Abstract
Objective
This systematic review aims to describe the value of saliva as a noninvasive sample for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in comparison with the current method for sample collection, the nasopharyngeal swab.
Study Design
We conducted a systematic review of the literature following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations. We searched in 5 databases (PubMed, Cochrane, EBSCO, Elsevier, and MEDLINE) and included articles published between December 2019 and July 2020.
Results
This review included 22 publications that met inclusion criteria, 17 of which were case series, 2 of which were case reports, and 3 of which were massive screenings. All articles compared saliva with nasopharyngeal swabs. The detection rate of SARS-CoV-2 in saliva was similar to that for nasopharyngeal swabs. The sensitivity ranged between 20% and 97%, and specificity ranged between 66% and 100%.
Conclusions
This systematic review found that saliva might be an appropriate, fast, painless, simple, and noninvasive sample for SARS-CoV-2 detection, making it ideal for massive screening of SARS-CoV-2 infection.
Statement of Clinical Relevance.
The results of this systematic review highlight the advantages of saliva as a sample for severe acute respiratory syndrome coronavirus 2 detection. As opposed to nasopharyngeal swabs, saliva is a painless, noninvasive, and fast test that can be used for diagnosis and massive screening.
Alt-text: Unlabelled box
Since the first outbreak was reported in Wuhan, China, in December 2019, more than 62 million cases of coronavirus disease 2019 (COVID-19) had been reported through the end of November 2020,1 and more than 1.4 million people had died worldwide, according to World Health Organization (WHO) statistics.1 The global pandemic was declared on March 11, 2020, and, since then, government regulations have been adopted to reduce the transmissibility. In addition, many countries declared quarantine, closing their borders.2 , 3 Since many regions have not controlled the outbreak to date, regulations are heavily affecting the economy, and incomes have been drastically reduced. These measures have been enforced and maintained in some countries up until now mainly because mass screening of the infected population is not yet available. Thus, traceability remains one of the biggest challenges in controlling the pandemic while newly available COVID-19 vaccines are deployed globally.4, 5, 6, 7 Mass screening for SARS-CoV-2 is defined as a high number of tests performed in a population, regardless of their status (symptomatic or asymptomatic), in order to identify and quarantine positive cases.6
According to WHO recommendations, case tracing and boosting testing capacity should be regarded as the main policy of disease surveillance, especially when cases begin to drop or when facing a second wave of infection, in order to rapidly detect and isolate positive cases. These measures, along with social distancing, are the foundations for a public health–based COVID-19 response.4 , 5 , 7
Determining the exact number of asymptomatic or mild cases, which until now seem to be underreported, is the utmost important factor to ensure case tracing, given that these cases are the ones driving the pandemic. Massive screening or early testing of suspicious asymptomatic cases may help in reducing the transmission—limiting the spread of the disease. For this purpose, widely available rapid diagnostic tests are necessary.8, 9, 10
COVID-19 diagnosis currently requires obtaining a sample by using a nasopharyngeal swab (NPS), which must be performed by medical or trained personnel. Afterward, a real-time reverse transcription–polymerase chain reaction (rRT-PCR) test is performed. The rRT-PCR test has high specificity for the detection of SARS-CoV-2, being the gold standard for SARS-CoV-2 infection diagnosis. However, the NPS is not the ideal sample collection method regarding patient compliance. Moreover, use of the NPS is an invasive technique that can expose health care personnel to aerosols due to patients’ coughing, and associated complications include epistaxis (which can be severe in anticoagulated patients).11 In this context, other sources of sample collection have been proposed, including saliva, which offers some advantages over NPSs.
Considering that massive screening will probably be adopted in more countries to control the pandemic, we propose that saliva could be a better sample than NPS because saliva collection can be performed by the patient himself, avoiding the need to attend a health care facility, thus diminishing crowding and health care personnel exposure.12 It has been reported that saliva sample collection is easy, fast, widely accepted by patients, and cost-effective—all conducive to massive testing.13 , 14 In addition, saliva is a feasible specimen type for respiratory virus diagnosis.15 To et al.16 reported a high overall agreement (93.3%) between saliva and nasopharyngeal aspirate specimens when other respiratory viruses were tested.
To date, several studies comparing the efficacy of rRT-PCR detection of SARS-CoV-2 between NPS and saliva samples have been published. Hence, the purpose of this review is to describe the value of saliva as a noninvasive sample for the detection of SARS-CoV-2 compared with the current method for sample collection (NPS), assessing if saliva offers adequate sensitivity for SARS-CoV-2 detection and providing a critical review of the selected articles.
METHODS
We conducted a systematic review of the literature following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations:
-
1.
Focused question: We formulated a focused question following the patient, intervention, comparison, outcome (PICO) strategy: Is saliva a reliable source for SARS-CoV-2 quantitative RT-PCR (RT-qPCR) detection compared with NPS in the general population?
-
2.
Inclusion criteria: We included case reports and case series that evaluated saliva as a sample for SARS-CoV-2 detection. We included articles published between December 2019 and October 2020 that were published in English and available in full text.
-
3.
Databases: We searched 5 databases: PubMed, Cochrane, EBSCO, Elsevier, and MEDLINE.
-
4.
Search strategy: A search strategy was developed according to the PICO strategy. Thus, we combined the following search terms: (saliva) AND (COVID-19), (saliva) AND (SARS-CoV-2), (saliva) AND (COVID-19) AND (diagnostic), (saliva) AND (COVID-19) AND (diagnosis), (saliva) AND (SARS-CoV-2) AND (diagnostic), (saliva) AND (SARS-CoV-2) AND (diagnosis).
We also screened the references of the selected articles to identify any relevant articles that were not included through the search strategy.
-
1.
Screening of selected articles and data extraction: The literature search was performed independently by 2 authors, and the results were then compared. First, the title and abstract were screened to evaluate if the inclusion criteria were met. Afterward, both authors compared the selected articles and discussed their inclusion for full-text review and final inclusion. All duplicate articles were excluded. All disagreements were settled by discussion between the 2 authors. All articles that did not answer the focused question were excluded.
Both authors extracted the relevant data from the selected articles, including first author, year of publication, country, number of subjects, sex and age of the included patients, sample collection method (methods, storage, and processing), and diagnostic method for SARS-CoV-2 detection.
RESULTS
Selected studies
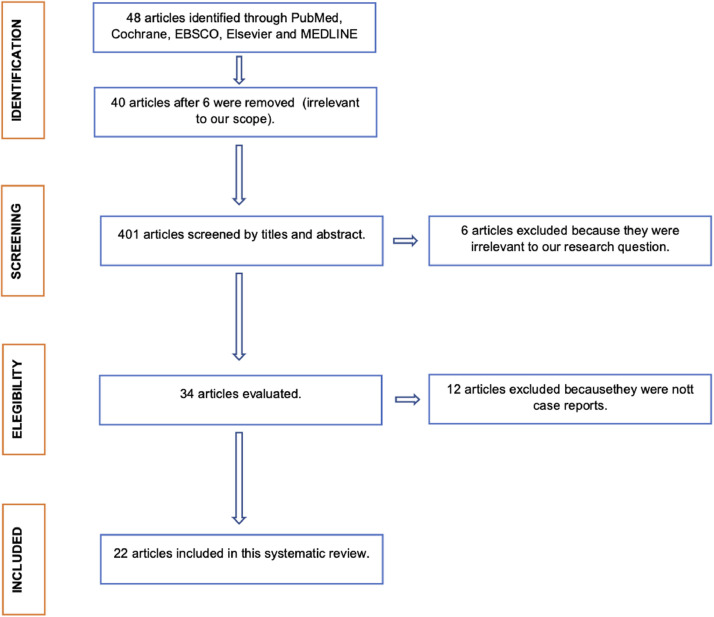
In total, 48 articles were found with the search strategies combining the 5 databases. Forty of them were selected by screening titles and abstracts. After a discussion between the 2 authors, 34 articles that met the inclusion criteria were included for full-text review. Manual screening was performed for the 34 articles; no new articles were included this way. After full-text review, an agreement was reached by the 2 reviewers to include 22 articles in the present review. The process of article selection is depicted in a flowchart (Figure 1 ).
Figure 1.
Flow chart of study selection process.
Main characteristics of included articles
The included articles are case series, case reports, and massive screenings published during 2020 in Australia, Belgium, Canada, China, Italy, France, Hong Kong, Japan, South Korea, Thailand, and the United States. The main characteristics of the included articles are summarized in Table I .
-
1.
Study design: This review included 17 case series, 2 case reports, and 3 massive screenings. Two articles were not peer reviewed.11, 12, 13, 14 , 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34
-
2.
Patients: A total of 7630 patients were tested and included in this review. Of these, 862 patients had positive test results for SARS-CoV-2. Only 16 articles reported the sex of the patients; in total, 1390 men and 1205 women were included in this review. Their mean age was 55.6 years (range, 1825 to 106 years27; Williams et al.,13 Becker et al.,23 Iwasaki et al.,24 Caulley et al.,31 Sakanashi et al.,33 and Mestdagh et al.34 did not report the sex of the patients, whereas Williams et al.,13 Becker et al.,23 Nagura-Ikeda et al.,28 Caulley et al.,31 Sakanashi et al.,33 and Mestdagh et al.34 did not report the age of the patients).
-
3.
Saliva sample collection: All included articles compared saliva with NPS for SARS-CoV-2 diagnosis. Saliva samples were self-collected by drooling (n = 1), spitting (n = 10), coughing up (n = 2), drooling or pipetting (n = 2), coughing up and spitting (n = 2), and spitting and saliva swabs (n = 1). Yoon et al.,14 Pasomsub et al.,11 McCormick-Baw et al.,22 and Yokota et al.32 did not report the saliva collection method.
-
4.
Obtention and preservation of the sample: Six articles documented the procedures and conditions necessary for the sample collection. To et al.18 obtained an early morning saliva sample, and the procedure was supervised by nurses. Zheng et al.17 requested patients not to drink water, eat, or gargle in the 30 minutes preceding the sample collection. In addition, all patients were asked to wear a mask and cough 3 to 5 times before spitting out saliva into a sterile container. Wyllie et al.21 instructed patients to avoid eating, brushing their teeth, and drinking water upon awakening and until samples were obtained. Nagura-Ikeda et al.28 collected saliva without restriction on timing or food intake. McCormick-Baw et al.22 and Mestdagh et al.34 recommended to patients that they not have any food, drink, tobacco, and gum at least 30 minutes before sample collection.
Table I.
Main characteristics of included articles
| Reference | Country, year | Study design | No. of patients | Sex | Mean age (years) | Saliva collection method | Saliva sample preservation | SARS-CoV-2 detection method | Sensitivity (%) for saliva | Specificity (%) for saliva | Viral load in saliva (copies per mL or Ct) | Viral load in NPS (copies per mL or Ct) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | To et al.12 | Hong Kong, China, February 2020 | CS | 12 | 7 M 5 W |
62.5 | Spit | VTM | RT-qPCR | – | – | 3.3 × 106 copies/mL (range, 9.9 × 102 to 1.2 × 108 copies/mL) | – |
| 2 | Zheng et al.17 | China, February 2020 | CS | 65 | 40 M 25 W |
54 | Coughing up and spitting | – | RT-qPCR | – | – | – | – |
| 3 | To et al.18 | Hong Kong, China, March 2020 | CS | 23 | 13 M 10 W |
62 | Coughed up | VTM | RT-qPCR | – | – | 2 log10 copies per mL to 6 log10 copies per mL | 5 × 2 log10 copies per mL (IQR, 4.1-7.0) |
| 4 | Azzi et al.19 | Italy, April 2020 | CS | 25 | 17 M 8 W |
61.5 | Drooling and pipetting | PBS (2 mL) | RT-qPCR | – | – | Ct 27.16 ± 3.07† | – |
| 5 | Azzi et al.20 | Italy, April 2020 | CR | 2 | 2 M | 67.5 | Drooling and pipetting | PBS (2 mL) | RT-qPCR | – | – | – | – |
| 6 | Williams et al.13 | Australia, April 2020 | CS | 522 | – | – | Spit | Liquid Amies media | RT-qPCR | 84.62% | 98% | – | – |
| 7 | Wyllie et al.21 | USA, April 2020 | CS | 44 | 23 M 21 W |
61 | Self-collected saliva and spit | – | RT-qPCR | – | – | – | – |
| 8 | Pasomsub et al.11 | Thailand, May 2020 | CS | 19 | 9 M 10 W |
33 | – | UTM | RT-qPCR | 84.21% | 98.9% | ORF1 ab gene Ct 32.7 (28-35.0) N gen Ct 31.8 (28.4-33.7)* | ORF1 ab gene Ct 32.0 (27.434.3) N gen Ct 30.5 (26.1-32.3)* |
| 9 | Yoon et al.14 | South Korea, May 2020 | CR | 2 | 2 W | 55.5 | – | VTM (2 mL) | RT-qPCR | – | – | Patient 1 = 6.63 log10 copies/mL Patient 2 = 7.10 log10 copies/mL | Patient 1: 8.41 log10 copies/mL Patient 2 = 7.49 log10 copies/mL |
| 10 | McCormick-Baw et al.22 | USA, May 2020 | CS | 156 | 90 M 66 W |
47.8 | – | Unpreserved | RT-qPCR | 95.92% | 99.06% | E gene Ct 26.10 ± 11.20; N2 gene Ct 30.40 ± 9.67† |
E gene Ct 23.83 ± 7.78; N2 gene Ct 26.70 ± 7.61† |
| 11 | Becker et al.23 | USA, May 2020 | CS | 112 | – | – | Self-collected and spit | Oragene·Dx Kit | RT-qPCR | 40%-60% 20%-50% |
97%-100% 75%-94% |
– | – |
| 12 | Iwasaki. et al.24 | Japan, June 2020 | CS | 76 | – | 69 | Self-collected saliva and spit | PBS (600 μL) | RT-qPCR | 88.89% | 98.51% | 4.1 ± 1.4 log10 gene copies/mL Ct 30.6 ± 4.6† |
5.4 ± 2.4 log10 gene copies/mL Ct 26.5 ± 8.1† |
| 13 | Hung et al.25 | Hong Kong, China, June 2020 | CS | 18 | 8 M 10 W |
39.1 | Coughed up | VTM (2 mL) | RT-qPCR | – | – | Early morning Ct 34.5 (32.5-41); before lunch Ct 38.2 (33.9-41); before teatime Ct 36.3 (34.5-41); before dinner Ct 41 (34.7-41); before bedtime Ct 41 (34.7-41)* | Ct on admission 28.09 (17.71-36.6)* |
| 14 | Chen et al.26 | Hong Kong, China, June 2020 | CS | 58 | 28 M 30 W |
38 | Coughing up followed by spit. | VTM (2 mL) | RT-qPCR | 89% | 66% | E gene Ct 29.7 (27.2-37.2); N2 gene Ct 32.3 (29.9-38.6)* | E gene Ct 26.8 (20.7-33.5); N2 gene Ct 29.3 (23.3-36.5)* |
| 15 | Jamal et al.27 | Canada, June 2020 | CS | 91 | 52 M 39 W |
66 | Spit | PBS (2.5 mL) | RT-qPCR | 68.75% | 70.37% | Ct 34.0 (31-37)* | Ct 30.0 (26-35)* |
| 16 | Nagura-Ikeda et al.28 | Japan, July 2020 | CS | 103 | 66 M 37 W |
– | Self-collected and spit | – | RT-qPCR RT-LAMP |
81.6% | – | – | – |
| 17 | Migueres et al.29 | France, August 2020 | CS | 123 | 49 M 74 W |
43 | Spit | – | RT-qPCR | 82.93% | 96.34% | RDrP gene Ct 29.5 (27.6-33.3)* |
RDrP gene Ct 24.9 (21.9-30.9)* |
| 18 | Kim et al.30 | South Korea, August 2020 | CS | 15 | 5 M 10 W |
59 | Spit | UTM | RT-qPCR | 64% | – | Ct 32.0 (28-38)* | Ct 33.0 (27-35)* |
| 19 | Caulley et al.31 | Canada, August 2020 | Mass screening | 1939 | – | – | Self-collected and spit | OMNIgene·ORAL, OM- 505 (DNA Genotek) |
RT-qPCR | 60.71% | 99.26% | – | – |
| 20 | Yokota et al.32 | Japan, September 2020 | Mass screening study | 1924 | 981 M 858 W 95 unknown |
Cohort 1: 44.9 Cohort 2: 33.5 | – | None | RT-qPCR RT-LAMP |
92% | >99.9% | – | – |
| 21 | Sakanashi et al.33 | Japan, September 2020 | CS | 12 | – | – | Self-collected (drooling) | VTM (3 mL) | RT-qPCR | 79% | 69% | – | – |
| 22 | Mestdagh et al.34 | Belgium, October 2020 | Mass screening | 2289 | – | – | Spit and saliva swab | Norgen Biotek's Saliva RNA Collection and Preservation Device Dx 53800 | RT-qPCR | 30.8% overall (spit) 22.4% Overall (swab) 97% in high viral load samples (spit) 76.7% in high viral load samples (swab) | – | – | – |
CR, case report; CS, Case-series; IQR, interquartile range; M, men; n, number of included patients, considers patients with PCR SARS-CoV-2 diagnosis; PBS, phosphate-buffered saline; RT-LAMP, reverse transcription loop-mediated isothermal amplification; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UTM, universal transport medium; VTM, viral transport medium; W, women.
Median (IQR).
Mean ± standard deviation.
Two articles documented the storage conditions and handling of the samples after obtention.21 , 27 Wyllie et al.21 stored the samples at room temperature and transported them to the laboratory for processing within 5 hours. Yokota et al.32 transported saliva at 4°C without transport media. Jamal et al.27 froze the samples at −80°C and transported them to the laboratory within 8 hours. Authors of 3 articles used commercial kits to collect and preserve nucleic acids: Oragene·Dx Kit (DNA Genotek, Ottawa, ON, Canada)23; OMNIgene·ORAL, OM-505 (DNA Genotek)31; and the Saliva RNA Collection and Preservation Device (Norgen Biotek, Thorold, ON, Canada).34 Most articles specified the handling for transportation of the sample. After collection, 2 to 2.5 mL of viral transport medium (VTM), phosphate-buffered saline (PBS), or universal transport medium (UTM) were added to the sample.
-
1.
SARS-CoV-2 detection method: Various RT-qPCR kits for SARS-CoV-2 detection in saliva samples were used in the included articles. Similarly, viral RNA extraction was performed using different commercial kits (only 3 articles did not report the RNA extraction kit used).
The amplification targets were different according to the authors. Within the reported amplified sequences are genetic regions belonging to genes E, S, N, N2, RDrP, ORFI ab, and 5′-untranslated region.
-
6.Salivary SARS-CoV2 detection: This review includes reports with small cohorts of patients with positive COVID-19 test results and large cohorts of asymptomatic close contacts and high-risk individuals. Moreover, some selected articles lacked information regarding SARS-CoV-2 viral load as well as sensitivity and specificity. Of the 22 included articles, 12 (54.5%) reported viral load as the number of copies and/or cycle threshold (Ct) values, and 14 (63.6%) of the included articles reported sensitivity and specificity.
-
a.SARS-CoV-2 viral load: To et al.18, Yoon et al.,14 Pasomsub et al.,11 and Iwasaki et al.24 reported the number of viral particles and found that the viral load was greater in NPS than in saliva samples. Also, Azzi et al.,19 Pasomsub et al.,11 McCormick-Baw et al.,22 Iwasaki et al.,24 Hung et al.,25 Chen et al.,26 Jamal et al.,27 Migueres et al.,29 and Kim et al.30 found that saliva samples showed higher Ct values than the NPS samples, indicating that the viral load was higher in NPS.
-
b.SARS-CoV-2 sensitivity and specificity: Eleven studies reported the sensitivity and specificity of saliva for SARS-CoV-2 detection. Three studies only reported sensitivity. Sensitivity ranged between 20%23 and 97%,34 with a mean of 79.47% for all included studies. Specificity ranged between 66%26 and 100%,23 with a mean of 90.48% for all included studies.
-
a.
Additionally, Iwasaki et al.24 reported that the concordance index between saliva and NPS was 97.4% with a kappa of 0.87%.
DISCUSSION
Salivary SARS-CoV-2 detection
According to To et al.,12 saliva is an alternative to NPS for SARS-CoV-2 detection and even other respiratory viruses. Xu et al.35 stated that saliva may be easily obtained from the patient, coincident with reports from Azzi et al.19 and Yoon et al.,14 who reported that the sample may be self-collected by the patient.
Viral load in saliva
Some studies have shown that epithelial cells from the oral mucosal lining, including those from the tongue dorsum, express a high amount of angiotensin-converting enzyme 2 (ACE2) receptors, critical for COVID-19 pathogenesis.36 , 37 Baghizadeh Fini38 proposed that SARS-CoV-2 can attach to ACE2 receptors on the epithelium of salivary glands, allowing the virus to enter the cell and replicate, consequently leading to their lysis and triggering an acute or chronic sialadenitis. This may explain the high concentrations of viral load detected in saliva.37 Interestingly, Zhu et al.39 reported a viral load peak in saliva (104 to 108 copies per mL) during the first week of symptoms that gradually declines over time. Moreover, no significant difference in salivary viral load between mild and severe cases of COVID-19 was found. On the other hand, To et al.12 proposed that SARS-CoV-2 detection in saliva may be due to nasopharyngeal epithelial debris accumulating in the oral cavity. However, studies in animals demonstrate that SARS-CoV-2 is capable of infecting ductal epithelial cells from salivary glands, which may explain the viral presence in saliva.40 It is important to point out that epithelial cells of the salivary glands may engulf viral particles or virus-containing exosomes on their basolateral portion, which may then be secreted to the acinar lumen, as suggested by Dawes et al.41
Accuracy of saliva for SARS-CoV-2 detection
Saliva has been proposed as a reliable sample for SARS-CoV-2 detection; thus, specificity and sensitivity must be greater than or equal to the those reported for NPS. So far, this seems to be the case, according to Pasomsub et al.11 and Williams et al.,13 although contingency tables are lacking. According to Zhu et al.,39 the sensitivity and specificity of saliva were 86.4% (95% confidence interval [CI], 82.8%-89.4%) and 97.0% (95% CI, 95.0%-98.3%), respectively, in 944 patients from 12 independent cohorts. Moreover, concordance analysis revealed a 92.1% observed virus detection accuracy between the respiratory tract and saliva samples (Cohen's kappa coefficient, 0.840; 95% CI, 0.805-0.874).39
Interestingly, the studies by Becker et al.,23 Caulley et al.,31 and Mestdagh et al.,34 who obtained the lowest sensitivities and specificities, used commercial kits to obtain and preserve saliva samples. On the one hand, it is possible to hypothesize that transfer of the sample in VTM, UTM, or PBS is preferable because the results have been better. Further studies are required to confirm this hypothesis. On the other hand, Mestdagh et al.34 mentioned that sensitivity improves dramatically in individuals with high viral load, which is greater at the onset of symptoms12 , 18 , 23 , 27 and which would allow the virus to be identified in asymptomatic individuals, with a consequent high viral load.
It has recently been reported that NPS has a significantly higher detection rate for SARS-CoV-2 than oropharyngeal swabs (OPSs),42 mainly due to the low viral load found in OPS. According to the results of this review, the viral load in saliva is high in symptomatic patients, as stated above37; thus, the detection rate is high and comparable to NPS in these patients. Even when the viral load in saliva is lower in asymptomatic patients, it may be detected with a salivary test, as reported by Chen et al.,26 Williams et al.,13 Pasomsub et al.,11 McCormick et al.,22 Iwasaki et al.,24 Nagura-Ikeda et al.,28 Migueres et al.,29 and Yokota et al.32 According to the results of Wyllie et al.,21 a salivary test detected all positive cases, including health care professionals who were screened.
Use of saliva for diagnostic and screening of SARS-CoV-2 infection
Countries such as China, Japan, Australia, Spain, and Germany have made some progress toward lifting COVID-19 containment measures, and these have unavoidably led to an increase in new positive cases.1 Results reported by López et al.,43 showed that early quarantine, social distancing measures, and self-care measures are the recommended interventions for the initial containment of the epidemic outbreaks.44 , 45 However, to safely lift containment and prevent the much-feared “second wave,” population-wide screening is needed to rapidly detect and isolate positive cases. The available tests for SARS-CoV-2 detection include serologic testing and rRT-PCR.11 , 43 Regarding rRT-PCR, SARS-CoV-2 RNA is usually detectable in nasopharyngeal samples, and, more recently, saliva sampling has been proposed as an alternative.
Saliva offers advantages over NPS regarding population-wide screening for SARS-CoV-2 and may also be helpful for regular screening for asymptomatic health care workers in health care facilities. Saliva is a noninvasive sample that can either be self-collected by the patient or collected by medical personal when the patient is unwilling or unable to cooperate. Most important, saliva reduces the risk of transmission between health care workers and shortens both the sample collection time and diagnosis time. Pasomsub et al.11 pointed out that, given the current complex economic situation in many countries, saliva is a viable alternative for reducing the costs of SARS-CoV-2 detection. Still, new investigations should focus on assessing the analytical and clinical sensitivity and specificity of saliva for SARS-CoV-2 detection; we were unable to retrieve these data from the included publications for this review.
General recommendations for saliva collection and preservation
According to the results of this systematic review, saliva is a feasible option for SARS-CoV-2 detection. The collection may be performed in either asymptomatic or symptomatic patients (including those intubated). Sample collection does not require a special setting or preparation. The following are recommendations for sample collection, storage, and handling of saliva for SARS-CoV-2 detection:
-
1.
Time of sample collection: It is always best to collect the sample in the morning.
-
2.
Before saliva collection (indications for patients): Do not eat, drink, wash your teeth, or rinse for at least 60 minutes before the sample collection.
-
3.
Sample collection: Samples may be obtained by the passive drooling method. Use stimulants only when necessary. For intubated patients, a pipette must be used to collect the sample. Label the vial with the patient's information.
-
4.
Sample volume: A sample volume of 1 to 3 mL is sufficient for SARS-CoV-2 detection.
-
5.
After saliva collection: If possible, freeze the sample immediately at −80°C and process it within 8 hours. Samples may be stored at room temperature for no longer than 5 hours before freezing at −80°C.
-
6.
Storage and handling: Resuspend the sample in 2 to 2.5 mL of VTM or PBS.
-
7.
Processing and RNA extraction: Samples are to be processed as soon as possible. Bring samples to room temperature, vortex, and then centrifuge for 15 minutes at 4°C at 20 000 × g. For RNA extraction, use the NucliSENS easyMAG (bioMérieux, Marcy l'Etoile, France) or the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany).
-
8.
SARS-CoV-2 detection method: rRT-qPCR.
The greatest viral load has been reported in the morning; thus, the sample must be obtained during the first hours of the day to ensure good results.25 In parallel, the patients must follow instructions before sample collection to avoid contamination.12 , 17 , 21 The sample may be obtained by drooling technique or pipetting if the patient is intubated or is unable to cooperate, and 1 to 3 mL should be enough to perform the assays.19 According to Sullivan et al.,46 it is advisable to rinse the mouth with water and discard the rinse, then wait 5 minutes before collecting the sample. Thick saliva may occur in dehydrated or hospitalized patients, making the pipetting difficult and risking contamination of the workspace47; therefore, precautions should be taken with such patients. In addition, considering that saliva contains proteins and peptides that have exhibited antiviral effects, hyposalivation could be a risk factor for SARS-CoV-2 infection.48
Sample preservation is another key point. Once the sample is obtained, it must be preserved in VTM or PBS19 , 27 to prevent salivary enzymes from damaging the viral RNA. The sample must be processed within the next hours. If the sample is stored at room temperature, it must be processed within the next 5 hours.21 If the sample is stored at −80°C, it can be processed within the next 8 hours or more.27 The subsequent RNA extraction can be performed with any available and U.S. Food and Drug Administration–approved commercial kit (such as NucliSENS easyMAG and QIAamp Viral RNA Mini Kit), and SARS-CoV-2 detection can be performed by using a validated rRT-qPCR kit. We suggest considering these key aspects of sample collection, storage, and handling of the saliva sample in order to obtain reliable results.
CONCLUSION
Saliva might be an appropriate, fast, painless, simple, and noninvasive sample for SARS-CoV-2 detection, making it ideal for massive screening of SARS-CoV-2 infection. The sample can be self-collected by the patient, thus reducing the infection risk among health care workers. Although sensitivity and specificity of saliva samples are not equivalent to NPS in all cases, values are relatively similar for symptomatic cases with high viral load and fairly acceptable for asymptomatic patients with low viral load. Thus, saliva could be considered a proper sample for massive screening or for the diagnosis of suspicious cases in health facilities where resources are scarce and NPS might not be widely available.
Funding
This work was supported by Fondecyt-Chile (1160015 to M.J.G., I.C.M., S.G.P.) and Fondecyt Iniciación (11170049 to I.C.M.).
References
- 1.World Health Organization (WHO). Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed November 15, 2020.
- 2.Liang XH, Tang X, Luo YT, Zhang M, Feng ZP. Effects of policies and containment measures on control of COVID19 epidemic in Chongqing. World J Clin Cases. 2020;8:2959–2976. doi: 10.12998/wjcc.v8.i14.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C, Romagnani P, von Brunn A, Anders HJ. SARS-CoV-2 and Europe: timing of containment measures for outbreak control. Infection. 2020;48:483–486. doi: 10.1007/s15010-020-01420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kucharski AJ, Klepac P, Conlan AJK, et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parvez M, Jagirdar R, Purty R, et al. COVID‑19 pandemic: understanding the emergence, pathogenesis and containment (Review) World Acad Sci J. 2020;2:18. [Google Scholar]
- 6.European Centre for Disease Prevention and Control (ECDC). Population-wide testing of SARS-CoV-2: country experiences and potential approaches in the EU/EEA and the United Kingdom. August 19, 2020. accesed November 09, 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-population-wide-testing-country-experiences.pdf.
- 7.World Health Organization (WHO). Contact tracing in the context of COVID-19. May 10, 2019. Accesed November 09, 2020. https://www.who.int/publications-detail/contact-tracing-in-the-context-of-covid-19.
- 8.Sun K, Viboud C. Impact of contact tracing on SARS-CoV-2 transmission. Lancet Infect Dis. 2020;20:876–877. doi: 10.1016/S1473-3099(20)30357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peeling RW, Wedderburn CJ, Garcia PJ, et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020;20:e245–e249. doi: 10.1016/S1473-3099(20)30517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellmunt JM, Caylà JA, Millet JP. Contact tracing in patients infected with SARS-CoV-2. The fundamental role of primary health care and public health. Semergen. 2020;46(Suppl 1):55–64. doi: 10.1016/j.semerg.2020.06.001. [in Spanish] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasomsub E, Watcharananan SP, Boonyawat K, et al. Saliva sample as a noninvasive specimen for the diagnosis of coronavirus disease 2019: A cross-sectional study [e-pub ahead of print] Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2020.05.001. Accessed June 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.To KKW, Tsang OTY, Yip CCY, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams E, Bond K, Zhang B, Putland M, Williamson DA. Saliva as a noninvasive specimen for detection of SARS-CoV-2 [e-pub ahead of print] J Clin Microbiol. 2021 doi: 10.1128/JCM.00776-20. Accessed June 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon JG, Yoon J, Song JY, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020;35(20):e195. doi: 10.3346/jkms.2020.35.e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.To KK, Lu L, Yip CC, et al. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg Microbes Infect. 2017;6:e49. doi: 10.1038/emi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.To KKW, Yip CCY, Lai CYW, et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25:372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Zheng S, Yu F, Fan J, et al. Saliva as a diagnostic specimen for SARS-CoV-2 by a PCR-based assay: a diagnostic validity study. accesed June 25, 2020. https://ssrn.com/abstract=3543605.2021 [DOI] [PMC free article] [PubMed]
- 18.To KKW, Tsang OTY, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azzi L, Carcano G, Gianfagna F, et al. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;81:e45–e50. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azzi L, Carcano G, Dalla Gasperina D, Sessa F, Maurino V, Baj A. Two cases of COVID-19 with positive salivary and negative pharyngeal or respiratory swabs at hospital discharge: a rising concern [e-pub ahead of print] Oral Dis. 2021 doi: 10.1111/odi.13368. Accessed July 04, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyllie AL, Fournier J, Casanovas-Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick-Baw C, Morgan K, Gaffney D, et al. Saliva as an alternate specimen source for detection of SARS-CoV-2 in symptomatic patients using Cepheid Xpert Xpress SARS-CoV-2. J Clin Microbiol. 2020;58:e01109–e01120. doi: 10.1128/JCM.01109-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker D, Sandoval E., Amin A., et al. Saliva is less sensitive than nasopharyngeal swabs for COVID-19 detection in the community setting. medRxiv, Infect Dis. 2020 doi: 10.1101/2020.05.11.20092338. published online May 17. [DOI] [Google Scholar]
- 24.Iwasaki S, Fujisawa S, Nakakubo S, et al. Comparison of SARS-CoV-2 detection in nasopharyngeal swab and saliva. J Infect. 2020;81:e145–e147. doi: 10.1016/j.jinf.2020.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung DL-L, Li X, Chiu KH-Y, et al. Early-morning vs spot posterior oropharyngeal saliva for diagnosis of SARS-CoV-2 infection: implication of timing of specimen collection for community-wide screening. Open Forum Infect Dis. 2020;7:ofaa210. doi: 10.1093/ofid/ofaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JHK, Yip CCY, Poon RWS, et al. Evaluating the use of posterior oropharyngeal saliva in a point-of-care assay for the detection of SARS-CoV-2. Emerg Microbes Infect. 2020;9:1356–1359. doi: 10.1080/22221751.2020.1775133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamal AJ, Mozafarihashjin M, Coomes E, et al. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [e-pub ahead of print] Clin Infect Dis. 2021 doi: 10.1093/cid/ciaa848. Accessed July 02, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagura-Ikeda M, Imai K, Tabata S, et al. Clinical evaluation of self-collected saliva by quantitative reverse transcription-PCR (RT-qPCR), Direct RT-qPCR, reverse transcription-loop-mediated isothermal amplification, and a rapid antigen test to diagnose COVID-19. J Clin Microbiol. 2020;58(9) doi: 10.1128/JCM.01438-20. e01438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migueres M. Saliva sampling for diagnosing SARS-CoV-2 infections in symptomatic patients and asymptomatic carriers. J Clin Virol. 2020;130 doi: 10.1016/j.jcv.2020.104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SE, Lee JY, Lee A, et al. Viral load kinetics of SARS-CoV-2 infection in saliva in Korean patients: a prospective multi-center comparative study. J Korean Med Sci. 2020;35:e287. doi: 10.3346/jkms.2020.35.e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caulley L, Corsten M, Eapen L, et al. Salivary detection of COVID-19. Ann Intern Med. 2020;2:2–3. doi: 10.7326/M20-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokota I, Shane P. Mass screening of asymptomatic persons for SARS-CoV-2 using saliva. Ann Med. 2020;53:151–159. [Google Scholar]
- 33.Sakanashi D, Asai N, Nakamura A, et al. Comparative evaluation of nasopharyngeal swab and saliva specimens for the molecular detection of SARS-CoV-2 RNA in Japanese patients with COVID-19. J Infect Chemother. 2021;27:126–129. doi: 10.1016/j.jiac.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mestdagh P, Gillard M, Arbyn M, et al. Evaluation of saliva sampling procedures for SARS-CoV-2 diagnostics reveals differential sensitivity and association with viral load. medRxiv. October 13, 2020 https://www.medrxiv.org/content/10.1101/2020.10.06.20207902v1 Published online. [Google Scholar]
- 35.Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12:11. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippi G, Mattiuzzi C, Bovo C, Plebani M. Current laboratory diagnostics of coronavirus disease 2019 (COVID-19) Acta Biomed. 2020;91:137–145. doi: 10.23750/abm.v91i2.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baghizadeh Fini M. Oral saliva and COVID-19. Oral Oncol. 2020;108 doi: 10.1016/j.oraloncology.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Guo J, Xu Y, Chen X. Viral dynamics of SARS-CoV-2 in saliva from infected patients. J Infect. 2020;81:e48–e50. doi: 10.1016/j.jinf.2020.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Wei Q, Alvarez X, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. 2011;85:4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dawes C, Wong DTW. Role of saliva and salivary diagnostics in the advancement of oral health. J Dent Res. 2019;98:133–141. doi: 10.1177/0022034518816961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Liu Q, Hu J, et al. Nasopharyngeal swabs are more sensitive than oropharyngeal swabs for COVID-19 diagnosis and monitoring the SARS-CoV-2 load. Front Med. 2020;7:334. doi: 10.3389/fmed.2020.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López L, Rodó X. The end of social confinement and COVID-19 re-emergence risk. Nat Hum Behav. 2020;4:746–755. doi: 10.1038/s41562-020-0908-8. [DOI] [PubMed] [Google Scholar]
- 44.Hoertel N, Blachier M, Blanco C, et al. A stochastic agent-based model of the SARS-CoV-2 epidemic in France. Nat Med. 2020;26:1417–1421. doi: 10.1038/s41591-020-1001-6. [DOI] [PubMed] [Google Scholar]
- 45.Tuite AR, Fisman DN, Greer AL. Mathematical modelling of COVID-19 transmission and mitigation strategies in the population of Ontario, Canada. CMAJ. 2020;192:E497–E505. doi: 10.1503/cmaj.200476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan PS, Sailey C, Guest JL, et al. Detection of SARS-CoV-2 RNA and antibodies in diverse samples: protocol to validate the sufficiency of provider-observed, home-collected blood, saliva, and oropharyngeal samples. JMIR Public Heal Surveill. 2020;6:e19054. doi: 10.2196/19054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landry ML, Criscuolo J, Peaper DR. Challenges in use of saliva for detection of SARS CoV-2 RNA in symptomatic outpatients. J Clin Virol. 2020;130 doi: 10.1016/j.jcv.2020.104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farshidfar N, Hamedani S. Hyposalivation as a potential risk for SARS-CoV-2 infection: Inhibitory role of saliva [e-pub ahead of print] Oral Dis. 2021 doi: 10.1111/odi.13375. Accessed September 01, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]