Abstract
Individuals with established cardiovascular disease or a high burden of cardiovascular risk factors may be particularly vulnerable to develop complications from coronavirus disease 2019 (COVID-19). We conducted a prospective cohort study at a tertiary care center to identify risk factors for in-hospital mortality and major adverse cardiovascular events (MACE; a composite of myocardial infarction, stroke, new acute decompensated heart failure, venous thromboembolism, ventricular or atrial arrhythmia, pericardial effusion, or aborted cardiac arrest) among consecutively hospitalized adults with COVID-19, using multivariable binary logistic regression analysis. The study population comprised 586 COVID-19 positive patients. Median age was 67 (IQR: 55 to 80) years, 47.4% were female, and 36.7% had cardiovascular disease. Considering risk factors, 60.2% had hypertension, 39.8% diabetes, and 38.6% hyperlipidemia. Eighty-two individuals (14.0%) died in-hospital, and 135 (23.0%) experienced MACE. In a model adjusted for demographic characteristics, clinical presentation, and laboratory findings, age (odds ratio [OR], 1.28 per 5 years; 95% confidence interval [CI], 1.13 to 1.45), previous ventricular arrhythmia (OR, 18.97; 95% CI, 3.68 to 97.88), use of P2Y12-inhibitors (OR, 7.91; 95% CI, 1.64 to 38.17), higher C-reactive protein (OR, 1.81: 95% CI, 1.18 to 2.78), lower albumin (OR, 0.64: 95% CI, 0.47 to 0.86), and higher troponin T (OR, 1.84; 95% CI, 1.39 to 2.46) were associated with mortality (p <0.05). After adjustment for demographics, presentation, and laboratory findings, predictors of MACE were higher respiratory rates, altered mental status, and laboratory abnormalities, including higher troponin T (p <0.05). In conclusion, poor prognostic markers among hospitalized patients with COVID-19 included older age, pre-existing cardiovascular disease, respiratory failure, altered mental status, and higher troponin T concentrations.
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) constitutes an ongoing global pandemic with considerable public health implications. As of January 7, 2021, >87 million cases had been reported in 191 countries, and ∼1.9 million deaths had been attributed to this condition.1 Similar to other common viral illnesses, individuals with established cardiovascular disease or a high burden of cardiovascular risk factors appear to be particularly vulnerable during infection.2, 3, 4, 5, 6, 7, 8 Concerns have also been raised that use of certain medications that affect the cardiovascular system, notably inhibitors of the renin-angiotensin-aldosterone system (RAAS) and nonsteroidal anti-inflammatory drugs (NSAIDs), may enhance infectivity and the likelihood of experiencing a severe disease course.9 , 10 Finally, the infection itself may increase the risk of cardiovascular complications, such as arrhythmia, heart failure, thromboembolic events, and myocarditis.11, 12, 13 Therefore, we designed this study to determine the prevalence of cardiovascular risk factors, established cardiovascular disease, and associated medications, and to identify risk factors for incident cardiovascular events and mortality, among hospitalized patients with COVID-19.
Methods
The study was conducted at Yale New Haven Hospital (YNHH), a nonprofit, 1,541-bed tertiary care medical center operated by the Yale New Haven Health System (YNHHS), and located in New Haven, Connecticut, USA. The Yale COVID-19 Cardiovascular Registry is an ongoing retrospective and prospective registry that is collecting data from all adult patients (age ≥18 years) admitted or transferred to YNHH with a positive test result for SARS-CoV-2. The diagnosis of SARS-CoV-2 infection was made using a reverse-transcriptase polymerase chain reaction assay or high-throughput sequencing, with nasopharyngeal or oropharyngeal swab specimens obtained at any point before or during hospitalization. However, patients could also be included to the registry if discharged with a diagnosis of COVID-19, using the International Classification of Diseases, Tenth Revision (ICD-10) emergency code, U07.1. For the present analysis, we included all participants admitted between March 1 and May 31, 2020 with a final disposition, that is, either in-hospital death or survival to hospital discharge.
On March 19, the YNHHS COVID-19 Treatment team, led by a multidisciplinary team of physicians, released the first version of its treatment algorithm for patients with nonsevere and severe disease (Appendix 1). Per this algorithm, proposed indications for active treatment included (1) respiratory failure with mechanical ventilation or extracorporeal membrane oxygenation, (2) an oxygen saturation ≤93% on room air or on chronic oxygen supplementation, or (3) fever and/or symptoms of respiratory disease plus abnormal chest imaging plus at least 1 risk factors for adverse outcomes (age >60 years, body mass index ≥40 kg/m2, chronic heart disease, chronic lung disease, or immunosuppressed state). Patients receiving >3 L/min of oxygen supplementation required evaluation by the intensive care unit (ICU). Interruption of ongoing treatment with RAAS-inhibitors or NSAIDs was not advised unless indicated for conventional reasons. Recommended laboratory studies included those related to hematology, inflammation, and circulatory function. The treatment algorithm has been updated numerous times since its release, and the latest version was published on November 25, 2020.
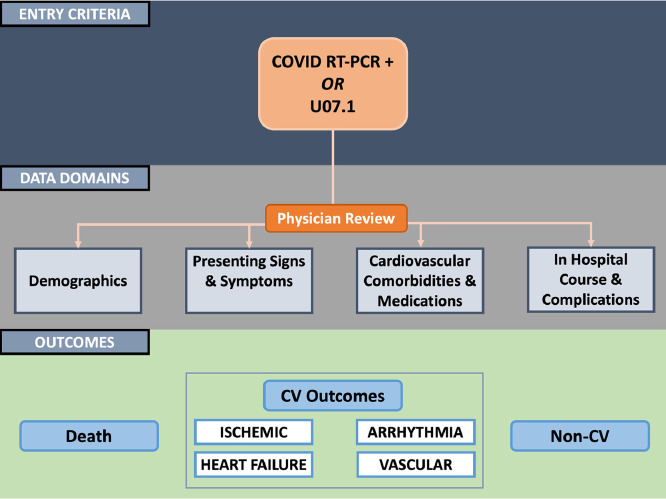
Information on hospitalized patients with a positive SARS-CoV-2 test result was acquired through the local Observational Medical Outcomes Partnership repository and the Joint Data Analytics Team at YNHHS, resources that provide customized reporting and data analysis from the electronic health record system, Epic. Study data were subsequently obtained through manual review of each patient's electronic health record by physicians. We collected data related to demographics, including prevalent cardiovascular risk factors, conditions, and medications, presenting symptoms, vital signs, laboratory test results, imaging findings, electrocardiograms, and in-hospital events, including cardiovascular and COVID-19-specific medication use, supportive measures, ICU admission, cardiovascular events, other pertinent clinical events, and mortality. The institutional review board at Yale University approved the study under an expedited review. The design of the registry is illustrated in Figure 1 .
Figure 1.
Design of the registry.
CV = cardiovascular; RT-PCR = reverse transcription polymerase chain reaction.
Cardiac troponin T was measured using a 4th generation electrochemiluminescence immunoassay (Elecsys, Roche Diagnostics, Basel, Switzerland). The lower limit of detection (99th percentile upper reference limit) was 0.010 μg/L, and the lowest concentration with a coefficient of variation ≤10% was 0.30 μg/L (intermediate within lot precision) or 0.060 μg/L (intermediate lot to lot precision).
The primary endpoint was in-hospital death from any cause. The secondary endpoint was in-hospital MACE, defined as a composite of type 1 or 2 myocardial infarction, stroke, new acute decompensated heart failure or cardiogenic shock, venous thromboembolism, new-onset ventricular arrhythmia, new-onset atrial fibrillation or flutter, pericardial effusion or cardiac tamponade, or aborted cardiac arrest. Myocardial infarction was defined according to the Fourth Universal Definition.14 Cardiac events were adjudicated by experienced physicians (Appendix 2). Other endpoints that were included for descriptive purposes were ICU admission, mechanical ventilation, and new renal replacement therapy.
Continuous variables are presented as medians and interquartile ranges (IQR). Categorical variables are presented as frequencies and corresponding percentages. Unadjusted differences in clinical and laboratory characteristics between survivors and nonsurvivors were examined using Mann-Whitney U test, Pearson's chi-squared test, or Fisher's exact test, as appropriate. We subsequently performed multivariable binary logistic regression with backward elimination (2-sided p-entry on univariable analysis: 0.10; 2-sided p-retention in the multivariable model: 0.10) to identify variables associated with the primary and secondary endpoints, respectively. Age and sex were forced into the regression models where necessary. For each endpoint, 3 subsets of models were rendered, using (1) demographic characteristics alone (model 1), (2) demographic characteristics and clinical presentation (model 2), and (3) demographic characteristics, clinical presentation, and laboratory findings (model 3). No collinearity of importance was detected in the final models (maximum variance inflation factor of 1.22). Adjusted odds ratios (ORs) with corresponding 95% confidence intervals (CIs) for variables that were retained were then shown using Forest plots. For laboratory studies, ORs were reported for 1 standard deviation increase in the logarithmically transformed concentrations. A 2-sided p-value <0.05 was considered statistically significant. No adjustments for multiple comparisons were made as the study was considered exploratory. Stata/IC 15 (StataCorp LP, College Station, TX, USA) was used for all computations.
Results
The study population comprised 586 COVID-19 positive patients who were admitted between March 1 and May 31, 2020 and had completed their hospital course. Median age was 67 (IQR: 55 to 80) years, 47.4% were female, and 49.0%, 30.7%, and 16.0% identified as Non-Hispanic White, Non-Hispanic Black, and Hispanic, respectively. A history of cardiovascular disease was reported in 36.7%, the most common of which were coronary artery disease (18.1%), heart failure (17.1%), and atrial arrhythmia (12.2%). In terms of risk factors, 60.2% had hypertension, 39.8% had diabetes mellitus, and 38.6% had hyperlipidemia. Angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor blockers were used by 32.9%, beta blockers by 30.0%, diuretics (including mineralocorticoid receptor antagonists) by 27.5%, aspirin by 29.5%, and NSAIDs by 11.3%. Demographic characteristics, co-morbidities, and medications, stratified according to vital status at discharge, are provided in Table 1 .
Table 1.
Demographic characteristics, co-morbidities, and medications in patients with COVID-19 who survived and did not survive to hospital discharge
| Survived to hospital discharge |
|||
|---|---|---|---|
| Characteristic | Yes (n = 504) |
No (n = 82) |
p-value for difference |
| Age (years) | 65 (54-78) | 79.5 (68-89) | <0.001 |
| Women (%) | 248 (49.2%) | 30 (37%) | 0.03 |
| Non-Hispanic White | 236 (46.8%) | 51 (62%) | 0.05 |
| Non-Hispanic Black | 162 (32.1%) | 18 (22%) | |
| Hispanic | 82 (16.3%) | 12 (15%) | |
| Other or unknown race | 24 (4.8%) | 1 (1%) | |
| Comorbidity | |||
| Coronary artery disease | 79 (15.7%) | 27 (33%) | <0.001 |
| Cerebrovascular disease | 47 (9.3%) | 17 (21%) | 0.002 |
| Peripheral artery disease | 15 (3.0%) | 8 (10%) | 0.003 |
| Heart failure or cardiomyopathy | 72 (14.3%) | 28 (34%) | <0.001 |
| Atrial fibrillation or flutter | 51 (10.1%) | 20 (24%) | <0.001 |
| Ventricular arrhythmia | 7 (1.4%) | 5 (6%) | 0.005 |
| Diabetes mellitus | 194 (38.5%) | 39 (48%) | 0.12 |
| Hypertension | 293 (58.1%) | 60 (73%) | 0.01 |
| Hyperlipidemia | 188 (37.3%) | 38 (46%) | 0.12 |
| Body mass index ≥30 kg/m2 | 234 (46.4%) | 40 (49%) | 0.69 |
| Venous thromboembolism | 40 (7.9%) | 6 (7%) | 0.85 |
| Chronic lung disease | 109 (21.6%) | 16 (20%) | 0.67 |
| Chronic kidney disease | 82 (16.3%) | 27 (33%) | <0.001 |
| Active or prior malignancy | 68 (13.5%) | 19 (23%) | 0.02 |
| HIV or organ transplantation | 20 (4.0%) | 4 (5%) | 0.70 |
| Medications | |||
| ACE inhibitor or ARB | 161 (31.9%) | 32 (39%) | 0.21 |
| Beta blocker | 138 (27.4%) | 36 (44%) | 0.002 |
| Calcium channel blocker | 121 (24.0%) | 31 (38%) | 0.008 |
| Diuretic | 125 (24.8%) | 36 (44%) | <0.001 |
| Aspirin | 146 (29.0%) | 27 (33%) | 0.47 |
| P2Y12 inhibitor | 11 (2.2%) | 7 (9%) | 0.002 |
| Statin | 183 (36.3%) | 40 (49%) | 0.03 |
| Anticoagulant | 56 (11.1%) | 13 (16%) | 0.22 |
| Antiarrhythmic | 13 (2.6%) | 4 (5%) | 0.25 |
| Nitrate or other antianginal | 14 (2.8%) | 2 (2%) | 0.86 |
| NSAID | 53 (10.5%) | 13 (16%) | 0.16 |
ACE = angiotensin converting enzyme; ARB = angiotensin II receptor blocker; HIV = human immunodeficiency virus; NSAID = nonsteroidal anti-inflammatory drug.
Median time from symptom onset to admission was 4 (IQR: 1 to 7) days, and median length of hospital stay was 13 (IQR: 7 to 21) days. The most common presenting symptom was cough (59.6%), followed by fever or chills (58.7%), dyspnea (54.5%), and fatigue or malaise (34.1%). Presenting symptoms and vital signs at admission are shown in Table 2 .
Table 2.
Presenting symptoms and vital signs at admission in patients with COVID-19 who survived and did not survive to hospital discharge
| Survived to hospital discharge |
|||
|---|---|---|---|
| Symptom or sign | Yes (n = 504) |
No (n = 82) |
p-value for difference |
| Symptom duration (days) | 4 (1-7) | 3 (0-6) | 0.06 |
| Length of hospital stay (days) | 13 (7-21) | 12 (6-21) | 0.63 |
| Specific symptoms | |||
| Fatigue or malaise | 169 (33.5%) | 31 (38%) | 0.45 |
| Fever or chills | 300 (59.5%) | 44 (54%) | 0.32 |
| Altered mental status | 48 (9.5%) | 14 (17%) | 0.04 |
| Headache | 57 (11.3%) | 5 (6%) | 0.16 |
| Nasal congestion | 35 (6.9%) | 6 (7%) | 0.90 |
| Anosmia | 20 (4.0%) | 0 | 0.09 |
| Ageusia | 15 (3.0%) | 0 | 0.25 |
| Cough | 292 (57.9%) | 57 (70%) | 0.05 |
| Sputum production | 52 (10.3%) | 11 (13%) | 0.40 |
| Hemoptysis | 3 (0.6%) | 3 (4%) | 0.04 |
| Sore throat | 35 (6.9%) | 2 (2%) | 0.15 |
| Shortness of breath | 261 (51.8%) | 58 (71%) | <0.001 |
| Chest discomfort | 67 (13.3%) | 5 (6%) | 0.07 |
| Palpitations | 2 (0.4%) | 0 | >0.99 |
| Nausea or vomiting | 100 (19.8%) | 10 (12%) | 0.10 |
| Diarrhea | 120 (23.8%) | 17 (21%) | 0.54 |
| Abdominal pain | 42 (8.3%) | 5 (6%) | 0.49 |
| Myalgia | 96 (19.1%) | 9 (11%) | 0.08 |
| Vital signs at admission | |||
| Respiratory rate (bpm) | 18 (18-22) | 20 (18-24) | 0.001 |
| Oxygen saturation (%) | 96 (95-98) | 96 (94-98) | 0.97 |
| Systolic blood pressure (mm Hg) | 127 (114-144) | 125.5 (106-141) | 0.26 |
| Diastolic blood pressure (mm Hg) | 74 (63-82) | 68 (57-80) | 0.04 |
| Heart rate (bpm) | 89 (76-103.5) | 89 (74-102) | 0.84 |
| Temperature (°F) | 99.8 (98.5-101.1) | 100.3 (98.4-101.3) | 0.85 |
| Oxygen therapy at admission | 207 (41.1%) | 58 (71%) | <0.001 |
bpm = breaths per minute (respiratory rate) and beats per minute (heart rate).
A total of 82 (14.0%) individuals died in the hospital. Patients who died were more likely to be older (p <0.001), males (p = 0.03), and have a history of cardiovascular disease (p <0.001), including all its individual components except venous thromboembolism, and of chronic kidney disease (p <0.001). Furthermore, beta blockers (p = 0.002), calcium channel blockers (p = 0.008), diuretics (p <0.001), P2Y12 inhibitors (p = 0.002), and statins (p = 0.03) were more commonly used by nonsurvivors than by survivors (Table 1).
Nonsurvivors more often presented with altered mental status (p = 0.04), hemoptysis (p = 0.04), and shortness of breath (p <0.001), and had higher respiratory rates (p = 0.001), lower diastolic blood pressures (p = 0.04), and more frequently required oxygen therapy (p <0.001) (Table 2).
White blood cell count (p = 0.005), absolute neutrophil count (p <0.001), creatinine (p <0.001), aspartate aminotransferase (p <0.001), total bilirubin (p = 0.002), international normalized ratio (INR) (p <0.001), C-reactive protein (p <0.001), procalcitonin (p <0.001), ferritin (p = 0.02), D-dimer (p <0.001), troponin T (p <0.001), and NT-proBNP (p <0.001) were generally higher, whereas hemoglobin (p = 0.03), absolute lymphocyte count (p <0.001), platelet count (p = 0.002), and albumin (p <0.001) were lower, in patients who did not survive to hospital discharge.
One-hundred and thirty-five (23.0%) patients experienced a MACE during their course of admission, most commonly new atrial fibrillation or flutter (7.9%), type 2 myocardial infarction (7.5%), venous thromboembolism (5.8%), or new acute decompensated heart failure (5.3%). Only 3 patients experienced a type 1 myocardial infarction, all of which were managed with percutaneous coronary intervention. Most patients with atrial fibrillation were managed with a rate control strategy. The incidence of composite and individual cardiovascular events stratified for survival status is presented in Table 3 .
Table 3.
Cardiovascular events in patients with COVID-19 who survived and did not survive to hospital discharge
| Survived to hospital discharge |
|||
|---|---|---|---|
| Endpoint | Yes (n = 504) |
No (n = 82) |
p-value for difference |
| MACE | 83 (16.5%) | 52 (63%) | <0.001 |
| Ischemic | |||
| Type 1 myocardial infarction | 1 (0.2%) | 2 (2%) | 0.05 |
| Type 2 myocardial infarction | 22 (4.4%) | 22 (27%) | <0.001 |
| Isolated myocardial injury | 46 (9.1%) | 18 (22%) | <0.001 |
| Stroke | 4 (0.8%) | 7 (9%) | <0.001 |
| Heart failure | |||
| New acute decompensated heart failure | 16 (3.2%) | 15 (18%) | <0.001 |
| Worsening acute decompensated heart failure | 19 (3.8%) | 16 (20%) | <0.001 |
| Cardiogenic shock | 4 (0.8%) | 8 (10%) | <0.001 |
| Myocarditis | 0 | 0 | - |
| Stress (takotsubo) cardiomyopathy | 4 (0.8%) | 0 | 0.55 |
| Arrhythmia | |||
| New-onset atrial fibrillation or atrial flutter | 27 (5.4%) | 19 (23%) | <0.001 |
| New-onset ventricular arrhythmia | 14 (2.8%) | 5 (6%) | 0.12 |
| Venous | |||
| Pulmonary embolism or deep vein thrombosis | 19 (3.8%) | 15 (18%) | <0.001 |
| Other | |||
| Pericardial effusion or cardiac tamponade | 2 (0.4%) | 3 (4%) | 0.02 |
| Aborted cardiac arrest | 5 (1.0%) | 16 (20%) | <0.001 |
MACE = major adverse cardiovascular events.
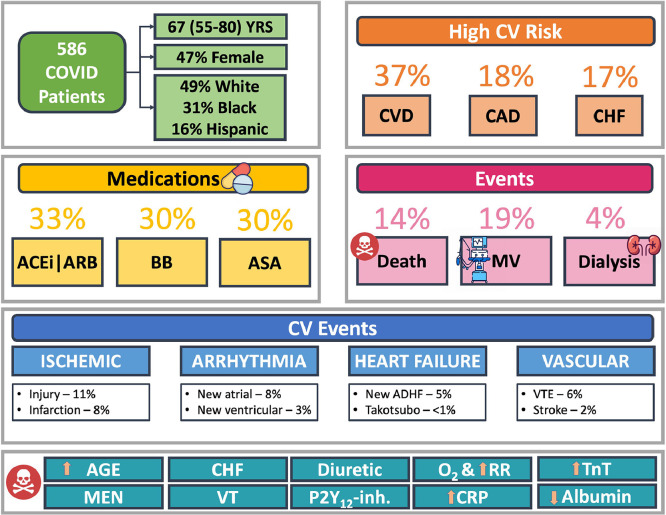
With respect to noncardiovascular events, 196 (33.5%) were admitted to the ICU, 111 (19.0%) required mechanical ventilation, and 24 of 557 (4.3%) underwent new renal replacement therapy. Importantly, 31 of 390 (8.0%) of patients who were not admitted to the ICU died, whereas 51 of 196 (26.0%) of patients admitted to the ICU died. Figure 2 provides a summary of cardiovascular risk and both cardiovascular and noncardiovascular events in the study population.
Figure 2.
Summary of cardiovascular risk and both cardiovascular and noncardiovascular events.
ACEi = angiotensin converting enzyme inhibitor; ADHF = acute decompensated heart failure; ARB = angiotensin II receptor blocker; ASA = aspirin; BB = beta blocker; CAD = coronary artery disease; CHF = congestive heart failure; CRP = C-reactive protein; CVD = any cardiovascular disease; MV = mechanical ventilation; O2 = oxygen supplementation; P2Y12-inh. = P2Y12 inhibitor; RR = respiratory rate; TnT = troponin T; VT = ventricular tachycardia; VTE = venous thromboembolism; YRS = years.
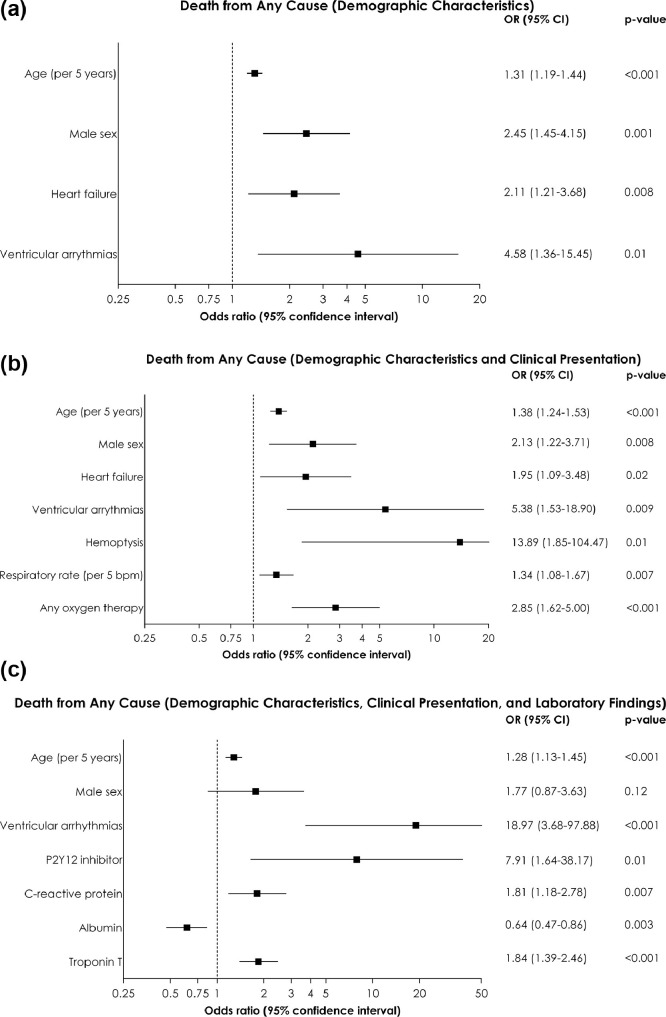
Figure 3 shows the predictors of in-hospital death from any cause, using the 3 regression model subsets. Demographic and clinical characteristics that were significantly associated with a higher risk of death in at least 1 model included older age, male sex, history of heart failure, history of ventricular arrhythmias, use of P2Y12 inhibitors, use of oxygen therapy at admission, and higher respiratory rates. Unfavorable laboratory findings included higher C-reactive protein, lower albumin, and higher troponin T.
Figure 3.
Predictors of in-hospital death from multivariable binary logistic regression analysis. (A) Based on demographic characteristics (model 1); (B) Based on demographic characteristics and clinical presentation (model 2); (C) Based on demographic characteristics, clinical presentation, and laboratory findings (model 3).
CI = confidence interval; OR = odds ratio.
For laboratory studies, odds ratios are reported for 1 standard deviation increase in the logarithmically transformed concentrations.
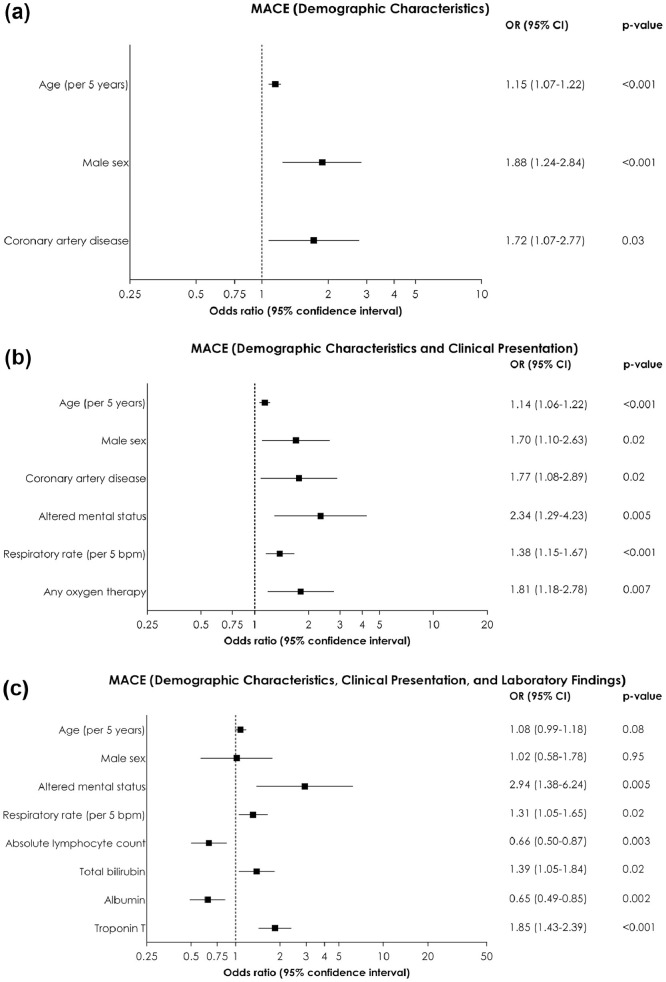
Factors associated with MACE in at least 1 model were older age, male sex, history of coronary artery disease, use of oxygen therapy at admission, higher respiratory rates, altered mental status, lower absolute lymphocyte count, higher total bilirubin, lower albumin, and higher troponin T (Figure 4 ).
Figure 4.
Predictors of in-hospital major adverse cardiovascular events from multivariable binary logistic regression analysis. (A) Based on demographic characteristics (model 1); (B) Based on demographic characteristics and clinical presentation (model 2); (C) Based on demographic characteristics, clinical presentation, and laboratory findings (model 3).
CI = confidence interval; OR = odds ratio.
For laboratory studies, odds ratios are reported for 1 standard deviation increase in the logarithmically transformed concentrations.
Discussion
Our observational study of patients hospitalized with COVID-19 at a tertiary care medical center in the United States showed high prevalence of cardiovascular risk factors and disease. Pre-existing cardiovascular disease, older age, male sex, early need for oxygen supplementation, higher respiratory rates, altered mental status, and laboratory abnormalities, including higher troponin T concentrations were among the characteristics related to poor outcomes. There were no associations of RAAS-inhibitors or NSAIDs with either mortality or cardiovascular events. Our study is particularly notable for its data acquisition through manual chart review and event adjudication by experienced physicians, approaches that provide more reliable information than use of administrative registries alone.15
Respiratory infections are known to increase the risk of major cardiovascular events and mortality.2 , 3 , 16 This is particularly well-established for seasonal influenza where vaccination appears to reduce cardiovascular morbidity and mortality by 15% to 20% among high-risk individuals.17 , 18 Both age and male sex predict adverse outcomes among patients with influenza, associations that may extend to those with COVID-19.2 , 4, 5, 6 , 19 , 20 Whereas the exact mechanism for male predominance in the context of SARS-CoV infections remains obscure, a possible explanation may be offered by sex-related differences in both innate and adaptive immunity related to estrogen receptor signaling.21 Our findings also support earlier reports that suggested pre-existing cardiovascular disease as an unfavorable prognostic factor.4, 5, 6, 7, 8 , 20 Indeed, the increased physiological demands imposed by severe infection affect persons with cardiovascular disease more seriously than those without.22 Poor cardiovascular reserve also negatively impacts upon the immune system, potentially leading to infection in itself.23
Importantly, we found no detrimental effects of RAAS-inhibitors or NSAIDs. Both received considerable attention early during the pandemic because of their potential ability to upregulate expression of ACE-2, the molecule used by SARS-CoV2 for endocytic internalization.9 , 10 Our results are in agreement with other observational studies of these drug classes7 , 10 , 24 and support the position statement of the European Society of Cardiology that treatment with RAAS-inhibitors should not be interrupted in patients with COVID-19.25 Considering another widely used medication, aspirin has been proposed to positively affect the disease course through inhibition of viral replication and reduced inflammation.26 We did not observe prognostic benefits of aspirin, although it may primarily have been used by individuals with established cardiovascular disease. Similarly, the associations between P2Y12 inhibitors (and calcium channel blockers) and mortality likely represented severity of co-morbid cardiovascular conditions rather than independent mechanistic effects.
Previous COVID-19 cohorts also reported cough, fever, dyspnea, and fatigue as the most common disease manifestations.4, 5, 6, 7 , 19 Altered mental status, hemoptysis and signs of respiratory failure were more frequent among patients who did not survive. On the other hand, detection of cardiovascular complications in patients with COVID-19 may be particularly challenging, exemplified by the high incidence of nonobstructive coronary disease despite ST-segment elevation.27 In the same way, although troponin elevations are common and have been associated with both mortality and cardiovascular events in this context, interpretation is difficult because they may not be reflective of a primary coronary event.12 , 13 , 28, 29, 30 Indeed, whereas our findings supported previous reports showing that several biomarkers of organ function, inflammation, and circulatory stress were associated with adverse outcomes, none of these are specific for COVID-19.4 , 5 , 8 , 19 , 30
In addition to our high-quality study data, systematic use of an institutional treatment algorithm also ensured collection of multiple variables, including laboratory tests, allowing for thorough adjustments. A notable limitation included the observational design that prevented us from making finite inferences regarding causality. Indeed, it remains unclear whether the infection is involved in the pathogenesis of, or mainly acts as a trigger for, cardiovascular events in individuals at elevated risk.11 The limited number of events and associated power for detailed exploration of individual cardiovascular outcomes are additional shortcomings, and the wide MACE-definition may have also made it difficult to infer which endpoints drove the various associations. Given multiplicity, the p-values, particularly those assessing univariable associations, should be interpreted cautiously. Because we only included hospitalized patients, they were older and had a higher co-morbidity burden and mortality than unselected patients with COVID-19.4 In addition, our case mix of patients admitted directly to the hospital or transferred from other institutions makes generalizability uncertain as the latter group would be expected to have a more severe form of the disease. Ongoing randomized studies of RAAS-inhibitors and aspirin are thus eagerly anticipated, as is examination of cardiovascular outcomes after vaccine introduction. In the meantime, vigilance is required regarding optimization of treatment of prevalent cardiovascular risk factors and known conditions, potentially leading to a reduced risk of complications in the setting of COVID-19.
In conclusion, consecutive patients hospitalized with COVID-19 had a high prevalence of cardiovascular risk factors and disease. Pre-existing cardiovascular disease, older age, male sex, clinical manifestations of respiratory failure, and laboratory findings indicative of circulatory stress were associated with cardiovascular events and mortality, whereas use of RAAS-inhibitors and NSAIDs were not.
Author Statement
Manan Pareek: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – Original Draft, Writing – Review & Editing, Visualization.
Avinainder Singh: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – Original Draft, Writing – Review & Editing, Visualization, Project administration.
Lina Vadlamani: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
Maxwell Eder: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
Justin Pacor: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
Jakob Park: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
Zaniar Ghazizadeh: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
Alex Heard: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
Ana Sofia Cruz-Solbes: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
Roozbeh Nikooie: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
Chad Gier: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
Zain V. Ahmed: Conceptualization, Methodology, Investigation, Writing – Review & Editing.
James V. Freeman: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Project administration.
Judith Meadows: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Project administration.
Kim G. E. Smolderen: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Project administration.
Rachel Lampert: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Project administration.
Eric J. Velazquez: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Project administration.
Tariq Ahmad: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Project administration.
Nihar R. Desai: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Supervision, Project administration.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
N/A
Disclosures
The other authors do not report any relevant disclosures.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2021.01.029.
Appendix. Supplementary materials
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1:274–281. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kytomaa S, Hegde S, Claggett B, Udell JA, Rosamond W, Temte J, Nichol K, Wright JD, Solomon SD, Vardeny O. Association of influenza-like illness activity with hospitalizations for heart failure: the atherosclerosis risk in communities study. JAMA Cardiol. 2019;4:363–369. doi: 10.1001/jamacardio.2019.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, Li Q, Li W, Yang S, Zhao X, Zhao Y, Wang H, Liu Y, Yin Z, Zhang R, Wang R, Yang M, Hui C, Wijns W, McEvoy JW, Soliman O, Onuma Y, Serruys PW, Tao L, Li F. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, Curnis A, Faggiano P, Gorga E, Lombardi CM, Milesi G, Vizzardi E, Volpini M, Nodari S, Specchia C, Maroldi R, Bezzi M, Metra M. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020;26 doi: 10.1016/j.cmi.2020.06.003. 1259.e5–1259.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Society of Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic.https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance#p00 (July 1, 2020)
- 12.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, ESC Scientific Document Group Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 15.Bensley RP, Yoshida S, Lo RC, Fokkema M, Hamdan AD, Wyers MC, Chaikof EL, Schermerhorn ML. Accuracy of administrative data versus clinical data to evaluate carotid endarterectomy and carotid stenting. J Vasc Surg. 2013;58:412–419. doi: 10.1016/j.jvs.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29:96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 17.Mohseni H, Kiran A, Khorshidi R, Rahimi K. Influenza vaccination and risk of hospitalization in patients with heart failure: a self-controlled case series study. Eur Heart J. 2017;38:326–333. doi: 10.1093/eurheartj/ehw411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modin D, Claggett B, Kober L, Schou M, Jensen JUS, Solomon SD, Vardeny O, Knop FK, Nielsen SD, Fralick M, Torp-Pedersen C, Gislason G, Biering-Sorensen T Influenza vaccination is associated with reduced cardiovascular mortality in adults with diabetes: a nationwide cohort study. Diabetes Care. 2020;43:2226–2233. doi: 10.2337/dc20-0229. [DOI] [PubMed] [Google Scholar]
- 19.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai PH, Lancet EA, Weiden MD, Webber MP, Zeig-Owens R, Hall CB, Prezant DJ. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5:1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford DW, Goodwin AJ, Simpson AN, Johnson E, Nadig N, Simpson KN. A severe sepsis mortality prediction model and score for use with administrative data. Crit Care Med. 2016;44:319–327. doi: 10.1097/CCM.0000000000001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker AMN, Drozd M, Hall M, Patel PA, Paton M, Lowry J, Gierula J, Byrom R, Kearney L, Sapsford RJ, Witte KK, Kearney MT, Cubbon RM. Prevalence and predictors of sepsis death in patients with chronic heart failure and reduced left ventricular ejection fraction. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fosbol EL, Butt JH, Ostergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, Torp-Pedersen C, Kober L. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324:168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Simone G. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers.https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang (July 19, 2020)
- 26.Glatthaar-Saalmuller B, Mair KH, Saalmuller A. Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study. Influenza Other Respir Viruses. 2017;11:85–92. doi: 10.1111/irv.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, Metra M, Curello S, Maffeo D, Pero G, Cacucci M, Assanelli E, Bellini B, Russo F, Ielasi A, Tespili M, Danzi GB, Vandoni P, Bollati M, Barbieri L, Oreglia J, Lettieri C, Cremonesi A, Carugo S, Reimers B, Condorelli G, Chieffo A. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V, Mount Sinai Covid Informatics Center Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, MC Aubry, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.