Abstract
Study Objectives:
Opioids have been reported to increase the risk for sleep-disordered breathing (SDB) in patients with noncancer chronic pain on opioid therapy. This study aims to determine the pooled prevalence of SDB in opioid users with chronic pain and compare it with patients with pain:no opioids and no pain:no opioids.
Methods:
A literature search of PubMed, Medline, Embase, and Cochrane Central Register of Controlled Trials was conducted. We included all observational studies that reported the prevalence of SDB in patients with chronic pain on long-term opioid therapy (≥3 months). The primary outcome was the pooled prevalence of SDB in opioid users with chronic pain (pain:opioids group) and a comparison with pain:no opioids and no pain:no opioids groups. The meta-analysis was performed using a random-effects model.
Results:
After screening 1,404 studies, 9 studies with 3,791 patients were included in the meta-analysis (pain:opioids group, n = 3181 [84%]; pain:no opioids group, n = 359 [9.4%]; no pain:no opioids group, n = 251 [6.6%]). The pooled prevalence of SDB in the pain:opioids, pain:no opioids, and no pain:no opioids groups were 91%, 83%, and 72% in sleep clinics and 63%, 10%, and 75% in pain clinics, respectively. Furthermore, in the pain: opioids group, central sleep apnea prevalence in sleep and pain clinics was 33% and 20%, respectively.
Conclusions:
The pooled prevalence of SDB in patients with chronic pain on opioid therapy is not significantly different compared with pain:no opioids and no pain:no opioids groups and varies considerably depending on the site of patient recruitment (ie, sleep vs pain clinics). The prevalence of central sleep apnea is high in sleep and pain clinics in the pain:opioids group.
Clinical Trial Registration: Registry: PROSPERO: International prospective register of systematic reviews; Name: Prevalence of sleep disordered breathing, hypoxemia and hypercapnia in patients on oral opioid therapy for chronic pain management; URL: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018103298; Identifier: CRD42018103298
Citation:
Mubashir T, Nagappa M, Esfahanian N, et al. Prevalence of sleep-disordered breathing in opioid users with chronic pain: a systematic review and meta-analysis. J Clin Sleep Med. 2020;16(6):961–969.
Keywords: opioids, prevalence, sleep-disordered breathing
INTRODUCTION
Opioids are effective in treating acute or chronic pain. Although opioids are widely used for managing chronic pain, reports of the associated opioid-related adverse effects continue to proliferate.1 A few studies have demonstrated an association between chronic opioid use and sleep-disordered breathing (SDB).2–4 SDB is a group of chronic sleep disorders that may result in a range of adverse sequelae, including an increased risk of death.5 Estimates of the prevalence of moderate-to-severe SDB (apnea-hypopnea index [AHI] ≥ 15 events/h) in the general population are 23.4% in women and 49.7% in men.6 SDB is further subclassified as obstructive sleep apnea (OSA), which is more commonly diagnosed in the general population compared with central sleep apnea (CSA): 47.6% versus 0.9%.7
OSA results in the intermittent narrowing of the pharyngeal airway with associated airflow limitation despite sustained respiratory effort. This contrasts to CSA, in which there is reduced airflow caused by an absence of respiratory effort. Both OSA and CSA have been described in opioid users. A recent meta-analysis showed that an increased risk for postoperative opioid-related respiratory depression was associated with preexisting OSA.8 Opioids have also been associated with abnormal breathing patterns, such as Biot or ataxic breathing,2 hypoxemia, and hypercapnia.9,10 The exact mechanism whereby opioids induce SDB has not been fully elucidated, although the inhibition of respiratory drive, dampening of the ventilatory response to CO2, and an indirect effect on the upper airway motor control may precipitate SDB and/or cause hypercapnea.11,12
The recent American Academy of Sleep Medicine position statement recommended appropriate screening, diagnostic testing, and treatment of opioid-associated SDB to improve patients’ health and quality of life.13 Therefore, knowledge of the prevalence of SDB in patients with chronic pain on opioids is essential for health care professionals. A previous review described a high prevalence of CSA of 24% in patients using opioids for chronic pain and addiction, but it did not compare the population of interest (pain:opioids) to controls (pain:no opioids or no pain:no opioids).12 Similarly, several studies in the literature investigated chronic opioid use as a risk factor for the development of SDB,9,14–21 showing a consistently positive association between SDB and chronic opioid use.
As the studies differed in type, prevalence of SDB, methodologies, sample sizes, recruitment place, dose of opioids, data limitations, and patient population, meaningful conclusions cannot be drawn. To overcome the data limitation in these studies, we choose to conduct a meta-analysis to determine the pooled prevalence of SDB in patients with chronic noncancer pain and compare its prevalence to patients with pain:no opioids and no pain:no opioids. The secondary objective is to determine the prevalence of hypoxemia and hypercapnia that may accompany patients in this population.
METHODS
Study registration
The protocol of this study was registered in the International Prospective Register of Systematic Reviews (CRD42018103298). This systematic review and meta-analysis were conducted in accordance with Cochrane systematic reviews.22 We reported all measures of assessment based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.23
Information sources and search strategy
An information specialist systematically searched for articles published in the following electronic databases: Medline, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and PubMed. The initial electronic searches were performed from database dates of inception to until June 21, 2018 and updated until May 1, 2019. The search strategy combined MeSH terms and keywords related to SDB (OSA OR CSA OR ataxic breathing) with those related to chronic pain and opioid use.
Inclusion and exclusion criteria
For inclusion, studies had to fulfill the following criteria: (1) adults ≥ 18 years on prescribed opioids with ≥3 months of noncancer chronic pain; (2) observational analytical (cross-sectional, case control, cohorts) or experimental (randomized controlled trials or, nonrandomized controlled trials) study design; and (3) one of the following outcomes were reported: prevalence of SDB, degree of hypoxemia, degree of hypercapnia. Only English language articles were included. The exclusion criteria were as follows: (1) observational descriptive studies (case reports, case series); (2) chronic pain secondary to cancer; (3) congestive heart failure and/or chronic obstructive pulmonary disease in >5% of the study population; and (4) greater than 5% of patients on opioids for addiction or withdrawal management.
Outcomes
The primary outcome was the prevalence of SDB in patients using opioid therapy for chronic pain (≥3 months) management and comparing it with pain:no opioids and no pain:no opioids groups in sleep and pain clinics. SDB included OSA, CSA, or abnormal breathing patterns such as ataxic breathing. The prevalence of SDB would be based on established diagnostic methods such as laboratory polysomnography or portable sleep study devices. The secondary outcome examined the risk of respiratory failure and ventilatory insufficiency by determining the degree of awake (PaO2 < 50 mm Hg) or nocturnal hypoxemia (SpO2 < 90% during sleep for >5 minutes with minimum value of SpO2 ≤ 85%, or SpO2 < 90% for >30% of total sleep duration) and/or awake (PaCO2 ≥ 45 mm Hg) or nocturnal (transcutaneous CO2 ≥ 45 mm Hg) hypercapnia.24,25 Measures of nocturnal and awake hypoxemia included pulse oximetry and arterial blood gas (ABG) analysis, whereas nocturnal and awake hypercapnia were measured by transcutaneous CO2 and ABG, respectively.
Study selection and data extraction
Two reviewers (NE and JB) independently screened the results of the electronic search by title and abstract to assess articles eligible for inclusion. The included full-text articles were considered for final eligibility in the systematic review. Any disagreements were resolved by consulting a third author (FC). A standardized data extraction Excel (Microsoft, Redmond, Washington) spreadsheet was used to collect any relevant information on study design, study quality assessment, demographics, pain management (eg, type of opioids, dose, morphine equivalent daily dose), and outcomes (eg, prevalence rates, degree of hypoxemia, and hypercapnia). Data were extracted from eligible full-text articles independently by 2 reviewers (TM and MN). Disagreements were resolved by a senior author (FC).
Quality of study assessment
Two authors (TM and AA) independently assessed the methodologic quality and the overall risk of bias in individual studies according to the Joanna Briggs Institute critical appraisal checklist for analytical cross-sectional studies.26 The key points of the checklist includes the following: (1) study population appropriately recruited, (2) study sample represents the target population, (3) standard criteria used for ascertaining the exposure (chronic opioid users) and the (4) outcome (SDB), (5) confounding factors identified and (6) adjusted for, and (7) appropriate statistical analysis carried out (if applicable). Each item was evaluated using yes/no/unclear or not applicable.
Data synthesis and analysis
We conducted a meta-analysis to investigate the pooled prevalence, which was estimated using the double arcsine transformation method to stabilize the variance as described by Barendregt et al.27 The prevalence of SDB was compared by the recruitment site of sleep clinics versus pain clinics among the pain:opioids, pain:no opioids, and no pain:no opioids groups. Similar comparisons were made for OSA and CSA. A random-effects model was used to perform the meta-analysis, which accounted for heterogeneity between studies. All statistical analyses were performed using the MetaXL (www.epigear.com) add-in for Microsoft Excel.
RESULTS
The search yielded 1,407 records in total. Of the 124 studies gathered for full-text screening, 115 were excluded (Figure 1). The most common reason for exclusion was the study population. Nine articles were included in the final systematic review and meta-analysis.9,14–21
Figure 1. Flow diagram of included studies.
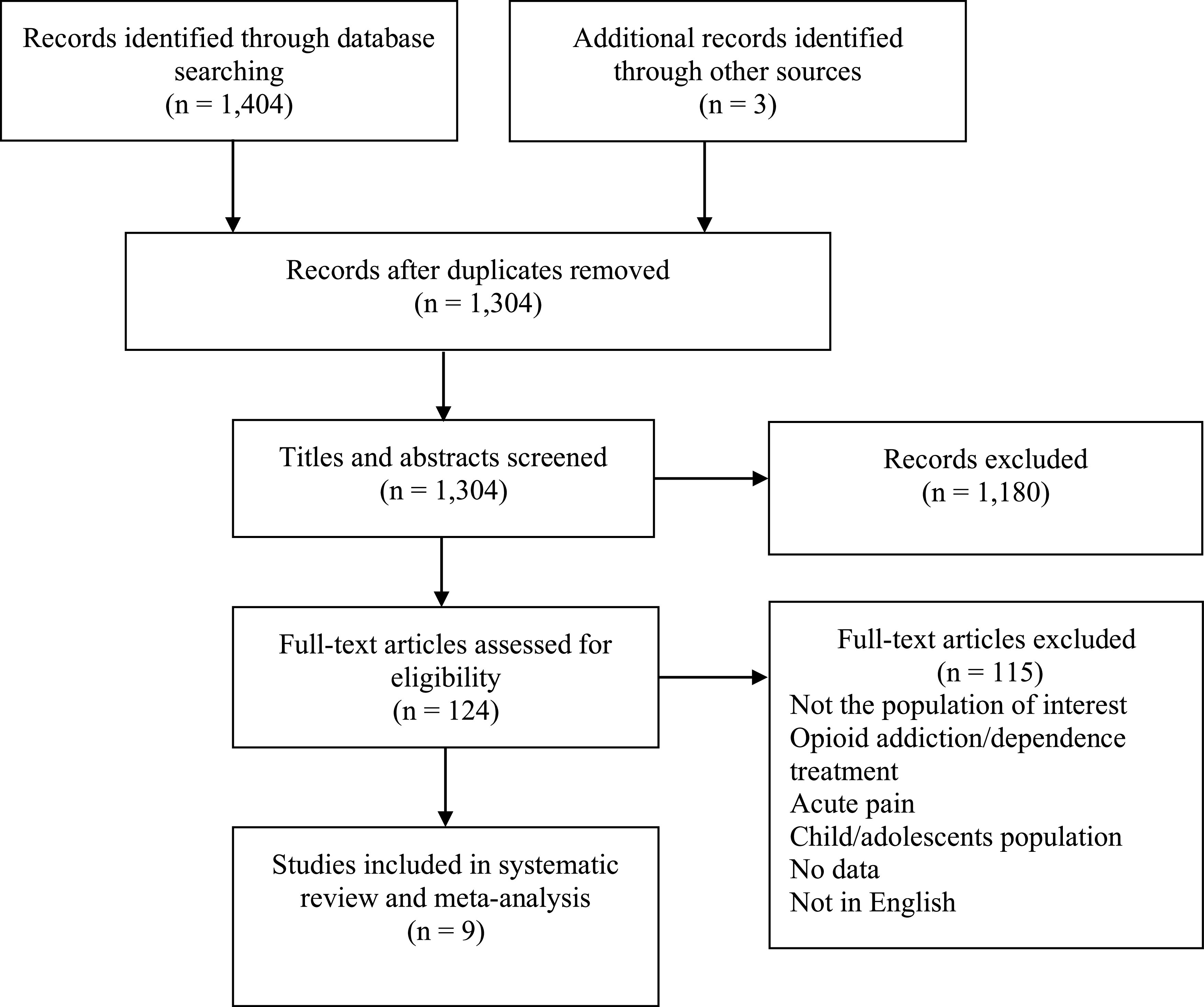
Characteristics of selected studies
The characteristics of the included studies are summarized in Table 1. Six studies were retrospective cohorts,9,14,15,17,18,21 2 were prospective cohorts,19,20 and 1 had a cross-sectional design.16 All studies used laboratory polysomnography to diagnose SDB. In 5 studies, patients from pain clinics were referred for polysomnography, whereas in 4 studies, patients from sleep clinics were recruited.14–17
Table 1.
Characteristics of studies grouped by patient intervention group and recruitment location.
| Study | Study Design | Groups | n | Age (Mean ± SD) (years) | Males (%) | BMI (Mean ± SD) (kg/m2) | MMEs per 24 hours |
|---|---|---|---|---|---|---|---|
| Recruitment location: sleep clinics | |||||||
| Walker 2007 | RC | Pain:opioids | 60 | 53 ± 13 | 33 | 32 ± 7.7 | 229 ± 161 |
| No pain:no opioids | 60 | 53 ± 13 | 33 | 33 ± 7.6 | ― | ||
| Farney 2008 | RC | Pain:opioids | 22 | 50 ± 13 | 41 | 33 ± 6.1 | NR |
| Jungquist 2012 | RC | Pain:opioids | 61 | 50 ± 11 | 36 | 33 ± 7 | 299 ± 164 |
| no pain:no opioids | 171 | 48 ± 12 | 63 | 33 ± 6 | 30.7 ± 10 | ||
| Pain:no opioids | 187 | 51 ± 12 | 45 | 35 ± 8 | 71 ± 26 | ||
| Javaheri 2014 | RC | Pain:opioids | 20 | 53 ± 10 | 65 | 33 ± 7 | 292 ± 241 |
| Recruitment location: pain clinics | |||||||
| Webster 2008 | RC | Pain:opioids | 140 | 52 ± 12 | 47 | 30 | 1,633 ± 1,141 |
| Mogri 2009 | RC | Pain:opioids | 98 | 50 ± 12 | 71 | 32 ± 5.3 | 326 ± 186 |
| Rose 2014 | PC | Pain:opioids | 24 | 52.4 ± 9.4 | 50 | 35 ± 9.4 | 120 ± 20 |
| No pain:no opioids | 20 | 51 ± 10 | 75 | 25 ± 2.6 | ― | ||
| Shapiro 2015 | PC | Pain:opioids | 74 | 51 ± 12 | 46 | 30 ± 5.4 | 390 ± 338 |
| Pampati 2016 | RC | Pain:opioids | 2,682 | 49 ± 13.6 | 43 | 31 + 7.9 | NR |
| Pain:no opioids | 172 | ||||||
BMI = body mass index, MME = morphine milligram equivalent, NR = not reported, PC = prospective cohort, RC = retrospective cohort.
All included studies had patients with noncancer chronic pain on long-term opioids classified as the pain:opioids group. Four studies compared this population to a control group.14,16,19,21 The control group included patients with pain and not taking opioids (pain:no opioids)16,21 or those without pain (no pain:no opioids).14,16,19 Among the 9 studies, 3,791 patients were included in the systematic review and meta-analysis (pain:opioids group, n = 3,181 [84%]; pain:no opioids group, n = 359 [9.4%]; no pain:no opioids group, n = 251 [6.6%]). Most individuals were middle-aged adults (50 ± 12 years), obese (33 ± 7 kg/m2), and taking multiple types of opioids. The morphine milligram equivalents (MMEs) per 24 hours varied considerably among studies, ranging from 7.5 to 5,985 mg. In the pain:opioids group, the mean MME was 691.9 ± 887 mg. The demographic factors of age (51 ± 12 vs 51 ± 12 vs 49 ± 12 years), sex (male: 53.1% vs 45% vs 64.5%), and body mass index (BMI; 31 ± 6 vs 35 ± 8.0 vs 32 ± 7 kg/m2) were comparable among all 3 groups.
Quality of studies
The appraisal of the included studies is presented in Table S1 in the supplemental material. All studies appropriately defined the inclusion criteria and described the patient population and setting in sufficient details. Although all included studies had patients with greater than 3 months of chronic pain on opioids, 2 studies did not report on an objective assessment of amount of opioid use in terms of MMEs.15,21 Standard criteria using polysomnography was used to diagnose the primary outcome, SDB, among all studies. Moreover, standard criteria using ABG analysis was used to diagnose the secondary outcomes, hypoxemia and hypercapnia, in 2 of 3 studies.17,19 Although important confounding factors such as age, sex, and BMI were identified by some studies, they were not always dealt with when comparing the population of interest with the control population.
Prevalence of SDB
The prevalence of SDB varied widely ranging from 14% to 100% in the pain:opioids group; 11% to 83% in the pain:no opioids group; and 50% to 85% in the no pain:no opioids group (Table 2). The pain:opioids group had no significant difference in the rate of SDB pooled prevalence between the sleep clinics and pain clinics (91%, 95% confidence interval [CI]: 74%–100% vs 63%, 95% CI: 18–100%; P = .57). The pain:no opioids group had significantly higher SDB pooled prevalence rates from sleep clinics than pain clinics (83%, 95% CI: 77–88% vs 10%, 95% CI: 6.3–16%; P < .01). No major differences in SDB prevalence were noted in the no pain:no opioids groups in either sleep or pain clinics (72%, 95% CI: 30–100% vs 75%, 95% CI: 53–92%; P = .95). In the pain:opioids group, the pooled prevalence of CSA was not significantly different between the sleep clinics and the pain clinics (33%, 95% CI: 3–72% vs 20%, 95% CI: 15–25%; P = .72; Table 2). Of the 6 studies that reported on the prevalence of OSA and CSA, all had a higher prevalence of CSA (range, 11.5–80%) in the pain:opioids group versus the pain:no opioids group (3.2%) and the no pain:no opioids group (range, 3.3–4.7%).
Table 2.
Proportion of patients with sleep-disordered breathing and subtypes in the included studies.
| Study | SDB (%) | OSA (%) | CSA (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | |
| Recruitment location: sleep clinics | |||||||||
| Walker 2007* | 73 | ― | 50 | 28 | ― | 23 | 22 | ― | 3.3 |
| Farney 2008 | 100 | ― | ― | NR | ― | ― | NR | ― | ― |
| Farney 2008 | 77 | 83 | 85 | 18 | NR | NR | 11.5 | 3.2 | 4.7 |
| Jungquist 2012 | 100 | ― | ― | 20 | ― | ― | 80 | ― | ― |
|
Pooled prevalence, % (95% CI) |
91 (74–100) | 83 (77–88) | 72 (30–100) | 23 (16–30) | NA | 23 (13–35) | 33 (3.0–72) | 3.2 (1.0–6.0) | 4.5 (2.2–7.6) |
| Recruitment location: pain clinics | |||||||||
| Webster 2008 | 75 | ― | ― | 29 | ― | ― | 18 | ― | ― |
| Mogri 2009 | 85 | ― | ― | 36 | ― | ― | 24 | ― | ― |
| Rose 2014 | 71 | ― | 75 | NR | ― | NR | 17 | ― | NR |
| Shapiro 2015 | 47 | ― | ― | NR | ― | ― | NR | ― | ― |
| Pampati 2016 | 14 | 11 | ― | 14 | 11 | ― | NR | NR | ― |
| Pooled prevalence %, (95% CI) | 63 (18–100) | 10 (6.3–16) | 75 (53–92)† | 25 (11–42) | 10 (6.3–16) | NA | 20 (15–25) | NA | NA |
*Data was obtained from authors. †P < .05 (no pain:no opioids vs pain:no opioids). CI = confidence interval, CSA = central sleep apnea, NA = not applicable, NR = not reported, OSA = obstructive sleep apnea, SDB = sleep-disordered breathing.
Polysomnography parameters
All included studies used polysomnography to diagnose SDB and reported on sleep parameters such as AHI, hypopnea index (HI), central apnea index (CAI), and obstructive apnea index (Table 3). The AHI and HI were not significantly different between the pain:opioids and no pain:no opioids groups, except in 1 study.19 In 3 studies, CAI was significantly higher in the pain:opioids group than the pain:no opioids and no pain:no opioids groups (P < .05; Table 3).
Table 3.
Polysomnography parameters in patients with sleep-disordered breathing.
| Study | AHI (events/h) | HI (events/h) | CAI (events/h) | OAI (events/h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | |
| Recruitment location: sleep clinics | ||||||||||||
| Walker 2007 | 44 ± 35 | ― | 30 ± 26 | 15 ± 14 | ― | 14 ± 13 | 13 ± 22* | ― | 2.1 ± 4 | 17 ± 24 | ― | 14 ± 18 |
| Farney 2008 | 67 ± 37 | ― | ― | 15 ± 14 | ― | ― | 26 ± 25 | ― | ― | 26 ± 23 | ― | ― |
| Farney 2008 | 23 ± 25 | 29 ± 26 | 26 ± 27 | 12 ± 13 | 16 ± 15 | 15 ± 18 | 5 ± 13† | 1.1 ± 4 | 1.6 ± 7 | 4.4 ± 9 | 9.4 ± 14 | 6.7 ± 14 |
| Jungquist 2012 | 61 ± 30 | ― | ― | NR | ― | ― | 32 ± 31 | ― | ― | 5 ± 5 | ― | ― |
| Recruitment location: pain clinics | ||||||||||||
| Webster 2008 | NR | ― | ― | NR | ― | ― | NR | ― | ― | NR | ― | ― |
| Mogri 2009 | NR | ― | ― | NR | ― | ― | NR | ― | ― | NR | ― | ― |
| Rose 2014 | 33 ± 25* | ― | 8.3 ± 4 | 26 ± 20* | ― | 7.1 ± 3 | 4 ± 8* | ― | 0.3 ± 0.6 | 3 ± 6 | ― | 1 ± 1.1 |
| Shapiro 2015 | 39 ± 31 | ― | ― | 15 ± 12 | ― | ― | 16 ± 18 | ― | ― | 9.7 ± 15 | ― | ― |
| Pampati 2016 | NR | NR | ― | NR | NR | ― | NR | NR | ― | NR | NR | ― |
Values are expressed as mean ± SD where appropriate. *P < .05 (pain:opioids vs no pain:no opioids). †P < .05 (pain:opioids vs no pain:no opioids). AHI = apnea-hypopnea index, CAI = central apnea index, HI = hypopnea index, NR = not reported, OAI = obstructive apnea index.
Biot’s respiration
Two of the 9 studies reported on Biot’s respiration within their study population.14,15 Walker et al14 found that 70% of patients taking chronic opioids for pain had breathing patterns consistent with ataxic or Biot’s breathing during non–rapid eye movement (NREM) sleep compared with only 5% in their control group (no pain:no opioids). Furthermore, 92% of patients taking a morphine dose equivalent of 200 mg or higher had Biot’s respiration versus only 5% of controls, which suggests a dose-dependent relationship may exist.14 Similarly, Farney et al15 reported that more than 95% of their patients taking chronic opioids had Biot’s respiration.
Hypoxemia and hypercapnia
Three studies reported on the overnight pulse oximetry parameters between the study groups.14,16,19 Although patients in the pain:opioids group spent a significantly greater amount of their total sleep duration with SpO2 < 90% compared with the no pain:no opioids group (10 ± 18% vs 0.1 ± 0.3%), the duration was less than 30% of total sleep.19 In 2 studies, the awake mean SpO2 was significantly lower in the pain:opioids group versus the no pain:no opioids population14,19 (Table 4), whereas 1 study demonstrated a lower nocturnal NREM SpO2.14 There were no significant differences between minimal SpO2 and oxygen desaturation index among the groups. In a subset of their patient population with chronic pain who were taking opioids, 2 studies conducted daytime ABG analysis and reported low mean PaO2 and higher level of PaCO217,19 (Table 4). In 1 study, 45% of the patients had an elevated PaCO2 (>45 mm Hg) and a higher awake PaCO2 correlated with sleep time spent with SpO2 < 90% (P = .04).19
Table 4.
Polysomnography parameters and arterial blood gas analysis as measures of hypoxia in patients with SDB.
| Study | Mean SpO2 (%) | Minimal SpO2 (%) | ODI (≥3%) | PaO2 and PaCO2 (mm Hg)* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | Pain:Opioids | Pain:No Opioids | No Pain:No Opioids | |
| Recruitment location: sleep clinics | ||||||||||||
| Walker 2007 | Awake: 91 ± 3,† NREM: 90 ± 3,† REM: 89 ± 7 | ― | Awake: 93 ± 2, NREM: 92 ± 2, REM: 91 ± 4 | NR | ― | NR | NR | ― | NR | ― | ― | ― |
| Farney 2008 | 90 ± 4 | ― | ― | 80 ± 8 | ― | ― | 31 ± 34 | ― | ― | ― | ― | ― |
| Farney 2008 | NR | NR | NR | 84 ± 6 | 83 ± 7 | 83 ± 7 | 20 ± 24 | 24 ± 27 | 25 ± 23 | ― | ― | ― |
| Jungquist 2012 | 94 ± 3 | ― | ― | 83 ± 8 | ― | ― | NR | ― | ― | PaO2: 80; range, 59–99; PaCO2: range, 42–53 | ― | ― |
| Recruitment location: pain clinics | ||||||||||||
| Webster 2008 | NR | ― | ― | NR | ― | ― | NR | ― | ― | ― | ― | ― |
| Mogri 2009 | NR | ― | ― | NR | ― | ― | NR | ― | ― | ― | ― | ― |
| Rose 2014 | Awake: 94 ± 1.6† | ― | Awake: 97 ± 1.3 | NR | ― | ― | NR | ― | ― | PaO2: 81.4 ± 9; range, 74–86; PaCO2: 44.8 ± 41; range: 42–47 | ― | ― |
| Shapiro 2015 | 93 ± 3 | ― | ― | 80 ± 8 | ― | ― | 33 ± 29 | ― | ― | ― | ― | ― |
| Pampati 2016 | NR | NR | ― | NR | NR | ― | NR | NR | ― | ― | ― | ― |
Values are expressed as mean ± SD where appropriate. *Data obtained from arterial blood gas analysis. †P < .05 vs no pain:no opioids. NR = not reported, NREM = non–rapid eye movement, ODI = oxygen desaturation index, PaCO2 = partial pressure of CO2 in arterial blood, PaO2 = partial pressure of O2 in arterial blood, REM = rapid eye movement, SpO2 = peripheral capillary O2 saturation.
DISCUSSION
To date, this systematic review and meta-analysis is the first to determine the prevalence of SDB, in patients with noncancer chronic pain who are on opioid therapy and compare it with patients with pain:no opioids and no pain:no opioids. In both sleep and pain clinics, the pooled prevalence of SDB in the pain:opioids group were high: 91% and 63%, respectively. The pooled prevalence of SDB in the pain:no opioids group was significantly higher in sleep clinics versus pain clinics (83% vs 10%). In the pain:opioids group, we demonstrated a high prevalence of CSA in both sleep (33%) and pain (20%) clinics, accounting for the higher AHI. The CAI was significantly higher in the pain:opioids group versus the pain:no opioids and no pain:no opioids groups (Table 3).14,16,19 These findings suggest that the differences in the prevalence rates between patients with chronic pain that use opioids versus patients not taking opioids is driven primarily by the increase in central events. Both hypoxic and hypercapnic responses are strongly affected by opioids and appear to be strongly mediated in the brainstem.28,29 The potential mechanisms of the increased CSA may be caused by an increased hypoxic ventilatory drive and the effects of centrally acting drugs on respiratory rhythm generation.28,29
There is evidence to indicate that a bidirectional relationship between sleep and pain exists. Pain disrupts sleep, and sleep disturbances can exacerbate pain.30 There is a great overlap between patients with chronic pain and those with SDB. Sleep is an important predictor of pain, and the management of sleep disorders such as sleep apnea may have benefits beyond sleep in patients with chronic pain. Effective treatment of SDB may have beneficial impacts on reducing pain.30 It is speculated that sleep fragmentation disrupts the pain inhibitory system, making patients with sleep apnea hyperalgesic that may be reversed through continuous positive pressure therapy.31,32 Hence, the American Academy of Sleep Medicine Position Statements recommended appropriate screening, diagnostic testing, and treatment of opioid-associated SDB to improve patients’ health and quality of life.13
Patient characteristics affecting SDB prevalence
The considerable variation in the prevalence rate of SDB between studies was likely because of differences in patient populations, opioid dosages and metabolism, and the coingestion of sedative medications.18,33 Patients with chronic pain referred to sleep clinics likely exhibit symptoms of sleep apnea, such as excessive daytime sleepiness and snoring, that prompt a referral and accounts for the higher SDB rates versus pain clinics. Other factors pertaining to population characteristics such as age, sex, BMI, and opioid dosage can also affect SDB rates.18,34 Increasing the daily opioid dose for patients with chronic pain has been shown to increase the number of obstructive apneas, central apneas, and hypopneas during sleep, with the greatest dose-dependent response observed with central apneas.14,16 A large case-cohort study demonstrated that the risk of fatal overdose was approximately 7-fold in individuals taking more than 100-mg MME for noncancer chronic pain.35 This review suggests an increased risk of SDB and CSA with chronic opioid use in patients without patients, whereby opioids may exacerbate or contribute to SDB by an indirect effect on the upper airway activity and/or respiratory pattern.36
Prevalence of hypoxemia and hypercapnia
It is difficult to interpret the clinical significance of our findings on the level of hypoxemia in patients with SDB taking opioids for several reasons. Because of the invasive nature of ABGs and the poor reliability and expense of other measures, only 2 studies objectively examined the level of hypoxemia and the presence of hypercapnia in chronic opioid users.17,19 Nonetheless, daytime ABG analysis showed normal oxygen saturation, but in a subset of chronic opioid users, daytime hypercapnia was demonstrated.17,19 Daytime ABG measurements do not reflect nighttime gas exchange with the exception of early morning testing (7:00 am).37 Unfortunately, blood gas sampling was conducted between 1400 and 1600 hours in 1 study,19 whereas the other17 did not report the time. In light of the relationship between opioids and state of arousal (sleep vs wake), the finding of normal daytime oxygen saturation is consistent with the theory of state-dependent mechanisms likely regulating respiratory depression.3,38 The presence of daytime hypercapnia in a subset of chronic opioid users suggests that there may be an impact of opioids on the ventilatory response to CO2.3
Limitations
This review has some limitations. Most of the included studies in the meta-analysis were observational studies with small sample sizes and differing population with regard to duration, type, and dosages of opioids used. The use of other centrally acting medications, such as benzodiazepines, underlying medical comorbidities, and genetic predispositions were not taken into consideration.39 Furthermore, the absence of a standardized guideline for the scoring of the irregular breathing pattern (eg, Biot’s) in opioid users precludes the accurate determination of the prevalence of SDB and other sleep-related breathing disorders such as sleep-related hypoventilation.40 The strength of this systematic review and meta-analysis is that it is the first pooled prevalence of SDB in patients with noncancer chronic pain on opioid therapy and compares patients with pain:no opioids and no pain:no opioids in both sleep clinics and pain clinics. The knowledge of the high prevalence of SDB in opioid users will increase awareness and alert health care professionals for further workup.
CONCLUSIONS
In patients with chronic pain on long-term opioid therapy, the prevalence of SDB is high: 91% in sleep clinics and 63% in pain clinics. In these patients, the prevalence of CSA was 33% in the sleep clinics and 20% in the pain clinics. The pooled prevalence of SDB in patients with chronic pain on opioid therapy is not significantly different compared with pain:no opioids and no pain:no opioids groups. Longitudinal prospective studies with larger community-based populations and comparable healthy controls are necessary to determine the true prevalence of SDB within chronic opioid users.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was supported by the University Health Network Foundation and the Department of Anesthesia and Pain Medicine, Toronto Western Hospital, University Health Network, University of Toronto. Financial disclosures: Talha Mubashir, none; Mahesh Nagappa, none; Nilufar Esfahanian, none; Joseph Botros, none; Abdul A. Arif, none; Colin Suen, none; Jean Wong, reports grants from the Ontario Ministry of Health and Long-Term Care, Anesthesia Patient Safety Foundation, and Acacia Pharma, outside of the submitted work; Clodagh M. Ryan, none; Frances Chung, reports research support from the Ontario Ministry of Health and Long-Term Care, University Health Network Foundation, Up-to-Date royalties, and STOP-Bang proprietary to University Health Network. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank Marina Englesakis, HBA, MLIS (Information Specialist, Health Sciences Library, University Health Network, Toronto, ON, Canada), for assistance with the literature search.
ABBREVIATIONS
- ABG
arterial blood gas
- AHI
apnea-hypopnea index
- BMI
body mass index
- CAI
central apnea index
- CI
confidence interval
- CSA
central sleep apnea
- HI
hypopnea index
- MME
morphine milligram equivalent
- NREM
non–rapid eye movement
- OR
odds ratio
- OSA
obstructive sleep apnea
- PaCO2
partial pressure of CO2 in artery
- PaO2
partial pressure of O2 in artery
- SDB
sleep-disordered breathing
- SpO2
pulsatile hemoglobin oxygen saturation
REFERENCES
- 1.Stannard C. Where now for opioids in chronic pain? Drug Ther Bull. 2018;56(10):118–122. 10.1136/dtb.2018.10.000007 [DOI] [PubMed] [Google Scholar]
- 2.Farney RJ, Walker JM, Cloward TV, Rhondeau S. Sleep-disordered breathing associated with long-term opioid therapy. Chest. 2003;123(2):632–639. 10.1378/chest.123.2.632 [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev. 2007;11(1):35–46. 10.1016/j.smrv.2006.03.006 [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Teichtahl H, Drummer OH, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348–1356. 10.1378/chest.128.3.1348 [DOI] [PubMed] [Google Scholar]
- 5.Young T, Finn L, Peppard EP, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 6.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study Cohort. Sleep. 2016;39(7):1353–1359. 10.5665/sleep.5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta K, Nagappa M, Prasad A, et al. Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open. 2018;8(12):e024086. 10.1136/bmjopen-2018-024086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mogri M, Desai H, Webster L, Grant BJ, Mador MJ. Hypoxemia in patients on chronic opiate therapy with and without sleep apnea. Sleep Breath. 2009;13(1):49–57. 10.1007/s11325-008-0208-4 [DOI] [PubMed] [Google Scholar]
- 10.Farney RJ, McDonald AM, Boyle KM, et al. Sleep disordered breathing in patients receiving therapy with buprenorphine/naloxone. Eur Respir J. 2013;42(2):394–403. 10.1183/09031936.00120012 [DOI] [PubMed] [Google Scholar]
- 11.Weil J, McCullough R, Kline J, Sodal I. Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl Med J. 1975;292(21):1103–1106. 10.1056/NEJM197505222922106 [DOI] [PubMed] [Google Scholar]
- 12.Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120(6):1273–1285. 10.1213/ANE.0000000000000672 [DOI] [PubMed] [Google Scholar]
- 13.Rosen I, Aurora RN, Kirsch D, et al. Chronic opioid therapy and sleep: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2019;15(11):1671–1673. 10.5664/jcsm.8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker JM, Farney RJ, Rhondeau SM, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med. 2007;3(5):455–461. 10.5664/jcsm.26908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farney RJ, Walker JM, Boyle KM, Cloward TV, Shilling KC. Adaptive servoventilation (ASV) in patients with sleep disordered breathing associated with chronic opioid medications for non-malignant pain. J Clin Sleep Med. 2008;4(4):311–319. 10.5664/jcsm.27230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jungquist CR, Flannery M, Perlis ML, Grace JT. Relationship of chronic pain and opioid use with respiratory disturbance during sleep. Pain Manag Nurs. 2012;13(2):70–79. 10.1016/j.pmn.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 17.Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637–643. 10.5664/jcsm.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9(4):425–432. 10.1111/j.1526-4637.2007.00343.x [DOI] [PubMed] [Google Scholar]
- 19.Rose AR, Catcheside PG, McEvoy RD, et al. Sleep disordered breathing and chronic respiratory failure in patients with chronic pain on long term opioid therapy. J Clin Sleep Med. 2014;10(8):847–852. 10.5664/jcsm.3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro CM, Chung SA, Wylie PE, et al. Home-use servo-ventilation therapy in chronic pain patients with central sleep apnea: initial and 3-month follow-up. Sleep Breath. 2015;19(4):1285–1292. 10.1007/s11325-015-1161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pampati S, Manchikanti L. What is the prevalence of symptomatic obstructive sleep apnea syndrome in chronic spinal pain patients? An assessment of the correlation of OSAS with chronic opioid therapy, obesity, and smoking. Pain Physician. 2016;19(4):E569–E579. [PubMed] [Google Scholar]
- 22.Higgins J, Green Se. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. https://handbook.cochrane.org/. Updated March 2011. Accessed August 22, 2018.
- 23.Moher D, Liberati A, Tetzlaff J, Altmanm D, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 24.Berry RB, Brooks R, Gamaldo CE, et al. ; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. Darien, IL: American Academy of Sleep Medicine; 2015. [Google Scholar]
- 25.American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manual. 1st ed-revised. Rochester, MN: American Academy of Sleep Medicine; 2001.
- 26.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute; 2017. https://reviewersmanual.joannabriggs.org/. Accessed August 22, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Barendregt J, Doi S, Lee Y, Norman R, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. [DOI] [PubMed] [Google Scholar]
- 28.Teichtahl H, Wang D, Cunnington D, et al. Ventilatory responses to hypoxia and hypercapnia in stable methadone maintenance treatment patients. Chest. 2005;128(3):1339–1347. 10.1378/chest.128.3.1339 [DOI] [PubMed] [Google Scholar]
- 29.Pattinson KT. Opioids and the control of respiration. Br J. Anaesth. 2008;100(6):747–758. 10.1093/bja/aen094 [DOI] [PubMed] [Google Scholar]
- 30.Smith M, Haythirnthwaite J. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. 10.1016/S1087-0792(03)00044-3 [DOI] [PubMed] [Google Scholar]
- 31.Khalid I, Roehrs T, Hudgel D, Roth T. Continuous positive airway pressure in severe obstructive sleep apnea reduces pain sensitivity. Sleep. 2011;34(12):1687–1691. 10.5665/sleep.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onen S, Onen F, Albrand G, Decullier E, Chapuis F, Dubray C. Pain tolerance and obstructive sleep apnea in the elderly. J Am Med Dir Assoc. 2010;11(9):612–616. 10.1016/j.jamda.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 33.Mason M, Cates CJ, Smith I. Effects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2015:CD011090. [DOI] [PubMed] [Google Scholar]
- 34.Peppard EP, Young T, Barnet J, Palta M, Hagen E, Hla K. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohnert ASB, Blow FC, Bair MJ. Opioid overdose-related deaths—reply.. JAMA. 2011;306(4):380–381. 10.1001/jama.2011.1039 [DOI] [PubMed] [Google Scholar]
- 36.Levitt E, Abdala A, Paton J, Bissonnette J, Williams J. μ Opioid receptor activation hyperpolarizes respiratory‐controlling Kölliker–Fuse neurons and suppresses post‐inspiratory drive. J Physiol. 2015;593(19):4453–4469. 10.1113/JP270822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarrega J, Guell R, Anton A, et al. Are daytime arterial blood gases a good reflection of nighttime gas exchange in patients on long-term oxygen therapy? Respir Care. 2002;47(8):882–886. [PubMed] [Google Scholar]
- 38.Montandon G, Cushing S, Campbell F, Propst E, Horner R, Narang I. Distinct cortical signatures associated with sedation and respiratory rate depression by morphine in a pediatric population. Anesthesiology. 2016;125(5):889–903. 10.1097/ALN.0000000000001303 [DOI] [PubMed] [Google Scholar]
- 39.Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68–78. 10.1093/pm/pnx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry RB, Budhiraja R, Gottlieb D, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


