Short abstract
Watch a video presentation of this article
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- CGHE
Center for Global Health Engagement
- COVID‐19
coronavirus disease 2019
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
The achievement of goals for elimination of hepatitis B virus (HBV) and hepatitis C virus (HCV) requires broad access to HBV and HCV testing and treatment services. 1 In response to the coronavirus disease 2019 (COVID‐19) pandemic, American Association for the Study of Liver Diseases (AASLD) and other liver associations recommended changes in hepatitis clinical care to mitigate transmission risks for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causative agent of COVID‐19. 2 In 2020, the Coalition for Global Hepatitis Elimination (CGHE) surveyed clinicians and program managers to assess the mitigation strategies deployed to reduce transmission and the changes in access to hepatitis testing and treatment services.
Methods
A 53‐question online survey was developed in SmartSheet by CGHE and partners,* translated into French and Spanish, and distributed via websites and e‐mail. Responses were received between August 12, 2020, and December 16, 2020. Respondents were not required to answer all questions.
The survey addressed four areas: (1) HBV and HCV services delivered before the emergence of COVID‐19, during the month most heavily impacted by the pandemic and the month of survey completion; (2) the mitigation strategies deployed to safely deliver hepatitis testing and treatment services; (3) respondents’ participation in the COVID‐19 response; and (4) perceived benefits to hepatitis systems from the response to COVID‐19. Program managers were asked about supply chain disruptions and training activities. These questions were added during the second iteration of the survey and were not administered to the first 32 respondents.
Results
The survey respondents included 103 clinicians and program managers from 44 countries.‡ Respondents were from six World Health Organization (WHO) regions, including the Americas (55 [53%]), the African Region (28 [27%]), and the European Region (15 [15%]). Most respondents, 86%, were physicians or nurses providing care in academic health centers (31%) or public hospitals (41%).
Changes in In‐Person Patient Care
All respondents reported February, March, April, or May as the month of highest COVID‐19 impact. In heavily impacted months, 60 of 92 (65%) respondents reported deferring >50% of in‐person clinic visits (Fig. 1). Most respondents (64%) reported improvements in delivery of in‐person hepatitis care in the current month. However, 80% reported the volume of in‐person care remained lower than pre‐COVID levels. The most common challenge hindering a return to pre‐COVID‐19 service levels was patients’ anxiety and fear (71%, 39/55) (Fig. 2).
FIG 1.
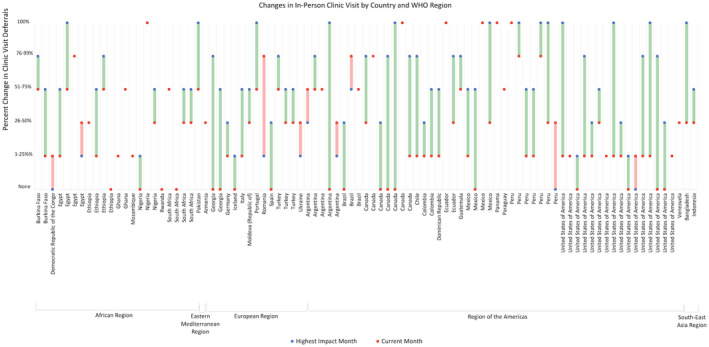
Percent of in‐person clinic visits deferred in the month of highest COVID‐19 impact and the current month. Length of the connecting line indicates the magnitude and direction of change between the two time points. Green indicates fewer clinic deferrals in the current month, and red indicates increases in deferral rates. Only respondents who reported deferral rates for the current month and highest impact month are included (N = 91).
FIG 2.
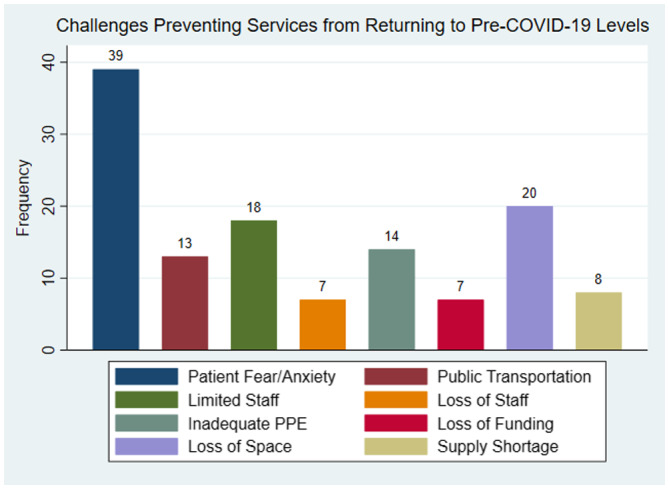
Most commonly reported challenges preventing facilities to return to normal pre‐COVID‐19 service levels among the 55 respondents identifying challenges.
To mitigate SARS‐CoV‐2 transmission risks, almost all respondents reported wearing masks (89%, 85/96), and 34% reported use of other personal protective equipment, that is, gowns and gloves. In addition, 51% reported monitoring patients for COVID‐19 symptoms/signs, including fever, and 59% required patients to wear masks (Table 1, mitigation strategies for distance‐based care). Providers used direct (82%) and public communications channels (18%) to inform patients of changes in clinical care (Table 1, communication strategies.
TABLE 1.
Impact of COVID‐19 on Hepatitis Service Delivery and Mitigation Strategies
| WHO Region | Total | ||||||
|---|---|---|---|---|---|---|---|
| Americas | African | European | South East Asian | Eastern Mediterranean | Western Pacific | ||
| Mitigation strategies for distance‐based care | |||||||
| Laboratory testing deferred | 71% (37) | 41% (9) | 62% (8) | 100% (2) | 0% (0) | 0% (0) | 63% (56) |
| Imaging deferred | 87% (46) | 50% (10) | 79% (11) | 100% (1) | 0% (0) | 0% (0) | 77% (68) |
| Increased pill count per prescription | 57% (31) | 95% (19) | 38% (5) | 100% (2) | 0% (0) | 0% (0) | 63% (57) |
| Use of telemedicine >75% of visits, high‐impact month | 40% (22) | 4% (1) | 36% (5) | 0% (0) | 0% (0) | 0% (0) | 29% (28) |
| Use of telemedicine >75% of visits, month of survey completion | 18% (9) | 0% (0) | 0% (0) | 0% (0) | 0% (0) | 0% (0) | 10% (9) |
| Infection‐control strategies for clinic‐based care | |||||||
| Staff masks | 86% (46) | 88% (21) | 93% (14) | 100% (2) | 100% (1) | 100% (1) | 89% (85) |
| Patient masks | 58% (31) | 58% (14) | 60% (9) | 50% (1) | 100% (1) | 100% (1) | 59% (57) |
| Gloves/Gown use | 38% (20) | 33% (8) | 20% (3) | 50% (1) | 100% (1) | 100% (1) | 35% (34) |
| Rigorous cleaning | 57% (30) | 54% (13) | 53% (8) | 50% (1) | 0% (0) | 0% (0) | 54% (52) |
| Spacing patient visits | 57% (30) | 38% (9) | 40% (6) | 50% (1) | 100% (1) | 0% (0) | 49% (47) |
| Patients checked for COVID symptoms | 58% (31) | 50% (12) | 33% (5) | 50% (1) | 0% (0) | 0% (0) | 51% (49) |
| Communication strategies | |||||||
| Individual outreach via phone, text, home visit | 88% (30) | 71% (10) | 77% (10) | 100% (2) | 100% (1) | 0% (0) | 82% (53) |
| Facility announcements | 21% (7) | 36% (5) | 23% (3) | 50% (1) | 0% (0) | 100% (1) | 26% (17) |
| Community outreach | 15% (5) | 21% (3) | 23% (3) | 50% (1) | 0% (0) | 0% (0) | 18% (12) |
Changes in Number of Patients Receiving HBV and HCV Testing and Treatment Services
Of respondents treating patients for HCV or HBV infection, 88% (59/67) and 80% (49/61) reported some level of disruption, and 39% (26/67) and 21% (13/61) reported a >50% decline in treatment volumes, respectively. Similarly, 28% (23/82) and 27% (19/70) of respondents reported >50% declines in HCV and HBV screening, respectively (Fig. 3). About 79% (19/24) of respondents implementing opioid substitution therapy reported disruptions, including 21% (5/24) with >50% decline in patients receiving opioid substitution therapy. Program managers also reported supply chain disruptions (Table 2).
FIG 3.
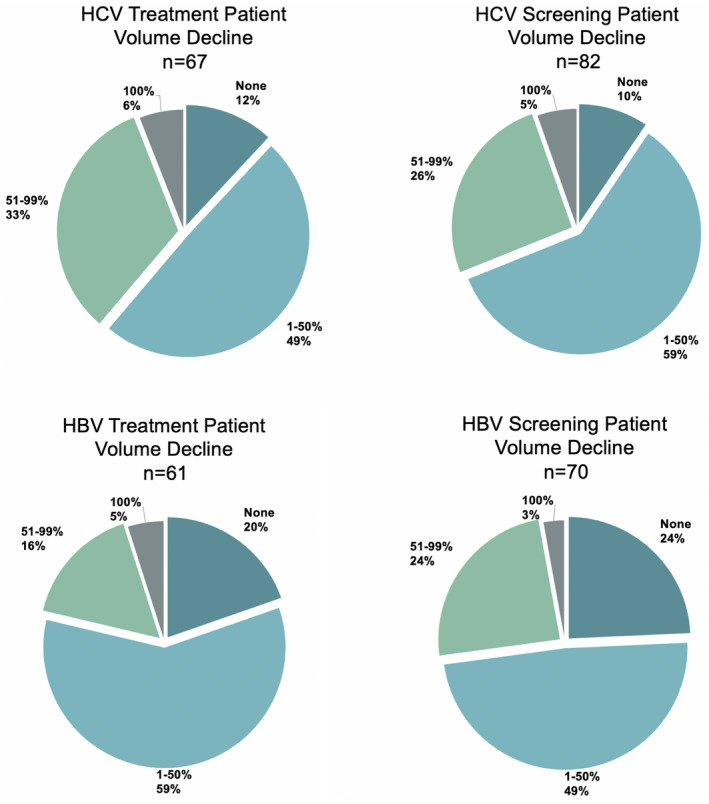
Decreases in patient volumes for select services comparing the month of highest COVID‐19 impact with a typical pre‐COVID‐19 month. n, respondents who reported baseline HCV/HBV treatment and screening volumes.
TABLE 2.
Supply Chain Disruptions Reported by Program Managers (N = 33)
| Supply Product | % (n) |
|---|---|
| HCV diagnostics | 27% (9) |
| HCV treatment | 27% (9) |
| HBV diagnostics | 21% (7) |
| HBV treatment | 15% (5) |
Mitigation Strategies
A variety of mitigation strategies were used to meet patients’ needs and limit the risk for SARS‐CoV‐2 transmission. During the month when care was most affected by the COVID‐19 pandemic, the majority of respondents (55/90) increased pill counts per prescription, 77% deferred imaging, 63% deferred laboratory work, and 30% (2/94) of respondents reported delivery of >75% of patient care via telemedicine (audio and/or video) (Table 1, mitigation strategies for distance‐based care); by the month of survey completion, only 10% (9/87) reported the use of telemedicine to this extent (Table 1, mitigation strategies for distance‐based care).
Provider Participation in the COVID‐19 Response
Most respondents (88%, 77/87) reported changes in their work activities as a result of COVID‐19. Activities included 31% (25/80) reporting greater than 25% of their time reallocated to COVID‐19 testing or care.
Potential Benefits of the COVID‐19 Response to Hepatitis Care
Respondents identified several aspects of the COVID‐19 response potentially improving hepatitis care. Increases in the capacity of laboratory testing (47%, 43/91) and improved health worker training (48%, 44/91) were the most commonly identified potential benefits (Fig. 4).
FIG 4.
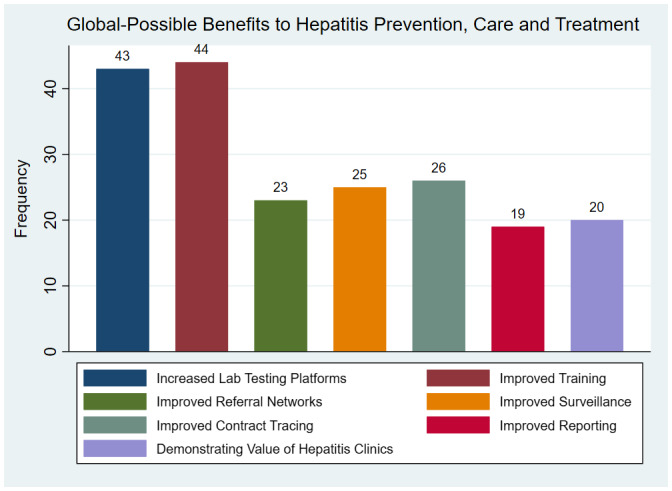
Most commonly reported benefits to hepatitis prevention, care, and treatment as a result of infrastructure and practice changes brought on by the COVID‐19 response among the 91 respondents identifying benefits.
Regional Differences
Risk mitigation strategies varied by region (Table 1, mitigation strategies for distance‐based care). Respondents in the Americas were mostly likely to report continued use of telemedicine strategies, while 95% (19/20) of African respondents reported increasing pill counts per prescription. Fewer respondents in the African region reported improvements in clinic visits deferrals between the month of highest COVID‐19 impact and the current month at only 45% (10/22) compared with 79% (11/14) in the European Region and 65% (34/52) in the region of the Americas. Respondents in the Americas also experienced the highest level of deferrals earlier on (Fig. 3). Conversely, fewer respondents in African countries reported deferrals in laboratory testing and imaging compared with the Americas and Europe (Table 1, mitigation strategies for distance‐based care). These differences were found to be significant when comparing the Americas with the African Region at the 95% confidence level (P = 0.029 and P = 0.001), but not between the European Region and the African Region.
Discussion
The results of this survey reveal challenges and opportunities for hepatitis care introduced by the response to the COVID‐19 pandemic. The emergence of COVID‐19 resulted in an immediate decrease in patients receiving HBV and HCV testing and treatment. Others have reported similar declines. 3 , 4 , 5 , 6 Although most respondents to our survey reported improvements in the number of patients receiving these services, ongoing monitoring can assess whether these improvements are sustained particularly during surges of COVID‐19 in communities.
Fortunately, the survey results suggest that many clinicians and program managers implemented recommended mitigation strategies to protect patients and providers from SARS‐CoV‐2 infection. Also, the response to the COVID‐19 pandemic has created new opportunities to strengthen hepatitis elimination efforts. For example, the strengthening of the telemedicine infrastructure, as well as expansion of diagnostic capacity can innovate hepatitis care, expanding access to hepatitis testing and treatment. 7 , 8 The varied use of mitigation strategies across regions reveals the value of sharing lessons learned to translate these innovative strategies into simpler models of routine hepatitis care.
The survey has limitations. Although respondents were from 44 countries, the survey is limited by responses coming from relatively small convenience sample of clinicians and program managers. The survey was translated into only French and Spanish, which may have limited participation.
In summary, the COVID‐19 pandemic resulted in decreases in hepatitis prevention, testing and treatment, slowing or halting, in the immediate term, progress toward goals for hepatitis elimination. However, mitigation strategies to stop transmission helped clinicians safely overcome these declines in hepatitis care during the pandemic response and hold promise for sustaining these strategies to simplify hepatitis testing and treatment particularly as cases of COVID‐19 wane. The hepatitis community can disseminate lessons learned for safely continuing service delivery, assist programs in introducing mitigation strategies, and capitalize on these opportunities for innovative changes in clinical care that advance progress toward hepatitis elimination.
Potential conflict of interest: The Task Force for Global Health receives funds for the general support of the Coalition for Global Hepatitis Elimination from Abbott, Gilead, AbbVie, Merck, Siemens, Cepheid, Roche, Pharco, Zydus‐Cadila, governmental agencies and philanthropic organizations.
Footnotes
The partners were Hepatitis ECHO network USA, Hepatitis ECHO network Africa, AASLD Hepatitis Elimination Task Force, Canadian Association for the Study of the Liver, European Association for the Study of the Liver, and CGHE. The Clinton Health Access Initiative also distributed the survey to program managers in seven countries.
Region of the Americas: Argentina (5), Brazil (4), Canada (7), Chile (1), Colombia (2), Dominican Republic (1), Ecuador (2), Grenada (1), Guatemala (1), Mexico (4), Panama (1), Paraguay (1), Peru (7), United States of America (16), Venezuela (1). African Region: Burkina Faso (2), Burundi (1), Congo (1), Egypt (5), Ethiopia (4), Ghana (2), Kenya (1), Mozambique (1), Nigeria (4), Rwanda (1), South Africa (4), Uganda (1). South East Asian Region: Bangladesh (1), Indonesia (1). European Region: Armenia (1), Georgia (2), Germany (1), Hungary (1), Iceland (1), Italy (1), Moldova (1), Portugal (1), Romania (1), Spain (1), Turkey (3), Ukraine (1). Eastern Mediterranean Region: Oman (1), Pakistan (1). Western Pacific Region: Vietnam (1).
References
- 1. World Health Organization . Global Hepatitis Report, 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 2. Lau GR, Ward JW. Synthesis of liver associations recommendations for hepatology and liver transplant care during the COVID‐19 pandemic. Clin Liver Dis 2020;15:204‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lemoine M, Kim J, Ndow G, et al. Effect of the COVID‐19 pandemic on viral hepatitis services in sub‐Saharan Africa. Gastroenterol Hepatol 2020;5:966‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wingrove C, Ferrier L, James C, Wang S. The impact of COVID‐19 on hepatitis elimination. Lancet Gastroenterol Hepatol 2020;5:792‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blach S, Kondili LA, Aghemo A, et al. Impact of COVID‐19 on global HCV elimination efforts. J Hepatol 2020;74:31‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexander C, Stoller K, Haffajee R, et al. An epidemic in the midst of a pandemic: opioid use disorder and COVID‐19. Ann Intern Med 2020. Available at: 10.7326/M20-1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serper M, Cubell AW, Deleener ME, et al. Telemedicine in liver disease and beyond: can the COVID‐19 crisis lead to action? Hepatology 2020;72:723‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arrese M. Telemedicine, COVID‐19 and liver diseases: revamping remote care initiatives in hepatology. Ann Hepatol 2020;19:339‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]


