection, unless seroreversion or a ≥4-fold decline in IgG antibody titer can be demonstrated by analyzing multiple serum samples collected longitudinally. Changes in antibody titer over time are best demonstrated using semiquantitative or quantitative EIAs. For accurate comparison, serially collected samples should be tested together at the same time, using the same assay; however, for the most part, frozen serially collected samples are only available in research settings. In clinical practice, earlier samples are usually not retained, sometimes prompting comparison of past and current laboratory reports, which is unreliable. Thus, if performed correctly, serial measurements of antibody titers can aid in monitoring the response to therapy but may be misleading if values from laboratory reports are compared from different assays or different assay runs.
If patients with a history of antimicrobial-treated erythema migrans are subsequently reinfected, as manifested by a new episode of erythema migrans, IgM-to-IgG isotype switching occurs more rapidly compared to early infections in naive hosts (131–133). IgM bands present on immunoblots during previous episodes remain and may intensify, but IgM antibodies against additional spirochetal antigens typically do not develop (35). Instead, additional IgG bands may sometimes appear early in the course of infection, indicating a more rapid expansion of the IgG antibody response than is typically associated with primary infection (35).
Conventional two-tiered serologic testing.
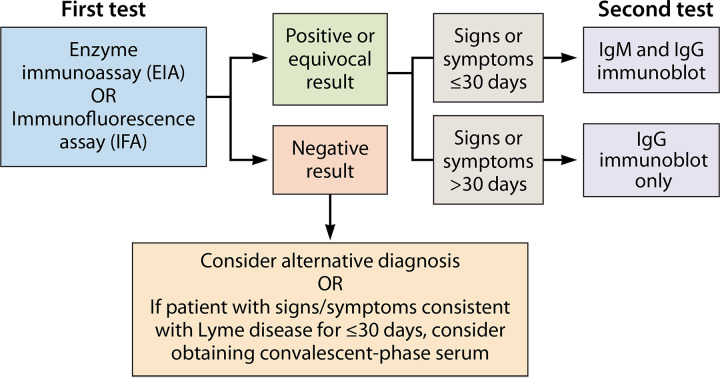
Current U.S. guidelines for the performance and interpretation of serologic tests for Lyme borreliosis were adopted in 1994, at the Second National Conference on Serologic Diagnosis of Lyme Borreliosis in Dearborn, Michigan, with support from the CDC, the U.S. Food and Drug Administration (FDA), the Association of State and Territorial Public Health Laboratory Directors (ASTPHLD), the National Institutes of Health (NIH), the Council of State and Territorial Epidemiologists, and the Clinical and Laboratory Standards Institute (CLSI; formerly the National Committee for Clinical Laboratory Standards) (134). These guidelines established and standardized a two-tiered testing protocol (Fig. 3), which was intended to maximize clinical sensitivity in the first tier, and to maximize specificity in the second tier. This has become known as conventional or standard two-tiered testing. In the first tier, serum is analyzed with a sensitive EIA or (less commonly) an indirect immunofluorescent antibody assay (IFA); specimens negative by the first-tier test need not be tested with a second-tier test (135). If the first-tier test is positive or equivocal, the serum sample is analyzed using standardized IgM and IgG immunoblots, and seropositivity is established only when one or both of the immunoblots is positive according to specific interpretive criteria (Table 1).
FIG 3.
Conventional two-tiered serologic testing protocol for the diagnosis of Lyme borreliosis. (Adapted from reference 228.)
As an added measure to enhance specificity, interpretive guidelines state that a positive IgM immunoblot alone (in the absence of a positive IgG immunoblot) should not be used as evidence of active disease in patients with illness greater than 1 month’s duration (135). Thus, a positive IgM response is meant primarily to support the diagnosis of Lyme borreliosis in patients with erythema migrans. The rationale for this caveat (sometimes termed the “1-month rule”) was that most patients with untreated active infection for >1 month were expected to have a well-developed IgG antibody response, but with more experience it has been learned that it may take up to 2 months for an expanded IgG response to develop. However, IgM reactivity alone lasting more than 1 to 2 months likely represents a false-positive result or indicates previous early Lyme borreliosis (136, 137).
(i) Performance characteristics.
(a) Clinical sensitivity in patients with erythema migrans.
In patients with untreated solitary erythema migrans lesions, the sensitivity of conventional two-tiered serologic testing is positively correlated with the duration of rash prior to serologic testing (114, 138). In a study by Wormser et al. (138), conventional two-tiered testing was 14% sensitive in patients with solitary erythema migrans lesions who were evaluated within 1 week after developing the rash, compared to 86% in patients with localized infection who were evaluated 22 to 30 days after symptom onset (P < 0.001). The duration of symptoms has less influence on sensitivity in patients presenting with multiple erythema migrans lesions, a clinical marker of disseminated infection, but at any given time point up to 3 weeks after onset of the rash, the sensitivity of two-tiered testing is higher in patients with multiple erythema migrans compared to those with solitary lesions (94, 138).
The clinical sensitivity of conventional two-tiered serologic testing in patients with erythema migrans is higher during convalescence (upon completion of 2 to 4 weeks of oral antimicrobial therapy) compared to acute-phase testing. In a large, prospective study of patients with culture-confirmed erythema migrans, Steere et al. reported that sensitivity increased to 53% in convalescence, compared to 17% at baseline, among patients with localized erythema migrans without evidence of dissemination (94). Among erythema migrans patients with evidence of dissemination, indicated either by multiple erythema migrans lesions and/or detection of B. burgdorferi DNA in peripheral blood by PCR, sensitivity in convalescence increased to 75%, compared to 43% prior to antibiotic therapy (94). Similar trends have been reported in several other studies (139, 140).
Besides the intrinsic delay in development of a detectable antibody response, the sensitivity of conventional two-tiered serologic testing in patients with erythema migrans is limited by the second-tier assay itself. Several studies have demonstrated that immunoblots are approximately half as sensitive compared to whole-cell sonicate EIAs, in the acute-phase of erythema migrans (138, 140, 141). Thus, when whole-cell sonicate EIAs are followed by immunoblots in a two-tiered testing algorithm, the overall performance is diminished by the insensitivity of immunoblots in early disease.
(b) Clinical sensitivity in patients with early Lyme neuroborreliosis or Lyme carditis.
Data defining the clinical sensitivity of conventional two-tiered serologic testing are less robust for these clinical manifestations, compared to erythema migrans. Not only are these manifestations relatively uncommon, but direct detection methods capable of confirming a diagnosis of erythema migrans (142) less often yield positive results in Lyme neuroborreliosis or carditis and are of little use as reference methods against which to compare the performance of conventional two-tiered testing. The most convincing studies have defined cases of early Lyme neuroborreliosis or Lyme carditis based on objective clinical features (e.g., the presence of cranial nerve palsy, meningitis, peripheral neuropathy, radiculoneuropathy, or atrioventricular nodal block) and, if applicable, characteristic laboratory abnormalities (e.g., cerebrospinal fluid lymphocytic pleocytosis), and sometimes either concomitant or recent erythema migrans (94, 139–141, 143).
According to such studies, approximately 60 to 90% of patients with early Lyme neuroborreliosis or Lyme carditis are positive by conventional two-tiered serologic testing at initial presentation, prior to the administration of antimicrobial therapy (94, 139–141). However, clinical sensitivity would be higher (90 to 100%) if immunoblot interpretive guidelines did not restrict the use of IgM reactivity in the diagnosis of Lyme borreliosis to an illness of ≤1 month’s duration (94, 139, 140). Some patients with these relatively early manifestations exhibit only IgM immunoblot seropositivity after 4 to 8 weeks of illness, whereas expansion of the IgG response is not yet robust enough to fulfill IgG immunoblot criteria—although some bands, primarily those corresponding to early antigens, are usually present on the IgG immunoblot (Fig. 2) (94, 139, 140). Thus, serologic assays (whole-cell sonicate EIAs and immunoblots) are highly sensitive in second-stage Lyme borreliosis, but the 1-month restriction degrades the calculated sensitivity of conventional two-tiered testing during this stage of the infection.
(c) Clinical sensitivity in patients with Lyme arthritis.
In the northeastern U.S., almost all cases of late-stage Lyme borreliosis manifest with Lyme arthritis, usually developing months to as long as 2 years after initial infection. After B. burgdorferi infection of this duration, a strong IgG antibody response is detected in almost all patients (135). Therefore, the reported sensitivity of conventional two-tiered serologic testing among U.S. patients with Lyme arthritis is usually 100%, or only slightly less (94, 139–141, 144).
(d) Specificity.
A major strength of conventional two-tiered serologic testing is its high specificity. When data from various control groups are combined, overall specificity is reported to be ≥99% (94, 114, 139–141, 145). In the largest study to date, the conventional two-tiered algorithm’s specificity among healthy blood donors (n = 512) who lived in regions of Lyme borreliosis nonendemicity was ≥99.8% (95% confidence interval [CI], 98.9 to 100%) (141). Among asymptomatic control subjects living in regions of endemicity for Lyme borreliosis (n = 1321), specificity in the largest study was ≥98% (95% CI, 98.8 to 99.7%) (141). The slightly lower specificity in comparison to control subjects from regions of nonendemicity likely reflects higher background seropositivity in individuals who have had past or asymptomatic infection. Among symptomatic subjects with no history of Lyme borreliosis but who have illnesses that can mimic Lyme borreliosis, or among subjects whose serum contains potentially interfering substances (Table 2), specificity is still ≥98% (94, 114, 139–141, 145).
TABLE 2.
Infections and inflammatory conditions associated with falsely positive Lyme EIA results
| Condition | Subcategory | Reference(s) |
|---|---|---|
| Infection | Spirochetal infections: syphilis, yaws, pinta, leptospirosis, relapsing fever, Borrelia miyamotoi infection | 143, 153, 175, 215, 375, 376 |
| Tick-borne infections: anaplasmosis, RMSF | 153, 233, 375 | |
| Viral infections: Epstein-Barr virus, cytomegalovirus, varicella, parvovirus B19 | 143, 375, 377–379 | |
| Bacterial endocarditis | 380 | |
| Inflammatory disorders | Autoimmune: rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis | 143, 215, 375, 380 |
| Periodontitis or ulcerative gingivitis | 376 | |
| Pain syndromes | Fibromyalgia | 239, 360, 381 |
| Vaccination | Lymerix (OspA) | 377 |
The high specificity of conventional two-tiered serologic testing is conferred primarily by the second-tier immunoblot, which is significantly more specific than the first-tier EIA (139–141). High immunoblot specificity is achieved by requiring evidence of multiple B. burgdorferi-specific antibodies (i.e., multiple specific bands) for a positive result. However, immunoblot specificity is also enhanced by testing only EIA-reactive samples; the specificity of immunoblots used as stand-alone tests is lower compared to conventional two-tiered testing (102, 146, 147). Also, the reported specificity of immunoblotting has usually been evaluated in the context of research investigations involving experienced laboratories. When immunoblots are performed by less experienced laboratories, weak bands are sometimes erroneously scored as “present,” when they should be ignored, resulting in falsely positive results. This is mostly a problem with IgM immunoblots (136, 137, 144), because the presence of only two specific bands is required for a positive result (Table 1). Incorrectly scoring just one or two weak IgM bands as “present” can change the outcome. In comparison, at least five specific bands must be present for a positive result in IgG immunoblotting.
The problems with visual interpretation of immunoblots can be partially overcome by the use of instrumentation to determine band intensity, but appropriate cutoff intensities have not been standardized or rigorously evaluated, and results must still be verified manually. Also, contrary to current interpretive guidelines (135), positive IgM immunoblots are sometimes inappropriately used as evidence of active disease in patients whose illness substantially exceeds 1 month’s duration. In a retrospective study of 182 patients referred to an infectious diseases subspecialist for presumptive Lyme borreliosis, 50 of 94 patients referred with a reportedly positive IgM Western blot (53%) were regarded as having a falsely positive IgM Western blot (137). Among those 50 patients, 45 (90%) had only a positive IgM Western blot (with a negative IgG Western blot) and symptoms >4 weeks duration at the time of testing; 39 (87%) had symptoms in excess of 8 weeks’ duration (137).
(e) Predictive value.
With any test, the predictive value of a positive or negative result depends on the sensitivity, specificity, and disease prevalence in the tested population, as follows:
| (1) |
where PPV is the positive predictive value and NPV is the negative predictive value. Whereas sensitivity and specificity are fixed characteristics of the test itself, disease prevalence can vary greatly between geographical regions. This is certainly the case with tick-borne infections such as Lyme borreliosis, in which background disease prevalence is heavily influenced by the local ecology. Large regions of the United States are not endemic for Lyme borreliosis, and transmission within these regions is either extremely rare or nonexistent. Thus, despite the high specificity of conventional two-tiered serologic testing, its positive predictive value is low outside regions of endemicity. For example, in a study of patients in North Carolina, the positive predictive value of conventional two-tiered testing was only 10% among individuals who had not traveled to regions of endemicity (148). In nonendemic settings, positive serologic test results—even using highly specific assays and testing algorithms—are usually falsely positive and should be viewed with appropriate skepticism.
(ii) First-tier assays.
(a) First-generation enzyme immunoassays.
First-tier serodiagnostic tests for Lyme borreliosis may be manufactured using several different assay formats and target antigen preparations. The prototypical first-tier tests are “whole-cell sonicate” enzyme immunoassays (WCS EIAs). These assays are constructed by cultivating one or more B. burgdorferi strains in broth medium and preparing a protein lysate from the cultured spirochetes, for use in binding and detecting human anti-B. burgdorferi antibodies present in serum or plasma, typically using an indirect EIA format. In some EIA methods, a preabsorption procedure is incorporated during sample preparation, which may improve specificity by reducing cross-reactive antibodies (149–152), but it also reduces titers of homologous antibodies and thus decreases sensitivity (153–155). EIAs may be polyvalent, meaning capable of detecting human anti-B. burgdorferi antibodies of any isotype (i.e., “total” antibody detection) or they may be isotype-specific, usually targeting IgM-, IgG-, or IgA-class antibodies. IgA antibody responses are common in Lyme disease (156), including in neuroborreliosis (157). However, diagnostic criteria have been based solely on IgM and/or IgG responses, and it has not yet been determined whether measurement of IgA responses could have a beneficial role in diagnostics.
(b) Second- and third-generation enzyme immunoassays.
First-generation EIAs, which are prepared from B. burgdorferi cellular lysates, contain innumerable different antigens and epitopes that bind human anti-B. burgdorferi antibodies, accounting (in part) for their higher sensitivity in comparison with immunoblotting. However, some immunodominant antigens (especially VlsE [100, 158–160]) are not expressed in appreciable quantity during in vitro B. burgdorferi culture and thus are absent or not well represented in assays prepared only from culture lysates. In addition, the use of a single B. burgdorferi isolate in first-tier assays (as is often done in the United States) may limit sensitivity, particularly for the detection of Lyme borreliosis acquired in Europe. In contrast with North America, several Bbsl species (B. afzelii, B. garinii, and B. burgdorferi) are prevalent in Europe, and there is significant heterogeneity among them with respect to the antigens most relevant in serodiagnosis (38, 39).
To maximize sensitivity, second-generation EIAs were developed in which whole-cell lysates are supplemented with particular recombinant or purified antigens, often including multiple forms of the same antigen derived from locally prevalent Bbsl genospecies and strains. While such assays have been commonly used in Europe, few are available on the U.S. market, presumably owing to the comparative homogeneity among B. burgdorferi strains prevalent in North America.
One drawback of first- and second-generation EIAs is that inclusion of numerous antigens in the form of whole-cell protein lysates increases the likelihood of false-positive results from cross-reactive antibodies. Many infectious and inflammatory conditions can cause cross-reactivity in these assays (Table 2), and falsely positive reactions are also common among healthy controls subjects (140, 141, 143).
Recently, EIAs have been developed that contain only one or just a few antigens for binding host antibodies. These third-generation (or “next-generation”) EIAs are prepared using recombinant proteins, synthetic peptides, or synthetic chimeric proteins, representing immunodominant antigens or epitopes important in the antibody response to B. burgdorferi infection (92). This eliminates many potentially cross-reactive cellular proteins that do not induce a strong host antibody response (109, 161). Furthermore, by engineering short, synthetic peptide antigens or chimeric proteins, the least cross-reactive, best-conserved, and most immunodominant epitopes can be selected for inclusion. This strategy is intended to maximize specificity without sacrificing sensitivity (162). Finally, the use of recombinant or synthetic antigens greatly reduces between-lot variability during assay manufacturing, simplifying the process of producing consistent quality and performance. Assays based on whole-cell sonicates of cultured spirochetes may vary in their antigen composition from lot to lot, because some key antigens are differentially expressed depending on the growth phase at the time of harvest and on the number of serial passages to which the cultured strain is subjected (163–167).
(c) VlsE-based enzyme immunoassays.
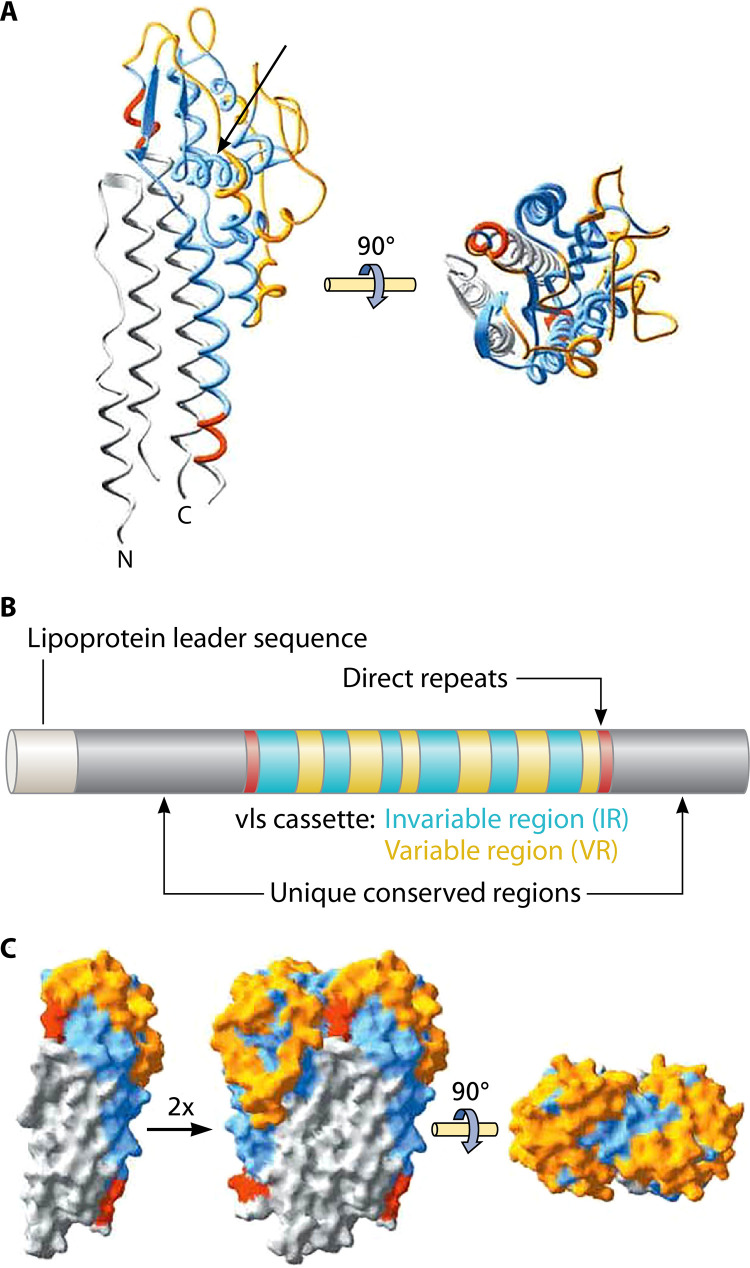
Several third-generation EIAs incorporate VlsE, or a portion of it, as an antigen target. VlsE (variable major protein [Vmp]-like sequence, expressed) is a 34- to 35-kDa surface lipoprotein that helps the spirochete evade the host immune response during infection by undergoing antigenic variation (168). The expressed portion of the vlsE gene contains six centrally located “variable regions” (VR-I through VR-VI) that recombine with multiple unexpressed cassette sequences located upstream on the same linear plasmid, resulting in a high degree of antigenic variation among B. burgdorferi clones infecting an individual host (159, 169).
The six variable regions of vlsE alternate with six conserved “invariable regions” (IR-I through IR-VI), and there are also invariable (but less well conserved) domains located at the 5′ and 3′ ends of the gene (159, 169, 170) (Fig. 4). During infection, VlsE stimulates a strong, early humoral immune response (168, 171), making it an attractive target for serodiagnostic assays. Interestingly, IgM-class antibodies do not bind well to VlsE epitopes and are less consistently detectable compared to IgG-class anti-VlsE antibodies, even at early time points; however, anti-VlsE IgG antibodies appear to develop very early during the course of infection, and IgM responses are usually not found in the absence of IgG reactivity (114, 161, 163). Thus, most single-antigen VlsE-based immunoassays are designed as polyvalent or IgG-specific assays, since there is little advantage in specifically targeting IgM-class antibodies.
FIG 4.
VlsE primary and tertiary structure. (A) The unique conserved N- and C-terminal regions are colored gray, direct repeats are red, and invariant regions of the cassette are blue, whereas variable cassette regions are orange. IR-VI (arrow) forms an alpha helix buried within the tertiary structure, with little surface exposure. (B) Schematic representation of the primary structure (color code as used in panel A). (C) Dimeric model of VlsE based on the crystal structure, illustrating how the formation of potential dimers could effectively shield invariant regions at the monomer-monomer interface. (Republished from reference 183 with permission of the American Society for Biochemistry and Molecular Biology. Note: The figure, as originally published, has been modified here with the addition of an arrow in panel A.)
Some third-generation assays use full-length, recombinant VlsE as an antigen target, whereas others use shorter peptide sequences corresponding to specific epitopes within the parent molecule. Among the latter, the best characterized is the C6 EIA, which employs a 25-mer oligopeptide (the “C6 peptide”) corresponding to the sixth invariable region (IR-VI) within VlsE. This peptide antigen has been a focus for serodiagnostic tests because, among the six invariant regions of VlsE, IR-VI is immunodominant and is the most conserved between strains and genospecies of Bbsl (101). Moreover, anti-C6 antibodies are elicited during infection with all three RST genetic subtypes of B. burgdorferi (172). However, antigenic conservation between Bbsl genospecies is only partial (20, 173). In addition, reactivity in C6 EIAs is frequently seen in patients with Borrelia miyamotoi infection (174), as with WCS EIAs (175). Surprisingly, B. miyamotoi—a relapsing fever borrelia rather than a Lyme-related borrelia—is also a pathogen sometimes found in I. scapularis ticks.
Numerous publications have described laboratory-developed C6 EIAs (94, 114, 163). An FDA-cleared commercial C6 EIA was on the U.S. market for many years but is not currently available. In patients with early- or late-stage Lyme borreliosis, the previously available commercial C6 EIA was comparably sensitive to WCS EIAs, although some studies have found that it was less sensitive in first-stage Lyme borreliosis (acute erythema migrans) (140, 141, 176, 177) and others more (178, 179). However, the commercial C6 EIA’s specificity was significantly greater compared to WCS EIAs in symptomatic or asymptomatic (healthy) control subjects (140, 141, 176, 180, 181).
Based on a limited number of head-to-head evaluations, serologic assays using full-length, recombinant VlsE (rVlsE) as the sole antigen target have similar sensitivity and specificity compared to C6 EIAs (114, 180, 182). However, assays employing full-length VlsE may not detect anti-C6 antibodies; more likely, they detect antibodies directed against other VlsE epitopes. When VlsE assumes its natural, tertiary structure, the IR-VI region (to which the C6 peptide corresponds) forms an α-helix that is buried within the molecule and is largely inaccessible to antibodies (Fig. 4) (183). Thus, based on the resolved crystal structure of VlsE, anti-IR-VI antibodies should have minimal interaction with the intact parent molecule (183), and this has been demonstrated experimentally (184). Although studies to date indicate that C6 EIAs and rVlsE EIAs perform comparably, additional head-to-head evaluations are needed as it is possible that important differences in performance could be revealed among certain patient subpopulations.
(d) Outer surface protein C-based enzyme immunoassays.
OspC is a surface lipoprotein that provokes a strong, early humoral immune response (98, 103, 185). Its immunodominance and usefulness in serodiagnostics have long been recognized; for example, OspC is one of the antigens scored when interpreting IgM or IgG Western blots according to CDC criteria established in 1995 (Table 1). The protein is encoded by a gene located on a circular plasmid (186). Unlike VlsE, which is not expressed until spirochetal transfer to a mammalian host, OspC is expressed while the spirochete is still contained within its tick vector during tick feeding (187). Thus, OspC is available to provoke a host immune response immediately upon initial infection, making it an attractive target for early antibody response detection in diagnostic assays.
Several EIAs have been developed using full-length recombinant OspC (rOspC) (21, 103, 118, 188–190). One complicating factor with this approach is the potential importance of matching the assay’s OspC antigen with that expressed by locally circulating Bbsl strains. In regions like Europe where Bbsl strain diversity is great, there is substantial OspC antigenic diversity and the choice of OspC genotype for immunoassay development may affect performance (21, 39, 191–195). However, this problem has only minor practical significance, since strain-restricted OspC antibody responses are uncommon (196, 197). Of note, the IgM response to OspC wanes during prolonged infection (93, 197–199) and IgG class-switching to OspC is typically not detected in early disease (198).
As an alternative to rOspC, serologic assays have been made using a short (10-amino-acid) synthetic peptide called pepC10, which corresponds to a conserved motif located at the carboxy terminus of OspC (114, 198, 200). Whereas full-length rOspC is known to contain cross-reactive epitopes (21, 105, 198), the use of a single epitope (pepC10) improves specificity by eliminating these cross-reactive epitopes, although the effect is minor (198). Using a conserved epitope also helps address concerns about universality. However, these advantages are balanced by evidence that pepC10 is not among the immunodominant epitopes of OspC (201). In a head-to-head comparison between a rOspC IgM EIA and a pepC10 IgM EIA, the latter was slightly less sensitive in patients with EM (44% versus 36%, respectively; P < 0.04) when assay cutoffs were set to produce equal specificity between the two assays (198). A different OspC epitope, OspC1, was recently discovered through linear epitope mapping and may produce improved EIA sensitivity and specificity compared to pepC10 (105), although further study is needed.
(e) Singleplex EIAs combining rVlsE or C6 peptide with rOspC or pepC10.
Multiantigen singleplex EIAs have been designed either by combining the OspC antigen (or pepC10) with VlsE (or C6) in a single assay, or running two single-antigen tests in parallel and combining results (i.e., requiring reactivity in both assays for an overall interpretation of seropositive) (114, 146). Using this combination of antigens/epitopes, sensitivity can be improved in early Lyme borreliosis compared with either antigen alone, although specificity is slightly decreased (114, 144).
(f) Other antigens and epitopes.
Although VlsE and OspC have been a focus of third-generation EIA development, assays based on many other antigens or epitopes have been developed or proposed. B-cell epitope mapping of specific B. burgdorferi protein antigens has accelerated this effort, helping to identify attractive peptide targets for assay development based on immunodominance, conservation across Bbsl genospecies and limited cross-reactivity (20, 105, 112, 113, 115, 202–204). Conversely, characterization of epitopes can show the nonutility of specific antigens due to extensive cross-reactivity in sera from control subjects (205). Epitope mapping has also shown that humoral responses against distinct epitopes within a particular protein antigen may develop asynchronously, with potential implications for staging of the infection (171, 206). Broader proteomic approaches have revealed undiscovered antigens, and confirmed the immunogenicity of proteins already established as antigens, defining additional possible targets for assay development (108, 110). Rather than using a single epitope or antigen, many novel assays are being developed using a few individual epitopes or antigens together, either fused into chimeric molecules (162, 207, 208) or separated in multiplexed configurations (92, 146, 209–213).
(g) Indirect immunofluorescent antibody assays.
EIAs have largely replaced IFAs as first-tier tests, although both are acceptable according to current guidelines (135). IFAs are typically manufactured by coating a multiwell microscope slide with whole B. burgdorferi spirochetes (214). After incubation with patient serum and staining, each slide well must be examined visually using a fluorescence microscope, a manual process which requires both skill and experience to avoid under- or overinterpretation. In contrast, the output of EIAs is measured objectively using a spectrophotometer or other instrumentation.
Several studies have demonstrated that EIAs are more specific and sensitive compared with IFAs (154, 215, 216). EIAs are also more convenient as first-tier assays, because in many cases they can be performed using semiautomated or fully automated instruments, enhancing throughput and allowing uni- or bidirectional interfaces with the laboratory information system. One advantage of IFAs, however, is that they are often designed as semiquantitative assays, producing an antibody titer; most commercial EIAs are qualitative tests. As with EIAs, IFAs may be designed as polyvalent assays or as isotype-specific assays.
(h) Capture enzyme immunoassays.
Capture EIAs are formatted as sandwich ELISAs, and are usually immunoglobulin class-specific. Although substantially more complex than the indirect EIA method, the capture EIA method may be more sensitive in early Lyme borreliosis for the detection of a B. burgdorferi IgM antibody response (126). This assay design also lends itself to semiquantification but is more cumbersome than the indirect EIA format.
(i) Quantifying the antibody response.
Although EIAs can be developed as qualitative, semiquantitative, or quantitative assays, most commercial B. burgdorferi EIAs are qualitative. Qualitative assays are interpreted categorically as positive, negative, or equivocal, whereas semiquantitative assays can determine relative antibody levels and may provide an antibody titer; quantitative assays provide the absolute antibody concentration. Although qualitative EIAs produce a numerical result—typically an optical density (OD) index value—this value is translated into a categorical result using predetermined cut-points, and the numerical value is not always reported in the medical record (depending on local preferences). OD index values can give a very rough idea of relative antibody levels (217), but this approach is imprecise. With a typical indirect EIA, the antibody concentration is related to OD by a logarithmic function (218) and when plotted on a logarithmic scale the dose-response curve is usually sigmoidal, with only the central region of the curve being linear.
Qualitative first-tier assays are usually adequate for testing individual samples, whereas semiquantitative or quantitative assays are particularly useful when comparing multiple serum samples collected from a single patient at different time points to assess for seroconversion, seroreversion, or reinfection. In some cases, these changes can be documented using qualitative assays, when serially collected samples transition from negative to positive or vice versa, but semiquantitative or quantitative assays are more informative since they reveal the amplitude of the antibody response at various time points. This is especially helpful when serially collected specimens are all reactive, but the antibody titer is rising or falling over time. Such changes cannot be assessed reliably using qualitative assays.
It has been suggested that first-tier EIA OD index values may be used to direct empirical antibiotic therapy in the absence of a second-tier assay result, if the value exceeds a certain threshold. In two studies using qualitative Lyme borreliosis EIAs, Nigrovic and colleagues reported that high OD values are strongly predictive of true disease (217, 219). These authors concluded that sufficiently high EIA values might obviate supplemental immunoblotting or at least could inform clinical decision-making while waiting for supplemental test results.
(iii) Second-tier assays.
(a) Western blots.
Currently, most FDA-cleared second-tier serodiagnostic tests are immunoblots. Early second-tier immunoblots were prepared by Western blotting, and the Western blot test format is still commonly used (220). Although high-quality B. burgdorferi Western blot test strips are commercially available, visual interpretation of Western blots is complex and subjective. It involves locating bands of interest and then determining whether each band’s intensity is sufficient to score it as present. In experienced hands, the method is reliable and reproducible (221), but in routine practice there is limited interlaboratory reproducibility (137). To reduce the subjectivity of visual interpretation, densitometric imaging techniques for measuring band intensity have been developed (102, 222, 223). These techniques are helpful, but standardized criteria for determining appropriate band intensity cutoffs using densitometry have not been established, and these interpretive aids are typically not FDA-cleared along with the test kits, meaning that imaging tools can be used as an aid but visual examination is still required to confirm preliminary results obtained in this manner.
(b) Line immunoblots and microarray immunoblots.
A newer generation of second-tier serologic tests has become available. Rather than starting with a relatively crude protein lysate and transferring all the proteins (within a certain size range) onto a membrane, as in Western blotting, individual purified or recombinant antigens of interest are directly applied to the membrane in specified locations. The membrane can be configured to resemble a Western blot, with protein antigens applied in lines or bands across a narrow strip and sorted by molecular weight (223) (Fig. 2), in which case the assay may be called a “line immunoblot,” “line immunoassay,” or “ladder immunoblot.” Initial IgM and IgG line immunoblot assays marketed in the United States have included the same 3 or 10 protein antigens, respectively, that are scored using standardized interpretive criteria (Table 1) (135).
Direct application of specific protein antigens to the membrane addresses an important drawback of Western blotting. Western blots prepared from culture lysates are frequently cluttered with uninformative bands that occur nearby those that are intended to be scored, because B. burgdorferi protein lysates contain numerous cross-reactive antigens that are common to, or similar to, antigens expressed by ubiquitous bacteria (155, 224). Antigen migration during electrophoresis of protein lysates can also vary from run to run or from one part of the gel to another, slightly altering band location from strip to strip. These features necessitate careful comparison of each test strip against a lot-specific band locator, to identify bands of interest—a subjective, time-intensive process that requires experience. Moreover, unrelated proteins of similar molecular weights can colocalize at an identical location on the strip, such that antibody cross-reactivity against uninformative protein antigens may produce bands that occur at the same location as those produced by reactivity against antigens of interest (110, 225). These potential pitfalls are avoided with line immunoblots, because only the antigens of interest are applied to the membrane, and the antigens are applied as bands that are well-separated from one another and evenly spaced across the test strip. This results in a less cluttered test strip that is easier to interpret.
The direct application of antigens to line immunoblots also allows more flexibility in optimizing their antigen composition. For example, OspC is an important early antigen in serodiagnostic testing but is not well conserved across Bbsl genospecies (105). Several commercial European line immunoblots therefore use a combination of OspC antigens derived from multiple genospecies in an attempt to improve sensitivity (226). Line immunoblots may also include antigens like VlsE that are not well represented in B. burgdorferi culture lysates (139, 226) (Fig. 2).
Although line immunoblots are formatted as membrane strips to resemble Western blots, antibody detection assays using selected antigens or epitopes can be designed in other configurations (211, 212, 227). One FDA-cleared second-tier test retains the membrane surface of line immunoblots but reduces it to a compact circle that fits into wells of a standard 96-well microtiter plate (227). Recombinant or purified B. burgdorferi antigens—the same antigens that are scored in conventional Western blotting—are applied at defined positions creating IgM or IgG “microarray immunoblots” (227). The main advantage of this format, compared to Western or line immunoblots, may be that immunoblots can be processed using standard automated microtiter plate ELISA instruments, allowing for full automation without the need for a dedicated processing instrument, although after processing the microarrays must be interpreted using a special optical reader coupled with proprietary software (227). In one study, this microarray immunoblot assay demonstrated >95% agreement with Western immunoblot and line immunoblot assays, using a consensus standard, and reduced technologist time (227).
(iv) Interpretation of results.
Conventional two-tiered testing can have three possible outcomes: (i) a negative first-tier test, in which case second-tier immunoblots should not be performed; (ii) a reactive (positive or equivocal) first-tier test, with negative IgM and IgG immunoblots; or (iii) a reactive first-tier test, with a positive IgM and/or IgG immunoblot. For each outcome, multiple potential interpretations exist. In an individual case, it is not always possible to determine which of the alternative interpretations is correct, but often the most likely possibility can be determined by correlating clinical features, patient history and risk factors with a detailed assessment of the laboratory findings (83, 84, 228).
(a) Negative first-tier test.
A negative first-tier test may represent a true-negative result or a false-negative result, depending on the circumstances. In general, a negative first-tier test in a patient who has been ill for longer than a few weeks is evidence against B. burgdorferi infection, since most individuals with Lyme borreliosis will have mounted a detectable, specific antibody response by that time. In contrast, falsely negative results are frequent in patients who have been infected for only days (Fig. 1). If the patient will not be treated empirically, one could repeat the first-tier test after an additional several weeks to assess for delayed seroconversion (Fig. 5). If the patient has recently traveled and could have acquired Lyme borreliosis in a region outside North America, testing may be repeated using an assay capable of detecting antibodies directed against European species and strains, particularly B. afzelii and B. garinii. This is less of a problem if a U.S. assay with the C6 peptide antigen is used (18, 19). In an immunosuppressed patient, the antibody response may be less robust but is usually still detectable. Although rare, a person with profoundly deficient humoral immunity, such as a patient on chemotherapy targeting B cells (229–232), would not be able to mount an antibody response.
FIG 5.
Recommended laboratory testing strategies for North American patients presenting with common manifestations of Lyme borreliosis.
(b) Reactive first-tier test, with negative IgM and IgG immunoblots.
Discordance between the first-tier and second-tier tests is frequent during early seroconversion in patients with erythema migrans or other early manifestations, because first-tier tests are typically more sensitive than immunoblots or similar second-tier assays. Similarly, a reactive (positive or equivocal) first-tier test with negative immunoblots or a negative first-tier test with a positive immunoblot is sometimes seen in patients with past, treated infection. In such cases, early antibiotic treatment may have blunted the humoral immune response, or an initial antibody response may have waned over time (partial seroreversion). Discordance between first- and second-tier tests should prompt additional workup if the probability of early Lyme borreliosis with partial seroconversion is high. In this scenario, antibody testing of paired serum samples collected at least 2 to 3 weeks apart may be useful.
First-tier tests are generally less specific than immunoblots, and false-positive first-tier tests with true-negative immunoblot results may be seen in patients with other illnesses, healthy individuals, or in those who received vaccination for Lyme disease with OspA (Table 2). In many cases, false reactivity in the first-tier test will produce an OD index measurement in the equivocal or low-positive range, but this is not a uniform finding (219). If the probability of active Lyme borreliosis is low, additional testing is not indicated when second-tier immunoblots are negative, especially if the duration of illness had exceeded 2 months at the time of sample collection.
(c) Reactive first-tier test, with positive IgM and/or IgG immunoblots.
When a first-tier test is reactive and an IgM and/or IgG immunoblot is positive by accepted criteria (Table 1), the findings can support a diagnosis of Lyme borreliosis with a few caveats. It should be remembered that the clinical picture of active infection associated with IgM reactivity alone is usually erythema migrans. Also, because a detectable antibody response can persist for years despite successful eradication of B. burgdorferi, it is not possible based on standard serologic testing of a sample collected at a single time point to differentiate between active and past infection (122, 129, 130, 233). Clinical correlation is necessary, and testing of serially collected samples may help. An increase in antibody titer with expansion of the antibody repertoire over time during the symptomatic period, and isotype switching from IgM to IgG antibodies supports the diagnosis of active B. burgdorferi infection. Conversely, demonstration of a contracting or waning antibody response is evidence of past (resolved) infection. Kinetic changes are best demonstrated using a semiquantitative or quantitative assay, if available. The immunoblot, a nonquantitative test, does not change much (or very slowly) after antibiotic therapy and resolution of infection (Fig. 2). Importantly, for valid comparisons of minor changes, it is necessary to analyze serially collected samples in the same assay run.
Although immunoblots are quite specific, false-positive immunoblot results do occur. Beyond detection of antibody reactivity related to past infection, other illnesses may produce falsely positive immunoblots. Cross-reactive antibodies may produce falsely positive first- and second-tier test results, since the tests are not independent (234). The problem is exacerbated by over-reading faint immunoblot bands and by the tendency of some laboratories to apply nonstandard interpretive criteria to Western blots (181). This disproportionately affects IgM blots (137), since only two specific bands are necessary to meet accepted criteria. Thus, nonstandard interpretive criteria should be avoided (181, 224).
Modified two-tiered serologic testing.
Modified two-tiered testing (MTTT) for Lyme borreliosis involves the sequential or concurrent use of two or more orthogonal EIAs without the use of immunoblots (Fig. 6 and 7). As with conventional two-tiered testing, reactivity in two assays is required for a positive result in order to improve overall specificity. The CDC has endorsed the MTTT approach assuming the use of EIAs that have been cleared by the FDA for use in MTTT algorithms (235).
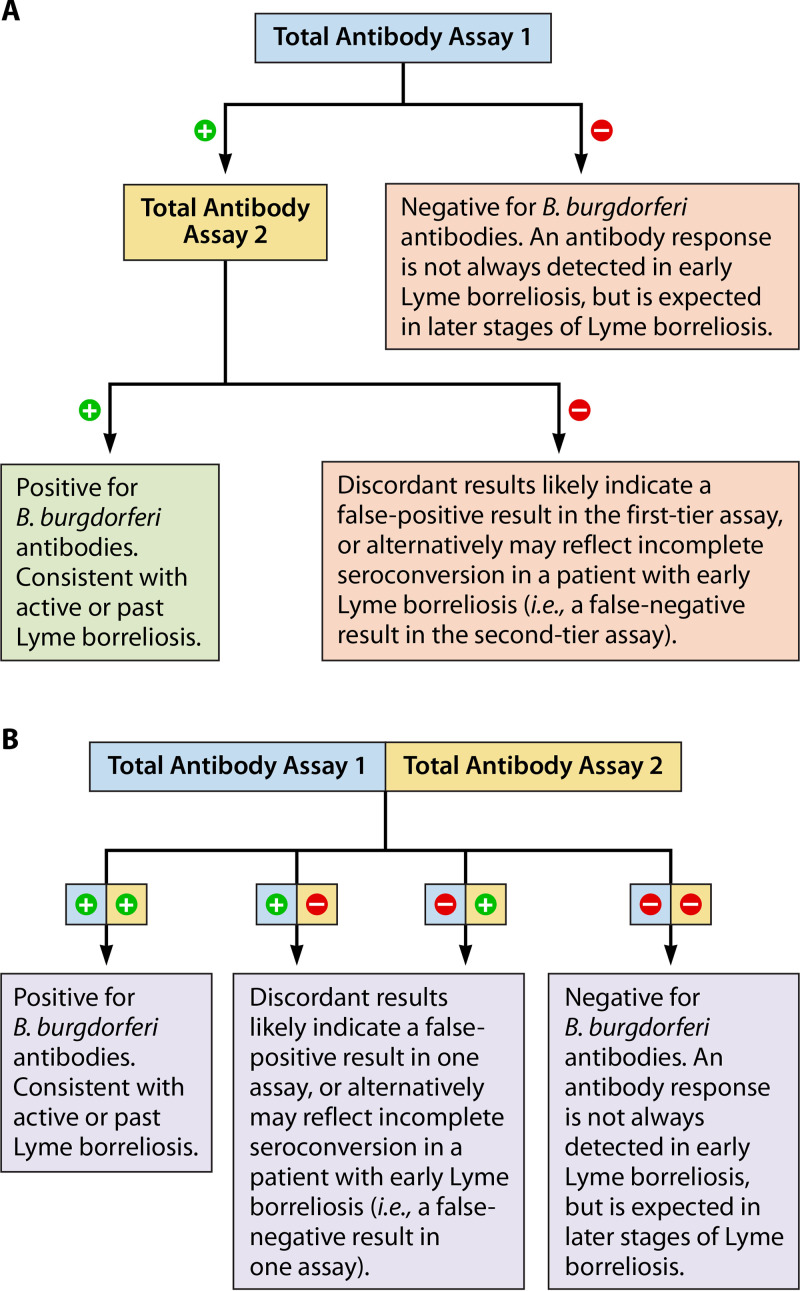
FIG 6.
Modified two-tiered serologic testing protocols for the diagnosis of Lyme borreliosis, using two polyvalent (total antibody) assays. Two orthogonal EIAs are used, either sequentially (A) or concurrently (B).
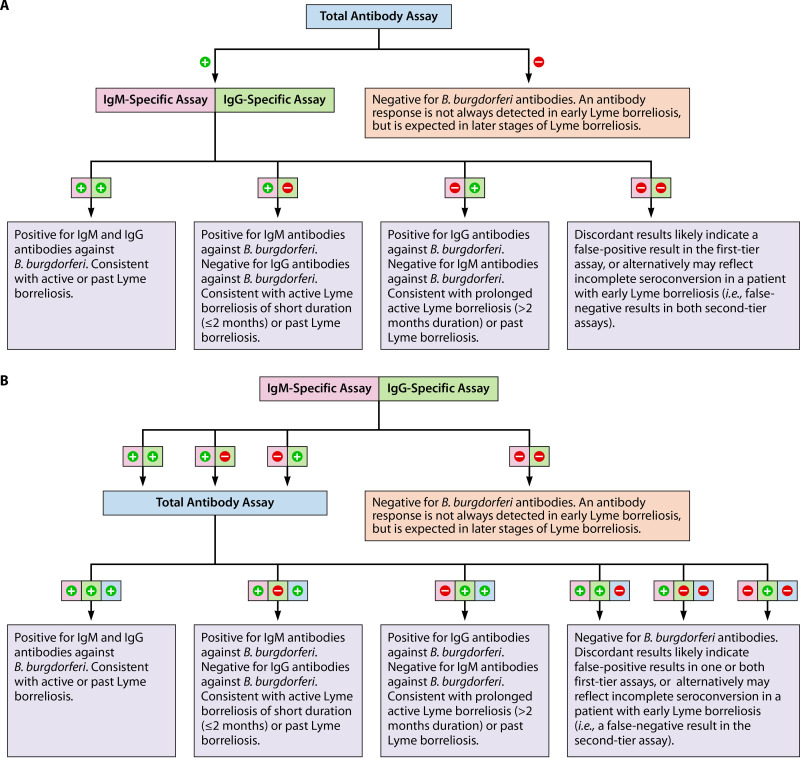
FIG 7.
Modified two-tiered serologic testing for the diagnosis of Lyme borreliosis, using one polyvalent (total antibody) EIA, one IgM-specific EIA, and one IgG-specific EIA. The polyvalent assay can be used in the first tier, followed by orthogonal IgM- and IgG-specific assays in the second tier (panel A) or, alternatively, the IgM- and IgG-specific assays can be used in the first tier, followed by an orthogonal polyvalent assay (panel B). If desired, all three tests could be performed in parallel (concurrently) rather than sequentially (not shown).
In the ideal situation, independent tests would be chosen for MTTT protocols to maximize overall specificity; by definition, independent tests are not susceptible to the same false-positive effects (236). Different EIAs for Lyme borreliosis are often not independent; however, pairing two nonindependent EIAs in an MTTT protocol may still improve specificity (237). Rather than independence, what is necessary is a sufficient degree of orthogonality between the tests, meaning that there are significant differences between the two tests in their format and design, and/or antigenic constituents (targets), that lead to a reduction in the probability of concurrent false-positive results (238). With the advent of third-generation EIAs for Lyme borreliosis, which contain only one or just a few antigen targets, the opportunity has arisen to formulate MTTT protocols involving paired EIAs with substantially different antigenic constituents and thus a sufficient degree of orthogonality to improve specificity compared to either test alone.
Although two polyvalent (total antibody) tests can be paired, additional diagnostic information can be obtained by using separate IgM- and IgG-class specific EIAs in at least one tier of the MTTT approach (Fig. 7) (239). For example, the U.S. FDA has cleared a test system in which a polyvalent third-generation EIA is used in the first tier, followed by separate IgM and IgG WCS EIAs (240).
(i) Performance characteristics.
One of the most validated MTTT protocols for Lyme borreliosis involves a WCS EIA (first-generation EIA) and a C6 EIA (third-generation EIA), performed sequentially or concurrently (18, 140, 141, 176, 177, 241, 242). Using this “2-EIA” protocol, specificity is greater than with either test alone and equal to the specificity of conventional two-tiered testing with a WCS EIA followed by immunoblots (92). This combination works well because the antigenic constituents of the two tests differ. The C6 peptide is either absent or minimally represented in WCS EIAs, since its parent molecule (VlsE) is not expressed well when B. burgdorferi is cultured in vitro (158). Furthermore, to the extent that the C6 parent molecule (VlsE) might be expressed in vitro, anti-C6 antibodies often do not react with VlsE and vice versa (184).
In patients with erythema migrans, MTTT is more sensitive compared to conventional two-tiered testing (92). This approach is also more sensitive in patients with early neuroborreliosis or acute Lyme carditis because of the “1-month rule,” after which IgM immunoblot reactivity is not used to support the diagnosis (92). In such patients, it is now known that it can take as long as 8 weeks for IgG antibodies to develop against enough antigens to meet IgG immunoblot criteria (140). The MTTT approach does not retain the 1-month rule, even if an IgM class-specific EIA is used in the algorithm (Fig. 7) and therefore patients who still have predominantly IgM reactivity during the 1- to 2-month period are nevertheless identified as seropositive. For example, if a polyvalent VlsE-based test is followed by separate IgM and IgG whole-cell sonicate tests, a reactive VlsE test and a positive IgM whole-cell test may be used to support the diagnosis of early Lyme disease even if symptoms have persisted for 2 months.
In modified two-tiered testing protocols, other third-generation assays can substitute for the C6 EIA, with equal sensitivity and specificity (176, 177, 239, 243). Similarly, two or more orthogonal third-generation EIAs can be used, without the use of a WCS EIA (177, 213, 239, 240, 243).
(ii) Advantages and limitations.
Beyond improved sensitivity in early Lyme borreliosis, the modified two-tiered testing offers a number of advantages compared to conventional two-tiered testing, owing to elimination of the immunoblot component in the second tier (92, 244). Whereas blot interpretation is subjective, EIA results are obtained using an instrumented reader, which is an objective process. This should improve reproducibility between tests performed in different laboratories. In addition, protocols involving immunoblotting are more expensive than MTTT protocols (245).
The techniques involved in performing EIAs are also well within the capabilities of most clinical laboratories, even small ones. This is not the case with immunoblotting; due to the complexity of immunoblot interpretation and the limited availability of automated instrumentation to perform the testing procedures, the majority of clinical laboratories do not perform immunoblots on-site, requiring the use of reference laboratories. Thus, with MTTT, more clinical laboratories should be capable of performing both elements of two-tiered testing, further lowering costs and shortening turnaround time associated with reliance on commercial reference laboratories. If the EIAs are performed concurrently, instead of sequentially, even greater improvements in turnaround time can be achieved.
Finally, the results of MTTT are simpler to understand than immunoblot results. For example, patients with other diseases or healthy individuals commonly have a few bands on immunoblots, but do not meet criteria for a positive result. Banding patterns that do not meet required criteria may cause confusion among physicians and patients. In contrast, for each tier of the MTTT algorithm, only a categorical result (positive, equivocal, or negative) is provided in the laboratory report, and the 1-month rule is eliminated. At least one test in each tier must be reactive (positive or equivocal) for an overall positive result. This should alleviate some of the confusion associated with serologic testing for Lyme borreliosis. For routine cases, in which objective signs consistent with a well-described Lyme borreliosis manifestation are present, with no history of Lyme borreliosis, a categorical result is likely sufficient.
The main limitation of MTTT is that, by eliminating the immunoblot component, detailed information about the host’s antibody response to an array of individual antigens is lost (92, 244). This detailed information can provide insight into the extent and maturity of the antibody response, which is important in the evaluation of nonspecific, complex or rare manifestations of the disease. Examples may include patients with monoarticular arthritis; patients with an unusual manifestation of Lyme borreliosis, such as ocular involvement; patients without current or previous objective signs of Lyme borreliosis, such as erythema migrans (EM) or seventh nerve palsy, presenting with a possible manifestation of the disease; or patients who have been treated empirically for potential Lyme borreliosis but who have not responded.
A detailed analysis of the antibody response is also helpful in evaluating patients for potential B. burgdorferi reinfection, or for evaluating patients without a known history of Lyme borreliosis but who are living in areas of hyperendemicity, where the background prevalence of Lyme borreliosis is high (>5 to 10%) and the potential for baseline seroreactivity is commensurately elevated. Patients with symptomatic or asymptomatic past infection are likely to remain seropositive, which complicates the interpretation of serologic testing. In such cases, conventional two-tiered testing with immunoblotting may be more informative, especially if samples collected at multiple time points are analyzed. Alternatively, the immunoblot component can be replaced by a validated multiplexed serologic assay that is assembled on a more convenient platform.
Evaluation for B. burgdorferi-specific intrathecal antibody synthesis in patients with suspected CNS Lyme neuroborreliosis
(i) Methodology.
Because IgG antibodies diffuse back and forth across the blood-brain barrier, and because inflammatory conditions may disrupt the blood-brain barrier allowing diffusion of any immunoglobulin subtype, a test for intrathecal antibody synthesis must account for transudation of B. burgdorferi-specific antibodies across the blood-brain barrier from serum into cerebrospinal fluid (CSF). Thus, to evaluate for intrathecal antibody production, concomitant serum and CSF samples must be tested; analysis of CSF without analysis of paired serum is uninformative (246). The usual approach is to calculate the Lyme antibody index, which is the ratio of CSF B. burgdorferi antibody concentration to serum B. burgdorferi antibody concentration, corrected to account for total (polyclonal) immunoglobulin transudation across the blood-brain barrier (246). Although the antibody index can be measured using a polyvalent (total antibody) assay, the use of immunoglobulin class-specific immunoassays is preferred because the kinetics of passive diffusion differ based on the size of the immunoglobulin molecule, and this influences correction factors. Also, the immunoassays must be quantitative or semiquantitative. Immunoblots, which are not easily adapted as quantitative or semiquantitative assays, do not work as well as EIAs or IFAs for this application (247), and interpretive criteria have not been established for CSF analysis using immunoblots.
For IgG-class antibody determinations, a typical method—although methods currently are not standardized—is to measure the total IgG concentration in paired CSF and serum samples, usually by nephelometry, and calculate the CSF/serum total IgG ratio. In parallel, the concentration of B. burgdorferi-specific IgG antibody is measured in the CSF and serum samples, often using an indirect EIA technique adapted for semiquantitation. The CSF/serum B. burgdorferi IgG ratio is then divided by the CSF/serum total IgG ratio to determine the Lyme IgG antibody index (247), as follows:
| (2) |
By including the total IgG ratio in the calculation, passive diffusion of IgG antibody across the blood-brain barrier—whether at a normal rate or at an accelerated rate due to barrier dysfunction—is accounted for. An alternative approach is to dilute the serum sample to a predetermined final dilution suitable for the immunoassay in use, and then dilute the CSF sample such that its total IgG concentration matches that of the diluted serum sample (218). The diluted serum and CSF samples are then assayed for B. burgdorferi antibodies in the same run, and the Lyme IgG antibody index is determined by calculating the ratio of the CSF value to the serum value.
Normalizing the CSF/serum B. burgdorferi IgG ratio to the CSF/serum total IgG ratio (see equation 2) is a sound approach unless there is intrathecal synthesis of total (polyclonal) IgG, in addition to total IgG antibody present in the CSF due to transudation; in this case, the calculated Lyme antibody index is artificially low unless corrected. The presence or absence of intrathecal total IgG production can be assessed by comparing the CSF/serum total IgG ratio with the CSF/serum albumin ratio, as albumin is never produced intrathecally and can only enter the CSF by passive diffusion (247–249). If intrathecal total IgG production is determined to be present, the usual calculation (see equation 2) can be corrected using an equation involving the CSF/serum albumin ratio, as described by Reiber et al. (246, 249).
An alternative approach to determining the Lyme IgG antibody index is to normalize the CSF/serum B. burgdorferi IgG ratio by dividing it by the tetanus toxoid IgG ratio (247). This method avoids a minor pitfall of the method shown in equation 1, namely, that abundant production of intrathecal B. burgdorferi-specific IgG antibody increases the total CSF IgG concentration, paradoxically lowering the Lyme IgG antibody index value (247). In contrast, tetanus toxoid antibodies are not produced intrathecally and are produced independently from B. burgdorferi antibodies (247).
Instead of using correction factors, one can substitute a capture EIA technique for an indirect EIA technique. This practical method, which involves separate antibody class-specific assays (IgM, IgA, and IgG), inherently compares the amount of IgM, IgA, or IgG B. burgdorferi-specific antibody to the total amount of IgM, IgA, or IgG antibody, respectively, and thus obviates the need separately to measure total antibody in the samples (246). Semiquantitative values obtained by testing CSF samples are simply compared with values obtained using paired serum samples to obtain the Lyme antibody index (157, 250).
(ii) Results interpretation.
If all B. burgdorferi antibody present in the CSF is due to transudation across the blood-brain barrier, the Lyme antibody index ratio for any immunoglobulin isotype should be 1.0. The higher the index ratio, the higher the likelihood that B. burgdorferi-specific antibody is being produced intrathecally (247). In our experience, we require an index of ≥1.3 before calling the test positive, although other cutoffs are used (249, 251), and a standard reference range has not been established. Although an elevated Lyme antibody index is evidence of intrathecal B. burgdorferi antibody production, it does not differentiate between active and past neuroborreliosis. As with serum antibody responses to B. burgdorferi, intrathecal antibody production can persist for months or years after treatment (252).
(iii) Clinical utility.
Neuroborreliosis may involve the central nervous system (primarily the meninges), the peripheral nervous system, or both. In patients without apparent central nervous system involvement, and without a CSF lymphocytic or monocytic pleocytosis, production of B. burgdorferi-specific intrathecal antibodies is not expected (253), although it occurs occasionally (218, 251). In contrast, one study demonstrated that 12 of 13 United States patients with Lyme meningitis (92%) had an elevated Lyme antibody index, most commonly IgA but usually with antibodies to multiple isotypes (157). Another study demonstrated 53% sensitivity among 15 U.S. patients with Lyme meningoradiculitis and 100% specificity, using measurement of the Lyme total antibody index and an index value cutoff of 1.3; the sensitivity was 87% and specificity was 93% when a cutoff of 0.9 was used (251). In this and a separate study (218), an elevated Lyme antibody index was also found in most patients with acute-onset or indolent Lyme encephalomyelitis, a rare entity. (Nearly all other studies of Lyme antibody index measurements have involved European patients.) Thus, evaluation of the Lyme antibody index is most useful in patients with potential CNS Lyme disease, especially acute Lyme meningitis—meaning those with exposure risk who have laboratory evidence of meningitis (CSF pleocytosis, with a lymphocyte or monocyte predominance) and detectable serum antibodies against B. burgdorferi. In U.S. patients, a serum antibody response is almost always detectable by the time signs or symptoms of neuroborreliosis have developed, at least with EIAs, and usually with immunoblots. Rarely, however, intrathecal antibody production of B. burgdorferi-specific antibodies precedes peripheral production. This phenomenon is well described in Europe (254) but has seldom been observed in North America (253).
Direct Detection Methods
Conventional or modified two-tiered serodiagnostic approaches have high sensitivity after the first several weeks of infection and are highly specific. Second- and third-generation tests have improved performance. However, this type of indirect test does not reliably distinguish active from past infection. In many infections, direct detection methods are available for that purpose, but such tests have been more problematic in Lyme borreliosis.
Direct microscopic visualization.
In contrast with relapsing fever borreliae, direct visualization of Lyme-related borreliae in blood or other infected tissues is very challenging and easily subject to misinterpretation. The in vivo organism burden is usually so low in primary tissue samples that direct visual detection is not sensitive or practical as a first-line diagnostic approach, or even an adjunctive test (255). Using light microscopy, B. burgdorferi spirochetes can be visualized using Romanowsky stains (e.g., Giemsa) (11, 256) or with silver impregnation or immunohistochemical staining techniques (256). Gram staining is usually unsuccessful (256). Among silver impregnation staining methods, the preferred methods include the modified Dieterle silver stain (256, 257) or Bosma’s modification of the Steiner stain (Bosma-Steiner) (256, 258). The Warthin-Starry stain will also highlight the organisms (256, 258, 259), but less sharply (258), and the routine (unmodified) Steiner stain does not work well (258). The spirochetes can be visualized using fluorescence microscopy, for example with the acridine orange stain (256) or with direct fluorescent antibody staining (260). Live, cultured organisms can be visualized using dark-field or phase-contrast microscopy (214).
In combination with serum antibody testing, microscopic tissue examination for B. burgdorferi spirochetes may serve an adjunctive diagnostic role, but direct examination requires considerable experience. Prior to antimicrobial treatment, Lyme-related borreliae can be visualized in skin samples from patients with erythema migrans (258, 261), occasionally in cardiac tissue from patients with Lyme carditis (262), or rarely in synovial tissue from patients with Lyme arthritis (258, 263). However, it is rare that one sees an entire organism, and it can be difficult to distinguish an organism from connective tissue fibers. Still, recognition of characteristic histopathological patterns can help support the diagnosis of erythema migrans or borrelial lymphocytoma, when the diagnosis is unclear, and is essential for the diagnosis of acrodermatitis chronica atrophicans (264–266). Direct microscopic examination of blood or cerebrospinal fluid is not useful in the diagnosis of Lyme borreliosis.
Borrelia culture.
Lyme-related borreliae can be cultivated in vivo using laboratory animals (e.g., mice, hamsters, or rabbits) or in vitro using artificial media (214). Their nutritional requirements are substantial, and borreliae will not grow in routine bacteriologic culture media. Complex, highly enriched artificial medium is required for in vitro culture. The most successful recipes have been modifications of Kelly’s medium, such as BSK-II medium (267), BSK-H medium (268), and Kelly-Pettenkofer (MKP) medium (269). Among these, BSK-H is the only commercially available formulation and is a more standardized medium, with less variability in its performance from batch to batch and a longer shelf-life with refrigeration (268). Various antibiotics may be added to provide selectivity against commensal flora when skin or other nonsterile sites are cultured (214).
Lyme-related borreliae can be cultivated at core body temperature (37°C), but a slightly lower temperature incubation is optimal (32 to 34°C) (214). The spirochetes are microaerophilic; culture tubes should be filled to near capacity, to minimize ambient air in the container, and capped tightly to prevent loss of CO2 from the medium (214). Although growth can frequently be detected within 1 or 2 weeks, 8 to 12 weeks of incubation should be allowed before termination (270). Growth detection may be performed using microscopy or PCR methods. Direct visualization of growing borreliae in culture, which is usually accomplished using dark-field microscopy or acridine orange staining, should be confirmed with PCR or a direct fluorescent antibody (DFA) staining method to improve specificity.
Although culture of erythema migrans skin lesions is the gold standard for research studies of early Lyme borreliosis, culture is rarely performed for diagnostic purposes. The need for expensive, complex media, technical expertise, and long incubation times makes this approach impractical for routine clinical use. The diagnostic yield of culture is also very low except in patients with erythema migrans, in whom moderate sensitivity may be obtained by culturing skin biopsy samples or blood. The sensitivity of skin biopsy culture in patients with untreated erythema migrans ranges from approximately 40 to 83% (142, 271–285). Skin biopsy samples should be taken from the leading edge (periphery) of the erythema migrans lesion (214) and must be obtained prior to initiation of antimicrobial therapy (283, 285).
Blood culture may be positive early in the infection, when erythema migrans is present, but is usually negative after that time. The yields are highest when plasma is cultured, as opposed to whole blood or serum (286), and when a high volume (at least 9 ml of plasma) is cultured (287). When a high-volume plasma culture is performed using microscopic examination for growth detection, the sensitivity in untreated patients with erythema migrans is approximately 40 to 50% (272, 286, 287). If culture growth detection is performed using PCR, the sensitivity can be increased to >70% (142, 288). A study reporting >90% sensitivity in incompletely characterized patients described as having Lyme borreliosis, using a novel blood culture method (289), was later invalidated (290, 291).
The sensitivity of plasma culture in untreated patients with extracutaneous objective manifestations of Lyme borreliosis is poor (<10%), unless there is concomitant erythema migrans. In a prospective study of U.S. patients with early Lyme neuroborreliosis, acute Lyme carditis, or Lyme-related arthritis or bursitis, the sensitivity of high-volume plasma culture with growth detection by microscopy was 6% (1 of 17, a patient with bilateral ankle bursitis of 21 days duration) among those without concomitant erythema migrans, compared to 44% (4 of 9) among patients with concomitant erythema migrans (292). All patients with a positive blood culture had been symptomatic for ≤30 days, and the authors of that study concluded that spirochetemia detectable by blood culture is mainly found in patients with a short duration of illness (292). Similarly, in a large European retrospective study, plasma cultures with growth detection by microscopy were positive in 1.9% of patients with borrelial lymphocytoma (1 of 53), 3.4% of patients with Lyme neuroborreliosis (6 of 176), 7.7% of patients with Lyme arthritis (1 of 13), and 1.5% of patients with acrodermatitis chronica atrophicans (3 of 200) (293). Among those with positive blood cultures, 36% (4 of 11) had concomitant erythema migrans. The median duration of symptoms prior to culture was 3.5 weeks, although the range was very wide (1 day to 2 years) (293). In a study of European patients with erythema migrans and concomitant signs and symptoms of nervous system involvement, the sensitivity of plasma culture was only 3% (4 of 127) prior to treatment (294). Another European study demonstrated 4% plasma culture sensitivity among patients with a clinical diagnosis of evident or suspected Lyme neuroborreliosis (295).
Culture of affected tissue sites in untreated patients with extracutaneous manifestations of Lyme borreliosis is also a low-yield test. Four European studies reported CSF culture sensitivity of ≤10% in patients with suspected Lyme neuroborreliosis (294–297), even though in one study, all patients had concomitant erythema migrans (294). Two studies involving culture of synovial fluid or tissue demonstrated 0% sensitivity in patients with untreated Lyme arthritis (298, 299). In contrast, nonviable spirochetes have occasionally been observed in synovial fluid (300, 301) and, as discussed below, PCR detection of B. burgdorferi nucleic acids in synovial fluid or tissue is often possible in Lyme arthritis. Thus, except in patients with erythema migrans, the low yield of culture even during active infection makes it an impractical test for diagnosis or to asses for cure at the conclusion of antimicrobial therapy.
Nucleic acid amplification tests.
In diagnostic microbiology, when an organism cannot be cultivated or directly visualized using convenient methods, nucleic acid amplification tests (NAATs) have often been an excellent alternative. Assays targeting several different B. burgdorferi genes have been developed and evaluated, including chromosomal targets (e.g., flaB [302], recA [303], 16S rRNA gene [304], oppA1 [9, 305]) and plasmid targets (e.g., ospA [306]). However, except for analysis of joint fluid prior to antimicrobial therapy in Lyme arthritis, PCR has been a low-yield procedure in Lyme borreliosis after the first weeks of infection. Also, NAATs do not work well as a test of cure. Bbsl nucleic acids may persist for weeks after antimicrobial therapy, and detection of nucleic acids (DNA or even mRNA) does not equate with the presence of viable spirochetes (271, 307–309).
(i) Blood PCR.
In patients with solitary erythema migrans, the most common clinical manifestation of Lyme borreliosis, spirochetemia with B. burgdorferi cannot be reliably detected by whole blood or plasma PCR. Reported clinical sensitivity in patients with untreated solitary erythema migrans is often in the range of 30 to 50% (46, 142, 302, 310, 311) and sometimes lower (312, 313), likely reflecting differences in methodology.
The best opportunity to detect spirochetemia in patients with EM using blood PCR seems to be in those who present with features of very early dissemination, namely, those with multiple erythema migrans lesions and/or those with numerous constitutional symptoms. Among studies that compared plasma PCR sensitivity in patients with solitary EM versus patients with multiple EM lesions, one showed a significantly higher rate of positivity among the latter group (312), and two showed a nonsignificant trend in this direction (142, 302). Similarly, patients with EM and numerous systemic symptoms are more likely have a positive plasma PCR result than those with few or no systemic symptoms (302, 312). However, even in this subset of patients with EM, plasma PCR is only approximately 60% sensitive or less (142, 302, 312). In studies that included patients with solitary and multiple erythema migrans but did not distinguish between them when calculating sensitivity or did not comment on whether erythema migrans lesions were single or multiple, the blood/plasma PCR positivity rate did not exceed 50% (46, 310, 311, 313), except in one study (62%) that used DNA extraction from large blood volumes plus target amplification prior to PCR (314).
Few studies have evaluated the clinical sensitivity of blood PCR in patients with noncutaneous manifestations of hematogenous dissemination. Two European studies reported sensitivity of 13 and 28% in patients with suspected Lyme neuroborreliosis, using plasma PCR assays (297, 315). Perhaps as an indication of the overall nonutility of blood PCR in the diagnosis of Lyme borreliosis and of the rampant overutilization of diagnostic testing when the pretest probability of infection is low, two retrospective studies conducted at a large Midwestern U.S. referral center reported blood PCR positivity rates of 0.1% (6 positive among 5,703 samples) and 0.4% (13 positive among 3,127 samples). Interestingly, in the latter study, 5 of the 13 positive samples contained Borrelia mayonii rather than B. burgdorferi, at copy numbers 50 to 8,000 times those of samples positive for B. burgdorferi during the same time period (9). These findings suggest that B. mayonii infection is more likely to present with detectable spirochetemia, and with a higher organism burden, although additional studies are needed. Currently, B. mayonii is only known to occur in the upper Midwestern United States, and there is little information regarding the clinical manifestations of this infection.
(ii) Solid-tissue or body fluid PCR.
PCR for B. burgdorferi can be performed on tissue or fluid samples, such as skin, CSF, or synovial fluid, depending on the suspected clinical manifestation. When applied to skin biopsy samples taken from erythema migrans lesions prior to antimicrobial therapy, the clinical sensitivity of various PCR assays has usually exceeded 50% (272, 274, 276, 278–281, 310, 316, 317) and sometimes reaches 70 to 80% (272, 274, 276, 278, 281, 316, 317). The wide range reflects differences in methodology, at least in part. For example, in one large study of 150 patients with erythema migrans, a PCR assay using primers targeting the flagellin gene, using agarose gel electrophoresis for amplicon detection, was 23% sensitive, while a nested PCR strategy targeting the intergenic rrf–rrl region (also with gel detection) was 71% sensitive when applied to the same skin samples (274). When directly compared to skin biopsy culture, PCR has usually provided superior sensitivity, although this is an inconsistent finding (142, 272, 274, 276, 278–281, 310, 316, 317). Most skin PCR methods yield superior sensitivity to that of conventional two-tiered serum antibody testing in patients with solitary erythema migrans, making skin PCR a reasonable alternative. However, many such patients present to primary care clinics or ambulatory clinics, where the necessary skin biopsy procedure may not be feasible. Furthermore, most clinical laboratories do not offer B. burgdorferi PCR on site, necessitating referral laboratory testing, which can be costly and may provide suboptimal turnaround time. Sample degradation during transport is also a risk. Thus, although PCR performed on skin samples of erythema migrans lesions provides good test performance in comparison to the reference method (antibody testing), there are practical impediments, and this approach is mainly used in research investigations.
B. burgdorferi PCR can be helpful clinically in cases of suspected Lyme arthritis. The sensitivity of PCR assays performed on synovial fluid or tissue prior to antimicrobial therapy exceeds 70% (271, 298, 318–321) in patients with Lyme arthritis. In contrast, the sensitivity of serum antibody testing approaches 100% (94, 114, 139, 141, 179). Therefore, serum antibody testing is the most appropriate first-line test in the diagnosis of Lyme arthritis (Fig. 5). However, in patients with possible past infection who are positive by two-tiered serum antibody testing, or in patients in whom one is considering a coexistent alternative diagnosis, a positive synovial fluid or tissue PCR result can improve diagnostic certainty, considering that seroreactivity can be detected for years in patients with past infection (122). On the other hand, PCR assays may remain positive for weeks or months after successful treatment for Lyme arthritis (271, 318, 321, 322). Therefore, a positive result does not prove active infection and, conversely, a negative result does not rule out active Lyme arthritis.
PCR for Bbsl is very insensitive when performed on CSF collected from patients with suspected Lyme neuroborreliosis. Clinical sensitivity was only 5% in a U.S. study of patients with Lyme meningitis (323). In patients with acute neuroborreliosis, including (but not limited to) Lyme meningitis, the median sensitivity of CSF PCR was 21% according to a meta-analysis of published studies (324). As with blood PCR, poor clinical sensitivity combined with overutilization in patients with a low pretest probability of infection leads to enormous waste. In a large retrospective study at a major referral center, only 14 of 15,939 CSF PCR tests were positive (305). Considering that the sensitivity is very low, a negative CSF PCR test does not rule Lyme neuroborreliosis and contributes little to the evaluation of patients.
TEST SELECTION AND INTERPRETATION IN CLINICAL PRACTICE
Although diagnostic testing for Lyme borreliosis is not always clinically indicated during the first several weeks of infection, the first-line test, regardless of the clinical manifestation, is two-tiered serum antibody testing (either conventional, with EIA and immunoblotting, or modified, with two or more EIAs). However, the selection of supplemental or alternative tests and the interpretation of test results are influenced by the clinical syndrome.
Erythema Migrans
When patients present with a slowly expanding, annular, erythematous skin lesion in the summer months, and there is established risk of Lyme borreliosis based on potential exposure to ixodid tick habitat in a region of endemicity, current guidelines recommend making a clinical diagnosis of erythema migrans rather than obtaining Lyme borreliosis diagnostic tests (84). This is because serologic testing is insensitive early in the infection and direct detection by culture or PCR requires a skin biopsy and specialized laboratory procedures. If serologic testing is pursued, there is a high risk of false-negative results, especially during the first week of infection. The risk of underdiagnosis stemming from falsely negative laboratory results is considered to outweigh the risk of overdiagnosis due to inaccurate clinical assessment, especially since treatment for erythema migrans usually works well and is relatively innocuous (84).
In some cases, patients may present with skin lesions that might be erythema migrans, but clinical suspicion is low. In such cases, diagnostic testing may be indicated, particularly if the patient will not be treated empirically for Lyme borreliosis. Paired serum samples should be obtained for serologic testing at the time of initial presentation and about 2 to 4 weeks later, since seroconversion may take several weeks (Fig. 5). However, if the acute-phase sample demonstrates seropositivity, the diagnosis of erythema migrans can be established and a convalescent-phase serum sample is not needed.
As adjunctive tests, B. burgdorferi culture or PCR testing of skin biopsy samples of EM skin lesions are often positive in true cases, and they provide direct, microbiologic proof of infection. However, many primary care providers are not credentialed to perform skin biopsies, necessitating referral to a dermatologist. Samples for high-volume plasma culture or blood PCR are not difficult to obtain (except in children), but these techniques are less sensitive than culture and PCR of skin biopsy samples in patients with EM. Moreover, culture of either skin biopsy specimens or plasma samples often requires incubation periods of several weeks in the laboratory before growth is detected, and Borrelia culture is not offered at most commercial reference laboratories. Thus, for practical reasons, patients with potential erythema migrans are usually treated on the basis of clinical features of the disease, but paired serum samples may be obtained for serologic analysis in an effort to support the diagnosis.
Early Lyme Neuroborreliosis
As with any evaluation for Lyme disease, it is important to assess whether the patient has had exposure to tick habitat in a region of endemicity. However, in contrast with erythema migrans, diagnostic testing is very important in the diagnosis of early Lyme neuroborreliosis (LNB) because signs and symptoms are insufficiently distinctive to allow for accurate clinical diagnosis. As with each manifestation of Lyme disease, the first-line test for LNB is two-tiered serologic testing, regardless of the anatomic site involved. As a supplemental test, measurement of the Lyme antibody index may be indicated if there is apparent central nervous system (CNS) involvement. Direct detection of B. burgdorferi spirochetes in CSF or blood, by PCR or culture, are low-yield procedures, so these tests are not routinely recommended.
Peripheral nervous system involvement.
The most common manifestations of early LNB involving the PNS are cranial neuritis and radiculoneuritis. When these clinical findings are present without apparent CNS involvement, intrathecal antibody production is not expected, though both manifestations are occasionally accompanied by a CSF pleocytosis. Therefore, the diagnosis of early LNB usually relies upon demonstration of seroreactivity using two-tiered serologic testing. At the time of initial clinical presentation, before antimicrobial administration, reactivity in serum EIAs and IgM or IgG Western blots can be demonstrated in most patients (94, 114). In uncomplicated cases, two or more EIAs (MTTT) may be preferable to EIA and immunoblotting (CTTT) due to higher sensitivity.
Central nervous system involvement.
Lyme meningitis is the most common CNS manifestation of early LNB in the United States. Patients with Lyme meningitis are expected to have a CSF pleocytosis with lymphocyte predominance, and the CSF protein concentration is commonly elevated. When these laboratory abnormalities are present in a patient with meningitis who has had plausible recent exposure to ixodid tick habitat in a region of endemicity, the diagnosis of Lyme meningitis may be established using two-tiered serologic testing alone (Fig. 5). In U.S. patients, a serum antibody response is almost always detectable by the time signs or symptoms of LNB have developed. However, in patients with early Lyme neuroborreliosis who are seen within the first 8 weeks of infection, only the IgM test may be positive (Fig. 2).
Measurement of the Lyme antibody index may help confirm the diagnosis, as specificity is very high for LNB when the index is elevated. However, as with serum antibody testing, intrathecal antibody production may persist for months or years after treated LNB, so the Lyme antibody index cannot differentiate between active and past LNB (218, 325, 326). Analysis of paired CSF and serum samples is necessary; B. burgdorferi antibody testing of CSF alone, without concurrent serum analysis, is diagnostically uninformative. Demonstration of B. burgdorferi-specific antibodies in CSF, whether IgG, IgM, or IgA, does not alone indicate intrathecal production of B. burgdorferi-specific antibodies. There are no interpretive criteria for immunoblot testing of CSF.
The sensitivity of Lyme antibody index determination is slightly lower compared to two-tiered serologic testing, and therefore normal Lyme antibody indices do not rule out Lyme meningitis. Because serum antibody testing is more sensitive and usually positive in patients with Lyme meningitis, measurement of the Lyme antibody index is most useful in patients with detectable serum antibodies against B. burgdorferi. Rarely, however, intrathecal antibody production of B. burgdorferi-specific antibodies precedes peripheral production. This phenomenon is well described in Europe but has seldom been observed North America (253).
Lyme Arthritis
As with LNB, the clinical manifestations of Lyme arthritis are not pathognomonic, and the diagnosis must be supported by laboratory testing. Two-tiered serologic testing is the mainstay approach, since it is highly sensitive and specific. In the United States, Lyme arthritis is usually a late manifestation of the infection, and therefore an expanded IgG antibody response is expected, with at least 5 of 10 specific bands (and usually more) present on IgG immunoblots (Fig. 2). The absence of an expanded, specific IgG antibody response meeting current immunoblot interpretive criteria should raise substantial doubt about a potential diagnosis of Lyme arthritis or other late manifestations of Lyme borreliosis. Occasionally, especially in young children or European patients, Lyme arthritis can present earlier in the course of infection, with predominantly IgM reactivity and incomplete IgM-to-IgG class switching. In such cases, the IgM immunoblot may be positive, while the IgG Western blot has fewer than five specific bands and is negative by criteria (241). However, this phenomenon is rare in the United States, whereas it is more frequently reported in Europe, both in children and adults (18). Thus, in the United States, an IgM response alone is usually evidence against the diagnosis of Lyme arthritis. Western blots should not be performed on synovial fluid; the viscous, sticky nature of the fluid commonly gives false-positive results.
The main limitation of serologic testing in the diagnosis of Lyme arthritis is the inability to differentiate reliably between active from past infection. To confirm that seroreactivity is related to active arthritis in an untreated patient, PCR testing for B. burgdorferi DNA can be performed on synovial fluid as a supplemental test. Although it may still help with diagnosis, synovial fluid PCR is less informative once a patient has received antimicrobial therapy, as PCR remains positive for weeks to months after successful treatment, and therefore positive results do not equate with active infection. Also, the sensitivity of synovial fluid PCR, although good, is significantly lower than that of serologic testing. Thus, a negative PCR result does not rule out Lyme arthritis, and synovial fluid PCR should only be pursued in seropositive patients.
Other direct detection methods, such as B. burgdorferi PCR of whole blood or plasma, or borrelia culture of synovial fluid or synovial tissue are much too insensitive in patients with Lyme arthritis to have any clinical utility. Also, while it is occasionally possible to detect B. burgdorferi spirochetes directly in synovial tissue using special staining techniques, the yield is low, the process is laborious, and it can be difficult to differentiate true spirochetes from artifact. Thus, direct microscopic detection for the routine diagnosis of Lyme arthritis is not a practical approach and is inferior to PCR as a supplement to serodiagnostic testing.
After successful treatment, the amount of antibody to B. burgdorferi declines gradually, as determined by EIA, but to obtain accurate results, serial samples need to be tested using the same quantitative or semiquantitative assay in the same run. Since commercial laboratories rarely save samples, repeat antibody testing has not been recommended. Immunoblots, which are nonquantitative tests, do not change much (or very slowly) after antibiotic therapy and are therefore not useful tests in assessing the response to antibiotic therapy.
OUTLOOKS
Insights recently gained through investigating the basic biology of Lyme-related borreliae, and the immune and inflammatory responses they elicit in the human host, are now being leveraged in attempts to improve diagnostic tests. The topics below address areas of active research, in which promising initial results have been reported but comprehensive validation is needed to assess analytical performance, clinical validity, and practical feasibility.
Indirect Detection Methods
Rapid, point-of-care serologic testing.
Currently, serologic tests for Lyme borreliosis are typically performed in central hospital laboratories or at commercial referral laboratories. Either way, this approach involves substantial delay in producing test results. Although subject to the same limitations as other serologic tests, an accurate, rapid assay that could be performed and interpreted near the point-of-care, during a patient visit, would be valuable in evaluating patients with suspected early Lyme neuroborreliosis, Lyme carditis (270), or Lyme arthritis, because a detectable antibody response is expected in most true cases and diagnosis is difficult to establish based on clinical features alone. Furthermore, emergency ward or urgent care providers must promptly make consequential decisions that would be better informed by a rapid serologic test. For example, physicians evaluating patients with facial nerve palsy must decide whether to provide antimicrobials directed against the agent of Lyme borreliosis or corticosteroids to treat Bell’s palsy. In children with new-onset arthritis affecting one or more large joints, physicians must determine whether to favor septic arthritis, in which case an urgent, operative joint washout may be indicated, along with antimicrobial therapy, or whether to favor Lyme arthritis, in which case a washout would be unnecessary.
Although rapid serologic assays in lateral flow format have been developed and marketed, so far they have been intended as first-tier tests in a two-tiered testing protocol, requiring second-tier immunoblots when the rapid assay is positive (327). The need for second-tier testing undermines the potential advantage of rapid assays. Thus, the availability of a stand-alone rapid assay will be necessary before rapid serologic tests can have much of an impact.
Stand-alone serologic tests.
As with rapid assays, all commercially available EIAs are intended to be used in two-tiered testing protocols, rather than as stand-alone tests. This is primarily because none has achieved equal specificity compared with two-tiered testing (92). The need to perform two separate tests before establishing seropositivity generally increases cost and extends turnaround time, especially when immunoblots are used as second-tier tests (245). Recent advances in assay development, especially the advent of assays using synthetic peptides, chimeric proteins or recombinant protein targets selected for minimal cross-reactivity, are likely to result in stand-alone serologic assays that match or exceed the performance (sensitivity and specificity) of two-tiered testing (92, 212).
Multiplexed serologic tests.
Unlike EIAs, Western blots and the newer line or microarray immunoblots are intended to provide detailed information about the humoral immune response, indicating the presence or absence of reactivity against multiple distinct antigen targets. Beyond the use of immunoblots, alternative solid-phase surfaces can substitute for the membrane, for example by the application of antigens to bead particles that are analyzed by flow cytometry (146, 209, 210) or using a microfluidics-based “lab on a chip” (212, 328). These alternative platforms are highly flexible and may facilitate efforts to reimagine the antigenic constituents of multiplexed serologic assays. Although it remains informative to assess antibody reactivity against the three antigens currently scored in IgM immunoblotting and the ten antigens currently scored in IgG immunoblotting, it is now recognized that some of these antigens are highly cross-reactive and lack specificity for B. burgdorferi infection (155, 205). An optimal multiplexed assay might retain some of the antigens currently scored on immunoblots but replace cross-reactive antigens with more informative antigens or epitopes that were not recognized at the time that current immunoblot interpretive criteria were developed (112, 113, 209, 329) and/or are not well expressed in cultured spirochetes (e.g., VlsE, DbpA, or BBK32) (325). One analysis indicated that more than 5 and possibly as many as 25 different antigens or epitopes may be needed in a multiplexed assay for optimal discrimination between Lyme borreliosis cases and control subjects (108). Such an assay could eventually replace immunoblotting as a second-tier test in a two-tiered testing protocol, or conceivably could work as a stand-alone assay, assuming sufficient clinical specificity (92).
Antibody avidity tests.
An important limitation of serologic testing is the difficulty in distinguishing antibody reactivity due to past (remote) infection from reactivity caused by active or recent infection. IgG avidity assays have been used to make this distinction when standard serologic assays have detected antibodies to cytomegalovirus (CMV), Toxoplasma, or rubella virus, and this approach has also been proposed for Lyme borreliosis. The principle of avidity testing is that specific IgG antibodies become more avid (i.e., bind their target with greater affinity) as the antibody response matures over time. Individuals with recently acquired CMV, toxoplasmosis, or rubella virus infection are expected to have lower IgG avidity compared to those whose infection was remote. However, a major difference is that reinfection is uncommon with these infections but is not uncommon after early Lyme borreliosis, likely complicating interpretation of antibody avidity tests. Furthermore, most patients with Lyme disease are treated at an early stage, and it is not known whether patients with previous (remote) early infection have high- or low-avidity B. burgdorferi IgG antibodies. Initial studies have not addressed these limitations (330–332), and a detailed understanding of changes in antibody avidity during the course of natural infection is lacking. Therefore, the potential utility of IgG avidity testing remains to be determined in Lyme borreliosis.
Gamma interferon release assays.
Studies using ex-vivo T-cell proliferation assays have demonstrated that B. burgdorferi antigens stimulate T-cell activation in patients with Lyme borreliosis (333, 334), but this proliferative response is fairly nonspecific (335). T-cell activation in Lyme borreliosis also leads to production of proinflammatory cytokines, especially interferon gamma, as measured using gamma interferon release assays (IGRAs) (336–339). In some cases, strong interferon gamma responses can be observed shortly after initial infection (336, 340, 341), and it is possible that IGRAs could be capable of detecting the infection earlier than antibody tests during the serologic window period. In addition, a recent study used an IGRA to demonstrate significant reductions in gamma interferon release after antimicrobial therapy in patients with erythema migrans (341). These findings suggest a potential use for IGRAs in differentiating between active infection and past (successfully treated) infection, although the authors of a separate study reached the opposite conclusion using a different IGRA (342) and further study is needed.
CXCL13.
In the setting of neuroinflammation, a chemokine called CXCL13 is upregulated by monocytes/macrophages and dendritic cells, attracting B cells to the central nervous system to promote a local humoral immune response (343–346). In patients with acute Lyme meningitis, there is strong correlation between elevated CSF CXCL13 levels and elevated Lyme antibody indices (295, 347–350), and CXCL13 elevation may develop earlier during the course of infection (346, 351–353). Thus, quantitative measurement of CSF CXCL13 concentrations using simple ELISAs has been suggested as a sensitive and convenient diagnostic tool. CXCL13 levels appear to decline rapidly following antimicrobial therapy (347, 348, 351, 352, 354), potentially making this biomarker more suitable for monitoring response to therapy than measurement of the intrathecal antibody response, which can persist for months to years (252, 354).
Importantly, however, an elevated CSF CXCL13 concentration is associated with many other medical conditions, including meningitis caused by other infectious agents, HIV infection, neurosyphilis, CNS lymphoma, and multiple sclerosis (347–351, 354–356). There is also overlap between CSF CXCL13 levels measured in patients with definite or possible Lyme neuroborreliosis and levels measured in patients with many of these other infectious or inflammatory conditions (350). Thus, it is not yet clear whether CXCL13 elevations due to Lyme meningitis can be differentiated from other causes based on determining CXCL13 concentration or other measurements. Moreover, methods and reference ranges have not been standardized. These are significant challenges, and it remains to be determined whether chemokine measurement will have a place in clinical diagnostics for Lyme borreliosis.
Metabolomics and proteomics.
Molins et al. recently applied an unbiased, iterative machine-learning strategy to discover signature metabolic biomarkers of early Lyme borreliosis (357). In this approach, a discovery set of serum samples from patients with untreated erythema migrans or healthy control subjects was analyzed by liquid chromatography-mass spectrometry for small-molecule metabolites. The data were used to develop and refine a metabolomics biosignature that could differentiate patients with early Lyme borreliosis (erythema migrans) from control subjects, based on shifts in the abundance of selected metabolites in one group compared to the other (357). When challenged with blinded samples, the biosignature achieved 88% sensitivity (95% CI, 84 to 95%) and 95% specificity (95% CI, 90 to 100%) in classifying cases and controls (357). Remarkably, this model could correctly classify most seronegative cases of confirmed early Lyme borreliosis, demonstrating its potential to reduce the serologic window period (357). Several biosignature constituents were known inflammatory mediators or markers (357), supporting other reports indicating that elements of the inflammatory response may be detectably altered in advance of seroconversion (358). Follow-up studies demonstrated that serum metabolic profiling could differentiate cases of EM from STARI, a Lone Star tick-associated illness that produces EM-like skin lesions (359), and that metabolic biosignatures of early Lyme disease are also identifiable in urine samples (360).
Another group reported on the use of proteomics rather than metabolomics in developing diagnostics for Lyme borreliosis (361). In this approach, targeted mass spectrometry was used to measure particular serum proteins of interest, which included acute-phase proteins involved in the innate immune response and proteins present only in specific organ systems that are frequently affected in Lyme borreliosis. The study identified 10 proteins whose serum levels were significantly altered in patients with acute erythema migrans compared to healthy control subjects. The sensitivity of the method in patients with erythema migrans was 75%, but the specificity was only 90% (361).
Although the experimental techniques used in proteomic and metabolomic studies are likely too complex and costly to be practical as routine diagnostic tests, it is possible that further studies will define and validate a manageable list of key diagnostic biomarkers, the measurement of which could be feasible on a large scale. Studies on the value of metabolomics or proteomics in later stages of Lyme borreliosis are also needed.
Direct Detection Methods
Nucleic acid amplification tests.
Nucleic acid amplification tests (NAATs) involving standard PCR or real-time PCR methods have been insensitive for the diagnosis of early or late Lyme borreliosis when applied to readily obtainable body fluid samples such as blood or CSF (228, 305, 362). To some extent, this may be a function of the infection’s pathophysiology, although this remains largely speculative. For example, in some cases of localized EM, it is possible that the infection is entirely confined to the skin, without any associated spirochetemia even at very low concentration. When there is involvement of the nervous system, heart or joints, spirochetemia must occur in order to seed these areas, but it is possible that it is only transient or intermittent and can only be detected if the timing of specimen collection is fortuitous. When there is central nervous system involvement, it is possible that spirochetes do not always enter the CSF or may not be present continuously. If any of these suppositions is true, then direct detection of B. burgdorferi in blood or CSF using NAATs will not be possible in a subset of cases no matter how low the assay’s limit of detection may be. On the other hand, the reported insensitivity of blood and CSF NAATs could in part be related to inadequate analytical sensitivity, and thus it is possible that method enhancements could improve performance.
Numerous variables can affect the analytical sensitivity of PCR assays, including the starting sample volume, the sample type (whole blood, plasma, serum, CSF, etc.), the extraction method, the amplification target and primer sequences, and the efficiency of the amplification reaction (which in turn is influenced by PCR chemistry and cycling conditions) (363). These variables have not been systematically evaluated in Lyme borreliosis to determine the optimal assay design. Nevertheless, a sufficient number of assay designs have been individually evaluated to suggest that a major improvement in analytical sensitivity is unlikely to arise from choosing novel PCR targets, further optimizing PCR chemistry, or combining multiple different PCR assays for use on a single sample. Rather, modifications to pre-PCR steps, such as increasing the volume of sample processed (302, 309, 311, 314) or amplifying B. burgdorferi DNA present in the sample prior to running the PCR test (314, 364), are more likely to have a significant impact on performance (362). It is also possible that new PCR technologies better equipped to detect low-abundance targets, such as digital PCR (365), will prove important.
Metagenomics.
A potential alternative to NAATs is detection of B. burgdorferi genomic DNA in plasma or CSF samples, using shotgun metagenomics. This method involves unbiased (“shotgun”) deep sequencing applied to primary samples, with the aim of detecting any microbial DNA present (366). It has been validated as a clinical diagnostic tool capable of detecting circulating fragments of genomic DNA from a wide variety of bacteria, fungi, viruses, and parasites in peripheral blood (plasma) (367, 368), and its utility in detecting B. burgdorferi infection was recently established (240).
An alternative to shotgun metagenomics is targeted deep sequencing, in which the sample is enriched for genomic DNA of a targeted pathogen using a NAAT or a hybrid capture technique prior to deep sequencing (362). Speculatively, this approach might be more sensitive compared than unbiased sequencing and would simplify data analysis to some extent. Either approach could also provide information about the species and strain type of the infecting agent (362).
Antigen detection.
As with nucleic acid detection, infection with various agents can sometimes be demonstrated by detection of signature microbial proteins or carbohydrates in body fluids such as urine, serum, or CSF. Although B. burgdorferi antigen detection methods have been reported (369–372) and are even available for clinical diagnostic use at some commercial reference laboratories, independent confirmation of reported study findings or validation of proposed assays is lacking (373). Also, the reported methods all involve detection of B. burgdorferi OspA in human body fluids, which is biologically implausible since OspA expression is suppressed during early human infection (119, 325). Nevertheless, advances in our understanding of the B. burgdorferi proteome (374) and in antigen concentration/enrichment methods (362) have prompted continued interest in antigen detection as a potential diagnostic tool for Lyme borreliosis.
Outlook Summary
As high-performing next-generation serologic assays and modified two-tiered testing algorithms become more widely adopted, the false-negative window period of serologic testing will be narrowed to its physiological limit. The remaining window, unavoidable due to the kinetics of the antibody response, will need to be closed either with improved direct diagnostic tests or with non-antibody-based indirect tests, such as cytokine release assays or metabolite-based assays. Direct detection tests involving serum or urine would be highly preferred, but components of the infecting spirochete (nucleic acids, protein antigens, etc.) may not be consistently present even in early infection and therefore may not be reliably detectable in those sample types even using the most sensitive methods. If not, indirect tests may be developed that can assist, but in general these are less likely than direct tests to provide clear-cut answers, particularly because indirect tests of any type rely on detecting responses in the host that take time to develop, even if the window is narrower compared to serology. Thus, the pathophysiology and kinetics of the infection may limit the ability to completely close the window period.
The other major problem in Lyme borreliosis diagnostics is the difficulty of differentiating active from past infection, especially when serologic test results from only a single time point are available. The solution will likely involve either improved direct detection or non-antibody-based indirect tests. It is possible that increasingly sensitive direct detection methods will be able to detect spirochetal components more consistently than current tests, but the limiting factor is whether such components are reliably present in readily obtainable fluids, particularly in later stages of the infection. Non-antibody indirect markers of infection may be developed, but identifying discriminatory cutoffs may prove difficult. Much research is now in progress to address these limitations (92, 362), which is likely to lead to further improvements in diagnostic testing for Lyme borreliosis.
ACKNOWLEDGMENTS
We thank Cassidy Lowell for technical assistance in preparing Fig. 2.
We did not receive any direct or indirect financial support for drafting this review article.
J.A.B. has received research support from Zeus Scientific, bioMérieux, Immunetics, Alere, DiaSorin, the Bay Area Lyme Foundation (BALF), and the National Institute of Allergy and Infectious Diseases (NIAID; award 1R21AI119457-01) for research projects related to the diagnosis of Lyme borreliosis. J.A.B. has also received consulting fees from Roche Diagnostics, T2 Biosystems, and DiaSorin. A.C.S. has received research support from NIH grants R01-AI-R01-101175 and R01-AI-144365, the Mathers Foundation, the Eshe Fund, and Zeus Scientific.
Biographies

John A. Branda, M.D., received his M.D. degree from Harvard Medical School in 2000. He completed residency training in Anatomic and Clinical Pathology at Massachusetts General Hospital (MGH), followed by a fellowship at MGH in Clinical Microbiology. He joined the MGH Pathology faculty in 2004 and is currently an Associate Pathologist and Associate Director of the Clinical Microbiology Laboratories. He also serves as Associate Professor of Pathology at Harvard Medical School. His research interests include improving patient care through the development of new diagnostic testing strategies and through utilization management of laboratory testing and antimicrobial agents. He has a special interest in diagnostic testing for tick-borne illnesses, such as borreliosis, babesiosis, anaplasmosis, and tularemia.

Allen C. Steere, M.D., serves as Professor of Medicine at Harvard Medical School and Director of Translational Research in Rheumatology at Massachusetts General Hospital. His research career has been centered on the elucidation of Lyme disease. Forty-five years ago, he led the team that evaluated a cluster of children with arthritis in Lyme, CT, which led to the discovery of Lyme disease. He has subsequently studied the clinical manifestations, epidemiology, pathogenesis, diagnosis, treatment, and prevention of this complex infection. During the 1990s, he was principal investigator of the SmithKline Beecham Lyme disease vaccine trial, which led to licensing of the first vaccine for Lyme disease. His current research is focused on infection-induced immune responses that stimulate autoimmune responses in various forms of chronic inflammatory arthritis, including postinfectious Lyme arthritis.
REFERENCES
- 1.Adeolu M, Gupta RS. 2014. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex. Antonie Van Leeuwenhoek 105:1049–1072. doi: 10.1007/s10482-014-0164-x. [DOI] [PubMed] [Google Scholar]
- 2.Margos G, Castillo-Ramirez S, Cutler S, Dessau RB, Eikeland R, Estrada-Pena A, Gofton A, Grana-Miraglia L, Hunfeld KP, Krause A, Lienhard R, Lindgren PE, Oskam C, Rudolf I, Schwartz I, Sing A, Stevenson B, Wormser GP, Fingerle V. 2020. Rejection of the name Borreliella and all proposed species comb. nov. placed therein. Int J Syst Evol Microbiol 70:3577–3581. doi: 10.1099/ijsem.0.004149. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson B, Fingerle V, Wormser GP, Margos G. 2019. Public health and patient safety concerns merit retention of Lyme borreliosis-associated spirochetes within the genus Borrelia, and rejection of the genus novum Borreliella. Ticks Tick Borne Dis 10:1–4. doi: 10.1016/j.ttbdis.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Steere AC. 1989. Lyme disease. N Engl J Med 321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 5.Margos G, Vollmer SA, Ogden NH, Fish D. 2011. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect Genet Evol 11:1545–1563. doi: 10.1016/j.meegid.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour AG, Adeolu M, Gupta RS. 2017. Division of the genus Borrelia into two genera (corresponding to Lyme disease and relapsing fever groups) reflects their genetic and phenotypic distinctiveness and will lead to a better understanding of these two groups of microbes (Margos et al. (2016): there is inadequate evidence to support the division of the genus Borrelia. Int J Syst Evol Microbiol 67:2058–2067. doi: 10.1099/ijsem.0.001815. [DOI] [PubMed] [Google Scholar]
- 7.Mead PS. 2015. Epidemiology of Lyme disease. Infect Dis Clin North Am 29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 9.Pritt BS, Mead PS, Johnson DK, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, Paskewitz SM, Ray JA, Bjork J, Steward CR, Deedon A, Lee X, Kingry LC, Miller TK, Feist MA, Theel ES, Patel R, Irish CL, Petersen JM. 2016. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis 16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, Liu G, Kingry LC, Mead PS, Neitzel DF, Schiffman E, Hoang Johnson DK, Davis JP, Paskewitz SM, Boxrud D, Deedon A, Lee X, Miller TK, Feist MA, Steward CR, Theel ES, Patel R, Irish CL, Petersen JM. 2016. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int J Syst Evol Microbiol 66:4878–4880. doi: 10.1099/ijsem.0.001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. 1982. Lyme disease-a tick-borne spirochetosis? Science 216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 12.van Dam AP. 2002. Diversity of Ixodes-borne Borrelia species: clinical, pathogenetic, and diagnostic implications and impact on vaccine development. Vector Borne Zoonotic Dis 2:249–254. doi: 10.1089/153036602321653833. [DOI] [PubMed] [Google Scholar]
- 13.Ornstein K, Berglund J, Nilsson I, Norrby R, Bergstrom S. 2001. Characterization of Lyme borreliosis isolates from patients with erythema migrans and neuroborreliosis in southern Sweden. J Clin Microbiol 39:1294–1298. doi: 10.1128/JCM.39.4.1294-1298.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strle F, Stanek G. 2009. Clinical manifestations and diagnosis of Lyme borreliosis. Curr Probl Dermatol 37:51–110. doi: 10.1159/000213070. [DOI] [PubMed] [Google Scholar]
- 15.Rijpkema SG, Tazelaar DJ, Molkenboer MJ, Noordhoek GT, Plantinga G, Schouls LM, Schellekens JF. 1997. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin Microbiol Infect 3:109–116. doi: 10.1111/j.1469-0691.1997.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, van Dam AP, Schwartz I, Dankert J. 1999. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin Microbiol Rev 12:633–653. doi: 10.1128/CMR.12.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. 2016. Lyme borreliosis. Nat Rev Dis Primers 2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branda JA, Strle F, Strle K, Sikand N, Ferraro MJ, Steere AC. 2013. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe. Clin Infect Dis 57:333–340. doi: 10.1093/cid/cit235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wormser GP, Tang AT, Schimmoeller NR, Bittker S, Cooper D, Visintainer P, Aguero-Rosenfeld ME, Ogrinc K, Strle F, Stanek G. 2014. Utility of serodiagnostics designed for use in the United States for detection of Lyme borreliosis acquired in Europe and vice versa. Med Microbiol Immunol 203:65–71. doi: 10.1007/s00430-013-0315-0. [DOI] [PubMed] [Google Scholar]
- 20.Gomes-Solecki MJ, Meirelles L, Glass J, Dattwyler RJ. 2007. Epitope length, genospecies dependency, and serum panel effect in the IR6 enzyme-linked immunosorbent assay for detection of antibodies to Borrelia burgdorferi. Clin Vaccine Immunol 14:875–879. doi: 10.1128/CVI.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panelius J, Lahdenne P, Heikkila T, Peltomaa M, Oksi J, Seppala I. 2002. Recombinant OspC from Borrelia burgdorferi sensu stricto, B. afzelii and B. garinii in the serodiagnosis of Lyme borreliosis. J Med Microbiol 51:731–739. doi: 10.1099/0022-1317-51-9-731. [DOI] [PubMed] [Google Scholar]
- 22.Dressler F, Ackermann R, Steere AC. 1994. Antibody responses to the three genomic groups of Borrelia burgdorferi in European Lyme borreliosis. J Infect Dis 169:313–318. doi: 10.1093/infdis/169.2.313. [DOI] [PubMed] [Google Scholar]
- 23.Wormser GP, Brisson D, Liveris D, Hanincova K, Sandigursky S, Nowakowski J, Nadelman RB, Ludin S, Schwartz I. 2008. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis 198:1358–1364. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. 2002. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the Northeastern United States. Genetics 160:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Wormser GP, Schriefer ME, Luft BJ. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun 67:3518–3524. doi: 10.1128/IAI.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanincova K, Mukherjee P, Ogden NH, Margos G, Wormser GP, Reed KD, Meece JK, Vandermause MF, Schwartz I. 2013. Multilocus sequence typing of Borrelia burgdorferi suggests existence of lineages with differential pathogenic properties in humans. PLoS One 8:e73066. doi: 10.1371/journal.pone.0073066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liveris D, Gazumyan A, Schwartz I. 1995. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol 33:589–595. doi: 10.1128/JCM.33.3.589-595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liveris D, Varde S, Iyer R, Koenig S, Bittker S, Cooper D, McKenna D, Nowakowski J, Nadelman RB, Wormser GP, Schwartz I. 1999. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol 37:565–569. doi: 10.1128/JCM.37.3.565-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margos G, Gatewood AG, Aanensen DM, Hanincova K, Terekhova D, Vollmer SA, Cornet M, Piesman J, Donaghy M, Bormane A, Hurn MA, Feil EJ, Fish D, Casjens S, Wormser GP, Schwartz I, Kurtenbach K. 2008. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc Natl Acad Sci U S A 105:8730–8735. doi: 10.1073/pnas.0800323105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatchikian CE, Nadelman RB, Nowakowski J, Schwartz I, Wormser GP, Brisson D. 2014. Evidence for strain-specific immunity in patients treated for early Lyme disease. Infect Immun 82:1408–1413. doi: 10.1128/IAI.01451-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro ED. 2014. Clinical practice: Lyme disease. N Engl J Med 370:1724–1731. doi: 10.1056/NEJMcp1314325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadelman RB, Hanincova K, Mukherjee P, Liveris D, Nowakowski J, McKenna D, Brisson D, Cooper D, Bittker S, Madison G, Holmgren D, Schwartz I, Wormser GP. 2012. Differentiation of reinfection from relapse in recurrent Lyme disease. N Engl J Med 367:1883–1890. doi: 10.1056/NEJMoa1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golde WT, Robinson-Dunn B, Stobierski MG, Dykhuizen D, Wang IN, Carlson V, Stiefel H, Shiflett S, Campbell GL. 1998. Culture-confirmed reinfection of a person with different strains of Borrelia burgdorferi sensu stricto. J Clin Microbiol 36:1015–1019. doi: 10.1128/JCM.36.4.1015-1019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nowakowski J, Schwartz I, Nadelman RB, Liveris D, Aguero-Rosenfeld M, Wormser GP. 1997. Culture-confirmed infection and reinfection with Borrelia burgdorferi. Ann Intern Med 127:130–132. doi: 10.7326/0003-4819-127-2-199707150-00006. [DOI] [PubMed] [Google Scholar]
- 36.Krause PJ, Foley DT, Burke GS, Christianson D, Closter L, Spielman A, Tick-Borne Disease Study Group . 2006. Reinfection and relapse in early Lyme disease. Am J Trop Med Hyg 75:1090–1094. doi: 10.4269/ajtmh.2006.75.1090. [DOI] [PubMed] [Google Scholar]
- 37.Nadelman RB, Wormser GP. 2007. Reinfection in patients with Lyme disease. Clin Infect Dis 45:1032–1038. doi: 10.1086/521256. [DOI] [PubMed] [Google Scholar]
- 38.Wilske B. 2003. Diagnosis of Lyme borreliosis in Europe. Vector Borne Zoonotic Dis 3:215–227. doi: 10.1089/153036603322662200. [DOI] [PubMed] [Google Scholar]
- 39.Hauser U, Krahl H, Peters H, Fingerle V, Wilske B. 1998. Impact of strain heterogeneity on Lyme disease serology in Europe: comparison of enzyme-linked immunosorbent assays using different species of Borrelia burgdorferi sensu lato. J Clin Microbiol 36:427–436. doi: 10.1128/JCM.36.2.427-436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norman GL, Antig JM, Bigaignon G, Hogrefe WR. 1996. Serodiagnosis of Lyme borreliosis by Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii Western blots (immunoblots). J Clin Microbiol 34:1732–1738. doi: 10.1128/JCM.34.7.1732-1738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanova L, Christova I, Neves V, Aroso M, Meirelles L, Brisson D, Gomes-Solecki M. 2009. Comprehensive seroprofiling of sixteen B. burgdorferi OspC: implications for Lyme disease diagnostics design. Clin Immunol 132:393–400. doi: 10.1016/j.clim.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wormser GP, Liveris D, Hanincova K, Brisson D, Ludin S, Stracuzzi VJ, Embers ME, Philipp MT, Levin A, Aguero-Rosenfeld M, Schwartz I. 2008. Effect of Borrelia burgdorferi genotype on the sensitivity of C6 and 2-tier testing in North American patients with culture-confirmed Lyme disease. Clin Infect Dis 47:910–914. doi: 10.1086/591529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strle K, Jones KL, Drouin EE, Li X, Steere AC. 2011. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol 178:2726–2739. doi: 10.1016/j.ajpath.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strle K, Shin JJ, Glickstein LJ, Steere AC. 2012. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum 64:1497–1507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wormser GP, Liveris D, Nowakowski J, Nadelman RB, Cavaliere LF, McKenna D, Holmgren D, Schwartz I. 1999. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis 180:720–725. doi: 10.1086/314922. [DOI] [PubMed] [Google Scholar]
- 46.Jones KL, Glickstein LJ, Damle N, Sikand VK, McHugh G, Steere AC. 2006. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J Clin Microbiol 44:4407–4413. doi: 10.1128/JCM.01077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones KL, McHugh GA, Glickstein LJ, Steere AC. 2009. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: high frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum 60:2174–2182. doi: 10.1002/art.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dykhuizen DE, Brisson D, Sandigursky S, Wormser GP, Nowakowski J, Nadelman RB, Schwartz I. 2008. The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am J Trop Med Hyg 78:806–810. doi: 10.4269/ajtmh.2008.78.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piesman J, Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129Suppl:S191–S220. doi: 10.1017/S0031182003004694. [DOI] [PubMed] [Google Scholar]
- 50.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, Fikrig E. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson SE, Swaminathan B, Moore P, Broome CV, Parvin M. 1990. Borrelia burgdorferi: survival in experimentally infected human blood processed for transfusion. J Infect Dis 162:557–559. doi: 10.1093/infdis/162.2.557. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. 2019. Lyme disease: transmission. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/Lyme/transmission/index.html. Accessed 8 September 2020. [Google Scholar]
- 53.Gerber MA, Zalneraitis EL. 1994. Childhood neurologic disorders and Lyme disease during pregnancy. Pediatr Neurol 11:41–43. doi: 10.1016/0887-8994(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 54.Strobino BA, Williams CL, Abid S, Chalson R, Spierling P. 1993. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol 169:367–374. doi: 10.1016/0002-9378(93)90088-Z. [DOI] [PubMed] [Google Scholar]
- 55.Lakos A, Solymosi N. 2010. Maternal Lyme borreliosis and pregnancy outcome. Int J Infect Dis 14:e494. doi: 10.1016/j.ijid.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro ED. 2014. Lyme disease. N Engl J Med 371:684. doi: 10.1056/NEJMc1407264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nadelman RB, Nowakowski J, Fish D, Falco RC, Freeman K, McKenna D, Welch P, Marcus R, Aguero-Rosenfeld ME, Dennis DT, Wormser GP, Tick Bite SG. 2001. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med 345:79–84. doi: 10.1056/NEJM200107123450201. [DOI] [PubMed] [Google Scholar]
- 58.Des Vignes F, Piesman J, Heffernan R, Schulze TL, Stafford KC, III, Fish D. 2001. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J Infect Dis 183:773–778. doi: 10.1086/318818. [DOI] [PubMed] [Google Scholar]
- 59.Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D, Wormser GP. 1999. Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am J Epidemiol 149:771–776. doi: 10.1093/oxfordjournals.aje.a009886. [DOI] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention. 2019. Lyme disease: confirmed cases by month of disease onset, United States, 2008–2018. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/lyme/stats/graphs.html. Accessed 8 September 2020. [Google Scholar]
- 61.Bacon RM, Kugeler KJ, Mead PS, Centers for Disease Control and Prevention . 2008. Surveillance for Lyme disease–United States, 1992–2006. MMWR Surveill Summ 57:1–9. [PubMed] [Google Scholar]
- 62.Hubalek Z. 2009. Epidemiology of Lyme borreliosis. Curr Probl Dermatol 37:31–50. [DOI] [PubMed] [Google Scholar]
- 63.Richard S, Oppliger A. 2015. Zoonotic occupational diseases in forestry workers: Lyme borreliosis, tularemia, and leptospirosis in Europe. Ann Agric Environ Med 22:43–50. doi: 10.5604/12321966.1141368. [DOI] [PubMed] [Google Scholar]
- 64.Wilking H, Fingerle V, Klier C, Thamm M, Stark K. 2015. Antibodies against Borrelia burgdorferi sensu lato among adults, Germany, 2008–2011. Emerg Infect Dis 21:107–110. doi: 10.3201/eid2101.140009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayes EB, Piesman J. 2003. How can we prevent Lyme disease? N Engl J Med 348:2424–2430. doi: 10.1056/NEJMra021397. [DOI] [PubMed] [Google Scholar]
- 66.Gasmi S, Ogden NH, Lindsay LR, Burns S, Fleming S, Badcock J, Hanan S, Gaulin C, Leblanc MA, Russell C, Nelder M, Hobbs L, Graham-Derham S, Lachance L, Scott AN, Galanis E, Koffi JK. 2017. Surveillance for Lyme disease in Canada: 2009–2015. Can Commun Dis Rep 43:194–199. doi: 10.14745/ccdr.v43i10a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogden NH, Bouchard C, Badcock J, Drebot MA, Elias SP, Hatchette TF, Koffi JK, Leighton PA, Lindsay LR, Lubelczyk CB, Peregrine AS, Smith RP, Webster D. 2019. What is the real number of Lyme disease cases in Canada? BMC Public Health 19:849. doi: 10.1186/s12889-019-7219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatchette TF, Johnston BL, Schleihauf E, Mask A, Haldane D, Drebot M, Baikie M, Cole TJ, Fleming S, Gould R, Lindsay R. 2015. Epidemiology of Lyme disease, Nova Scotia, Canada, 2002–2013. Emerg Infect Dis 21:1751–1758. doi: 10.3201/eid2110.141640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention. 2020. Reported cases of Lyme disease by state or locality, 2009–2018. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/lyme/stats/tables.html. Accessed 8 September 2020. [Google Scholar]
- 70.Ogden NH, Barker IK, Beauchamp G, Brazeau S, Charron DF, Maarouf A, Morshed MG, O’Callaghan CJ, Thompson RA, Waltner-Toews D, Waltner-Toews M, Lindsay LR. 2006. Investigation of ground level and remote-sensed data for habitat classification and prediction of survival of Ixodes scapularis in habitats of southeastern Canada. J Med Entomol 43:403–414. doi: 10.1603/0022-2585(2006)043[0403:IOGLAR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 71.Lantos PM, Tsao J, Nigrovic LE, Auwaerter PG, Fowler VG, Ruffin F, Foster E, Hickling G. 2017. Geographic expansion of Lyme disease in Michigan, 2000–2014. Open Forum Infect Dis 4:ofw269. doi: 10.1093/ofid/ofw269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lantos PM, Nigrovic LE, Auwaerter PG, Fowler VG, Jr, Ruffin F, Brinkerhoff RJ, Reber J, Williams C, Broyhill J, Pan WK, Gaines DN. 2015. Geographic expansion of Lyme disease in the southeastern United States, 2000–2014. Open Forum Infect Dis 2:ofv143. doi: 10.1093/ofid/ofv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kugeler KJ, Farley GM, Forrester JD, Mead PS. 2015. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis 21:1455–1457. doi: 10.3201/eid2108.141878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eisen RJ, Eisen L, Beard CB. 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol 53:349–386. doi: 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steere AC, Sikand VK. 2003. The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med 348:2472–2474. doi: 10.1056/NEJM200306123482423. [DOI] [PubMed] [Google Scholar]
- 76.Steere AC. 2001. Lyme disease. N Engl J Med 345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 77.Steere AC, Bartenhagen NH, Craft JE, Hutchinson GJ, Newman JH, Rahn DW, Sigal LH, Spieler PN, Stenn KS, Malawista SE. 1983. The early clinical manifestations of Lyme disease. Ann Intern Med 99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 78.Smith RP, Schoen RT, Rahn DW, Sikand VK, Nowakowski J, Parenti DL, Holman MS, Persing DH, Steere AC. 2002. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 136:421–428. doi: 10.7326/0003-4819-136-6-200203190-00005. [DOI] [PubMed] [Google Scholar]
- 79.Wormser GP, Masters E, Nowakowski J, McKenna D, Holmgren D, Ma K, Ihde L, Cavaliere LF, Nadelman RB. 2005. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 41:958–965. doi: 10.1086/432935. [DOI] [PubMed] [Google Scholar]
- 80.Wormser GP, Aguero-Rosenfeld ME, Cox ME, Nowakowski J, Nadelman RB, Holmgren D, McKenna D, Bittker S, Zentmaier L, Cooper D, Liveris D, Schwartz I, Horowitz HW. 2013. Differences and similarities between culture-confirmed human granulocytic anaplasmosis and early Lyme disease. J Clin Microbiol 51:954–958. doi: 10.1128/JCM.02929-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nadelman RB, Nowakowski J, Forseter G, Goldberg NS, Bittker S, Cooper D, Aguero-Rosenfeld M, Wormser GP. 1996. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med 100:502–508. doi: 10.1016/S0002-9343(95)99915-9. [DOI] [PubMed] [Google Scholar]
- 82.Wormser GP, McKenna D, Carlin J, Nadelman RB, Cavaliere LF, Holmgren D, Byrne DW, Nowakowski J. 2005. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 142:751–755. doi: 10.7326/0003-4819-142-9-200505030-00011. [DOI] [PubMed] [Google Scholar]
- 83.Wormser GP, McKenna D, Nowakowski J. 2018. Management approaches for suspected and established Lyme disease used at the Lyme disease diagnostic center. Wien Klin Wochenschr 130:463–467. doi: 10.1007/s00508-015-0936-y. [DOI] [PubMed] [Google Scholar]
- 84.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 85.Forrester JD, Meiman J, Mullins J, Nelson R, Ertel SH, Cartter M, Brown CM, Lijewski V, Schiffman E, Neitzel D, Daly ER, Mathewson AA, Howe W, Lowe LA, Kratz NR, Semple S, Backenson PB, White JL, Kurpiel PM, Rockwell R, Waller K, Johnson DH, Steward C, Batten B, Blau D, DeLeon-Carnes M, Drew C, Muehlenbachs A, Ritter J, Sanders J, Zaki SR, Molins C, Schriefer M, Perea A, Kugeler K, Nelson C, Hinckley A, Mead P, Centers for Disease Control and Prevention . 2014. Notes from the field: update on Lyme carditis, groups at high risk, and frequency of associated sudden cardiac death–United States. MMWR Morb Mortal Wkly Rep 63:982–983. [PMC free article] [PubMed] [Google Scholar]
- 86.Steere AC, Schoen RT, Taylor E. 1987. The clinical evolution of Lyme arthritis. Ann Intern Med 107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 87.Steere AC, Malawista SE, Hardin JA, Ruddy S, Askenase W, Andiman WA. 1977. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med 86:685–698. doi: 10.7326/0003-4819-86-6-685. [DOI] [PubMed] [Google Scholar]
- 88.Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. 2017. Surveillance for Lyme Disease: United States, 2008–2015. MMWR Surveill Summ 66:1–12. doi: 10.15585/mmwr.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steere AC, Sikand VK, Schoen RT, Nowakowski J. 2003. Asymptomatic infection with Borrelia burgdorferi. Clin Infect Dis 37:528–532. doi: 10.1086/376914. [DOI] [PubMed] [Google Scholar]
- 90.Gustafson R, Svenungsson B, Gardulf A, Stiernstedt G, Forsgren M. 1990. Prevalence of tick-borne encephalitis and Lyme borreliosis in a defined Swedish population. Scand J Infect Dis 22:297–306. doi: 10.3109/00365549009027051. [DOI] [PubMed] [Google Scholar]
- 91.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. 1983. The spirochetal etiology of Lyme disease. N Engl J Med 308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 92.Branda JA, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, Gerald NJ, Gomes-Solecki M, Kintrup M, Ledizet M, Levin AE, Lewinski M, Liotta LA, Marques A, Mead PS, Mongodin EF, Pillai S, Rao P, Robinson WH, Roth KM, Schriefer ME, Slezak T, Snyder J, Steere AC, Witkowski J, Wong SJ, Schutzer SE. 2018. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 66:1133–1139. doi: 10.1093/cid/cix943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aguero-Rosenfeld ME, Nowakowski J, McKenna DF, Carbonaro CA, Wormser GP. 1993. Serodiagnosis in early Lyme disease. J Clin Microbiol 31:3090–3095. doi: 10.1128/JCM.31.12.3090-3095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steere AC, McHugh G, Damle N, Sikand VK. 2008. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 47:188–195. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aguero-Rosenfeld ME, Nowakowski J, Bittker S, Cooper D, Nadelman RB, Wormser GP. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol 34:1–9. doi: 10.1128/JCM.34.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Craft JE, Fischer DK, Shimamoto GT, Steere AC. 1986. Antigens of Borrelia burgdorferi recognized during Lyme disease: appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest 78:934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coleman JL, Benach JL. 1987. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis 155:756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- 98.Dressler F, Whalen JA, Reinhardt BN, Steere AC. 1993. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis 167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 99.Grodzicki RL, Steere AC. 1988. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J Infect Dis 157:790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- 100.Lawrenz MB, Hardham JM, Owens RT, Nowakowski J, Steere AC, Wormser GP, Norris SJ. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J Clin Microbiol 37:3997–4004. doi: 10.1128/JCM.37.12.3997-4004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang FT, Alvarez AL, Gu Y, Nowling JM, Ramamoorthy R, Philipp MT. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J Immunol 163:5566–5573. [PubMed] [Google Scholar]
- 102.Engstrom SM, Shoop E, Johnson RC. 1995. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol 33:419–427. doi: 10.1128/JCM.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Padula SJ, Dias F, Sampieri A, Craven RB, Ryan RW. 1994. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J Clin Microbiol 32:1733–1738. doi: 10.1128/JCM.32.7.1733-1738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilske B, Preac-Mursic V, Schierz G, Busch KV. 1986. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A 263:92–102. doi: 10.1016/S0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- 105.Arnaboldi PM, Seedarnee R, Sambir M, Callister SM, Imparato JA, Dattwyler RJ. 2013. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early Lyme disease. Clin Vaccine Immunol 20:474–481. doi: 10.1128/CVI.00608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gilmore RD, Jr, Murphree RL, James AM, Sullivan SA, Johnson BJ. 1999. The Borrelia burgdorferi 37-kilodalton immunoblot band (P37) used in serodiagnosis of early Lyme disease is the flaA gene product. J Clin Microbiol 37:548–552. doi: 10.1128/JCM.37.3.548-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Panelius J, Lahdenne P, Saxen H, Heikkila T, Seppala I. 2001. Recombinant flagellin A proteins from Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii in serodiagnosis of Lyme borreliosis. J Clin Microbiol 39:4013–4019. doi: 10.1128/JCM.39.11.4013-4019.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, Baldi P, Felgner PL. 2008. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun 76:3374–3389. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Magnarelli LA, Ijdo JW, Padula SJ, Flavell RA, Fikrig E. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J Clin Microbiol 38:1735–1739. doi: 10.1128/JCM.38.5.1735-1739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nowalk AJ, Gilmore RD, Jr, Carroll JA. 2006. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect Immun 74:3864–3873. doi: 10.1128/IAI.00189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brissette CA, Rossmann E, Bowman A, Cooley AE, Riley SP, Hunfeld KP, Bechtel M, Kraiczy P, Stevenson B. 2010. The borrelial fibronectin-binding protein RevA is an early antigen of human Lyme disease. Clin Vaccine Immunol 17:274–280. doi: 10.1128/CVI.00437-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Signorino G, Arnaboldi PM, Petzke MM, Dattwyler RJ. 2014. Identification of OppA2 linear epitopes as serodiagnostic markers for Lyme disease. Clin Vaccine Immunol 21:704–711. doi: 10.1128/CVI.00792-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arnaboldi PM, Sambir M, Dattwyler RJ. 2014. Decorin binding proteins A and B in the serodiagnosis of Lyme disease in North America. Clin Vaccine Immunol 21:1426–1436. doi: 10.1128/CVI.00383-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bacon RM, Biggerstaff BJ, Schriefer ME, Gilmore RD, Jr, Philipp MT, Steere AC, Wormser GP, Marques AR, Johnson BJ. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J Infect Dis 187:1187–1199. doi: 10.1086/374395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coleman AS, Rossmann E, Yang X, Song H, Lamichhane CM, Iyer R, Schwartz I, Pal U. 2011. BBK07 immunodominant peptides as serodiagnostic markers of Lyme disease. Clin Vaccine Immunol 18:406–413. doi: 10.1128/CVI.00461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coleman AS, Pal U. 2009. BBK07, a dominant in vivo antigen of Borrelia burgdorferi, is a potential marker for serodiagnosis of Lyme disease. Clin Vaccine Immunol 16:1569–1575. doi: 10.1128/CVI.00301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lahdenne P, Panelius J, Saxen H, Heikkila T, Sillanpaa H, Peltomaa M, Arnez M, Huppertz HI, Seppala IJ. 2003. Improved serodiagnosis of erythema migrans using novel recombinant borrelial BBK32 antigens. J Med Microbiol 52:563–567. doi: 10.1099/jmm.0.05095-0. [DOI] [PubMed] [Google Scholar]
- 118.Fung BP, McHugh GL, Leong JM, Steere AC. 1994. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun 62:3213–3221. doi: 10.1128/IAI.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li X, Strle K, Wang P, Acosta DI, McHugh GA, Sikand N, Strle F, Steere AC. 2013. Tick-specific borrelial antigens appear to be upregulated in American but not European patients with Lyme arthritis, a late manifestation of Lyme borreliosis. J Infect Dis 208:934–941. doi: 10.1093/infdis/jit269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Crowley H, Huber BT. 2003. Host-adapted Borrelia burgdorferi in mice expresses OspA during inflammation. Infect Immun 71:4003–4010. doi: 10.1128/IAI.71.7.4003-4010.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Szer IS, Taylor E, Steere AC. 1991. The long-term course of Lyme arthritis in children. N Engl J Med 325:159–163. doi: 10.1056/NEJM199107183250304. [DOI] [PubMed] [Google Scholar]
- 122.Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. 2001. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis 33:780–785. doi: 10.1086/322669. [DOI] [PubMed] [Google Scholar]
- 123.Kannian P, McHugh G, Johnson BJ, Bacon RM, Glickstein LJ, Steere AC. 2007. Antibody responses to Borrelia burgdorferi in patients with antibiotic-refractory, antibiotic-responsive, or non-antibiotic-treated Lyme arthritis. Arthritis Rheum 56:4216–4225. doi: 10.1002/art.23135. [DOI] [PubMed] [Google Scholar]
- 124.Shrestha M, Grodzicki RL, Steere AC. 1985. Diagnosing early Lyme disease. Am J Med 78:235–240. doi: 10.1016/0002-9343(85)90432-2. [DOI] [PubMed] [Google Scholar]
- 125.Glatz M, Golestani M, Kerl H, Mullegger RR. 2006. Clinical relevance of different IgG and IgM serum antibody responses to Borrelia burgdorferi after antibiotic therapy for erythema migrans: long-term follow-up study of 113 patients. Arch Dermatol 142:862–868. doi: 10.1001/archderm.142.7.862. [DOI] [PubMed] [Google Scholar]
- 126.Berardi VP, Weeks KE, Steere AC. 1988. Serodiagnosis of early Lyme disease: analysis of IgM and IgG antibody responses by using an antibody-capture enzyme immunoassay. J Infect Dis 158:754–760. doi: 10.1093/infdis/158.4.754. [DOI] [PubMed] [Google Scholar]
- 127.Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, Duray PH, Larson MG, Wright EA, Ginsburg KS, Katz JN, Liang MH. 1994. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med 121:560–567. doi: 10.7326/0003-4819-121-8-199410150-00002. [DOI] [PubMed] [Google Scholar]
- 128.Shadick NA, Phillips CB, Sangha O, Logigian EL, Kaplan RF, Wright EA, Fossel AH, Fossel K, Berardi V, Lew RA, Liang MH. 1999. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med 131:919–926. doi: 10.7326/0003-4819-131-12-199912210-00003. [DOI] [PubMed] [Google Scholar]
- 129.Feder HM, Jr, Gerber MA, Luger SW, Ryan RW. 1992. Persistence of serum antibodies to Borrelia burgdorferi in patients treated for Lyme disease. Clin Infect Dis 15:788–793. doi: 10.1093/clind/15.5.788. [DOI] [PubMed] [Google Scholar]
- 130.Peltomaa M, McHugh G, Steere AC. 2003. Persistence of the antibody response to the VlsE sixth invariant region (IR6) peptide of Borrelia burgdorferi after successful antibiotic treatment of Lyme disease. J Infect Dis 187:1178–1186. doi: 10.1086/374376. [DOI] [PubMed] [Google Scholar]
- 131.Weber K. 1986. Lyme borreliosis. Hautarzt 37:583–586. [PubMed] [Google Scholar]
- 132.Pfister HW, Neubert U, Wilske B, Preac-Mursic V, Einhaupl KM, Borasio GD. 1986. Reinfection with Borrelia burgdorferi. Lancet 2:984–985. doi: 10.1016/S0140-6736(86)90640-9. [DOI] [PubMed] [Google Scholar]
- 133.Hassler D, Maiwald M. 2008. Reinfection with Borrelia burgdorferi in an immunocompetent patient. Dtsch Med Wochenschr 119:338–342. doi: 10.1055/s-2008-1058700. [DOI] [PubMed] [Google Scholar]
- 134.Association of State and Territorial Public Health Laboratory Directors. 1994. Proceedings of the Second National Conference on Serologic Diagnosis of Lyme Disease: October 27–29, 1994. Conference publication. Association of State and Territorial Public Health Laboratory Directors, Washington, DC. [Google Scholar]
- 135.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 44:590–591. [PubMed] [Google Scholar]
- 136.Lantos PM, Lipsett SC, Nigrovic LE. 2016. False-positive Lyme disease IgM immunoblots in children. J Pediatr 174:267–269. doi: 10.1016/j.jpeds.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. 2012. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 18:1236–1240. doi: 10.1111/j.1469-0691.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 138.Wormser GP, Nowakowski J, Nadelman RB, Visintainer P, Levin A, Aguero-Rosenfeld ME. 2008. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. CVI 15:1519–1522. doi: 10.1128/CVI.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Branda JA, Aguero-Rosenfeld ME, Ferraro MJ, Johnson BJ, Wormser GP, Steere AC. 2010. 2-tiered antibody testing for early and late Lyme disease using only an immunoglobulin G blot with the addition of a VlsE band as the second-tier test. Clin Infect Dis 50:20–26. doi: 10.1086/648674. [DOI] [PubMed] [Google Scholar]
- 140.Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. 2011. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 53:541–547. doi: 10.1093/cid/cir464. [DOI] [PubMed] [Google Scholar]
- 141.Wormser GP, Schriefer M, Aguero-Rosenfeld ME, Levin A, Steere AC, Nadelman RB, Nowakowski J, Marques A, Johnson BJ, Dumler JS. 2013. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis 75:9–15. doi: 10.1016/j.diagmicrobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liveris D, Schwartz I, McKenna D, Nowakowski J, Nadelman R, Demarco J, Iyer R, Bittker S, Cooper D, Holmgren D, Wormser GP. 2012. Comparison of five diagnostic modalities for direct detection of Borrelia burgdorferi in patients with early Lyme disease. Diagn Microbiol Infect Dis 73:243–245. doi: 10.1016/j.diagmicrobio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Molins CR, Sexton C, Young JW, Ashton LV, Pappert R, Beard CB, Schriefer ME. 2014. Collection and characterization of samples for establishment of a serum repository for Lyme disease diagnostic test development and evaluation. J Clin Microbiol 52:3755–3762. doi: 10.1128/JCM.01409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Theel ES. 2016. The past, present, and (possible) future of serologic testing for Lyme disease. J Clin Microbiol 54:1191–1196. doi: 10.1128/JCM.03394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Trevejo RT, Krause PJ, Schriefer ME, Dennis DT. 2001. Evaluation of a two-test serodiagnostic method for community assessment of Lyme disease in an endemic area. Am J Trop Med Hyg 65:563–566. doi: 10.4269/ajtmh.2001.65.563. [DOI] [PubMed] [Google Scholar]
- 146.Porwancher RB, Hagerty CG, Fan J, Landsberg L, Johnson BJ, Kopnitsky M, Steere AC, Kulas K, Wong SJ. 2011. Multiplex immunoassay for Lyme disease using VlsE1-IgG and pepC10-IgM antibodies: improving test performance through bioinformatics. Clin Vaccine Immunol 18:851–859. doi: 10.1128/CVI.00409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Johnson BJ, Robbins KE, Bailey RE, Cao BL, Sviat SL, Craven RB, Mayer LW, Dennis DT. 1996. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis 174:346–353. doi: 10.1093/infdis/174.2.346. [DOI] [PubMed] [Google Scholar]
- 148.Lantos PM, Branda JA, Boggan JC, Chudgar SM, Wilson EA, Ruffin F, Fowler V, Auwaerter PG, Nigrovic LE. 2015. Poor positive predictive value of Lyme disease serologic testing in an area of low disease incidence. Clin Infect Dis 61:1374–1380. doi: 10.1093/cid/civ584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fawcett PT, Gibney KM, Rose CD, Klein JD, Doughty RA. 1991. Adsorption with a soluble Escherichia coli antigen fraction improves the specificity of ELISA tests for Lyme disease. J Rheumatol 18:705–708. [PubMed] [Google Scholar]
- 150.Fawcett PT, O’Brien AE, Doughty RA. 1989. An adsorption procedure to increase the specificity of enzyme-linked immunosorbent assays for Lyme disease without decreasing sensitivity. Arthritis Rheum 32:1041–1044. doi: 10.1002/anr.1780320814. [DOI] [PubMed] [Google Scholar]
- 151.Wilske B, Schierz G, Preac-Mursic V, Weber K, Pfister HW, Einhaupl K. 1984. Serological diagnosis of erythema migrans disease and related disorders. Infection 12:331–337. doi: 10.1007/BF01651147. [DOI] [PubMed] [Google Scholar]
- 152.Fawcett PT, Rose CD, Gibney KM. 1995. Comparative evaluation of adsorption with E. coli on ELISA tests for Lyme borreliosis. J Rheumatol 22:684–688. [PubMed] [Google Scholar]
- 153.Magnarelli LA, Anderson JF, Johnson RC. 1987. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis 156:183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- 154.Craft JE, Grodzicki RL, Steere AC. 1984. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis 149:789–795. doi: 10.1093/infdis/149.5.789. [DOI] [PubMed] [Google Scholar]
- 155.Bruckbauer HR, Preac-Mursic V, Fuchs R, Wilske B. 1992. Cross-reactive proteins of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis 11:224–232. doi: 10.1007/BF02098084. [DOI] [PubMed] [Google Scholar]
- 156.D’Arco C, Dattwyler RJ, Arnaboldi PM. 2017. Borrelia burgdorferi-specific IgA in Lyme disease. EBioMedicine 19:91–97. doi: 10.1016/j.ebiom.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Steere AC, Berardi VP, Weeks KE, Logigian EL, Ackermann R. 1990. Evaluation of the intrathecal antibody response to Borrelia burgdorferi as a diagnostic test for Lyme neuroborreliosis. J Infect Dis 161:1203–1209. doi: 10.1093/infdis/161.6.1203. [DOI] [PubMed] [Google Scholar]
- 158.Crother TR, Champion CI, Wu XY, Blanco DR, Miller JN, Lovett MA. 2003. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect Immun 71:3419–3428. doi: 10.1128/IAI.71.6.3419-3428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang JR, Hardham JM, Barbour AG, Norris SJ. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275–285. doi: 10.1016/S0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 160.Gottner G, Schulte-Spechtel U, Wilske B. 2004. Heterogeneity of the immunodominant surface protein VlsE among the three genospecies of Borrelia burgdorferi pathogenic for humans. Int J Med Microbiol 293:172–173. doi: 10.1016/S1433-1128(04)80034-2. [DOI] [PubMed] [Google Scholar]
- 161.Magnarelli LA, Lawrenz M, Norris SJ, Fikrig E. 2002. Comparative reactivity of human sera to recombinant VlsE and other Borrelia burgdorferi antigens in class-specific enzyme-linked immunosorbent assays for Lyme borreliosis. J Med Microbiol 51:649–655. doi: 10.1099/0022-1317-51-8-649. [DOI] [PubMed] [Google Scholar]
- 162.Gomes-Solecki MJ, Dunn JJ, Luft BJ, Castillo J, Dykhuizen DE, Yang X, Glass JD, Dattwyler RJ. 2000. Recombinant chimeric Borrelia proteins for diagnosis of Lyme disease. J Clin Microbiol 38:2530–2535. doi: 10.1128/JCM.38.7.2530-2535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liang FT, Steere AC, Marques AR, Johnson BJ, Miller JN, Philipp MT. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J Clin Microbiol 37:3990–3996. doi: 10.1128/JCM.37.12.3990-3996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Indest KJ, Ramamoorthy R, Sole M, Gilmore RD, Johnson BJ, Philipp MT. 1997. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun 65:1165–1171. doi: 10.1128/IAI.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ramamoorthy R, Philipp MT. 1998. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect Immun 66:5119–5124. doi: 10.1128/IAI.66.11.5119-5124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Margolis N, Rosa PA. 1993. Regulation of expression of major outer surface proteins in Borrelia burgdorferi. Infect Immun 61:2207–2210. doi: 10.1128/IAI.61.5.2207-2210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Norris SJ, Carter CJ, Howell JK, Barbour AG. 1992. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun 60:4662–4672. doi: 10.1128/IAI.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Palmer GH, Bankhead T, Lukehart SA. 2009. ‘Nothing is permanent but change’: antigenic variation in persistent bacterial pathogens. Cell Microbiol 11:1697–1705. doi: 10.1111/j.1462-5822.2009.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang JR, Norris SJ. 1998. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect Immun 66:3698–3704. doi: 10.1128/IAI.66.8.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liang FT, Bowers LC, Philipp MT. 2001. C-terminal invariable domain of VlsE is immunodominant but its antigenicity is scarcely conserved among strains of Lyme disease spirochetes. Infect Immun 69:3224–3231. doi: 10.1128/IAI.69.5.3224-3231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jacek E, Tang KS, Komorowski L, Ajamian M, Probst C, Stevenson B, Wormser GP, Marques AR, Alaedini A. 2016. Epitope-specific evolution of human B cell responses to Borrelia burgdorferi VlsE protein from early to late stages of Lyme disease. J Immunol 196:1036–1043. doi: 10.4049/jimmunol.1501861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Embers ME, Wormser GP, Schwartz I, Martin DS, Philipp MT. 2007. Borrelia burgdorferi spirochetes that harbor only a portion of the lp28-1 plasmid elicit antibody responses detectable with the C6 test for Lyme disease. CVI 14:90–93. doi: 10.1128/CVI.00266-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kingry LC, Batra D, Replogle A, Rowe LA, Pritt BS, Petersen JM. 2016. Whole genome sequence and comparative genomics of the novel Lyme borreliosis-causing pathogen, Borrelia mayonii. PLoS One 11:e0168994. doi: 10.1371/journal.pone.0168994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Molloy PJ, Weeks KE, Todd B, Wormser GP. 2018. Seroreactivity to the C6 peptide in Borrelia miyamotoi infections occurring in the northeastern United States. Clin Infect Dis 66:1407–1410. doi: 10.1093/cid/cix1023. [DOI] [PubMed] [Google Scholar]
- 175.Molloy PJ, Telford SR, III, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. 2015. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med 163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 176.Molins CR, Delorey MJ, Sexton C, Schriefer ME. 2016. Lyme borreliosis serology: performance of several commonly used laboratory diagnostic tests and a large resource panel of well-characterized patient samples. J Clin Microbiol 54:2726–2734. doi: 10.1128/JCM.00874-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Branda JA, Strle K, Nigrovic LE, Lantos PM, Lepore TJ, Damle NS, Ferraro MJ, Steere AC. 2017. Evaluation of modified 2-tiered serodiagnostic testing algorithms for early Lyme disease. Clin Infect Dis 64:1074–1080. doi: 10.1093/cid/cix043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Philipp MT, Wormser GP, Marques AR, Bittker S, Martin DS, Nowakowski J, Dally LG. 2005. A decline in C6 antibody titer occurs in successfully treated patients with culture-confirmed early localized or early disseminated Lyme borreliosis. CVI 12:1069–1074. doi: 10.1128/CDLI.12.9.1069-1074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Waddell LA, Greig J, Mascarenhas M, Harding S, Lindsay R, Ogden N. 2016. The accuracy of diagnostic tests for Lyme disease in humans, a systematic review and meta-analysis of North American research. PLoS One 11:e0168613. doi: 10.1371/journal.pone.0168613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ledue TB, Collins MF, Young J, Schriefer ME. 2008. Evaluation of the recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin Vaccine Immunol 15:1796–1804. doi: 10.1128/CVI.00195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fallon BA, Pavlicova M, Coffino SW, Brenner C. 2014. A comparison of Lyme disease serologic test results from 4 laboratories in patients with persistent symptoms after antibiotic treatment. Clin Infect Dis 59:1705–1710. doi: 10.1093/cid/ciu703. [DOI] [PubMed] [Google Scholar]
- 182.Riesbeck K, Hammas B. 2007. Comparison of an automated Borrelia indirect chemiluminescent immunoassay (CLIA) with a VlsE/C6 ELISA and Immunoblot. Eur J Clin Microbiol Infect Dis 26:517–519. doi: 10.1007/s10096-007-0329-x. [DOI] [PubMed] [Google Scholar]
- 183.Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem 277:21691–21696. doi: 10.1074/jbc.M201547200. [DOI] [PubMed] [Google Scholar]
- 184.Embers ME, Jacobs MB, Johnson BJ, Philipp MT. 2007. Dominant epitopes of the C6 diagnostic peptide of Borrelia burgdorferi are largely inaccessible to antibody on the parent VlsE molecule. Clin Vaccine Immunol 14:931–936. doi: 10.1128/CVI.00075-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Padula SJ, Sampieri A, Dias F, Szczepanski A, Ryan RW. 1993. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect Immun 61:5097–5105. doi: 10.1128/IAI.61.12.5097-5105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sadziene A, Wilske B, Ferdows MS, Barbour AG. 1993. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun 61:2192–2195. doi: 10.1128/IAI.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schwan TG. 2003. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans 31:108–112. doi: 10.1042/bst0310108. [DOI] [PubMed] [Google Scholar]
- 188.Magnarelli LA, Fikrig E, Padula SJ, Anderson JF, Flavell RA. 1996. Use of recombinant antigens of Borrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J Clin Microbiol 34:237–240. doi: 10.1128/JCM.34.2.237-240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gerber MA, Shapiro ED, Bell GL, Sampieri A, Padula SJ. 1995. Recombinant outer surface protein C ELISA for the diagnosis of early Lyme disease. J Infect Dis 171:724–727. doi: 10.1093/infdis/171.3.724. [DOI] [PubMed] [Google Scholar]
- 190.Kaiser R, Rauer S. 1999. Advantage of recombinant borrelial proteins for serodiagnosis of neuroborreliosis. J Med Microbiol 48:5–10. doi: 10.1099/00222615-48-1-5. [DOI] [PubMed] [Google Scholar]
- 191.Hauser U, Lehnert G, Wilske B. 1998. Diagnostic value of proteins of three Borrelia species (Borrelia burgdorferi sensu lato) and implications for development and use of recombinant antigens for serodiagnosis of Lyme borreliosis in Europe. Clin Diagn Lab Immunol 5:456–462. doi: 10.1128/CDLI.5.4.456-462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wilske B, Jauris-Heipke S, Lobentanzer R, Pradel I, Preac-Mursic V, Rossler D, Soutschek E, Johnson RC. 1995. Phenotypic analysis of outer surface protein C (OspC) of Borrelia burgdorferi sensu lato by monoclonal antibodies: relationship to genospecies and OspA serotype. J Clin Microbiol 33:103–109. doi: 10.1128/JCM.33.1.103-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun 61:2182–2191. doi: 10.1128/IAI.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jauris-Heipke S, Fuchs R, Motz M, Preac-Mursic V, Schwab E, Soutschek E, Will G, Wilske B. 1993. Genetic heterogenity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med Microbiol Immunol 182:37–50. doi: 10.1007/BF00195949. [DOI] [PubMed] [Google Scholar]
- 195.Theisen M, Frederiksen B, Lebech AM, Vuust J, Hansen K. 1993. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol 31:2570–2576. doi: 10.1128/JCM.31.10.2570-2576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mathiesen MJ, Hansen K, Axelsen N, Halkier-Sorensen L, Theisen M. 1996. Analysis of the human antibody response to outer surface protein C (OspC) of Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii. Med Microbiol Immunol 185:121–129. doi: 10.1007/s004300050021. [DOI] [PubMed] [Google Scholar]
- 197.Wilske B, Fingerle V, Preac-Mursic V, Jauris-Heipke S, Hofmann A, Loy H, Pfister HW, Rossler D, Soutschek E. 1994. Immunoblot using recombinant antigens derived from different genospecies of Borrelia burgdorferi sensu lato. Med Microbiol Immunol 183:43–59. doi: 10.1007/BF00193630. [DOI] [PubMed] [Google Scholar]
- 198.Mathiesen MJ, Christiansen M, Hansen K, Holm A, Åsbrink E, Theisen M. 1998. Peptide-based OspC enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J Clin Microbiol 36:3474–3479. doi: 10.1128/JCM.36.12.3474-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wilske B, Fingerle V, Herzer P, Hofmann A, Lehnert G, Peters H, Pfister HW, Preac-Mursic V, Soutschek E, Weber K. 1993. Recombinant immunoblot in the serodiagnosis of Lyme borreliosis: comparison with indirect immunofluorescence and enzyme-linked immunosorbent assay. Med Microbiol Immunol 182:255–270. doi: 10.1007/BF00579624. [DOI] [PubMed] [Google Scholar]
- 200.Mathiesen MJ, Holm A, Christiansen M, Blom J, Hansen K, Ostergaard S, Theisen M. 1998. The dominant epitope of Borrelia garinii outer surface protein C recognized by sera from patients with neuroborreliosis has a surface-exposed conserved structural motif. Infect Immun 66:4073–4079. doi: 10.1128/.66.9.4073-4079.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Izac JR, Camire AC, Earnhart CG, Embers ME, Funk RA, Breitschwerdt EB, Marconi RT. 2019. Analysis of the antigenic determinants of the OspC protein of the Lyme disease spirochetes: evidence that the C10 motif is not immunodominant or required to elicit bactericidal antibody responses. Vaccine 37:2401–2407. doi: 10.1016/j.vaccine.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pulzova L, Flachbartova Z, Bencurova E, Potocnakova L, Comor L, Schreterova E, Bhide M. 2016. Identification of B-cell epitopes of Borrelia burgdorferi outer surface protein C by screening a phage-displayed gene fragment library. Microbiol Immunol 60:669–677. doi: 10.1111/1348-0421.12438. [DOI] [PubMed] [Google Scholar]
- 203.Baum E, Randall AZ, Zeller M, Barbour AG. 2013. Inferring epitopes of a polymorphic antigen amidst broadly cross-reactive antibodies using protein microarrays: a study of OspC proteins of Borrelia burgdorferi. PLoS One 8:e67445. doi: 10.1371/journal.pone.0067445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yu Z, Carter JM, Sigal LH, Stein S. 1996. Multi-well ELISA based on independent peptide antigens for antibody capture: application to Lyme disease serodiagnosis. J Immunol Methods 198:25–33. doi: 10.1016/0022-1759(96)00140-8. [DOI] [PubMed] [Google Scholar]
- 205.Arnaboldi PM, Dattwyler RJ. 2015. Cross-reactive epitopes in Borrelia burgdorferi p66. Clin Vaccine Immunol 22:840–843. doi: 10.1128/CVI.00217-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chandra A, Latov N, Wormser GP, Marques AR, Alaedini A. 2011. Epitope mapping of antibodies to VlsE protein of Borrelia burgdorferi in post-Lyme disease syndrome. Clin Immunol 141:103–110. doi: 10.1016/j.clim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Burbelo PD, Issa AT, Ching KH, Cohen JI, Iadarola MJ, Marques A. 2010. Rapid, simple, quantitative, and highly sensitive antibody detection for Lyme disease. CVI 17:904–909. doi: 10.1128/CVI.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gomes-Solecki MJ, Wormser GP, Schriefer M, Neuman G, Hannafey L, Glass JD, Dattwyler RJ. 2002. Recombinant assay for serodiagnosis of Lyme disease regardless of OspA vaccination status. J Clin Microbiol 40:193–197. doi: 10.1128/JCM.40.1.193-197.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lahey LJ, Panas MW, Mao R, Delanoy M, Flanagan JJ, Binder SR, Rebman AW, Montoya JG, Soloski MJ, Steere AC, Dattwyler RJ, Arnaboldi PM, Aucott JN, Robinson WH. 2015. Development of a multiantigen panel for improved detection of Borrelia burgdorferi infection in early Lyme disease. J Clin Microbiol 53:3834–3841. doi: 10.1128/JCM.02111-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Embers ME, Hasenkampf NR, Barnes MB, Didier ES, Philipp MT, Tardo AC. 2016. Five-Antigen fluorescent bead-based assay for diagnosis of Lyme disease. Clin Vaccine Immunol 23:294–303. doi: 10.1128/CVI.00685-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Joung HA, Ballard ZS, Wu J, Tseng DK, Teshome H, Zhang L, Horn EJ, Arnaboldi PM, Dattwyler RJ, Garner OB, Di Carlo D, Ozcan A. 2020. Point-of-care serodiagnostic test for early-stage Lyme disease using a multiplexed paper-based immunoassay and machine learning. ACS Nano 14:229–240. doi: 10.1021/acsnano.9b08151. [DOI] [PubMed] [Google Scholar]
- 212.Arumugam S, Nayak S, Williams T, di Santa Maria FS, Guedes MS, Chaves RC, Linder V, Marques AR, Horn EJ, Wong SJ, Sia SK, Gomes-Solecki M. 2019. A multiplexed serologic test for diagnosis of Lyme disease for point-of-care use. J Clin Microbiol 57:e01142-19. doi: 10.1128/JCM.01142-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hahm JB, Breneman JWt, Liu J, Rabkina S, Zheng W, Zhou S, Walker RP, Kaul R. 2020. A fully automated multiplex assay for diagnosis of Lyme disease with high specificity and improved early sensitivity. J Clin Microbiol 58:e01785-19. doi: 10.1128/JCM.01785-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Barbour AG. 1988. Laboratory aspects of Lyme borreliosis. Clin Microbiol Rev 1:399–414. doi: 10.1128/CMR.1.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Russell H, Sampson JS, Schmid GP, Wilkinson HW, Plikaytis B. 1984. Enzyme-linked immunosorbent assay and indirect immunofluorescence assay for Lyme disease. J Infect Dis 149:465–470. doi: 10.1093/infdis/149.3.465. [DOI] [PubMed] [Google Scholar]
- 216.Magnarelli LA, Meegan JM, Anderson JF, Chappell WA. 1984. Comparison of an indirect fluorescent-antibody test with an enzyme-linked immunosorbent assay for serological studies of Lyme disease. J Clin Microbiol 20:181–184. doi: 10.1128/JCM.20.2.181-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nigrovic LE, Lipsett SC, Molins CR, Wormser GP, Bennett JE, Garro AC, Levas MN, Balamuth F, Neville D, Lingampalli N, Robinson WH, Branda JA. 2019. Higher C6 enzyme immunoassay index values correlate with a diagnosis of noncutaneous Lyme disease. Diagn Microbiol Infect Dis 94:160–164. doi: 10.1016/j.diagmicrobio.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 218.Halperin JJ, Luft BJ, Anand AK, Roque CT, Alvarez O, Volkman DJ, Dattwyler RJ. 1989. Lyme neuroborreliosis: central nervous system manifestations. Neurology 39:753–759. doi: 10.1212/WNL.39.6.753. [DOI] [PubMed] [Google Scholar]
- 219.Lipsett SC, Pollock NR, Branda JA, Gordon CD, Gordon CR, Lantos PM, Nigrovic LE. 2015. The positive predictive value of Lyme ELISA for the diagnosis of Lyme disease in children. Pediatr Infect Dis J 34:1260–1262. doi: 10.1097/INF.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Klempner MS, Schmid CH, Hu L, Steere AC, Johnson G, McCloud B, Noring R, Weinstein A. 2001. Intralaboratory reliability of serologic and urine testing for Lyme disease. Am J Med 110:217–219. doi: 10.1016/S0002-9343(00)00701-4. [DOI] [PubMed] [Google Scholar]
- 222.Zoller L, Burkard S, Schafer H. 1991. Validity of western immunoblot band patterns in the serodiagnosis of Lyme borreliosis. J Clin Microbiol 29:174–182. doi: 10.1128/JCM.29.1.174-182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Binnicker MJ, Jespersen DJ, Harring JA, Rollins LO, Bryant SC, Beito EM. 2008. Evaluation of two commercial systems for automated processing, reading, and interpretation of Lyme borreliosis Western blots. J Clin Microbiol 46:2216–2221. doi: 10.1128/JCM.00200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dattwyler RJ, Arnaboldi PM. 2014. Comparison of Lyme disease serologic assays and Lyme specialty laboratories. Clin Infect Dis 59:1711–1713. doi: 10.1093/cid/ciu705. [DOI] [PubMed] [Google Scholar]
- 225.Feng S, Hodzic E, Stevenson B, Barthold SW. 1998. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun 66:2827–2835. doi: 10.1128/IAI.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Busson L, Reynders M, Van den Wijngaert S, Dahma H, Decolvenaer M, Vasseur L, Vandenberg O. 2012. Evaluation of commercial screening tests and blot assays for the diagnosis of Lyme borreliosis. Diagn Microbiol Infect Dis 73:246–251. doi: 10.1016/j.diagmicrobio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 227.Theel ES, Sorenson M, Granger D. 2018. Evaluation of a novel microarray immunoblot assay for detection of IgM- and IgG-class antibodies to Borrelia burgdorferi. J Clin Microbiol 56:e00992-18. doi: 10.1128/JCM.00992-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Moore A, Nelson C, Molins C, Mead P, Schriefer M. 2016. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme Disease, United States. Emerg Infect Dis 22:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Harrer T, Geißdörfer W, Schoerner C, Lang E, Helm G. 2007. Seronegative Lyme neuroborreliosis in a patient on treatment for chronic lymphatic leukemia. Infection 35:110–113. doi: 10.1007/s15010-007-6121-0. [DOI] [PubMed] [Google Scholar]
- 230.van Dop WA, Kersten MJ, de Wever B, Hovius JW. 2013. Seronegative Lyme neuroborreliosis in a patient using rituximab. BMJ Case Rep 2013:bcr2012007627. doi: 10.1136/bcr-2012-007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wagemakers A, Visser MC, de Wever B, Hovius JW, van de Donk N, Hendriks EJ, Peferoen L, Muller FF, Ang CW. 2018. Case report: persistently seronegative neuroborreliosis in an immunocompromised patient. BMC Infect Dis 18:362. doi: 10.1186/s12879-018-3273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gampourou F, Taithe F, Moisset X, Clavelou P. 2016. Seronegative Lyme neuroborreliosis in a patient treated by rituximab. Rev Neurol 172:166–167. doi: 10.1016/j.neurol.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 233.Wormser GP, Horowitz HW, Nowakowski J, McKenna D, Dumler JS, Varde S, Schwartz I, Carbonaro C, Aguero-Rosenfeld M. 1997. Positive Lyme disease serology in patients with clinical and laboratory evidence of human granulocytic ehrlichiosis. Am J Clin Pathol 107:142–147. doi: 10.1093/ajcp/107.2.142. [DOI] [PubMed] [Google Scholar]
- 234.Wormser GP, Carbonaro C, Miller S, Nowakowski J, Nadelman RB, Sivak S, Aguero-Rosenfeld ME. 2000. A limitation of 2-stage serological testing for Lyme disease: enzyme immunoassay and immunoblot assay are not independent tests. Clin Infect Dis 30:545–548. doi: 10.1086/313688. [DOI] [PubMed] [Google Scholar]
- 235.Mead P, Petersen J, Hinckley A. 2019. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep 68:703. doi: 10.15585/mmwr.mm6832a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shen Y, Wu D, Zelen M. 2001. Testing the independence of two diagnostic tests. Biometrics 57:1009–1017. doi: 10.1111/j.0006-341X.2001.01009.x. [DOI] [PubMed] [Google Scholar]
- 237.Wormser GP, Molins CR, Levin A, Lipsett SC, Nigrovic LE, Schriefer ME, Branda JA. 2018. Evaluation of a sequential enzyme immunoassay testing algorithm for Lyme disease demonstrates lack of test independence but high diagnostic specificity. Diagn Microbiol Infect Dis 91:217–219. doi: 10.1016/j.diagmicrobio.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Centers for Disease Control and Prevention. 2014. Revised surveillance case definition for HIV infection–United States, 2014. MMWR Recomm Rep 63:1–10. [PubMed] [Google Scholar]
- 239.Molins CR, Delorey MJ, Replogle A, Sexton C, Schriefer ME. 2017. Evaluation of bioMerieux’s dissociated VIDAS Lyme IgM II (LYM) and IgG II (LYG) as a first-tier diagnostic assay for Lyme disease. J Clin Microbiol 55:1698–1706. doi: 10.1128/JCM.02407-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Branda JA, Lemieux JE, Blair L, Ahmed AA, Hong DK, Bercovici S, Blauwkamp TA, Hollemon D, Ho C, Strle K, Damle NS, Lepore TJ, Pollock NR. 2020. Detection of Borrelia burgdorferi cell-free DNA in human plasma samples for improved diagnosis of early Lyme borreliosis. Clin Infect Dis doi: 10.1093/cid/ciaa858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lipsett SC, Branda JA, Nigrovic LE. 2019. Evaluation of the Modified Two-Tiered Testing Method for Diagnosis of Lyme Disease in Children. J Clin Microbiol 57:e00547-19. doi: 10.1128/JCM.00547-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Davis IRC, McNeil SA, Allen W, MacKinnon-Cameron D, Lindsay LR, Bernat K, Dibernardo A, LeBlanc JJ, Hatchette TF. 2020. Performance of a modified two-tiered testing enzyme immunoassay algorithm for serologic diagnosis of Lyme disease in Nova Scotia. J Clin Microbiol 58:e01841-19. doi: 10.1128/JCM.01841-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pegalajar-Jurado A, Schriefer ME, Welch RJ, Couturier MR, MacKenzie T, Clark RJ, Ashton LV, Delorey MJ, Molins CR. 2018. Evaluation of modified two-tiered testing algorithms for Lyme disease laboratory diagnosis using well-characterized serum samples. J Clin Microbiol 56:e01943-17. doi: 10.1128/JCM.01943-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Marques AR. 2018. Revisiting the Lyme disease serodiagnostic algorithm: the momentum gathers. J Clin Microbiol 56:e00749-18. doi: 10.1128/JCM.00749-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wormser GP, Levin A, Soman S, Adenikinju O, Longo MV, Branda JA. 2013. Comparative cost-effectiveness of two-tiered testing strategies for serodiagnosis of Lyme disease with noncutaneous manifestations. J Clin Microbiol 51:4045–4049. doi: 10.1128/JCM.01853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Theel ES, Aguero-Rosenfeld ME, Pritt B, Adem PV, Wormser GP. 2018. Limitations and confusing aspects of diagnostic testing for neurologic Lyme disease in the United States. J Clin Microbiol 57:e01406-18. doi: 10.1128/JCM.01406-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wilske B, Schierz G, Preac-Mursic V, von Buscb K, Kuhbeck R, Pfister H-W, Einhaupl K. 1986. Intrathecal production of specific antibodies against Borrelia burgdorferi in patients with lymphocytic meningoradiculitis (Bannwarth’s syndrome). J Infect Dis 153:304–314. doi: 10.1093/infdis/153.2.304. [DOI] [PubMed] [Google Scholar]
- 248.Reiber H. 1980. The discrimination between different blood-CSF barrier dysfunctions and inflammatory reactions of the CNS by a recent evaluation graph for the protein profile of cerebrospinal fluid. J Neurol 224:89–99. doi: 10.1007/BF00313347. [DOI] [PubMed] [Google Scholar]
- 249.Reiber H, Lange P. 1991. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem 37:1153–1160. doi: 10.1093/clinchem/37.7.1153. [DOI] [PubMed] [Google Scholar]
- 250.Hansen K, Lebech AM. 1991. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi-specific immunoglobulin G, A, and M. Ann Neurol 30:197–205. doi: 10.1002/ana.410300212. [DOI] [PubMed] [Google Scholar]
- 251.Halperin JJ, Volkman DJ, Wu P. 1991. Central nervous system abnormalities in Lyme neuroborreliosis. Neurology 41:1571–1582. doi: 10.1212/WNL.41.10.1571. [DOI] [PubMed] [Google Scholar]
- 252.Hammers-Berggren S, Hansen K, Lebech AM, Karlsson M. 1993. Borrelia burgdorferi-specific intrathecal antibody production in neuroborreliosis: a follow-up study. Neurology 43:169–175. doi: 10.1212/WNL.43.1_Part_1.169. [DOI] [PubMed] [Google Scholar]
- 253.Halperin JJ. 2015. Nervous system Lyme disease. Infect Dis Clin North Am 29:241–253. doi: 10.1016/j.idc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 254.Knudtzen FC, Andersen NS, Jensen TG, Skarphedinsson S. 2017. Characteristics and clinical outcome of Lyme neuroborreliosis in a high endemic area, 1995–2014: a retrospective cohort study in Denmark. Clin Infect Dis 65:1489–1495. doi: 10.1093/cid/cix568. [DOI] [PubMed] [Google Scholar]
- 255.Schwan TG, Battisti JM, Porcella SF, Raffel SJ, Schrumpf ME, Fischer ER, Carroll JA, Stewart PE, Rosa P, Somerville GA. 2003. Glycerol-3-phosphate acquisition in spirochetes: distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. J Bacteriol 185:1346–1356. doi: 10.1128/JB.185.4.1346-1356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Aberer E, Duray PH. 1991. Morphology of Borrelia burgdorferi: structural patterns of cultured borreliae in relation to staining methods. J Clin Microbiol 29:764–772. doi: 10.1128/JCM.29.4.764-772.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Duray PH. 1987. The surgical pathology of human Lyme disease. An enlarging picture. Am J Surg Pathol 11:47–60. doi: 10.1097/00000478-198700111-00005. [DOI] [PubMed] [Google Scholar]
- 258.De Koning J, Bosma RB, Hoogkamp-Korstanje JA. 1987. Demonstration of spirochaetes in patients with Lyme disease with a modified silver stain. J Med Microbiol 23:261–267. doi: 10.1099/00222615-23-3-261. [DOI] [PubMed] [Google Scholar]
- 259.Berger BW. 1989. Dermatologic manifestations of Lyme disease. Rev Infect Dis 11:S1475–S1481. doi: 10.1093/clinids/11.Supplement_6.S1475. [DOI] [PubMed] [Google Scholar]
- 260.Park HK, Jones BE, Barbour AG. 1986. Erythema chronicum migrans of Lyme disease: diagnosis by monoclonal antibodies. J Am Acad Dermatol 15:406–410. doi: 10.1016/S0190-9622(86)70190-4. [DOI] [PubMed] [Google Scholar]
- 261.Berger BW, Clemmensen OJ, Ackerman AB. 1983. Lyme disease is a spirochetosis: a review of the disease and evidence for its cause. Am J Dermatopathol 5:111–124. doi: 10.1097/00000372-198304000-00008. [DOI] [PubMed] [Google Scholar]
- 262.Centers for Disease Control and Prevention. 2013. Three sudden cardiac deaths associated with Lyme carditis—United States, November 2012-July 2013. MMWR Morb Mortal Wkly Rep 62:993–996. [PMC free article] [PubMed] [Google Scholar]
- 263.Johnston YE, Duray PH, Steere AC, Kashgarian M, Buza J, Malawista SE, Askenase PW. 1985. Lyme arthritis: spirochetes found in synovial microangiopathic lesions. Am J Pathol 118:26–34. [PMC free article] [PubMed] [Google Scholar]
- 264.Mullegger RR, Glatz M. 2008. Skin manifestations of Lyme borreliosis: diagnosis and management. Am J Clin Dermatol 9:355–368. doi: 10.2165/0128071-200809060-00002. [DOI] [PubMed] [Google Scholar]
- 265.Asbrink E. 1993. Acrodermatitis chronica atrophicans. Clin Dermatol 11:369–375. doi: 10.1016/0738-081x(93)90092-q. [DOI] [PubMed] [Google Scholar]
- 266.Brehmer-Andersson E, Hovmark A, Asbrink E. 1998. Acrodermatitis chronica atrophicans: histopathologic findings and clinical correlations in 111 cases. Acta Derm Venereol 78:207–213. doi: 10.1080/000155598441558. [DOI] [PubMed] [Google Scholar]
- 267.Barbour AG. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 268.Pollack RJ, Telford SR, III, Spielman A. 1993. Standardization of medium for culturing Lyme disease spirochetes. J Clin Microbiol 31:1251–1255. doi: 10.1128/JCM.31.5.1251-1255.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Preac-Mursic V, Wilske B, Schierz G. 1986. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralbl Bakteriol Mikrobiol Hyg A 263:112–118. doi: 10.1016/S0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- 270.Marques AR. 2015. Laboratory diagnosis of Lyme disease: advances and challenges. Infect Dis Clin North Am 29:295–307. doi: 10.1016/j.idc.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. 2011. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum 63:2238–2247. doi: 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nowakowski J, Schwartz I, Liveris D, Wang G, Aguero-Rosenfeld ME, Girao G, McKenna D, Nadelman RB, Cavaliere LF, Wormser GP, Lyme Disease Study Group . 2001. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin Infect Dis 33:2023–2027. doi: 10.1086/324490. [DOI] [PubMed] [Google Scholar]
- 273.Berger BW, Kaplan MH, Rothenberg IR, Barbour AG. 1985. Isolation and characterization of the Lyme disease spirochete from the skin of patients with erythema chronicum migrans. J Am Acad Dermatol 13:444–449. doi: 10.1016/S0190-9622(85)70187-9. [DOI] [PubMed] [Google Scholar]
- 274.Cerar T, Ruzic-Sabljic E, Glinsek U, Zore A, Strle F. 2008. Comparison of PCR methods and culture for the detection of Borrelia spp. in patients with erythema migrans. Clin Microbiol Infect 14:653–658. doi: 10.1111/j.1469-0691.2008.02013.x. [DOI] [PubMed] [Google Scholar]
- 275.Jurca T, Ruzic-Sabljic E, Lotric-Furlan S, Maraspin V, Cimperman J, Picken RN, Strle F. 1998. Comparison of peripheral and central biopsy sites for the isolation of Borrelia burgdorferi sensu lato from erythema migrans skin lesions. Clin Infect Dis 27:636–638. doi: 10.1086/514715. [DOI] [PubMed] [Google Scholar]
- 276.O’Rourke M, Traweger A, Lusa L, Stupica D, Maraspin V, Barrett PN, Strle F, Livey I. 2013. Quantitative detection of Borrelia burgdorferi sensu lato in erythema migrans skin lesions using internally controlled duplex real time PCR. PLoS One 8:e63968. doi: 10.1371/journal.pone.0063968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ruzic-Sabljic E, Maraspin V, Cimperman J, Strle F, Lotric-Furlan S, Stupica D, Cerar T. 2014. Comparison of isolation rate of Borrelia burgdorferi sensu lato in two different culture media, MKP and BSK-H. Clin Microbiol Infect 20:636–641. doi: 10.1111/1469-0691.12457. [DOI] [PubMed] [Google Scholar]
- 278.Stupica D, Lusa L, Maraspin V, Bogovič P, Vidmar D, O’Rourke M, Traweger A, Livey I, Strle F. 2015. Correlation of culture positivity, PCR positivity, and burden of Borrelia burgdorferi sensu lato in skin samples of erythema migrans patients with clinical findings. PLoS One 10:e0136600. doi: 10.1371/journal.pone.0136600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zore A, Ruzic-Sabljic E, Maraspin V, Cimperman J, Lotric-Furlan S, Pikelj A, Jurca T, Logar M, Strle F. 2002. Sensitivity of culture and polymerase chain reaction for the etiologic diagnosis of erythema migrans. Wien Klin Wochenschr 114:606–609. [PubMed] [Google Scholar]
- 280.Schwartz I, Wormser GP, Schwartz JJ, Cooper D, Weissensee P, Gazumyan A, Zimmermann E, Goldberg NS, Bittker S, Campbell GL, Pavia CS. 1992. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J Clin Microbiol 30:3082–3088. doi: 10.1128/JCM.30.12.3082-3088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Liveris D, Wang G, Girao G, Byrne DW, Nowakowski J, McKenna D, Nadelman R, Wormser GP, Schwartz I. 2002. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: correlation of results with clinical and laboratory findings. J Clin Microbiol 40:1249–1253. doi: 10.1128/JCM.40.4.1249-1253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mitchell PD, Reed KD, Vandermause MF, Melski JW. 1993. Isolation of Borrelia burgdorferi from skin biopsy specimens of patients with erythema migrans. Am J Clin Pathol 99:104–107. doi: 10.1093/ajcp/99.1.104. [DOI] [PubMed] [Google Scholar]
- 283.Nadelman RB, Nowakowski J, Forseter G, Bittker S, Cooper D, Goldberg N, McKenna D, Wormser GP. 1993. Failure to isolate Borrelia burgdorferi after antimicrobial therapy in culture-documented Lyme borreliosis associated with erythema migrans: report of a prospective study. Am J Med 94:583–588. doi: 10.1016/0002-9343(93)90208-7. [DOI] [PubMed] [Google Scholar]
- 284.Berger BW, Johnson RC, Kodner C, Coleman L. 1992. Cultivation of Borrelia burgdorferi from erythema migrans lesions and perilesional skin. J Clin Microbiol 30:359–361. doi: 10.1128/JCM.30.2.359-361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Picken MM, Picken RN, Han D, Cheng Y, Ruzic-Sabljic E, Cimperman J, Maraspin V, Lotric-Furlan S, Strle F. 1997. A two year prospective study to compare culture and polymerase chain reaction amplification for the detection and diagnosis of Lyme borreliosis. Mol Pathol 50:186–193. doi: 10.1136/mp.50.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wormser GP, Bittker S, Cooper D, Nowakowski J, Nadelman RB, Pavia C. 2000. Comparison of the yields of blood cultures using serum or plasma from patients with early Lyme disease. J Clin Microbiol 38:1648–1650. doi: 10.1128/JCM.38.4.1648-1650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wormser GP, Bittker S, Cooper D, Nowakowski J, Nadelman RB, Pavia C. 2001. Yield of large-volume blood cultures in patients with early Lyme disease. J Infect Dis 184:1070–1072. doi: 10.1086/323424. [DOI] [PubMed] [Google Scholar]
- 288.Liveris D, Schwartz I, Bittker S, Cooper D, Iyer R, Cox ME, Wormser GP. 2011. Improving the yield of blood cultures from patients with early Lyme disease. J Clin Microbiol 49:2166–2168. doi: 10.1128/JCM.00350-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sapi E, Pabbati N, Datar A, Davies EM, Rattelle A, Kuo BA. 2013. Improved culture conditions for the growth and detection of Borrelia from human serum. Int J Med Sci 10:362–376. doi: 10.7150/ijms.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Johnson BJ, Pilgard MA, Russell TM. 2014. Assessment of new culture method for detection of Borrelia species from serum of Lyme disease patients. J Clin Microbiol 52:721–724. doi: 10.1128/JCM.01674-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wormser GP, Shapiro ED, Strle F. 2017. Studies that report unexpected positive blood cultures for Lyme borrelia: are they valid? Diagn Microbiol Infect Dis 89:178–181. doi: 10.1016/j.diagmicrobio.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Nowakowski J, McKenna D, Nadelman RB, Bittker S, Cooper D, Pavia C, Holmgren D, Visintainer P, Wormser GP. 2009. Blood cultures for patients with extracutaneous manifestations of Lyme disease in the United States. Clin Infect Dis 49:1733–1735. doi: 10.1086/648076. [DOI] [PubMed] [Google Scholar]
- 293.Maraspin V, Ogrinc K, Ruzic-Sabljic E, Lotric-Furlan S, Strle F. 2011. Isolation of Borrelia burgdorferi sensu lato from blood of adult patients with borrelial lymphocytoma, Lyme neuroborreliosis, Lyme arthritis, and acrodermatitis chronica atrophicans. Infection 39:35–40. doi: 10.1007/s15010-010-0062-8. [DOI] [PubMed] [Google Scholar]
- 294.Ogrinc K, Lotric-Furlan S, Maraspin V, Lusa L, Cerar T, Ruzic-Sabljic E, Strle F. 2013. Suspected early Lyme neuroborreliosis in patients with erythema migrans. Clin Infect Dis 57:501–509. doi: 10.1093/cid/cit317. [DOI] [PubMed] [Google Scholar]
- 295.Cerar T, Ogrinc K, Lotric-Furlan S, Kobal J, Levicnik-Stezinar S, Strle F, Ruzic-Sabljic E. 2013. Diagnostic value of cytokines and chemokines in Lyme neuroborreliosis. Clin Vaccine Immunol 20:1578–1584. doi: 10.1128/CVI.00353-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Strle F, Ruzic-Sabljic E, Cimperman J, Lotric-Furlan S, Maraspin V. 2006. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin Infect Dis 43:704–710. doi: 10.1086/506936. [DOI] [PubMed] [Google Scholar]
- 297.Cerar T, Ogrinc K, Cimperman J, Lotric-Furlan S, Strle F, Ruzic-Sabljic E. 2008. Validation of cultivation and PCR methods for diagnosis of Lyme neuroborreliosis. J Clin Microbiol 46:3375–3379. doi: 10.1128/JCM.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bradley JF, Johnson RC, Goodman JL. 1994. The persistence of spirochetal nucleic acids in active Lyme arthritis. Ann Intern Med 120:487–489. doi: 10.7326/0003-4819-120-6-199403150-00007. [DOI] [PubMed] [Google Scholar]
- 299.Steere AC, Grodzicki RL, Craft JE, Shrestha M, Kornblatt AN, Malawista SE. 1984. Recovery of Lyme disease spirochetes from patients. Yale J Biol Med 57:557–560. [PMC free article] [PubMed] [Google Scholar]
- 300.Wormser GP, Nadelman RB, Schwartz I. 2012. The amber theory of Lyme arthritis: initial description and clinical implications. Clin Rheumatol 31:989–994. doi: 10.1007/s10067-012-1964-x. [DOI] [PubMed] [Google Scholar]
- 301.Snydman DR, Schenkein DP, Berardi VP, Lastavica CC, Pariser KM. 1986. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann Intern Med 104:798–800. doi: 10.7326/0003-4819-104-6-798. [DOI] [PubMed] [Google Scholar]
- 302.Liveris D, Schwartz I, McKenna D, Nowakowski J, Nadelman RB, DeMarco J, Iyer R, Cox ME, Holmgren D, Wormser GP. 2012. Quantitation of cell-associated borrelial DNA in the blood of Lyme disease patients with erythema migrans. Eur J Clin Microbiol Infect Dis 31:791–795. doi: 10.1007/s10096-011-1376-x. [DOI] [PubMed] [Google Scholar]
- 303.Liu W, Liu HX, Zhang L, Hou XX, Wan KL, Hao Q. 2016. A novel isothermal assay of Borrelia burgdorferi by recombinase polymerase amplification with lateral flow detection. Int J Mol Sci 17:1250. doi: 10.3390/ijms17081250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kingry LC, Anacker M, Pritt B, Bjork J, Respicio-Kingry L, Liu G, Sheldon S, Boxrud D, Strain A, Oatman S, Berry J, Sloan L, Mead P, Neitzel D, Kugeler KJ, Petersen JM. 2018. Surveillance for and discovery of Borrelia species in US patients suspected of tickborne illness. Clin Infect Dis 66:1864–1871. doi: 10.1093/cid/cix1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Babady NE, Sloan LM, Vetter EA, Patel R, Binnicker MJ. 2008. Percent positive rate of Lyme real-time poLymerase chain reaction in blood, cerebrospinal fluid, synovial fluid, and tissue. Diagn Microbiol Infect Dis 62:464–466. doi: 10.1016/j.diagmicrobio.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 306.Guy EC, Stanek G. 1991. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J Clin Pathol 44:610–611. doi: 10.1136/jcp.44.7.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Iyer R, Mukherjee P, Wang K, Simons J, Wormser GP, Schwartz I. 2013. Detection of Borrelia burgdorferi nucleic acids after antibiotic treatment does not confirm viability. J Clin Microbiol 51:857–862. doi: 10.1128/JCM.02785-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Picha D, Moravcova L, Vanousova D, Hercogova J, Blechova Z. 2014. DNA persistence after treatment of Lyme borreliosis. Folia Microbiol (Praha) 59:115–125. doi: 10.1007/s12223-013-0272-4. [DOI] [PubMed] [Google Scholar]
- 309.Mosel MR, Carolan HE, Rebman AW, Castro S, Massire C, Ecker DJ, Soloski MJ, Aucott JN, Eshoo MW. 2019. Molecular testing of serial blood specimens from patients with early Lyme disease during treatment reveals changing coinfection with mixtures of Borrelia burgdorferi genotypes. Antimicrob Agents Chemother 63:e00237-19. doi: 10.1128/AAC.00237-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Coulter P, Lema C, Flayhart D, Linhardt AS, Aucott JN, Auwaerter PG, Dumler JS. 2005. Two-year evaluation of Borrelia burgdorferi culture and supplemental tests for definitive diagnosis of Lyme disease. J Clin Microbiol 43:5080–5084. doi: 10.1128/JCM.43.10.5080-5084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Snyder JL, Giese H, Bandoski-Gralinski C, Townsend J, Jacobson BE, Shivers R, Schotthoefer AM, Fritsche TR, Green C, Callister SM, Branda JA, Lowery TJ. 2017. T2 magnetic resonance assay-based direct detection of three Lyme disease-related Borrelia species in whole-blood samples. J Clin Microbiol 55:2453–2461. doi: 10.1128/JCM.00510-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Goodman JL, Bradley JF, Ross AE, Goellner P, Lagus A, Vitale B, Berger BW, Luger S, Johnson RC. 1995. Bloodstream invasion in early Lyme disease: results from a prospective, controlled, blinded study using the polymerase chain reaction. Am J Med 99:6–12. doi: 10.1016/S0002-9343(99)80097-7. [DOI] [PubMed] [Google Scholar]
- 313.Nadelman RB, Schwartz I, Wormser GP. 1994. Detecting Borrelia burgdorferi in blood from patients with Lyme disease. J Infect Dis 169:1410–1411. doi: 10.1093/infdis/169.6.1410. [DOI] [PubMed] [Google Scholar]
- 314.Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, Soloski MJ, Ecker DJ, Schutzer SE, Aucott JN. 2012. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One 7:e36825. doi: 10.1371/journal.pone.0036825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Picha D, Moravcova L, Zdarsky E, Maresova V, Hulinsky V. 2005. PCR in Lyme neuroborreliosis: a prospective study. Acta Neurol Scand 112:287–292. doi: 10.1111/j.1600-0404.2005.00482.x. [DOI] [PubMed] [Google Scholar]
- 316.Lebech AM, Hansen K, Brandrup F, Clemmensen O, Halkier-Sorensen L. 2000. Diagnostic value of PCR for detection of Borrelia burgdorferi DNA in clinical specimens from patients with erythema migrans and Lyme neuroborreliosis. Mol Diagn 5:139–150. doi: 10.2165/00066982-200005020-00007. [DOI] [PubMed] [Google Scholar]
- 317.Moter SE, Hofmann H, Wallich R, Simon MM, Kramer MD. 1994. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospA-specific PCR. J Clin Microbiol 32:2980–2988. doi: 10.1128/JCM.32.12.2980-2988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 319.Persing DH, Rutledge BJ, Rys PN, Podzorski DS, Mitchell PD, Reed KD, Liu B, Fikrig E, Malawista SE. 1994. Target imbalance: disparity of Borrelia burgdorferi genetic material in synovial fluid from Lyme arthritis patients. J Infect Dis 169:668–672. doi: 10.1093/infdis/169.3.668. [DOI] [PubMed] [Google Scholar]
- 320.Lipowsky C, Altwegg M, Michel BA, Bruhlmann P. 2003. Detection of Borrelia burgdorferi by species-specific and broad-range PCR of synovial fluid and synovial tissue of Lyme arthritis patients before and after antibiotic treatment. Clin Exp Rheumatol 21:271–272. [PubMed] [Google Scholar]
- 321.Jaulhac B, Chary-Valckenaere I, Sibilia J, Javier RM, Piemont Y, Kuntz JL, Monteil H, Pourel J. 1996. Detection of Borrelia burgdorferi by DNA amplification in synovial tissue samples from patients with Lyme arthritis. Arthritis Rheum 39:736–745. doi: 10.1002/art.1780390505. [DOI] [PubMed] [Google Scholar]
- 322.Priem S, Burmester GR, Kamradt T, Wolbart K, Rittig MG, Krause A. 1998. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann Rheum Dis 57:118–121. doi: 10.1136/ard.57.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Avery RA, Frank G, Eppes SC. 2005. Diagnostic utility of Borrelia burgdorferi cerebrospinal fluid polymerase chain reaction in children with Lyme meningitis. Pediatr Infect Dis J 24:705–708. doi: 10.1097/01.inf.0000172903.14077.4c. [DOI] [PubMed] [Google Scholar]
- 324.Dumler JS. 2001. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 6:1–11. doi: 10.2165/00066982-200106010-00001. [DOI] [PubMed] [Google Scholar]
- 325.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. 2005. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mygland A, Ljostad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I, European Federation of Neurological S . 2010. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol 17:8–16. doi: 10.1111/j.1468-1331.2009.02862.x. [DOI] [PubMed] [Google Scholar]
- 327.Gomes-Solecki MJ, Wormser GP, Persing DH, Berger BW, Glass JD, Yang X, Dattwyler RJ. 2001. A first-tier rapid assay for the serodiagnosis of Borrelia burgdorferi infection. Arch Intern Med 161:2015–2020. doi: 10.1001/archinte.161.16.2015. [DOI] [PubMed] [Google Scholar]
- 328.Nayak S, Sridhara A, Melo R, Richer L, Chee NH, Kim J, Linder V, Steinmiller D, Sia SK, Gomes-Solecki M. 2016. Microfluidics-based point-of-care test for serodiagnosis of Lyme disease. Sci Rep 6:35069. doi: 10.1038/srep35069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Toumanios C, Prisco L, Dattwyler RJ, Arnaboldi PM. 2019. Linear B cell epitopes derived from the multifunctional surface lipoprotein BBK32 as targets for the serodiagnosis of Lyme disease. mSphere 4:e00111-19. doi: 10.1128/mSphere.00111-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Guerineau AL, Dhote R, Christiann F, Rayet P, Assous MV. 1999. Differentiation between early and late complicated Lyme borreliosis by specific IgG avidity. Lancet 354:1096–1097. doi: 10.1016/S0140-6736(99)03198-0. [DOI] [PubMed] [Google Scholar]
- 331.Mavin S, Evans R, Cornulier T, Bowman AS. 2018. The development of an IgG avidity Western blot with potential to differentiate patients with active Lyme borreliosis from those with past infection. J Microbiol Methods 146:71–76. doi: 10.1016/j.mimet.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 332.Rauer S, Beitlich P, Neubert U, Rasiah C, Kaiser R. 2001. Avidity determination of Borrelia burgdorferi-specific IgG antibodies in Lyme disease. Scand J Infect Dis 33:809–811. doi: 10.1080/00365540110027321. [DOI] [PubMed] [Google Scholar]
- 333.Benach JL, Coleman JL, Garcia-Monco JC, Deponte PC. 1988. Biological activity of Borrelia burgdorferi antigens. Ann N Y Acad Sci 539:115–125. doi: 10.1111/j.1749-6632.1988.tb31845.x. [DOI] [PubMed] [Google Scholar]
- 334.Dattwyler RJ, Volkman DJ, Luft BJ, Halperin JJ, Thomas J, Golightly MG. 1988. Seronegative Lyme disease: dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med 319:1441–1446. doi: 10.1056/NEJM198812013192203. [DOI] [PubMed] [Google Scholar]
- 335.Zoschke DC, Skemp AA, Defosse DL. 1991. Lymphoproliferative responses to Borrelia burgdorferi in Lyme disease. Ann Intern Med 114:285–289. doi: 10.7326/0003-4819-114-4-285. [DOI] [PubMed] [Google Scholar]
- 336.Glickstein L, Moore B, Bledsoe T, Damle N, Sikand V, Steere AC. 2003. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect Immun 71:6051–6053. doi: 10.1128/IAI.71.10.6051-6053.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Forsberg P, Ernerudh J, Ekerfelt C, Roberg M, Vrethem M, Bergstrom S. 1995. The outer surface proteins of Lyme disease borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFN-gamma): diagnostic and pathogenic implications. Clin Exp Immunol 101:453–460. doi: 10.1111/j.1365-2249.1995.tb03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Pohl-Koppe A, Balashov KE, Steere AC, Logigian EL, Hafler DA. 1998. Identification of a T cell subset capable of both IFN-gamma and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J Immunol 160:1804–1810. [PubMed] [Google Scholar]
- 339.Jin C, Roen DR, Lehmann PV, Kellermann GH. 2013. An enhanced ELISPOT assay for sensitive detection of antigen-specific T cell responses to Borrelia burgdorferi. Cells 2:607–620. doi: 10.3390/cells2030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bouquet J, Soloski MJ, Swei A, Cheadle C, Federman S, Billaud JN, Rebman AW, Kabre B, Halpert R, Boorgula M, Aucott JN, Chiu CY. 2016. Longitudinal transcriptome analysis reveals a sustained differential gene expression signature in patients treated for acute Lyme disease. mBio 7:e00100-16. doi: 10.1128/mBio.00100-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Callister SM, Jobe DA, Stuparic-Stancic A, Miyamasu M, Boyle J, Dattwyler RJ, Arnaboldi PM. 2016. Detection of IFN-gamma secretion by T cells collected before and after successful treatment of early Lyme disease. Clin Infect Dis 62:1235–1241. doi: 10.1093/cid/ciw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.van Gorkom T, Sankatsing SUC, Voet W, Ismail DM, Muilwijk RH, Salomons M, Vlaminckx BJM, Bossink AWJ, Notermans DW, Bouwman JJM, Kremer K, Thijsen SFT. 2018. An enzyme-linked immunosorbent spot assay measuring Borrelia burgdorferi B31-specific interferon gamma-secreting T cells cannot discriminate active Lyme neuroborreliosis from past Lyme borreliosis: a prospective study in the Netherlands. J Clin Microbiol 56:e01695-17. doi: 10.1128/JCM.01695-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kowarik MC, Cepok S, Sellner J, Grummel V, Weber MS, Korn T, Berthele A, Hemmer B. 2012. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation 9:93. doi: 10.1186/1742-2094-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rupprecht TA, Plate A, Adam M, Wick M, Kastenbauer S, Schmidt C, Klein M, Pfister HW, Koedel U. 2009. The chemokine CXCL13 is a key regulator of B cell recruitment to the cerebrospinal fluid in acute Lyme neuroborreliosis. J Neuroinflammation 6:42. doi: 10.1186/1742-2094-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Narayan K, Dail D, Li L, Cadavid D, Amrute S, Fitzgerald-Bocarsly P, Pachner AR. 2005. The nervous system as ectopic germinal center: CXCL13 and IgG in Lyme neuroborreliosis. Ann Neurol 57:813–823. doi: 10.1002/ana.20486. [DOI] [PubMed] [Google Scholar]
- 346.Rupprecht TA, Kirschning CJ, Popp B, Kastenbauer S, Fingerle V, Pfister HW, Koedel U. 2007. Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infect Immun 75:4351–4356. doi: 10.1128/IAI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Hytonen J, Kortela E, Waris M, Puustinen J, Salo J, Oksi J. 2014. CXCL13 and neopterin concentrations in cerebrospinal fluid of patients with Lyme neuroborreliosis and other diseases that cause neuroinflammation. J Neuroinflammation 11:103. doi: 10.1186/1742-2094-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Bremell D, Mattsson N, Edsbagge M, Blennow K, Andreasson U, Wikkelso C, Zetterberg H, Hagberg L. 2013. Cerebrospinal fluid CXCL13 in Lyme neuroborreliosis and asymptomatic HIV infection. BMC Neurol 13:2. doi: 10.1186/1471-2377-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wutte N, Berghold A, Loffler S, Zenz W, Daghofer E, Krainberger I, Kleinert G, Aberer E. 2011. CXCL13 chemokine in pediatric and adult neuroborreliosis. Acta Neurol Scand 124:321–328. doi: 10.1111/j.1600-0404.2010.01477.x. [DOI] [PubMed] [Google Scholar]
- 350.Eckman EA, Pacheco-Quinto J, Herdt AR, Halperin JJ. 2018. Neuroimmunomodulators in neuroborreliosis and Lyme encephalopathy. Clin Infect Dis 67:80–88. doi: 10.1093/cid/ciy019. [DOI] [PubMed] [Google Scholar]
- 351.Schmidt C, Plate A, Angele B, Pfister HW, Wick M, Koedel U, Rupprecht TA. 2011. A prospective study on the role of CXCL13 in Lyme neuroborreliosis. Neurology 76:1051–1058. doi: 10.1212/WNL.0b013e318211c39a. [DOI] [PubMed] [Google Scholar]
- 352.Ljostad U, Mygland A. 2008. CSF B—lymphocyte chemoattractant (CXCL13) in the early diagnosis of acute Lyme neuroborreliosis. J Neurol 255:732–737. doi: 10.1007/s00415-008-0785-y. [DOI] [PubMed] [Google Scholar]
- 353.Rupprecht TA, Koedel U, Angele B, Fingerle V, Pfister HW. 2006. Cytokine CXCL13: a possible early CSF marker for neuroborreliosis. Nervenarzt 77:470–473. doi: 10.1007/s00115-005-2021-7. [DOI] [PubMed] [Google Scholar]
- 354.Senel M, Rupprecht TA, Tumani H, Pfister HW, Ludolph AC, Brettschneider J. 2010. The chemokine CXCL13 in acute neuroborreliosis. J Neurol Neurosurg Psychiatry 81:929–933. doi: 10.1136/jnnp.2009.195438. [DOI] [PubMed] [Google Scholar]
- 355.Marra CM, Tantalo LC, Sahi SK, Maxwell CL, Lukehart SA. 2010. CXCL13 as a cerebrospinal fluid marker for neurosyphilis in HIV-infected patients with syphilis. Sex Transm Dis 37:283–287. doi: 10.1097/OLQ.0b013e3181d877a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.van Burgel ND, Bakels F, Kroes AC, van Dam AP. 2011. Discriminating Lyme neuroborreliosis from other neuroinflammatory diseases by levels of CXCL13 in cerebrospinal fluid. J Clin Microbiol 49:2027–2030. doi: 10.1128/JCM.00084-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Molins CR, Ashton LV, Wormser GP, Hess AM, Delorey MJ, Mahapatra S, Schriefer ME, Belisle JT. 2015. Development of a metabolic biosignature for detection of early Lyme disease. Clin Infect Dis 60:1767–1775. doi: 10.1093/cid/civ185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Soloski MJ, Crowder LA, Lahey LJ, Wagner CA, Robinson WH, Aucott JN. 2014. Serum inflammatory mediators as markers of human Lyme disease activity. PLoS One 9:e93243. doi: 10.1371/journal.pone.0093243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Molins CR, Ashton LV, Wormser GP, Andre BG, Hess AM, Delorey MJ, Pilgard MA, Johnson BJ, Webb K, Islam MN, Pegalajar-Jurado A, Molla I, Jewett MW, Belisle JT. 2017. Metabolic differentiation of early Lyme disease from southern tick-associated rash illness (STARI). Sci Transl Med 9:eaal2717. doi: 10.1126/scitranslmed.aal2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Pegalajar-Jurado A, Fitzgerald BL, Islam MN, Belisle JT, Wormser GP, Waller KS, Ashton LV, Webb KJ, Delorey MJ, Clark RJ, Molins CR. 2018. Identification of urine metabolites as biomarkers of early Lyme disease. Sci Rep 8:12204. doi: 10.1038/s41598-018-29713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zhou Y, Qin S, Sun M, Tang L, Yan X, Kim TK, Caballero J, Glusman G, Brunkow ME, Soloski MJ, Rebman AW, Scavarda C, Cooper D, Omenn GS, Moritz RL, Wormser GP, Price ND, Aucott JN, Hood L. 2020. Measurement of organ-specific and acute-phase blood protein levels in early Lyme disease. J Proteome Res 19:346–359. doi: 10.1021/acs.jproteome.9b00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Schutzer SE, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, Gerald NJ, Gomes-Solecki M, Kintrup M, Ledizet M, Levin AE, Lewinski M, Liotta LA, Marques A, Mead PS, Mongodin EF, Pillai S, Rao P, Robinson WH, Roth KM, Schriefer ME, Slezak T, Snyder JL, Steere AC, Witkowski J, Wong SJ, Branda JA. 2019. Direct diagnostic tests for Lyme disease. Clin Infect Dis 68:1052–1057. doi: 10.1093/cid/ciy614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Nolte O. 2012. Nucleic acid amplification based diagnostic of Lyme (neuro-)borreliosis: lost in the jungle of methods, targets, and assays? Open Neurol J 6:129–139. doi: 10.2174/1874205X01206010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hosono S, Faruqi AF, Dean FB, Du Y, Sun Z, Wu X, Du J, Kingsmore SF, Egholm M, Lasken RS. 2003. Unbiased whole-genome amplification directly from clinical samples. Genome Res 13:954–964. doi: 10.1101/gr.816903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Li H, Bai R, Zhao Z, Tao L, Ma M, Ji Z, Jian M, Ding Z, Dai X, Bao F, Liu A. 2018. Application of droplet digital PCR to detect the pathogens of infectious diseases. Biosci Rep 38:BSR20181170. doi: 10.1042/BSR20181170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. 2017. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol 35:833–844. doi: 10.1038/nbt.3935. [DOI] [PubMed] [Google Scholar]
- 367.Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, Kawli T, Christians FC, Venkatasubrahmanyam S, Wall GD, Cheung A, Rogers ZN, Meshulam-Simon G, Huijse L, Balakrishnan S, Quinn JV, Hollemon D, Hong DK, Vaughn ML, Kertesz M, Bercovici S, Wilber JC, Yang S. 2019. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol 4:663–674. doi: 10.1038/s41564-018-0349-6. [DOI] [PubMed] [Google Scholar]
- 368.Abril MK, Barnett AS, Wegermann K, Fountain E, Strand A, Heyman BM, Blough BA, Swaminathan AC, Sharma-Kuinkel B, Ruffin F, Alexander BD, McCall CM, Costa SF, Arcasoy MO, Hong DK, Blauwkamp TA, Kertesz M, Fowler VG, Jr, Kraft BD. 2016. Diagnosis of Capnocytophaga canimorsus sepsis by whole-genome next-generation sequencing. Open Forum Infect Dis 3:ofw144. doi: 10.1093/ofid/ofw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Hyde FW, Johnson RC, White TJ, Shelburne CE. 1989. Detection of antigens in urine of mice and humans infected with Borrelia burgdorferi, etiologic agent of Lyme disease. J Clin Microbiol 27:58–61. doi: 10.1128/JCM.27.1.58-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Douglas TA, Tamburro D, Fredolini C, Espina BH, Lepene BS, Ilag L, Espina V, Petricoin EF, III, Liotta LA, Luchini A. 2011. The use of hydrogel microparticles to sequester and concentrate bacterial antigens in a urine test for Lyme disease. Biomaterials 32:1157–1166. doi: 10.1016/j.biomaterials.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Magni R, Espina BH, Shah K, Lepene B, Mayuga C, Douglas TA, Espina V, Rucker S, Dunlap R, Petricoin EF, Kilavos MF, Poretz DM, Irwin GR, Shor SM, Liotta LA, Luchini A. 2015. Application of Nanotrap technology for high sensitivity measurement of urinary outer surface protein A carboxyl terminus domain in early stage Lyme borreliosis. J Transl Med 13:346. doi: 10.1186/s12967-015-0701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Cheung CS, Anderson KW, Benitez KY, Soloski MJ, Aucott JN, Phinney KW, Turko IV. 2015. Quantification of Borrelia burgdorferi membrane proteins in human serum: a new concept for detection of bacterial infection. Anal Chem 87:11383–11388. doi: 10.1021/acs.analchem.5b02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Raffetin A, Saunier A, Bouiller K, Caraux-Paz P, Eldin C, Gallien S, Jouenne R, Belkacem A, Salomon J, Patey O, Talagrand-Reboul E, Jaulhac B, Grillon A. 2020. Unconventional diagnostic tests for Lyme borreliosis: a systematic review. Clin Microbiol Infect 26:51–59. doi: 10.1016/j.cmi.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 374.Angel TE, Luft BJ, Yang X, Nicora CD, Camp DG, II, Jacobs JM, Smith RD. 2010. Proteome analysis of Borrelia burgdorferi response to environmental change. PLoS One 5:e13800. doi: 10.1371/journal.pone.0013800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Sayahtaheri-Altaie S, Meier FA, Dalton HP. 1993. Identification of species-specific, non-cross-reactive proteins of Borrelia burgdorferi. Diagn Microbiol Infect Dis 16:43–51. doi: 10.1016/0732-8893(93)90129-U. [DOI] [PubMed] [Google Scholar]
- 376.Magnarelli LA, Miller JN, Anderson JF, Riviere GR. 1990. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol 28:1276–1279. doi: 10.1128/JCM.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wieneke CA, Lovrich SD, Callister SM, Jobe DA, Marks JA, Schell RF. 2000. Evaluation of whole-cell and OspC enzyme-linked immunosorbent assays for discrimination of early Lyme borreliosis from OspA vaccination. J Clin Microbiol 38:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Feder HM, Jr, Gerber MA, Luger SW, Ryan RW. 1991. False-positive serologic tests for Lyme disease after varicella infection. N Engl J Med 325:1886–1887. doi: 10.1056/nejm199112263252615. [DOI] [PubMed] [Google Scholar]
- 379.Tuuminen T, Hedman K, Soderlund-Venermo M, Seppala I. 2011. Acute parvovirus B19 infection causes nonspecificity frequently in Borrelia and less often in Salmonella and Campylobacter serology, posing a problem in diagnosis of infectious arthropathy. Clin Vaccine Immunol 18:167–172. doi: 10.1128/CVI.00367-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Kaell AT, Redecha PR, Elkon KB, Golightly MG, Schulman PE, Dattwyler RJ, Kaell DL, Inman RD, Christian CL, Volkman DJ. 1993. Occurrence of antibodies to Borrelia burgdorferi in patients with nonspirochetal subacute bacterial endocarditis. Ann Intern Med 119:1079–1083. doi: 10.7326/0003-4819-119-11-199312010-00004. [DOI] [PubMed] [Google Scholar]
- 381.Dinerman H, Steere AC. 1992. Lyme disease associated with fibromyalgia. Ann Intern Med 117:281–285. doi: 10.7326/0003-4819-117-4-281. [DOI] [PubMed] [Google Scholar]