In this review, Akay et al. discuss the physiological roles of oligodendrocyte precursor cells (OPCs) in the brain beyond their contribution to myelination. The authors also explore how sex and age may influence OPC activity, and discuss how OPC dysfunction may play a primary role in numerous neurological and neuropsychiatric diseases.
Keywords: Alzheimer's disease, NG2 glia, blood–brain barrier, immunity, neurodevelopment, oligodendrocyte precursor, senescence, sex differences, synapse
Abstract
Oligodendrocyte precursor cells (OPCs) are not merely a transitory progenitor cell type, but rather a distinct and heterogeneous population of glia with various functions in the developing and adult central nervous system. In this review, we discuss the fate and function of OPCs in the brain beyond their contribution to myelination. OPCs are electrically sensitive, form synapses with neurons, support blood–brain barrier integrity, and mediate neuroinflammation. We explore how sex and age may influence OPC activity, and we review how OPC dysfunction may play a primary role in numerous neurological and neuropsychiatric diseases. Finally, we highlight areas of future research.
Oligodendrocyte precursor cells (OPCs) are a heterogeneous, multipotent population that emerges during embryogenesis and persists as resident cells of the adult brain parenchyma. Although their role as progenitors of oligodendrocytes is well understood (Nishiyama et al. 1999), their additional capacities remain relatively unexplored as compared with other brain cells. In this review, we highlight the unique properties of OPCs (summarized in Fig. 1). The biology of myelination and mature oligodendrocytes lies beyond our scope and has been thoroughly reviewed by others (e.g., Bradl and Lassmann 2010; Michalski and Kothary 2015; Philips and Rothstein 2017).
Figure 1.
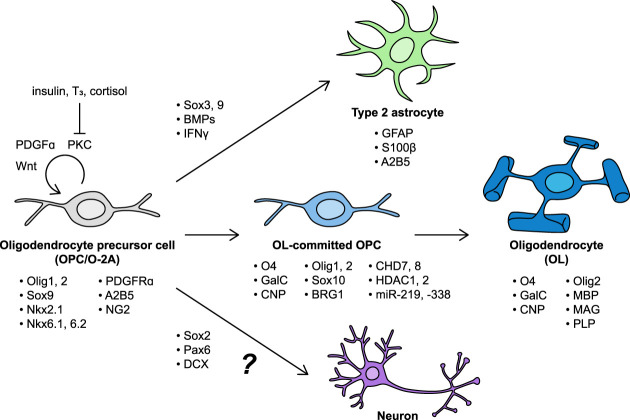
Transcription factors, growth factors, signaling pathways, and markers of cell identity associated with different stages of oligodendrocyte precursor cell (OPC) maintenance and differentiation. OPC proliferation and self-renewal depend on PDGFα–PDGFRα signaling, high Wnt tone, and PKC activation. Insulin, T3, and cortisol disinhibit differentiation of OPCs into mature oligodendrocytes (OLs), likely through counteracting PKC activation. OPCs can be induced to differentiate into Type 2 astrocytes by BMPs and IFNγ. Although some evidence suggests that OPCs express proneural transcription factors such as Sox2 and Pax6, and neuronal precursor markers such as doublecortin (DCX), it is unknown whether OPCs give rise to appreciable numbers of neurons in vivo.
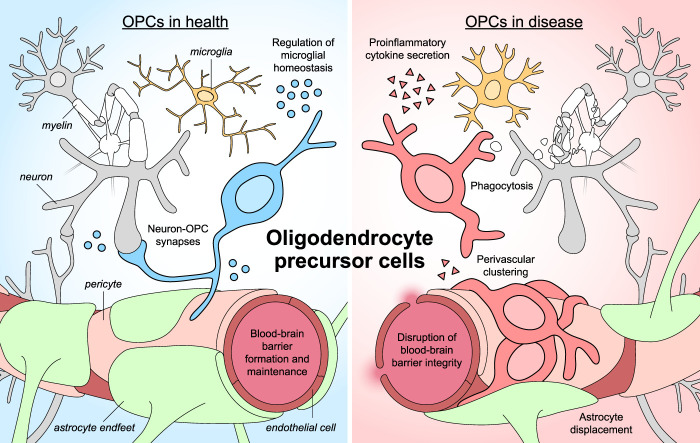
Over the past few decades, OPCs have emerged as active participants in multiple aspects of brain structure and function. OPCs receive synaptic input from neurons (Lin and Bergles 2004) and may generate action potentials (Káradóttir et al. 2008). They can assume the role of immune cells, monitoring the environment, presenting antigens, and releasing immunomodulatory factors (Falcão et al. 2018; Kirby et al. 2019). OPCs associate closely with the blood–brain barrier and help maintain its integrity (Seo et al. 2014). Finally, biological sex influences OPC development, resilience, and response to pathology. As recent studies have implicated OPCs in CNS disorders ranging from Alzheimer's disease to major depressive disorder, understanding the physiological roles of OPCs and how they may become dysregulated, is imperative to more fully understanding the brain.
OPCs in the developing brain
In mice, OPC formation is thought to occur in three waves, with OPCs first derived from Nkx2.1-expressing precursors around embryonic days E11.5–E12.5 in the ventricular zone of the medial ganglionic eminence and anterior entopeduncular area (Kessaris et al. 2006). A second wave of OPC generation has been described at E16.5 in the Gsh2-expressing ventricular zone of the lateral and central ganglionic eminences (Kessaris et al. 2006). However, recent single-cell transcriptomic analyses have suggested that these embryonic cells may constitute “primitive” or “preceding” OPCs; that is, OPC precursors with a distinct transcriptional identity (Marques et al. 2016; Weng et al. 2019; Huang et al. 2020). Human prenatal OPC generation occurs around the embryonic-fetal transition, approximately gestational weeks GW10–15 (Jakovcevski et al. 2009).
Like neuronal progenitors, OPCs are generated through asymmetric division of radial glia, the neural stem cells (NSCs) that line the ventricular zone of the developing brain. Whereas the apical daughter cell maintains stemness through contact with both apical and basal surfaces of the neuroepithelium, the prospective OPC detaches from the luminal surface and resides in the ventricular zone as it begins to transform (Kriegstein and Alvarez-Buylla 2009). Sonic hedgehog (Shh), which is also expressed in the medial ganglionic eminence, up-regulates expression of the class II transcription factors Nkx6.1 and Nkx6.2 in differentiating neuroepithelial stem cells. Together, Shh and the Nkx6 family transcription factors induce expression of the basic helix–loop–helix transcription factors Olig1 and Olig2 (Nery et al. 2001; Wegner 2008). Olig2 is essential for normal OL lineage specification and dimerizes with E2A proteins to induce Nkx2.2, Sox9, and Sox10 expression (Zhou et al. 2001; Lu et al. 2002; Takebayashi et al. 2002; Wegner 2008). OPCs develop a characteristic bipolar cellular morphology and express NG2 chondroitin sulphate proteoglycan and platelet-derived growth factor receptor α (PDGFRα), part of an essential signaling axis for OPC survival, proliferation, and migration (Hart et al. 1989; Polito and Reynolds 2005; Finzsch et al. 2008; Bergles and Richardson 2016).
Newly formed OPCs subsequently migrate radially and tangentially throughout the developing forebrain and optic nerve. Numerous secreted signals such as Shh, C-X-C motif chemokine ligand 1 (CXCL1), PDGFRα, fibroblast growth factors (FGFs), class 3 semaphorins, bone morphogenetic proteins (BMPs), and chemotactic netrins attract or repel OPCs at different times and in different spatial contexts (Simpson and Armstrong 1999; Tsai et al. 2002; Merchán et al. 2007; Furusho et al. 2011; Piaton et al. 2011; Choe et al. 2014; Tsai et al. 2016). OPCs in the optic nerve may express ephrin ligands for contact-dependent migration along optic nerve axons expressing Eph receptors (Prestoz et al. 2004). Although prenatal OPCs appear to populate the brain indiscriminately, early and late waves of prenatally generated OPCs may be differentially sensitive to certain chemoattractants such as neuregulin-1, which could fine-tune their final destination in the brain (Ortega et al. 2012). As they migrate, OPCs continually adjust their trajectories to avoid other OPCs by extending and retracting their processes, resulting in an even, nonoverlapping distribution of cells (Kirby et al. 2006; Hughes et al. 2013).
Evidence suggests that the developing vasculature plays a dual role in OPC migration. First, because the vascular endothelium has substantially infiltrated the parenchyma by E11.5 (Daneman et al. 2009), it serves as a physical scaffold for migration. OPCs can be observed in close association with blood vessels as early as E12 in mice and GW14 in humans (Tsai et al. 2016). Time-lapse and live imaging suggest that OPCs move along and between blood vessels. Second, juxtacrine signaling between chemokine receptor 4 (CXCR4) on OPCs and SDF1 (CXCL12) on endothelial cells promotes MEK/ERK- and PI3K/AKT-dependent migration (Tian et al. 2018), as well as maintains Wnt activation in OPCs to block premature differentiation (Tsai et al. 2016). Endothelial cells also secrete trophic factors such as BDNF and FGF, which activate Src/Akt signaling and promote PDGFRα expression in OPCs to sustain proliferation (McKinnon et al. 1990; Arai and Lo 2009). These results suggest that OPCs develop an intimate relationship with the vasculature at an early developmental stage, which they exploit to travel throughout the growing brain.
In addition to early patterns of OPC generation and migration described above, a third wave of pre-OPCs emerges late in embryonic development (E17.5 in mice) and later constitutes the majority of oligodendrocytes in the neocortex (Winkler et al. 2018). Unlike the first two populations, which emerge from the ventral forebrain, the third wave of pre-OPCs is derived from Emx1-expressing cortical progenitors of the dorsal ventricular zone (Kessaris et al. 2006). These late-born OPCs were initially thought to replace the early-born OPCs, eliminated by an unknown mechanism. However, more recent work has challenged this hypothesis by showing a specified population of early-born OPCs survives in the developing cortex, eventually transcriptionally converging with later-born OPCs after birth (Marques et al. 2016; Orduz et al. 2019).
The functional significance of neocortical OPC turnover is not well understood, but it may result from the changing molecular environment of the developing brain. For example, interneurons that infiltrate the neocortex from E14.5–16.5 secrete Shh as part of interneuron subtype specification; in turn, Shh might stimulate proliferation of late-born dorsal OPCs (Winkler et al. 2018).
Though PDGFRɑ is commonly used as a marker of OPC identity, it has been hypothesized that some late-born cortical OPCs do not require PDGFRɑ for survival (Zheng et al. 2018). PDGFRɑ-negative OPCs express other markers of OPC identity such as Olig1, Olig2, and Sox10, and they give rise to mature myelin basic protein (MBP)-expressing oligodendrocytes. However, these populations are restricted in number, range, and ability to form myelin (Zheng et al. 2018), underscoring PDGFRɑ’s importance in OPC proliferation, migration, and potential for differentiation.
The fate of OPCs
Oligodendrocytes and other glial cells have been described since the early 1900s, but it was only in the 1980s that OPCs were appreciated to form a distinct cell population (Doering and Fedoroff 1984; Hirayama et al. 1984; Ffrench-Constant and Raff 1986). Best known for their crucial role in oligodendrocyte production, OPCs can also differentiate into Schwann cells of the CNS, as well as “type 2” astrocytes, which express markers such as GFAP and S100β while retaining some OPC markers such as A2B5 (Sawamura et al. 1995; Windrem et al. 2004; Zawadzka et al. 2010; Tabata 2015; Assinck et al. 2017). Thus, OPCs were dubbed “O-2A cells,” and this label was used by some through the turn of the 21st century. Because type 2 astrocytes were relatively rare and elusive in vivo, OPC gradually superseded O-2A as the term of choice (Richardson et al. 2011). Utility of the O-2A label remains for emphasizing distinctions between completely uncommitted glial progenitor cells, bipotent O-2A cells, and oligodendrocyte-committed OPCs (Baracskay et al. 2007). The term “NG2-glia” has also been used to describe OPCs, due to their surface expression of the chondroitin sulfate proteoglycan; however, as pericytes also express NG2, this nomenclature is potentially problematic. In this review, we use the term “OPC” to refer to bipotent progenitors of oligodendrocytes and astrocytes. We also discuss possible contributions of OPCs to neurogenesis. These cell fates, key regulatory factors, and common markers are summarized in Figure 2.
Figure 2.
Transcription factors, growth factors, signaling pathways, and markers of cell identity associated with different stages of oligodendrocyte precursor cell (OPC) maintenance and differentiation. OPC proliferation and self-renewal depend on PDGFα–PDGFRα signaling, high Wnt tone, and PKC activation. Insulin, T3, and cortisol disinhibit differentiation of OPCs into mature oligodendrocytes (OLs), likely through counteracting PKC activation. OPCs can be induced to differentiate into Type 2 astrocytes by BMPs and IFNγ. Although some evidence suggests that OPCs express proneural transcription factors such as Sox2 and Pax6, and neuronal precursor markers such as doublecortin (DCX), it is unknown whether OPCs give rise to appreciable numbers of neurons in vivo.
Differentiation of OPCs into oligodendrocytes
The differentiation of OPCs into mature, myelinating oligodendrocytes begins around E18.5 in mice and continues throughout adulthood in both mice and humans (McCarthy and Leblond 1988; Dimou et al. 2008; Rivers et al. 2008). Differentiation consists of at least two discrete waves of gene expression (Dugas et al. 2006) and is tightly regulated by a combination of transcriptional, epigenetic, and extrinsic factors (Emery 2010). Many extrinsic signals, such as astrocyte-derived cues in the extracellular matrix (ECM), serve to block OPC differentiation (Mayoral and Chan 2016). Other factors such as triiodothyronine (T3), cortisol, and insulin may be required to unblock oligodendrocyte differentiation by counteracting PKC activation and enhancing cholesterol synthesis, which is required to support myelination (Baron et al. 1998; Cardona et al. 2019). OPCs committing to the oligodendrocyte lineage will exit the cell cycle, express myelin-associated genes, and associate intimately with axons. Committed OPCs express surface proteins including O4, galactocerebroside (GalC), and 2′,3′-cyclic nucleotide 3′ phosphodiesterase (CNP) (Wegner 2008). They also exhibit branching of cell processes and increased lipid biosynthesis to support the creation of myelin membranes (Pfeiffer et al. 1993; Dugas et al. 2006; Wegner 2008). Oligodendrocyte maturation and myelination is reviewed in more depth by Bradl and Lassmann (2010) and Michalski and Kothary (2015).
The sequential action of several key transcription factors guide oligodendrocyte differentiation. Although Sox9 is essential for early commitment to a glial fate and OPC survival, its expression vanishes as OPCs mature and commit to oligodendrogenesis (Stolt et al. 2003). Olig1, which is important for early differentiation, is acetylated and translocated to the cytoplasm as OPCs progress toward more mature, myelinating states, where it may continue to indirectly regulate gene expression (Dai et al. 2015). Sox10 and Olig2 promote the expression of myelin genes such as MBP, myelin-associated glycoprotein (MAG), and proteolipid protein (PLP) (Stolt et al. 2003). Other transcription factors and nuclear receptors that participate in oligodendrocyte development are extensively reviewed by Emery and Lu (2015) and Elbaz and Popko (2019).
Oligodendrocyte differentiation is also regulated on the epigenetic level. Whole-genome wide chromatin immunoprecipitation sequencing (CHIP-seq) against RNA polymerase II (RNAPII) identified Smarca4 (encoding BRG1) as the most prominent RNAPII binding target, indicating that it is an actively transcribed gene at the onset of oligodendrocyte differentiation (Yu et al. 2013). Made accessible by the chromatin remodeler CHD8, BRG1 forms chromatin remodeling complexes that activate CHD7, inducing a cascade of chromatin remodeling and gene transcription that inhibits nonoligodendroglial fates (Liu et al. 2007; Zhao et al. 2018). Complementing CHD activity, histone deacetylases HDAC1 and HDAC2 drive oligodendrocyte differentiation by blocking stabilization and nuclear translocation of the β-catenin/TCF7L2 complex, thus lowering Wnt tone and enabling differentiation (Ye et al. 2009). MicroRNAs (miR) 219 and 338 have also been reported to repress negative regulators of oligodendrocyte differentiation (Zhao et al. 2010).
Finally, the transition from immature to myelinating oligodendrocyte appears to be regulated both internally and externally by proteins such as γ-secretase (Watkins et al. 2008), G protein-coupled receptor Gpr17 (Chen et al. 2009), and transforming growth factor β (TGFβ) signaling (Palazuelos et al. 2014). Oligodendrocytes have also been reported to preferentially myelinate specific classes of neurons. These preferences may emerge during differentiation, but the molecular mechanisms of neuron class recognition in oligodendrocytes is unknown (Zonouzi et al. 2019).
The complex regulation of oligodendrocyte production underscores the challenge of (re)generating these cells. Fully understanding the mechanisms of OPC to oligodendrocyte differentiation and myelination would lay the groundwork for a new era of neuro-regenerative medicine, from mobilizing OPCs to repair demyelination to preventing age-associated cognitive decline (Wang et al. 2020).
Differentiation of OPCs into astrocytes
The potential of OPCs to give rise to type 2 astrocytes was initially discovered in the rat optic nerve when isolated progenitors were demonstrated to generate either cell type in vitro if cultured in appropriate media (Raff et al. 1983). Subsequent studies confirmed that isolated OPCs could differentiate into either cell type (Temple and Raff 1985; Raff 1989; Bögler et al. 1990). OPC differentiation into astrocytes appears to be regulated predominantly by extrinsic cues. Early in vitro studies suggested that whereas OPC differentiation into oligodendrocytes followed an “intrinsic clock” that counted cell divisions, type 2 astrocytes could be generated at any time by adding fetal calf serum to OPC cultures (Raff et al. 1985). Later in vitro experiments identified BMPs and IFNγ as extrinsic factors that push OPCs toward the astrocyte lineage (Tanner et al. 2011; Tabata 2015; Suzuki et al. 2017). These findings are reflected in vivo, as the changing microenvironment of the developing brain directs OPCs to take on different fates over time. In one study, OPCs transplanted into P7 mouse telencephalon differentiated largely into MBP-expressing oligodendrocyte lineage cells; the majority of OPCs transplanted into 6-mo-old mouse cortex generated GFAP-positive astrocytes (Sawamura et al. 1995).
At the level of transcriptional regulation, cytoplasmic translocation of Olig2 corresponds to the adoption of an astrocytic fate (Zhao et al. 2009). Sequential Sox protein expression may also regulate which transcriptional program is activated. Across neuronal and glial progenitors, Sox3 binds to mature cell type-specific genes to prevent premature activation. In OPCs that maintain Sox3 expression, Sox3 and Sox9 prebind to astrocyte-specific genes that are enriched for Nfi motifs. Sox9 then acts synergistically with the astrocyte-associated transcription factor NFIA to drive their expression and promote an astrocytic fate (Klum et al. 2018). In the absence of Sox3, Sox9 binds to both classes of glial genes; Sox10 then targets oligodendrocyte-specific genes to drive an oligodendrocyte fate (Klum et al. 2018). Presumably, expression of these transcription factors can be influenced by extrinsic signals, but direct evidence connecting growth factors or cytokines to Sox proteins in the context of OPC fate remains to be described.
Differentiation of OPCs into neurons
From its earliest identification in a retroviral labeling study, the neuropotent OPC has garnered mixed evidence for its existence (Price et al. 1992). One early study used transgenic mice expressing GFP under the control of the CNP promoter to mark oligodendrocyte lineage cells. These CNP-GFP-expressing cells were positive for NG2; when isolated in vitro, they could be observed to lose CNP-GFP expression and differentiate into NeuN-positive neurons (Belachew et al. 2003). In vivo, CNP-GFP cells in the hippocampus endured into early adulthood (P30) and gained markers of differentiation and functional specialization such as TOAD-64 (DPYSL3) and GAD-67 (GAD1) (Belachew et al. 2003). Most promisingly, these cells were electrically excitable and fired action potentials in vitro and in situ. Later studies found that OPCs express low levels of neuronal precursor markers such as Sox2, Pax6, and doublecortin (DCX), suggesting they may be intrinsically competent for neuronal differentiation (Dayer et al. 2005; Guo et al. 2010; Robins et al. 2013).
Using transgenic mice that allow for Cre-recombinase-dependent fate mapping, various studies have identified neurons presumably birthed from NG2-positive or PDGFRɑ-positive OPCs in the piriform cortex and hypothalamus (Rivers et al. 2008; Guo et al. 2010; Robins et al. 2013). In contrast, other research groups using similar Cre reporter systems found that NG2-positive cells did not give rise to neurons (Kang et al. 2010; Zhu et al. 2011; Huang et al. 2014, 2019). Evaluation of these findings is complicated by the presence of neural stem cells (NSCs), which could exhibit ectopic Cre-lox recombination as well as some electrophysiological similarities between OPCs and neurons described later in this review. An alternative hypothesis based on in vitro findings suggests that OPCs can appropriate an NSC-like state as a result of external signals (Kondo and Raff 2000). Taken together, the degree to which true OPC-derived neurons exist remains to be determined.
OPCs in the adult brain
Following their rapid expansion and diffusion in the developing brain, OPCs reduce their rate of proliferation and migration significantly, though they remain one of the most mitotically active cell types in the adult CNS (Rao and Jacobson 2006). OPCs constitute ∼5% of total cells and 70% of BrdU-positive dividing cells in the brain, residing both in the neurogenic niche of the adult subventricular zone as well as throughout the brain and optic nerve (Dawson et al. 2003; Domingues et al. 2016).
As a cell population, OPCs possess a remarkable degree of functional, transcriptional, and regional heterogeneity. Differences in the degree of calcium signaling activity, phagocytosis, and electrical activity have been documented within OPCs (Falcão et al 2018; Kirby et al. 2019; Marisca et al. 2020). This functional diversity may be described at a transcriptional level, as single-cell RNA sequencing has revealed distinct clusters of OPCs, marked by unique expression of genes related to migration, cell-cycle, or astrocytic fate commitment (Marques et al. 2016). Transcriptional analysis of human postmortem brain tissue from multiple sclerosis patients has revealed diversity within oligodendrocytes, raising the intriguing possibility that certain subpopulations of OPCs may be more likely to survive or differentiate under disease states (Jäkel et al. 2019). Whether all OPCs are equally able to participate in different functions, how these roles are assumed, and relevance to human disease are all certainly interesting questions to pursue.
OPCs self-renew through asymmetric division and can generate differentiating oligodendrocytes throughout the CNS in support of myelin remodeling and repair (Young et al. 2013). However, new oligodendrocyte generation from adult OPCs is limited. In humans, the majority of oligodendrocytes are generated by 5 yr of age, and only 0.3% of the population is replaced annually (Yeung et al. 2014). Oligodendrocytes in the mouse brain possess a similar longevity; for example, 90% of oligodendrocytes at P60 were found to persist 8 mo later (Tripathi et al. 2017). Although acute disease processes as seen in aggressive multiple sclerosis (MS) can trigger an increase in oligodendrocyte generation during adulthood, 14C analysis suggests that OPC proliferation and differentiation into mature oligodendrocytes is rare in the majority of individuals (Yeung et al. 2019). The longevity of oligodendrocytes, and the relatively large population of OPCs in the adult brain, raises the possibility that most OPCs may hold responsibilities beyond replacing oligodendrocytes.
Electrophysiological properties of OPCs
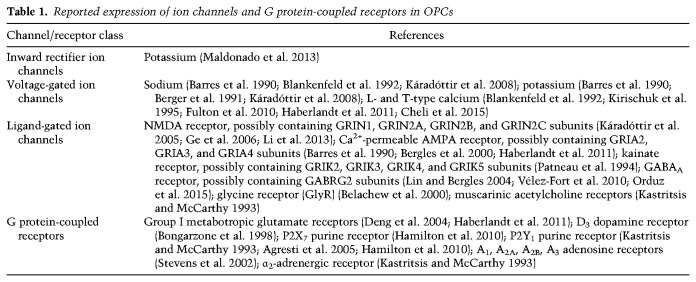
The advent of patch-clamp technology enabled electrophysiological recording from glial cells for the first time in the 1980s and 1990s (Walz and MacVicar 1988; Berger et al. 1991). Combined with immunohistochemical labeling of NG2 or O4, these recordings revealed that OPCs express a multitude of ion channels and respond to neuroligands, listed in Table 1 (for reviews, see also Káradóttir and Attwell 2007; Paez and Lyons 2020).
Table 1.
Reported expression of ion channels and G protein-coupled receptors in OPCs
OPCs are heterogeneous in their electrophysiological properties, which also change with age. For example, adult OPCs are more sensitive to local increases in extracellular potassium by expressing the inwardly rectifying potassium channel Kir1.4 (Maldonado et al. 2013). OPCs that are more committed to an oligodendrocyte fate reduce their expression of receptor and ion channel genes (Fröhlich et al. 2011). Transcriptional profiling revealed increased expression of particular ion channel subunits in OPCs during developmental myelination, suggesting that electrical and chemical signaling may regulate OPC fate (Spitzer et al. 2019). Other reviews describe the role of neurotransmitter signaling in OPC differentiation during activity-dependent myelination, also referred to as myelin plasticity (for reviews, see Fernández-Castañeda and Gaultier 2016; de Faria et al. 2019; Bonetto et al. 2020).
In several model systems, two functional classes of OPCs have been observed. Live-cell imaging, single-cell transcriptomics, and calcium imaging in zebrafish spinal cord identified one OPC population that engaged in high rates of calcium signaling, and one that was more likely to differentiate into myelinating oligodendrocytes, suggesting that signaling in highly connected OPCs coordinates the positioning and differentiation of more mature OPCs (Marisca et al. 2020). Furthermore, some have shown that the electrically active subpopulation of OPCs is capable of generating action potentials. Káradóttir et al. (2008) found that 46% of rat OPCs recorded in situ expressed voltage-gated channels and generated both spontaneous and evoked action potentials, in contrast to remaining OPCs that were electrically inactive and did not express ion channels. In another study, 19% of recorded NG2-positive mouse OPCs fired all-or-nothing action potentials when depolarized to −30 mV, and 76% fired graded spikes proportional to stimulus magnitude (Ge et al. 2009). Further study is required to interrogate the potential relevance of OPC-initiated electrical activity to neuronal signaling.
The neuron-OPC synapse
While OPCs were known to express ion channels, whether they formed bonafide synapses remained unclear until the landmark discovery that, unlike other glia, OPCs form functional excitatory synapses with neurons (Bergles et al. 2000). Electrophysiological recordings demonstrated that hippocampal OPCs experienced excitatory postsynaptic currents in response to stimulation of CA1 neurons. Ultrastructural imaging revealed neuronal presynaptic junctions onto OPCs and close association between OPC processes and neuronal postsynaptic densities (Bergles et al. 2000).
Excitatory neuron-OPC synapses have since been found in mice during development and adulthood, suggesting that their function likely extends beyond the establishment of developmental, activity-dependent myelination cues (Ziskin et al. 2007). More recently, monosynaptic tracing has revealed that OPCs in the corpus callosum receive synaptic inputs from many functionally connected cortical and thalamic regions, suggesting that OPCs are well positioned to integrate signals from brain-wide circuits (Mount et al. 2019). Finally, calcium-dependent AMPA-receptor neuron-OPC synapses have been reported to undergo long-term potentiation (Ge et al. 2006), inviting speculation on the role of neuron-OPC synaptic plasticity in learning and memory. Whether other forms of synaptic plasticity exist at neuron-OPC synapses, and potential functional consequences, remains unknown.
OPCs have also been shown to form inhibitory synapses with GABAergic interneurons in the P14 rat hippocampus. OPCs exhibited miniature inhibitory postsynaptic currents (mIPSCs) consistent with quantal GABA release at synapses, and increased their spontaneous activity when interneurons were pharmacologically depolarized in acute slices (Lin and Bergles 2004). GABAergic interneurons have been shown to form a synaptic network with OPCs most active at the onset of myelination in the second postnatal week (Vélez-Fort et al. 2010; Orduz et al. 2015). Both fast-spiking interneurons (FSIs) and non-fast-spiking interneurons (NFSIs) synapse onto OPCs, but FSIs are more extensively connected to OPCs and target the soma and proximal processes (Orduz et al. 2015). As FSIs are responsible for establishing cortical γ oscillations (Cardin et al. 2009; Sohal et al. 2009), it could be of interest to determine whether OPCs, through their intimate relationship with FSIs, help to coordinate cortical oscillations and thus contribute to higher-order cognitive function.
Beyond GABA and glutamate, OPCs are sensitive to glycine, histamine, adenosine, and many other signaling molecules that can trigger a rise in intracellular calcium levels (Kastritsis and McCarthy 1993; Belachew et al. 2000; Stevens et al. 2002). How analogous the intracellular second messenger signaling cascades are to those observed in neurons remains incompletely known. Responsiveness to histamine also suggests that OPCs could be monitoring the immune environment, a topic explored later in this review. OPCs have also been reported to possess dopaminergic D3 receptors, potentially allowing them to respond to changes in dopaminergic tone (Bongarzone et al. 1998).
Neuromodulation by OPCs
In addition to receiving synaptic input, OPCs engage with neurons along multiple signaling axes to regulate neuronal signaling. NG2, one of the hallmark OPC membrane proteins, may directly modulate neuron-neuron synapses when it is cleaved by α- and γ-secretases to release its ectodomain into the ECM. Either genetic ablation of NG2 or pharmacological inhibition of the α-secretase ADAM10 resulted in significantly impaired NMDAR-dependent LTP and sensorimotor gating (Sakry et al. 2014). However, because NG2 is also expressed by mural cells such as pericytes and vascular smooth muscle cells, NG2 knockout mice cannot be used to exclude the possibility that other NG2-expressing cells contribute to this effect.
OPCs also secrete neuromodulatory factors such as prostaglandin D2 synthase (PTGDS), neuronal pentraxin 2 (NP2), and FGF2 (Birey et al. 2015; Sakry et al. 2015). Partial ablation of OPCs in stress-susceptible mice and subsequent loss of OPC-secreted FGF2 has been shown to dampen glutamatergic signaling in prefrontal cortex, suppress glutamate uptake by astrocytes, and induce MDD-like behavioral deficits on open-field and micro-defeat tests (Birey et al. 2015). A recent transcriptomic study of male patients with major depressive disorder (MDD) identified OPCs and deep-layer excitatory neurons as the most differentially regulated cell types, constituting almost half of all gene expression changes (Nagy et al. 2020). Analysis of receptor-ligand interactions between these two cell types revealed disrupted FGF and neurexin-neuroligin signaling, suggesting that OPCs are required for normal excitatory neurite outgrowth and synaptic maintenance (Nagy et al. 2020). In another example, loss of NG2-positive OPCs in the arcuate nucleus of the median eminence results in a striking loss of neuronal sensitivity to leptin, leading to overeating behavior and weight gain in mice (Djogo et al. 2016). Taken together, these data suggest that OPCs are intertwined with neurons and other glia at neuron-neuron synapses to enable normal signal transmission and synaptic plasticity.
The highly communicative nature of OPCs raises many interesting questions. Could spiking OPCs influence neuronal properties and activity? Does electrical input to OPCs influence nonmyelinating functions such as immune surveillance? How does electrical activity in OPCs affect cortical interneuron development? How do neuron-OPC synapses change in response to disease or injury, and could OPCs sense and respond to neural circuit disruption via their synaptic connections with neurons? These questions highlight a rich area of future study that could lend unique insight into normal and pathological brain signaling.
OPCs at the blood–brain barrier
The blood–brain barrier (BBB) is responsible for regulating nutrient uptake from peripheral blood, clearing toxic waste products, and protecting the CNS from pathogenic insult. Maintaining BBB integrity is therefore crucial for ensuring brain health, and OPCs have been shown to play a critical role in both development and maintenance of the BBB. First, OPCs may be required for normal embryonic brain vascularization, as genetic ablation of NG2-positive OPCs in embryonic mice leads to reduced density and branching of the vascular network (Minocha et al. 2015). After birth, OPCs continue to promote angiogenesis in white matter through HIF signaling and paracrine Wnt signaling, coupling postnatal myelination and angiogenesis (Yuen et al. 2014). In addition to facilitating the penetration of blood vessels into the brain, OPCs also promote endothelial cell junction tightness within the BBB by secreting the cytokine TGFβ. Consequently, inhibiting OPC-specific TGFβ secretion causes massive cerebrovascular disruption and hemorrhage (Seo et al. 2014). However, these results should be interpreted with some caution, as NG2 is also expressed in pericytes, and therefore expressing Cre under the NG2 promoter may directly affect pericytes, a critical cellular component of the BBB.
In both mice and humans, OPCs associate closely with pericytes, another cell type of the BBB; they wrap processes around pericytes and attach to them via the abluminal basal lamina (Maki et al. 2015). Media conditioned by one cell type increases proliferation of the other in vitro, suggesting that OPCs and pericytes support and regulate each other's growth (Maki et al. 2015). Pericytes also appear to be essential for white matter maintenance. In a mouse model of pericyte loss in adulthood, myelin sheaths become thinner, white matter tracts become disorganized and vacuolized, and oligodendrocytes undergo apoptosis (Montagne et al. 2018). Interestingly, OPCs do not significantly proliferate or facilitate remyelination in this model, despite loss of oligodendrocytes. In conjunction with previous findings that pericytes stimulate OPC differentiation after CNS demyelination (De La Fuente et al. 2017), this suggests that pericyte loss can both initiate oligodendrocyte loss and affect subsequent regeneration by OPCs in white matter diseases (Montagne et al. 2018).
Due to their close relationship with the vasculature, OPCs are not only victims of BBB disruption, but may also participate in BBB pathogenesis. OPCs proliferate and up-regulate NG2 in response to macrophage, platelet, or cytokine exposure, but not other lesions that do not violate BBB integrity, suggesting that OPCs are specifically responsive to BBB opening (Rhodes et al. 2006). These activated OPCs migrate to areas of injury using the vasculature as a scaffold (Bonfanti et al. 2017). While this process is essential for remyelination following ischaemic injury, dysregulated OPCs can actually drive further BBB degradation. OPCs exposed to fibrinogen, which activates BMP signaling, are more likely to differentiate into astrocytes instead of oligodendrocytes, fueling gliosis and hindering remyelination (Petersen et al. 2017). Wnt signaling dysfunction in MS can cause OPCs to fail at migration along blood vessels and instead cluster around the vasculature (Niu et al. 2019). This dysfunctional clustering leads OPCs to physically evict astrocyte endfeet from the BBB, disrupt tight junctions between endothelial cells, and increase BBB permeability (Niu et al. 2019).
In addition to physically disrupting the BBB, OPCs may actively degrade the ECM. Following injury to the white matter via cerebral hypoperfusion, OPCs were shown to secrete MMP9, a matrix metalloproteinase that cleaves ECM proteins (Seo et al. 2013). In vitro treatment of OPC cultures with IL1β also induced secretion of MMP9. Exposing brain endothelial cell cultures to this OPC-conditioned media resulted in degradation of ZO1, the tight junction protein responsible for maintaining BBB integrity (Seo et al. 2013). Further study is required to understand the pathological influences of OPCs on the BBB and may yield novel therapeutic strategies for neuroinflammatory or neurovascular diseases.
OPCs as immunomodulatory cells
In concert with immune cells like microglia, OPCs may play a significant role in modulating the immune response. Early observations found that OPCs near inflammatory lesions in experimental autoimmune encephalitis (EAE) undergo morphological changes, including shortening and thickening of processes reminiscent of microglial or astrocytic activation (Nishiyama et al. 1999). In a rat model of contusive spinal cord injury, a small number of NG2-positive cells expressed CD11b, a canonical marker of macrophages and microglia, and assumed an unusual ameboid morphology with short processes and endfeet (Lytle et al. 2006). Following cortical cryo-injury, NG2-positive OPCs at the lesion site preferentially differentiated into bushy GFAP-positive astrocytes, suggesting a role in glial scar formation (Tatsumi et al. 2008). A study of spinal cord injury in postmortem human samples revealed high levels of NG2 around lesion sites up to 24 d after injury (Buss et al. 2009). Notably, some cells were positive for both NG2 and CD68, a protein typically expressed by phagocytic immune cells. OPCs are also highly sensitive to proinflammatory cytokines. IFNγ treatment decreases OPC differentiation into myelinating oligodendrocytes and prevents remyelination following cuprizone treatment (Lin et al. 2006). Similarly, exposure to TNFɑ inhibited differentiation and induced apoptosis (Su et al. 2011). Concurrent IFNγ and TNFɑ exposure in cultures of differentiating OPCs resulted in lower levels of MBP production (Feldhaus et al. 2004). Immune-mediated OPC dysfunction and death is reviewed in more detail by Antel et al. (2019). Although one clear effect of proinflammatory stimulation is cell death, the failed differentiation of surviving cells introduces the possibility that they are forsaking their oligodendrocyte fate to assume an immune cell-like phenotype.
Together, these earlier studies suggested that OPCs are not only present at sites of brain inflammation but likely also have unique roles as reactive and immunomodulatory cells. This has since been observed across many disease contexts. OPCs increase migratory speed and proliferation rate after cuprizone-mediated demyelination, releasing IL1β and CCL2 in the process (Moyon et al. 2015). OPCs in both active MS white matter lesions and tissue samples from patients with schizophrenia exhibit clustering behavior, unlike their typical nonoverlapping spatial arrangement (Hughes et al. 2013; Kolomeets et al. 2013; Niu et al. 2019). In human postmortem brain samples from AD patients, proximity to amyloid-β plaques was associated with retracted processes in OPCs (Nielsen et al. 2013). These morphological changes could be recapitulated in vitro by the addition of amyloid-β oligomers, suggesting that OPCs react directly to toxic protein aggregates in a primary inflammatory response (Nielsen et al. 2013). In models of synucleinopathies like multiple system atrophy or Parkinson's disease (PD), OPCs have been shown to accumulate endogenous α-synuclein and internalize exogenous α-synuclein, leading to impaired differentiation to oligodendrocytes and reduced trophic and metabolic support of neurons (Kaji et al. 2018).
OPCs have been shown to express numerous immunomodulatory molecules, including chemokines, cytokines, complement and complement receptors, and regulatory ligands (Zeis et al. 2016). They may play a critical role in amplifying peripheral immune cell recruitment in EAE via IL17-Act1 signaling (Kang et al. 2013). Some activated OPCs may even be phagocytes. Falcão et al. (2018) found that half of OPCs in vitro could phagocytose myelin particles, suggesting significant functional heterogeneity among OPCs. Characterizing the difference between phagocytic and nonphagocytic OPCs could yield insight into mechanisms regulating the conversion of OPCs into immune-responsive cells.
Stereotyped changes to OPC activity and morphology in disease environments suggest the existence of a distinct disease-associated cell state that can be identified transcriptomically. Indeed, inducing EAE in mice led to the generation of a transcriptionally distinct OPC population absent from untreated animals (Falcão et al. 2018). These OPCs expressed genes related to the innate immune response, including the pattern recognition receptor Toll-like receptor 3 (Tlr3) as well as members of the Serpina gene family. OPCs expressing MHC class II proteins have been found in both mouse models and human patients with MS, indicating that disease conditions can drive OPCs to become antigen-processing and antigen-presenting cells (Falcão et al. 2018; Kirby et al. 2019). Other human single-nucleus and single-cell RNA sequencing datasets also suggest that OPCs skew toward proinflammatory fates in various neurological diseases. In multiple sclerosis patients, OPCs are less abundant, and oligodendrocytes are enriched for a subcluster of intermediate oligodendrocytes expressing genes associated with antigen presentation (Jäkel et al. 2019). In individuals with high Alzheimer's disease pathology, OPCs express more genes related to granulocyte activation (Mathys et al. 2019). How these gene expression patterns converge or diverge across inflammatory CNS diseases remains unknown, but they may serve as a framework for exploring how disease-associated OPCs arise and behave in vivo, similar to disease-associated microglia (DAMs) in AD (Keren-Shaul et al. 2017).
The decision to become a proinflammatory OPC may depend on signal transduction via low-density lipoprotein receptor-related protein 1 (LRP1). Mice with LRP1 conditionally knocked out under control of the Olig1 promoter had a reduced transcriptional and phenotypic inflammatory response to both EAE and cuprizone, accompanied by increased myelination and decreased CD8+ cell proliferation (Fernández-Castañeda et al. 2020). Deletion of LRP1 under the Pdgfra promoter also reduced cuprizone-induced pathology (Auderset et al. 2020, preprint), suggesting that LRP1 signaling pushes OPCs toward a nonmyelinating, proinflammatory phenotype. As LRP1 has been linked to clearance of the pathological protein amyloid-β in Alzheimer's disease, understanding which binding partner of LRP1 mediates this effect would be of high interest (Kanekiyo and Bu 2014).
In contrast to their detrimental effect on brain health in some contexts, OPCs may play an important beneficial role in immune homeostasis. Ablation of NG2-positive cells in conjunction with lipopolysaccharide (LPS) treatment led to microglia overactivation and neuronal death from massive neuroinflammation (Nakano et al. 2017). Intriguingly, while inhibition of microglia activation attenuated neuronal death, treatment with an NG2-cell-derived cytokine provided even more significant neuroprotection (Nakano et al. 2017). These findings suggest that OPCs not only regulate the activity of immune cells but also mediate the sensitivity and vulnerability of neurons to an inflammatory environment.
This relationship between OPCs and microglia may be mediated in large part by TGFβ2 signaling (Zhang et al. 2019b). The cytokine TGFβ is well known for its essential role in the induction and maintenance of a homeostatic or quiescent phenotype in microglia, which is characterized by expression of the CX3C motif chemokine receptor 1 (CX3CR1) (Zujovic et al. 2000; Abutbul et al. 2012; Butovsky et al. 2014; Limatola and Ransohoff 2014). Culturing primary rat microglia with OPCs or OPC-conditioned media not only up-regulated CX3CR1 in the microglia, but also suppressed their response to LPS-induced inflammation. OPC-derived TGFβ2 was demonstrated to be responsible for this homeostatic effect (Zhang et al. 2019b). Whether OPCs release other cytokines to regulate other aspects of microglia function or even peripheral immune cells remains unknown.
Oxidative stress and OPC senescence
Under physiological conditions, OPCs play a critical role in maintaining the homeostasis of neurons, endothelial cells, and other glia, and are highly reactive to damage or dysfunction in other brain cell types. However, OPCs may be the primary targets of some pathologies, namely, oxidative stressors. OPCs depend on high pentose phosphate pathway activity to produce the antioxidant glutathione and are sensitive to NADPH depletion (Kilanczyk et al. 2016). Compared with astrocytes, OPCs are under higher baseline oxidative stress and have higher free intracellular iron, meaning a poorer ability to scavenge reactive oxidative species (ROS) and increased susceptibility to cell death following hypoxia or blue light exposure (Husain and Juurlink 1995; Thorburne and Juurlink 1996). When subject to oxidative stress, both rodent and human OPCs exhibit increased degrees of free radicals, mitochondrial swelling, and chromatin margination and condensation compared with mature oligodendrocytes (Back et al. 1998). Because of their metabolic sensitivity, OPCs may serve as sentinels and active participants in early disease progression. This property may be of great relevance in PD, which is characterized by oxidative stress and disease-causing genetic variants related to mitochondrial impairment.
Deeply connected to oxidative stress and metabolic dysfunction is senescence, a cellular state characterized by permanent cell cycle cessation and apoptosis resistance (Di Leonardo et al. 1994). It was first described by Leonard Hayflick and Paul Moorhead, who noticed that cultured human cells ceased proliferating after 40–60 cycles of cell division due to progressive telomere shortening (1961). Although arresting the proliferation of cells with DNA damage is beneficial for tumor suppression, the resulting senescent cells remain metabolically active and can release inflammatory cytokines, reactive oxidative species, and ECM-degrading enzymes. This senescence-associated secretory phenotype (SASP) can drive inflammation in the cellular milieu and induce senescence in neighboring cells (Hubackova et al. 2012; Nelson et al. 2018). Evidence is rapidly accumulating that senescence may be a primary event in many neurodegenerative diseases (Golde and Miller 2009; Streit et al. 2009; Bussian et al. 2018).
Early in vitro studies suggested that OPCs are a nonsenescent cell type, as OPCs derived from P7 rats did not undergo replicative senescence even after 20 mo in culture (Tang et al. 2000). The cells retained p53-dependent checkpoint responses and were able to assume a senescent phenotype after treatment with known genotoxic agents, indicating that these OPCs had the ability to undergo senescence but simply did not do so under normal culture conditions (Tang et al. 2001). However, subsequent studies found that OPCs cultured from aged rodents are impaired in multiple ways compared with those from young animals, including a diminished ability to differentiate into mature oligodendrocytes in vitro (Neumann et al. 2019). “Old” OPCs exhibited double-stranded DNA breaks and up-regulation of senescence-associated genes such as Cdkn2a (Neumann et al. 2019). Conversely, experimental models of DNA damage also generate OPCs with a senescent-like phenotype in vivo. Following focal X-irradiation at T13 of the adult mouse spinal cord, up to one-third of OPCs persisted in the irradiated zone for up to 6 wk. Surviving OPCs were mitotically inert, prevented infiltration of new OPCs into the area, and were significantly less capable of remyelination following a second demyelinating insult (Chari et al. 2003). Taken together, these findings indicate that damaged OPCs may become senescent or senescent-like, and stressors ranging from natural aging to acute injury can induce these dysfunctional phenotypes.
Because aging in humans and rodents is associated with reduced white matter volume and reduced remyelination potential, it is appealing to speculate that conversion of OPCs into a senescent state may contribute to these deficits through reduced proliferation and differentiation into myelinating oligodendrocytes (Gilson and Blakemore 1993; Sim et al. 2002; Cox et al. 2016; Farokhian et al. 2017). These deficits may be exacerbated by age-associated neurodegenerative diseases such as AD. In the triple transgenic mouse model of AD, which develops amyloid plaques by 6 mo of age, OPCs exhibit a pattern of early morphological atrophy but do not decrease significantly in numbers, suggesting that rapid and early loss of MBP in the hippocampus is due to OPC disruption rather than exhaustion or depletion (Vanzulli et al. 2020). Thus, OPC senescence may be responsible for some of the earliest neuropathological changes that occur long before overt cell loss in AD.
Senescent OPCs also change in their capacity as immune modulators. Recent work has provided evidence for senescent OPCs associated with amyloid-β plaques in human patients with AD and mouse models, consistent with a general hypothesis that OPCs may be primary responders to amyloid-β itself (Zhang et al. 2019a; Vanzulli et al. 2020). Notably, pharmacologically ablating senescent OPCs in a transgenic amyloid-β mouse model reduced microglial activation and decreased levels of IL6, IL1β, and TNFɑ, which are molecular components of the SASP, suggesting that senescent OPCs create an inflammatory environment that drives responses from other glial cell types. However, ablation of senescent cells also decreased the overall amyloid burden, so it is unclear whether reduced inflammation is a primary or secondary result of OPC senolysis (Zhang et al. 2019a).
One conserved mechanism of OPC senescence could be pathological intracellular lipid droplet (LD) accumulation, a hallmark of many diseases and risk factors such as aging, AD, atherosclerosis, cancer, inflammation, and mitochondrial dysfunction (Bozza and Viola 2010; Boren and Brindle 2012; Yuan et al. 2012; Cantuti-Castelvetri et al. 2018; Marschallinger et al. 2020). LD accumulation has been characterized in the brain for microglia, which develop LDs and suffers lysosomal damage when cholesterol from phagocytosed myelin oversaturates the cell's lipid efflux capacity, leading to lysosomal damage and release of proinflammatory molecules via the NLRP3 inflammasome (Cantuti-Castelvetri et al. 2018). Conversely, chronic inflammation can induce microglial LD accumulation, leading to reduced phagocytic activity and lysosome dysfunction (Marschallinger et al. 2020). Stressed astrocytes also form more LDs and exhibit dysfunctional phenotypes such as glucose hypometabolism and impaired endocytosis (Farmer et al. 2019). Whether OPCs accumulate LDs under stress and how they may respond is unknown. Because a subset of OPCs may be able to phagocytose myelin particles (Falcão et al. 2018), it is possible that LD formation and inflammation analogous to that in microglia could drive similar senescent-like, proinflammatory phenotypes in OPCs.
Roles of biological sex in OPC pathology and resilience
Sex hormones are known to broadly modulate both glial cell activity and immune responses (for review, see Schwartz et al. 2012), and molecular evidence indicates that OPCs are no exception. Estrogen ɑ and β-receptors are expressed on the nucleus and in the cytosol of both male and female OPCs (Takao et al. 2004). In culture, OPCs respond to progesterone treatment by proliferating, up-regulating key transcription factors, producing myelin, and increasing cellular branching, suggesting that steroid cues can influence OPC differentiation into oligodendrocytes (Chan et al. 1998; Marin-Husstege et al. 2004; Labombarda et al. 2009). While direct evidence for progesterone receptor expression on OPCs has not been reported, it is indirectly implicated through the process of myelination. For example, progesterone treatment in rats increases OPC proliferation and MBP expression following spinal cord injury, and global ablation of the progesterone receptor in mice abolishes progesterone-mediated OPC proliferation (Labombarda et al. 2006, 2015). Blocking progesterone in the periphery also inhibits remyelination of the mouse sciatic nerve (Baulieu and Schumacher 2000).
As a result of their sensitivity to sex hormones, OPCs may exhibit functional sex differences. OPC primary cultures derived from female rats were reported to have increased proliferation and migration compared with those derived from males, whereas male-derived OPCs differentiated more readily and exhibited increased cytotoxicity in response to cell stress (Yasuda et al. 2020). Single-cell RNA sequencing revealed that OPCs from female mice showed up-regulation of proliferation-associated genes including Olig1, Olig2, Pdgfa, and Nf1 as well as genes associated with BBB regulation such as Tgfβ1 and Igf1 (Yasuda et al. 2020). In a separate single-cell RNA sequencing study, the proportions of OPC subtypes defined by the study differed by sex (Beiter et al. 2020). Male OPCs were overrepresented in a cluster enriched in genes associated with cellular respiration, and female OPCs were overrepresented in a cluster enriched in genes associated with neuronal differentiation and synapse organization, potentially suggesting that a larger proportion of OPCs contribute to synaptic function in females. It remains to be studied whether these differences functionally impact neuron-OPC synapses.
Regional sex differences in OPCs have also been documented. OPCs in male mice were found to be enriched in the septal wall of the SVZ compared with OPCs in female mice (Mizrak et al. 2019). Such a difference may reflect a bias in male animals toward generating OPCs or oligodendrocyte lineage cells from neural stem cells in the adult brain. Whether this may impact sex differences in remyelination is certainly an interesting area for future research.
Such differences in developing OPCs could be responsible for the differences in white matter structure and volume observed in both humans and animal models. Males are reported to possess a higher overall volume of white matter than females, but females appear to have larger corpus callosum area (Holloway and de Lacoste 1986; Gur et al. 1999). Females reach peak myelination earlier and have more myelin immunoreactivity than males in the first three decades of life, suggesting differences in the dynamics of OPC proliferation and differentiation (Benes et al. 1994; Yurgelun-Todd et al. 2002). Interestingly, slower myelination in males may prolong a window of vulnerability in OPCs, leading to the increased prevalence of certain neurodevelopmental disorders such as autism spectrum disorder and schizophrenia, which are additionally characterized by white matter defects (Bartzokis 2004).
Together, sex differences and age also affect the efficiency and extent of remyelination following injury to the white matter, as this process largely depends on the proliferation, migration, and differentiation of OPCs into oligodendrocytes. Although MS is more prevalent in women, their overall prognosis is improved and may be due in part to more extensive remyelination (Nicot 2009). In mice, gonadectomy and supplementation of 17-β-estradiol (estrogen) increased remyelination in both male and female mice that were 11–13 wk of age. Estrogen treatment also increased the numbers of oligodendrocytes and OPCs without affecting other glia types, suggesting that increased OPC proliferation drives this sex difference in remyelination (Patel et al. 2013). Other studies have shown that though young animals do not differ significantly in remyelination due to the rapid pace of repair, aged female rats are able to remyelinate more efficiently than males. This effect is not abolished through gonadectomy in old animals, suggesting that this age-dependent sex difference may depend on nongonadal sex differences or epigenetic changes (Li et al. 2006).
By the seventh decade of life, both males and females are reported to suffer a decline in white matter volume of between 16%–20% (Meier-Ruge et al. 1992; Salat et al. 1999). However, responses to age-associated disease may diverge. Single-cell RNA sequencing from postmortem human brain tissue has revealed that both OPCs and oligodendrocytes exhibit sex-specific differences in their transcriptional response to AD pathology (Mathys et al. 2019). Male oligodendrocytes largely up-regulated genes in response to pathology, and female oligodendrocytes did not. Moreover, female OPCs tended to down-regulate genes in response to pathology, whereas male OPCs did not. A negative correlation between white matter lesions and cognition was reported in females but not males, which may imply that OPCs and oligodendrocytes in females do not produce as strong of a compensatory transcriptional response to preserve cognition following insult (Mathys et al. 2019). Understanding the mechanisms of sexual dimorphism in OPCs and oligodendrocytes would improve our ability to disentangle the complicated progression of this disease. Ultimately, the functional significance of sex differences in glia is a now flourishing research area (Hanamsagar et al. 2017; Kodama and Gan 2019; VanRyzin et al. 2019). Given the relevance of OPCs to many aspects of CNS function and disease, extending this work to these cells would be of high interest to the field.
Conclusions
Research over the last two decades has clearly established OPCs as a distinct brain cell type that plays numerous roles in the developing and mature CNS. Beyond serving as a self-renewing oligodendrocyte progenitor population, OPCs form an intricate and widespread network of neuronal activity sensors, immune-responsive cells, and vascular regulators. Nevertheless, OPC function and regulation remain relatively poorly understood. Transcriptomic and epigenetic profiling and cluster analysis of OPCs across different brain regions in mice could reveal how OPCs functionally specialize based on the demands of their microenvironment. The interactions between OPCs and neurons or other glia constitute another major area of future research. For example, cell type-specific application of optogenetic or chemogenetic tools in mice could provide an in vivo model system to understand the flow of information between neurons and OPCs, and how OPCs may influence neural circuit function.
OPCs are an understudied component of many neurological diseases. Because of their dynamic, multipotent nature, they are poised to initiate or perpetuate disease pathology in several contexts, including neuroinflammation and neurodegeneration. Here, human models are essential to understand human-specific mechanisms that are most likely to inform successful clinical advances. Human induced pluripotent stem cell (iPSC)-derived models are thus a powerful avenue for dissecting the roles that genetic risk variants play in OPCs, as well as crosstalk between OPCs and other brain cell types (Penney et al. 2020). Combined with improved high-throughput methods, these models have immense translational potential to identify novel therapeutic strategies that target OPCs (Skaper 2019).
Furthermore, sex differences in OPCs may play significant roles in susceptibility and response to neurological disorders. Certain neurodevelopmental or neuropsychiatric disorders with significant sex biases in incidence such as ASD may be partly explained by differences in OPC proliferation and differentiation caused by sex hormones or biological sex. For complex disorders with significant environmental contributions, the study of OPC population dynamics in mouse models of social stress or toxicant exposure (Bolton et al. 2017) could delineate whether oligodendrocyte lineage dysfunction is a primary mechanism or secondary consequence of sex-specific resilience or vulnerability. Ultimately, a deeper understanding of the many factors that influence OPC biology will prove essential to our understanding of the brain's complexities in health and disease.
Acknowledgments
We thank Joel Blanchard, Mitchell Murdock, Jay Penney, William Ralvenius, and Matheus Victor for providing critical feedback during manuscript preparation. This work was supported by the Robert A. and Renee E. Belfer Family Foundation, the Cure Alzheimer's Fund, the Ludwig Family Foundation, and the National Institutes of Health (RF1 AG062377, RF1 AG054012-01, U54HG008097, and UG3NS115064). L.A.A. was supported by the Henry E. Singleton Fellowship, and A.H.E. was supported by the Schoemaker Graduate Fellowship.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.344218.120.
References
- Abutbul S, Shapiro J, Szaingurten-Solodkin I, Levy N, Carmy Y, Baron R, Jung S, Monsonego A. 2012. TGF-β signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia 60: 1160–1171. 10.1002/glia.22343 [DOI] [PubMed] [Google Scholar]
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonté C, Aloisi F, Visentin S. 2005. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia 50: 132–144. 10.1002/glia.20160 [DOI] [PubMed] [Google Scholar]
- Antel JP, Lin YH, Cui Q-L, Pernin F, Kennedy TE, Ludwin SK, Healy LM. 2019. Immunology of oligodendrocyte precursor cells in vivo and in vitro. J Neuroimmunol 331: 28–35. 10.1016/j.jneuroim.2018.03.006 [DOI] [PubMed] [Google Scholar]
- Arai K, Lo EH. 2009. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci 29: 4351–4355. 10.1523/JNEUROSCI.0035-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assinck P, Duncan GJ, Plemel JR, Lee MJ, Stratton JS, Manesh SB, Liu J, Ramer LM, Kang SH, Bergles DE, et al. 2017. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J Neurosci 37: 8635–8654. 10.1523/JNEUROSCI.2409-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auderset L, Pitman KA, Cullen CL, Pepper RE, Taylor BV, Foa L, Young KM. 2020. Low-density lipoprotein receptor-related protein 1 (LRP1) is a negative regulator of oligodendrocyte progenitor cell differentiation in the adult mouse brain. Front Cell Dev Biol 8: 564351 10.3389/fcell.2020.564351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. 1998. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci 18: 6241–6253. 10.1523/JNEUROSCI.18-16-06241.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracskay KL, Kidd GJ, Miller RH, Trapp BD. 2007. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia 55: 1001–1010. 10.1002/glia.20519 [DOI] [PubMed] [Google Scholar]
- Baron W, Jonge JCD, Vries HD, Hoekstra D. 1998. Regulation of oligodendrocyte differentiation: protein kinase C activation prevents differentiation of O2A progenitor cells toward oligodendrocytes. Glia 22: 121–129. <121::AID-GLIA3>3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- Barres BA, Koroshetz WJ, Swartz KJ, Chun LL, Corey DP. 1990. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron 4: 507–524. 10.1016/0896-6273(90)90109-S [DOI] [PubMed] [Google Scholar]
- Bartzokis G. 2004. Quadratic trajectories of brain myelin content: unifying construct for neuropsychiatric disorders. Neurobiol Aging 25: 49–62. 10.1016/j.neurobiolaging.2003.08.001 [DOI] [Google Scholar]
- Baulieu E, Schumacher M. 2000. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Steroids 65: 605–612. 10.1016/S0039-128X(00)00173-2 [DOI] [PubMed] [Google Scholar]
- Beiter RM, Fernández-Castañeda A, Rivet-Noor C, Merchak A, Bai R, Slogar E, Seki SM, Rosen DA, Overall CC, Gaultier A. 2020. Evidence for oligodendrocyte progenitor cell heterogeneity in the adult mouse brain. bioRxiv 10.1101/2020.03.06.981373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Malgrange B, Rigo JM, Rogister B, Leprince P, Hans G, Nguyen L, Moonen G. 2000. Glycine triggers an intracellular calcium influx in oligodendrocyte progenitor cells which is mediated by the activation of both the ionotropic glycine receptor and Na+-dependent transporters. Eur J Neurosci 12: 1924–1930. 10.1046/j.1460-9568.2000.00085.x [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. 2003. Postnatal NG2 proteoglycan–expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol 161: 169–186. 10.1083/jcb.200210110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. 1994. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry 51: 477–484. 10.1001/archpsyc.1994.03950060041004 [DOI] [PubMed] [Google Scholar]
- Berger T, Schnitzer J, Kettenmann H. 1991. Developmental changes in the membrane current pattern, K+ buffer capacity, and morphology of glial cells in the corpus callosum slice. J Neurosci 11: 3008–3024. 10.1523/JNEUROSCI.11-10-03008.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Richardson WD. 2016. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol 8: a020453 10.1101/cshperspect.a020453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JDB, Somogyi P, Jahr CE. 2000. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405: 187–191. 10.1038/35012083 [DOI] [PubMed] [Google Scholar]
- Birey F, Kloc M, Chavali M, Hussein I, Wilson M, Christoffel DJ, Chen T, Frohman MA, Robinson JK, Russo SJ, et al. 2015. Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 88: 941–956. 10.1016/j.neuron.2015.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenfeld G v, Verkhratsky AN, Kettenmann H. 1992. Ca2+ channel expression in the oligodendrocyte lineage. Eur J Neurosci 4: 1035–1048. 10.1111/j.1460-9568.1992.tb00130.x [DOI] [PubMed] [Google Scholar]
- Bögler O, Wren D, Barnett SC, Land H, Noble M. 1990. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. PNAS 87: 6368–6372. 10.1073/pnas.87.16.6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD. 2017. Gestational exposure to Air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Front Synaptic Neurosci 9: 10 10.3389/fnsyn.2017.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetto G, Kamen Y, Evans KA, Káradóttir RT. 2020. Unraveling myelin plasticity. Front Cell Neurosci 14: 156 10.3389/fncel.2020.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti E, Gelosa P, Fumagalli M, Dimou L, Viganò F, Tremoli E, Cimino M, Sironi L, Abbracchio MP. 2017. The role of oligodendrocyte precursor cells expressing the GPR17 receptor in brain remodeling after stroke. Cell Death Dis 8: e2871 10.1038/cddis.2017.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongarzone ER, Howard SG, Schonmann V, Campagnoni AT. 1998. Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation. J Neurosci 18: 5344–5353. 10.1523/JNEUROSCI.18-14-05344.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren J, Brindle KM. 2012. Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation. Cell Death Differ 19: 1561–1570. 10.1038/cdd.2012.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza PT, Viola JPB. 2010. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids 82: 243–250. 10.1016/j.plefa.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. 2010. Oligodendrocytes: biology and pathology. Acta Neuropathol 119: 37–53. 10.1007/s00401-009-0601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, Brook GA. 2009. NG2 and phosphacan are present in the astroglial scar after human traumatic spinal cord injury. BMC Neurol 9: 32 10.1186/1471-2377-9-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. 2018. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562: 578–582. 10.1038/s41586-018-0543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. 2014. Identification of a unique TGF-β dependent molecular and functional signature in microglia. Nat Neurosci 17: 131–143. 10.1038/nn.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil M-T, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lütjohann D, et al. 2018. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359: 684–688. 10.1126/science.aan4183 [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI. 2009. Driving fast-spiking cells induces γ rhythm and controls sensory responses. Nature 459: 663–667. 10.1038/nature08002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona JG, Smith MD, Wang J, Kirby L, Schott JT, Davidson T, Karnell JL, Whartenby KA, Calabresi PA. 2019. Quetiapine has an additive effect to triiodothyronine in inducing differentiation of oligodendrocyte precursor cells through induction of cholesterol biosynthesis. PLoS One 14: e0221747 10.1371/journal.pone.0221747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Phillips LJ 2nd, Glaser M. 1998. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci 95: 10459–10464. 10.1073/pnas.95.18.10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari DM, Huang WL, Blakemore WF. 2003. Dysfunctional oligodendrocyte progenitor cell (OPC) populations may inhibit repopulation of OPC depleted tissue. J Neurosci Res 73: 787–793. 10.1002/jnr.10700 [DOI] [PubMed] [Google Scholar]
- Cheli V, Santiago González D, Spreuer V, Paez P. 2015. Voltage-gated Ca++ entry promotes oligodendrocyte progenitor cells maturation and myelination in vitro. Exp Neurol 265: 69–83. 10.1016/j.expneurol.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA, et al. 2009. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci 12: 1398–1406. 10.1038/nn.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y, Huynh T, Pleasure SJ. 2014. Migration of oligodendrocyte progenitor cells is controlled by transforming growth factor β family proteins during corticogenesis. J Neurosci 34: 14973–14983. 10.1523/JNEUROSCI.1156-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, Wardlaw JM, Gale CR, Bastin ME, Deary IJ. 2016. Ageing and brain white matter structure in 3,513 UK biobank participants. Nat Commun 7: 13629 10.1038/ncomms13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Bercury KK, Jin W, Macklin WB. 2015. Olig1 acetylation and nuclear export mediate oligodendrocyte development. J Neurosci 35: 15875–15893. 10.1523/JNEUROSCI.0882-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. 2009. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci 106: 641–646. 10.1073/pnas.0805165106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MRL, Polito A, Levine JM, Reynolds R. 2003. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24: 476–488. 10.1016/S1044-7431(03)00210-0 [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. 2005. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol 168: 415–427. 10.1083/jcb.200407053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Faria O, Gonsalvez DG, Nicholson M, Xiao J. 2019. Activity-dependent central nervous system myelination throughout life. J Neurochem 148: 447–461. 10.1111/jnc.14592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente AG, Lange S, Silva ME, Gonzalez GA, Tempfer H, van Wijngaarden P, Zhao C, Di Canio L, Trost A, Bieler L, et al. 2017. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep 20: 1755–1764. 10.1016/j.celrep.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Wang H, Rosenberg PA, Volpe JJ, Jensen FE. 2004. Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc Natl Acad Sci 101: 7751–7756. 10.1073/pnas.0307850101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leonardo A, Linke SP, Clarkin K, Wahl GM. 1994. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev 8: 2540–2551. 10.1101/gad.8.21.2540 [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. 2008. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci 28: 10434–10442. 10.1523/JNEUROSCI.2831-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djogo T, Robins SC, Schneider S, Kryzskaya D, Liu X, Mingay A, Gillon CJ, Kim JH, Storch K-F, Boehm U, et al. 2016. Adult NG2-glia Are required for median eminence-mediated leptin sensing and body weight control. Cell Metab 23: 797–810. 10.1016/j.cmet.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Doering LC, Fedoroff S. 1984. Isolation and transplantation of oligodendrocyte precursor cells. J Neurol Sci 63: 183–196. 10.1016/0022-510X(84)90195-3 [DOI] [PubMed] [Google Scholar]
- Domingues HS, Portugal CC, Socodato R, Relvas JB. 2016. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front Cell Dev Biol 4: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. 2006. Functional genomic analysis of oligodendrocyte differentiation. J Neurosci 26: 10967–10983. 10.1523/JNEUROSCI.2572-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz B, Popko B. 2019. Molecular control of oligodendrocyte development. Trends Neurosci 42: 263–277. 10.1016/j.tins.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. 2010. Regulation of oligodendrocyte differentiation and myelination. Science 330: 779–782. 10.1126/science.1190927 [DOI] [PubMed] [Google Scholar]
- Emery B, Lu QR. 2015. Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb Perspect Biol 7: a020461 10.1101/cshperspect.a020461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcão AM, van Bruggen D, Marques S, Meijer M, Jäkel S, Agirre E, Samudyata, Floriddia EM, Vanichkina DP, ffrench-Constant C, et al. 2018. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat Med 24: 1837–1844. 10.1038/s41591-018-0236-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer BC, Kluemper J, Johnson LA. 2019. Apolipoprotein E4 alters astrocyte fatty acid metabolism and lipid droplet formation. Cells 8: 182 10.3390/cells8020182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhian F, Yang C, Beheshti I, Matsuda H, Wu S. 2017. Age-Related gray and white matter changes in normal adult brains. Aging Dis 8: 899–909. 10.14336/AD.2017.0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaus B, Dietzel ID, Heumann R, Berger B. 2004. Kortikoide schützen oligodendrozyten-vorläuferzellen vor zytokin-induzierten schäden [Corticoids protect oligodendrocyte precursor cells against cytokine-induced damage]. Zentralbl Gynakol 126: 282–285. 10.1055/s-2004-822759 [DOI] [PubMed] [Google Scholar]
- Fernández-Castañeda A, Gaultier A. 2016. Adult oligodendrocyte progenitor cells—multifaceted regulators of the CNS in health and disease. Brain Behav Immun 57: 1–7. 10.1016/j.bbi.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Castañeda A, Chappell MS, Rosen DA, Seki SM, Beiter RM, Johanson DM, Liskey D, Farber E, Onengut-Gumuscu S, Overall CC, et al. 2020. The active contribution of OPCs to neuroinflammation is mediated by LRP1. Acta Neuropathol 139: 365–382. 10.1007/s00401-019-02073-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant C, Raff MC. 1986. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature 319: 499–502. 10.1038/319499a0 [DOI] [PubMed] [Google Scholar]
- Finzsch M, Stolt CC, Lommes P, Wegner M. 2008. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor α expression. Development 135: 637–646. 10.1242/dev.010454 [DOI] [PubMed] [Google Scholar]
- Fröhlich N, Nagy B, Hovhannisyan A, Kukley M. 2011. Fate of neuron–glia synapses during proliferation and differentiation of NG2 cells. J Anat 219: 18–32. 10.1111/j.1469-7580.2011.01392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Paez PM, Fisher R, Handley V, Colwell CS, Campagnoni AT. 2010. Regulation of L-type Ca++ currents and process morphology in white matter oligodendrocyte precursor cells by golli-myelin proteins. Glia 58: 1292–1303. 10.1002/glia.21008 [DOI] [PubMed] [Google Scholar]
- Furusho M, Kaga Y, Ishii A, Hébert JM, Bansal R. 2011. Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. J Neurosci 31: 5055–5066. 10.1523/JNEUROSCI.4800-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W-P, Yang X-J, Zhang Z, Wang H-K, Shen W, Deng Q-D, Duan S. 2006. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science 312: 1533–1537. 10.1126/science.1124669 [DOI] [PubMed] [Google Scholar]
- Ge W-P, Zhou W, Luo Q, Jan LY, Jan YN. 2009. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc Natl Acad Sci 106: 328–333. 10.1073/pnas.0811353106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson J, Blakemore WF. 1993. Failure of remyelination in areas of demyelination produced in the spinal cord of old rats. Neuropathol Appl Neurobiol 19: 173–181. 10.1111/j.1365-2990.1993.tb00424.x [DOI] [PubMed] [Google Scholar]
- Golde TE, Miller VM. 2009. Proteinopathy-induced neuronal senescence: a hypothesis for brain failure in Alzheimer's and other neurodegenerative diseases. Alzheimers Res Ther 1: 5 10.1186/alzrt5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. 2010. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci 30: 12036–12049. 10.1523/JNEUROSCI.1360-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. 1999. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci 19: 4065–4072. 10.1523/JNEUROSCI.19-10-04065.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlandt C, Derouiche A, Wyczynski A, Haseleu J, Pohle J, Karram K, Trotter J, Seifert G, Frotscher M, Steinhäuser C, et al. 2011. Gray matter NG2 cells display multiple Ca2+-signaling pathways and highly motile processes. PLoS One 6: e17575 10.1371/journal.pone.0017575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Wigley R, Butt AM. 2010. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia 58: 66–79. 10.1002/glia.20902 [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD. 2017. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 65: 1504–1520. 10.1002/glia.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart IK, Richardson WD, Heldin CH, Westermark B, Raff MC. 1989. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development 105: 595–603. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. 1961. The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621. 10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]
- Hirayama M, Eccleston PA, Silberberg DH. 1984. The mitotic history and radiosensitivity of developing oligodendrocytes in vitro. Dev Biol 104: 413–420. 10.1016/0012-1606(84)90096-4 [DOI] [PubMed] [Google Scholar]
- Holloway RL, de Lacoste MC. 1986. Sexual dimorphism in the human corpus callosum: an extension and replication study. Hum Neurobiol 5: 87–91. [PubMed] [Google Scholar]
- Huang W, Zhao N, Bai X, Karram K, Trotter J, Goebbels S, Scheller A, Kirchhoff F. 2014. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia 62: 896–913. 10.1002/glia.22648 [DOI] [PubMed] [Google Scholar]
- Huang W, Guo Q, Bai X, Scheller A, Kirchhoff F. 2019. Early embryonic NG2 glia are exclusively gliogenic and do not generate neurons in the brain. Glia 67: 1094–1103. 10.1002/glia.23590 [DOI] [PubMed] [Google Scholar]
- Huang W, Bhaduri A, Velmeshev D, Wang S, Wang L, Rottkamp C, Alvarez-Buylla A, Rowitch DH, Kriegstein AR. 2020. Origins and proliferative states of human oligodendrocyte precursor cells. Cell 182: 594–608.e11. 10.1016/j.cell.2020s.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubackova S, Krejcikova K, Bartek J, Hodny Z. 2012. IL1- and TGFβ-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘bystander senescence’. Aging 4: 932–951. 10.18632/aging.100520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. 2013. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci 16: 668–676. 10.1038/nn.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain J, Juurlink BH. 1995. Oligodendroglial precursor cell susceptibility to hypoxia is related to poor ability to cope with reactive oxygen species. Brain Res 698: 86–94. 10.1016/0006-8993(95)00832-B [DOI] [PubMed] [Google Scholar]
- Jäkel S, Agirre E, Mendanha Falcão A, van Bruggen D, Lee KW, Knuesel I, Malhotra D, ffrench-Constant C, Williams A, Castelo-Branco G. 2019. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566: 543–547. 10.1038/s41586-019-0903-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. 2009. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat 3: 5 10.3389/neuro.05.005.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji S, Maki T, Kinoshita H, Uemura N, Ayaki T, Kawamoto Y, Furuta T, Urushitani M, Hasegawa M, Kinoshita Y, et al. 2018. Pathological endogenous α-synuclein accumulation in oligodendrocyte precursor cells potentially induces inclusions in multiple system atrophy. Stem Cell Reports 10: 356–365. 10.1016/j.stemcr.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Bu G. 2014. The low-density lipoprotein receptor-related protein 1 and amyloid-β clearance in Alzheimer's disease. Front Aging Neurosci 6: 93 10.3389/fnagi.2014.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. 2010. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68: 668–681. 10.1016/j.neuron.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM, et al. 2013. Act1 mediates IL-17–induced EAE pathogenesis selectively in NG2+glial cells. Nat Neurosci 16: 1401–1408. 10.1038/nn.3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Attwell D. 2007. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience 145: 1426–1438. 10.1016/j.neuroscience.2006.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Cavelier P, Bergersen LH, Attwell D. 2005. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438: 1162–1166. 10.1038/nature04302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Hamilton NB, Bakiri Y, Attwell D. 2008. Spiking and non-spiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci 11: 450–456. 10.1038/nn2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastritsis CH, McCarthy KD. 1993. Oligodendroglial lineage cells express neuroligand receptors. Glia 8: 106–113. 10.1002/glia.440080206 [DOI] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. 2017. A unique microglia type associated with restricting development of Alzheimer's disease. Cell 169: 1276–1290.e17. 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. 2006. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci 9: 173–179. 10.1038/nn1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilanczyk E, Saraswat Ohri S, Whittemore SR, Hetman M. 2016. Antioxidant protection of NADPH-depleted oligodendrocyte precursor cells Is dependent on supply of reduced glutathione. ASN Neuro 8: 175909141666040 10.1177/1759091416660404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. 2006. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci 9: 1506–1511. 10.1038/nn1803 [DOI] [PubMed] [Google Scholar]
- Kirby L, Jin J, Cardona JG, Smith MD, Martin KA, Wang J, Strasburger H, Herbst L, Alexis M, Karnell J, et al. 2019. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 10: 1–20. 10.1038/s41467-019-11638-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Scherer J, Möller T, Verkhratsky A, Kettenmann H. 1995. Subcellular heterogeneity of voltage-gated Ca2+ channels in cells of the oligodendrocyte lineage. Glia 13: 1–12. 10.1002/glia.440130102 [DOI] [PubMed] [Google Scholar]
- Klum S, Zaouter C, Alekseenko Z, Björklund ÅK, Hagey DW, Ericson J, Muhr J, Bergsland M. 2018. Sequentially acting SOX proteins orchestrate astrocyte- and oligodendrocyte-specific gene expression. EMBO Rep 19: e46635 10.15252/embr.201846635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama L, Gan L. 2019. Do microglial sex differences contribute to sex differences in neurodegenerative diseases? Trends Mol Med 25: 741–749. 10.1016/j.molmed.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomeets NS, Vostrikov VM, Uranova NA. 2013. Abnormalities in oligodendrocyte clusters in the inferior parietal cortex in schizophrenia are associated with insight. Eur J Psychiatry 27: 248–258. 10.4321/S0213-61632013000400003 [DOI] [Google Scholar]
- Kondo T, Raff M. 2000. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289: 1754–1757. 10.1126/science.289.5485.1754 [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. 2009. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32: 149–184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labombarda F, Gonzalez S, Deniselle MCG, Garay L, Guennoun R, Schumacher M, Nicola AFD. 2006. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J Neurotrauma 23: 181–192. 10.1089/neu.2006.23.181 [DOI] [PubMed] [Google Scholar]
- Labombarda F, González SL, Lima A, Roig P, Guennoun R, Schumacher M, Nicola AF de. 2009. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia 57: 884–897. 10.1002/glia.20814 [DOI] [PubMed] [Google Scholar]
- Labombarda F, Jure I, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF. 2015. A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J Steroid Biochem Mol Biol 154: 274–284. 10.1016/j.jsbmb.2015.09.011 [DOI] [PubMed] [Google Scholar]
- Li W-W, Penderis J, Zhao C, Schumacher M, Franklin RJM. 2006. Females remyelinate more efficiently than males following demyelination in the aged but not young adult CNS. Exp Neurol 202: 250–254. 10.1016/j.expneurol.2006.05.012 [DOI] [PubMed] [Google Scholar]
- Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C. 2013. A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61: 732–749. 10.1002/glia.22469 [DOI] [PubMed] [Google Scholar]
- Limatola C, Ransohoff RM. 2014. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci 8: 229 10.3389/fncel.2014.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Bergles DE. 2004. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci 7: 24–32. 10.1038/nn1162 [DOI] [PubMed] [Google Scholar]
- Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. 2006. Interferon-γ inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain 129: 1306–1318. 10.1093/brain/awl044 [DOI] [PubMed] [Google Scholar]
- Liu A, Han YR, Li J, Sun D, Ouyang M, Plummer MR, Casaccia-Bonnefil P. 2007. The glial or neuronal fate choice of oligodendrocyte progenitors is modulated by their ability to acquire an epigenetic memory. J Neurosci 27: 7339–7343. 10.1523/JNEUROSCI.1226-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu RQ, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. 2002. Common developmental requirement for olig function indicates a motor neuron/oligodendrocyte connection. Cell 109: 75–86. 10.1016/S0092-8674(02)00678-5 [DOI] [PubMed] [Google Scholar]
- Lytle JM, Vicini S, Wrathall JR. 2006. Phenotypic changes in NG2+ cells after spinal cord injury. J Neurotrauma 23: 1726–1738. 10.1089/neu.2006.23.1726 [DOI] [PubMed] [Google Scholar]
- Maki T, Maeda M, Uemura M, Lo EK, Terasaki Y, Liang AC, Shindo A, Choi YK, Taguchi A, Matsuyama T, et al. 2015. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett 597: 164–169. 10.1016/j.neulet.2015.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado PP, Vélez-Fort M, Levavasseur F, Angulo MC. 2013. Oligodendrocyte precursor cells Are accurate sensors of local K+ in mature gray matter. J Neurosci 33: 2432–2442. 10.1523/JNEUROSCI.1961-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. 2004. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. DNE 26: 245–254. [DOI] [PubMed] [Google Scholar]
- Marisca R, Hoche T, Agirre E, Hoodless LJ, Barkey W, Auer F, Castelo-Branco G, Czopka T. 2020. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat Neurosci 23: 363–374. 10.1038/s41593-019-0581-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcão A, Xiao L, Li H, Häring M, Hochgerner H, Romanov RA, et al. 2016. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352: 1326–1329. 10.1126/science.aaf6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, et al. 2020. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci 23: 194–208. 10.1038/s41593-019-0566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, et al. 2019. Single-cell transcriptomic analysis of Alzheimer's disease. Nature 570: 332–337. 10.1038/s41586-019-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral SR, Chan JR. 2016. The environment rules: spatiotemporal regulation of oligodendrocyte differentiation. Curr Opin Neurobiol 39: 47–52. 10.1016/j.conb.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy GF, Leblond CP. 1988. Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J Comp Neurol 271: 589–603. 10.1002/cne.902710409 [DOI] [PubMed] [Google Scholar]
- McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronsont SA. 1990. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron 5: 603–614. 10.1016/0896-6273(90)90215-2 [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Ulrich J, Brühlmann M, Meier E. 1992. Age-related white matter atrophy in the human brain. Ann N Y Acad Sci 673: 260–269. 10.1111/j.1749-6632.1992.tb27462.x [DOI] [PubMed] [Google Scholar]
- Merchán P, Bribián A, Sánchez-Camacho C, Lezameta M, Bovolenta P, de Castro F. 2007. Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. Mol Cell Neurosci 36: 355–368. 10.1016/j.mcn.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Michalski J-P, Kothary R. 2015. Oligodendrocytes in a nutshell. Front Cell Neurosci 9: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha S, Valloton D, Brunet I, Eichmann A, Hornung J-P, Lebrand C. 2015. NG2 glia are required for vessel network formation during embryonic development. Elife 4: e09102 10.7554/eLife.09102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak D, Mendes Levitin H, Delgado AC, Crotet V, Yuan J, Chaker Z, Silva-Vargas V, Sims PA, Doetsch F. 2019. Single-cell analysis of regional differences in adult V-SVZ neural stem cell lineages. Cell Rep 26: 394–406.e5. 10.1016/j.celrep.2018.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, Barnes SR, Daianu M, Ramanathan A, Go A, et al. 2018. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 24: 326–337. 10.1038/nm.4482 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mount CW, Yalçın B, Cunliffe-Koehler K, Sundaresh S, Monje M. 2019. Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. Elife 8: e49291 10.7554/eLife.49291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyon S, Dubessy AL, Aigrot MS, Trotter M, Huang JK, Dauphinot L, Potier MC, Kerninon C, Melik Parsadaniantz S, Franklin RJM, et al. 2015. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J Neurosci 35: 4–20. 10.1523/JNEUROSCI.0849-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy C, Maitra M, Tanti A, Suderman M, Théroux J-F, Davoli MA, Perlman K, Yerko V, Wang YC, Tripathy SJ, et al. 2020. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat Neurosci 23: 771–781. 10.1038/s41593-020-0621-y [DOI] [PubMed] [Google Scholar]
- Nakano M, Tamura Y, Yamato M, Kume S, Eguchi A, Takata K, Watanabe Y, Kataoka Y. 2017. NG2 glial cells regulate neuroimmunological responses to maintain neuronal function and survival. Sci Rep 7: 42041 10.1038/srep42041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Kucheryavenko O, Wordsworth J, von Zglinicki T. 2018. The senescent bystander effect is caused by ROS-activated NF-κB signalling. Mech Ageing Dev 170: 30–36. 10.1016/j.mad.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G. 2001. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development 128: 527–540. [DOI] [PubMed] [Google Scholar]
- Neumann B, Baror R, Zhao C, Segel M, Dietmann S, Rawji KS, Foerster S, McClain CR, Chalut K, van Wijngaarden P, et al. 2019. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 25: 473–485.e8. 10.1016/j.stem.2019.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot AB. 2009. Gender and sex hormones in multiple sclerosis pathology and therapy. Front Biosci (Landmark Ed) 14: 4477–4515. 10.2741/3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HM, Ek D, Avdic U, Orbjörn C, Hansson O, Veerhuis R, Rozemuller AJ, Brun A, Minthon L, Wennström M. 2013. NG2 cells, a new trail for Alzheimer's disease mechanisms? Acta Neuropathol Commun 1: 7 10.1186/2051-5960-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. 1999. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol 58: 1113–1124. 10.1097/00005072-199911000-00001 [DOI] [PubMed] [Google Scholar]
- Niu J, Tsai H-H, Hoi KK, Huang N, Yu G, Kim K, Baranzini SE, Xiao L, Chan JR, Fancy SPJ. 2019. Aberrant oligodendroglial–vascular interactions disrupt the blood–brain barrier, triggering CNS inflammation. Nat Neurosci 22: 709–718. 10.1038/s41593-019-0369-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orduz D, Maldonado PP, Balia M, Vélez-Fort M, de Sars V, Yanagawa Y, Emiliani V, Angulo MC. 2015. Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex. Elife 4: e06953 10.7554/eLife.06953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orduz D, Benamer N, Ortolani D, Coppola E, Vigier L, Pierani A, Angulo MC. 2019. Developmental cell death regulates lineage-related interneuron-oligodendroglia functional clusters and oligodendrocyte homeostasis. Nat Commun 10: 4249 10.1038/s41467-019-11904-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega MC, Bribián A, Peregrín S, Gil MT, Marín O, de Castro F. 2012. Neuregulin-1/ErbB4 signaling controls the migration of oligodendrocyte precursor cells during development. Exp Neurol 235: 610–620. 10.1016/j.expneurol.2012.03.015 [DOI] [PubMed] [Google Scholar]
- Paez PM, Lyons DA. 2020. Calcium signaling in the oligodendrocyte lineage: regulators and consequences. Annu Rev Neurosci 43: 163– 186. 10.1146/annurev-neuro-100719-093305 [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Klingener M, Aguirre A. 2014. TGFβ signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci 34: 7917–7930. 10.1523/JNEURsOSCI.0363-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Moore S, Crawford DK, Hannsun G, Sasidhar MV, Tan K, Molaie D, Tiwari-Woodruff SK. 2013. Attenuation of corpus callosum axon myelination and remyelination in the absence of circulating sex hormones. Brain Pathology 23: 462–475. 10.1111/bpa.12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. 1994. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron 12: 357–371. 10.1016/0896-6273(94)90277-1 [DOI] [PubMed] [Google Scholar]
- Penney J, Ralvenius WT, Tsai L-H. 2020. Modeling Alzheimer's disease with iPSC-derived brain cells. Mol Psychiatry 25: 148–167. 10.1038/s41380-019-0468-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MA, Ryu JK, Chang K-J, Etxeberria A, Bardehle S, Mendiola AS, Kamau-Devers W, Fancy SPJ, Thor A, Bushong EA, et al. 2017. Fibrinogen activates BMP signaling in oligodendrocyte progenitor cells and inhibits remyelination after vascular damage. Neuron 96: 1003–1012.e7. 10.1016/j.neuron.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. 1993. The oligodendrocyte and its many cellular processes. Trends Cell Biol 3: 191–197. 10.1016/0962-8924(93)90213-K [DOI] [PubMed] [Google Scholar]
- Philips T, Rothstein JD. 2017. Oligodendroglia: metabolic supporters of neurons. J Clin Invest 127: 3271–3280. 10.1172/JCI90610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaton G, Aigrot M-S, Williams A, Moyon S, Tepavcevic V, Moutkine I, Gras J, Matho KS, Schmitt A, Soellner H, et al. 2011. Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain 134: 1156–1167. 10.1093/brain/awr022 [DOI] [PubMed] [Google Scholar]
- Polito A, Reynolds R. 2005. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat 207: 707–716. 10.1111/j.1469-7580.2005.00454.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestoz L, Chatzopoulou E, Lemkine G, Spassky N, Lebras B, Kagawa T, Ikenaka K, Zalc B, Thomas J-L. 2004. Control of axonophilic migration of oligodendrocyte precursor cells by Eph–ephrin interaction. Neuron Glia Biol 1: 73–83. 10.1017/S1740925X04000109 [DOI] [PubMed] [Google Scholar]
- Price J, Williams B, Grove E. 1992. The generation of cellular diversity in the cerebral cortex. Brain Pathol 2: 23–29. [PubMed] [Google Scholar]
- Raff MC. 1989. Glial cell diversification in the rat optic nerve. Science 243: 1450–1455. 10.1126/science.2648568 [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. 1983. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303: 390–396. 10.1038/303390a0 [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Fok-Seang J. 1985. Reconstitution of a developmental clock in vitro: a critical role for astrocytes in the timing of oligodendrocyte differentiation. Cell 42: 61–69. 10.1016/S0092-8674(85)80101-X [DOI] [PubMed] [Google Scholar]
- Rhodes KE, Raivich G, Fawcett JW. 2006. The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience 140: 87–100. 10.1016/j.neuroscience.2006.01.055 [DOI] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I. 2011. NG2-glia as multipotent neural stem cells – fact or fantasy? Neuron 70: 661–673. 10.1016/j.neuron.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. 2008. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 11: 1392–1401. 10.1038/nn.2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins SC, Trudel E, Rotondi O, Liu X, Djogo T, Kryzskaya D, Bourque CW, Kokoeva MV. 2013. Evidence for NG2-glia derived, adult-born functional neurons in the hypothalamus. PLoS One 8: e78236 10.1371/journal.pone.0078236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D, Neitz A, Singh J, Frischknecht R, Marongiu D, Binamé F, Perera SS, Endres K, Lutz B, Radyushkin K, et al. 2014. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol 12: e1001993 10.1371/journal.pbio.1001993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D, Yigit H, Dimou L, Trotter J. 2015. Oligodendrocyte precursor cells synthesize neuromodulatory factors. PLoS One 10: e0127222 10.1371/journal.pone.0127222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. 1999. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol 56: 338–344. 10.1001/archneur.56.3.338 [DOI] [PubMed] [Google Scholar]
- Sawamura S, Sawada M, Ito M, Nagatsu T, Nagatsu I, Suzumura A, Shibuya M, Sugita K, Marunouchi T. 1995. The bipotential glial progenitor cell line can develop into both oligodendrocytes and astrocytes in the mouse forebrain. Neurosci Lett 188: 1–4. 10.1016/0304-3940(95)11378-A [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Sholar PW, Bilbo SD. 2012. Sex differences in microglial colonization of the developing rat brain. J Neurochem 120: 948–963. 10.1111/j.1471-4159.2011.07630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Miyamoto N, Hayakawa K, Pham L-DD, Maki T, Ayata C, Kim K-W, Lo EH, Arai K. 2013. Oligodendrocyte precursors induce early blood–brain barrier opening after white matter injury. J Clin Invest 123: 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Maki T, Maeda M, Miyamoto N, Liang AC, Hayakawa K, Pham L-DD, Suwa F, Taguchi A, Matsuyama T, et al. 2014. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-β signaling. PLoS One 9: e103174 10.1371/journal.pone.0103174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJM. 2002. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 22: 2451–2459. 10.1523/JNEUROSCI.22-07-02451.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PB, Armstrong RC. 1999. Intracellular signals and cytoskeletal elements involved in oligodendrocyte progenitor migration. Glia 26: 22–35. [DOI] [PubMed] [Google Scholar]
- Skaper SD. 2019. Oligodendrocyte precursor cells as a therapeutic target for demyelinating diseases. Prog Brain Res 245: 119–144. 10.1016/bs.pbr.2019.03.013 [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. 2009. Parvalbumin neurons and γ rhythms enhance cortical circuit performance. Nature 459: 698–702. 10.1038/nature07991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer SO, Sitnikov S, Kamen Y, Evans KA, Kronenberg-Versteeg D, Dietmann S, de Faria O, Agathou S, Káradóttir RT. 2019. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron 101: 459–471.e5. 10.1016/j.neuron.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. 2002. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36: 855–868. 10.1016/S0896-6273(02)01067-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Sock E, Chaboissier M-C, Schedl A, Wegner M. 2003. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev 17: 1677–1689. 10.1101/gad.259003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue Q-S, Bechmann I. 2009. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol 118: 475–485. 10.1007/s00401-009-0556-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Yuan Y, Chen J, Zhu Y, Qiu Y, Zhu F, Huang A, He C. 2011. Reactive astrocytes inhibit the survival and differentiation of oligodendrocyte precursor cells by secreted TNF-α. J Neurotrauma 28: 1089–1100. 10.1089/neu.2010.1597 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Sekimoto K, Hayashi C, Mabuchi Y, Nakamura T, Akazawa C. 2017. Differentiation of oligodendrocyte precursor cells from Sox10 -Venus mice to oligodendrocytes and astrocytes. Sci Rep 7: 14133 10.1038/s41598-017-14207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H. 2015. Diverse subtypes of astrocytes and their development during corticogenesis. Front Neurosci 9: 114 10.3389/fnins.2015.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao T, Flint N, Lee L, Ying X, Merrill J, Chandross KJ. 2004. 17β-estradiol protects oligodendrocytes from cytotoxicity induced cell death. J Neurochem 89: 660–673. 10.1111/j.1471-4159.2004.02370.x [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. 2002. The basic helix–loop–helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol 12: 1157–1163. 10.1016/S0960-9822(02)00926-0 [DOI] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Raff MC. 2000. Long-Term culture of purified postnatal oligodendrocyte precursor cells. J Cell Biol 148: 971–984. 10.1083/jcb.148.5.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Apperly JA, Lloyd AC, Raff MC. 2001. Lack of replicative senescence in cultured rat oligodendrocyte precursor cells. Science 291: 868–871. 10.1126/science.1056780 [DOI] [PubMed] [Google Scholar]
- Tanner DC, Cherry JD, Mayer-Pröschel M. 2011. Oligodendrocyte progenitors reversibly exit the cell cycle and give rise to astrocytes in response to interferon-γ. J Neurosci 31: 6235–6246. 10.1523/JNEUROSCI.5905-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi K, Takebayashi H, Manabe T, Tanaka KF, Makinodan M, Yamauchi T, Makinodan E, Matsuyoshi H, Okuda H, Ikenaka K, et al. 2008. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J Neurosci Res 86: 3494–3502. 10.1002/jnr.21862 [DOI] [PubMed] [Google Scholar]
- Temple S, Raff MC. 1985. Differentiation of a bipotential glial progenitor cell in single cell microculture. Nature 313: 223–225. 10.1038/313223a0 [DOI] [PubMed] [Google Scholar]
- Thorburne SK, Juurlink BH. 1996. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem 67: 1014–1022. 10.1046/j.1471-4159.1996.67031014.x [DOI] [PubMed] [Google Scholar]
- Tian Y, Yin H, Deng X, Tang B, Ren X, Jiang T. 2018. CXCL12 induces migration of oligodendrocyte precursor cells through the CXCR4-activated MEK/ERK and PI3K/AKT pathways. Mol Med Rep 18: 4374–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RB, Jackiewicz M, McKenzie IA, Kougioumtzidou E, Grist M, Richardson WD. 2017. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep 21: 316–323. 10.1016/j.celrep.2017.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-H, Frost E, To V, Robinson S, ffrench-Constant C, Geertman R, Ransohoff RM, Miller RH. 2002. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell 110: 373–383. 10.1016/S0092-8674(02)00838-3 [DOI] [PubMed] [Google Scholar]
- Tsai H-H, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien A-C, Kuo CJ, Chan JR, Daneman R, et al. 2016. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 351: 379–384. 10.1126/science.aad3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, Argue KJ, Vecchiarelli HA, Ashton SE, Arambula SE, Hill MN, McCarthy MM. 2019. Microglial phagocytosis of newborn cells Is induced by endocannabinoids and sculpts Sex differences in juvenile Rat social play. Neuron 102: 435–449.e6. 10.1016/j.neuron.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzulli I, Papanikolaou M, De-La-Rocha IC, Pieropan F, Rivera AD, Gomez-Nicola D, Verkhratsky A, Rodríguez JJ, Butt AM. 2020. Disruption of oligodendrocyte progenitor cells is an early sign of pathology in the triple transgenic mouse model of Alzheimer's disease. Neurobiol Aging 94: 130–139. 10.1016/j.neurobiolaging.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Fort M, Maldonado PP, Butt AM, Audinat E, Angulo MC. 2010. Postnatal switch from synaptic to extrasynaptic transmission between interneurons and NG2 cells. J Neurosci 30: 6921–6929. 10.1523/JNEUROSCI.0238-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W, MacVicar B. 1988. Electrophysiological properties of glial cells: comparison of brain slices with primary cultures. Brain Res 443: 321–324. 10.1016/0006-8993(88)91626-5 [DOI] [PubMed] [Google Scholar]
- Wang F, Ren S-Y, Chen J-F, Liu K, Li R-X, Li Z-F, Hu B, Niu J-Q, Xiao L, Chan JR, et al. 2020. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat Neurosci 23: 481–486. 10.1038/s41593-020-0588-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TA, Emery B, Mulinyawe S, Barres BA. 2008. Distinct stages of myelination regulated by γ-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60: 555–569. 10.1016/j.neuron.2008.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M. 2008. A matter of identity: transcriptional control in oligodendrocytes. J Mol Neurosci 35: 3–12. 10.1007/s12031-007-9008-8 [DOI] [PubMed] [Google Scholar]
- Weng Q, Wang J, Wang J, He D, Cheng Z, Zhang F, Verma R, Xu L, Dong X, Liao Y, et al. 2019. Single-cell transcriptomics uncovers glial progenitor diversity and cell fate determinants during development and gliomagenesis. Cell Stem Cell 24: 707–723.e8. 10.1016/j.stem.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G II, Roy NS, Goldman SA. 2004. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med 10: 93–97. 10.1038/nm974 [DOI] [PubMed] [Google Scholar]
- Winkler CC, Yabut OR, Fregoso SP, Gomez HG, Dwyer BE, Pleasure SJ, Franco SJ. 2018. The dorsal wave of neocortical oligodendrogenesis begins embryonically and requires multiple sources of sonic hedgehog. J Neurosci 38: 5237–5250. 10.1523/JNEUROSCI.3392-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Maki T, Kinoshita H, Kaji S, Toyokawa M, Nishigori R, Kinoshita Y, Ono Y, Kinoshita A, Takahashi R. 2020. Sex-specific differences in transcriptomic profiles and cellular characteristics of oligodendrocyte precursor cells. Stem Cell Res 46: 101866 10.1016/j.scr.2020.101866 [DOI] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao X, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, et al. 2009. HDAC1 and HDAC2 regulate oligodendrocyte differentiation By disrupting β-catenin-TCF interaction. Nat Neurosci 12: 829–838. 10.1038/nn.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MSY, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, et al. 2014. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159: 766–774. 10.1016/j.cell.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Yeung MSY, Djelloul M, Steiner E, Bernard S, Salehpour M, Possnert G, Brundin L, Frisén J. 2019. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566: 538–542. 10.1038/s41586-018-0842-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn S-J, Cossell L, Attwell D, Tohyama K, Richardson WD. 2013. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77: 873–885. 10.1016/j.neuron.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Chen Y, Kim B, Wang H, Zhao C, He X, Liu L, Liu W, Wu LMN, Mao M, et al. 2013. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 152: 248–261. 10.1016/j.cell.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Li P, Ye J. 2012. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell 3: 173–181. 10.1007/s13238-012-2025-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SPJ, Zahed H, Maltepe E, Rowitch DH. 2014. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158: 383–396. 10.1016/j.cell.2014.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WDS, Young AD. 2002. Sex differences in cerebral tissue volume and cognitive performance during adolescence. Psychol Rep 91: 743–757. 10.2466/pr0.2002.91.3.743 [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SPJ, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, et al. 2010. CNS-resident glial progenitor/stem cells produce schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6: 578–590. 10.1016/j.stem.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeis T, Enz L, Schaeren-Wiemers N. 2016. The immunomodulatory oligodendrocyte. Brain Res 1641: 139–148. 10.1016/j.brainres.2015.09.021 [DOI] [PubMed] [Google Scholar]
- Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, et al. 2019a. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci 22: 719–728. 10.1038/s41593-019-0372-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang Q, Yang Q, Gu H, Yin Y, Li Y, Hou J, Chen R, Sun Q, Sun Y, et al. 2019b. NG2 glia regulate brain innate immunity via TGF-β2/TGFBR2 axis. BMC Med 17: 204 10.1186/s12916-019-1439-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J-W, Raha-Chowdhury R, Fawcett JW, Watts C. 2009. Astrocytes and oligodendrocytes can be generated from NG2+ progenitors after acute brain injury: intracellular localization of oligodendrocyte transcription factor 2 is associated with their fate choice. Eur J Neurosci 29: 1853–1869. 10.1111/j.1460-9568.2009.06736.x [DOI] [PubMed] [Google Scholar]
- Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Li H, Xin M, et al. 2010. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65: 612–626. 10.1016/j.neuron.2010.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Dong C, Frah M, Deng Y, Marie C, Zhang F, Xu L, Ma Z, Dong X, Lin Y, et al. 2018. Dual requirement of CHD8 for chromatin landscape establishment and histone methyltransferase recruitment to promote CNS myelination and repair. Dev Cell 45: 753–768.e8. 10.1016/j.devcel.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Wang C, Yang J, Huang H, Zhao X, Zhang Z, Qiu M. 2018. Molecular and genetic evidence for the PDGFRα-independent population of oligodendrocyte progenitor cells in the developing mouse brain. J Neurosci 38: 9505–9513. 10.1523/JNEUROSCI.1510-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. 2001. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron 31: 791–807. 10.1016/S0896-6273(01)00414-7 [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. 2011. Age-dependent fate and lineage restriction of single NG2 cells. Development 138: 745–753. 10.1242/dev.047951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. 2007. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci 10: 321–330. 10.1038/nn1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonouzi M, Berger D, Jokhi V, Kedaigle A, Lichtman J, Arlotta P. 2019. Individual oligodendrocytes show bias for inhibitory axons in the neocortex. Cell Rep 27: 2799–2808.e3. 10.1016/j.celrep.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zujovic V, Benavides J, Vigé X, Carter C, Taupin V. 2000. Fractalkine modulates TNF-α secretion and neurotoxicity induced by microglial activation. Glia 29: 305–315. <305::AID-GLIA2>3.0.CO;2-V [PubMed] [Google Scholar]