Abstract
Introduction
To analyze COVID-19 mortality in cancer patients and associated factors such as age, sex, type of insurance, situation at COVID-19 diagnosis, and cancer histology during the pandemic at a cancer center in Brazil.
Methods
Cross-sectional study carried out from April 02, 2020 to August 31, 2020 at A.C. Camargo Cancer Center (ACCCC), in São Paulo, Brazil. Cases were extracted from the Hospital Cancer Registry. COVID-19 lethality rates by histology were calculated; multiple logistic regression was used to identify factors associated with COVID-19 mortality. The log-rank test was applied to compare the survival curves for each variable.
Results
Of the 411 patients analyzed, 51 (12.4%) died due to COVID-19. Death occurred at an average age of 63 years. The fatality rate was higher for lung (0.333) and hematological (0.213) cancers and was associated with age over 60 years. The greatest chances of death from COVID-19 were in cases of lung (odds ratio, OR, 4.05, 95% confidence interval, CI 1.33–12.34) and hematological (OR 2.17, 95% CI 0.96–4.90) cancers, and in patients currently undergoing cancer treatment (OR 2.77, 95% CI 1.25–6.13). There were no statistical differences in survival by sex, age group, type of insurance, situation at the diagnosis of COVID-19, and histology of cancer for COVID-19.
Conclusions
Mortality due to COVID-19 in cancer patients is heterogeneous. These findings reinforce the need for individualized strategies for the management of different types of cancer that reduce the risk of death from COVID-19.
Keywords: COVID-19, Cancer, Patients, Mortality, Risk factor
Introduction
COVID-19 is a disease caused by a novel coronavirus, SARS-CoV-2, first reported in China in late 2019 [1]. Its rapid spread led the World Health Organization (WHO) to declare it a pandemic in March 2020 [2].
In Brazil, the first case was reported on February 26 and the first death on March 12, 2020, in São Paulo [3,4]. By May of 2020, Brazil was considered one of the epicenters of the global COVID-19 pandemic, while the state of São Paulo was considered the national epicenter, with the highest number of deaths [5].
Epidemiological and clinical evidence points to a greater risk of serious outcomes of COVID-19 in elderly patients, men, smokers, and patients with comorbidities such as hypertension, diabetes, obesity, and cancer [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22] [See Text Box and Table 1 ]. Cancer patients are more vulnerable to complications and death when infected with SARS-CoV-2. This is due to a combination of factors including immunosuppressive therapies, age over 60, use of corticosteroids, presence of comorbidities, advanced metastatic cancer, and coexisting lung cancer. [23,24,25]. However, as mortality due to COVID-19 is different for each type of cancer, it is not accurate to classify all cancer patients as equally susceptible [6]. Some studies have shown higher mortality from COVID-19 in patients with lung and hematological cancers [6,7,8,9,13,14,16,17,21]; others have not [10,11].
Table 1.
Key references describing patients with a diagnosis of cancer and COVID-19.
| Reference | Description | Results | Comments |
|---|---|---|---|
| Lee et al. [6] |
|
|
|
| Dai et al. [7] |
|
|
|
| Garassino et al. [8] |
|
|
|
| Mehta et al. [9] |
|
|
|
| Kuderer et al. [10] |
|
|
|
| Melo et al. [11] |
|
|
|
| Robilotti et al. [12] |
|
|
|
| Barlesi et al. [13] |
|
|
|
| Venkatesulu et al. [14] |
|
|
|
| Liang et al. [15] |
|
|
|
| Luo et al. [16] |
|
|
|
| Jee et al. [17] |
|
|
|
| Yekedüz et al. [18] |
|
|
|
| Elkrief et al. [19] |
|
|
|
| Zhang et al. [20] |
|
|
|
| Mato et al. [21] |
|
|
|
| Saini et al. [22] |
|
|
|
CFR = case fatality rate; CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group performance status; HR = hazard ratio; ICIs = immune checkpoint inhibitors; NCLC = nonsmall cell lung cancer; OR = odds ratio; SCLC = small cell lung cancer; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
In Brazil, few studies have evaluated COVID-19 mortality in cancer patients. An analysis carried out at the National Cancer Institute (INCA) from April 30, 2020 to May 26, 2020 identified a COVID-19 mortality rate of 33.1% in patients with a diagnosis of cancer [11]. In New York, the Montefiore Health System identified a 28% mortality rate [9], while at the Memorial Sloan Kettering Cancer Center mortality was reported to be 9% [12]. In France, the 15% death rate among cancer patients at Gustave Roussy was similar to that seen in the general population [13]. Therefore, the mortality of COVID-19 patients appears to differ between cancer treatment centers.
TEXT BOX.
-
•
Patients with cancer have a higher risk of coronavirus disease 2019 (COVID-19) than noncancer patients – although all analyses confounded by older age of cancer patients and their attendant co-morbidities [7], [11].
-
•
Patients with a diagnosis of cancer appear to do worse [7], [9], [12], [14], [17], [19], [22].
-
•
Different tumor types appear to confer differing susceptibility to SARS-CoV-2 infection and COVID-19 phenotypes [6].
-
•
Advancing age is associated with worse outcomes [8], [9], [10], [11], [12], [15], [19], this study.
-
•
Patient with a diagnosis of hematological malignancies seem to do worse [6], [9], [11], [13], [14], [17], [21], this study.
-
•
Amongst solid tumors, lung cancer appears to do worse [7], [11], [13], [14], [16], [27], this study.
-
•
Amongst solid tumors, advanced cancer appears to do worse [7], [11], [13], [19].
-
•
Data suggests chemotherapy may worsen outcomes [6], [8], [13], [15], [17], [18], [20], this study, but not all studies agree [12].
-
•
Data suggests surgery may worsen outcomes [7], [15], [17] but not all studies agree [10].
-
•
Radiotherapy does not appear to worsen outcomes [7].
Alt-text: Unlabelled box
The A.C. Camargo Cancer Center (ACCCC) is a collaborative private-public oncology treatment center in the state of São Paulo, with treatment, teaching, and research units. Having been in operation for over 60 years, it is the primary oncologic reference in South America and attends an average of 8,000 new cases of cancer annually [26]. An ACCCC crisis committee was created on March 13, 2020 to implement a safe flow for coping with COVID-19, with the objective of treating patients and ensuring the safety of the professionals involved [27].
Therefore, analyzing COVID-19 mortality in cancer patients, identifying risk topographies, the situation at COVID-19 diagnosis, and factors such as age and sex in patients treated at a cancer center, can outline the profile of Brazilian patients in one of the largest treatment centers in the country and thus form health policies adapted to the Brazilian reality.
Methods
This is a retrospective cross-sectional study that analyzed mortality, age, sex, type of insurance, situation at the diagnosis of COVID-19, and histology of COVID-19 positive cancer patients. The period of analysis was from April 2 to August 31, 2020. The cases were extracted from the ACCCC Hospital Cancer Registry. The ACCCC is a private hospital, considered a tertiary referral center for cancer that maintains a partnership contract with both public and private health systems.
The variables analyzed were sex, categorized age (<60 years and ≥60 years), type of insurance (public or private), situation at diagnosis of COVID-19 (in follow-up, in treatment, or palliative), and cancer histology. Histology was classified using the International Classification of Diseases for Oncology, Third Edition (ICD-O3) codes as follows: hematological (C42, C77), breast (C50), digestive organs (C15-C17, C22-C26), lung (C34), colorectal (C18-C21), prostate (C61), urinary tract (C64-C68), organs respiratory and intrathoracic, except lung (C30-C39), female genitals (C51-C58), soft tissue (C49), lip, oral cavity and pharynx (C00-C14), and central nervous system (CNS) (C69-C72).
According to the WHO a death from COVID-19 is defined for surveillance purposes as a death resulting from a clinically compatible disease, in a confirmed case of COVID-19 by RT-PCR, unless there is a clear alternative cause of death that cannot be associated with COVID-19 [28]. This was the COVID-19 mortality criterion defined for the cases described here.
Statistical analysis
Absolute and relative frequencies were calculated for each variable. Categorical variables were compared using the chi-squared test, using a significance level of 5%. The mortality rate for COVID-19 was calculated for each cancer histology by dividing the number of COVID-19 deaths by the total number of cases. Lethality graphics by age group were made using Microsoft Excel.
Survival rates were calculated considering the dates of diagnosis and death by COVID-19. Survival analyses at 10, 20, and 30 days were applied to the following variables: sex, age group, type of insurance, situation at the diagnosis of COVID-19, and cancer histology. The log-rank test was performed to compare the survival curves for each variable, using a significance level of 0.05.
To estimate the odds ratio (OR) and 95% confidence interval by histology (hematological and lung) and situation at the diagnosis of COVID-19, adjusted for potential confounding factors age and sex, multiple logistic regression was performed. Solid tumors were used as reference, and a P-value <0.05 was defined as significant. The type of insurance variable did not enter the multiple analysis because it presented a value of P > 0.20 in the univariate analysis. Analyses were performed using STATA 15 (College Station, Texas, 2017) and Statistical Package for the Social Sciences (SPSS) version 23 (IBM Corp., Armonk, NY, USA).
Ethical approval
This study was approved by the Research Ethics Committee of the Antônio Prudente Foundation, A. C. Camargo Cancer Center, reference number 2462/17.
Results
In the studied period, 411 patients were diagnosed with COVID-19, 51 of whom died (12.4%), and 16 of whom were excluded from analysis because they presented nonmelanoma skin cancer. The average age of patients with COVID-19 was 56.9 years; 52.3% (215/411) were aged <60 years, 56.9% (234/411) were female, 68% (281/411) were attended by the private health system, and 64.7% (266/411) were undergoing cancer treatment at the time of COVID-19 diagnosis.
The demographic and clinical data of patients who died from COVID-19 were compared with those who survived. It was observed that the majority of patients were female in both groups 52.9% (27) and 57.5% (207), respectively. Of the patients who died, 66.7% (34) were ≥60 years old, mean age 63 years, while in the surviving group 55.0% (198) were <60 years old with a mean age of 56 years. In both groups, the majority of patients were covered by private health insurance 64.7% (33) and 68.9% (248), and 74.5% (38), and 63.3% (228) were currently undergoing cancer treatment. Significant differences were observed between deaths and survivors for age group, histology, and treatment situation (Table 2 ).
Table 2.
Sociodemographic and clinical data of the A.C. Camargo Cancer Center COVID-19 cohort, from April to August, 2020, São Paulo – SP, Brazil.
| Died from COVID-19 (n = 51) |
Survived COVID-19 (n = 360) | Pa | |
|---|---|---|---|
| Sex | |||
| Female | 27 (52.9%) | 207 (57.5%) | .538 |
| Male | 24 (47.1%) | 153 (42.5%) | |
| Age group | |||
| Average (± SD) | 63.0 (±13.0) | 56.0 (±15.1) | .004 |
| <60 | 17 (33.3%) | 198 (55.0%) | |
| ≥60 | 34 (66.7%) | 162 (45.0%) | |
| Histology (ICD-O3) | |||
| Hematological (C42, C77) | 10 (19.6%) | 37 (10.3%) | .032 |
| Breast (C50) | 8 (15.7%) | 85 (23.6%) | |
| Digestive organs (C15-C17, C22-C26) | 8 (15.7%) | 32 (8.9%) | |
| Lung (C34) | 6 (11.8%) | 12 (3.3%) | |
| Colorectal (C18-C21) | 4 (7.8%) | 48 (13.3%) | |
| Prostate (C61) | 4 (7.8%) | 36 (10.0%) | |
| Urinary tract (C64-C68) | 4 (7.8%) | 23 (6.4%) | |
| Respiratory and intrathoracic organs, not lung (C30-C39) | 2 (3.9%) | 2 (0.6%) | |
| Female genital organs (C51-C58) | 2 (3.9%) | 24 (6.7%) | |
| Soft tissues (C49) | 1 (1.9%) | 11 (3.1%) | |
| Lip, oral cavity, and pharynx (C00-C14) | 1 (1.9%) | 18 (5.0%) | |
| SNC (C69-C72) | 1 (1.9%) | 5 (1.4%) | |
| Bone and articular cartilage (C40-C41) | - | 2 (0.6%) | |
| Male genital organs (C60-C63) | - | 9 (2.5%) | |
| Endocrine glands (C73-C75) | - | 16 (4.4%) | |
| Type of insurance | |||
| Private | 33 (64.7%) | 248 (68.9%) | .548 |
| Public | 18 (35.3%) | 112 (31.1%) | |
| Situation at diagnosis of COVID-19 | |||
| In follow-up | 9 (17.7%) | 129 (35.8%) | <.001 |
| In treatment | 38 (74.5%) | 228 (63.3%) | |
| Palliative | 4 (7.8%) | 3 (0.8%) |
Pearson Chi-squared test.
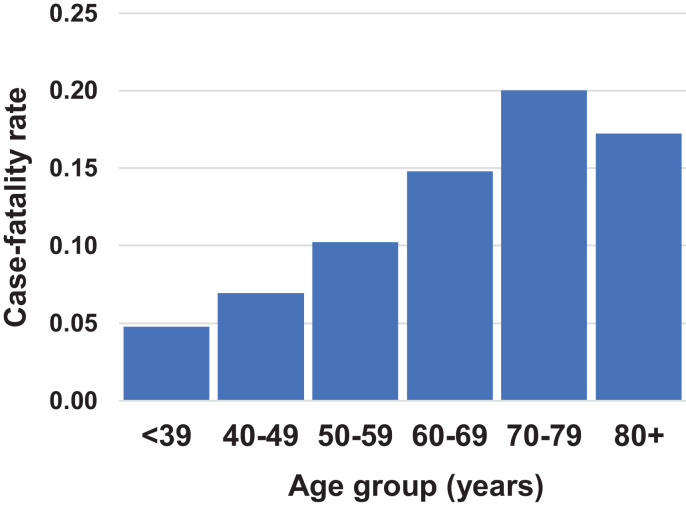
The fatality rate in cancer patients after COVID-19 infection was highest among patients with lung cancer (0.333) and hematological cancers (0.213) (Table 3 ), and in the 70–79 age group (Fig. 1 ).
Table 3.
COVID-19 case-fatality rate by cancer histology (ICD-O3) at the A.C. Camargo Cancer Center, from April to August, 2020, São Paulo – SP, Brazil.
| Histology (ICD-O3) | Deaths | Total | Case–fatality rate |
|---|---|---|---|
| Hematological (C42, C77) | 10 | 47 | 0.213 |
| Breast (C50) | 8 | 93 | 0.086 |
| Digestive organs (C15-C17, C22-C26) | 8 | 40 | 0.200 |
| Lung (C34) | 6 | 18 | 0.333 |
| Colorectal (C18-C21) | 4 | 52 | 0.077 |
| Prostate (C61) | 4 | 40 | 0.100 |
| Urinary tract (C64-C68) | 4 | 27 | 0.148 |
| Respiratory and intrathoracic Organs, not lung (C30-C39) | 2 | 4 | 0.500 |
| Female genital organs (C51-C58) | 2 | 26 | 0.077 |
| Soft tissues (C49) | 1 | 12 | 0.083 |
| Lip, oral cavity, and pharynx (C00-C14) | 1 | 19 | 0.053 |
| SNC (C69-C72) | 1 | 6 | 0.167 |
*COVID-19 topographies without deaths were excluded from the analysis.
Fig. 1.
Case-fatality rate by COVID-19 in cancer patients by age group, in the A.C. Camargo Cancer Center from April to August, 2020, São Paulo – SP, Brazil.
In univariate analysis, a greater chance of death from COVID-19 was observed in patients over 60 years of age (OR 2.44 [1.31; 4.53]), those undergoing cancer treatment (OR 2.38 [1.11; 5.09]) or in palliative care (OR 19.01 [3.69; 98.75]), and in those with lung cancer (OR 4.44 [1.56; 12.57]), or hematological malignancies (OR 2.40 [1.09; 5.24]). In the multiple analysis, a greater chance of death from COVID-19 was observed in patients over 60 years of age (OR 2.26 [1.18; 4.35]), in cancer treatment (OR 2.77 [1.25; 6.13]) and in palliative care (OR 17.66 [3.13; 99.59]), with lung cancer (OR 4.05 [1.33; 12.34]) and hematological malignancies (OR 2.17 [0.96; 4.90]). Sex and type of treatment were not associated with COVID-19 mortality in either analysis (Table 4 ).
Table 4.
Univariate and adjusted analysis of COVID-19 mortality, in the A.C. Camargo Cancer Center, from April to August, 2020, São Paulo – SP, Brazil.
| Variables | ORa univariate (95% CI) | P | ORa adjusted (95% CI) | P |
|---|---|---|---|---|
| Histology (ICD-O3) | ||||
| Other Solid Tumors | 1 | 1 | ||
| Hematological (C42, C77) | 2.40 (1.09–5.24) | .029 | 2.17 (0.96–4.90) | .062 |
| Lung (C34) | 4.44 (1.56–12.57) | .005 | 4.05 (1.33–12.34) | .014 |
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 0.83 (0.46–1.49) | .539 | 0.88 (0.47–1.66) | .709 |
| Age group | ||||
| <60 | 1 | 1 | ||
| ≥60 | 2.44 (1.31–4.53) | .005 | 2.26 (1.18–4.35) | .014 |
| Type of insurance | ||||
| Public | 1 | - | - | |
| Private | 0.83 (0.45–1.53) | .548 | - | - |
| Situation at diagnosis of COVID-19 | ||||
| In follow-up | 1 | - | - | |
| In treatment | 2.38 (1.11–5.09) | .024 | 2.77 (1.25–6.13) | .011 |
| Palliative | 19.01 (3.69–98.75) | .001 | 17.66 (3.13–99.59) | .001 |
Other Solid Tumors = (C50, C15-C17, C22-C26, C18-C21, C61, C64-C68, C30-C39, C51-C58, C49, C00-C14, C69-C72).
The values in bold are significant.
OR = Odds Ratio. Multivariable corrections were made for patient age and sex.
The highest overall survival in cancer patients with COVID-19 were in women, other solid tumors, and in those undergoing follow-up. However, no significant differences were observed in survival by sex, age group, tumor site, type of insurance, and situation at diagnosis of COVID-19 (Supplemental Table 1; Supplemental Figure 1).
Discussion
In the present study, mortality due to COVID-19 in cancer patients was most associated with lung and hematological cancers, and particularly for patients undergoing cancer treatment in the age group above 60 years. There were no differences in survival by sex, age group, histology, type of insurance, or situation at diagnosis of COVID-19. A systematic review with meta-analysis showed that cancer patients affected by COVID-19 have a higher chance of death (OR 2.54 [1.47; 4.42]) and are 10 years older, than the general population. The most frequent neoplasms observed in that study were hematological (34.3%), breast (29%), and lung (27.3%) [14].
Other studies have also demonstrated higher mortality from COVID-19 in cancer patients, with reported mortality ranging from 9% to 50% [7,9,12,14,15,17,19,22]. The results of the present study fall on the lower end of this spectrum, with a 12.4% mortality. The highest prevalence of mortality was observed in studies of Liang et al. [15]. who analyzed 18 patients (50% died), Garassino et al. [8] 200 (33,3%), Melo et al. [11] 181 (33.1%), Mehta et al. [9] 218 (28%), and Luo et al. [16] 69 (24%). Whereas, the lowest prevalence of mortality was found in studies of Barlesi et al. [13] 137 (15%), Dai et al. [7] 154 (11,4%), and Robilotti et al. [12] 423 (9%), as well, in this study in which 411 patients were analyzed and 12.4% (51) died due to COVID-19 (Table 5 ). Given the diversity of the population studied one cannot and should not infer any signifcance to these differences.
Table 5.
Case-fatality by COVID-19 in cancer patients in selected studies.
| COVID-19/Cancer | N patients (% death) |
|---|---|
| Liang et al. [15] | 18 (50%) |
| Garassino et al. [8] | 200 (33,3%) |
| Melo et al. [11] | 181 (33,1%) |
| Mehta et al. [9] | 218 (28%) |
| Luo et al. [16] | 69 (24%) |
| Barlesi et al. [13] | 137 (15%) |
| Fernandes et al. (2021) | 411 (12,4%) |
| Dai et al. [7] | 154 (11,4%) |
| Robilotti et al. [12] | 423 (9%) |
The values in bold are significant.
In this study we reported that patients with hematological cancers had 2.17 times the chance of death from COVID-19, which paralleles results of a UK study (OR 1.57) [6]; as well as those of a systematic review (OR 2.39) [14]. However, in the USA, Canada and Spain (OR 1.40) [10]; as in a Brazilian study (OR 1.0) [11] there was no increase in mortality in COVID-19 patients with hematological cancers. It is thought that patients with hematological cancers are more vulnerable to severe outcomes due to immunosuppression caused by intense treatment with myelosuppressants or in the cae of chronic lymphocytic leukemia, their intrinsic immunosuppression [21]. Lymphopenia and basal neutropenia are additional factors that can increase the risk of worse outcomes and are frequently observed in individuals with hematological cancers [6,17,29].
Lung cancer patients are more vulnerable to COVID-19 and the data suggests their outcomes are worse [16]. The chances of death were reported here to be increased by 4.05 times, somewhat higher than the chances reported by a meta-analysis (OR 1.83) [14]. In contrast, a study in the United Kingdom found no increase in lung cancer mortality (OR 1.41) [6]. In this study, 66% of patients diagnosed with lung cancer and who died of COVID-19 were smokers or ex-smokers. The high prevalence of smoking can be attributed as a risk factor associated with mortality. The TERAVOLT study, which analyzed data from patients with chest cancer (76% nonsmall cell lung cancer), found that smoking history was associated with an increased risk of death [8]. According to the THOCOoP cooperative group, patients with lung cancer represent a population particularly vulnerable to COVID-19, due to smoking, low immunity, and the presence of comorbidities such as chronic obstructive pulmonary disease (COPD). They suggest that treatment should be maintained with special care and that avoiding patient exposure to SARS-CoV-2 is paramount [30].
A systematic review revealed that patients undergoing cancer treatment, such as chemotherapy, had a higher risk of death from COVID-19 [18], which aligns with our own findings that patients who had received chemotherapy in the previous 30 days were more vulnerable to death from COVID-19. However, another systematic review found no association between the receipt of any type of cancer therapy and mortality from SARS-CoV-2 [14]. Different cancer therapies are believed to have different effects on the risk of serious outcomes for COVID-19 [31].
The patients in the present study had a mean age (56.9 years) lower than that of a recent systematic review (65.1 years) [14] and similar to that of another study conducted in Brazil (55.3 years) [11]. This lower average age is due to a higher prevalence of visits by young women with breast cancer at the ACCCC. However, even in our cohort, age over 60 years was associated with higher mortality from COVID-19, similar to the findings of other studies [6,11,32], and may be due to a less efficient immune response in the elderly [33,34].
Male cancer patients have been reported to have more severe COVID-19 outcomes [10,23,24]. This increase in mortality in men is related to a higher number of comorbidities, prevalence of smoking, alcohol consumption, and occupational exposures [35,36,37]. In the present study, differences in mortality due to COVID-19 between the sexes were not identified, as reported in other studies [9,11,19]. Sex hormones are believed to be important in the immune response; estrogen is known to act as an immune booster, but androgens need to be further investigated for their interaction with COVID-19 [38,39].
Cancer patients develop severe COVID-19 outcomes in less time than cancer-free patients do [15]. Studies in China have reported lower survival in patients with lung cancer and COVID-19 compared to people without cancer [7], as well as in patients undergoing cancer treatment compared to patients who are in the follow-up [20]. A study carried out in Canada identified a lower survival rate in elderly patients with advanced stage IV disease [19], with age greater than or equal to 75 years associated with low survival in a patient with chronic lymphocytic leukemia [21]. However, in the present study there were no differences in survival by sex, age group, tumor site, type of insurance, or situation at the diagnosis of COVID-19.
The ACCCC is a public-private treatment center in which increased COVID-19 related mortality from lung and hematological cancer has been observed. These findings are unlike what was observed in a study carried out at INCA, a public referral center for cancer treatment in Brazil, supervised by the Ministry of Health [11]. At the ACCCC, no difference in mortality was observed in terms of access to public versus private care, which demonstrates equity in treatment, without a difference in patient survival.
These results demonstrate the importance of studies in cancer patients since it is a population at higher risk of death from COVID-19. A total of 411 patients were analyzed at a cancer center where most of the care is provided by the private system. Detailed studies on the clinical profiles, comorbidities, and staging should be investigated to better understand mortality and mechanisms of COVID-19 in this risk group. Our study has limitations, as it is a cross-sectional study limited in time, but still presents useful information on mortality due to COVID-19 in cancer patients at the largest cancer center in Latin America.
Conclusion
We observed that cancer patients have a greater chance of death associated with COVID-19 if they have hematological or lung cancers, are in the age group above 60 years, and if they are currently undergoing cancer treatment. There was no difference in mortality regarding sex or type of insurance. From these data we conclude that cancer patients are not affected equally by COVID-19. Consequently one can envision that deployment of different strategies for individual cancer patients will be needed to reduce the chance of death while also maintaining oncologic care.
Author contributions
Gisele A. Fernandes: Conceptualization, Methodology, Software, Writing- Original draft preparation, Writing- Reviewing and Editing, Visualization, Investigation. Maria P. Curado: Conceptualization, Methodology, Writing- Original draft preparation, Supervision, Writing- Reviewing and Editing, Visualization, Investigation. Diego R. M. Silva: Data curation, Methodology, Software. Ivan L.A.F. Silva: Data curation, Writing- Original draft preparation, Supervision, Visualization, Investigation. Diego Feriani: Data curation, Writing- Original draft preparation, Visualization, Investigation. Juliana S. Canteras: Data curation. Rodrigo R. Silva: Data curation. Paola E. Arantes: Data curation.
Funding
None.
Declaration of competing interest
None.
Biographies

Ms. Gisele Aparecida Fernandes graduated in Nursing from the Federal University of Alfenas (UNIFAL). Master's degree from the Federal University of Itajubá (UNIFEI). PhD in Public Health, Epidemiology, at the Faculty of Public Health, University of São Paulo (FSP - USP). She is currently an Epidemiologist in the group of Epidemiology and Statistics in Cancer at A.C. Camargo Cancer Center (Fundação Antônio Prudente). Has experience in Collective Health, with emphasis on Epidemiology, acting mainly on the following themes: cancer, epidemiology, lung cancer.

Mr. Diego Feriani Infectious diseases Medical Doctor. Works with infection prevention and control. Currently is in a professional master's program at University of São Paulo Medical School, focusing on prevention and control of healthcare associated infections.

Mr. Ivan Leonardo Avelino França e Silva PhD in Medical Sciences at the Faculty of Medicine, University of São Paulo, He is currently an infectious disease physician at the Instituto de Infectologia Emílio Ribas, director of the Department of Infectious Diseases, Coordinator of the Hospital Infection Control Service and Risk Manager at Hospital A.C. Camargo, Head of infectious back-up team at Hospital Alemão Oswaldo Cruz. Acting mainly on the following topics: Infection in immunocompromised patients, infection in cancer patients, AIDS, hospital infection control.

Mr. Diego Rodrigues Mendonça e Silva Biologist from Faculdade Araguaia (2009), Master in Health Sciences from Universidade Federal de Goiás (2012). He is currently a Research Analyst in the Cancer Epidemiology and Statistics Group; and Supervisor of the Hospital Registry of Cancer at A.C.Camargo Cancer Center (Fundação Antônio Prudente). Experience in Epidemiological Surveillance of cancer, performance in Cancer Registry, database treatment, preparation of reports on research data, scientific production and basic statistical analysis.

Ms. Paola Engelmann Arantes Biomedical Scientist with experiences in clinical research, focusing on epidemiology and biostatistics. Currently working on the epidemiological profile project of COVID-19 at A.C.Camargo Cancer Center and digital marketing for the translational project “Headspace study” for head and neck cancer in partnership with the International Cancer Research Agency (IARC). Working daily with platforms like Stata 12 and Excel for data analysis.

Ms. Juliana da Silva Canteras graduated in Nursing from the School of Nursing at the University of São Paulo (2011). Specialization in Infection Control by the Professional Improvement Program of the School of Continuing Education of Hospital das Clínicas, Faculty of Medicine, University of São Paulo. She is currently Nurse at the Infection Control and Prevention Service of AC Camargo Cancer Center.

Mr. Rodrigo Reghini da Silva Graduated in Nursing. Specialization in Infection Control by the Professional Improvement Program of the School of Continuing Education of Hospital das Clínicas, Faculty of Medicine, University of São Paulo, HC-FMUSP. He is currently a Nurse at the Hospital Infection Control Service of the AC Camargo Cancer Center and a graduate student in Oncology at the Faculty of Education in Health Sciences – FECS. Has experience in the area of Nursing with an emphasis on Epidemiological Surveillance and Health Care Related Infections.

Ms. Maria Paula Curado graduated in Medicine from the Federal University of Goiás (1977), Master in Medicine (Head and Neck Surgery) from Hospital Heliópolis - PhD in Oncology Area at - AC Camargo Cancer Center (2004). Since 2016 a collaborator of Global Burden of Disease, also a collaborator Professor of pos Graduate ciencias da saude at the Federal University of Goiás, Professor of the Graduate Program in Oncology Sciences at the Antônio Prudente Foundation. Since 2015 head of the group epidemiology cancer group the Cipe ACCamargo Cancer Center. I have experience in cancer epidemiology, case control studies and multicentric. Studies on the head and neck cancer, gastric and breast cancer.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1053/j.seminoncol.2021.01.003.
Appendix. Supplementary materials
References
- 1.Whisenant J.G., Trama A., Torri V., et al. TERAVOLT: Thoracic Cancers International COVID-19 Collaboration. Cancer Cell. 2020;37:742–745. doi: 10.1016/j.ccell.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva: 2020. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-mediabriefing-on-covid-19 March 11. —11-march-2020. [Accessed October 7, 2020] [Google Scholar]
- 3.Johns Hopkins Coronavirus Resource Center, 2020. COVID-19 Map. https://coronavirus.jhu.edu/map.html. [Accessed September 24, 2020].
- 4.Newspaper Agência Brasil. https://agenciabrasil.ebc.com.br/saude/noticia/2020-06/primeira-morte-por-covid-19-no-brasil-aconteceu-em-12-de-marco. https://agenciabrasil.ebc.com.br/saude/noticia/2020-06/primeira-morte-por-covid-19-no-brasil-aconteceu-em-12-de-marco [Accessed September 30, 2020].
- 5.Newspaper Agência Brasil. COVID-19: Brazil has 438,238 cases; death toll up to 26,754. https://agenciabrasil.ebc.com.br/en/saude/noticia/2020-05/covid-19-brazil-has-438238-cases-death-toll-26754. [Accessed September 24, 2020].
- 6.Lee L.Y.W., Cazier J.-B., Starkey T., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garassino M.C., Whisenant J.G., Huang L.-C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;20:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta V., Goel S., Kabarriti R., et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melo A.C., Thuler L.C.S., Silva J.L., et al. Cancer inpatient with COVID-19: a report from the Brazilian National Cancer Institute. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0241261. bioRxiv Preprint 2020. Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robilotti E.V., Babady N.E., Mead P.A., et al. Determinants of severity in cancer patients with COVID-19 Illness. medRxiv. 2020 medRxiv 2020;1-19. May 8:2020.05.04.20086322. [Google Scholar]
- 13.Barlesi F., Foulon S., Bayle A., et al. Outcome of cancer patients infected with COVID-19, including toxicity of cancer treatments. Proceedings of the Annual Meeting of the American Association for Cancer Research 2020; 2020 Apr 27-28 and Jun 22-24; Philadelphia (PA): AACR; 2020. [Google Scholar]
- 14.Venkatesulu B.P., Chandrasekar V.T., Girdhar P., et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. medRxiv. 2020 doi: 10.1093/jncics/pkaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J., Rizvi H., Preeshagul I.R., et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jee J., Foote M.B., Lumish M., et al. Chemotherapy and COVID-19 Outcomes in Patients With Cancer. J Clin Oncol. 2020;38:3538–3546. doi: 10.1200/JCO.20.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yekedüz A., Utkan G., Ürüna Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer. 2020;141:92–104. doi: 10.1016/j.ejca.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkrief A., Desilets A., Papneja N., et al. High mortality among hospital-acquired COVID-19 infection in patients with cancer: a multicentre observational cohort study. Eur J Cancer. 2020;139:181–187. doi: 10.1016/j.ejca.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H., Wang L., Chen Y., et al. Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan. China Cancer. 2020;126:4023–4031. doi: 10.1002/cncr.33042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mato A.R., Roeker L.E., Lamanna N., et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saini K.S., Tagliamento M., Lambertini M., et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai A., Sachdeva S., Parekh T., et al. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:557–559. doi: 10.1200/GO.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang K., Sheng Y., Huang C., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X., Zhang L., Chen L., et al. Does COVID-2019 have an Impact on the Purchase Intention of Commercial Long-Term Care Insurance among the elderly in China? Healthcare (Basel) 2020;8:126. doi: 10.3390/healthcare8020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camargo AC, Cancer Center. https://www.accamargo.org.br/. [Accessed October 7, 2020].
- 27.Leite F.P.M., Curi C., Sanches S.M., et al. How to maintain elective treatment of breast cancer during the COVID-19 pandemic—a cancer center experience. J Surg Oncol. 2021;123(1):9–11. doi: 10.1002/jso.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization, 2020. https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19.pdf?ua=1. [Accessed September 24, 2020].
- 29.Addeo A., Friedlaender A. Cancer and COVID-19: Unmasking their ties. Cancer Treat Rev. 2020;88:9–11. doi: 10.1016/j.ctrv.2020.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arrieta O., Cardona A.F., Lara-Mejía L., et al. Recommendations for detection, prioritization, and treatment of thoracic oncology patients during the COVID-19 pandemic: the THOCOoP cooperative group. Crit Rev Oncol Hematol. 2020;153 doi: 10.1016/j.critrevonc.2020.103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The UK Coronavirus Cancer Monitoring Project: protecting patients with cancer in the era of COVID-19. Lancet Oncol. 2020;21:622–624. doi: 10.1016/S1470-2045(20)30230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhopal R. Covid-19 worldwide: we need precise data by age group and sex urgently. BMJ. 2020;369:m1366. doi: 10.1136/bmj.m1366. [DOI] [PubMed] [Google Scholar]
- 33.Goronzy J.J., Fang F., Cavanagh M.M., et al. Naive T Cell maintenance and function in human aging. J Immunol. 2015;194:4073–4080. doi: 10.4049/jimmunol.1500046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019. Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma G., Volgman A.S., Michos E.D. Sex differences in mortality from COVID-19 pandemic are men vulnerable and women protected? JACC Case Rep. 2020;2:1407–1410. doi: 10.1016/j.jaccas.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakravarty D., Nair S.S., Hammouda N., et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol. 2020;3:374. doi: 10.1038/s42003-020-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhopal S.S., Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet. 2020;396:532–533. doi: 10.1016/S0140-6736(20)31748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strope J.D., Chau C.H., Figg W.D. Are sex discordant outcomes in COVID-19 related to sex hormones? Semin Oncol. 2020;47:335–340. doi: 10.1053/j.seminoncol.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cattrini C., Bersanelli M., Latocca M.M., et al. Sex hormones and hormone therapy during COVID-19 pandemic: implications for patients with cancer. Cancers (Basel) 2020;12:2325. doi: 10.3390/cancers12082325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.