Abstract
The US is confronted with a rise in opioid use disorder (OUD), opioid misuse, and opioid-associated harms. Medication treatment for opioid use disorder (MOUD)—including methadone, buprenorphine and naltrexone—is the gold standard treatment for OUD. MOUD reduces illicit opioid use, mortality, criminal activity, healthcare costs, and high-risk behaviors. The Veterans Health Administration (VHA) has invested in several national initiatives to encourage access to MOUD treatment. Despite these efforts, by 2017, just over a third of all Veterans diagnosed with OUD received MOUD. VHA OUD specialty care is often concentrated in major hospitals throughout the nation and access to this care can be difficult due to geography or patient choice. Recognizing the urgent need to improve access to MOUD care, in the Spring of 2018, the VHA initiated the Stepped Care for Opioid Use Disorder, Train the Trainer (SCOUTT) Initiative to facilitate access to MOUD in VHA non-SUD care settings. The SCOUTT Initiative’s primary goal is to increase MOUD prescribing in VHA primary care, mental health, and pain clinics by training providers working in those settings on how to provide MOUD and to facilitate implementation by providing an ongoing learning collaborative. Thirteen healthcare providers from each of the 18 VHA regional networks across the VHA were invited to implement the SCOUTT Initiative within one facility in each network. We describe the goals and initial activities of the SCOUTT Initiative leading up to a two-day national SCOUTT Initiative conference attended by 246 participants from all 18 regional networks in the VHA. We also discuss subsequent implementation facilitation and evaluation plans for the SCOUTT Initiative. The VHA SCOUTT Initiative could be a model strategy to implement MOUD within large, diverse health care systems.
INTRODUCTION
The US is confronted with a rise in opioid use disorder (OUD), opioid misuse, and opioid-associated harms. Medication treatment for opioid use disorder (MOUD)—including methadone, buprenorphine and naltrexone—is the gold standard treatment for OUD. MOUD reduces illicit opioid use, mortality, criminal activity, healthcare costs, and high-risk behaviors.2-8 In addition, MOUD improves patients’ quality of life.9-12 Patient and system outcomes generally improve with longer treatment duration and relapse to illicit use and mortality increases when MOUD ceases.6,13-19 Thus, it is imperative to increase patients access to MOUD care.20,21
The US Department of Veterans Affairs’ (VA) Veterans Health Administration (VHA) is the largest direct provider of substance use disorder (SUD) care in the US.22 Since 2003, VHA has established an expectation that MOUD be offered to all patients with OUD.22,23 Despite extensive efforts to facilitate provision of MOUD and VHA policy requiring that it be available, relatively few VHA patients with OUD receive MOUD. Since offered as a non-formulary medication in 2003 and a formulary medication in 2005, access to buprenorphine MOUD has risen steadily: by 2017 just over a third (34.8%) of all Veterans diagnosed with OUD received MOUD.22,24 In addition, a wide variation in MOUD prescribing rates occurs across the VHA; many VA facilities provide MOUD to fewer than 10% of their patients with OUD.25
There exists substantial literature regarding the barriers of access to MOUD treatment in VHA and non-VHA environments. In the VHA, system-, provider-, and patient-level barriers reduce access to MOUD care.22,24-31 Within the VHA, significant provider barriers to prescribing MOUD have been reported, including lack of interest, stigma, and education about MOUD treatment and how to deliver this treatment in VHA settings. However, facilitators of improving access to MOUD treatment in the VHA include recognized need in access of OUD care, health care provider interest, and being provided resources and time to provide MOUD care. Another recognized facilitator to improve access to MOUD care is local, regional, and national leadership directions and support. In prior studies, facilities with a high number of prescribers who provided MOUD care had a recognized clinical champion who advocated for this treatment.28 Thus, engendering national, regional, and local VHA leaders to prioritize MOUD care and provide resources for this care as well as training potential local clinical champions to advocate and provide MOUD care facilities may be optimal strategies to facilitate more MOUD care in local clinical environments.
The VHA has invested in several national initiatives to encourage patient access to MOUD. These include the Buprenorphine in the VA (BIV) Initiative and the Medication Addiction Treatment in the VA (MAT-VA) Initiative—two national educational and consult services with the stated goal to educate, advise, and mentor VHA providers to assess and mitigate opioid-related risks and improve access to quality MOUD care across the VHA. These—and other national VHA initiatives—have concentrated on access to MOUD in any VHA clinic environment with less attention to improve access to MOUD care specifically in non-SUD care settings like primary care, mental health, and pain clinic environments. These three clinical environments are often where patients with OUD access ongoing, longitudinal care and could be prime locations to engage patients who are unable or unwilling to access SUD specialty care. The Veterans Affairs/Department of Defense Clinical Practice Guidelines for the Management of Substance Use Disorders, which were revised in 2015, was one of the first attempts by the VHA to specifically recommend primary care and other office based clinicians to engage patients in SUD treatment within environments where they practice.
Provider-level factors may account for the limited uptake of buprenorphine in non-specialty care settings.25,29,34 In the VHA, too few providers are waivered to prescribe buprenorphine: during one six-month period from 2017 to early 2018 only 2% of 72,272 VHA clinical prescribers were credentialed to prescribe buprenorphine.26 In addition, few of those waivered clinicians are prescribing buprenorphine; of the providers who were credentialed to prescribe buprenorphine, over half (56.4%) of non-mental health providers and nearly one-third (28.8%) of mental health providers, had not prescribed buprenorphine in the past 180 days. Most (89.1%) VHA MOUD prescriptions came from mental health prescribers who worked predominately in VHA SUD specialty care settings. Only 6.8% of these buprenorphine prescriptions came from primary care providers. This contrasts with non-VA OUD care, where a vast majority of patients who receive MOUD care receive that care from non-mental health prescribers.35,36
Prescriber type and clinical setting are important for patient access and outcomes. Patients are more likely to have ready access to primary care providers and to lack access to mental health and SUD providers. This is especially true in rural areas, where Veterans are more likely to live. Indeed, a recent Government Accountability Office (GAO) report indicated only 27% of Veterans living in rural areas received MOUD compared to 34% of Veterans living in urban areas. To improve specialty care access for rural Veterans, VHA has a national network of approximately 1,400 community-based outpatient clinics (CBOCs), primarily staffed by primary care providers who are affiliated with mental health providers at larger VHA Medical Centers. However, CBOCs often direct patients needing SUD care to a VHA Medical Center specialty clinic. These VHA Medical Centers are usually in urban centers, often geographically challenging for patients to access. Additionally, many patients prefer to seek treatment for SUDs and mental health conditions in primary care to avoid the perceived stigma of seeking addiction care. Providing more SUD care in primary care settings, including CBOCs, could improve Veterans’ access to any SUD treatment.
Several initiatives over the years have targeted the VHA to improve Veterans’ ready access to all health care services, especially specialty care services. For instance, the MISSION Act, a recently enacted law, charges the VHA to provide non-VHA care to eligible Veterans due to lack of timely access to VHA health care services or long travel distances to the nearest VHA facility.38 The Act allows Veterans to seek care, reimbursed by the VHA, in non-VHA healthcare environments. The MISSION act applies to mental health and SUD care in the community—care that may not be as integrated, as effective, or efficient, as VHA care. Thus, providing more ready access to addiction care to be contained within VHA environment may improve integration and patient- and system-level outcomes.
We describe the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) Initiative, a national VHA program intending to improve access to MOUD in primary care, mental health, and pain clinic environments throughout the VHA. By improving access to addiction care in these unique environments, Veterans could have more ready access to addiction care regardless of their distance to VHA addiction specialty care and allow integration of care within longitudinal care environments. The intention of this SCOUTT Initiative description is not to show efficacy and effectiveness of the Initiative, but to describe the purpose, the methods of implementing the program, and the activities of the Initiative through the start-up phase.
Thus, we describe the purpose as well as the activities of the Initiative through the official start of the Initiative, which occurred in August, 2018, when a national meeting brought nearly 300 VHA staff from 18 regions of the country to learn about the Initiative and “kick off” implementation of the SCOUTT Initiative in their local facilities. This Initiative could be replicable and applied to non-VHA settings, such as State-wide initiatives, health systems of care, and local community environments. Subsequent implementation facilitation processes to support implementation as well as a formal evaluation of the SCOUTT Initiative will be described elsewhere. Thus, this description provides a roadmap to potentially replicate this or similar Initiatives in other health care and community environments.
THE STEPPED CARE FOR OPIOID USE DISORDER TRAIN THE TRAINER (SCOUTT) INITIATIVE
Recognizing the urgent need to improve access to MOUD care, in the Spring of 2018, the VHA Office of Mental Health & Suicide Prevention (OMHSP) initiated the Stepped Care for Opioid Use Disorder, Train the Trainer (SCOUTT) Initiative to facilitate access to MOUD in VHA non-SUD care settings. OMHSP collaborated with a wide representation of VHA stakeholders in originating, planning, and implementing the SCOUTT Initiative, including leaders representing (among others) national pain, primary care, mental health, SUD specialty-care, primary care mental health integration, pharmacy, nursing, and education services. These leaders formed the multidisciplinary Planning Committee for the SCOUTT Initiative that began meeting monthly in January 2018 and continues to meet today.
The SCOUTT Initiative’s primary goal is to increase MOUD prescribing in VHA primary care, mental health, and pain clinics by training providers working in those settings on how to provide MOUD and to facilitate implementation by providing an ongoing learning collaborative. While not specifically targeting rural CBOCs or rural facilities within the VHA, the SCOUTT Initiative’s outcomes may be most applicable to these environments by bridging a huge gap of VHA OUD treatment for Veterans in rural settings.
OUD is a chronic medical condition, and like other chronic diseases, should be treated using a chronic care model.39 The SCOUTT Initiative leadership recognized, that like other medical illnesses, patients transition from acute, high intensity care environments (like SUD specialty care settings) to non-acute, lower intensity care environments (like primary care, mental health, and pain clinics) where longitudinal care can be provided. This is not atypical for other chronic medical disorders. For example, patients with a new diagnosis of HIV are often seen, assessed, and then started on antiretroviral medications by HIV/infectious disease providers. Once stabilized on an appropriate regimen, patients return to their primary care providers to continue a medication regimen. If patients have a crisis, or need more intensive care, they can transition back to their HIV/infectious disease specialist for stabilization. Patients then return to their primary care provider for ongoing care once the crisis has stabilized. Patients thus “step up” or “step down” in care based on severity of the illness or instability of the disease process.
Similarly, in OUD treatment, patients often initially present to SUD clinics or programs where they are assessed and treated. Often these programs and/or clinics provide episodes of care that are finite in duration. Patients, once stable, may be discharged from SUD specialty care to a lower intensity of care, such as primary care, mental health, or pain clinic environments. In addition, for patients with mild to moderate OUD who will not seek or cannot access SUD specialty care, primary care, mental health, and pain clinic office-based environments may be optimal clinical environments to initiate and maintain patients in care. This is akin to patients with mild to moderate major depressive disorder or diabetes; diseases that can be successfully treated in primary care environments without specialty mental health or endocrinology care.
A stepped care approach to increase access to care and improve the quality of care is not a foreign concept for a variety of mental health or physical health conditions. For example, stepped care is an approach used in depression care where patients often typically start with low-intensity evidence-based treatment and “step up” to higher intensity care if needed.40 A stepped care approach has been used and advocated for diseases such as hypertension and osteoarthritis.41-43 A recent literature review indicates some promise in the feasibility, implementation, and efficacy of stepped care models in primary care settings for the management of chronic pain and opioid use, including patients with OUD and at risk for development of OUD.
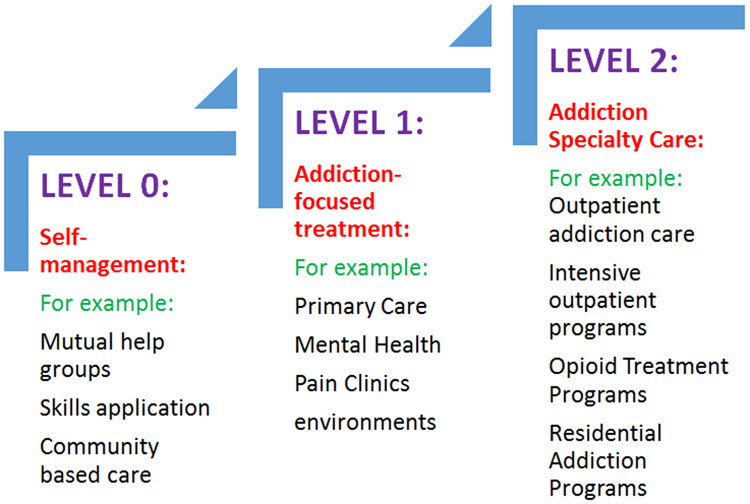
Recognizing that there are different “steps” in environments of care for many chronic medical conditions, the SCOUTT Initiative designated three increasingly specialized levels, or steps, of OUD care. SCOUTT designated STEP-0 as self-directed care/self-management for OUD including the use of mutual support groups; STEP-1 environments as office based primary care, mental health, and pain clinics; and STEP-2 environments as SUD specialty care settings (FIGURE 1). In the VHA Stepped Care Model for OUD, the steps of care were not mutually exclusive: patients could engage in STEP-0 care along with STEP-1 or STEP-2 care. However, an important element of the Stepped Care Model is that every patient would have longitudinal care in office-based clinical settings, with a primary care, mental health, or pain clinician. Thus, having clinicians with knowledge of OUD, particularly expertise to assess and treat OUD, and systems to promote referral to STEP-2 care in these clinics are essential. For this reason, SCOUTT targeted clinicians in STEP-1 clinics for training about OUD assessment and treatment.
FIGURE:
The Veteran Health Administration’s Stepped Care Model for Medication Treatment for Opioid Use Disorder
The SCOUTT Initiative intended to provide: 1) education on VA/DoD Clinical Practice Guidelines and national policy requirements which recommend MOUD as primary treatment for OUD and reinforce that psychosocial interventions alone are not recommended; 2) means to address patient resistance to referral to SUD specialty care, which impedes access to treatment; 3) promotion of care in settings where Veterans are most likely to present; and 4) recognition that MOUD saves lives and can be successfully implemented in non-SUD specialty settings. In fact, the majority of clinical research in and outside the VA indicates that MOUD can be safely, effectively and efficiently provided in non-SUD specialty care settings such as office based primary care, mental health, and pain clinic environments.
Upon the suggestion of the SCOUTT Initiative’s multidisciplinary planning committee of VHA stakeholders, leaders, and clinicians, in May 2018, VA directed regional VHA Veterans Integrated Service Network (VISN) Directors to nominate interdisciplinary SCOUTT Facility Implementation Teams (SCOUTT FITs) to lead and implement SCOUTT activities in STEP-1 clinics in each of the VISNs and eventually spread this model across their networks. In the VHA, each of the 18 VISNs within the VHA is responsible for the planning, budgeting, and service delivery of healthcare to Veterans within its region and each VISN Director is ultimately in charge of this mission in each VISN. For the SCOUTT Initiative, the VISN Directors were directed to identify and nominate VISN teams of 13 VHA health care staff members from each VISN: four VISN leaders with decisional authority within 1) mental health, 2) substance use disorder, 3) primary care, and 4) pain services as well as a facility team consisting of: a physician-leader from a STEP-2 clinic, a physician-leader from a STEP-1 clinic, and 7 associated interdisciplinary team members (2 physicians, nurse practitioners, or physician assistants; 1 pharmacist; 2 nurses; 2 therapists) including members from SUD specialty care and one of three targeted clinics (general mental health, primary care, pain). Nominated teams—SCOUTT FITs—were directed to attend several webinars in the Spring and early Summer 2018 regarding MOUD care and attend the national SCOUTT conference in August 2018.
The charge of these SCOUTT FITs was to ensure the SCOUTT Initiative’s implementation at two STEP 1 clinics at their facility within 12 months (PHASE 1) and another STEP 1 clinic at another facility within the VISN within 24 months (PHASE 2). PHASE 1 was divided into three components which charged the SCOUTT FITs to 1) participate in community of practice calls and the national learning community of the SCOUTT Initiative (0-6 months), 2) implement the Stepped Care Model within a Step 1 clinic at their facility (6-9 months), and 3) implement the Stepped Care Model within another Step 1 clinic at their facility (9-12 months).
Based on feedback from the planning committee and at the direction of VHA, in the spring of 2018, several national webinars introduced the 18 SCOUTT FITs to the SCOUTT Initiative and to two models of care with evidence of effect outside of the VA for integrating MOUD within STEP 1 clinics: the medication management (physician-led) and collaborative care (nurse-led) models.45,46 In May 2018, a kickoff national webinar occurred where Karen Drexler, MD, Director of SUD Services within the OMHSP, introduced the SCOUTT FITs to the SCOUTT Initiative including the Initiative’s need, goals, and processes. During this seminar, a discussant (David Fiellin, MD) described the role of the physician/prescriber in providing medical management for MOUD and the utility of non-pharmacologic approaches as an adjunct treatment for MOUD in office based settings; there exists evidence that non-pharmacologic treatment may not be needed to improve patient, addiction-specific outcomes for all patients.47-51 In addition, another discussant (Colleen Labelle, MSN, RN-BC, CARN) described how nurses and other allied health care providers can assume a central role in the management of MOUD within office based settings.45 Prior to the SCOUTT Initiative Conference, additional webinars occurred to further orient the SCOUTT FITs to the SCOUTT Initiative.
After these national webinars and before the subsequent SCOUTT Initiative Conference it was identified by SCOUTT leaders that the SCOUTT FITs faced several barriers to implementing SCOUTT. Many of these barriers have been described in prior studies.25,28,30,31,52 Perceived barriers included providers’ and administrators’ lack of knowledge about MOUD, lack of education about MOUD care, workload concerns, length of treatment and diagnosis and coding questions for OUD, tele-health prescribing concerns, and issues with credentialing and privileging of buprenorphine prescribers. The pre-conference webinars started to address these concerns; the SCOUTT Initiative Planning Committee modified the SCOUTT Initiative Conference agenda to also address these perceived barriers to MOUD implementation.
The 2-day SCOUTT Initiative Conference was held in August 2018 in Hartford, Connecticut. All members of the 18 SCOUTT FITs were invited to participate (total of 234 staff members). The SCOUTT Conference was organized to address the barriers identified by the SCOUTT FITs in the preceding months, further promote the evidence base of the two models of care (medical management and collaborative care), and give providers knowledge and skills to implement these models within their local SCOUTT STEP-1 clinics. The SCOUTT Initiative Conference covered a range of topics including sessions focused on stepped care for OUD; diagnosis of OUD in patients with chronic pain; medical management of OUD; essential counseling skills for medical management; implementation challenges; facilitators of Stepped Care; examples of VA models that work in Step-1 care; VA resources to assist the SCOUTT FITs in implementing SCOUTT, and verbal patient testimonials regarding the value of MOUD care in primary care (TABLE: SCOUTT Conference agenda). Presenters included both non-VHA and VHA scholars and clinicians and a patient who described his experience on MOUD care in VHA primary care setting. During the SCOUTT Initiative Conference, dedicated time was set aside for SCOUTT FITs to network with other providers and facilitators to discuss local challenges in STEP-1 Stepped Care and to facilitate discussion to overcome those challenges. SCOUTT FITs presented their ideas and plans for implementation at their facilities to the rest of the SCOUTT Initiative Conference attendees.
TABLE.
Agenda: Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) Initiative Conference
| DAY 1 | ||
|---|---|---|
| Time | Title of Presentation | Type of Presentation |
| 7:00 – 7:45 a.m. | Registration | |
| 7:45 – 8:00 a.m. | National Anthem | |
| 8:00 – 8:15 a.m. | Overview and Introductions Welcome |
Lecture |
| 8:15 – 9:00 a.m. | Stepped Care for Opioid Use Disorder: One VA’s Experience | Panel Discussion |
| 9:00 – 9:30 a.m. | Diagnosis of OUD in Patients with Chronic Pain | Lecture |
| 9:45 – 10:45 a.m. | Medical Management of OUD | Lecture |
| 10:45 – 11:45 a.m. | Essential Counseling Skills for Medical Management | Lecture |
| 12:45 – 2:45 p.m. | Essentials of Office-based Treatment: Screening/Intake; Induction; Stabilization; Maintenance; Red Flags |
Lecture |
| 3:00 – 4:00 p.m. | Action Team Implementation Session #1 | Breakout |
| 4:00 – 4:30 p.m. | Wrap-up/adjourn | Lecture |
| DAY 2 | ||
| Time | Title of Presentation | Type of Presentation |
| 7:00 – 7:45 a.m. | Sign-In | |
| 7:45 – 8:10 a.m. | Welcome to Day 2 Summary and Plan for the day A few words from VHA Executive in Charge |
Lecture |
| 8:10 – 9:00 a.m. | Implementation Challenges, Facilitators, and Models That Work | Panel Discussion |
| 9:00 – 10:30 a.m. | VA Resources that Can Help
|
Lecture |
| 10:45 – 11:15 a.m. | Research to Inform Implementation: 2018-2019 projects | Panel Discussion |
| 11:15 – 12:30 p.m. | Action Team Implementation Session #2 Networking: Charter and Statement of Work |
Breakout |
| 1:30 – 2:30 p.m. | Action Team Implementation Session #3 Networking: Charter and Statement of Work |
Breakout |
| 2:45 – 4:00 p.m. | Action Team Implementation: Plan Sharing | Group Discussion |
| 4:00 – 4:30 p.m. | Wrap-up/adjourn | Lecture |
The attendees of the SCOUTT Initiative Conference included 246 participants, including the SCOUTT FIT members, lecturers/panelists, and national stakeholders from all 18 VISNs. Each SCOUTT FIT developed problem statements, aim statements, project scope, and project deliverable plans to implement SCOUTT within their facility and regional network. Informal post-conference evaluations among 168 respondents (68.3% of total) indicated 95% overall satisfaction with the SCOUTT Conference. Respondents identified themselves as physicians (n=68), nurses (28), psychologists (26), pharmacists (25), social workers (13), physician assistants (2), counselor (1), and/or others (n=5 no selection). Drug Addiction Treatment Act of 2000 (DATA 2000) half-half optional buprenorphine x-waiver trainings were also offered to participants prior to and after the SCOUTT Conference.30 Fifty-seven participants also attended one of two optional, in-person buprenorphine x-waiver trainings provided before and after the SCOUTT Initiative Conference. After the Conference, the SCOUTT FITs returned to their facilities with their action steps to implement SCOUTT.
While the SCOUTT Initiative was guided by the belief that OUD is a chronic medical condition that should be addressed within the confines of longitudinal settings (primary care, mental health, and pain clinic environments), it was not grounded in any particular implementation science framework. However, recognizing that education alone will not change health care providers behaviors or facilitate overcoming emerging barriers, the VHA subsequently funded two implementation research projects after the SCOUTT Initiative Conference to facilitate implementation and evaluate the SCOUTT Initiative, which were guided by the Promoting Action on Research Implementation in Health Services (i-PARIHS) and the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) frameworks.53-58
The SCOUTT Initiative leadership was concerned that members of the SCOUTT FITs may return to their facilities to have competing time demands, have lingering stigma of OUD and MOUD, and have little incentive for real practice change.59-62 In addition, considering that primary care, mental health, and pain clinic providers are often busy, overworked, and confronted with several multiple competing mandates to accomplish, there existed a concern about innovation fatigue after the SCOUTT Initiative Conference. Thus, the SCOUTT Initiative leadership advocated for, and received, additional longitudinal implementation intervention and evaluation support after the SCOUTT Initiative Conference. These projects continue to assist the SCOUTT Initiative and are briefly described herein.
The grant “Facilitation of the Stepped Care Model and Medication Treatment for Opioid Use Disorder” (PEC 19-001, Gordon principal investigator) was funded to facilitate the SCOUTT Initiative through intensive external implementation facilitation, ongoing education, and 1:1 concierge consultation mechanism. The external implementation facilitation intervention consists of assigning an implementation specialist to each SCOUTT FIT team. In this project, a national implementation specialist is assigned to each SCOUTT FIT team and tasked with convening monthly telephone contacts with the team to track progress on action plans, identify barriers and assist in problem solving solutions to barriers. On an as-needed basis, the implementation specialists connect SCOUTT FIT teams to available resources, expert buprenorphine prescribers, and to national leadership to support their efforts and assist in resolving barriers. Site visits from two members of the implementation team were made available to SCOUTT FIT teams. All implementation specialist contacts with SCOUTT FIT teams are recorded in an electronic tracking database.
This database is also utilized by the second implementation research project which was funded to evaluate the SCOUTT Initiative, “Evaluating the Implementation of the VA Stepped Care for Opioid Use Disorder Train-the-Trainer (SCOUTT)” (PEC 18-203, Hawkins principal investigator). This project utilizes both quantitative (patient and providers) and qualitative (providers) data to evaluate the impact of SCOUTT on number of providers prescribing MOUD, number of patients with OUD receiving MOUD and to document barriers to, facilitators of, and strategies for implementation. These ongoing projects are synergistic and, to date, continue to meet weekly with national leaders and stakeholders as the SCOUTT Initiative enters Phase 2. Further descriptions and results of these two projects will be more fully described and reported elsewhere.
The SCOUTT Initiative works to empower primary care, mental health, and pain clinic champions to introduce the SCOUTT Initiative to their facilities and another facility in their VISN. Improving MOUD care in STEP 1 clinics is expected to increase access to MOUD care throughout the VHA. Early results indicate increased access to MOUD care within SCOUTT STEP-1 clinics, increased access to MOUD care within SCOUTT facilities, and increased numbers of providers who are prescribing MOUD in both SCOUTT clinics and at SCOUTT facilities. Patient and provider perspectives of the SCOUTT Initiative appear to be positive.
Further research is needed to quantify SCOUTT Initiative progress and impact upon the VHA and the two ongoing projects should elucidate the progress and outcomes of the SCOUTT Initiative. Nevertheless, the SCOUTT Initiative is a large investment for the VHA to improve the access to quality SUD treatment in non-SUD care settings for Veterans with OUD. Time will tell whether the SCOUTT Initiative improves integration of SUD care in non-addiction care settings, the retention of Veterans in VHA care, and SUD care coordination in a variety of settings.
Ongoing evaluations will examine whether the SCOUTT Initiative is effective at improving the access to MOUD treatment in primary care, mental health, and pain clinic environments. If proven successful at improving access to MOUD, the Initiative could be a reproducible model of implementation for other large healthcare systems to emulate. This is especially true if the SCOUTT Initiative reduces health care emergency room visits and hospitalization for patients with OUD and proves to be a cost-effective evidence-based implementation strategy. If so, it may be that the SCOUTT Initiative could be replicated by State-wide initiatives, communities, health care systems, and insurers and be a model of implementation to improve access to care and disease and health system metrics for other, non-OUD, diseases.
ACKNOWLEDGEMENTS
This material is based upon work supported by the U.S. Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the Program for Addiction Research, Clinical Care, Knowledge, and Advocacy (PARCKA) at the University of Utah; the Vulnerable Veteran Innovative PACT (VIP) Initiative at the VA Salt Lake City Health Care System; the VA Center of Excellence in Substance Addiction Treatment and Education (CESATE); the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001 and #18-203. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors thank Carrie Edlund, MS for assistance with editing the manuscript. In addition, the authors wish to acknowledge the leadership and collaboration of the following leaders (alphabetically listed) on the SCOUTT Initiative: Lucille Burgo, Melissa Christopher, Steve Hunt, Terri Jorgenson, Jenny Knoeppel, Tera Moore, Andy Pomerantz, Beverly Randolph, Friedhelm Sandbrink, the entire SCOUTT Initiative Planning Committee, and the Veteran Health Administration clinicians who contributed to the vision and success of the implementation of the SCOUTT Initiative.
Footnotes
CONFLICTS OF INTEREST
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or any of its academic affiliates.
REFERENCES
- 1.Kertesz SG, Gordon AJ. A crisis of opioids and the limits of prescription control: United States. Addiction. 2019;114(1):169–180. [DOI] [PubMed] [Google Scholar]
- 2.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014(2):CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–2066. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med. 2017;377(4):391–394. [DOI] [PubMed] [Google Scholar]
- 5.Thomas CP, Fullerton CA, Kim M, et al. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatr Serv. 2014;65(2):158–170. [DOI] [PubMed] [Google Scholar]
- 6.Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94(1-3):151–157. [DOI] [PubMed] [Google Scholar]
- 7.Tkacz J, Volpicelli J, Un H, Ruetsch C. Relationship between buprenorphine adherence and health service utilization and costs among opioid dependent patients. J Subst Abuse Treat. 2014;46(4):456–462. [DOI] [PubMed] [Google Scholar]
- 8.Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011(8):CD004145. [DOI] [PubMed] [Google Scholar]
- 9.Giacomuzzi SM, Ertl M, Kemmler G, Riemer Y, Vigl A. Sublingual buprenorphine and methadone maintenance treatment: a three-year follow-up of quality of life assessment. ScientificWorldJournal. 2005;5:452–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacomuzzi SM, Riemer Y, Ertl M, et al. Buprenorphine versus methadone maintenance treatment in an ambulant setting: a health-related quality of life assessment. Addiction. 2003;98(5):693–702. [DOI] [PubMed] [Google Scholar]
- 11.Ponizovsky AM, Grinshpoon A. Quality of life among heroin users on buprenorphine versus methadone maintenance. Am J Drug Alcohol Abuse. 2007;33(5):631–642. [DOI] [PubMed] [Google Scholar]
- 12.Ponizovsky AM, Margolis A, Heled L, Rosca P, Radomislensky I, Grinshpoon A. Improved quality of life, clinical, and psychosocial outcomes among heroin-dependent patients on ambulatory buprenorphine maintenance. Subst Use Misuse. 2010;45(1-2):288–313. [DOI] [PubMed] [Google Scholar]
- 13.Lo-Ciganic WH, Gellad WF, Gordon AJ, et al. Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization. Addiction. 2016;111(5):892–902. [DOI] [PubMed] [Google Scholar]
- 14.Gordon AJ, Lo-Ciganic WH, Cochran G, et al. Patterns and Quality of Buprenorphine Opioid Agonist Treatment in a Large Medicaid Program. J Addict Med. 2015;9(6):470–477. [DOI] [PubMed] [Google Scholar]
- 15.Bentzley BS, Barth KS, Back SE, Aronson G, Book SW. Patient Perspectives Associated with Intended Duration of Buprenorphine Maintenance Therapy. J Subst Abuse Treat. 2015;56:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentzley BS, Barth KS, Back SE, Book SW. Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J Subst Abuse Treat. 2015;52:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug Alcohol Depend. 2011;119(1-2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend. 2009;105(1-2):9–15. [DOI] [PubMed] [Google Scholar]
- 19.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361(9358):662–668. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Blanco C. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkow ND. Personalizing the Treatment of Substance Use Disorders. Am J Psychiatry. 2020;177(2):113–116. [DOI] [PubMed] [Google Scholar]
- 22.Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veterans Health Administration/Department of Defense. VA/DoD Clinical Practice Guideline: Management of Opioid Therapy for Chronic Pain. Veterans Health Administration and Department of Defense; www.healthquality.va.gov/guidelines/Pain/cot/. Published 2017. Accessed March 21, 2017, 2017. [Google Scholar]
- 24.Oliva EM, Trafton JA, Harris AH, Gordon AJ. Trends in opioid agonist therapy in the Veterans Health Administration: is supply keeping up with demand? Am J Drug Alcohol Abuse. 2013;39(2):103–107. [DOI] [PubMed] [Google Scholar]
- 25.Oliva EM, Harris AH, Trafton JA, Gordon AJ. Receipt of opioid agonist treatment in the Veterans Health Administration: facility and patient factors. Drug Alcohol Depend. 2012;122(3):241–246. [DOI] [PubMed] [Google Scholar]
- 26.Valenstein-Mah H, Hagedorn H, Kay CL, Christopher ML, Gordon AJ. Underutilization of the current clinical capacity to provide buprenorphine treatment for opioid use disorders within the Veterans Health Administration. Subst Abus. 2018;39(3):286–288. [DOI] [PubMed] [Google Scholar]
- 27.Hagedorn H, Kenny M, Gordon AJ, et al. Advancing pharmacological treatments for opioid use disorder (ADaPT-OUD): protocol for testing a novel strategy to improve implementation of medication-assisted treatment for veterans with opioid use disorders in low-performing facilities. Addict Sci Clin Pract. 2018;13(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon AJ, Kavanagh G, Krumm M, et al. Facilitators and barriers in implementing buprenorphine in the Veterans Health Administration. Psychol Addict Behav. 2011;25(2):215–224. [DOI] [PubMed] [Google Scholar]
- 29.Oliva EM, Maisel NC, Gordon AJ, Harris AH. Barriers to use of pharmacotherapy for addiction disorders and how to overcome them. Curr Psychiatry Rep. 2011;13(5):374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon AJ, Liberto J, Granda S, Salmon-Cox S, Andree T, McNicholas L. Outcomes of DATA 2000 certification trainings for the provision of buprenorphine treatment in the Veterans Health Administration. Am J Addict. 2008;17(6):459–462. [DOI] [PubMed] [Google Scholar]
- 31.Gordon AJ, Trafton JA, Saxon AJ, et al. Implementation of buprenorphine in the Veterans Health Administration: results of the first 3 years. Drug Alcohol Depend. 2007;90(2-3):292–296. [DOI] [PubMed] [Google Scholar]
- 32.Gellad WF, Good CB, Shulkin DJ. Addressing the Opioid Epidemic in the United States: Lessons From the Department of Veterans Affairs. JAMA Intern Med. 2017. [DOI] [PubMed] [Google Scholar]
- 33.United States Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders (Version 3.0). https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf Published 2015. Accessed.
- 34.Haffajee RL, Bohnert ASB, Lagisetty PA. Policy Pathways to Address Provider Workforce Barriers to Buprenorphine Treatment. Am J Prev Med. 2018;54(6S3):S230–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kermack A, Flannery M, Tofighi B, McNeely J, Lee JD. Buprenorphine prescribing practice trends and attitudes among New York providers. J Subst Abuse Treat. 2017;74:1–6. [DOI] [PubMed] [Google Scholar]
- 36.Wen H, Borders TF, Cummings JR. Trends In Buprenorphine Prescribing By Physician Specialty. Health Aff (Millwood). 2019;38(1):24–28. [DOI] [PubMed] [Google Scholar]
- 37.Rubin R. Rural Veterans Less Likely to Get Medication for Opioid Use Disorder. JAMA. 2020;323(4):300. [DOI] [PubMed] [Google Scholar]
- 38.Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a Blueprint for Helping Veterans Make the Most of New Choices. J Gen Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLellan AT, Starrels JL, Tai B, et al. Can Substance Use Disorders be Managed Using the Chronic Care Model? Review and Recommendations from a NIDA Consensus Group. Public Health Rev. 2014;35(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Straten A, Hill J, Richards DA, Cuijpers P. Stepped care treatment delivery for depression: a systematic review and meta-analysis. Psychol Med. 2015;45(2):231–246. [DOI] [PubMed] [Google Scholar]
- 41.Moser M. Is the stepped-care approach to the management of hypertension still appropriate? Chest. 1985;88(4):629. [DOI] [PubMed] [Google Scholar]
- 42.Moser M. Initial treatment of adult patients with essential hypertension: I. Why conventional stepped-care therapy of hypertension is still indicated. Pharmacotherapy. 1985;5(4):189–195. [DOI] [PubMed] [Google Scholar]
- 43.Smink AJ, van den Ende CH, Vliet Vlieland TP, et al. "Beating osteoARThritis": development of a stepped care strategy to optimize utilization and timing of non-surgical treatment modalities for patients with hip or knee osteoarthritis. Clin Rheumatol. 2011;30(12):1623–1629. [DOI] [PubMed] [Google Scholar]
- 44.Speed TJ, Parekh V, Coe W, Antoine D. Comorbid chronic pain and opioid use disorder: literature review and potential treatment innovations. Int Rev Psychiatry. 2018;30(5):136–146. [DOI] [PubMed] [Google Scholar]
- 45.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171(5):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaBelle CT, Han SC, Bergeron A, Samet JH. Office-Based Opioid Treatment with Buprenorphine (OBOT-B): Statewide Implementation of the Massachusetts Collaborative Care Model in Community Health Centers. J Subst Abuse Treat. 2016;60:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss RD, Griffin ML, Potter JS, et al. Who benefits from additional drug counseling among prescription opioid-dependent patients receiving buprenorphine-naloxone and standard medical management? Drug Alcohol Depend. 2014;140:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss RD. Behavioural treatment combined with buprenorphine does not reduce opioid use compared with buprenorphine alone. Evid Based Ment Health. 2014;17(2):e2. [DOI] [PubMed] [Google Scholar]
- 49.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiellin DA, Barry DT, Sullivan LE, et al. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. Am J Med. 2013;126(1):74 e11–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365–374. [DOI] [PubMed] [Google Scholar]
- 52.Harris AH, Ellerbe L, Reeder RN, et al. Pharmacotherapy for alcohol dependence: perceived treatment barriers and action strategies among Veterans Health Administration service providers. Psychol Serv. 2013;10(4):410–419. [DOI] [PubMed] [Google Scholar]
- 53.Stetler CB, Damschroder LJ, Helfrich CD, Hagedorn HJ. A Guide for applying a revised version of the PARIHS framework for implementation. Implement Sci. 2011;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helfrich CD, Damschroder LJ, Hagedorn HJ, et al. A critical synthesis of literature on the promoting action on research implementation in health services (PARIHS) framework. Implement Sci. 2010;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donaldson NE, Rutledge DN, Ashley J. Outcomes of adoption: measuring evidence uptake by individuals and organizations. Worldviews Evid Based Nurs. 2004;1 Suppl 1:S41–51. [DOI] [PubMed] [Google Scholar]
- 56.Rycroft-Malone J. The PARIHS framework--a framework for guiding the implementation of evidence-based practice. J Nurs Care Qual. 2004;19(4):297–304. [DOI] [PubMed] [Google Scholar]
- 57.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagedorn H, Hogan M, Smith JL, et al. Lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI. J Gen Intern Med. 2006;21 Suppl 2:S21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haley SJ, Pinsker EA, Gerould H, Wisdom JP, Hagedorn HJ. Patient perspectives on alcohol use disorder pharmacotherapy and integration of treatment into primary care settings. Subst Abus. 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 61.Hagedorn HJ, Brown R, Dawes M, et al. Enhancing access to alcohol use disorder pharmacotherapy and treatment in primary care settings: ADaPT-PC. Implement Sci. 2016;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris AH, Bowe T, Hagedorn H, et al. Multifaceted academic detailing program to increase pharmacotherapy for alcohol use disorder: interrupted time series evaluation of effectiveness. Addict Sci Clin Pract. 2016;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]