Abstract
Study Objectives:
The COVID-19 pandemic required sleep centers to consider and implement infection control strategies to mitigate viral transmission to patients and staff. Our aim was to assess measures taken by sleep centers due to the COVID-19 pandemic and plans surrounding reinstatement of sleep services.
Methods:
We distributed an anonymous online survey to health care providers in sleep medicine on April 29, 2020. From responders, we identified a subset of unique centers by region and demographic variables.
Results:
We obtained 379 individual responses, which represented 297 unique centers. A total of 93.6% of unique centers reported stopping all or nearly all sleep testing of at least one type, without significant differences between adult and pediatric labs, geographic region, or surrounding population density. By contrast, a greater proportion of respondents continued home sleep apnea testing services. A total of 60.3% reduced home sleep apnea testing volume by at least 90%, compared to 90.4% that reduced in-laboratory testing by at least 90%. Respondents acknowledged that they implemented a wide variety of mitigation strategies. While no respondents reported virtual visits to be ≥ 25% of clinical visits prior to the pandemic, more than half (51.9%) anticipated maintaining ≥ 25% virtual visits after the pandemic.
Conclusions:
Among surveyed sleep centers, the vast majority reported near-cessation of in-laboratory sleep studies, while a smaller proportion reported reductions in home sleep apnea tests. A large increase in the use of telemedicine was reported, with the majority of respondents expecting the use of telehealth to endure in the future.
Citation:
Johnson KG, Sullivan SS, Nti A, Rastegar V, Gurubhagavatula I. The impact of the COVID-19 pandemic on sleep medicine practices. J Clin Sleep Med. 2021;17(1):79–87.
Keywords: COVID-19, polysomnography, sleep centers, telemedicine
BRIEF SUMMARY
Current Knowledge/Study Rationale: To understand changes in sleep medicine practices due to COVID-19, we distributed an anonymous online survey to sleep medicine health care workers and received 397 responses, primarily from sleep technologists and physicians, at 297 unique centers.
Study Impact: The vast majority of respondents reported implementing radical changes to their practices, with 93.6% of centers reporting cessation of all or nearly all sleep testing of at least one type. Pediatric studies (82.2%) and home sleep apnea testing services (60.3%) were less frequently discontinued or reduced. Centers reported utilizing a variety of mitigation strategies. Though rarely used prior to the pandemic, virtual visits were frequently implemented, and 51.9% of respondents anticipate that ≥ 25% of their encounters will continue to occur through telemedicine after the pandemic.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic, a result of the novel coronavirus SARS-CoV-2, has demanded that health care practices adapt quickly to limit the possible spread of this infection, which has caused tremendous morbidity and mortality worldwide. Although the spread of the virus was deemed to occur largely through infectious droplets of varying sizes, evidence demonstrating viral existence in aerosol droplets in room air samples has raised questions regarding aerosol transmissibility. Both the World Health Organization and the Centers for Disease Control (CDC) acknowledged the potential for viral transmission via “aerosol-generating procedures,” including noninvasive ventilation such as positive airway pressure (PAP) therapy and high-flow oxygen.1–6 Such advisories resulted in sudden disruption in the provision of health care services, with recommendations by some professional societies to stop certain elective services entirely, such as upper airway surgeries.7 The CDC emphasized the role of social distancing as a vital measure in mitigating the risk of viral transmission.8
Diagnostic testing for sleep-related respiratory disorders and therapies utilizing PAP are widely utilized in the sleep medicine field and constitute gold standard care in many cases. Patients receiving such services often have comorbidities associated with risk of severe COVID-19, including obesity, hypertension, and diabetes mellitus.9 Therefore, the COVID-19 pandemic inflicted immediate challenges for the safe provision of these core diagnostic and therapeutic services.
In response, the American Academy of Sleep Medicine (AASM) published guidance on March 19, 2020, regarding mitigation strategies for the spread of COVID-19 in sleep practices.10 Updated on April 8, 2020, this document recommended postponement and rescheduling of in-laboratory polysomnography and PAP administration except in emergencies, with continued postponement of all other nonurgent care until at least April 30. This recommendation resulted in the closure of many attended sleep laboratories and a reduction in home sleep apnea testing (HSAT), but the extent of the response to and consistency with these recommendations across regions remained unknown. Follow-up recommendations about mitigation strategies to consider when reopening laboratories was published on April 27, 2020.11
Clinical care was also heavily affected by limitations on face-to-face visits. The use of virtual platforms to provide clinical care for patients with sleep disorders has existed for nearly 2 decades and has been rising recently.12 The AASM has defined key standards with regard to its application.12 Although data suggest that telemedicine in sleep medicine may improve adherence to PAP,13 and increase efficiency of care14 while maintaining patient satisfaction,15 use of sleep telemedicine remained limited by lack of payer coverage, state licensing restrictions, and specific payer requirements for face-to-face visits. During the COVID-19 pandemic, however, many insurance providers, including the Centers for Medicare Services, have increased coverage for phone and video virtual visits, waived face-to-face requirements, and permitted provision of care across state lines during the COVID-19 pandemic.16
To understand the extent of the COVID-19 pandemic’s impact on sleep medicine health care services, we surveyed a broad range of individuals working in this field regarding closure of sleep diagnostic laboratories, policies for screening patients for COVID-19, strategies to guide laboratory reopening, and other opinions regarding procedures implemented to mitigate viral transmission in the acute stages of the pandemic. We hypothesized that regional differences in the changes of practice may relate to the degree of local COVID-19 transmission.
METHODS
Study design
On April 29, 2020, we distributed the link to a 139-item, anonymous, and confidential survey to a large group of health care workers in sleep medicine by electronic mail using distribution lists provided by the American Academy of Sleep Medicine’s weekly bulletin (n = 7,609 recipients). The link was also posted on the American Academy of Neurology sleep medicine Synapse group as well as on several physician and sleep technologist Facebook groups, and could also be shared through word-of-mouth using email or social media accounts. The Tufts Research Electronic Data Capture platform housed the survey designed with branching logic based on provider and sleep study types.17 Only some demographic responses were mandatory, and no personal identifying information was requested. For example, providers were asked questions only about their clinics and telemedicine. The protocol and survey were submitted to the Institutional Review Board of Baystate Medical Center and determined to be exempt from review. The link was active from April 29, 2020, through May 8, 2020.
Survey development
The survey was developed based on clinical experience of sleep medicine physicians who were working in the northeast, midwest, and West Coast of the United States, near regions that were then experiencing a high or increasing prevalence of COVID-19. A draft version of the survey was created by the author (KJ), who then solicited input from an expert panel including representatives from AASM, American Academy of Sleep Technologists, and other practicing sleep experts (see Acknowledgments). Co-authors IG and SS served on the AASM Public Safety Committee and helped write AASM’s COVID-19 mitigation strategies. Group consensus was used to structure the questions. Through interactive discussions and review of current Centers for Disease Control and AASM recommendations, the group prioritized key areas of assessment, including: (1) use of telemedicine; (2) reduction of lab services; (3) transmission concerns; (4) preferred mitigation strategies; and (5) anticipated reopening strategies. The wording and formatting of the survey questions were further revised by a research team member with experience in survey design at Baystate Medical Center to enhance readability.
Participants
Health care providers in sleep medicine were the intended respondents.
Data collected
We collected demographic variables about the respondents’ sleep laboratories, including country, state, primary laboratory location, laboratory type, number of beds, adult/pediatric, and HSAT use. We also collected information concerning changes in the volume of laboratory procedures and in the adoption of telemedicine during the COVID-19 pandemic, as well as anticipated changes in coming months. The full questionnaire is in the supplemental material.
Inclusion/exclusion criteria
We excluded respondents if they did not fill in survey data beyond the first page (demographics) or had incomplete demographic data. Surveys that were incomplete otherwise were still included. We created a “unique center” cohort by analyzing 7 laboratory characteristics (Table 2). Entries were defined as replicates if they agreed on all 7 variables. When this occurred, we chose the medical director, if available, for inclusion in the unique center cohort. Otherwise, we included the first respondent from the group of respondents presumed to be from the same center in the unique cohort. We used the resulting unique center cohort to mitigate replication bias from multiple respondents from the same laboratory.
Table 2.
Sleep laboratory characteristics.
| All (n = 379) | Unique Center (n = 297) | |
|---|---|---|
| Accredited lab | 322 (86.3) | 252 (84.8) |
| Perform HSAT | 339 (89.4) | 262 (88.2) |
| Primary lab location | ||
| Hospital | 202 (53.4) | 152 (51.2) |
| Other medical building | 98 (25.9) | 83 (27.9) |
| Stand-alone sleep lab | 53 (14.0) | 47 (15.8) |
| Research lab | 5 (1.3) | 4 (1.3) |
| Hotel | 11 (2.9) | 7 (2.4) |
| HSAT only | 5 (1.3) | 4 (2.4) |
| NA/unknown | 5 (1.4) | 0 (0.0) |
| Primary lab setting | ||
| Urban | 164 (44.0) | 125 (42.1) |
| Suburban | 147 (39.4) | 122 (41.1) |
| Rural | 62 (16.6) | 50 (16.8) |
| Unknown | 6 (1.6) | 0 (0.0) |
| Number of beds | ||
| 1 | 4 (1.1) | 4 (1.3) |
| 2 | 50 (13.4) | 46 (15.5) |
| 3–5 | 120 (32.2) | 98 (33.0) |
| 6–9 | 95 (25.5) | 83 (27.9) |
| >10 | 104 (27.9) | 66 (22.2) |
| Unknown | 6 (1.6) | 0 (0.0) |
| Population served | ||
| Both | 188 (50.3) | 135 (45.5) |
| Adult only | 163 (43.6) | 140 (47.1) |
| Pediatric only | 23 (6.1) | 22 (7.4) |
| Unknown | 5 (1.3) | 0 (0.0) |
| Region | ||
| United States | 349 (92.1) | 268 (90.2) |
| Northeast | 127 (33.5) | 80 (26.9) |
| Southeast | 66 (17.4) | 63 (21.2) |
| Midwest | 85 (22.4) | 66 (22.2) |
| Southwest | 38 (10.0) | 31 (10.4) |
| Western | 31 (8.2) | 27 (9.1) |
| Puerto Rico | 1 (0.3) | 1 (0.3) |
| Unknown | 1 (0.3) | 0 (0.0) |
| Outside of United States* | 28 (7.5) | 29 (9.8) |
| Unknown | 1 (0.3) | 0 (0.0) |
Data are presented as N (%). Accredited labs: if United States, accredited by American Academy of Sleep Medicine or by national accrediting body if outside United States. *Non–US respondents included the following: Canada (8), Europe (7), Australia (7), Asia (6), Africa (1). HSAT = home sleep apnea testing.
Statistical analysis
We reported summary statistics on a participant level as well as the unique cohort level. For data about HSAT or pediatric testing, we limited the responses only to those who reported that their lab performed those type of studies. For calculating sleep study activity rates, we used the appropriate corresponding denominator, based on whether the laboratory performed in-laboratory adult, in-laboratory pediatric, or home studies. We used Fisher’s exact test to compare categorical variables between groups. Significance testing was 2-sided at a critical test level of 5%. All data management and statistical analysis were performed using Stata version 16.0.
RESULTS
Respondents
We received a total of 446 survey responses. We excluded 51 respondents who provided only demographic information and another 16 surveys that were blank. Therefore, we included a total of 379/446 (85%) total respondents in the analysis. A nonresponder analysis confirmed that excluded surveys (n = 51) did not differ significantly in demographics or survey date from those that were included. Our unique center cohort contained n = 297 surveys.
Table 1 summarizes demographic information in all 379 included respondents and 297 respondents in the unique center cohort. In brief, the majority of respondents were either physicians (75, 19.8%) or sleep technologists (283, 74.7%). Other professions included administrative coordinator (1), nontechnologist sleep manager (3), registered nurse (1), registered health care scientist (1), and clinical coordinator (1). Physicians were able to choose multiple subspecialties as their primary specialty and 57 (76.0%) physicians reported sleep medicine as one of their primary specialties. Responses reflected a wide range of experience in sleep medicine, with the majority reporting 10–20 years (162, 58.1%).
Table 1.
Respondent characteristics.
| All | Unique Center† | |
|---|---|---|
| Education and training | n = 379 | n = 297 |
| Sleep technologists | 283 (74.7) | 217 (73.1) |
| RPSGT only | 204 (72.1) | 159 (73.3) |
| RRT only | 40 (14.1) | 32 (14.7) |
| Both RRT and RPSGT | 28 (9.9) | 18 (8.3) |
| Unregistered PSGT | 11 (3.9) | 8 (3.7) |
| Physicians/DMD/PhD | 78 (20.6) | 68 (22.9) |
| Sleep medicine* | 57 | 51 |
| Neurology | 18 | 18 |
| Pediatrics | 16 | 13 |
| Pulmonology | 12 | 10 |
| Pulmonary/critical care | 6 | 4 |
| Internal medicine | 9 | 7 |
| Psychiatry | 3 | 2 |
| Family medicine | 2 | 2 |
| Psychologist | 2 | 2 |
| Dentist | 1 | 0 |
| Other | 1 | 0 |
| Advanced practitioners | 10 (2.6) | 7 (2.4) |
| Other | 8 (2.1) | 5 (1.7) |
| Administrative responsibility | n = 375 | n = 297 |
| Lab medical director | 48 (12.8) | 47 (15.8) |
| Experience in sleep medicine | n = 379 | n = 297 |
| In training | 4 (1.1) | 2 (0.7) |
| 0–5 years | 71 (18.7) | 56 (18.9) |
| 6–10 years | 57 (15.0) | 46 (15.5) |
| 10–20 years | 162 (42.7) | 124 (41.8) |
| > 20 years | 85 (22.4) | 69 (23.2) |
| Practice setting | n = 84 | n = 73 |
| Academic hospital | 52 (61.9) | 43 (58.9) |
| Nonacademic MC or HMO | 15 (17.9) | 13 (17.8) |
| Private practice | 13 (15.5) | 13 (17.8) |
| Veterans Affairs | 4 (4.8) | 4 (5.5) |
Data are presented as N (%). *Allowed to choose as many specialties as applied. †Unique center cohort chosen by keeping only 1 respondent (medical director, if available) from multiple responses that matched for 7 sleep lab characteristics. MC = medical center, PSGT = polysomnographic technologist, RRT = registered respiratory therapist, RPSGT = registered polysomnographic therapist.
Table 2 details sleep laboratory characteristics, including types of testing offered, ages of patients served, and region. Respondents were from 13 countries and 46 different US states or territories. Respondents were most likely to endorse being from a hospital-based laboratory (53.4%), in an urban setting (44.0%), and having a laboratory size of 3–5 beds (32.2%). Among the unique centers, the majority of laboratories were involved in pediatric testing (52.8%), with nearly 86.0% of these laboratories testing both adults and children. Of unique center respondents working in the United States, 233 (86.6%) worked at an AASM-accredited laboratory. Compared to the characteristics of all AASM-accredited facilities, our unique cohort had more adult-only laboratories (47.1% vs 19.2%), higher representation in the northeastern United States (26.9% vs 17.6%), and lower representation from the southeastern United States (21.2% vs 32.3%) and western United States (9.1% vs 15.7%), but a similar number of hospital-affiliated facilities (58.9% of AASM-accredited).18
Reduction in sleep testing due to COVID-19
Table 3 summarizes the changes in sleep laboratory testing due to the COVID-19 pandemic by laboratory characteristics and region. A total of 278 (93.6%) of unique responses reported stopping or reducing at least 1 type of testing by at least 90% due to the COVID-19 pandemic. A total of 90.4% of unique centers reported halting or reducing in-laboratory studies by at least 90%, while only 60.3% of centers performing HSAT reported similar reductions in HSATs. There was no statistical difference in polysomnography reductions in study volume by region. There was a trend toward less polysomnography reductions in testing in south and central United States, but the number of respondents was too small. Fewer centers in the northeast and western United States stopped or reduced HSAT than in central and southern United States (P = .017). Centers located in medical buildings were less likely to reduce all types of testing compared to laboratories located in hospitals or other locations. Of centers that serve only pediatric patients, 90.9% reported stopping or significantly reducing any type of testing compared to 96% of adult-only laboratories, but the difference was not significant (P = .126).
Table 3.
For unique centers (n = 297), proportion with testing type reduced by > 90%.
| Any Testing | Adult Diagnostic PSG | Adult Titration PSG | Pediatric PSG | HSAT | |
|---|---|---|---|---|---|
| All | 278/297 (93.6) | 265/293 (90.4) | 265/293 (90.4) | 129/157 (82.2) | 158/262 (60.3) |
| Accredited labs | 237/252 (94.0) | 225/240 (93.8) | 226/241 (93.8) | 111/135 (82.2) | 131/223 (58.7) |
| Primary laboratory location | |||||
| Hospital | 147/152 (96.7) | 145/150 (96.7) | 141/146 (96.6) | 67/76 (88.8) | 91/131 (69.5) |
| Medical building | 73/83 (88.0)† | 68/78 (87.2)† | 71/81 (87.7)† | 34/48 (70.8)† | 33/76 (43.4)† |
| Other | 58/62 (93.5) | 50/54 (92.6) | 51/55 (92.7) | 28/33 (84.8) | 34/58 (58.6) |
| Number of beds | |||||
| ≤ 5 beds | 136/148 (91.9) | 128/140 (85.9) | 127/139 (91.4) | 50/66 (89.3) | 84/133 (63.2) |
| > 5 beds | 142/149 (95.3) | 135/142 (95.1) | 136/143 (95.1) | 79/91 (86.8) | 74/132 (56.3) |
| Primary laboratory setting | |||||
| Urban/suburban | 230/247 (93.1) | 220/237 (92.8) | 216/233 (92.7) | 111/137 (81.0) | 131/217 (60.4) |
| Rural | 48/50 (96.0) | 43/45 (95.6) | 47/49 (95.9) | 18/20 (0.0) | 27/48 (56.3) |
| Region | |||||
| United States | 252/269 (93.7) | 240/257 (93.4) | 241/258 (93.4) | 121/145 (83.4) | 143/245 (58.4) |
| Outside of US | 26/28 (92.9) | 23/25 (92.0) | 22/24 (91.7) | 8/12 (66.7) | 15/20 (75.0) |
| NE and western US | 102/107 (95.3) | 98/103 (95.1) | 96/102 (95.0) | 46/57 (80.7) | 49/100 (49.0)† |
| SE, MW, SW US | 149/161 (92.5) | 140/152 (92.1) | 143/155 (92.3) | 73/87 (84.9) | 93/143 (65.0) |
Data are presented as n/# respondents performing test type (%). †Effect of lab location on any testing reduction: P = .036; adult diagnostic PSG: P = .023; adult titration reduction: P = .032; pediatric testing reduction: P = .053; HSAT reduction: P = .001; effect of US region on HSAT reduction: P = .017. PSG = polysomnography, HSAT = home sleep apnea testing, NE = northeast, SE = southeast, MW = midwest, SW = southwest; the one respondent from Puerto Rico was not included in the regional US analysis.
Many respondents reported reducing mask fittings (168, 61%) and oximetry (101, 36%), but data regarding the total percentage of centers offering these services before the pandemic is unknown, so the overall prevalence of change in these practices is not clear. In an open question about stopping other services, some centers also reported significant reductions in actigraphy, administration of the Multiple Sleep Latency Test, studies on children under age 13 years and adults in subgroups at high risk for severe COVID-19 (over age 65 years or having comorbidities), education sessions, PAP naps, and loaner services for PAP devices, but since these services were not specifically queried it is unclear how often they were affected.
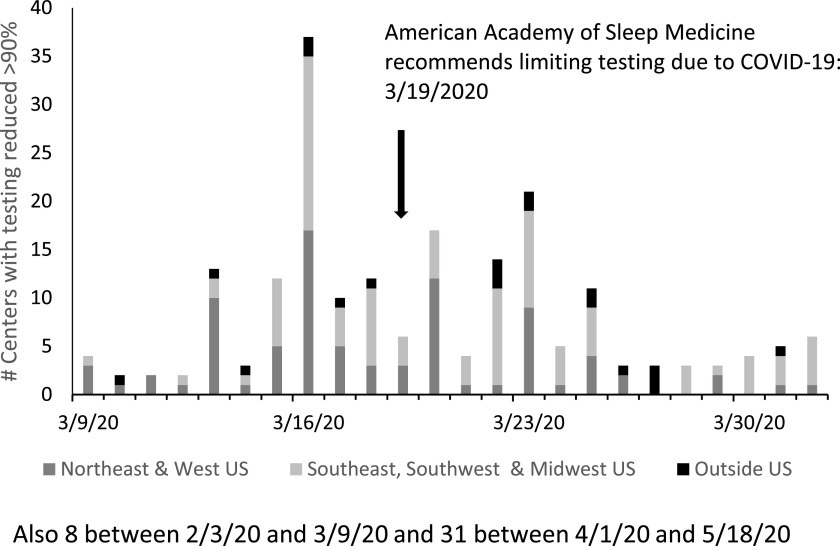
A total of 261 unique centers reported an exact date for service reduction by > 90%. The most frequently reported closing date was March 16, 2020, (n = 37), and the median date was March 20, 2020, (Figure 1). The median closure date was March 18, 2020, in northeast or western United States; March 20, 2020, in southeast, southwest, or midwestern United States, and March 23, 2020, for respondents from countries outside of the United States. Over 75% of sleep laboratories stopped or reduced services March 14–31, and 55.6% did so after the AASM issued its initial recommendation to limit testing on March 19th.
Figure 1. Date of laboratory closure or > 90% reduction in testing by region.
Among the subset of the unique center respondents from the United States, the proportion reporting > 90% reduction in any type of testing did not differ according to accreditation status (accredited laboratories [210/224 (93.8%)] vs nonaccredited laboratories (31/34 [91.2%]). A greater proportion of nonaccredited laboratories 22/35 (62.9%) reduced HSAT compared to accredited laboratories (121/210 (57.6%), but the difference was not significant (P = .585).
Sleep laboratories’ prearrival screening for COVID-19
Screening for symptoms (77.1%) and taking the patient’s temperature on arrival (64.3%) were the most commonly reported screening techniques implemented or expected to be implemented prior to in-laboratory testing. A total of 17.8% had implemented or expected to implement SARS-CoV-2 polymerase chain reaction testing (Figure 2A). Responses to open-ended questions added other screening that included review of recent travel and known exposure to COVID-19, presence of comorbidities that confer high risk of severe disease, and living in a group environment. Pulse oximetry on arrival was also implemented by some locations. Other mitigation strategies included having patients wear face masks and ensuring single-person bathrooms. Screening at the time of making an appointment, by previsit reminder calls, and upon arrival to the laboratory were all mentioned, as well as asking patients to self-quarantine for 7 days prior to the laboratory testing date.
Figure 2. Sleep study screening, setup, and processing mitigation strategies.
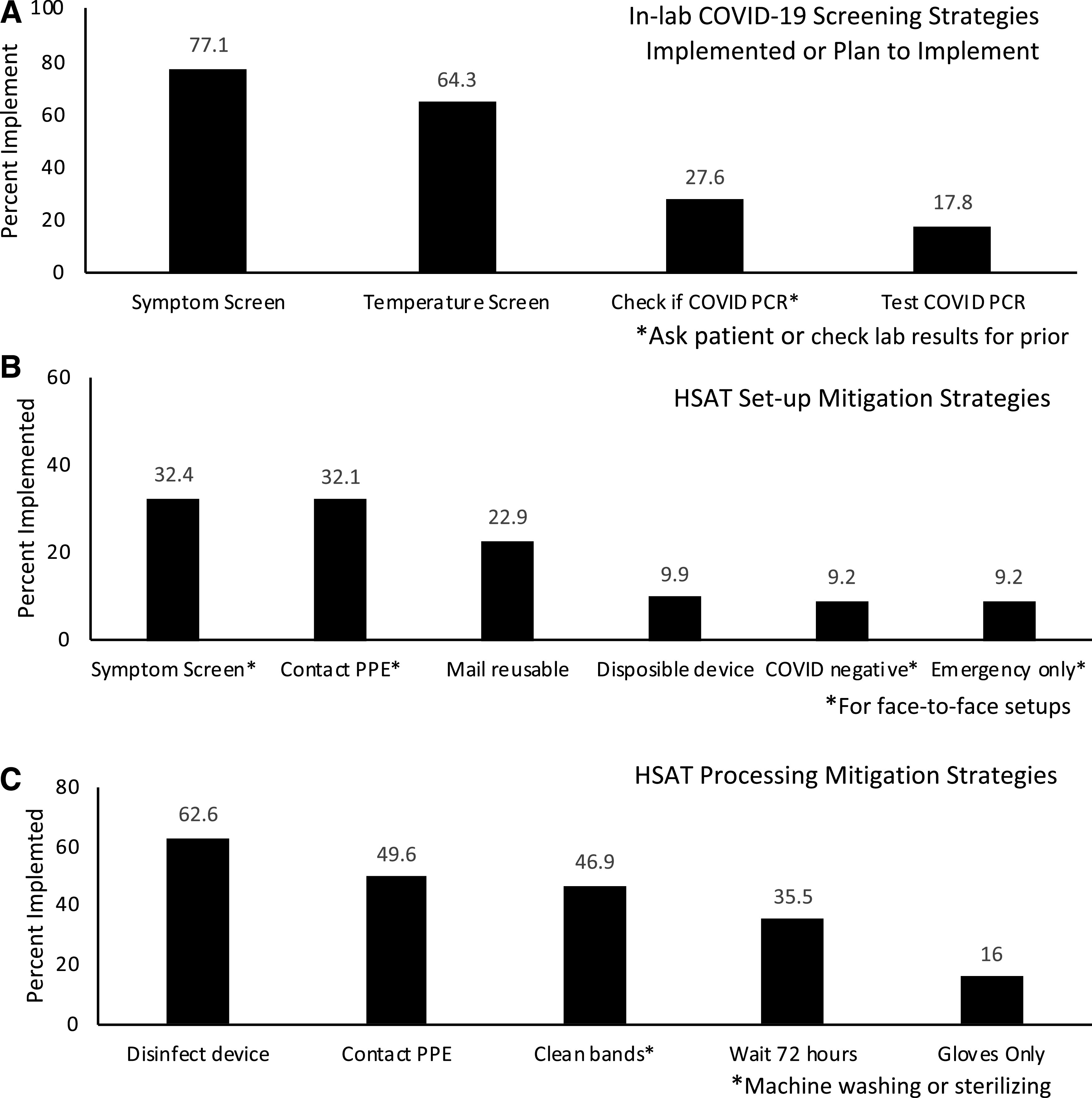
(A) In-laboratory sleep study screening mitigation strategies implemented or expected to be implemented due to COVID-19 (n = 292). (B) Home sleep apnea testing (HSAT) setup mitigation strategies implemented due to COVID-19 (n = 244). (C) HSAT processing mitigation strategies implemented due to COVID-19 (n = 224). PCR = polymerase chain reaction, PPE = personal protective equipment.
Technologist staffing and responsibilities during HSAT setups
Figure 2B summarizes mitigation strategies for setting up patients with HSAT; only 5% of 244 respondents reported no change in their processes. Of the 170 respondents that did not mail devices, 44% screened patients and 9% performed viral testing prior to face-to-face setups. Other set-up strategies included screening patients and others who were in the home prior to mailing studies, set up at the office using video instruction as much as possible to limit face-to-face time, curbside pickup, home drop-off, virtual phone or video calls for instruction regarding set-up, written instructions with link to online video, staggering appointment times, limiting appointments to emergency or “low-risk” patients only (ie, patients at lower risk for more severe health consequences if they were to become infected by SARS-CoV-2), and contracting with a commercial HSAT service provider.
Figure 2C summarizes other mitigation strategies for processing HSAT from 224 respondents. Of those not using disposable devices, 72% reported wearing contact personal protective equipment (PPE) and 19% reported wearing gloves without contact PPE when handling devices. Other mitigation strategies included having a drop-box for returns, curbside handoff to a technologist wearing PPE, using disposable bags to house equipment, using a filter for the nasal cannula, using disposable oximeters, having patients discard the cannula before returning the device, and cleaning devices with ozone or ultraviolet light.
Sleep medicine outpatient practice mitigation strategies implemented
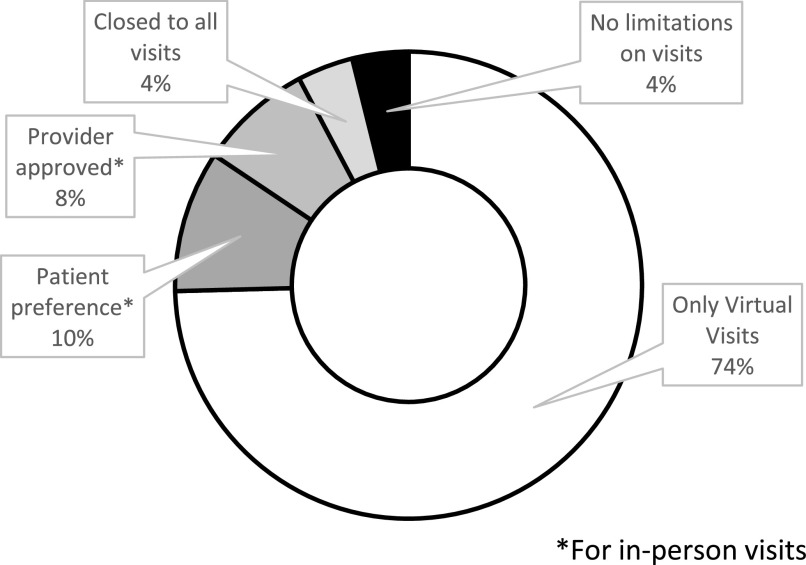
Among the unique center cohort, 52 physicians and advanced practitioners reported data on sleep medicine clinic changes due to COVID-19 (Figure 3). A total of 73.1% reported utilizing only virtual visits with 22/26 (84.6%) in northeast and western United States and 14/19 (73.7%) in southeast, midwestern, and southwestern United States. A total of 20/29 (69.0%) urban and 18/23 (78.3%) suburban practices reported using only virtual visits, but these numbers were too small to assess statistical significance.
Figure 3. Sleep clinic mitigation strategies.
Virtual clinic visits before and after the pandemic
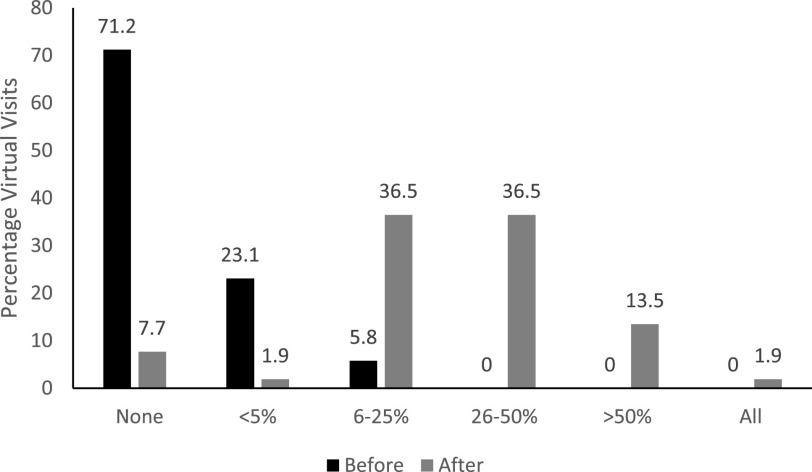
None of the 52 respondents reported that virtual visits were more than 25% prior to the COVID pandemic, with the highest rates of virtual visits in the northeast and southeast United States. Sleep medicine providers expected much higher rates of virtual visits after the pandemic (36%, 36%, and 14% expecting 6–25%, 26–50%, and > 50% of visits, respectively) (Figure 4).
Figure 4. Estimated percentage of virtual clinic visits before and after COVID-19.
Anticipated strategies for reopening sleep laboratories
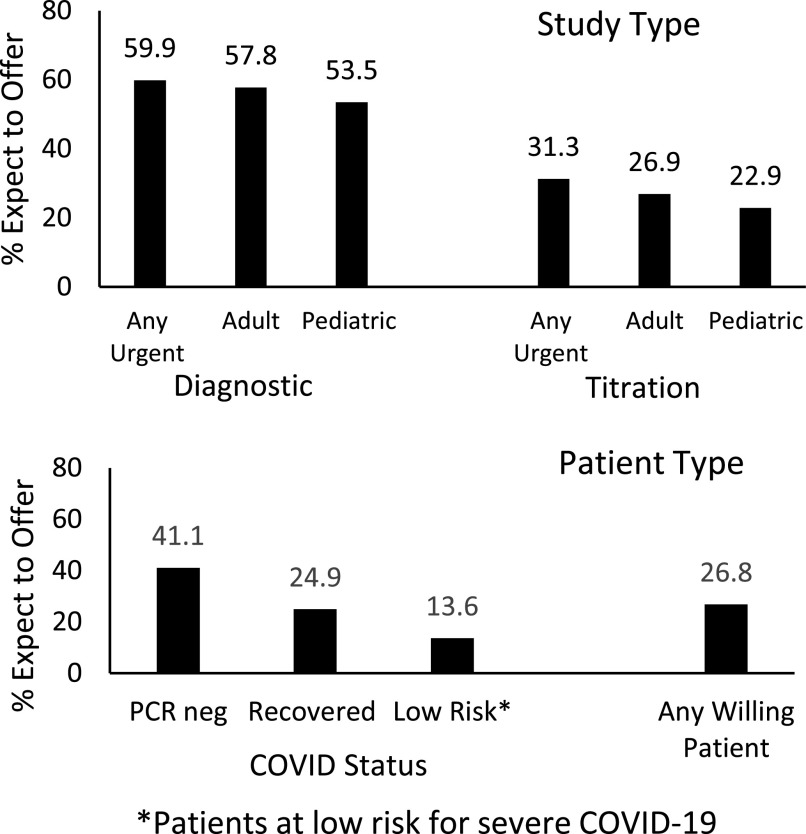
Among the unique center cohort, approximately 60% of laboratories expected to offer adult diagnostic testing and 30% adult titration studies in the next month without much regional difference (Table S1 in the supplemental material). Fewer laboratories expected to offer pediatric diagnostic (53%) or titration studies (23%). Approximately 40% expected to include COVID-19 diagnostic testing for patients prior to sleep studies, while others endorsed the use of specified criteria to restrict access to sleep studies, such as conducting studies only for “low-risk” patients and 57% limiting to urgent-only patients (Figure 5). Of the unique centers, 27% expected to offer studies to any willing patient, with highest rates in southeast (32%) and southwest (45%) United States; and lowest rates in northeast (21%), midwest (19%), and outside (8%) the United States. Tactics mentioned by respondents who noted other mitigation strategies included allowing titration or diagnostic studies only for urgent adult patients with no high-risk comorbidities and either known negative status or low risk based on screening.
Figure 5. Reopening within the next month.
Expected types of testing to be offered upon reopening and COVID-19 status needed to proceed with testing.
Other mitigation strategies expected to be used for in-laboratory studies included asking technologists not to reuse PAP masks, taking the technologist’s temperature with each shift, and supporting social distancing by reducing staffing and maintaining a 1:1 technologist/patient ratio.
DISCUSSION
Conducted during the COVID-19 pandemic, this study demonstrates several key findings. First, responding sleep medicine centers in the United States and worldwide quickly adopted widespread changes in their practices that supported social distancing, a strategy central to mitigating the spread of SARS-CoV-2.19 Indeed, 93.6% of centers reported stopping all or nearly all testing of at least one type, with little difference between adult and pediatric laboratories. Second, HSAT services were also curtailed, but less frequently than in-laboratory testing, independently of region or population density. Third, the majority of respondents (78%) indicated screening patients for active COVID-19 infection by asking about symptoms or checking temperature. In further support of social distancing goals, the use of telemedicine services expanded significantly and is anticipated by respondents to continue even after the pandemic, indicating benefits of this approach beyond infection control. The greater reduction of in-laboratory testing vs HSAT is consistent with the increased risk of viral spread in the laboratory setting, due to the potential for close contacts between patients and technologists, and the potential for viral aerosolization during in-laboratory PAP administration.6
Fortunately, the last few years have seen significant growth not only in HSAT use but also an increase in availability of PAP devices connected by cloud-based technologies to monitor PAP adherence, as well as remote-access availability of diagnostic data, and some growth in sleep telemedicine.20–22 The adaptations that were indicated by our respondents within a short period of time suggest that sleep medicine practices were poised to make these changes quickly and efficiently. Many labs reported stopping in-lab testing even before AASM’s recommendation to stop or limit testing was posted on March 19, 2020.
Our study had several key strengths. First, the survey was distributed during early phases of the COVID-19 pandemic, rather than retrospectively, which may have reduced the likelihood of recall bias. The survey covered not only steps already taken by some centers but also queried anticipated next steps. The proportion of returned surveys with usable responses was large, and the respondent group represented a variety of provider perspectives, training backgrounds, practice experience, administrative responsibility, patient types, and geographic regions, including 13 countries, 45 states, and Puerto Rico. Despite the wide variety, our sample may not accurately represent all centers, as our sample had different characteristics than all AASM-accredited facilities and had relatively low international representation.
Additionally, good representation existed from centers serving both adult and pediatric populations, which provided a unique opportunity to identify similarities between how these entities responded to the pandemic. There are few, if any, studies in the literature evaluating pediatric and adult sleep medicine practices side-by-side in conditions of a “natural experiment” such as those imposed by the COVID-19 pandemic. Finally, the study had a robust response from sleep technologists, who in this crisis are often first-line providers in sleep centers.
We note some limitations as well. This study uses a convenience sample design, which is vulnerable to response and selection bias. Laboratories with greater safety concerns may have been more likely to respond, resulting in an overestimation of those who reduced their services. We also do not have a way to estimate overall response rate, since we did not track the numbers of individuals who received our survey link. While we suspect that the length of the survey precluded the likelihood of nonunique responses from individuals, we cannot verify that every respondent is unique nor that every respondent in the unique cohort represents a unique center. We attempted to control for this by analyzing a total of 7 demographic and laboratory characteristics to create a cohort that excluded likely repeats from the same center; in doing so, some unique centers that matched on all 7 variables may have been excluded; given the large number of matched variables, we suspect this number to be exceedingly low. Another limitation is the reliance on self-reports, rather than objectively verified data. We identified 51 respondents who reported their demographics and nothing further in the survey. These respondents, however, did not differ significantly in demographics or survey date from those completing the survey; we suspect that the platform they used to complete the survey did not allow for them to advance to the next page. Finally, to preserve anonymity, we did not collect data regarding the exact locations of sleep practices. Therefore, we were unable to fully account for variance in responses due to local rates of spread of COVID-19. We did request information regarding population density (urban, suburban, rural) and region of the United States (supplemental material).
Future investigations should include an assessment of patient-centered and disease-specific outcomes of these new modes of care delivery, including the increased reliance on HSAT and the use of telemedicine technologies for initial evaluation, education, treatment delivery, and ongoing care management. Particular attention should also be given to groups at high risk for health care disparities, which includes individuals and communities who lack access to electricity and internet, telephone, or tele-video services. Another subgroup that requires careful attention are those who require more advanced modes of PAP therapy such as bilevel and noninvasive ventilation; with the exception of respiratory failure, current guidelines for payer coverage for advanced PAP devices require in-laboratory testing. New recommendations for managing care in the absence of testing will be needed, and these may include non-PAP approaches.23 Additionally, new guidelines about when face-to-face visits are recommended to optimize care and possible roles for sleep educators/technologists to enhance virtual interactions will be needed. Whether the inability to perform in-person mask fittings and PAP education impacts short- and longer-term adherence to PAP is unknown. Given that adherence is a requirement for third-party payer coverage, long-term health outcomes may be adversely impacted, unless novel ways of providing these services are devised, including video-education and facial analysis technologies to select masks,24 or payer rules are amended in the long term,16 in the setting of limited access to in-laboratory and face-to-face services.
In addition to monitoring outcomes related to sleep disorders, ongoing attention to viral transmission rates among sleep medicine patients and staff would help assess the efficacy of risk-mitigation strategies. We note that during the pandemic, many centers halted other services that carried high risk of viral transmission and were deemed elective, and these included upper airway surgeries and fitting for mandibular advancement devices for sleep apnea. The impact of these decisions on PAP adherence rates, costs of care, and clinical outcomes deserves evaluation. Some have called for a cessation of PAP and noninvasive ventilation use unless medically necessary to support life.25 Given the dearth of specific data, a recent symposium26 revealed that sleep medicine practitioners are seeking guidance and assistance in formulating strategies for risk-mitigation of COVID-19 transmission. Given the heterogeneity of logistical and financial challenges faced by various practice settings, these strategies would not be one-size-fits-all but should take into account a host of factors that impact risk. Some of these include local rate of community spread; the availability of adequate personnel protective equipment; the availability and reliability of COVID-19 viral RNA and antibody testing; the availability of contact tracing; engineering controls in the study environment, such as air exchange rates, filtration, and ventilation; health status of the technologist; and patient-related factors. These factors call for a nuanced, informed decision-making process, as well as health care policy and third-party payer rules that take into account patient-care needs and the health and safety of the workforce.
In summary, a large number of sleep health providers who responded to our survey reported that they curtailed in-laboratory sleep studies by at least 90%, while a majority curtailed HSAT and expanded the use of telemedicine visits in the early days of the COVID-19 pandemic. What remains to be seen is how these changes impact patient and health care provider acceptability, health, and safety in the long term, and whether regulatory and payment models will continue to support these changes in the months and years to come. Whether these changes are a transient response to a worldwide emergency or a permanent transformation in sleep medicine health care delivery remains to be seen.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Baystate Medical Center, Springfield, MA. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank the American Academy of Sleep Medicine and American Association of Sleep Technologists for helping to distribute the survey to their members. We also thank Brendan Duffy, RRT, Craig Canapari, MD, Michelle Cao, MD, Peter Gay, MD, Meir Kryger, MD, Robert Thomas, MD, and Lisa Wolfe, MD, for help designing survey questions.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- COVID-19
coronavirus disease 19
- HSAT
home sleep apnea testing
- PAP
positive airway pressure
REFERENCES
- 1.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 2.Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5(5):e10717. 10.1371/journal.pone.0010717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797. 10.1371/journal.pone.0035797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonds AK, Hanak A, Chatwin M, et al. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14(46):131–172. 10.3310/hta14460-02 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected: Interim Guidance. https://www.who.int/publications/i/item/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125; 2020. Published March 19, 2020. Accessed September 29, 2020.
- 6.Centers for Disease Control and Prevention . Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Published 2020. Accessed May 14, 2020.
- 7.American Academy of Otolarangology-Head and Neck Surgery AAoO-HaN . Otolaryngologists and the COVID-19 Pandemic. https://www.entnet.org/content/otolaryngologists-and-covid-19-pandemic. Published 2020. Accessed March 23, 2020.
- 8.Centers for Disease Control and Prevention . Social Distancing: Keep a Safe Distance to Slow the Spread. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html. Published 2020. Accessed June 8, 2020.
- 9.Centers for Disease Control and Prevention . People at Increased Risk: And Other People Who Need to Take Extra Precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html. Published 2020. Accessed May 24, 2020.
- 10.American Academy of Sleep Medicine . COVID-19 Mitigation Strategies for Sleep Clinics and Labs. https://j2vjt3dnbra3ps7ll1clb4q2-wpengine.netdna-ssl.com/wp-content/uploads/2020/03/covid_mitigation-sleep-clinics-labs.pdf. Published 2020. Accessed September 29, 2020.
- 11.American Academy of Sleep Medicine . Summary of CDC Recommendations Relevant for Sleep Practices During COVID-19. https://aasm.org/covid-19-resources/covid-19-mitigation-strategies-sleep-clinics-labs. Published 2020. Accessed May 6, 2020.
- 12.Singh J, Badr MS, Diebert W, et al. American Academy of Sleep Medicine (AASM) position paper for the use of telemedicine for the diagnosis and treatment of sleep disorders. J Clin Sleep Med. 2015;11(10):1187–1198. 10.5664/jcsm.5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox N, Hirsch-Allen AJ, Goodfellow E, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2012;35(4):477–481. 10.5665/sleep.1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor Y, Eliasson A, Andrada T, Kristo D, Howard R. The role of telemedicine in CPAP compliance for patients with obstructive sleep apnea syndrome. Sleep Breath. 2006;10(3):132–138. 10.1007/s11325-006-0059-9 [DOI] [PubMed] [Google Scholar]
- 15.Parikh R, Touvelle MN, Wang H, Zallek SN. Sleep telemedicine: patient satisfaction and treatment adherence. Telemed J E Health. 2011;17(8):609–614. 10.1089/tmj.2011.0025 [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services. Medicare and Medicaid Programs; Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published 2020. Accessed May 14, 2020.
- 17.Harris PA, Taylor R, Minor BL, et al. REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KG, Gurubhagavatula I, Kryger M. Emerging from the Lockdown: What We Have Learned about the Effect of COVID-19 on Sleep Medicine. Presented at: Connecticut State Virtual Sleep Grand Rounds. June 3,2020. https://medicine.yale.edu/intmed/pulmonary/clinical/excellence/sleep-medicine/. Accessed June 8, 2020.
- 19.Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020;20(5):e102–e107. 10.1016/S1473-3099(20)30129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields BG, Dholakia SA, Ioachimescu OC. Sleep telemedicine training in fellowship programs: a survey of program directors. J Clin Sleep Med. 2020;16(4):575–581. 10.5664/jcsm.8280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of sleep telemedicine for veterans: the TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355–1364. 10.5664/jcsm.7934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphie P, Little S, McKinstry B, Pinnock H. Remote consulting with telemonitoring of continuous positive airway pressure usage data for the routine review of people with obstructive sleep apnoea hypopnoea syndrome: a systematic review. J Telemed Telecare. 2019;25(1):17–25. 10.1177/1357633X17735618 [DOI] [PubMed] [Google Scholar]
- 23.Thomas RJ. Alternative approaches to treatment of central sleep apnea. Sleep Med Clin. 2014;9(1):87–104. 10.1016/j.jsmc.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spear L. How to Get Sleep Apnea Patients into Well-Fitting Interfaces…Remotely. Sleep Review. Published August 14, 2019. https://www.sleepreviewmag.com/sleep-treatments/therapy-devices/cpap-pap-devices/how-to-get-sleep-apnea-patients-into-well-fitting-interfaces-remotely/. Accessed September 30, 2020.
- 25.Barker J, Oyefeso O, Koeckerling D, Mudalige NL, Pan D. COVID-19: community CPAP and NIV should be stopped unless medically necessary to support life. Thorax. 2020;75:367. 10.1136/thoraxjnl-2020-214890 [DOI] [PubMed] [Google Scholar]
- 26.Johnson KG, Gurubhagavatula I, Kryger M, et al. The Post Pandemic Sleep Lab. Presented at: Connecticut State Virtual Sleep Grand Rounds; April 29, 2020; https://medicine.yale.edu/intmed/pulmonary/clinical/excellence/sleep-medicine/. Accessed June 6, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.