Abstract
High-pathogenicity avian influenza (HPAI) viruses have arisen from low-pathogenicity avian influenza (LPAI) viruses via changes in the hemagglutinin proteolytic cleavage site, which include mutation of multiple nonbasic to basic amino acids, duplication of basic amino acids, or recombination with insertion of cellular or viral amino acids. Between 1959 and 2019, a total of 42 natural, independent H5 (n = 15) and H7 (n = 27) LPAI to HPAI virus conversion events have occurred in Europe (n = 16), North America (n = 9), Oceania (n = 7), Asia (n = 5), Africa (n = 4), and South America (n = 1). Thirty-eight of these HPAI outbreaks were limited in the number of poultry premises affected and were eradicated. However, poultry outbreaks caused by A/goose/Guangdong/1/1996 (H5Nx), Mexican H7N3, and Chinese H7N9 HPAI lineages have continued. Active surveillance and molecular detection and characterization efforts will provide the best opportunity for early detection and eradication from domestic birds.
Avian influenza (AI) viruses have their ancestry within the ecological niches occupied by aquatic birds, predominately of the orders Anseriformes and Charadriiformes, and these aquatic birds are the primordial reservoir of genes for all Influenzavirus A strains with the exception of the genetic material of H17N11 and H18N12 influenza A viruses, which have been detected only in bats (Webster et al. 1992; Mehle 2014). Currently, AI viruses have surface proteins of any of the 16 different hemagglutinin (H1–16) and nine different neuraminidase (N1–9) subtypes (Swayne et al. 2020). Based on their pathogenicity in chickens, most H1–H16 AI viruses cause mild respiratory, enteric, or reproductive diseases (i.e., low-pathogenicity avian influenza [LPAI]), whereas some of the H5 and H7 AI viruses cause deadly, systemic disease (high-pathogenicity avian influenza [HPAI]).
Historically, the first identification of an AI virus in birds was an HPAI virus, termed fowl plague virus, which was initially identified in Northern Italy during the 1880s (Kaleta and Rulke 2008). Most fowl plague cases were in gallinaceous poultry, principally chicken and turkeys, but some cases also affected geese (Kaleta and Rulke 2008). The affected poultry were housed close to migratory waterfowl on river banks such as the River Po. The initial clinical presentation in poultry had a mild course with an abrupt change to highly lethal, rapidly spreading disease very similar to our contemporary understanding of the origins of a HPAI virus through changes in the hemagglutinin (HA) proteolytic cleavage site of a H5 or H7 LPAI virus (Bosch et al. 1979; Kawaoka and Webster 1985; Perdue et al. 1996; Kaleta and Rulke 2008). The fowl plague cases from the 1880s to 1959 were exclusively H7N7 and H7N1 HPAI viruses, but in 1959, the first H5 HPAI virus appeared in chickens in Scotland, H5N1 (Kaleta and Rulke 2008; Swayne et al. 2020). From the 1880s to 1959, fowl plague spread throughout Europe, Asia, and Africa and was reported from North and South America. However, it is unclear if these fowl plague cases were a single HPAI virus lineage that was maintained and widely spread geographically among poultry populations or if there were multiple local occurrences of LPAI viruses giving rise to HPAI viruses, but with a more restricted geographic spread. The lack of knowledge related to uniqueness of virus isolates prior to 1959 severely limited the maintenance of diverse and unique fowl plague isolates, which are available today for genetic analysis (Swayne 2008a). However, since 1959, with well-maintained unique virus isolates in archives, outbreaks caused by HPAI viruses have mostly arisen from virulence shifts by LPAI viruses with limited spread and eradication from poultry via stamping out programs. The major exception has been the A/goose/Guangdong/1/1996 (Gs/GD) lineage of H5, which arose in 1996 and has spread and been maintained in poultry and wild aquatic bird reservoirs until the current date, producing infections in poultry, wild birds, or humans in 84 countries in Asia, Africa, Europe, and North America (Röhm et al. 1995; Swayne et al. 2020). The first LPAI virus from poultry was identified in chickens in Germany in 1949 (Dinter virus, H10N7) (Swayne 2008a), and the first LPAI viruses in wild waterfowl (Slemons et al. 1974) and pelagic seabirds (Downie and Laver 1973) were identified in 1972, although antibodies to Influenzavirus A had been detected in migratory waterfowl as early as 1968 (Easterday et al. 1968).
This review will discuss the mechanisms and genetic changes in the HA gene that are responsible for the shift from LPAI to HPAI viruses and analyze the genetic information, demonstrating that HPAI viruses belong to distinct lineages (Table 1).
Table 1.
Forty-two documented pandemics, epidemics, or limited outbreaks of HPAI since discovery of AI viruses as cause of fowl plague in 1955
| No. | Year | Country | Host species | Prototype AI virus | Subtype | Accession No. (NCBI GenBank or GISAID Epiflu database) | HA cleavage site | Number affected with high mortality or depopulateda |
|---|---|---|---|---|---|---|---|---|
| 1 | 1959 | Scotland | Domestic poultry | A/chicken/Scotland/1959 | H5N1 | GU052518 | PQRKKR/GLF | Aberdeen: 1 premise, unknown number of chickens (Gallus gallus domesticus) affected (Pereira et al. 1965; Alexander et al. 2008) |
| 2 | 1961 | South Africa | Wild bird | A/tern/South Africa/1961 | H5N3 | GU052822 | PQRETRRQKR/GLF | Western and Eastern Cape provinces (coastline from Port Elizabeth to Lambert's Bay): 1300 common terns (Sterna hirundo) (Rowan 1962; Becker 1966; Alexander et al. 2008) |
| 3 | 1963 | England | Domestic poultry | A/turkey/England/1963 | H7N3 | AF202238 | PETPKRRRR/GLF | Norfolk County: 2 farms; 29,000 breeder turkeys (Meleagridis gallopavo) in outdoor and indoor pens (Wells 1963; Alexander et al. 2008) |
| 4 | 1966 | Canada | Domestic poultry | A/turkey/Ontario/7732/1966 | H5N9 | CY107859 | PQRRRKKR/GLF | Ontario province: 2 indoor farms; 8,100 breeder turkeys (Lang et al. 1968; Swayne 2008b) |
| 5 | 1976 | Australia | Domestic poultry | A/chicken/Victoria/1976 | H7N7 | CY024786 | PEIPKKREKR/GLF | Victoria province: 2 farms; 25,000 indoor laying chickens, 17,000 indoor broilers, and 16,000 indoor and outdoor ducks (Anas platyrhyncos) (Anonymous 1976; Turner 1976; Bashiruddin et al. 1992; Sims and Turner 2008b) |
| 6 | 1979 | Germany | Domestic poultry | A/chicken/Germany/01/1979 A/goose/Leipzig/187_7/1979 A/goose/Leipzig/137/8/1979 A/chicken/Leipzig/79 A/goose/Leipzig/192/7/1979 |
H7N7 |
CY107844 L43914 L43913 U20459 L43915 |
PEIPKKKKKKR/GLF PETPKKKKKKR/GLF PEIPKRKKR/GLF PEIPKKKKR/GLF PEIPKKRKKR/GLF |
Saxony: 2 farms: 600,000 chickens, 80 geese (Röhm et al. 1996; Harder and Werner 2006; Alexander et al. 2008) |
| 7 | 1979 | England | Domestic poultry | A/turkey/England/199/1979 | H7N7 | N/A | PEIPKKRKR/GLF, PEIP KRRRR/GLF, PEIP KKREKR/GLF | Norfolk county: 3 commercial farms, 9262 turkeys (Alexander et al. 2008) |
| 8 | 1983–84 | USA | Domestic poultry | A/chicken/Pennsylvania/1/1983 (LP) A/chicken/Pennsylvania/1370/1983 (HP) | H5N2 |
J04325 GU052771 |
PQKKKR/GLF (LP) PQKKKR/GLF (HP) – lost a glycosylation site on amino acid 13 |
Pennsylvania, Maryland, and Virginia: 452 flocks, 17 million birds; mostly chickens or turkeys, a few chukar partridges (Alectoris chukar) and guinea fowl (Numida meleagris) (Kawaoka and Webster 1985; USAHA 1985; Eckroade and Silverman-Bachin 1986; Easterday et al. 1997) |
| 9 | 1983 | Ireland | Domestic poultry | A/turkey/Ireland/1378/1983 | H5N8 | M18451 | PQRKRKKR/GLF | Monaghan County: 4 farms; 8,120 turkeys, 28,020 chickens, and 270,000 ducks (McNulty et al. 1985; Alexander et al. 2008) |
| 10 | 1985 | Australia | Domestic poultry | A/chicken/Victoria/1/1985 | H7N7 | M17735 | PEIPKKREKR/GLF | Victoria province: 1 farm; 24,000 broiler breeders, 27,000 laying chickens, and 61,000 broilers (Barr et al. 1986; Cross 1987; Senne et al. 1996a; Sims and Turner 2008b) |
| 11 | 1991 | England | Domestic poultry | A/turkey/England/50-92/1991 | H5N1 | GU052510 | PQRKRKTR/GLF | Norfolk County: 1 farm; 8000 turkeys (Alexander et al. 1993; Alexander and Wood 1993; Wood et al. 1993; Senne et al. 1996) |
| 12 | 1992 | Australia | Domestic poultry | A/chicken/Victoria/1/1992 | H7N3 | AF202227 | PEIPKKKKR/GLF | Victoria province: 2 farms, 1 backyard flock and 1 hatchery; 17,000 broiler breeders, 5,700 ducks, 105,000 day-old chicks, 540,000 hatching eggs (Selleck et al. 1997; Westbury 1998; Sims and Turner 2008b) |
| 13 | 1994–95 | Australia | Domestic poultry | A/chicken/Queensland/1994 A/chicken/Queensland/667/1995 |
H7N3 |
CY022685 AF202231 |
PEIPRKRKR/GLF | Queensland province: 1 farm; 22,000 laying chickens (Westbury 1998; Perdue et al. 1999) |
| 14 | 1994–95 | Mexico | Domestic poultry | A/chicken/Mexico/31381-7/1994 (LP) A/chicken/Puebla/8623-607/1994 (HP) |
H5N2 |
GU186573 AB558473 |
PQRETR/GLF (LP) PQRKRKTR/GLF (HP) |
Puebla and Queretaro: Chickens—concurrent circulation of LP (1993–) and HPAI virus (late 1994 to mid-1995) strains. 360 commercial chicken flocks “depopulated” (1995) via vaccination and controlled marketing. Unknown number of HP-infected birds (García et al. 1996; Easterday et al. 1997; Perdue et al. 1997; Villareal and Flores 1998; Smith 2006) |
| A/chicken/Queretaro/14588_19/1995 A/chicken/Queretaro/7653_20/1995 |
H5N2 |
AB558474 U85390 |
PQRKRKTR/GLF PQRKRKRKTR/GLF |
|||||
| 15 | 1994–95, 2004 | Pakistan | Domestic poultry | A/chicken/Pakistan/447/1995 A/chicken/Pakistan/CR2/95 A/chicken/Karachi/NARC-23/2003 |
H7N3 |
AF202226 AF202230 HM346493 |
PETPKRKRKR/GLF PETPKRRKR/GLF |
Two incursions: (1) 3.2 million broilers and broiler breeder chickens (northern part of country, 1994–1995), and (2) 2.52 million layers (Karachi, 2004). Vaccination and controlled marketing (Naeem and Hussain 1995; Easterday et al. 1997; Naeem 1998; Banks et al. 2000) |
| 16 | 1996– cont. | 84 countries in Asia, Africa, Europe and North America | Domestic poultry and wild bird | A/goose/Guangdong/1/1996 (Gs/GD) | H5Nx |
NC_007362 | PQRERRRKKR/GLF (majority), Variations: 1) Clade 1, PQREGRRKKR/GLF; 2) Clade 2.1, PQRESRRKK/GLF; 3) Clade 2.2, QGERRRKKR/GLF; 4) Clade 2.3, PQRERRRKR/GLF, PLRERRRKR/GLF; 5) Clade 7, PQIEGRRRKR/GLF |
Unknown number of commercial and noncommercial flocks (principally village poultry); more than 400 million birds dead or culled from 2003 to early 2012, mostly chickens, but also ducks, geese, Japanese quail, and some wild birds (Sims et al. 2003a; Sims et al. 2003b; FAO 2006; Sims and Brown 2008a; Sims and Brown 2017). Largest HPAI outbreak since 1959 with more birds and countries affected than the other 41 outbreaks since 1959. |
| 17 | 1997 | Australia | Domestic poultry | A/chicken/NSW/1/1997 A/chicken/New_South_Wales/327/1997 |
H7N4 |
AY943924 CY022701 |
PEIPRKRKR/GLF PEIPRRRKR/GLF |
New South Wales province: 3 farms; 160,000 indoor broiler breeders and 261 outdoor emu (Dromaius novaehollandiae) (Perdue et al. 1999; Sims and Turner 2008b) |
| 18 | 1997 | Italy | Domestic poultry | A/poultry/Italy/330/1997 | H5N2 | CY017403 | PQRRRKKR/GLF | Veneto and Fruili–Venezia–Giulia Regions: 8 flocks (hobby/backyard only); 2116 chickens, 1501 turkeys, 731 guinea fowl, 2322 ducks, 204 quail (species unknown), 45 pigeons (Columbia livia), 45 geese (species unknown), 1 pheasant (species unknown) (Capua et al. 1999; Alexander et al. 2008) |
| 19 | 1999–2000 | Italy | Domestic poultry | A/turkey/Italy/977/1999 (LP) A/turkey/Italy/4580/1999 (HP) | H7N1 |
GU052999 CY021405 |
PEIPKGR/GLF (LP) PEIPKGSRVRR/GLF (HP, majority), PEIPKGSRMRR/GLF (HP, minor), PEIPKRSRVRR/GLF (HP, minor) |
Veneto and Lombardia Regions: 413 farms, 8.1 million laying chickens; 2.7 million meat and breeder turkeys; 2.4 million broiler breeders and broilers; 247,000 guinea fowl; 260,000 quail, ducks, and pheasants; 1,737 backyard poultry and 387 ostriches (Banks et al. 2001; Capua et al. 2003; Alexander et al. 2008) |
| 20 | 2002 | Chile | Domestic poultry | A/chicken/Chile/176822/2002 (LP) A/chicken/Chile/4322/2002 (HP) A/chicken/Chile/4957/2002 (HP) |
H7N3 |
AY303630 AY303631 AY303632 |
PEKPKTR/GLF (LP) PEKPKTCSPLSRCRETR/GLF (HP) PEKPKTCSPLSRCRKTR/GLF (HP) |
Valparaíso region: Two farms of one company, multiple houses; 617,800 broiler breeders, 18,500 turkey breeders (2 houses) (Rojas et al. 2002; Suarez et al. 2004; Max et al. 2007; Swayne 2008b) |
| 21 | 2003 | Netherlands Belgium Germany |
Domestic poultry | A/chicken/Netherlands/1/03 | H7N7 | AY338458 | PEIPKRRRR/GLF | (1) Netherlands, Gelderse Vallei and Limburg region: 255 infected flocks, and 1381 commercial and 16,521 backyard/smallholder flocks depopulated—30 million affected, mostly chickens. (2) Belgium (Limburg and Antwerp provinces), 8 farms, 2.3 million chickens. (3) Germany (Nordrhein–Westfalen State), 1 farm, 419,000 chickens (Elbers et al. 2004; Harder and Werner 2006; Alexander et al. 2008) |
| 22 | 2004 | Canada | Domestic poultry | A/chicken/Canada/AVFV1/2004 (LP) A/chicken/Canada/AVFV2/2004 (HP) |
H7N3 |
AY650270 AY648287 |
LP: PENPKTR/GLF HP: PENPKQAYRKRMTR/GLF PENPKQAYQKRMTR/GLF, PENPKQAYKKRMTR/GLF, PENPKQAYHKRMTR/GLF, PENPKQAHQKRMTR/GLF, PENPRQAYRKRMTR/GLF, PENPKQACQKRMTR/GLF |
British Columbia province: 42 commercial and 11 backyard flocks infected (1.2 million poultry)—approximately 16 million commercial poultry depopulated, most were chickens (Hirst et al. 2004; Pasick et al. 2005) |
| 23 | 2004 | USA | Domestic poultry | A/chicken/Texas/298313/2004 | H5N2 | AY849793 | PQRKKR/GLF | Texas state: 1 noncommercial farm (6608 chickens), 2 LPM affected; 3 dangerous LPM contacts culled (Lee et al. 2005) |
| 24 | 2004 | South Africa | Domestic poultry | A/ostrich/South Africa/N227/2004 | H5N2 | FJ519983 | PQREKRRKKR/GLF | East Cape province: 2004–2008 farms, culled. 23,625 ostriches, 3,550 other poultry (chickens, turkeys, geese, ducks, and pigeons), 1594 ostrich eggs and 1707 other farmed bird eggs (Abolnik 2007; Alexander et al. 2008; Abolnik et al. 2009) |
| 25 | 2006 | South Africa | Domestic poultry | A/ostrich/South_Africa/AI1160/2006 (LP) A/ostrich/South Africa/AI1091/2006 (HP) |
H5N2 |
EF591757 EF591749 |
PQRRKKR/GLF | Western Cape province: 24 farms, 7334 ostriches culled (Abolnik 2007; Alexander et al. 2008; Brown et al. 2017) |
| 26 | 2005 | North Korea | Domestic poultry | A/chicken/North Korea/1/2005 | H7N7 | N/A | PEIPKGRHRRPKR/GLF | 3 farms, 218,882 layer chickens culled; number dead unknown (Alexander et al. 2008) |
| 27 | 2007 | Canada | Domestic poultry | A/chicken/Saskatchewan/HR-00011/2007 | H7N3 | EU500860 | PENPKTTKPRPRR/GLF | Saskatchewan province: 1 farm, 10 barns, 49,500 broiler breeder hens and roosters (Swayne 2008b; Berhane et al. 2009) |
| 28 | 2008 | England | Domestic poultry | A/chicken/England/1158-114061/2008 | H7N7 | FJ476173 | PEIPKRKKR/GLF | Oxfordshire county: 1 farm, 25,000 free-range layer chickens (Brown et al. 2017) |
| 29 | 2009 | Spain | Domestic poultry | A/chicken/Spain/6279-2/2009 | H7N7 | GU121458 | PELPKGTKPRPRR/GLF | Guadalajara province: 1 farm, 5 barns; 308,640 layer chickens (Iglesias et al. 2010; Brown et al. 2017) |
| 30 | 2011–2013 | South Africa | Domestic poultry | A/ostrich/SA/AI2114/2011 A/ostrich/SA/AI2512/2011 |
H5N2 |
JX069081 JX069097 |
PQRRKKR/GLF PQRRRKR/GLF |
Western Cape province: 45,343 ostriches on 50 premises (Brown et al. 2017) |
| 31 | 2012–2013 | Taiwan | Domestic poultry | A/chicken/Taiwan/A1997/2012 A/chicken/Taiwan/1680/2013 |
H5N2 |
KF193394 KJ162620 |
PQRRKR/GLF PQRKKR/GLF |
Chang-Hua, Pingtung, Yunlin, and Penghu counties: 6 premises (4 native chickens, 1 broiler breeder, 1 layer chicken); 47,151 chickens (Lee et al. 2014; Brown et al. 2017) |
| 32 | 2012- present | Mexico | Domestic poultry | A/chicken/Jalisco/12283/2012 | H7N3 | JX908509 | PENPKDRKSRHRRTR/GLF | Jalisco, Aguascalientes, Guanajuato, Tlaxcala, and Puebla states: 110 premises, 18,906,702 poultry. Two waves of disease: (1) 6/13/2012–9/29/2012, and (2) 1/3/2013–ongoing (Maurer-Stroh et al. 2013; Brown et al. 2017) |
| 33 | 2012 | Australia | Domestic poultry | A/chicken/New South Wales/12-3121-1/2012 | H7N7 | N/A | PEIPRKRKR/GLF | New South Wales province: 1 premise, free-range layers, 50,000 chickens (Brown et al. 2017) |
| 34 | 2013 | Italy | Domestic poultry | A/chicken/Italy/13VIR4527-11/2013 | H7N7 | KF569186 | PETPKRRERR/GLF | Emilia-Romagna Region: 6 premises, layers, 952,658 chickens (Brown et al. 2017) |
| 35 | 2013 | Australia | Domestic poultry | A/chicken/New South Wales/13-02811-1/2013 | H7N2 | N/A | PEIPRKRKR/GLF | New South Wales province: 2 premises, free-range and caged layers, 490,000 chickens (Brown et al. 2017) |
| 36 | 2015 | England | Domestic poultry | A/chicken/England/26352/2015 (H7N7) | H7N7 | EPI623939 | PEIPRHRKGR/GLF | Lancashire county: 1 premise, colony and free-range laying chickens, 179,865 affected (Brown et al. 2017) |
| 37 | 2015 | Germany | Domestic poultry | A/chicken/Germany/AR1385/2015 (H7N7) | H7N7 | EPI634885 | PEIPKRKRR/GLF | Lower Saxony state: 1 premise, laying chickens, 10,104 affected (Brown et al. 2017) |
| 38 | 2015–2016 | France | Domestic poultry | A/chicken/France/150169a/2015 A/duck/France/150233/2015 A/duck/France/150236/2015 |
H5N1 H5N2 H5N9 |
KU310447 KX014878 KX014886 |
HQRRKR/GLF | 8 southwest/southcentral Departments: 81 premises, 155,415 poultry affected; primarily affected fattening ducks, and some guinea fowl, geese, and layer and meat chickens in small farms and backyard operations. H5N1 virus reassorted with Eurasian LPAI viruses to produce H5N2 and H5N9 HPAI viruses (OIE 2016b; Briand et al. 2017) |
| 39 | 2016 | USA | Domestic poultry | A/turkey/Indiana/16-001403-1/2016 | H7N8 | KU558906 | PENPKKRKTR/GLF | Indiana state, Dubois county: 1 premise, 43,500 meat turkeys. 1 dangerous contact layer farm (156,158 layers), and 9 LP-affected turkey farms (195,937 birds) in control zone depopulated (ISBOAH 2016; Lee et al. 2017b; Swayne et al. 2017) |
| 40 | 2016 | Italy | Domestic poultry | A/chicken/Italy/16VIR-1873/2016 | H7N7 | EPI756028* | PELPKGRKRR/GLF | Emilia-Romagna region: 1 premise, 17,500 organic/free range layers (OIE 2016a; OIE 2016b) |
| 41 | 2016–2019 | China | Domestic poultry | A/chicken/Huizhou/HZ-3/2016 A/chicken/Huizhou/HZ04/2016 A/chicken/Heyuan/16876/2016 |
H7N9 | EPI917102 EPI918826 EPI919533 |
PEVPKRKRTAR/GLF; PEVPKGKRTAR/GLF |
Initially Guangdong province: Since 10 January 2017, HP H7N9 virus was reported in a total of 58 poultry or environmental samples (46 chickens, 2 duck, and 10 environmental samples); H7N9 virus isolates from 32 human cases were found to be HP virus (as of 07 August 2019). HP derived from LP virus circulating in live poultry market system since early 2013 (OIE 2017a; Qi et al. 2018) |
| 42 | 2017 | USA | Domestic poultry | A/chicken/Tennessee/17-007147-1/2017 | H7N9 | MF357740 | PENPKTDRKSRHRRIR/GLF | Tennessee, Lincoln county: 2 premise, 128,000 chicken broiler breeders with HPAI virus. LPAI precursor virus on 12 premises (6 backyard and 6 commercial, 125,000 birds) in Tennessee, Alabama, Kentucky and Georgia (Lee et al. 2017a; OIE 2017b; USDA 2017) |
Data modified from Alexander (2000), Swayne and Suarez (2000), Swayne (2017), OFFLU (2019), and Swayne et al. (2020).
(AI) Avian influenza, (GISAID) Global Initiative on Sharing All Influenza Data, (HA) hemagglutinin, (HP) high pathogenicity, (LP) low pathogenicity, (LPM) live poultry market, (N/A) not available, (NCBI) National Center for Biotechnology Information.
aMost outbreaks were controlled by “stamping-out” or depopulation policies for infected and/or exposed populations of poultry. Chickens, turkeys, and poultry in the order Galliformes had clinical signs and mortality patterns consistent with HPAI, whereas ducks, geese, and other aquatic poultry lacked or had low mortality rates or infrequent presence of clinical signs.
MECHANISMS OF THE EMERGENCE OF H5 AND H7 HPAI VIRUSES
The phenotype classification of avian influenza into LPAI (H1–16) and HPAI (H5 and H7) viruses is based on in vivo testing (i.e., the ability to produce severe lethal disease in chickens on intravenous inoculation) and molecular characteristics of the HA protein, more specifically as changes in the proteolytic cleavage site (OIE 2019b). According to the 12th Organisation for Animal Health (OIE) Terrestrial Animal Health Code, the H5 and H7 AI viruses classified as HPAI virus should have an intravenous pathogenicity index (IVPI) in 6-wk-old chickens of >1.2 (OIE 2002). Beginning with the 13th edition of the Terrestrial Animal Health Code (OIE 2004), H5 and H7 AI viruses with IVPI of ≤1.2 or lethality of <75% should be sequenced to determine whether multiple basic amino acids are present at the cleavage site of the HA. If similar sequences of the cleavage site have been observed for other previously reported HPAI viruses, the virus should be classified as HPAI virus. These regulatory definitions are still in place in 2019 (OIE 2019b). However, the presence of any insertion that lengthens the cleavage site but has not been previously reported should be discussed with an OIE Avian Influenza Reference Laboratory or Collaborating Centre before classifying the virus as LPAI or HPAI virus (OFFLU 2019). Although most of the HPAI chicken viruses emerged from LPAI precursors in aquatic wild birds, the emergence of HPAI viruses does not always occur in gallinaceous host (Swayne et al. 2020). The detailed steps of how LPAI viruses evolve into HPAI viruses are not completely understood, but molecular techniques such as next-generation sequencing and reverse genetics have helped to identify the HA cleavage site changes associated with high virulence. In addition, changes in other AIV gene segments can affect the phenotype, usually through increased replication efficiency and virus particle release.
The mechanisms by which H5/H7 LPAI viruses change into HPAI virus include mutation, insertion, and recombination in the proteolytic cleavage site of the HA. The HA is the AI virus surface glycoprotein responsible to attach the virus to the host cell receptor to initiate the infection life cycle. After the virus enters the cell the new viruses start to be produced, including the inactive precursor HA protein (HA0). The HA0 is cleaved at the proteolytic cleavage site by cellular proteases of the host to form the functional subunits HA1 and HA2, resulting in an infectious virus. The cleavage site region in the HA0 consists of an arginine (R) residue adjacent to a conserved glycine (G) (Garten and Klenk 1983; Swayne et al. 2020). The number of basic amino acids at the HA cleavage site plays a critical role in virulence by determining which proteases cleave the HA0 and consequently in which cell types and tissues in the host the AI viruses can replicate (Bosch et al. 1981; Kawaoka et al. 1987; Horimoto and Kawaoka 1994; Swayne et al. 2020). First, the AI virus HA cleavage site can be classified as a monobasic (e.g., PEKQTR/GLF) or multibasic (e.g., PQRKKR/GLF). The monobasic cleavage site usually contains one or two nonconsecutive basic amino acids, arginine (R) or lysine (K), in the critical position and is cleaved by trypsin or trypsin-like proteases that confines virus replication in the epithelial cells of the respiratory and gastrointestinal tracts (Suarez 2016; Swayne et al. 2020). The H5/H7 LPAI viruses maintain the general principle of monobasic cleavage site, although in the wild birds, these subtypes have varying patterns of amino acids at the cleavage site (Suarez 2016). The H5 LPAI viruses usually encode the QRETR/G sequence, whereas H7 LPAI viruses typically possess the NPKTR/G sequence at the cleavage site. An increased number of basic amino acids and/or a lengthening of the proteolytic cleavage site to a minimum motif of four basic amino acids change the virus to high virulence by allowing the HA0 to be cleaved by several ubiquitous cellular proteases (furin-like proteases), which permit the virus to replicate in cells of multiple tissues, increasing the potential to cause a systemic disease and lethal infection in gallinaceous host (OIE 2019b; Swayne et al. 2020). More specifically, the changes observed in the HA cleavage site of HPAI viruses occurred because of (a) substitutions of nonbasic with basic amino acids and in some situations accompanied by loss of a shielding glycosylation site, (b) insertions of multiple basic amino acids from codons duplicated, (c) short inserts of basic and nonbasic amino acids from unknown source, or (d) nonhomologous recombination with cellular (e.g., host 28S RNA) or viral RNAs (e.g., RNA coding NP or M protein) that lengthen the proteolytic cleavage site. The changes (a), (b), and (c) have been observed in the H5/H7 HPAI viruses, whereas (d) has only been observed in H7 HPAI viruses (Suarez et al. 2004; Pasick et al. 2005; Maurer-Stroh et al. 2013; Swayne et al. 2020).
As illustrated in Table 1, the length of the multibasic cleavage site varies between different HPAI viruses within or between different outbreaks. Analysis of naturally occurring H5 HPAI viruses shows a preference of the cleavage site to harbor two additional basic amino acids residues for a total of four basic amino acids (Table 1), but additional basic amino acids do not increase the pathogenicity in chickens (Alexander et al. 1986; Suarez 2016). Experiments by reverse genetics confirmed that five or more basic amino acids in the cleavage site are preferentially selected with high viral fitness in chicken over those with fewer insertions (Luczo et al. 2018). Some studies suggest that the potential role of the polymerase slippage facilitates the incorporation of additional basic residues into the HA cleavage site (Perdue et al. 1997; Abolnik 2017; Nao et al. 2017). Other studies indicate that increased spacing in the cleavage site loop appears to play an important role in the virulence instead of just adding basic amino acids (Suarez 2016). Furthermore, in silico analysis suggests that potential RNA secondary structures in the cleavage site regions of HA segments, having conserved stem-loop structures with cleavage site codons in the hairpin loops, may facilitate evolution toward an multibasic cleavage site, although future experimental studies should be done to clarify the structure–function relationships in these domains for H5 and H7 subtypes compared to other subtypes that have not had LPAI to HPAI phenotype changes (Gultyaev et al. 2019). Interestingly, reverse genetic approaches show that the virus loses the ability to cause severe disease when the multibasic antigenic site motif or specific basic residues of HPAI viruses are removed (Kawaoka and Webster 1988; Walker and Kawaoka 1993; Horimoto and Kawaoka 1994, 1997; Walker et al. 1994; Lu et al. 2006; Bogs et al. 2010; Gohrbandt et al. 2011). In general, the mechanisms for amino acid insertions in the cleavage site appear to be different in many cases, and for this reason the genetic basis to predict which LPAI viruses will result in the emergence of a HPAI virus remains unclear.
Other important mechanisms that alter the AI virus phenotype include the presence or absence of N-linked glycans and changes in histidine 184 residue or the adjacent E216(R/K) in HA. The glycosylation sites, especially in the HA stalk, can influence the protease accessibility to the cleavage site. Apart from the important glycosylation site at the position 10–12 (Asn-Asn-Ser) in the HA of the H5N2 HPAIV outbreak in Pennsylvania in 1983 (Kawaoka et al. 1984), other potential glycosylation sites have been demonstrated to affect the AI viruses’ pathogenicity (Kawaoka and Webster 1989; Hulse et al. 2004; Zhang et al. 2015). The glycosylation sites in the HA are also shown to contribute to the virus replication and chicken-to-chicken transmission, which consequently affect the AI virus virulence (Abdelwhab et al. 2016; Swayne et al. 2020). The presence of critical key histidine residues in the HA can affect the conformational changes postcleavage. Reports show that mutations at or close to histidine 184 at the HA1–HA1 interface modulate the pH dependence and consequently the HA conformation (Mair et al. 2014). Alignments of the HA sequences from H5N1 HPAI viruses show that a Glu-to-Arg mutation at position 216 close to His184 can possibly contribute to AI virus adaptation to hosts (Mair et al. 2014). Other studies demonstrated that mutations altering the pH of fusion modulate H5N1 virus pathogenicity in avian and mammalian host (Reed et al. 2010; DuBois et al. 2011; Zaraket et al. 2013a,b; Russell 2014).
Although the HA is the major determinant of virulence in AI viruses, the modulation of pathogenesis from LPAI to HPAI virus in domestic chickens may involve multiples genes. Comparison of the genetic changes between LPAI virus precursors and HPAI viruses in outbreaks between 1966 and 2016 show that, even though mutations were observed in other genes, changes mainly occur in the HA and polymerase (e.g., PA) gene (Richard et al. 2017). The number of changes varies from 7 to 68 substitutions without any association as a prerequisite of HPAI virus emergence (Richard et al. 2017). It is still unknown if the changes naturally observed in the evolution from LPAI to HPAI viruses in the field are only a selection of viruses adapted to replicate in several tissues. Most of the time, specific selection procedures (i.e., virus isolation without exogenous trypsin, etc.) are necessary to recover HPAI virus, which likely represent minority variants present in the field. Moreover, experimental studies show that mutation in the carboxy terminal at the nonstructural 1 (NS1) protein and neuraminidase (NA) stalk deletion during the change from LPAI to HPAI virus may contribute to virulence, increasing virus shedding and poultry adaptation (Senne et al. 1996; Keiner et al. 2010; Munier et al. 2010).
Therefore, besides all the available data, further studies are still necessary to better understand the modulation of virulence from LPAI to HPAI virus.
UNIQUE GENETIC LINEAGES ASSOCIATED WITH AVIAN HPAI OUTBREAKS
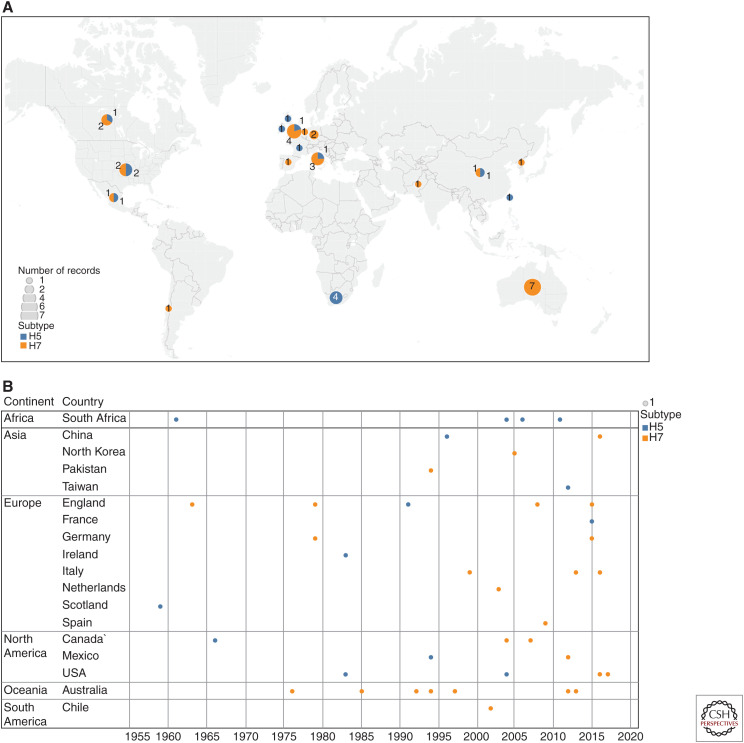
Table 1 lists the summaries and references of HPAI outbreaks between 1959 and 2017 based on epidemiological data, peer-reviewed publication, and published OIE reports. As of August 2019, a total of 42 independent H5 and H7 LPAI to HPAI conversion events have been documented since the discovery of AI viruses as the cause of fowl plague in 1955 (i.e., HPAI [Swayne 2017; Dhingra et al. 2018; Swayne et al. 2020]). More specifically, for these 42 conversion events, 27 were H7 subtype (11 in Europe, seven in Oceania, five in North America, three in Asia, and one in South America) and 15 were H5 subtype (five in Europe, four in Africa, four in North America, and two in Asia) (Fig. 1). The highest number of conversions were documented in Europe (n = 16), followed by North America (n = 9), Oceania (n = 7), Asia (n = 5), Africa (n = 4), and South America (n = 1). Among these 42 conversions, 40 outbreaks involved domestic poultry (principally chickens and turkeys), the South Africa outbreak (1961) involved exclusively wild birds, and the outbreak of Gs/GD lineage of H5 (1996–present) involved both domestic poultry and wild birds.
Figure 1.
Geographic origin of H5 and H7 high-pathogenicity avian influenza viruses by country from 1959 to 2019. (A) Map based on average of longitude and average of latitude. Color shows details about subtype. Size shows sum of number of records. The marks are labeled by sum of number of records. Details are shown for each country. (B) Year for each country broken down by continent. Color shows details about subtype. Size shows number of records.
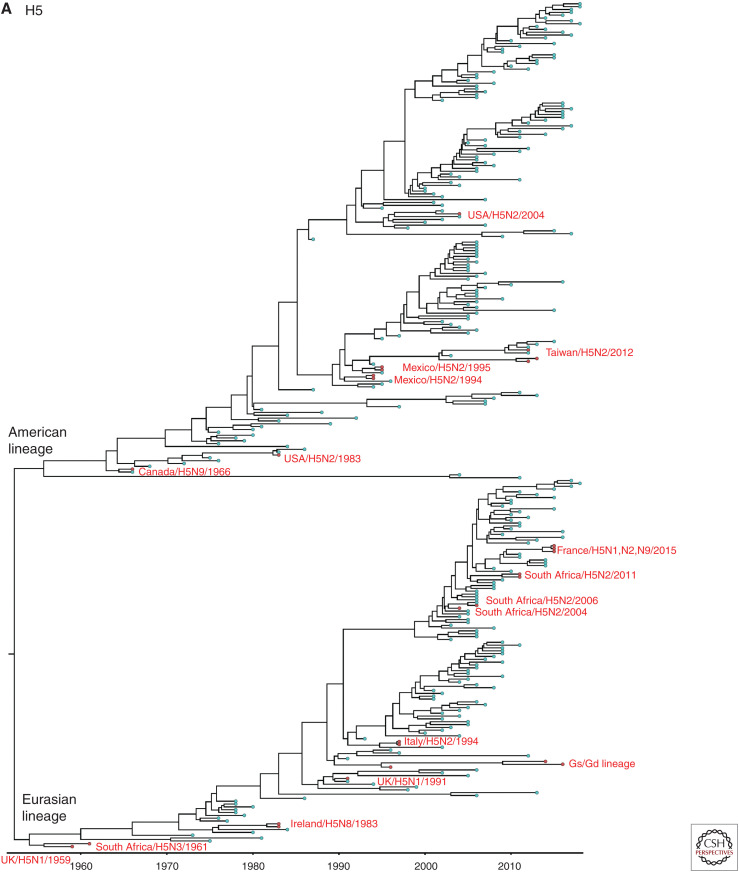
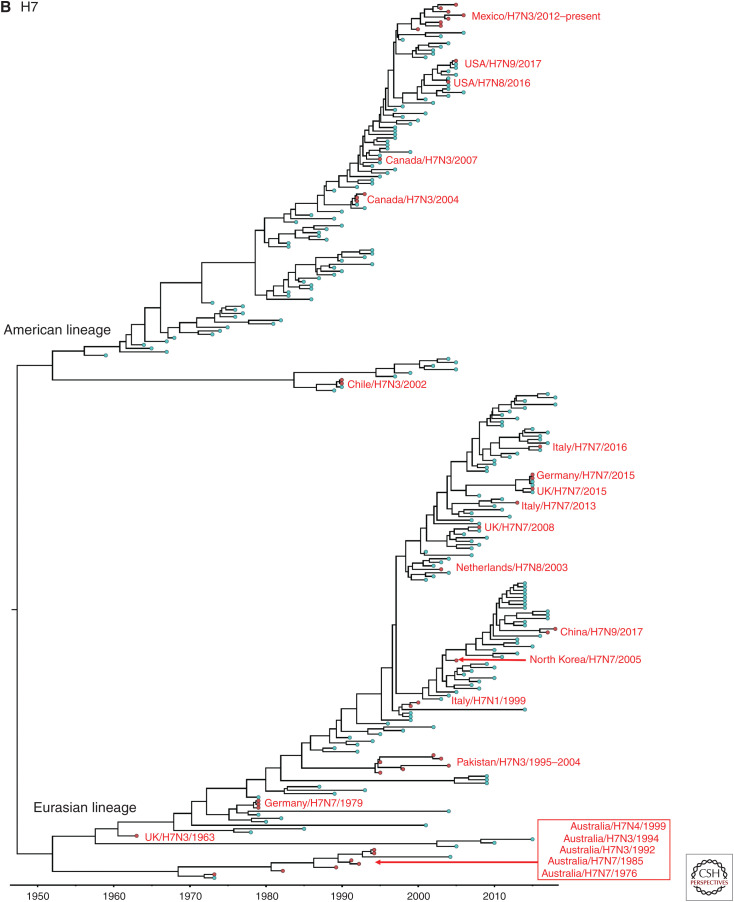
Genome sequencing data has been used to determine the pathogenicity based on the genetic sequences of the HA cleavage site and to trace the origin of viruses using a molecular epidemiological approach. Particularly, phylogenetic analysis and the use of direct sequence comparisons between isolates have provided important clues about the ancestry of outbreak strains. We were able to obtain all HA gene sequences of LPAI to HPAI conversions events, except three H7 outbreaks: 1979 H7N7 outbreak in England, and 2012 and 2013 H7N7 outbreaks in Australia. All available recorded conversions of the H5 and H7 HPAI viruses since 1959 were phylogenetically analyzed. All H7 and non-Gs/GD H5 nucleotide sequences from avian species were downloaded from the Influenza Research Database (www.fludb.org). We then removed identical nucleotide sequences with ≥98% (H7) and 98.5% (non Gs/Gs H5) identity using the Cluster Database at High Identity with Tolerance (CD-HIT) (Li and Godzik 2006), resulting in representative strains that form the final set of nonredundant sequences. Representative Gs/GD H5 nucleotide sequences were added in the H5 data set. Sequences (H5: n = 298, H7: n = 250) were aligned using multiple alignment with fast Fourier transformation (MAFFT) in the Geneious v8.1.2 program and trimmed to remove nucleotides that were outside the coding region. The Bayesian relaxed clock phylogenetic analysis of the HA gene was conducted using BEAST v1.10.4. with an uncorrelated lognormal distribution relaxed clock method, the HKY nucleotide substitution model and the Gaussian Markov random field (GMRF) Bayesian skyride coalescent prior (Minin et al. 2008). All 39 documented HPAI outbreaks were phylogenetically different from each other (Fig. 2A,B). Each branch of documented HPAI virus reflects the independent conversion from a LPAI ancestor to HPAI virus.
Figure 2.
Bayesian relaxed clock phylogenetic analysis of the H5 (A) and H7 (B) HA genes for 39 high-pathogenicity avian influenza viruses that emerged since 1959.
In Europe, the emergence of HPAI viruses was recorded on 16 occasions in England (n = 5; H5 in 1991, H7 in 1963, 1979, 2008, 2015), Italy (n = 4; H5 in 1997, H7 in 1999, 2013, 2016), Germany (n = 2; H7 in 1979, 2015), Scotland (n = 1; H5 in 1959), Ireland (n = 1; H5 in 1983), Netherland (n = 1; H7 in 2003), Spain (n = 1; H7 in 2009), and France (n = 1; H5 in 2015) between 1959 and 2015. It has been suggested that the two outbreaks in 1979, in Germany and England, may have represented a single emergence of H7N7 HPAI virus that was spread by wild birds (Alexander and Brown 2009). It was not possible to genetically compare the two outbreaks in Germany and England during 1979 because the genetic sequence of 1979 H7N7 viruses in England was not available in public databases. The phylogenetic tree in a previous study suggested that they have emerged from a same close LPAI common ancestor (Banks and Plowright 2003). However, the HA cleavage sites of the Germany and England viruses have a slightly different multibasic cleavage site as shown in Table 1. These findings suggest that these viruses were converted from LPAI to HPAI viruses independently from the same close common ancestor circulating in Western Europe during 1979.
In North America, the emergence of HPAI viruses was recorded on nine occasions during 1966–2017, including the United States (n = 4; H5 in 1983, 2004; H7 in 2016, 2017), Canada (n = 3; H5 in 1966; H7 in 2004, 2007), and Mexico (n = 2; H5 in 1994, H7 in 2012). On the other hand, there was only one occasion in South America (Chile, H7 in 2002). All of these HPAI outbreaks were eradicated from poultry, except the Mexican HPAI H7N3 outbreak. The Mexican HPAI H7N3 has continued to cause outbreaks in commercial chickens in Mexico since the first case in 2012 (OIE 2019a).
In Oceania, only Australia has had seven emergences of HPAI in poultry during 1976–2013, all of them the H7 subtype (H7N7 in 1976 and 1985, H7N3 in 1992 and 1994, H7N4 in 1997, H7N7 in 2012, and H7N2 in 2013). In addition, the devastating Gs/GD H5Nx HPAI viruses have not been detected in Oceania. All H7 HPAI strains identified in Australia belong to the Australian lineage (Fig. 2). The two recent outbreaks in Australia were not included in the phylogenetic analysis because the HA gene sequences of the 2012 H7N7 and 2013 H7N2 strains were not available in public databases. It has been documented that H7 LPAI viruses similar to that found in the H7N7 outbreak in 2012 and the H7N2 outbreak in 2013 were unrelated samples from Australian wild waterfowl that have been circulating in Australia for many years (Wong and Daniels 2013).
In Asia, a total of five conversions have been recorded, including China (n = 2; Gs/GD H5N1 in 1996 and H7N9 in 2016), Pakistan (n = 1; H7N3 in 1994), North Korea (n = 1; H7N7 in 2005), and Taiwan (n = 1; H5N2 in 2012). Phylogenetic analysis shows that all HPAI strains that were identified in Asia emerged from Eurasian LPAI viruses, except the H5N2 HPAI identified in Taiwan during 2012, which clustered with Mexican H5N2 viruses (Fig. 2). The high similarity of the Taiwanese H5N2 viruses to the Mexican H5N2 vaccine strain suggests that the Mexican H5N2 viruses might have been introduced to Taiwan by using inadequately inactivated or attenuated Mexican H5N2 vaccines (Lee et al. 2014).
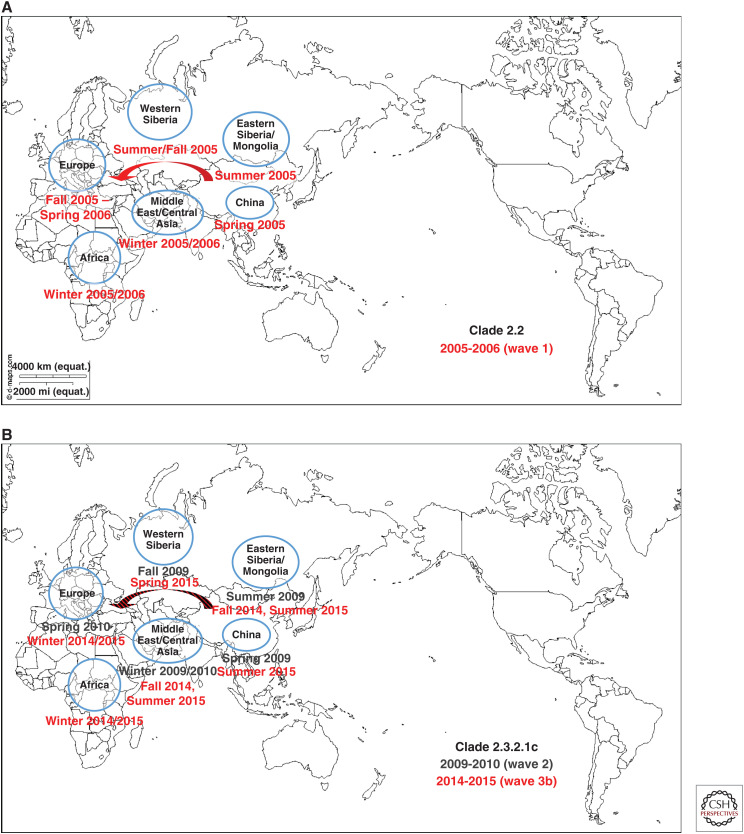
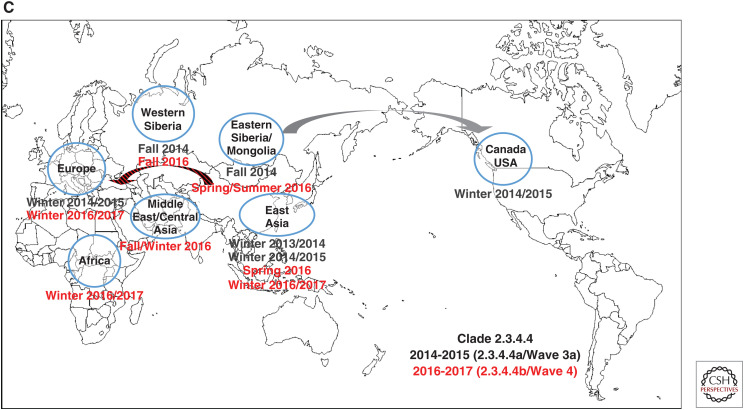
Unlike most previous HPAI virus epizootics that have been geographically limited, involving sporadic interfarm transmissions, and were eradicated from poultry by stamping-out programs, the Gs/GD lineage has caused deaths in wild birds, poultry, and mammals including humans and has spread to 84 countries in Asia, Europe, Africa, and North America as of August 2019. The first Gs/GD lineage H5N1 HPAI virus was identified in a domestic goose in southern Guangdong province of China in 1996 and this lineage has evolved into 10 genetically distinct virus clades (0–9) and multiple subclades (WHO/OIE/FAO_H5N1_Evolution_Working_Group 2008, 2014). There have been four waves of intercontinental transmission of Gs/GD lineage H5Nx virus (Figs. 3A–C; Sims et al. 2017). Briefly, the first intercontinental wave in 2005–2006 caused by a clade 2.2 H5N1 HPAI virus involved China, Mongolia, Russia, Central Asia, Europe (widespread: over 20 European countries), Middle East, West Africa, Japan, and Republic of Korea (Fig. 3A). The second intercontinental wave in 2009–2010 caused by a clade 2.3.2.1c H5N1 HPAI virus involved mostly East Asia (China, Mongolia, Japan, and Republic of Korea), but was also found in Eastern Europe (Russia, Romania, and Bulgaria) and Nepal (Fig. 3B). The third intercontinental wave in 2014–2015 involved two separate viral lineages, clade 2.3.4.4a H5Nx (wave 3a) (Fig. 3C) and clade 2.3.2.1c H5N1 that differed from the 2009–2010 isolate (wave 3b) (Fig. 3B). The wave 3a was caused by a clade 2.3.4.4a H5N8 HPAI virus originated from East Asia that had spread rapidly and globally through wild birds and evolved through reassortment with prevailing local LPAI viruses to produce multiple H5Nx viruses. The wave 3b caused by a clade 2.3.2.1c H5N1 involved Russia, China, Middle East, West Africa, Cameroon, Romania, Bulgaria, and Central Asia. The fourth intercontinental wave in 2016–2017 caused by a clade 2.3.4.4b H5Nx involved Asia (East, Central, and South), Middle East, Europe (widespread), and Africa (West Central, East, and Southern) (Fig. 3C). The Gs/GD lineage has not been eradicated and still poses a serious threat to the poultry industry and public health as well. Additionally, LPAI H7N9 viruses have been a threat to public health since their emergence in 2013 in China (see Chen 2019). It has mutated into HPAI and caused human infections and outbreaks among poultry since 2016 (Yang et al. 2017).
Figure 3.
Four waves of intercontinental transmission of Gs/GD lineage H5Nx virus: (A) Clade 2.2, 2005-2006, wave 1. (B) Clade 2.3.2.1c, 2009-2010, wave 2; Clade 2.3.2.1.c, 2014-2015, wave 3b. (C) Clade 2.3.4.4a, 2014-2015, wave 3a; Clade 2.3.4.4b, 2016-2017, wave 4. (Maps modified, with permission, from https://d-maps.com/carte.php?num_car=3503&lang=en.)
In Africa, only South Africa has had four emergences of HPAI during 1961–2013, which were caused by viruses of the H5 subtype (H5N3 in 1961 and H5N2 in 2004, 2006, and 2011). The phylogenetic tree suggests that the H5N2 identified in 2004 and 2006 have emerged from the same close LPAI common ancestor. In a previous study, molecular and phylogenetic characterization was performed to determine whether the 2006 outbreak strain was supposedly derived from the eradicated 2004 H5N2 strain (Abolnik 2007). It was demonstrated that although the 2004 and 2006 H5N2 strains shared a common ancestor, the two outbreaks were not related. Not only were extensive reassortments with wild bird viruses involved in the evolution of the 2006 strains, but also the HA cleavage site sequence of the 2006 H5N2 virus contained fewer monobasic amino acid insertions (Abolnik 2007).
CONCLUDING REMARKS
Historically, the first AI virus in birds was identified in the 1880s as an HPAI virus (i.e., fowl plague virus). Forty-two documented epidemics or limited outbreaks of HPAI have occurred since the discovery of AI viruses as the cause of fowl plague in 1955. Since 1959 with well-maintained virus archives, most HPAI virus have arisen from changes in LPAI viruses with limited spread of outbreaks and eradication from poultry. However, the Gs/GD H5Nx, Mexican H7N3, and Chinese H7N9 HPAI viruses are concerning because these strains have not been eradicated and still cause poultry outbreaks. In particular, the Gs/GD H5Nx and Chinese H7N9 viruses pose a significant pandemic threat to human health. The Gs/GD lineage of H5 has a broad One Health impact as it continues to cause infections, disease, and death in poultry, wild birds, and humans. Furthermore, the Gs/GD lineage outbreak has affected more poultry and countries than the other 41 HPAI outbreaks combined.
Although chicken HPAI viruses are derived from LPAI viruses of aquatic wild birds, the emergence of HPAI virus does not always happen. The mechanisms by which H5/H7 HPAI virus has emerged is via mutation, insertion, or recombination within the HA—more specifically changes in the proteolytic cleavage site. The number of basic amino acids in the HA cleavage site plays a critical role in virulence determining which proteases can cleave HA and in which tissues the AI viruses can replicate. In general, the mechanisms for amino acid insertions in the cleavage site appear to be different in many cases, and for this reason the genetic basis to predict which LPAI viruses will result in the emergence of a HPAI virus remains unclear. The presence or absence of the N-linked glycans and key histidine residues in the HA are reported to influence the AI virus phenotype. In addition, changes in other AIV gene segments can increase or decrease the maximal phenotypic expression of the HA usually through increasing replication efficiency and release. In general, molecular techniques such as next-generation sequencing and reverse genetics have helped to identify changes associated with high virulence. However, significant emphasis on basic research is needed to understand why changes occur only in certain LPAI precursors that increase their pathogenicity, which, once understood, could enhance our ability to predict and subsequently prevent conversion events and potentially lead to a reduced number of HPAI outbreaks. With the ultimate goal of eradication, enhanced active surveillance, education, biosecurity, rapid molecular, and characterization efforts will provide the best opportunity for early detection and elimination of HPAI in domestic poultry.
ACKNOWLEDGMENTS
The authors acknowledge Jung-Hoon Kwon for technical assistance.
This article has been made freely available online courtesy of TAUNS Laboratories.
Footnotes
Editors: Gabriele Neumann and Yoshihiro Kawaoka
Additional Perspectives on Influenza: The Cutting Edge available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Abdelwhab EM, Veits J, Tauscher K, Ziller M, Grund C, Hassan MK, Shaheen M, Harder TC, Teifke J, Stech J, et al. 2016. Progressive glycosylation of the haemagglutinin of avian influenza H5N1 modulates virus replication, virulence and chicken-to-chicken transmission without significant impact on antigenic drift. J Gen Virol 97: 3193–3204. 10.1099/jgv.0.000648 [DOI] [PubMed] [Google Scholar]
- Abolnik C. 2007. Molecular characterization of H5N2 avian influenza viruses isolated from South African ostriches in 2006. Avian Dis 51: 873–879. 10.1637/7953-022107-REGR.1 [DOI] [PubMed] [Google Scholar]
- Abolnik C. 2017. Evolution of H5 highly pathogenic avian influenza: sequence data indicate stepwise changes in the cleavage site. Arch Virol 162: 2219–2230. 10.1007/s00705-017-3337-x [DOI] [PubMed] [Google Scholar]
- Abolnik C, Londt BZ, Manvell RJ, Shell W, Banks J, Gerdes GH, Akol G, Brown IH. 2009. Characterisation of a highly pathogenic influenza A virus of subtype H5N2 isolated from ostriches in South Africa in 2004. Influenza Other Respir Viruses 3: 63–68. 10.1111/j.1750-2659.2009.00074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DJ. 2000. The history of avian influenza in poultry. World Poultry Nov: 7–8. [Google Scholar]
- Alexander DJ, Brown IH. 2009. History of highly pathogenic avian influenza. Rev Sci Tech 28: 19–38. 10.20506/rst.28.1.1856. [DOI] [PubMed] [Google Scholar]
- Alexander DJ, Wood GW. 1993. Highly pathogenic avian influenza diagnosis: past experience, and future problems. In Proceedings of the European Commission Meeting on Virus Diseases of Poultry—New and Evolving Pathogens, Brussels, 1992, pp. 3–13. CEC, Brussels. [Google Scholar]
- Alexander DJ, Parsons G, Manvell RJ. 1986. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol 15: 647–662. 10.1080/03079458608436328 [DOI] [PubMed] [Google Scholar]
- Alexander DJ, Lister SA, Johnson MJ, Randall CJ, Thomas PJ. 1993. An outbreak of highly pathogenic avian influenza in turkeys in Great Britain in 1991. Vet Rec 132: 535–536. 10.1136/vr.132.21.535 [DOI] [PubMed] [Google Scholar]
- Alexander DJ, Capua I, Koch G. 2008. Highly pathogenic avian influenza outbreaks in Europe, Africa and Asia since 1959, excluding the Asian H5N1 virus outbreaks. In Avian influenza (ed. Swayne DE), pp. 217–237. Blackwell Publishing, Ames, Iowa. [Google Scholar]
- Anonymous. 1976. The outbreak of fowl plague in Victoria. In Annual report, pp. 4–6. Division of Animal Health, Department of Agriculture, Victoria. [Google Scholar]
- Banks J, Plowright L. 2003. Additional glycosylation at the receptor binding site of the hemagglutinin (HA) for H5 and H7 viruses may be an adaptation to poultry hosts, but does it influence pathogenicity? Avian Dis 47: 942–950. 10.1637/0005-2086-47.s3.942 [DOI] [PubMed] [Google Scholar]
- Banks J, Speidel EC, McCauley JW, Alexander DJ. 2000. Phylogenetic analysis of H7 haemagglutinin subtype influenza A viruses. Arch Virol 145: 1047–1058. 10.1007/s007050050695 [DOI] [PubMed] [Google Scholar]
- Banks J, Speidel ES, Moore E, Plowright L, Piccirillo A, Capua I, Cordioli P, Fioretti A, Alexander DJ. 2001. Changes in the haemagglutinin and the neuraminidase genes prior to the emergence of highly pathogenic H7N1 avian influenza viruses in Italy. Arch Virol 146: 963–973. 10.1007/s007050170128 [DOI] [PubMed] [Google Scholar]
- Barr DA, Kelly AP, Badman RT, Campey AR, O'Rourke MD, Grix DC, Reece RL. 1986. Avian influenza on a multi-age chicken farm. Aust Vet J 63: 195–196. 10.1111/j.1751-0813.1986.tb02976.x [DOI] [PubMed] [Google Scholar]
- Bashiruddin JB, Gould AR, Westbury HA. 1992. Molecular pathotyping of two avian influenza viruses isolated during the Victoria 1976 outbreak. Aust Vet J 69: 140–142. 10.1111/j.1751-0813.1992.tb07485.x [DOI] [PubMed] [Google Scholar]
- Becker WB. 1966. The isolation and classification of Tern virus: influenza A-Tern South Africa—1961. J Hyg (Lond) 64: 309–320. 10.1017/S0022172400040596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhane Y, Hisanaga T, Kehler H, Neufeld J, Manning L, Argue C, Handel K, Hooper-McGrevy K, Jonas M, Robinson J, et al. 2009. Highly pathogenic avian influenza virus a (H7N3) in domestic poultry, Saskatchewan, Canada, 2007. Emerg Infect Dis 15: 1492–1495. 10.3201/eid1509.080231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogs J, Veits J, Gohrbandt S, Hundt J, Stech O, Breithaupt A, Teifke JP, Mettenleiter TC, Stech J. 2010. Highly pathogenic H5N1 influenza viruses carry virulence determinants beyond the polybasic hemagglutinin cleavage site. PLoS ONE 5: e11826 10.1371/journal.pone.0011826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch FX, Orlich M, Klenk HD, Rott R. 1979. The structure of the hemagglutinin, a determinant for the pathogenicity of influenza viruses. Virology 95: 197–207. 10.1016/0042-6822(79)90414-8 [DOI] [PubMed] [Google Scholar]
- Bosch FX, Garten W, Klenk HD, Rott R. 1981. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of Avian influenza viruses. Virology 113: 725–735. 10.1016/0042-6822(81)90201-4 [DOI] [PubMed] [Google Scholar]
- Briand FX, Schmitz A, Ogor K, Le Prioux A, Guillou-Cloarec C, Guillemoto C, Allée C, Le Bras MO, Hirchaud E, Quenault H, et al. 2017. Emerging highly pathogenic H5 avian influenza viruses in France during winter 2015/16: phylogenetic analyses and markers for zoonotic potential. Euro Surveill 22 10.2807/1560-7917.ES.2017.22.9.30473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I, Abolnik C, Garcia-Garcia J, McCullough S, Swayne DE, Cattoli G. 2017. High-pathogenicity avian influenza outbreaks since 2008, excluding multi-continental panzootic of H5 Goose/Guangdong-lineage viruses. In Animal influenza (ed. Swayne DE), pp. 248–270. Wiley-Blackwell, Ames, IA [Google Scholar]
- Capua I, Marangon S, Selli L, Alexander DJ, Swayne DE, Pozza MD, Parenti E, Cancellotti FM. 1999. Outbreaks of highly pathogenic avian influenza (H5N2) in Italy during October 1997 to January 1998. Avian Pathol 28: 455–460. 10.1080/03079459994470 [DOI] [PubMed] [Google Scholar]
- Capua I, Marangon S, Cancellotti FM. 2003. The 1999-2000 avian influenza (H7N1) epidemic in Italy. Vet Res Commun 27: 123–127. 10.1023/B:VERC.0000014128.68876.31 [DOI] [PubMed] [Google Scholar]
- *.Chen H. 2019. H7N9 viruses. Cold Spring Harb Perspect Med 10.1101/cshperspect.a038349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross GM. 1987. The status of avian influenza in poultry in Australia. In Proceedings of the Second International Symposium on Avian Influenza (ed. Easterday BC), pp. 96–103. U.S. Animal Health Association, Richmond, VA. [Google Scholar]
- Dhingra MS, Artois J, Dellicour S, Lemey P, Dauphin G, Von Dobschuetz S, Van Boeckel TP, Castellan DM, Morzaria S, Gilbert M. 2018. Geographical and historical patterns in the emergences of novel highly pathogenic avian influenza (HPAI) H5 and H7 viruses in poultry. Front Vet Sci 5: 84 10.3389/fvets.2018.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie JC, Laver WG. 1973. Isolation of a type A influenza virus from an Australian pelagic bird. Virology 51: 259–269. 10.1016/0042-6822(73)90426-1 [DOI] [PubMed] [Google Scholar]
- DuBois RM, Zaraket H, Reddivari M, Heath RJ, White SW, Russell CJ. 2011. Acid stability of the hemagglutinin protein regulates H5N1 influenza virus pathogenicity. PLoS Pathog 7: e1002398 10.1371/journal.ppat.1002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterday BC, Trainer DO, Tůmová B, Pereira HG. 1968. Evidence of infection with influenza viruses in migratory waterfowl. Nature 219: 523–524. 10.1038/219523a0 [DOI] [PubMed] [Google Scholar]
- Easterday BC, Hinshaw VS, Halvorson DA. 1997. Influenza. In Diseases of poultry (ed. Calnek BW, Barnes HJ, Beard CW, et al. ), pp. 583–605. Iowa State University Press, Ames, IA. [Google Scholar]
- Eckroade RJ, Silverman-Bachin LA. 1986. Avian influenza in Pennsylvania. The beginning. In Proceedings of the Second International Symposium on Avian Influenza (ed. Easterday BC), pp. 22–32. U.S. Animal Health Association, Richmond, VA. [Google Scholar]
- Elbers ARW, Fabri THF, de Vries TS, de Wit JJ, Pijpers A, Koch G. 2004. The highly pathogenic avian influenza A (H7N7) virus epidemic in The Netherlands in 2003—lessons learned from the first five outbreaks. Avian Dis 48: 691–705. 10.1637/7149 [DOI] [PubMed] [Google Scholar]
- FAO. 2006. Summary of confirmed HPAI outbreaks in affected countries. FAO AIDE News—AI Bulletin 41: 9–10. [Google Scholar]
- García M, Crawford JM, Latimer JW, Rivera-Cruz MVZE, Perdue ML. 1996. Heterogeneity in the haemagglutinin gene and emergence of the highly pathogenic phenotype among recent H5N2 avian influenza viruses from Mexico. J Gen Virol 77: 1493–1504. 10.1099/0022-1317-77-7-1493 [DOI] [PubMed] [Google Scholar]
- Garten W, Klenk HD. 1983. Characterization of the carboxypeptidase involved in the proteolytic cleavage of the influenza haemagglutinin. J Gen Virol 64: 2127–2137. 10.1099/0022-1317-64-10-2127 [DOI] [PubMed] [Google Scholar]
- Gohrbandt S, Veits J, Hundt J, Bogs J, Breithaupt A, Teifke JP, Weber S, Mettenleiter TC, Stech J. 2011. Amino acids adjacent to the haemagglutinin cleavage site are relevant for virulence of avian influenza viruses of subtype H5. J Gen Virol 92: 51–59. 10.1099/vir.0.023887-0 [DOI] [PubMed] [Google Scholar]
- Gultyaev AP, Richard M, Spronken MI, Olsthoorn RCL, Fouchier RAM. 2019. Conserved structural RNA domains in regions coding for cleavage site motifs in hemagglutinin genes of influenza viruses. Virus Evol 5: vez034 10.1093/ve/vez034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder TC, Werner O. 2006. Avian influenza. In Influenza report (ed. Kamps BS, Hoffmann C, Preiser W). http://www.influenzareport.com/ir/ai.htm
- Hirst M, Astell CR, Griffith M, Coughlin SM, Moksa M, Zeng T, Smailus DE, Holt RA, Jones S, Marra MA, et al. 2004. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg Infect Dis 10: 2192–2195. 10.3201/eid1012.040743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto T, Kawaoka Y. 1994. Reverse genetics provides direct evidence for a correlation of hemagglutinin cleavability and virulence of an avian influenza A virus. J Virol 68: 3120–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto T, Kawaoka Y. 1997. Biologic effects of introducing additional basic amino acid residues into the hemagglutinin cleavage site of a virulent avian influenza virus. Virus Res 50: 35–40. 10.1016/S0168-1702(97)00050-6 [DOI] [PubMed] [Google Scholar]
- Hulse DJ, Webster RG, Russell RJ, Perez DR. 2004. Molecular determinants within the surface proteins involved in the pathogenicity of H5N1 influenza viruses in chickens. J Virol 78: 9954–9964. 10.1128/JVI.78.18.9954-9964.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias I, Martínez M, Muñoz MJ, de la Torre A, Sánchez-Vizcaíno JM. 2010. First case of highly pathogenic avian influenza in poultry in Spain. Transbound Emerg Dis 57: 282–285. 10.1111/j.1865-1682.2010.01145.x [DOI] [PubMed] [Google Scholar]
- ISBOAH. 2016. Highly pathogenic avian influenza. Indiana State Board of Animal Health; www.gov/boah/2390.htm. [Google Scholar]
- Kaleta EF, Rulke CPA. 2008. The beginning and spread of fowl plague (H7 high pathogenicity avian influenza) across Europe and Asia (1878–1955). In Avian influenza (ed. Swayne DE), pp. 145–189. Blackwell Publishers, Ames, IA. [Google Scholar]
- Kawaoka Y, Webster RG. 1985. Evolution of the A/Chicken/Pennsylvania/83 (H5N2) influenza virus. Virology 146: 130–137. 10.1016/0042-6822(85)90059-5 [DOI] [PubMed] [Google Scholar]
- Kawaoka Y, Webster RG. 1988. Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci 85: 324–328. 10.1073/pnas.85.2.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y, Webster RG. 1989. Interplay between carbohydrate in the stalk and the length of the connecting peptide determines the cleavability of influenza virus hemagglutinin. J Virol 63: 3296–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y, Naeve CW, Webster RG. 1984. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology 139: 303–316. 10.1016/0042-6822(84)90376-3 [DOI] [PubMed] [Google Scholar]
- Kawaoka Y, Nestorowicz A, Alexander DJ, Webster RG. 1987. Molecular analyses of the hemagglutinin genes of H5 influenza viruses: origin of a virulent turkey strain. Virology 158: 218–227. 10.1016/0042-6822(87)90256-X [DOI] [PubMed] [Google Scholar]
- Keiner B, Maenz B, Wagner R, Cattoli G, Capua I, Klenk HD. 2010. Intracellular distribution of NS1 correlates with the infectivity and interferon antagonism of an avian influenza virus (H7N1). J Virol 84: 11858–11865. 10.1128/JVI.01011-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G, Narayan O, Rouse BT, Ferguson AE, Connell MC. 1968. A new influenza A virus infection in turkeys II. A highly pathogenic variant, A/turkey/Ontario/7732/66. Can Vet J 9: 151–160. [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Swayne DE, Linares JA, Senne DA, Suarez DL. 2005. H5N2 avian influenza outbreak in Texas in 2004: the first highly pathogenic strain in the United States in 20 years? J Virol 79: 3692–3702. 10.1128/JVI.79.17.11412-11421.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Zhu H, Huang PY, Peng L, Chang YC, Yip CH, Li YT, Cheung CL, Compans R, Yang C, et al. 2014. Emergence and evolution of avian H5N2 influenza viruses in chickens in Taiwan. J Virol 88: 5677–5686. 10.1128/JVI.00139-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Torchetti MK, Killian ML, Berhane Y, Swayne DE. 2017a. Highly pathogenic avian influenza A(H7N9) virus, Tennessee, USA, March 2017. Emerg Infect Dis 23: 1860–1863. 10.3201/eid2311.171013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Torchetti MK, Killian ML, Swayne DE. 2017b. Deep sequencing of H7N8 avian influenza viruses from surveillance zone supports H7N8 high pathogenicity avian influenza was limited to a single outbreak farm in Indiana during 2016. Virology 507: 216–219. 10.1016/j.virol.2017.04.025 [DOI] [PubMed] [Google Scholar]
- Li W, Godzik A. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22: 1658–1659. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- Lu JH, Long JX, Jia LJ, Liu YL, Shao WX, Zhang YM, Liu XF. 2006. Reassortment and modification of hemagglutinin cleavage motif of avian/WSN influenza viruses generated by reverse genetics that correlate with attenuation. Acta Virol 50: 243–249. [PubMed] [Google Scholar]
- Luczo JM, Tachedjian M, Harper JA, Payne JS, Butler JM, Sapats SI, Lowther SL, Michalski WP, Stambas J, Bingham J. 2018. Evolution of high pathogenicity of H5 avian influenza virus: haemagglutinin cleavage site selection of reverse-genetics mutants during passage in chickens. Sci Rep 8: 11518 10.1038/s41598-018-29944-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair CM, Meyer T, Schneider K, Huang Q, Veit M, Herrmann A. 2014. A histidine residue of the influenza virus hemagglutinin controls the pH dependence of the conformational change mediating membrane fusion. J Virol 88: 13189–13200. 10.1128/JVI.01704-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer-Stroh S, Lee RT, Gunalan V, Eisenhaber F. 2013. The highly pathogenic H7N3 avian influenza strain from July 2012 in Mexico acquired an extended cleavage site through recombination with host 28S rRNA. Virol J 10: e139 10.1186/1743-422X-10-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max VK, Herrera JR, Moreira RZ, Rojas HO. 2007. Avian influenza in Chile: a successful experience. Avian Dis 51: 363–365. 10.1637/7631-042806R1.1 [DOI] [PubMed] [Google Scholar]
- McNulty MS, Allan GM, McCracken RM, McParland PJ. 1985. Isolation of a highly pathogenic influenza virus from turkeys. Avian Pathol 14: 173–176. 10.1080/03079458508436216 [DOI] [PubMed] [Google Scholar]
- Mehle A. 2014. Unusual influenza A viruses in bats. Viruses 6: 3438–3449. 10.3390/v6093438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minin VN, Bloomquist EW, Suchard MA. 2008. Smooth skyride through a rough skyline: Bayesian coalescent-based inference of population dynamics. Mol Biol Evol 25: 1459–1471. 10.1093/molbev/msn090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munier S, Larcher T, Cormier-Aline F, Soubieux D, Su B, Guigand L, Labrosse B, Cherel Y, Quere P, Marc D, et al. 2010. A genetically engineered waterfowl influenza virus with a deletion in the stalk of the neuraminidase has increased virulence for chickens. J Virol 84: 940–952. 10.1128/JVI.01581-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem K. 1998. The avian influenza H7N3 outbreak in South Central Asia. In Proceedings of the Fourth International Symposium on Avian Influenza (ed. Swayne DE, Slemons RD), pp. 31–35. USAHA, Richmond, VA. [Google Scholar]
- Naeem K, Hussain M. 1995. An outbreak of avian influenza in poultry in Pakistan. Vet Rec 137: 439–439. 10.1136/vr.137.17.439 [DOI] [PubMed] [Google Scholar]
- Nao N, Yamagishi J, Miyamoto H, Igarashi M, Manzoor R, Ohnuma A, Tsuda Y, Furuyama W, Shigeno A, Kajihara M, et al. 2017. Genetic predisposition to acquire a polybasic cleavage site for highly pathogenic avian influenza virus hemagglutinin. MBio 8 10.1128/mBio.02298-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OFFLU. 2019. Influenza A cleavage sites. wwwofflunet/fileadmin/home/en/resource-centre/pdf/Influenza_A_Cleavage_Sites.pdf. [Google Scholar]
- OIE. 2002. International animal health code. www.oieint/eng/normes/MCode/A_00003htm. [Google Scholar]
- OIE. 2004. Avian influenza. In International animal health code—2004. OIE, Paris: www.oie.int/eng/normes/mcode/en_chapitre_2.7.12.htm. [Google Scholar]
- OIE. 2016a. Highly pathogenic avian influenza, Italy. Immediate Notification. In OIE disease information. OIE, Paris. [Google Scholar]
- OIE. 2016b. Influenza a cleavage site. www.offlu.net/fileadmin/home/en/resource-centre/pdf/Influenza_A_Cleavage_Sites.pdf OIE. [Google Scholar]
- OIE. 2017a. Highly pathogenic avian influenza, China. Immediate Notification. In OIE disease information. OIE, Paris. [Google Scholar]
- OIE. 2017b. Highly pathogenic avian influenza, United States of America. Immediate Notification. In OIE disease information. OIE, Paris. [Google Scholar]
- OIE. 2019a. Highly pathogenic avian influenza, Mexico. Immediate Notification. In OIE disease information. OIE, Paris. [Google Scholar]
- OIE. 2019b. Influenza A cleavage site. OFFLU-OIE/FAO; http://www.offlu.net/fileadmin/home/en/resource-centre/pdf/Influenza_A_Cleavage_Sites.pdf [Google Scholar]
- Pasick J, Handel K, Robinson J, Copps J, Ridd D, Hills K, Kehler H, Cottam-Birt C, Neufeld J, Berhane Y, et al. 2005. Intersegmental recombination between the haemagglutinin and matrix genes was responsible for the emergence of a highly pathogenic H7N3 avian influenza virus in British Columbia. J Gen Virol 86: 727–731. 10.1099/vir.0.80478-0 [DOI] [PubMed] [Google Scholar]
- Perdue ML, García M, Beck J, Brugh M, Swayne DE. 1996. An Arg-Lys insertion at the hemagglutinin cleavage site of an H5N2 avian influenza isolate. Virus Genes 12: 77–84. 10.1007/BF00370003 [DOI] [PubMed] [Google Scholar]
- Perdue ML, Garcı´a M, Senne D, Fraire M. 1997. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res 49: 173–186. 10.1016/S0168-1702(97)01468-8 [DOI] [PubMed] [Google Scholar]
- Perdue ML, Suarez DL, Swayne DE. 1999. Avian Influenza in the 1990s. Poultry Avian Biol Rev 11: 1–20. [Google Scholar]
- Pereira HG, Tumova B, Law VG. 1965. Avian influenza A viruses. Bull World Health Org 32: 855–860. [PMC free article] [PubMed] [Google Scholar]
- Qi W, Jia W, Liu D, Li J, Bi Y, Xie S, Li B, Hu T, Du Y, Xing L, et al. 2018. Emergence and adaptation of a novel highly pathogenic H7N9 influenza virus in birds and humans from a 2013 human-infecting low-pathogenic ancestor. J Virol 92 10.1128/JVI.00921-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed ML, Bridges OA, Seiler P, Kim JK, Yen HL, Salomon R, Govorkova EA, Webster RG, Russell CJ. 2010. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus pathogenicity and transmissibility in ducks. J Virol 84: 1527–1535. 10.1128/JVI.02069-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Fouchier R, Monne I, Kuiken T. 2017. Mechanisms and risk factors for mutation from low to highly pathogenic avian influenza virus. Eur Food Saf Auth 14: EN–1287. [Google Scholar]
- Röhm C, Horimoto T, Kawaoka Y, Süss J, Webster RG. 1995. Do hemagglutinin genes of highly pathogenic avian influenza viruses constitute unique phylogenetic lineages? Virology 209: 664–670. 10.1006/viro.1995.1301 [DOI] [PubMed] [Google Scholar]
- Röhm C, Süss J, Pohle V, Webster RG. 1996. Different hemagglutinin cleavage site variants of H7N7 in an influenza outbreak in chickens in Leipzig, Germany. Virology 218: 253–257. 10.1006/viro.1996.0187 [DOI] [PubMed] [Google Scholar]
- Rojas H, Moreira R, Avalos P, Capua I, Marangon S. 2002. Avian influenza in poultry in Chile. Vet Rec 151: 188. [PubMed] [Google Scholar]
- Rowan MK. 1962. Mass mortality among European Common Terns in South Africa in April–May 1961. British Birds 55: 103–114. [Google Scholar]
- Russell CJ. 2014. Acid-induced membrane fusion by the hemagglutinin protein and its role in influenza virus biology. Curr Top Microbiol Immunol 385: 93–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleck PW, Gleeson LJ, Hooper PT, Westbury HA, Hansson E. 1997. Identification and characterisation of an H7N3 influenza A virus from an outbreak of virulent avian influenza in Victoria. Aust Vet J 75: 289–292. 10.1111/j.1751-0813.1997.tb10099.x [DOI] [PubMed] [Google Scholar]
- Senne DA, Panigrahy B, Kawaoka Y, Pearson JE, Suss J, Lipkind M, Kida H, Webster RG. 1996. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis 40: 425–437. 10.2307/1592241 [DOI] [PubMed] [Google Scholar]
- Sims LD, Brown IH. 2008a. Multi-continental epidemic of H5N1 high pathogenicity avian influenza (1996–2007). In Avian influenza (ed. Swayne DE), pp. 251–286. Blackwell, Ames, IA. [Google Scholar]
- Sims LD, Turner AJ. 2008b. Avian influenza in Australia. In Avian influenza (ed. Swayne DE), pp. 239–250. Blackwell Publishing, Ames, IA. [Google Scholar]
- Sims LD, Brown IH. 2017. Multi-continental epidemic of H5N1 high pathogenicity avian influenza (1996-2015). In Avian influenza (ed. Swayne DE), pp. 202–247. Wiley, Ames, IA. [Google Scholar]
- Sims LD, Ellis TM, Liu KK, Dyrting K, Wong H, Peiris M, Guan Y, Shortridge KE. 2003a. Avian influenza in Hong Kong 1997-2002. Avian Dis 47: 832–838. 10.1637/0005-2086-47.s3.832 [DOI] [PubMed] [Google Scholar]
- Sims LD, Guan Y, Ellis TM, Liu KK, Dyrting K, Wong H, Kung NYH, Shortridge KF, Peiris M. 2003b. An update on avian influenza in Hong Kong 2002. Avian Dis 47: 1083–1086. 10.1637/0005-2086-47.s3.1083 [DOI] [PubMed] [Google Scholar]
- Sims L, Harder T, Brown I, Gaidet N, Belot G, Dobschuetz Sv, Kamata A, Kivaria F, Palamara E, Bruni M, et al. 2017. Highly pathogenic H5 avian influenza in 2016 and 2017—observations and future perspectives. FOCUS ON, No. 11, November 2017. http://www.fao.org/3/a-i8068e.pdf
- Slemons RD, Johnson DC, Osborn JS, Hayes F. 1974. Type-A influenza viruses isolated from wild free-flying ducks in California. Avian Dis 18: 119–124. 10.2307/1589250 [DOI] [PubMed] [Google Scholar]
- Smith DJ. 2006. Predictability and preparedness in influenza control. Science 312: 392–394. 10.1126/science.1122665 [DOI] [PubMed] [Google Scholar]
- Suarez DL. 2016. Common aspects of animal influenza. In Animal influenza (ed. Swayne DE). Wiley-Blackwell, Ames, IA. [Google Scholar]
- Suarez DL, Senne DA, Banks J, Brown IH, Essen SC, Lee CW, Manvell RJ, Mathieu-Benson C, Moreno V, Pedersen JC, et al. 2004. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg Infect Dis 10: 693–699. 10.3201/eid1004.030396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne DE. 2008a. The global nature of avian influenza. In Avian influenza (ed. Swayne DE), pp. 123–143. Blackwell Publishers, Ames, IA. [Google Scholar]
- Swayne DE. 2008b. High pathogenicity avian influenza in the Americas. In Avian influenza (ed. Swayne DE), pp. 191–216. Blackwell Publishing, Ames, IA. [Google Scholar]
- Swayne DE. 2017. The global nature of avian influenza. In Animal influenza (ed. Swayne DE), pp. 177–201. Wiley-Blackwell, Ames, IA. [Google Scholar]
- Swayne DE, Suarez DL. 2000. Highly pathogenic avian influenza. Rev Sci Tech 19: 463–482. 10.20506/rst.19.2.1230 [DOI] [PubMed] [Google Scholar]
- Swayne DE, Hill RE, Clifford J. 2017. Safe application of regionalization for trade in poultry and poultry products during highly pathogenic avian influenza outbreaks in the USA. Avian Pathol 46: 125–130. 10.1080/03079457.2016.1257775 [DOI] [PubMed] [Google Scholar]
- Swayne DE, Suarez DL, Sims L. 2020. Influenza. In Diseases of poultry (ed. Swayne DE, Boulianne M, Logue C, McDougald LD, Nair V, Suarez DL), pp. 210–256. Wiley, Ames, IA. [Google Scholar]
- Turner AJ. 1976. The isolation of fowl plague virus in Victoria. Aust Vet J 52: 384 10.1111/j.1751-0813.1976.tb09503.x [DOI] [PubMed] [Google Scholar]
- USAHA. 1985. Report of the Committee on Transmissible Diseases of Poultry and Other Species. In Proceedings of the United States Animal Health Association. Proceedings of the 89th Annual Meeting of the US Animal Health Association, pp. 296–305. Richmond, VA. [PubMed] [Google Scholar]
- USDA. 2017. Final report for the 2017 outbreak of highly pathogenic avian influenza (HPAI)/low pathogenicity avian influenza (LPAI) in the Southeastern United States https://www.aphis.usda.gov/animal_health/emergency_management/downloads/hpai/h7-hpai-lpai-finalreport.pdf, pp. 1–46.
- Villareal CL, Flores AO. 1998. The Mexican avian influenza (H5N2) outbreak. In Proceedings of the Fourth International Symposium on Avian Influenza (ed. Swayne DE, Slemons RD), pp. 18–22. U.S. Animal Health Association, Richmond, VA. [Google Scholar]
- Walker JA, Kawaoka Y. 1993. Importance of conserved amino acids at the cleavage site of the haemagglutinin of a virulent avian influenza A virus. J Gen Virol 74: 311–314. 10.1099/0022-1317-74-2-311 [DOI] [PubMed] [Google Scholar]
- Walker JA, Molloy SS, Thomas G, Sakaguchi T, Yoshida T, Chambers TM, Kawaoka Y. 1994. Sequence specificity of furin, a proprotein-processing endoprotease, for the hemagglutinin of a virulent avian influenza virus. J Virol 68: 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol Rev 56: 152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RJH. 1963. An outbreak of fowl plague in turkeys. Vet Rec 75: 783–786. [Google Scholar]
- Westbury HA. 1998. History of highly pathogenic avian influenza in Australia. In Proceedings of the Fourth International Symposium on Avian Influenza (ed. Swayne DE, Slemons RD), pp. 23–30. U.S. Animal Health Association, Richmond, VA. [Google Scholar]
- WHO/OIE/FAO_H5N1_Evolution_Working_Group. 2008. Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 14: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/OIE/FAO_H5N1_Evolution_Working_Group. 2014. Revised and updated nomenclature for highly pathogenic avian influenza A (H5N1) viruses. Influenza Other Respir Viruses 8: 384–388. 10.1111/irv.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Daniels P. 2013. Avian influenza—why it's not going away. The Conversation. http://theconversation.com/avian-influenza-why-its-not-going-away-20038
- Wood GW, McCauley JW, Bashiruddin JB, Alexander DJ. 1993. Deduced amino acid sequences at the haemagglutinin cleavage site of avian influenza A viruses of H5 and H7 subtypes. Arch Virol 130: 209–217. 10.1007/BF01319010 [DOI] [PubMed] [Google Scholar]
- Yang L, Zhu W, Li X, Chen M, Wu J, Yu P, Qi S, Huang Y, Shi W, Dong J, et al. 2017. Genesis and spread of newly emerged highly pathogenic H7N9 avian viruses in Mainland China. J Virol 91: e01277–17. 10.1128/JVI.01277-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaraket H, Bridges OA, Duan S, Baranovich T, Yoon SW, Reed ML, Salomon R, Webby RJ, Webster RG, Russell CJ. 2013a. Increased acid stability of the hemagglutinin protein enhances H5N1 influenza virus growth in the upper respiratory tract but is insufficient for transmission in ferrets. J Virol 87: 9911–9922. 10.1128/JVI.01175-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaraket H, Bridges OA, Russell CJ. 2013b. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus replication and pathogenesis in mice. J Virol 87: 4826–4834. 10.1128/JVI.03110-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen S, Yang D, Wang X, Zhu J, Peng D, Liu X. 2015. Role of stem glycans attached to haemagglutinin in the biological characteristics of H5N1 avian influenza virus. J Gen Virol 96: 1248–1257. 10.1099/vir.0.000082 [DOI] [PubMed] [Google Scholar]