Short abstract
Watch a video presentation of this article
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- Asia‐Pacific
Asia‐Pacific Working Party on Nonalcoholic Fatty Liver Disease
- DM
diabetes mellitus
- EASL
European Association for the Study of the Liver
- ETOH
alcohol
- F2
stage 2 fibrosis
- F3
stage 3 fibrosis
- FDA
US Food and Drug Administration
- FIB‐4
fibrosis‐4 score
- GRADE
grading of recommendation assessment, development, and evaluation
- HCC
hepatocellular carcinoma
- HS
hepatic steatosis
- IR
insulin resistance
- MAFLD
metabolic (dysfunction)‐associated fatty liver disease
- MetS
metabolic syndrome
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFS
NAFLD fibrosis score
- T2DM
type 2 diabetes mellitus
- TBW
total body weight
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States and in other industrialized nations. Its increase in prevalence and severity correlates with the rise in obesity and the metabolic syndrome, and NAFLD now represents a leading indication for liver transplantation in the United States. 1 The rising clinical and economic burden of NAFLD has highlighted the need for a streamlined approach to prevention, diagnosis, and treatment of the disease. In this review, we will summarize updated guideline and guidance recommendations for the management of adult NAFLD; highlight key difference between US, Asian, and European recommendations; and provide key updates.
Key Updates to US Gastroenterology and Hepatology Society Recommendations For Adult NAFLD
In 2012, the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology, and the American Gastroenterological Association published a joint practice guideline on NAFLD. 2 The diagnosis of NAFLD currently requires: (1) evidence of hepatic steatosis (HS) by imaging or histology, (2) no significant alcohol consumption, (3) no competing causes of HS, and (4) no coexisting causes of chronic liver disease. Research efforts have led to significant progress in our understanding of the disease. An updated practice guidance, based on expert consensus rather than by systematic review of the literature, was published by AASLD in 2018 to help clinicians navigate the most recent evidence into clinical practice. 3 The guidance should be used in conjunction with the graded recommendations from previously published guidelines.
One notable change in guidance is a stronger emphasis on assessment for metabolic risk factors in patients with incidental findings of HS and normal liver chemistries but lacking liver‐related symptoms. Growing evidence supports that patients with NAFLD have increased cardiovascular morbidity and mortality. 4 Moreover, advanced liver fibrosis is associated with increasing number of metabolic comorbidities. 5 Thus, early identification and treatment of individual components of the metabolic syndrome are critical in preventing both cardiovascular and liver‐related mortality.
The importance of identifying and staging the degree of fibrosis in patients with NAFLD is underscored in the updated guidance because it is thought to be the main driver of overall and liver‐related mortality. 6 In the original guideline, NAFLD fibrosis score was the only recommended tool to assess fibrosis noninvasively because imaging modalities were not yet readily available in the United States. Fibrosis‐4 score (FIB‐4), ultrasound‐based elastography, and magnetic resonance elastography have now been added to the arsenal of clinically useful tools to assess fibrosis staging. Accessibility to advanced imaging tools vary across institutions, and no guidance is provided for the optimal sequence of diagnostic testing.
More recently, a consensus of international experts proposed changing the name of NAFLD to metabolic (dysfunction)‐associated fatty liver disease (MAFLD). 7 The paradigm shift to MAFLD would reflect the underlying pathogenesis, eliminate the “negative” nomenclature, and allow for the coexistence of other chronic liver diseases, including alcoholic liver disease. One concern of the use of MAFLD would be an inclusive definition that would not specifically address the population with nonalcoholic steatohepatitis (NASH) who are at highest risk for complications. Future research and guidelines will likely address this ongoing conversation within the field currently.
Similarities and Differences in Guidelines From Europe, Asia, and The United States
In today’s increasingly globalized world, awareness of international differences in the approach to NAFLD is important to provide high‐quality care to patients of all backgrounds. The European Association for the Study of the Liver (EASL), in a joint effort with the European Association for the Study of Diabetes and European Association for the Study of Obesity, published a NAFLD clinical practice guideline in 2016. 8 The Asia‐Pacific Working Party on NAFLD published its guideline in 2017. 9 , 10 Both the European and Asian guidelines use the grading of recommendation assessment, development, and evaluation (GRADE) approach to rate the quality of evidence and the strength of each recommendation. Although many similarities exist across guidelines, there are several key areas of divergence that will be outlined later (Table 1).
TABLE 1.
Similarities and Differences in Guidelines from Europe, Asia, and the United States
| AASLD (2018) | EASL (2016) | Asia‐Pacific (2017) | |
|---|---|---|---|
| Definition of significant alcohol consumption |
|
|
|
| Screening for NAFLD | Systematic screening of the general population not recommended | ||
|
|
|
|
| Fibrosis assessment |
|
|
|
| Liver biopsy remains the gold standard for differentiating NAFL from NASH and staging liver fibrosis. Proceed with liver biopsy if: (1) suspicion for NAFLD advanced fibrosis (2), or concern for coexisting or competing etiology of chronic liver disease (B2). | |||
| Lifestyle intervention | Target weight loss of 7% to 10% TBW (B1). Achieve with 500‐1000 daily caloric deficit and moderate‐intensity exercise, preferably in a structured weight loss program (C2). | ||
|
|
|
|
| Pharmacological intervention | There are currently no approved drugs to treat NAFLD or NASH. However, multiple drugs are in phase 3 development. In patients with cardiovascular indications, statins can be safely used in patients with NASH and compensated cirrhosis (B1) | ||
|
|
|
|
GRADE scores, when available, are listed in parentheses.
What Is the Definition of “Significant” Alcohol Use?
All society guidelines characterize NAFLD by the presence of HS in the absence of significant alcohol consumption. However, there is no international consensus as to the amount of alcohol considered “significant.” The Asian guideline has the most conservative alcohol threshold and mirrors the exclusion criteria for alcohol use defined in the National Institutes of Health Nonalcoholic Steatohepatitis Research Network database protocol. It is important to keep in mind that alcohol thresholds are oversimplified because the duration of significant alcohol exposure, drinking pattern, and individual susceptibility all play a role in alcohol‐induced liver injury.
Who Should Be Screened for NAFLD?
All societies recommend against systematic screening for NAFLD in the general population. AASLD currently recommends against screening even in high‐risk populations because of the lack of effective drug treatment, cost‐effectiveness analysis, and unclear long‐term benefits to screening. A “high index of suspicion” for NAFLD is advised in patients with type 2 diabetes.
The European guideline acknowledges the lack of validated cost‐utility studies and the need to be cognizant of regional variations in available health care resources but recommends that all patients with obesity or the metabolic syndrome be screened for NAFLD because of the prognostic implications of progressive disease. The Asian guidelines state that screening may be considered in at‐risk groups, such as patients with diabetes and obesity. Lean NAFLD is prevalent in Asia, where almost a quarter of patients with NAFLD are not obese. 11 Thus, insulin resistance (IR) and altered body fat distribution rather than body mass index per se may be better indicators of NAFLD in such patients. In patients without diabetes, the homeostatic model assessment for IR (HOMA‐IR) provides an acceptable estimate of IR. Ultrasound remains the first‐line assessment for HS because of its wide availability and low cost. However, it is less reliable when HS is <20% 12 and raises concerns of underestimating the prevalence of NAFLD. Magnetic resonance imaging–derived proton density fat fraction is highly sensitive but is not widely available outside of research settings. Controlled attenuation parameter is available with the FibroScan system and may be more sensitive than ultrasound. Its point‐of‐care nature makes it appealing as a tool to monitor disease progression and treatment response, but more studies are needed to assess its validity.
How Should NAFLD Be Diagnosed, and How Should It Be Monitored?
Liver histology remains the gold standard for differentiating steatohepatitis from simple steatosis and for assessing fibrosis staging. Due to its invasive nature and associated costs, all guidelines agree that liver biopsy should be considered only in select individuals. The American and European guidelines agree that patients with NAFLD and suspicion for advanced fibrosis should have a liver biopsy to confirm findings because this would have prognostic implications and lead to management changes. The Asian guidelines differ in that they recommend biopsy only if the presence and/or the severity of coexisting chronic liver disease cannot be excluded or if assessment of fibrosis using noninvasive testing is inconclusive. All guidelines agree that noninvasive tools should be used to stratify patients as low or high risk for advanced fibrosis, but a preferred sequence of testing is not provided in the American and Asian guidelines. The European guideline provides a proposed diagnostic algorithm with suggestions to guide referral to hepatology. In addition, it provides a proposed follow‐up strategy to monitor for disease progression with the caveat that optimal follow‐up has yet to be determined.
The identification of NASH is clinically important because it indicates an increased risk for fibrosis progression and the need for aggressive treatment and closer follow‐up. There are currently no acceptable noninvasive modalities to differentiate between bland steatosis and steatohepatitis. The presence of the metabolic syndrome increases the risk for steatohepatitis, and the US guidelines suggest performing liver biopsy in these patients. However, because most patients with NAFLD have at least one component of the metabolic syndrome, such an approach is clinically impractical. Furthermore, without the availability of a US Food and Drug Administration (FDA)–approved pharmacological therapy for NASH, many clinicians remain hesitant to proceed with biopsy.
Once NASH is diagnosed, therapies recommended by the AASLD guidelines include vitamin E for patients with advanced fibrosis and without diabetes mellitus (DM) and pioglitazone, a thiazolidinedione that may be used in patients with NASH and diabetes. More recently, liraglutide, a glucagon‐like peptide‐1 receptor agonist, was shown to be of benefit in patients with NASH and DM. Pharmacological therapy for NASH is an area of significant ongoing investigation.
Key Outcomes of Consideration in Patients With NAFLD
Hepatocellular carcinoma (HCC) related to NAFLD is of growing concern, particularly because it can occur in the absence of cirrhosis. 13 Obesity, type 2 diabetes, advanced age, male sex, and certain gene polymorphisms are associated with increased risk for HCC. However, the mortality benefit and cost‐effectiveness of surveillance for HCC in patients with noncirrhotic NAFLD is yet to be determined and is not recommended at this time by any of the guidelines.
Early recognition and intervention are key to improving clinical outcomes and reducing the economic and health care burden of NAFLD. Despite this, widespread awareness of NAFLD in the primary care setting is lacking and remains underdiagnosed in real‐world settings. 14 , 15
Once drugs specifically targeting NAFLD obtain FDA approval, there will most likely be a surge of interest in NAFLD by the key health care stakeholders: patients, providers, payors, and policymakers. NAFLD is a fast‐moving field, and current guidelines will soon be outdated. Future guideline updates should outline a practical strategy for the identification of high‐risk patients with NAFLD who would benefit most from hepatology referral and targeted therapy (Fig. 1). There remains a pressing need to establish the optimal assessment of steatosis, steatohepatitis, and fibrosis in a cost‐effective and minimally invasive manner.
FIG 1.
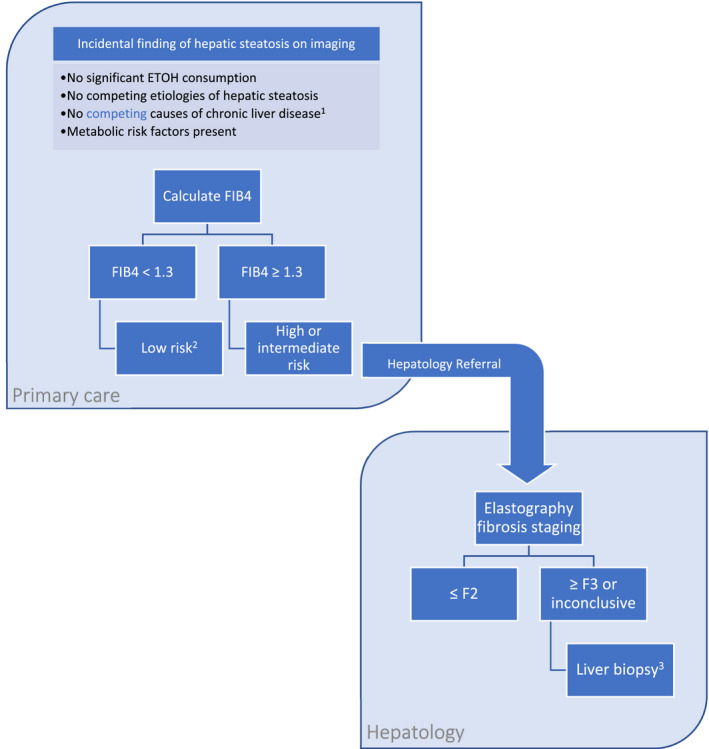
Proposed diagnostic and risk stratification algorithm for patients with suspected NAFLD. 1HBV and HCV serological workup should be completed in the primary care setting, with subsequent workup tailored to the individual patient by hepatology. Note that NAFLD may coexist with other chronic liver diseases. 2Evidence‐based optimal follow‐up of patients with NAFLD has not been established. The EASL recommends monitoring low‐risk patients with NAFLD without worsening metabolic risk factors every 2 to 3 years. 3Biopsy should also be considered in patients with increasing number of metabolic diseases who are at high risk for steatohepatitis.
Potential conflict of interest: Nothing to report.
References
- 1. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 2. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005‐2023. [DOI] [PubMed] [Google Scholar]
- 3. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 4. Targher G, Byrne CD, Lonardo A, et al. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 5. Wong RJ, Tran T, Kaufman H, et al. Increasing metabolic co‐morbidities are associated with higher risk of advanced fibrosis in nonalcoholic steatohepatitis. PLoS One 2019;14:e0220612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angulo P, Kleiner DE, Dam‐Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eslam M, Sanyal AJ, George J, International Consensus Panel . MAFLD: a consensus‐driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999‐2014.e1. [DOI] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 9. Wong VW, Chan WK, Chitturi S, et al. Asia‐Pacific Working Party on Non‐alcoholic Fatty Liver Disease guidelines 2017‐Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018;33:70‐85. [DOI] [PubMed] [Google Scholar]
- 10. Chitturi S, Wong VW, Chan WK, et al. The Asia‐Pacific Working Party on Non‐alcoholic Fatty Liver Disease guidelines 2017‐Part 2: management and special groups. J Gastroenterol Hepatol 2018;33:86‐98. [DOI] [PubMed] [Google Scholar]
- 11. Liu CJ. Prevalence and risk factors for non‐alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol 2012;27:1555‐1560. [DOI] [PubMed] [Google Scholar]
- 12. Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 2009;51:1061‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mittal S, El‐Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in united states veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2016;14:124‐131.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blais P, Husain N, Kramer JR, et al. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol 2015;110:10‐14. [DOI] [PubMed] [Google Scholar]
- 15. Alexander M, Loomis AK, Fairburn‐Beech J, et al. Real‐world data reveal a diagnostic gap in non‐alcoholic fatty liver disease. BMC Med 2018;16:130. [DOI] [PMC free article] [PubMed] [Google Scholar]


