Abstract
Objectives
To avoid the significant risks posed by the use of COVID-19 serology tests with supply chain constraints or poor performance characteristics, we developed an in-house SARS-CoV-2 total antibody test. Our test was compared with three commercial methods, and was used to determine COVID-19 seroprevalence among healthcare workers and outpatients in Minnesota.
Methods
Seventy-nine plasma and serum samples from 50 patients 4–69 days after symptom onset who tested positive by a SARS-CoV-2 PCR method using a nasopharyngeal (NP) swab were used to evaluate our test’s clinical performance. Seropositive samples were analyzed for IgG titers in a follow-up assay. Thirty plasma and serum from 12 patients who tested negative by a SARS-CoV-2 PCR method using a nasopharyngeal (NP) swab and 210 negative pre-pandemic serum samples were also analyzed. Among samples from patients > 14 days after symptom onset, the assay had 100% clinical sensitivity and 100% clinical specificity, 100% positive predictive value and 100% negative predictive value. Analytical specificity was 99.8%, indicating minimal cross-reactivity. A screening study was conducted to ascertain COVID-19 seroprevalence among healthcare workers and outpatients in Minnesota.
Results
Analysis of serum collected between April 13 and May 21, 2020 indicated a COVID-19 seroprevalence of 2.96% among 1,282 healthcare workers and 4.46% among 2,379 outpatients.
Conclusions
Our in-house SARS-CoV-2 total antibody test can be used to conduct reliable epidemiological studies to inform public health decisions during the COVID-19 pandemic.
Keywords: COVID-19, SARS-CoV-2, Serology, Antibody, ELISA, Seroprevalence
1. Introduction
SARS-CoV-2 is the viral causative agent of COVID-19, which is currently a global pandemic.[1] COVID-19 serology or antibody tests are a key tool for elucidating an individual’s or community’s exposure history to SARS-CoV-2, through detection of an immune response from a current or past infection with SARS-CoV-2. Identifying this population is important because SARS-CoV-2 polymerase chain reaction (PCR) tests only detect the presence of viral nucleic acid in individuals with active infections. It is currently estimated that as many as 25% of those who are infected are asymptomatic; therefore antibody testing can provide comprehensive data on true rates of exposure to SARS-CoV-2.[2], [3] Potential uses of serology assays include community screening, contact tracing, epidemiological studies, and screening convalescent plasma collected from individuals who have recovered from COVID-19.[4]
In response to the public health emergency related to COVID-19, the Food and Drug Administration (FDA) invoked the Emergency Use Authorization (EUA) pathway to accelerate the availability of COVID-19 tests. Although FDA EUA is required for clinical applications of SARS-CoV-2 molecular tests, until May 4, 2020, manufacturers of serologic assays were submitting for EUA only on a voluntary basis.[5] As a result, several serology tests were marketed that were not approved under the EUA, did not provide accurate results, had high false positive rates, and were not independently validated.[6]
To avoid the significant risks posed by the use of commercially available tests during the early stages of the pandemic – some of which had poor performance characteristics and did not have EUA designation – and potential associated supply chain disruptions, we pursued a multi-departmental effort within the University of Minnesota to develop a SARS-CoV-2 total antibody test using an enzyme-linked immunoassay (ELISA) format. First, we examined what is known about SARS-CoV-2 and other coronaviruses. Coronaviruses share structural similarities and are composed of 16 non-structural and four structural proteins, which are the spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins.[7] Common to all coronaviruses is a receptor binding domain (RBD) within the spike protein; prior studies have found that the RBD is a common target for neutralizing antibodies with the closely related SARS and MERS viruses.[8] For SARS-CoV-2, the S protein and RBD have unique features relative to other coronaviruses for enhanced cell entry, including an RBD within the S protein that can undergo furin pre-activation for cell entry and the RBD’s high angiotensin converting enzyme 2 (ACE2) binding affinity.[9] Given the key role of the RBD in viral pathogenesis and known antigenicity among other closely related coronaviruses, we utilized a recombinant SARS-CoV-2 RBD protein fragment to produce antigen for the development of a manual ELISA within the University of Minnesota’s Center for Immunology.[10], [11] The ELISA was then transferred to the University of Minnesota’s Advanced Research and Diagnostic Laboratory where clinical validation was performed to enable the assay to be used for patient testing with a current capacity of 2,000 tests per day.
As part of the assay validation, we conducted a method comparison study with three commercial SARS-CoV-2 antibody assays that utilize various SARS-CoV-2 antigens (N, S protein), detect different antibody classes (IgG, total antibody), and have different assay formats (ELISA and chemiluminescent microparticle immunoassay). Although these commercial assays have been used in large-scale seroprevalence studies in metropolitan cities worldwide, there is a critical lack of data demonstrating their comparative diagnostic performance. The use of seroprevalence data in guiding public policy decisions underscores the importance of comprehensively delineating the relative clinical sensitivity and specificity of these SARS-CoV-2 serology tests. Our method comparison study was designed to provide this critical data.
With our robustly validated laboratory-developed serology test, we set forth to establish an initial determination of COVID-19 seroprevalence among healthcare workers and outpatients in Minnesota. Here, we present the results from the first 38 days that this test was available for the afore-mentioned populations.
2. Materials and methods
2.1. Study approval and specimen acquisition
The University of Minnesota Institutional Review Board (IRB) determined that this study was not considered human research. Clinical validation of the ELISA assay entailed use of remnant serum, lithium heparin plasma and EDTA plasma specimens with associated clinical identification obtained from M Health Fairview acute care clinical laboratories serving hospitalized COVID-19 patients. COVID-19 positive (n = 79 samples from 50 patients: 38 Lithium Heparin plasma, 32 serum, 9 EDTA plasma) or negative (n = 30 samples from 12 patients: 28 Lithium Heparin plasma, 2 serum) patients were categorized based on SARS-CoV-2 PCR test results (Fig. 1 ). SARS-CoV-2 testing was conducted using four methods. Three of these methods have FDA EUA designation (CDC 2019-nCoV RT-PCR Diagnostic Panel, Initial EUA issued 02/02/2020; Roche Molecular Systems cobas SARS-CoV-2, EUA issued 03/12/2020; DiaSorin Molecular Simplexa COVID-19 Direct, EUA issued 03/19/2020), and one method is under evaluation for EUA approval (EUA 200,153 University of Minnesota Genomics Center (UMGC) COVID-19 qRT-qPCR assay). Blood samples from patients meeting these criteria were identified using the laboratory information system (Sunquest, Sunquest Information Systems, Tucson, AZ).
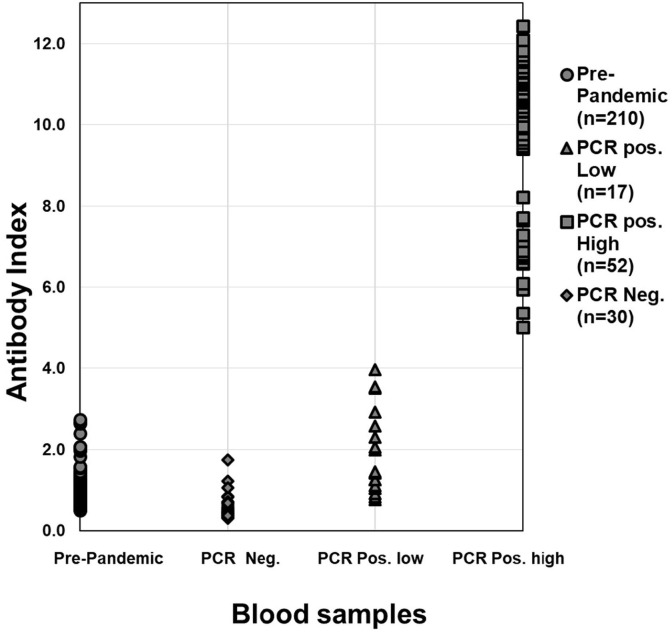
Fig. 1.
Distribution of SARS-CoV-2 total antibody assay index values among pre-pandemic, SARS-CoV-2 PCR negative, and PCR positive blood samples. A total of 210 serum samples collected from 210 presumed SARS-CoV-2 negative patients between September 2018 and March 2019 (prior to the emergence of the COVID-19 pandemic), 30 plasma and serum samples from 12 patients who tested negative by an NP swab-based SARS-CoV-2 PCR method, 17 plasma and serum samples from 11 patients 4–15 days post-COVID-19 symptom onset (“Low” antibody index) who tested positive by an NP swab-based SARS-CoV-2 PCR method, and 52 plasma and serum samples from 25 positive patients 7–35 days post-COVID-19 symptom onset who tested positive by an NP swab-based SARS-CoV-2 PCR method (“High” antibody index) were analyzed using our laboratory-developed SARS-CoV-2 total antibody assay.
Serology testing is prioritized by the M Health Fairview healthcare system to ensure patients are tested in a manner that most significantly impacts the system’s ability to understand disease spread and evaluate higher-risk individuals. For our seroprevalence study, we analyzed 3,661 serum samples received during the first 38 days that our assay was available as an orderable clinical test. These samples were obtained from healthcare workers with confirmed and non-confirmed COVID-19 exposures ≥ 14 days prior, and asymptomatic outpatients with potential COVID-19 exposures or history of prior symptoms consistent with COVID-19 ≥ 14 days prior.
3. Preparation of antigen for ELISA
The RBD fragment from the previously published literature [10], [11] was expressed in insect cells. To improve the antigenicity of the RBD fragment, we re-expressed the same RBD fragment in HEK293T cells. To this end, HEK293T cells stably expressing RBD (containing a human Fc tag) were made according to the E and F section of the pLKO.1 Protocol from Addgene (http://www.addgene.org/protocols/plko/). The Fc-tagged RBD was then purified as previously described.[10], [11]
4. SARS-CoV-2 total antibody ELISA
The SARS-CoV-2 total antibody screening assay employed an indirect enzyme immunoassay technique. Method validation studies included analytical sensitivity, interference, precision around the cutoffs, matrix equivalency, intra- and inter-assay precision, linearity, and stability (Supplemental Methods). The assay was validated for use with serum, lithium heparin plasma, and EDTA plasma (Supplemental Fig. 1). Quality control material included a negative control (cell culture supernatant from non-transfected HEK293T cells), pooled serum negative control (commercially available serum from Solomon Park Research Laboratories), low and high spike control (cell culture supernatant from HEK293T cells secreting anti-SARS-CoV-2 monoclonal antibody CR3022), and bovine serum albumin (BSA) blanks. The SARS-CoV-2 specific monoclonal antibody CR3022 (kindly provided by Dr. Florian Krammer) was used as the spike control.[12] ELISA plates were coated with recombinant SARS-CoV-2 spike RBD antigen.[10] 3,3′,5,5′-tetramethylbenzidine (TMB) was used as the substrate and Surmodics BioFX 450 nm stop reagent for TMB microwell substrates was used as the stop solution. Patient total RBD protein antibodies were recognized by goat anti-human IgG H + L-HRP. The Antibody Index (AI) was calculated by dividing each sample’s OD450nm by the serum pooled control mean. Antibody indices were categorized as follows: Negative, ≤2.5; Equivocal, 2.51 – 4.0; Positive > 4.0 (Fig. 2 ). Samples that were positive using the initial screening assay were analyzed for IgG titers. The SARS-CoV-2 antibody titration procedure was similar to the screening procedure with the exception of the use of a Rabbit anti-Human IgG antibody. The following dilutions were reported: 1:50, 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:6400, 1:12800, 1:25600, and ≥ 1:51200.
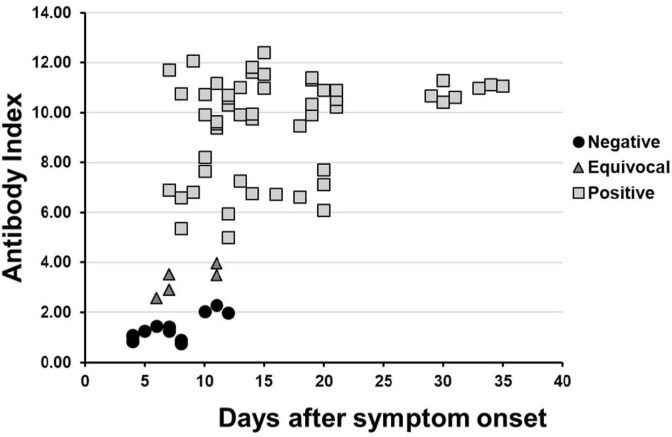
Fig. 2.
Antibody index vs. days after symptom onset for SARS-CoV-2 PCR positive patients. Antibody indices were established as follows: Negative ≤ 2.50, Equivocal 2.51 – 4.0, and Positive > 4.0. The mean number of days after symptom onset for the 12 samples (n = 8 patients) with negative antibody results was 7 (range: 4–12 days), the mean number of days after symptom onset for the 5 samples (n = 5 patients) with equivocal antibody results was 8 (range: 6–11 days), and the mean number of days after symptom onset for the 52 samples (n = 25 patients) with positive antibody results was 16 (range: 7–35 days).
5. Commercial SARS-CoV-2 antibody tests
Method comparison studies were performed using three commercial SARS-CoV-2 antibody tests: Abbott Architect SARS-CoV-2 IgG (Abbott Laboratories, Abbott Park, IL), Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Mountain Lakes, NJ), and Epitope Diagnostics, Inc. Novel Coronavirus COVID-19 IgG ELISA (EDI, San Diego, CA). Each product was used according to the manufacturer’s instructions.[13], [14], [15]
5.1. Data analysis
Patient demographic data were extracted using Sunquest and clinical data including patient-reported COVID-19 symptoms and date of symptom onset were extracted from electronic health records (EPIC, EPIC Systems Corporation, Verona, WI). COVID-19 symptoms included fever, cough, fatigue, shortness of breath and myalgia.[16] Serum samples from 210 individuals collected between September 2018 and March 2019 (prior to the emergence of the COVID-19 pandemic) and stored at −80 °C were used as presumed COVID-19 true negative specimens, and an additional 310 specimens that were positive for other infectious agents or autoimmune antibodies were used to determine the analytical specificity/cross-reactivity. Specimens from patients who tested negative or positive by a SARS-CoV-2 PCR method using a nasopharyngeal (NP) source were considered as true negatives or true positives, respectively. The diagnostic performance of the SARS-CoV-2 total antibody ELISA was determined based on sensitivity [(true positives)/(true positives + false negatives)], specificity [(true negatives)/(true negatives + false positives)], negative predictive value [(true negatives)/(true negatives + false negatives)] and positive predictive value [(true positives)/(true positives + false positives)]. Three of the 210 pre-pandemic samples with equivocal SARS-CoV-2 antibody indices were excluded from the diagnostic performance calculations. Statistical analyses were conducted using the data analysis ToolPak in Excel. The statistical significance of p-values was assessed at an alpha of 0.05. Positive, negative, and overall percent agreement, 95% confidence intervals (CIs), and Cohen’s kappa values were calculated for method comparison. Cohen’s kappa statistic is a measure of agreement between categorical variables that takes into account the possibility of the agreement occurring by chance. A kappa value of 0 indicates random agreement, whereas a value of 1 indicates complete agreement. Cohen’s kappa values of <0.40, 0.40–0.75, and >0.75 were interpreted to indicate poor, fair, and excellent agreement, respectively.[17], [18]
6. Results
6.1. Analytical assay performance
Our in-house SARS-CoV-2 total antibody ELISA is a qualitative screen for total antibodies to the spike RBD antigen, with the semi-quantitative measurement of IgG antibodies by endpoint titer (Supplemental Fig. 2). Serum, lithium heparin and EDTA plasma are acceptable specimen types (Supplemental Methods). The limit of quantification (functional sensitivity) is 10 ng/mL. The screening test was designed to recognize IgG, IgM and IgA antibodies as the combined detection of these immunoglobulins has a higher sensitivity than the detection of each antibody alone in the setting of COVID-19.[19], [20], [21] Although it has been shown that heat inactivation of serum interferes with the detection of antibodies to SARS-CoV-2 potentially causing false-negative results, we did not observe a significant difference in the antibody indices of 10 samples that were analyzed with and without heat inactivation (56 °C for 1 h; p > 0.05; t-test).[22]
Analytical specificity/cross-reactivity was evaluated by testing 520 blood specimens from patients with antibodies to 17 other common viral infections and autoantibodies, which could potentially cause false positive results: Herpes Simplex Virus IgG (n = 10), Mitochondrial IgG (n = 10), Cytomegalovirus IgG (n = 12), Varicella Zoster IgG (n = 15), Epstein Barr Virus IgG (n = 5), Rubella IgG (n = 13), Mumps IgG (n = 3), Rubeola IgG (n = 3), Human immunodeficiency virus 1/2 antibody (n = 10), Hepatitis B core antibody (n = 5), Hepatitis B surface antibody (n = 13), Hepatitis C antibody (n = 2), Haemophilus influenza type B IgG (n = 23), Influenza A IgG (n = 88), and Influenza B IgG (n = 77), and a pre-pandemic cohort used to assess for cross-reactivity to common cold coronavirus antibodies (n = 210). Samples were also obtained from 21 patients who tested positive for Hepatitis B virus DNA. Samples with direct evidence of antibodies to the common cold coronaviruses were not available for testing. However, the seroprevalence for the common cold coronaviruses is high (60–90%); therefore, cross-reactivity was indirectly ruled out through testing 210 pre-pandemic samples.[23], [24], [25] A total of 519 out of 520 samples (99.8%; 95% CI: 98.92 – 99.97%) showed no cross-reactivity (Table 1 ) indicating high analytical specificity.
Table 1.
Cross-reactivity of SARS-CoV-2 total antibody ELISA.
|
SARS-CoV-2 total antibody test result |
|||
|---|---|---|---|
| Antibody | Positive | Negative | Total |
| Pre-pandemic cohort, to assess common cold coronavirus antibodies (HKU1, NL63, OC43, 229E)* | 0 | 210 | 210 |
| Cytomegalovirus IgG | 0 | 12 | 12 |
| Epstein Barr Virus IgG | 0 | 5 | 5 |
| Haemophilus Influenzae type B IgG | 0 | 23 | 23 |
| Hepatitis B Virus## | 1# | 20 | 21 |
| Hepatitis B Virus core antibody | 0 | 5 | 5 |
| Hepatitis B Virus surface antibody | 0 | 13 | 13 |
| Hepatitis C Virus | 0 | 2 | 2 |
| Herpes Simplex Virus type 1/2 IgG | 0 | 10 | 10 |
| Influenza A IgG | 0 | 88 | 88 |
| Influenza B IgG | 0 | 77 | 77 |
| HIV-1/2 | 0 | 10 | 10 |
| Mitochondrial M2 IgG | 0 | 10 | 10 |
| Mumps IgG | 0 | 3 | 3 |
| Rubella IgG | 0 | 13 | 13 |
| Rubeola IgG | 0 | 3 | 3 |
| Varicella Zoster IgG | 0 | 15 | 15 |
| Total | 1 | 519 | 520 |
Samples with direct evidence of antibodies to the common cold coronaviruses (HKU1, NL63, OC43, and 229E) were not available for testing. However, the known seroprevalence for these coronaviruses is 60–90%23-25; therefore, SARS-CoV-2 antibody cross-reactivity was indirectly ruled out through the testing of 210 pre-pandemic samples collected between September 2018 – March 2019.
The antibody index of this sample was 5.3.
These samples were obtained from patients who tested positive for Hepatitis B Virus (HBV) DNA and therefore either have early acute HBV infection or chronic HBV infection. The one SARS-CoV-2 antibody positive sample was from a patient diagnosed with chronic HBV infection.
7. Clinical assay performance
The diagnostic performance of the SARS-CoV-2 total antibody ELISA was assessed using plasma and serum samples from patients with clinical presentations suggestive of COVID-19 infection who had positive or negative SARS-CoV-2 PCR tests, and serum from presumed true negative samples that were collected between September 2018 and March 2019 (prior to the emergence of the COVID-19 pandemic). A total of 79 blood samples (38 Lithium Heparin plasma, 32 serum, 9 EDTA) were analyzed from 50 patients 4–69 days after symptom onset who tested positive by a SARS-CoV-2 PCR method using an NP swab as determined by review of the electronic medical record. Blood samples with negative or equivocal antibody indices ≤ 4.0 from patients with PCR positive NP specimens were classified as “PCR positive Low”, whereas blood samples with positive antibody indices > 4.0 from patients with PCR positive NP specimens were classified as “PCR positive High”. A total of 237 plasma and serum samples were analyzed from 12 patients with SARS-CoV-2 PCR negative NP specimens (28 Lithium Heparin plasma, 2 serum) and 207 presumed true negative pre-pandemic samples (all sera) from 207 patients (Fig. 1). The SARS-CoV-2 total antibody ELISA has an overall 86.1% clinical sensitivity (95% CI: 76.4% – 92.8%), and 100% clinical specificity (95% CI: 98.5%–100.0%). The positive and negative predictive values based on a disease prevalence of 1.5% or 5% are indicated in Table 2 . Among patients with PCR positive NP specimens, stratification by days after symptom onset yields a clinical sensitivity of 71.8% (95% CI: 55.1%–85.0%), 100% clinical specificity (95% CI: 98.5% – 100.0%), for patients between 4 and 14 days after symptom onset. The assay has the most robust performance characteristics when used with patients > 14 days after symptom onset: 100% clinical sensitivity (95% CI: 91.2%–100.0%) and 100% clinical specificity (95% CI: 98.5% – 100.0%).
Table 2.
Diagnostic performance of SARS-CoV-2 total antibody ELISA.
| 4 – 69 days post symptom onset | 4 – 14 days post symptom onset | >14 days post symptom onset | |
|---|---|---|---|
| Sensitivity (95% CI) | 86.1% (76.4–92.8%)n = 316§ | 71.8% (55.1–85.0%)n = 276§§ | 100% (91.2–100.0%)n = 277§§§ |
| Specificity (95% CI) | 100% (98.5–100.0%) | ||
| Positive predictive value# | 100%; 100% | ||
| Negative predictive value (95% CI)# | 99.8% (99.6–99.9%);99.3% (98.8–99.9%) | 99.6% (99.3–99.7%);98.5% (97.6–99.1%) | 100%; 100% |
Three of the 210 pre-pandemic samples with equivocal SARS-CoV-2 antibody indices were excluded from the diagnostic performance calculations.
PCR, polymerase chain reaction; CI, confidence interval.
Values assuming 1.5% disease prevalence; 5% disease prevalence.
79 PCR positive, 30 PCR negative, 207 pre-pandemic (presumed PCR negative).
39 PCR positive, 30 PCR negative, 207 pre-pandemic (presumed PCR negative).
40 PCR positive; 30 PCR negative, 207 pre-pandemic (presumed PCR negative).
The clinical sensitivity of our assay increases with the number of days post-COVID-19 symptom onset (Supplemental Fig. 3). Both patients whose SARS-CoV-2 antibody temporal profiles are depicted in Supplemental Fig. 3 had positive diagnostic PCR tests six days after symptom onset. Patient 1 had a 31-day hospital course after ICU admission prior to being discharged, whereas patient 2 had a 16-day hospital course from ICU admission to discharge.
Whereas SARS-CoV-2 viral RNA is detectable even prior to symptom onset and reaches its peak at day 5 after symptoms, antibody responses begin near day 7 and most patients exhibit rising IgG and IgM antibody titers 10 days after symptom onset.[20], [26] Because of what is known to-date regarding the viral kinetics and antibody responses in patients with COVID-19,[27] our SARS-CoV-2 total antibody ELISA is not recommended for use in patients within 10 days of symptom onset as they may produce insufficient levels of detectable antibodies.
Equivocal results indicate that antibodies were detected at a level close to the threshold of the limit of detection for the assay. Such results could represent an early stage of SARS-CoV-2 infection, detection of decreasing antibody levels, cross-reactivity with viral antibodies not included in the method validation studies, or a weak antibody response among immunosuppressed patients or patients with an underlying immune disorder. Repeat testing of patients with equivocal results with additional blood samples at a later date is recommended if clinically indicated.
8. Comparison of laboratory-developed SARS-CoV-2 total antibody ELISA with commercial serology assays
We compared our laboratory-developed total antibody ELISA to three commercially available serology assays using plasma and serum samples from a total of 35 SARS-CoV-2 patients 4–35 days after symptom onset who tested positive by a SARS-CoV-2 PCR method using an NP swab and 68 presumed true negative pre-pandemic samples. A total of 20, 43, or 60 samples were used for each comparison. The positive samples included seroconversion samples that were obtained from the same patient across multiple days. The 20 samples used for the comparison with the Epitope Diagnostics assay were a subset of the 43 samples used for the comparison with the Euroimmun assay. The 60 samples used for the comparison with the Abbott Architect assay included seroconversion samples that did not overlap with the samples used for the Epitope Diagnostics and Euroimmun assay comparisons. The technical and performance specifications of these assays, as indicated in each assay’s Instructions for Use document, are provided in Table 3 . Our laboratory-developed ELISA assay demonstrated excellent agreement with the compared commercial assays (Table 4 ). Given that the range of antibody indices corresponding with the presence or absence of antibodies differs for each assay, the results were evaluated based on the categorical variables of “positive” and “negative”; equivocal results were omitted from the analysis. The results showed that our laboratory-developed total antibody ELISA exhibited 100% overall agreement (20/20; Cohen’s kappa = 0.99) with the Epitope Diagnostics SARS-CoV-2 nucleocapsid protein IgG assay, 94.8% (55/58; Cohen’s kappa = 0.89) with the Abbott Architect SARS-CoV-2 nucleocapsid protein IgG method, and 92.1% (35/38; Cohen’s kappa = 0.83) with the Euroimmun SARS-CoV-2 spike protein IgG assay. For the three discordant results between our laboratory-developed ELISA and the Euroimmun assay, the negative results from our assay are likely correct as these samples were from the pre-pandemic cohort. The one sample with a negative result from our laboratory-developed ELISA that had a positive result when using the Abbott assay was also from the pre-pandemic cohort. The two samples with positive results from our laboratory-developed ELISA that had negative results when using the Abbott assay were obtained from SARS-CoV-2 patients who tested positive by a PCR method using an NP swab. These discordant results could be explained by the difference in the epitopes and isotypes targeted by the different assays (the Abbott assay detects IgG whereas our laboratory-developed ELISA detects IgG, IgM, and IgA) and the sensitivity of the assays when testing patients who are within the early stages of disease progression.
Table 3.
Technical and performance specifications of assays used in method comparison study.
| Laboratory-developed | Abbott Architect | Euroimmun | Epitope Diagnostics | |
|---|---|---|---|---|
| Format | ELISA | CMIA | ELISA | ELISA |
| Detection | Total antibodies | IgG | IgG | IgG |
| Antigen | SARS-CoV-2 spike protein RBD | SARS-CoV-2 nucleocapsid protein | SARS-CoV-2 spike protein | SARS-CoV-2 nucleocapsid protein |
| Sensitivity (PPA) | 100%*(24/24) | 100%**(88/88) | 90.0%^(27/30) | 98.4%^^(184/187) |
| Specificity (NPA) | 100%(237/237) | 99.6% (1066/1070) | 100%(80/80) | 99.8%(623/624) |
| Data source | In-house | Manufacturer-provided | Manufacturer-provided | Manufacturer-provided |
| Result interpretation (AI) | Negative ≤ 2.5; Equivocal2.51 – 4.0;Positive > 4.0 | Negative < 1.4; Positive ≥ 1.4 | Negative < 0.8; Borderline≥0.8 to < 1.1;Positive ≥ 1.1 | Negative < 0.25; Positive > 0.3 (OD; not antibody index) |
| FDA EUA approval | Submitted on 05/04/2020 | 04/26/2020 | 05/04/2020 | Submitted on 03/05/2020 |
CMIA, chemiluminescent microparticle immunoassay; ELISA, Enzyme-linked immunosorbent assay; PPA, positive predictive agreement, NPA, negative predictive agreement; RBD, receptor binding domain; AI, antibody index; OD, optical density; FDA, Food and Drug Administration; EUA, emergency use authorization
* > 14 days, ** ≥ 14 days, ^ ≥ 21 days, ^^ RT-PCR confirmed positive patients. Information was collected from assays’ instructions for use.
Table 4.
Concordance between the laboratory-developed total antibody ELISA and commercially available serology assays.
| In-house ELISA |
Euroimmun (n = 43) |
% Agreement (95% CI) |
Kappa value (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| POS | NEG | EQV | Positive | Negative | Overall | ||
| POS | 13 | 0 | 0 | 81.3%(57.0–93.4) | 100.0%(85.1–100.0) | 92.1%(79.2–97.3) | 0.83(0.65–1.00) |
| NEG | 3# | 22 | 5* | ||||
| EQV | 0 | 0 | 0 | ||||
| In-house ELISA |
Epitope Diagnostics (n = 20) |
% Agreement (95% CI) |
Kappa value (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| POS | NEG | EQV | Positive | Negative | Overall | ||
| POS | 13 | 0 | 0 | 100.0%(77.2–100.0) | 100.0%(64.6–100.0) | 100.0%(83.9–100.0) | 0.99(0.99–0.99) |
| NEG | 0 | 7 | 0 | ||||
| EQV | 0 | 0 | 0 | ||||
| In-house ELISA |
Abbott Architect (n = 60) |
% Agreement (95% CI) |
Kappa value(95% CI) | ||||
|---|---|---|---|---|---|---|---|
| POS | NEG | EQV | Positive | Negative | Overall | ||
| POS | 20 | 2## | 0 | 95.2%(77.3–99.2) | 94.6%(82.3–98.5) | 94.8%(85.9–98.2) | 0.89(0.77–1.00) |
| NEG | 1### | 35 | 0 | ||||
| EQV | 0 | 2* | 0 | ||||
ELISA, Enzyme-linked immunosorbent assay; POS, positive; NEG, negative; EQV, equivocal; CI, confidence interval.
Seven samples with EQV results were not included in the analysis: 5 from the Euroimmun assay and 2 from the in-house ELISA.
Antibody indices from in-house ELISA: 0.1 1.1, 0.9 (Negative: ≤2.5) vs. antibody indices from Euroimmun assay: 1.32, 2.30, 1.84 (Positive: ≥1.1).
Antibody indices from in-house ELISA: 4.4, 4.6 (Positive: >4.0) vs. antibody indices from Abbott Architect assay: 1.09, 0.02 (Negative: <1.4).
Antibody index from in-house ELISA: 0.7 (Negative: ≤2.5) vs. antibody index from Abbott Architect assay: 1.8 (Positive: ≥1.4).
9. Seroprevalence among healthcare workers and outpatients
The M Health Fairview healthcare system includes 10 hospitals and 60 clinics predominantly located in the Twin Cities, but inclusive of outlying cities throughout the state as well. As an initial step to a statewide COVID-19 testing initiative announced by the Governor of Minnesota to establish an estimate of the percentage of the population that has been exposed to COVID-19, we deployed our SARS-CoV-2 total antibody assay to test 1,282 healthcare workers and 2,379 outpatients within the M Health Fairview system during the first 38 days that our assay was available as an orderable clinical test (Table 5 ).
Table 5.
Stratified seroprevalence data.
| Category | Number (%) |
|---|---|
| Outpatients | 2,379 |
| Sex | |
| Female | 1,460 (61.4%) |
| Male | 916 (38.5%) |
| Not reported | 3 (0.1%) |
| Median age, years (range) | 49 (0.17–93) |
| Seroprevalence (95% CI) | 4.46% (3.63–5.28%) |
| Healthcare workers | 1,282 |
| Sex | |
| Female | 1,000 (78%) |
| Male | 280 (21.8%) |
| Not reported | 2 (0.2%) |
| Median age, years (range) | 41 (18–73) |
| Seroprevalence (95% CI) | 2.96% (2.04–3.89%) |
The specimens from outpatients were collected at 35 clinics from 2,379 individuals (1,460 females, 916 males, 3 gender unreported) with a median age of 49 years (range: 2 months – 93 years). The seroprevalence among this population was 4.46% (95% CI: 3.63 – 5.28%); 1.13% of outpatients had equivocal antibody levels (95% CI: 0.71–1.56%).
The healthcare worker specimens were collected from 1,282 employees (1,000 females, 280 males, 2 gender unreported) providing patient care services supporting four hospitals. The cohort of employees included those with confirmed and non-confirmed COVID-19 exposures ≥ 14 days prior. The median age of employees was 41 years (range: 18 – 73) and the rate of seroprevalence was 2.96% (95% CI: 2.04 – 3.89%); 1.17% of healthcare workers had equivocal antibody levels (95% CI: 0.58 – 1.76%).
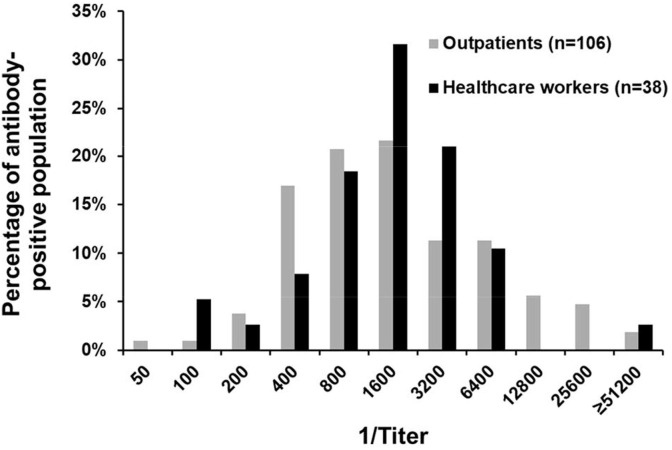
The distribution of IgG titers in the outpatient population was not significantly different from the range of IgG titers among the healthcare workers (p = 0.60, Chi-square test) (Fig. 3 ). Additionally, there were no significant age- or sex-specific differences in seroprevalence in these two populations.
Fig. 3.
Distribution of SARS-CoV-2 S1-RBD IgG titers among outpatients and healthcare workers. SARS-CoV-2 S1-RBD total antibody positive sera from outpatients (n = 106) and healthcare workers (n = 38) were analyzed by endpoint IgG titer using our laboratory-developed ELISA. The distributions of IgG titers in these populations were not significantly different (p = 0.60, Chi-square test).
10. Discussion
Our study provides the first determination of COVID-19 seroprevalence among healthcare workers and outpatients in Minnesota. Although the 4.46% seroprevalence among this cohort of patients suggests that individuals with mild presentation of COVID-19 develop an antibody response, we do not have data regarding the percentage of these outpatients who required subsequent hospitalization due to COVID-19 related symptoms. As serology tests are deployed on a broader scale, the relationship between asymptomatic or mild forms of disease presentation and the development of an immune response will be more clearly defined.
Potential sources of variability among serologic assays include differences in acceptable specimen types (serum, plasma, whole blood), formats (ELISAs, chemiluminescent immunoassay, lateral flow immunoassay), detected antibody classes (IgA, IgM, IgG, total), and SARS-CoV-2 antigen(s) used to design the assay.[28] There is particular debate on whether assays should target the N or S protein - whereas the N protein might be expressed earlier in the viral lytic cycle and may have greater sensitivity earlier in the disease process than the S protein, the S protein could be more immunologically relevant due to its role as a target for neutralizing antibodies and vaccine development.[29] Despite these potential sources of variability, the results from our method comparison study demonstrated excellent concordance between our in-house method and three commercial antibody methods. Importantly, our data suggest that the target SARS-CoV-2 antigen does not appear to significantly impact the accuracy or concordance of serology test results. This could have important implications for correlating various antibody test methods to protective neutralizing antibodies that primarily target the S protein RBD. In these cases, ELISAs using the SARS-CoV-2 N protein as the antigen could be a reasonable surrogate for measuring protective neutralizing antibodies, although additional confirmatory studies are needed. In a recently published study, antibodies against the N protein showed 100% sensitivity and specificity in patients ≥ 15 days after symptom onset, whereas antibodies against the S protein were associated with 91% sensitivity and 100% specificity.[29] However, our data demonstrated that our assay, which detects total antibodies to the S protein RBD, exhibited equivalent diagnostic agreement with a commercial assay that detects IgG antibodies against the N protein (Epitope Diagnostics) even when samples were collected < 14 days of symptom onset.
The population of healthcare workers we tested for our seroprevalence study represents potential workplace COVID-19 exposures. The low seroprevalence (2.96%) in this population suggests that guidelines regarding the use of personal protective equipment (PPE) for these healthcare workers are effective at mitigating the spread of COVID-19. A seroprevalence study in Germany came to the same conclusion regarding the effectiveness of PPE, with a low seroprevalence rate of 1.6% among healthcare workers at a tertiary care hospital.[30] Acknowledging that a sampling of the outpatients within one healthcare system is not a random community sampling, our data suggest that healthcare workers could have a lower overall rate of infection compared to the general population in Minnesota. The results from a study investigating the rate of COVID-19 infection among healthcare workers in downstate New York demonstrated a similar trend.[31]
Large-scale geographic COVID-19 seroprevalence surveys have been conducted in major metropolitan cities throughout the U.S. The seroprevalence in Santa Clara County, CA was 2.8% as of April 3–4, 2020.[32] Preliminary data suggest that COVID-19 infections are far more widespread — and the fatality rate much lower — in Los Angeles County than previously thought. The seroprevalence in this region was determined to be 4.1%.[33] Although these studies provide informative benchmarks for local disease prevalence, the commercial serology tests used in these studies have questionable performance characteristics, which impedes the reliability of the pursuant comparative studies. As additional seroprevalence data are obtained to support public health decision-making during the COVID-19 pandemic, it is imperative that these data are acquired using serologic test platforms with reliable performance characteristics.
Given the high sensitivity and specificity of our in-house developed test, the predictive value of our test will be robust even among populations with a low prevalence of exposure. Extrapolating the 4.46% seroprevalence in our sample of 2,379 outpatients to the population of Minnesota (5.64 million),[34] 251,500 individuals would be expected to have been exposed to COVID-19. However, this number is 10-times greater than the current number of laboratory-confirmed COVID-19 cases in the state as reported by the Minnesota Department of Health,[35] suggesting that COVID-19 is more widespread than reported.
Although our study focused on the use of serology tests for seroprevalence determinations, the detection of SARS-CoV-2 antibodies also has an integral role in identifying protective antibodies with neutralization assays. Such assays provide quantitative information on the ability of patient antibodies to confer protective immunity based on the antibody-mediated inhibition of virus growth ex vivo. RBD-specific antibodies have previously been shown to exhibit neutralizing functions against SARS-CoV-2, supporting the likelihood for protective immunity.[36], [37] However, future studies are needed to more rigorously demonstrate correlation between laboratory-developed and commercial antibody test results and neutralizing antibody titers.
Our study has limitations that should be considered when interpreting the results. First, remnant blood specimens were utilized for the assay validation studies. The majority of the specimens were analyzed 24–48 h following their initial collection after which time they were stored at 4 °C. However, the data from our stability studies indicate that these storage conditions did not cause significant changes in the levels of SARS-CoV-2 total antibody detected by our ELISA method. Second, the initial date of symptom onset was determined from subjective reports obtained from the patients at their time of hospital admission, as noted in the electronic medical record. However, this is a limitation that is common to several COVID-19 studies. Third, our method validation, which demonstrated 100% sensitivity and specificity after 14 days from symptom onset, was performed primarily with samples from PCR positive COVID-19 patients who required hospitalization. While our data on healthcare workers and outpatients demonstrates the ability of our method to detect antibody responses in milder cases, it is not clear if these sensitivity and specificity metrics apply for non-hospitalized PCR positive COVID-19 patients.
Preliminary studies suggest a correlation between serum antibody levels and clinical severity of disease.[38], [39] Therefore, additional studies utilizing quantitative or semi-quantitative antibody methods like ours are needed to definitively establish the relationship between antibody levels and severity. Additional unanswered questions remain including the influence of co-morbidities on patients’ COVID-19 immune responses, the impact of immunogenetic determinants on immune response, the time course of antibody production in the context of naturally acquired immunity, the kinetics of a protective antibody response stimulated by a safe and effective vaccine, and the identification and mechanism of action of neutralizing antibodies as prophylactic and therapeutic COVID-19 treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the following individuals for their contributions to this study: Dr. Fang Li (Professor, Department of Veterinary and Biomedical Sciences, University of Minnesota) for donating the RBD antigen used for the ELISA method; members of the labs of Drs. Marc Jenkins, Tyler Bold, Fang Li, and Bharat Thyagarajan; Steve J. Story (Technical Specialist, M Health Fairview Special Chemistry Laboratory), Christine K. Senn (Laboratory Manager, M Health Fairview University of Minnesota Medical Center West Bank Laboratory), Robert Janicek (Business Manager, University of Minnesota Advanced Research and Diagnostic Laboratory), Emily Kokaisel (Chemistry Technical Specialist, M Health Fairview University of Minnesota Medical Center East Bank Laboratory), Stephanie McGlone (Manager of Laboratory Services, M Health Fairview Bethesda and St. Joseph’s Hospital), and the M Health Fairview COVID-19 System Command Center.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinbiochem.2021.01.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Xu Y. Unveiling the Origin and Transmission of 2019-nCoV. Trends Microbiol. 2020;28:239–240. doi: 10.1016/j.tim.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease, (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2019;2020:25. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., Wang M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.R. Patel, E. Babady, E.S. Theel, G.A. Storch, B.A. Pinsky, K. St George, T.C. Smith, S. Bertuzzi, Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: Value of Diagnostic Testing for SARS-CoV-2/COVID-19. mBio 2020, 11. [DOI] [PMC free article] [PubMed]
- 5.Guidance Document: Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised). U.S. Department of Health and Human Services: Food and Drug Administration Center for Devices and Radiological Health, FDA-2020-D-0987.
- 6.Tuzman KT: NCI takes lead on serological test validation as cancer center joins COVID-19 fight. Biocentury.
- 7.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang S., Hillyer C., Du L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Nat.l Acad. Sci. U.S.A. 2020 doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang J., Wan Y., Liu C., Yount B., Gully K., Yang Y., Auerbach A., Peng G., Baric R., Li F. Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ter Meulen J., van den Brink E.N., Poon L.L., Marissen W.E., Leung C.S., Cox F., Cheung C.Y., Bakker A.Q., Bogaards J.A., van Deventer E., Preiser W., Doerr H.W., Chow V.T., de Kruif J., Peiris J.S., Goudsmit J. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott Laboratories. SARS-CoV-2 IgG assay Instructions for Use. April 2020.
- 14.Euroimmun. Anti-SARS-CoV-2 ELISA (IgG) Instructions for Use. March 24, 2020.
- 15.Epitope Diagnostics. Novel Coronavirus COVID-19 IgG ELISA Kit. Instructions for Use. March 2020.
- 16.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Covid-19 CMTEGf: Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleiss J.L. Measuring nominal scale agreement among many raters. Psychol. Bull. 1971;76:378–382. [Google Scholar]
- 18.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 19.Cai X.F., Chen J., Hu J.L., Long Q.X., Deng H.J., Fan K., Liao P., Liu B.Z., Wu G.C., Chen Y.K., Li Z.J., Wang K., Zhang X.L., Tian W.G., Xiang J.L., Du H.X., Wang J., Hu Y., Tang N., Lin Y., Ren J.H., Huang L.Y., Wei J., Gan C.Y., Chen Y.M., Gao Q.Z., Chen A.M., He C.L., Wang D.X., Hu P., Zhou F.C., Huang A.L., Liu P., Wang D.Q. A Peptide-based Magnetic Chemiluminescence Enzyme Immunoassay for Serological Diagnosis of Coronavirus Disease 2019 (COVID-19) J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., Wang D.Q., Hu Y., Ren J.H., Tang N., Xu Y.Y., Yu L.H., Mo Z., Gong F., Zhang X.L., Tian W.G., Hu L., Zhang X.X., Xiang J.L., Du H.X., Liu H.W., Lang C.H., Luo X.H., Wu S.B., Cui X.P., Zhou Z., Zhu M.M., Wang J., Xue C.J., Li X.F., Wang L., Li Z.J., Wang K., Niu C.C., Yang Q.J., Tang X.J., Zhang Y., Liu X.M., Li J.J., Zhang D.C., Zhang F., Liu P., Yuan J., Li Q., Hu J.L., Chen J., Huang A.L. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020 doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 21.Padoan A., Sciacovelli L., Basso D., Negrini D., Zuin S., Cosma C., Faggian D., Matricardi P., Plebani M. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin. Chim. Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, An T, Situ B, Hu y, Ou Z, Li Q, He X, Zhang Y, Tian P, Sun D, Rui Y, Wang Q, Ding D, Zheng L: Heat inactivation of serum interferes with the immunoanalysis of antibodies to SARS-CoV-2. May 16, 2020, https://doi.org/10.1101/2020.03.12.20034231. [DOI] [PMC free article] [PubMed]
- 23.Severance E.G., Bossis I., Dickerson F.B., Stallings C.R., Origoni A.E., Sullens A., Yolken R.H., Viscidi R.P. Development of a nucleocapsid-based human coronavirus immunoassay and estimates of individuals exposed to coronavirus in a U.S. metropolitan population. Clin. Vaccine Immunol. 2008;15:1805–1810. doi: 10.1128/CVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou W., Wang W., Wang H., Lu R., Tan W. First infection by all four non-severe acute respiratory syndrome human coronaviruses takes place during childhood. BMC Infect. Dis. 2013;13:433. doi: 10.1186/1471-2334-13-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorse G.J., Patel G.B., Vitale J.N., O'Connor T.Z. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin. Vaccine Immunol. 2010;17:1875–1880. doi: 10.1128/CVI.00278-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C., Cai J.P., Chan J.M., Chik T.S., Lau D.P., Choi C.Y., Chen L.L., Chan W.M., Chan K.H., Ip J.D., Ng A.C., Poon R.W., Luo C.T., Cheng V.C., Chan J.F., Hung I.F., Chen Z., Chen H., Yuen K.Y. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., Lamers M.M., Sikkema R.S., de Bruin E., Chandler F.D., Yazdanpanah Y., Le Hingrat Q., Descamps D., Houhou-Fidouh N., Reusken C.B.E.M., Bosch B.J., Drosten C., Koopmans M.P.G., Haagmans B.L. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease, Patients. Emerg. Infect. Dis. 2019;2020:26. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassaniti I., Novazzi F., Giardina F., Salinaro F., Sachs M., Perlini S., Bruno R., Mojoli F., Baldanti F. Force MotSMPC-T: Performance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J. Med. Virol. 2020 doi: 10.1002/jmv.25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.P.D. Burbelo, F.X. Riedo, C. Morishima, S. Rawlings, D. Smith, S. Das, J.R. Strich, D.S. Chertow, R.T. Davey, J.I. Cohen, Detection of Nucleocapsid Antibody to SARS-CoV-2 is More Sensitive than Antibody to Spike Protein in COVID-19 Patients. medRxiv April 24, 2020, https://doi.org/10.1101/2020.04.20.20071423.
- 30.Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M., Cordes S., Ross B., Esser S., Lindemann M., Kribben A., Dittmer U., Witzke O., Herrmann A. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.L. Voytko, Fewer NY healthcare workers are being infected with COVID-19 compared to public, Cuomo says. Forbes, May 7, 2020.
- 32.E. Bendavid, B. Mulaney, N. Sood, S. Shah, E. Ling, R. Bromley-Dulfano, C. Lai, Z. Weissberg, R. Saavedra-Walker, J. Tedrow, D. Tversky, A. Bogan, T. Kupiec, D. Eichner, R. Gupta, J. Ioannidis, J. Bhattacharya, COVID-19 Antibody Seroprevalence in Santa Clara County, California. medRxiv April 30, 2020, https://doi.org/10.1101/2020.04.14.20062463.
- 33.Public Health. 2020 [Google Scholar]
- 34.United States Census Bureau. Quick Facts for Minnesota.
- 35.Minnesota Department of Health. Situation Update for COVID-19. Edited by Health MDo. https://www.health.state.mn.us/diseases/coronavirus/situation.html ed.
- 36.Wu Y., Wnag F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y., Gong Y., Xiao H., Fan Z., Tan S., Wu G., Tan W., Lu X., Fan C., Wang Q., Liu Y., Zhang C., Qi J., Gao G.F., Gao F., Liu L. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020:eabc2241. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.B. Ju, Q. Zhang, X. Ge, R. Wang, J. Yu, S. Shan, B. Zhou, S. Song, X. Tang, J. Yu, J. Ge, J. Lan, J. Yuan, H. Wang, J. Zhao, S. Zhang, Y. Wang, X. Shi, L. Liu, X. Wang, Z. Zhang, L. Zhang, Potent human neutralizing antibodies elicited by SARS-CoV-2 infection. bioRxiv March 26, 2020, https://www.biorxiv.org/content/10.1101/2020.03.21.990770v2. [DOI] [PubMed]
- 38.B. Zhang, X. Zhou, C. Zhu, F. Feng, Y. Qiu, J. Feng, Q. Jia, Q. Song, B. Zhu, J. Wang, Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. March 16, 2020:https://doi-org.ezp3.lib.umn.edu/10.1101/2020.03.12.20035048. [DOI] [PMC free article] [PubMed]
- 39.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.