Abstract
Importance:
Catamenial epilepsy (CE) is exacerbated by hormonal fluctuations during the menstrual cycle. Approximately 1.7 million women have epilepsy in the United States. CE affects more than 40% of women with epilepsy. There is a paucity of literature addressing this condition from a clinical standpoint, and the literature that does exist is limited to the neurological community. This article reviews the diagnosis and management of CE for the non-neurologist. Women with CE have early touch points in their care with numerous health care providers before ever consulting with a specialist, including OB/GYNs, pediatricians, emergency department physicians, and family medicine providers. In addition, women affected by CE have seizures that are more recalcitrant to traditional epilepsy treatment regimens. To optimize management in patients affected by CE, menstrual physiology must be understood, individualized hormonal contraception treatment considered, and adjustments and interactions with antiepileptic drugs addressed.
Observations:
CE is a unique subset of seizure disorders affected by menstrual fluctuations of progesterone and estrogen. The diagnosis of CE has been refined and clarified. There is an ever-increasing understanding of the importance and variety of options of hormonal contraception available to help manage CE. Furthermore, antiepileptic drugs and contraception can interact, so attention must be directed to optimizing both regimens to prevent uncontrolled seizures and pregnancy.
Conclusion and Relevance:
CE can be diagnosed with charting of menstrual cycles and seizure activity. Hormonal treatments that induce amenorrhea have been shown to reduce CE. Optimizing antiepileptic drug dosing and contraceptive methods also can minimize unplanned pregnancies in women affected by CE.
Keywords: Adolescent Gynecology, Birth Control, Catamenial Epilespy, Clincal, Seizures, Depomedroxyprogesterone acetate, Contraception, Epilepsy, Gynecology, LNG-IUD, Menstrual cycle, Menstrual suppression, Oral birth control pills, Premenstrual, Progesterone, Seizures, Women's Health
INTRODUCTION
Catamenial epilepsy (CE) is a prevalent and serious seizure pattern characterized by periodic fluctuations in seizure frequency corresponding to the menstrual cycle.1 Studies estimate that the prevalence of CE ranges from 10% to 70% in women with epilepsy (WWE).1-7 The wide range in prevalence depicts the early ambiguity in its classification. Herzog defined CE as the point of inflection on a graph of multiples of greater seizure frequency versus percentage of women with this increase in seizure frequency.1 This was used to define the 3 distinct subtypes of CE: perimenstrual (C1), periovulatory (C2), and inadequate luteal phase (C3). Today, a ≥twofold increase in seizure frequency during a specific period in the menstrual cycle is used as the clinical diagnosis.1 A large-scale National Institutes of Health (NIH) trial found 44.2% (130/294) of their randomized female patients with seizure have CE.8
The purpose of this review was to provide a comprehensive clinical understanding of CE and to outline treatment options for all clinicians who care for women affected by this disorder. We first give a brief description of the pathophysiology of this disorder, which is commonly associated with fluctuations in serum estrogen and progesterone levels. Second, we discuss common presentations and best practices for evaluation and diagnosis. Third, we summarize the most effective modern-day treatments for CE. We review the evidence supporting the role of hormonal management in CE. Last, contraception management in patients with CE is reviewed.
DISCUSSION
Pathophysiology
CE is strongly associated with the neuroendocrine system. These seizure fluctuations are modulated by the predominately proconvulsant properties of estrogen and anticonvulsant properties of progesterone and its metabolites.
Estrogen
Estrogens, specifically estradiol (E2), have been shown to have excitatory proconvulsant effects on the brain. In neurons, specifically excitatory CA1 pyramidal neurons in the hippocampus, E2 acts as a posttranscriptional modulator to positively regulate the density of spines and of excitatory N-methyl-D-aspartate receptors, leading to increased excitability.9-13 Other proposed actions of E2 are the genetic repression of inhibitory neurotransmitters such as gamma-aminobutyric acid (GABA),14 and short-acting membrane-mediated current induction.15,16 Multiple female rat studies have found activation of spike discharges and a decrease in the seizure threshold when rats are administered E2.10,13,17-21 In female subjects with epilepsy, intravenously administered conjugated estrogen (a mixture including estrone sulfate, equilin sulfate, delta 8,9-dehydroestrone sulfate, 17-alpha estradiol sulfate, and 17-alpha dihydroequilin sulfate) produced seizures in 11 of 16 subjects and clinical seizures in 4 subjects.22 The role of estrogen in seizure activity is complex, and studies have suggested that the effects are “dependent on dose, route of administration, acute versus chronic administration, natural hormonal environment, and estrogenic species.”16
Progesterone
Conversely, progesterone has been found to have anticonvulsant effects. Most of the anticonvulsant actions occur via the reduced metabolites of progesterone, most notably allopregnanolone (AP). Studies have found that AP has sedative-hypnotic and anticonvulsant properties.13,23,24 AP may exert these inhibitory effects by aiding the potentiation GABAergic neurons.13,23-26 AP levels rise and fall based on a woman’s serum progesterone levels.13,23 Several studies have demonstrated that progesterone acts via a genetic pathway in which it controls the synthesis of various neurotransmitters. In multiple mouse studies, increased progesterone levels had an overall inhibitory effect on the brain, leading to decreases in seizure occurrence.13,21,27-29 In human models, progesterone injections that produce luteal-phase levels of progesterone led to significant reduction in the frequency of characteristic seizure brain wave spikes in 4 of 7 women with partial epilepsy.30
Diagnosis
CE is the occurrence or worsening of seizure activity related in timing to a woman’s menstrual cycle. CE is commonly diagnosed in women after no other explanation of seizure patterns can be made. Awareness of the menstrual hormonal fluctuations and their impact on seizure activity can help clinicians provide an earlier and more precise diagnosis. Important questions to ask include the following: Are seizures continuing despite traditional antiepileptic drugs (AEDs)? Are the seizures occurring in a cyclic fashion? Has the patient charted her menstrual cycle and noted increase in seizure activity during particular times?
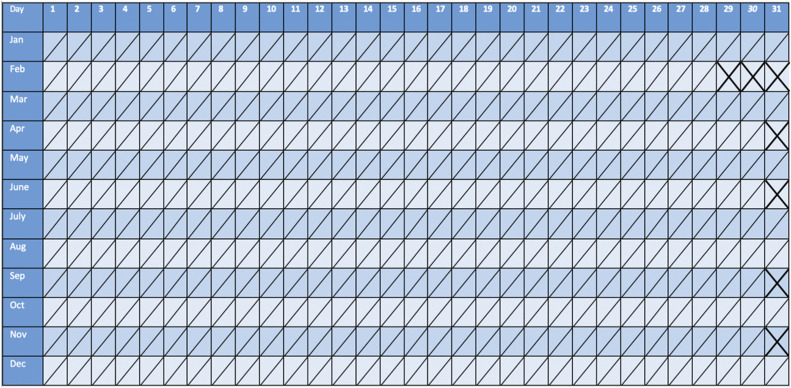
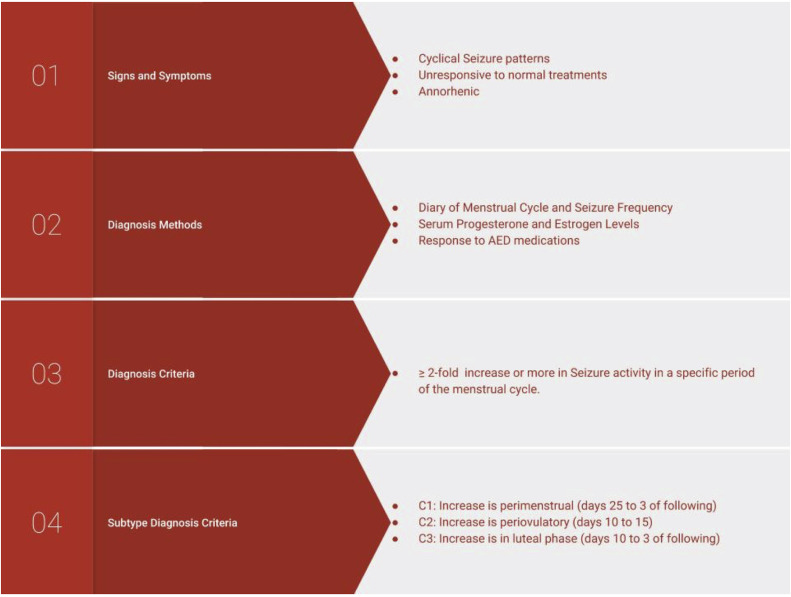
For clinicians, the first step in diagnosis of CE is to advise the patient to track menstrual cycles and seizures. This is commonly done by having the patient keep a seizure and menstrual diary (see Figure 1).31 In addition to tracking bleeding, physicians also may consider tracking basal body temperature. Temperatures should be taken orally each morning. A ≥0.7 °F change signifies the beginning of the postovulatory phase.31 Serum progesterone measurements also can be made, with >3 ng/mL marking the ovulatory phase.31 Physicians also can track serum progesterone levels. For patients with irregular or anovulatory cycles, hormone levels and the use of ovulation kits can better elucidate cyclic relationship of seizures to the menstrual cycle. The specific increase in seizure frequency to make a diagnosis of CE is specific to the subtype (C1: 1.69-fold; C2: 1.83-fold; C3: 1.62-fold). In practice, a diagnosis of CE can be made if the patient presents with ≥twofold increase in seizure frequency during one of the menstrual times noted as follows, or if the increase is simply repeated at similar times in the patient’s menstrual cycle. The 3 subtypes, in which the seizure frequency typically increases ≥twofold, are perimenstrual (C1), periovulatory (C2), or inadequate luteal phase (C3) (see Figure 2).1
Figure 1.
Diagnosis calendar. This calendar tracks the menstrual cycle and seizure cycle to help with diagnosis.
Figure 2.
Diagnosis for catamenial epilepsy. This 4-step diagnosis process begins with signs and symptoms of cyclic seizure patterns that should raise suspicions. When clinicians become aware of these, they should attain more information with the Diagnosis Methods. With this information, they can make a diagnosis and subtype diagnosis. AED = antiepileptic drug.
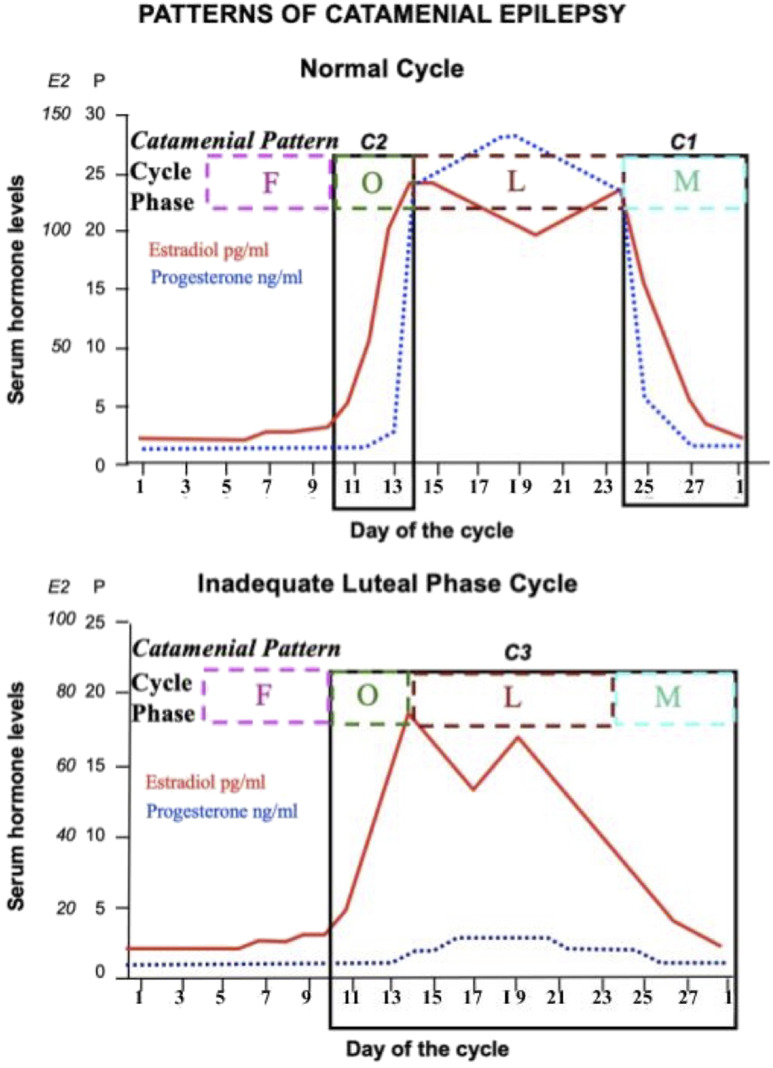
Classification is based on a 28-day cycle, with day 1 being the onset of menstrual flow. The follicular phase is during days 1 to 14, and the luteal phase is during days 15 to 28. The perimenstrual subtype (C1) is characterized by the rapid drop in progesterone during menstruation (days 25 to 3 of the following cycle). Although the proconvulsant estrogen does drop as well in this period, the progesterone experiences a more rapid decrease. This pattern has been the most responsive to treatment. The periovulatory subtype (C2) is characterized by the rapid surge of estrogen at day 10 to 15. The inadequate luteal phase subtype (C3) is characterized by an inadequate rise of progesterone during the luteal phase (days 10 to 3 of the following cycle).
This classification is applicable only to patients with anovulatory cycles. This is because of an inadequate development of the corpus luteum, which causes reduced levels of progesterone, but normal levels of estrogen (see Figure 3).1,16 A 1997 study found that 42.3% of WWE presented with at least one of these classifications (C1: 35.7%, C2: 28.5%, C3: 41.4%).1 Similarly, a 2015 NIH study found that of the 47.1% of WWE who presented with at least one of these classifications, 39.8% are C1, 33.9% are C2, and 47.1% are C3.32
Figure 3.
Hormone cycle and catamenial epilepsy (CE) classification. Estradiol and progesterone follow a consistent cyclical pattern during the menstrual cycle. Hormone levels and CE subtypes are shown for normal cycles and inadequate luteal-phase cycles. Because menstrual cycle intervals vary but, in the general population, most women ovulate 14 days before menstrual onset, this image transcribes the patient calendars to ones that designate the last 14 days as −14, −13, −12, and so on. The following stages are shown in this figure: follicular (F), ovulatory (O), luteal (L), and menstrual (M).62
Treatment
The role of the hormonal milieu in women with CE and the impact of hormones via contraceptives is extremely complex. Hormone treatment for CE should be considered after traditional methods have been tried. The most promising hormonal treatments for CE in studies are not hormonal contraception, but are actually natural progesterones (for example, a well-known hormone used in hormone replacement therapy: Prometrium) and amenorrhea-inducing drugs, such as gonadotropin-releasing hormone (GnRH) analogues and medroxyprogesterone acetate (MPA). However, studies are limited, as neither GnRH analogues nor MPA have undergone large-scale clinical trials for use in patients with CE.
Natural Progesterone
Natural progesterone can reduce the frequency of seizures. To date, there have been 5 important studies using natural progesterone as a treatment. Natural progesterone is sold in compound pharmacies in lozenge, suppository, lotion, and pill form (Prometrium). A large-scale, randomized, double-blind NIH study in 2015 concluded that natural progesterone in lozenge form given 3 times a day in 200-mg doses during day 14 through day 25 can decrease seizure frequency in C1-type CE. For a the subset of those with C1 type who normally had a ≥threefold increase in seizures during the perimenstrual period, the percentage of patients with a greater than 50% decrease in seizure rates increased from 21.3% to 57.1% when given natural progesterone lozenges versus 19.6% to 20.0% for those administered placebo.32,33 In a second study of 8 women with C3 class CE, 6 of 8 women receiving 50 to 400 mg of natural progesterone suppositories twice a day had an average 68% decreased seizure activity.34 A small earlier study on 25 patients with C1 and C3 pattern using cyclic natural progesterone treatment resulted in 72% of the subjects having reduced seizure activity, which persisted at a 3-year follow-up study.35,36
In addition, a randomized study of 38 women with C1 or C3 seizures found that the number of seizures after treatment was significantly decreased compared with placebo state (p = 0.024) when treated with 200-mg lozenges 3 times per day.37 Based on these findings, natural progesterone has been shown to be an effective treatment for those with CE with a subset of C1 patterns, and possibly for C3. The advisable treatment based on these studies is 50 to 200 mg natural progesterone in lozenge for suppository form 3 times per day. It is recommended that this treatment is tapered during the final 3 days of the cycle to prevent withdrawal seizures. For example, Herzog13,32,33,34,35,36 recommends one-half to 1 dose 3 times a day for days 14 to 25; then one-half dose 3 times a day on days 26 to 27, and one-quarter dose on day 28 before stopping. The limitation to these studies is the small number of patients and the equal efficacy of the easy-to-obtain Prometrium (natural progesterone in pill form vs. lozenge).
GnRH Analogues
GnRH analogues may be an effective treatment because they reduce hormone variations in patients with CE. These GnRH agonists work by blocking pituitary gonadotropin secretions such as follicle-stimulating hormone and luteinizing hormone by desensitizing the pituitary through constant stimulus.38 In 1 study of 10 patients with CE, patients were given 3.75 mg of intramuscular triptorelin (a synthetic GnRH analogue) every 4 weeks for an average of 11.8 months. Once all were amenorrhoeic, 3 were seizure free, 4 had nearly a 50% reduction in seizure frequency, 2 experienced no benefit, and none had an increase in seizure activity (p < 0.02).39 In 1 case study, a woman with frequent catamenial status epilepticus, who was unresponsive to combined oral contraceptive pills (COCs), received 3.6 mg of goserelin (a synthetic GnRH analogue) every 4 weeks. Her inpatient hospital admissions, due to status epilepticus, decreased from 10 admissions in a 4-week period to only 3 admissions in a 4-week period.40 One study found that during the first 3 weeks of GnRH analogue treatment, there can be an increase in seizure activity, as there is an initial stimulation of estrogen production before its production is inhibited. Experts recommend daily progesterone use for 2 to 3 weeks following the first injection to minimize this risk of transient flare of seizures that are mitigated by the progesterone.41 Side effects of GnRH analogues include hot flashes and vaginal dryness. Serious potential long-term use can increase risks of osteoporosis and cardiovascular disease.13 Common measures to prevent bone loss in patients using GnRH analogues include the addition of MPA.42 Regular exercise and calcium with vitamin D supplementation are also recommended in women using GnRH agonists. GnRH analogues are effective and important treatment considerations in these patients, as they suppress menses, diminish hormone fluctuations, and reduce seizure activity in women with CE.
MPA Pills and Injections
MPA, in doses to produce amenorrhea, has been another successful hormonal treatment for CE, to date. In 1 study, 11 patients were administered 10-mg MPA pills 2 to 4 times per day. Of these 11, 7 became amenorrheic. The 4 who continued to have menses, received an additional 120 to 150 mg of intramuscular Depo Medroxyprogesterone acetate (DMPA) every 6 to 12 weeks to induce amenorrhea. Once amenorrheic, a total of 7 of 11 patients experienced a clear improvement in seizure activity, with an average seizure activity reduction of 30% (p = 0.02).43 The Birth Control Registry study of Herzog and colleagues44,45 found that DMPA had the highest rate of decreased seizure frequencies compared with any other hormonal contraception (COC, vaginal ring, hormonal patch, progestin-only pill [POP], and progestin implant) (p = 0.0008). Of the 200 patients taking DMPA, 19% had increased seizure frequency, 17.5% had decreased seizure frequency, and 63.5% saw no change.44,45 Those surveyed in this study were not diagnosed with CE or a CE subtype specifically, only with epilepsy. This could suggest that those unaffected by treatment were unaffected because they are WWE, but not specifically women with cyclic exacerbations of seizures, or in other words, diagnosed with CE. Studies have demonstrated that inducing amenorrhea with MPA or DMPA may lead to reductions in seizures in WWE. It is possible that the women who benefit most from the reduction in hormone variations that comes with amenorrhea are those affected by CE, thus their seizures are most responsive to suppression of hormonal fluctuations. This is an area in which further research is needed to better optimize treatment for patients with CE.
Other Treatment Options for CE
Last, acetazolamide (also known as Diamox), commonly used to treat glaucoma, epilepsy, and edema, has long been used as a nonhormonal treatment for women with CE. A retrospective study of women admitted to the Cleveland Clinic Emergency Department who presented with seizures and were given acetazolamide found that 40% of the women reported decreased seizure frequency.46 Large-scale studies still are needed.
Hormonal Contraception Considerations
Oral Contraceptive Pills
COCs are birth control pills containing both progestin and ethinyl estradiol. Several studies of WWE using COCs have shown that seizure frequency is unaffected. The Birth Control Registry study, by Herzog and colleagues44,45 in 2016, surveyed 1144 WWE who were not specifically diagnosed with CE.
Of those surveyed, 635 were taking COCs. The survey did not separate cyclical and continuous COC use. Eighteen percent had increased seizure frequency when taking COCs, whereas 9% had decreased frequency. However, COC use was associated with the lowest overall increase of seizure frequency compared with all other hormonal contraceptive methods (COC, vaginal ring, hormonal patch, POP, and progestin implant).44,45 It is important to note that nearly 75% of all COC users had no change in seizure frequency. These studies show that COC use in patients with CE does not significantly increase risk of seizures and can possibly reduce such seizures; however, more research is needed to address COC patterns of use (specifically menstrual suppression) in women with CE.
Progestin-only Pills
Little research has been done on POPs in WWE and CE. Herzog and colleagues44,45 found patients taking POPs had a higher incidence of seizures. For those using POPs, the percentage of those with increased and decreased seizure frequencies were 29.3% and 8.6%, respectively. This result suggests a clear difference in the effect of natural progesterone and synthetic progestin taken orally. Natural progesterone increases AP levels in the brain (GABA agonist), but synthetic progestins lower AP levels. Synthetic progestins do not seem to suppress seizures unless administered in doses to suppress menses.44,45 Clearly, not all progesterones have similar effects.
Progesterone Intrauterine Device
Some studies found no change and others found a slight decrease in seizures for patients with a progesterone (levonorgestrel or LNG) intrauterine device (IUD). In addition to being one of the most effective contraceptive modalities available, the IUD is a top-tier choice for women with complex medical conditions, as it has no known AED interactions.47,48 The LNG IUD is an effective modality to suppress menses. Thirty percent to 40% and in some studies up to 70% of patients experience amenorrhea after 1 year of use.49 Of the 228 patients in the Birth Control Registry using an IUD, 6.1% reported an increase in seizure frequency, 13.2% reported a decrease, and 80.7% reported no change. Interestingly, there were no significant differences between the effect of LNG IUDs and the copper IUDs on seizure activity. The percentage of patients who saw increases and decreases in seizure frequency was 6.7%/14.7% for the LNG IUD and 5.1%/10.3% for copper T-IUD, respectively.44,45
Other Hormonal Contraception Options
More research is needed regarding other contraceptive options. Women who used the (etonogestrel) implant (Nexplanon) saw increased and decreased seizure frequencies of 24.3% and 5.4%, respectively.44,45 The contraceptive patch and ring have had few studies evaluating their impact on WWE and CE. Although the implant has fewer patients with amenorrhea over time (20%), the ring and patch can effectively suppress menses when used continuously. All 3 options warrant further investigation to determine if they, too, reduce seizure frequency in women with CE.
AED Considerations and Contraceptive Efficacy
It is important for clinicians to consider how AEDs and contraceptives will interact for 2 significant reasons. First, contraceptive interactions with AEDs can reduce the effectiveness of hormonal contraceptives (HCs), and lead to unplanned pregnancy. Second, interactions between HCs and AEDs can reduce the levels of the AED itself, thus potentially inadequately treating WWE and leading to more seizure activity. Studies have found that a proper balance of hormone levels (birth control) and AED levels is paramount in controlling CE and optimizing the effectiveness of birth control.
Enzyme-Inducing and Non–Enzyme-Inducing AEDs
For patients taking both AEDs and HCs, the levels of serum progesterone and estrogen can be reduced if hepatic enzyme-inducing AEDs (EIAED) are used. Non-EIAEDs do not cause a reduction in hormone levels of the HC and therefore do not pose a risk in decreased HC efficacy (see Table 1). EIAEDs increase the levels of P450 3A4 enzymes in the liver, which also metabolize estradiol and progestin. This metabolism has been shown to reduce serum estradiol and progestin levels by 50%.50-52 This significant drop in hormone levels in HCs can lead to birth control failures and probably unwanted side effects (unscheduled bleeding and spotting) for women taking EIAEDs.50 One double-blind study found a 42% decrease in serum progestin levels, and no decrease in estradiol in patients given felbamate and a COC with 30 µg ethinyl estradiol.50,53 Similarly, topiramate use was associated with a reduced concentration of estradiol in 2 studies of women taking COCs with 35 µg of ethinyl estradiol. Both found no effect on estradiol below 200 mg of topiramate use daily, and no decrease in progestin levels.50,54,55 Both felbamate and topiramate are considered moderately enzymatic inducing, whereas others reduce levels more drastically. Clobazam has been found to be an effective treatment for CE. It, however, has enzyme-inducing activity much like an EIAED. One study found that of the 18 patients given 20 to 30 mg of clobazam, none had increased seizure frequency and 50% reported a reduction.56 Clobazam has gained a growing acceptance as an intermittent treatment for CE, but given its enzyme-inducing activity, caution is indicated for patients using COCs, as it may decrease contraceptive efficacy. EIAEDs can also reduce levels of folic acid. Health care providers recommend that all women considering having children take folic acid supplements to reduce the incidence of birth defects. This is particularly important in WWE who may have particularly lower folic acid levels because of their medication use. Folic acid is often recommended to be taken at prescription dosage (0.8 mg or 1 mg).57
Table 1.
Antiepileptic drug and hormonal contraception interactions
| When AED is taken with hormonal contraception | ||
|---|---|---|
| Effect | Type | Name |
| No change in contraceptive efficacy | NEIAED | Gabapentin |
| Levetiracetam | ||
| Tiagabine | ||
| Vigabatrin | ||
| Zonisamide | ||
| Pregabalin | ||
| Lacosamide | ||
| Ethosuximide | ||
| Possible decreased contraceptive efficacy | EIAED | Carbamazepine |
| Felbamate | ||
| Oxcarbazepine | ||
| Phenobarbital | ||
| Phenytoin | ||
| Primidone | ||
| Weak EIAED | Topiramate | |
| Clobazam | ||
| Decreased AED levels, no change in contraceptive efficacy | Glucuronidated AED | Lamotrigine |
| Decreased AED levels and contraceptive efficacy | Enzyme-inhibiting AED | Valproate |
Non–enzyme-inducing AEDs (NEIAEDs) do not interact with hormonal contraceptives. Enzyme-inducing AEDs (EIAEDs) have been found to reduce levels of systemic hormonal contraception, leading to potentially reduced contraceptive efficacy. Lamotrigine and valproate levels also can be reduced in patients taking hormonal contraceptives.
AED = antiepileptic drug.
WWE, Contraception, and Pregnancy Planning
As shown previously, WWE can have their seizures exacerbated by hormonal variations. It is important to have proper consideration of HCs for all WWE to prevent catamenial exacerbation, as well as providing reliable contraception for those patients who do not desire a pregnancy. All birth control options can be considered safe and as options for patients with WWE. It is important for the provider to review their individual risks and benefits of the methods and help in shared decision making. All WWE who are not planning a pregnancy should be offered and counseled about the full range of contraceptive options available. Studies have shown that seizure activity is not increased by the use of hormonal contraception, and depending on the method chosen, HC may benefit CE by reducing seizure activity. At least preliminarily, this seems to be the case particularly in WWE who use DMPA and those with CE who experience amenorrhea on their birth control method. Contraception counseling for reproductive-age WWE should occur at every medical touch point. WWE have particular pregnancy risks: many medications used for epilepsy are teratogenic, extra folic acid supplementation is recommended preconceptually, and optimizing health and medication regimen, before planning a pregnancy, is paramount to a healthy pregnancy and infant. The full range of contraceptive methods for women currently include implantable rods (Nexplanon); IUDs, progesterone containing (all durations and doses) and Copper T; the shot or injection (Depo Provera), the contraceptive patch; vaginal contraceptive ring; combined oral contraceptive pills (extended or continuous use); progestin-only contraceptive pills; female and male sterilization; female and male condoms; diaphragms; sponges; cervical caps; and spermicide. In addition, instruction infertility awareness-based methods, including the lactation amenorrhea method, although less effective, should be provided for women desiring alternative contraceptive methods. WWE who use non-EIAEDs have not been shown to experience an increase in unplanned pregnancy. However, for those WWE who take EIAEDs, special consideration to choosing a birth control method and dose is critical to contraceptive efficacy. WWE who prefer to use a COC as their contraceptive of choice, and have no medical contraindications, should be prescribed a birth control pill that contains at least 50 µg of ethinyl estradiol. That is higher than the low-dose pills (20-35 μg routinely prescribed). If they have contraindications to COCs or experience untoward side effects, they should be counseled about other HC options and collaborate with their provider in shared decision making for another contraceptive option. An underutilized and superb contraceptive method for WWE is the hormonal and nonhormonal IUD. In women with CE, menstrual suppression as a result of their contraceptive method may have dual benefits of preventing an unplanned pregnancy and reducing seizure activity. Consideration to DMPA and nonoral contraceptive methods may minimize disruption of AED dose and optimize contraceptive efficacy. Further resources and patient-friendly graphics can be found in the US Medical for Contraceptive Use of the Centers for Disease Control and Prevention.58
HCs and Lamotrigine and Valproic Acid: Special Circumstances
In the presence of HCs, levels of the AED lamotrigine have repeatedly been found to be reduced (see Table 1). This is significant because patients taking both lamotrigine and HCs have increased seizure activity requiring adjustments to dosing. In a study of 22 women taking both lamotrigine and a COC, lamotrigine levels were decreased by 50% compared with those taking lamotrigine only.59 In the Birth Control Registry study, 18.2% of those taking HCs and glucuronidated AEDs, such as lamotrigine, reported increased seizure frequency as a result of the decreased AED levels compared with those who used AEDs alone. Estradiol, not progestins, reduce lamotrigine levels.60 Much like lamotrigine, one study found valproate levels are decreased by 23.4% when used with HCs.61 Interestingly, lamotrigine is the most commonly used AED among WWE.44 In the Birth Control Registry study, valproate had the highest increased seizure frequencies in patients taking HCs.45 Women taking both COCs and lamotrigine or valproate require close AED-level monitoring after starting their COC regimen. Adjustments should be made and monitored periodically to ensure effective levels. In addition, doses should be adjusted during the COC placebo period if continuous use is not prescribed.50
CONCLUSIONS
CE is a unique condition that affects a subset of WWE. Clinicians should be aware of this subset of epilepsy and understand the patients’ seizure activity in the context of their menstrual cycle. Using menstrual calendars, the clinician can identify patients with CE, and ideally classify the pattern. Once a diagnosis is made, clinicians can optimize treatments for patients with CE. In general, traditional AEDs with fluctuating doses throughout the menstrual cycle are a common antiseizure regimen. Unlike traditional epilepsy, for which AED dosage is steady and consistent, AED dosage for patients with CE are increased and decreased during their menstrual cycle. The classification as C1, C2, or C3 allows the provider to individualize and optimize their medication. Initial trials have shown promise for hormonal treatments for CE. To date, the most consistently effective hormonal treatments are natural progesterone, MPA (pills and injection), and GnRH analogues. The studies do have several limitations. First, use of natural progesterone alone for premenopausal women is less common in practice and is not an option for effective pregnancy prevention. The studies of natural progesterone, to date, were small and did not use common or available-to-prescribe delivery systems (ie, lozenges, not oral or vaginal Prometrium). It is likely that oral and vaginal preparations are analogous to the regimens used in the study protocols, but randomized controlled trials are needed. WWE of reproductive age also should be counseled about contraception at their medical visits. Contraceptive counseling and joint decision making should be made with special consideration to their medications, pregnancy plans, and special consideration to the role of menstrual suppression as a result of their contraceptive method. Their contraceptive method of choice may have additional health benefits of pregnancy prevention and mitigating seizure activity. Further research in this area is needed. The ideal study would mirror the Birth Control Registry by recruiting patients with CE delineating their specific birth control methods and controlling for their AED (drug and dose). In addition, it would be helpful to tier the results based on contraceptive method and regimen, such as cyclic, extended, or continuous use. CE is a fascinating subset of epilepsy, and future research in this arena can offer promising treatments, a better understanding of HC and AED interactions, and further reduce unplanned and often high-risk pregnancies for patients with this complex condition.
Acknowledgments
A.G. Herzog, MD, of the Harvard Neuroendocrine unit at the Beth Israel Deaconess Medical Center reviewed the article and offered comments prior to manuscript. The diagnosis, treatment, and contraceptive options for patients with catamenial epilepsy should be well understood for optimal patient outcomes.
Footnotes
Disclosure Statement: The author(s) have no conflicts of interest to disclose.
Authors’ Contributions: Mr. Frank performed a detailed literature review and wrote the manuscript. Dr. Tyson performed a brief initial literature review, provided expert input, and edited the manuscript.
References
- 1.Herzog AG, Klein P, Rand BJ. Three patterns of catamenial epilepsy. Epilepsia Oct, 1997;38:1082-–8.. DOI: 10.1111/j.1528-1157.1997.tb01197.x [DOI] [PubMed] [Google Scholar]
- 2.Tauball E, Lundervold A, Gjerstad L. Temporal distribution of seizures in epilepsy. Epilepsy Research 1991 Mar;8:153-–65.. DOI: 10.1016/0920-1211(91)90084-s [DOI] [PubMed] [Google Scholar]
- 3.Laidlaw J. Catamenial epilepsy. Lancet 1956 Dec;268:1235-7. DOI: https://doi.org/10.1016/s0140- 6736(56)90003-4 [DOI] [PubMed] [Google Scholar]
- 4.Rosciszewska D, Buntner B, Guz I, Zawisza L. Ovarian hormones, anticonvulsant drugs, and seizures during the menstrual cycle in women with epilepsy. J Neurol Neurosurg Psychiatry 1986 Jan;49:47-51. DOI: 10.1136/jnnp.49.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansell B, Clarke E. Epilepsy and menstruation; the role of water retention. Lancet 1956 Dec;271:1232-5. DOI: 10.1016/s0140-6736(56)90002-2 [DOI] [PubMed] [Google Scholar]
- 6.Duncan S, Read CL, Brodie MJ. How common is catamenial epilepsy? Epilepsia 1993 Sep-Oct;34:827-31. DOI: 10.1111/j.1528-1157.1993.tb02097.x [DOI] [PubMed] [Google Scholar]
- 7.Dickerson WW. The effect of menstruation on seizure incidence. J Nerv Ment Dis 1941 Aug;94:160-9. DOI: 10.1097/00005053-194108000-00003 [DOI] [Google Scholar]
- 8.Herzog AG, Fowler KM, Smithson SD, et al. Progesterone vs placebo therapy for women with epilepsy: a randomized clinical trial. Neurology 2012 Jun;78:1959-66. DOI: 10.1212/wnl.0b013e318259e1f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SS. Estrogen administration increases neuronal responses to excitatory amino acids as a long-term effect. Brain Res 1989 Dec;503:354-7. DOI: 10.1016/0006-8993(89)91691-0 [DOI] [PubMed] [Google Scholar]
- 10.Wong M, Moss R. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci Aug, 1992;12:3217-25. DOI: 10.1523/jneurosci.12-08-03217.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woolley CS, Mcewen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 1993 Oct;336:293-306. DOI: 10.1002/cne.903360210 [DOI] [PubMed] [Google Scholar]
- 12.Woolley C, Mcewen B. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci 1994 Dec;14:7680-7. DOI: 10.1523/jneurosci.14-12-07680.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzog AG. Catamenial epilepsy: Definition, prevalence pathophysiology and treatment. Seizure 2008 Mar;17:151-9. DOI: 10.1016/j.seizure.2007.11.014 [DOI] [PubMed] [Google Scholar]
- 14.Wallis CJ, Luttge WG. Influence of estrogen and progesterone on glutamic acid decarboxylase activity in discrete regions of rat brain. J Neurochem Mar, 1980;34:609-13. DOI: 10.1111/j.1471-4159.1980.tb11187.x [DOI] [PubMed] [Google Scholar]
- 15.Gu Q, Moss RL. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620-9. DOI: 10.1523/JNEUROSCI.16-11-03620.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harden CL, Pennell PB. Neuroendocrine considerations in the treatment of men and women with epilepsy. Lancet Neurol 2013 Jan;12:72-83. DOI: 10.1016/s1474-4422(12)70239-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami M, Terasawa E, Ibuki T. Changes in multiple unit activity of the brain during the estrous cycle. Neuroendocrinology 1970;6:30-48. DOI: 10.1159/000121900. [DOI] [PubMed] [Google Scholar]
- 18.Logothetis J, Harner R. Electrocortical activation by estrogens. Arch Neurol 1960 Sep;3:290-7. DOI: 10.1001/archneur.1960.00450030068007 [DOI] [PubMed] [Google Scholar]
- 19.Marcus EM. Effects of steroids on cerebral electrical activity. Arch Neurol 1966 Nov;15:521-32. DOI: 10.1001/archneur.1966.00470170075008 [DOI] [PubMed] [Google Scholar]
- 20.Hom AC, Buterbaugh GG. Estrogen alters the acquisition of seizures kindled by repeated amygdala stimulation or pentylenetetrazol administration in ovariectomized female rats. Epilepsia 1986 Mar-Apr;27:103-8. DOI: 10.1111/j.1528-1157.1986.tb03510.x [DOI] [PubMed] [Google Scholar]
- 21.Nicoletti F, Speciale C, Sortino MA, et al. Comparative effects of estradiol benzoate, the antiestrogen clomiphene citrate, and the progestin medroxyprogesterone acetate on kainic acid-induced seizures in male and female rats. Epilepsia 1985;26:252-7. DOI: 10.1111/j.1528-1157.1985.tb05414.x [DOI] [PubMed] [Google Scholar]
- 22.Logothetis J, Harner R, Morrell F, Torres F. The role of estrogens in catamenial exacerbation of epilepsy. Neurology 1959 May;9:352-60. DOI: 10.1212/wnl.9.5.352 [DOI] [PubMed] [Google Scholar]
- 23.Paul SM, Purdy RH. Neuroactive steroids. FASEB J 1992;6:2311-22. DOI: 10.1096/fasebj.6.6.1347506 [DOI] [PubMed] [Google Scholar]
- 24.Gee KW, McCauley LD, Lan NC. A Putative receptor for neurosteroids on the GABa receptor complex: The pharmacological properties of therapeutic potential of epalons. Crit Rev Neurobiol 1995;9:207-27. [PubMed] [Google Scholar]
- 25.Majewska M, Harrison N, Schwartz R, Barker J, Paul S. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986 May;232:1004-7. DOI: 10.1126/science.2422758 [DOI] [PubMed] [Google Scholar]
- 26.Navis A, Harden C. A treatment approach to catamenial epilepsy. Curr Treat Options Neurol 2016 Jul;18:30. DOI: 10.1007/s11940-016-0413-6 [DOI] [PubMed] [Google Scholar]
- 27.Spiegel E, Wycis H. Anticonvulsant effects of steroids. J Lab Clin Med 1945 Nov;30:947-53. [Google Scholar]
- 28.Woolley DE, Timiras PS. The gonad-brain relationship: effects of female sex hormones on electroshock convulsions in the rat. Endocrinology 1962 Feb;70:196-209. DOI: 10.1210/endo-70-2-196 [DOI] [PubMed] [Google Scholar]
- 29.Frye C. The neurosteroid 3α,5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res 1995 Oct;696:113-20. DOI: https://doi.org/10.1016/0006- 8993(95)00793-p [DOI] [PubMed] [Google Scholar]
- 30.Bäckström T, Zetterlund B, Blom S, Romano M. Effects of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol Scand 1984 Apr;69:240-8. DOI: 10.1111/j.1600-0404.1984.tb07807.x [DOI] [PubMed] [Google Scholar]
- 31.Foldvary-Schaefer N, Falcone T. Catamenial epilepsy: pathophysiology, diagnosis, and management. Neurology 2003 Sep;61:S2-15. DOI: 10.1212/wnl.61.6_suppl_2.s2 [DOI] [PubMed] [Google Scholar]
- 32.Herzog AG. Catamenial epilepsy: update on prevalence, pathophysiology and treatment from the findings of the NIH Progesterone Treatment Trial. Seizure 2015 May;28:18-25. DOI: 10.1016/j.seizure.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 33.Herzog AG, Fowler KM, Smithson SD, et al. Progesterone vs placebo therapy for women with epilepsy: A randomized clinical trial. Neurology 2012 Jun;78:1959-66. DOI: 10.1212/wnl.0b013e318259e1f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herzog AG. Intermittent progesterone therapy and frequency of complex partial seizures in women with menstrual disorders. Neurology 1986 Dec;36:1607-10. DOI: 10.1212/wnl.36.12.1607 [DOI] [PubMed] [Google Scholar]
- 35.Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology 1995 Sep;45:1660-2. DOI: 10.1212/wnl.45.9.1660 [DOI] [PubMed] [Google Scholar]
- 36.Herzog AG. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology 1999 Jun;52:1917-8. DOI: 10.1212/wnl.52.9.1917-a [DOI] [PubMed] [Google Scholar]
- 37.Najafi M, Mehvari J, Zare M, Akbari M, Sadeghi M. Progesterone therapy in women with intractable catamenial epilepsy. Adv Biomed Res 2013 Mar;2:8. DOI: 10.4103/2277-9175.107974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar P, Sharma A. Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. J Hum Reprod Sci 2014 Jul; 7:170-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer J, Wildt L, Flügel D, Stefan H. The effect of a synthetic GnRH analogue on catamenial epilepsy: a study in ten patients. J Neurol 1992 May;239:284-6. DOI: 10.1007/BF00810354 [DOI] [PubMed] [Google Scholar]
- 40.Haider Y, Barnett D. Catamenial epilepsy and goserelin. Lancet 1991 Dec;338:1530. DOI: 10.1016/0140-6736(91)92354-5 [DOI] [PubMed] [Google Scholar]
- 41.Herzog AG. Reproductive endocrine considerations and hormonal therapy for women with epilepsy. Epilepsia 1991 Dec;32:S27-33. DOI: 10.1111/j.1528-1157.1991.tb05889.x [DOI] [PubMed] [Google Scholar]
- 42.Reid B, Gangar KF. Catamenial epilepsy and goserelin. Lancet 1992 Jan;339:253. DOI: 10.1016/0140-6736(92)90066-C [DOI] [PubMed] [Google Scholar]
- 43.Mattson RH, Cramer JA, Caldwell BV, Siconolfi BC. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology 1984 Sep;34:1255-8. DOI: 10.1212/wnl.34.9.1255 [DOI] [PubMed] [Google Scholar]
- 44.Herzog AG, Mandle HB, Cahill KE, Fowler KM, Hauser WA, Davis AR. Contraceptive practices of women with epilepsy: findings of the epilepsy birth control registry. Epilepsia 2016 Jul;57:630-7. DOI: 10.1111/epi.13320 [DOI] [PubMed] [Google Scholar]
- 45.Herzog AG, Mandle HB, Cahill KE, Fowler KM, Hauser WA. Differential impact of contraceptive methods on seizures varies by antiepileptic drug category: findings of the epilepsy birth control registry. Epilepsy Behav 2016 Jul;60:112-7. DOI: 10.1016/j.yebeh.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 46.Lim L-L, Foldvary N, Mascha E, Lee J. Acetazolamide in women with catamenial epilepsy. Epilepsia 2001 Jun;42:746-9. DOI: 10.1046/j.1528-1157.2001.33600.x [DOI] [PubMed] [Google Scholar]
- 47.Pennell PB. Hormonal aspects of epilepsy. Neurol Clin 2009 Nov;27:941-65. DOI: 10.1016/j.ncl.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy DS. Do oral contraceptives increase epileptic seizures? Expert Rev Neurother 2017 Feb;17:129-34. DOI: 10.1080/14737175.2016.1243472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altshuler AL, Hillard PJA. Menstrual suppression for adolescents. Curr Opin Obstet Gynecol 2014 Oct;26:323-31. DOI: 10.1097/gco.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 50.Harden CL, Leppik I. Optimizing therapy of seizures in women who use oral contraceptives. Neurology 2006 Dec;67:S56-8. DOI: 10.1212/wnl.67.12_suppl_4.s56 [DOI] [PubMed] [Google Scholar]
- 51.Back D, Bates M, Bowden A, et al. The interaction of phenobarbital and other anticonvulsants with oral contraceptive steroid therapy. Contraception 1980 Nov;22:495-503. DOI: 10.1016/0010-7824(80)90102-x [DOI] [PubMed] [Google Scholar]
- 52.Orme M, Back D, Chadwick D, Crawford P, Martin C, Tjia J. The interaction of phenytoin and carbamazepine with oral contraceptive steroids. Eur J Pharmacol 1990 Jul;183:1029-30. DOI: 10.1016/0014-2999(90)92884-l [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saano V, Glue P, Banfield CR, et al. Effects of felbamate on the pharmacokinetics of a low- dose combination oral contraceptive. Clin Pharmacol Ther 1995 Nov;58:523-31. DOI: 10.1016/0009-9236(95)90172-8 [DOI] [PubMed] [Google Scholar]
- 54.Rosenfeld WE, Doose DR, Walker SA, Nayak RK. Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia 1997 Mar;38:317-23. DOI: https://doi.org/10.1111/j.1528- 1157.1997.tb01123.x [DOI] [PubMed] [Google Scholar]
- 55.Doose DR, Wang S-S, Padmanabhan M, Schwabe S, Jacobs D, Bialer M. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia 2003 Apr;44:540-9. DOI: 10.1046/j.1528-1157.2003.55602.x [DOI] [PubMed] [Google Scholar]
- 56.Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy: a model for evaluating anticonvulsants. Lancet 1982 Jul;2:71-3 [DOI] [PubMed] [Google Scholar]
- 57.Morrell M. Folic acid and epilepsy. Epilepsy Curr 2002 Mar;2(2):31-4. DOI: 10.1046/j.1535-7597.2002.00017.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curtis K, Tepper N, Jatlaoui T, et al. US medical eligibility criteria (US MEC) for contraceptive use. Recommendations and Reports 2016; 65(3):1-104 [DOI] [PubMed] [Google Scholar]
- 59.Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology 2003 Aug;61:570-1. DOI: 10.1212/01.wnl.0000076485.09353.7a [DOI] [PubMed] [Google Scholar]
- 60.Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia 2005 Sep;46:1414-7. DOI: 10.1111/j.1528-601 [DOI] [PubMed] [Google Scholar]
- 61.Herzog AG, Blum AS, Farina EL, et al. Valproate and lamotrigine level variation with menstrual cycle phase and oral contraceptive use. Neurology 2009 Mar;72:911-4. DOI: 10.1212/01.wnl.0000344167.78102.f0 [DOI] [PubMed] [Google Scholar]
- 62.Herzog AG, Harden CL, Liporace J, et al. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol 2004 Sep;56:431-4. DOI: 10.1002/ana.20214 [DOI] [PubMed] [Google Scholar]