Abstract
Study Objectives:
The objective of this study was to describe the feasibility, acceptability, and preliminary efficacy of a novel Sleep Intervention for Kids and Parents (SKIP). Parent and child primary sleep outcomes were total sleep time, wake after sleep onset (WASO), sleep efficiency (SE), and bedtime range.
Methods:
Children 6–11 years of age with asthma and 1 parent, both with behavioral sleep disturbance, enrolled in this single-group pilot. The 8-week shared management intervention included weekly online educational modules, goal setting, and progress reporting. Feasibility was measured by the number of dyads who were eligible, enrolled, and retained. Acceptability was measured by survey and semistructured interview. Total sleep time, WASO, SE, and bedtime range were measured by actigraphy at baseline, after the intervention, and 12-week follow-up. Mixed-effects regression models were used to determine change in sleep outcomes from baseline.
Results:
Thirty-three of 39 eligible dyads enrolled; of 29 dyads that started the intervention, 25 (86%) completed all study visits. SKIP was acceptable for 61% of children and 92% of parents. Compared with baseline, at follow-up, children had significantly improved WASO (−37 minutes; 95% confidence interval [CI], −44.5 to −29.7; P < .001), SE (5.4%; 95% CI, 4.2–6.5; P < .001), and bedtime range (−35.2 minutes; 95% CI, −42.9 to −27.5; P < .001). Parents also had significantly improved WASO (−13.9 minutes; 95% CI, −19.5 to −8.2; P < .001), SE (2.7%; 95% CI, 1.7–.7; P < .001), and bedtime range (−35.3 minutes; 95% CI, −51.0 to −19.7; P < .001).
Conclusions:
SKIP was feasible, acceptable, and we observed improved child and parent sleep outcomes except total sleep time. Following refinements, further testing of SKIP in a controlled clinical trial is warranted.
Clinical Trial Registration: Registry: ClinicalTrials.gov; Name: Sleep Intervention for Kids and Parents: A Self-Management Pilot Study; URL: https://www.clinicaltrials.gov/ct2/show/study/NCT03144531; Identifier: NCT03144531.
Citation:
Sonney JT, Thompson HJ, Landis CA, et al. Sleep intervention for children with asthma and their parents (SKIP Study): a novel web-based shared management pilot study. J Clin Sleep Med. 2020;16(6):925–936.
Keywords: asthma, bedtime routine, behavioral sleep intervention, school-age children, shared management, sleep disturbance, sleep hygiene
BRIEF SUMMARY
Current Knowledge/Study Rationale: School-age children with asthma and their parents experience behavioral sleep disturbance, yet no known intervention addresses this problem for both parents and children. This study examined the feasibility, acceptability, and preliminary efficacy of the Sleep Intervention for Kids and Parents, a novel shared management intervention for school-age children with asthma and their parents.
Study Impact: Findings from this study indicate that Sleep Intervention for Kids and Parents was feasible, acceptable, and potentially efficacious for children with asthma and their parents. Sleep Intervention for Kids and Parents holds promise as a scalable intervention that engages parents and children together as a team to improve sleep, and a larger, controlled clinical trial is warranted.
INTRODUCTION
Asthma is one of the most common chronic conditions of childhood, affecting more than 6 million children in the United States.1 Symptoms of asthma include cough, wheeze, shortness of breath, activity intolerance, and nighttime sleep disturbance, particularly among children with poor asthma control.2,3 An estimated 30–40% of children with asthma experience sleep disturbances in comparison to 20–30% of their healthy peers.4–6 The most common behavioral sleep disturbances among children with asthma include bedtime resistance and variability, prolonged night awakenings, and sleep-related anxiety.5,7 Consequences of behavioral sleep disturbances include impaired daytime functioning, such as daytime sleepiness, difficulties with self-regulation and problem solving, and poorer health outcomes, including quality of life and asthma morbidity.6,8–10 Compounding this problem is the feedback loop between sleep and asthma whereby worsened asthma morbidity impairs sleep, which worsens asthma status even further.2–4,11
Parent sleep is also affected: an estimated 20–40% of parents of children with asthma experience an inadequate amount of sleep and/or poor quality sleep in comparison to 10% of parents of healthy children.2 Although some parent sleep disturbances are attributed to frequent awakenings to attend to their child’s nighttime needs, parents may also experience bedtime variability and sleep-related anxiety caused by either their child’s asthma and/or the related child sleep problems.12–15 Inadequate amount of sleep and/or poor-quality sleep in parents has been associated with poorer quality of life, decreased work productivity, stress, depression, and anxiety.16,17
Although common, behavioral sleep disturbances are also modifiable.7,18–21 Customary behavioral sleep intervention components include stimulus control (eg, reserving bed for sleep only) and sleep hygiene, including reducing light exposure, consistent bedtime and wake time, relaxation, and establishing a consistent bedtime routine.18–24 Interestingly, online behavioral sleep intervention approaches have shown similar efficacy to more traditional in-person and/or group formats in children and adults, with the added benefit of improved accessibility and scalability.6,7,19,25 Despite the prevalence of behavioral sleep disturbances among both children with asthma and their parents, to our knowledge, no interventions jointly target parent and child together. To address this gap, we developed the Sleep Intervention for Kids and Parents (SKIP), a web-based, shared management intervention for 6- to 11-year-old children with asthma and their parents with behavioral sleep disturbances.
The SKIP intervention targets both children and parents because the school-age years are a critical time when children begin sharing responsibility for their health with their parents and establish life-long health behaviors.26,27 The SKIP intervention was guided by the Common Sense Model of Parent-Child Shared Regulation (CSM-PC)26 that postulates parents and children have unique interpretations of health scenarios, and the way in which they merge these individual interpretations will determine how they share management of their health. The parent-child dyad’s appraisal of their actions will influence future health management decisions, either by reinforcing or prompting revision of future actions. As such, shared management interventions that engage both parent and child as equal participants to improve sleep hold promise as a potentially sustainable strategy for improving sleep. Unfortunately, no known interventions specifically target parent-child shared management of behavioral sleep disturbance.7,20,21
Given the prevalence of sleep disturbances among children with asthma and their parents, this study sought to (1) describe the feasibility and acceptability of SKIP and (2) explore changes in sleep outcomes, including total sleep time (TST), wake after sleep onset (WASO), sleep efficiency (SE), and bedtime range as measured by actigraphy both in children and in one of their parents. Outcome variables were measured at baseline, immediately after the 8-week intervention, and 12 weeks after intervention.
METHODS
This study used a prospective single group design to determine the feasibility, acceptability, and preliminary efficacy of the SKIP intervention. The SKIP website was used both for delivering the intervention and for data collection during the intervention. The University of Washington Institutional Review Board approved this study.
Participants
Study recruitment efforts targeted parents of children 6–11 years of age with asthma. Recruitment flyers were distributed from May 2017 to November 2017 through social media postings and study flyers distributed in pediatric practices and by school nurses within 3 counties in western Washington. Interested parents were directed to the SKIP study website, which described the study as aiming to understand and improve sleep in children with asthma and their parents. Child eligibility included the following: (1) age 6–11 years; (2) persistent asthma, defined as a prescription for daily asthma medication; (3) able to speak and understand English; and (4) presence of a sleep disturbance defined as a score of greater than 41 on the Child Sleep Habits Questionnaire (CSHQ).28 Children were excluded if their parents reported (1) developmental delay, (2) a chronic health condition that would interfere with intervention participation (traumatic brain injury, autism spectrum disorder, attention deficit hyperactivity disorder, cancer), (3) a diagnosed sleep disorder (eg, obstructive sleep apnea) and/or a score above the cutoff point on the Sleep-Related Breathing Disorder Subscale of the Pediatric Sleep Questionnaire (SRBD/PSQ),29 or (4) current use of sleep medication (eg, melatonin). Parent eligibility included the following: (1) 18 years or older; (2) able to speak and read English; (3) reside with the child at least 50% of the time; (4) legal guardian for the child; (5) have reliable internet access; and (6) presence of sleep disturbance defined as a score of 5 or greater on the Pittsburgh Sleep Quality Index (PSQI).30 Parents were excluded if (1) they self-reported a diagnosed sleep disorder (eg, obstructive sleep apnea), (2) they were a current night shift worker, or (3) if they had current sleep medication use. Both parent and child needed to meet eligibility criteria to enroll in the study. If families had more than 1 eligible child, the child with the higher sleep deficiency score was included in the study. No exclusions based on family structure were made.
Procedures
Screening
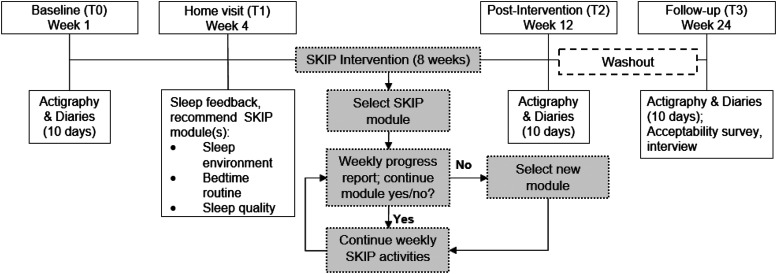
Parents completed eligibility screening surveys on behalf of their child and themselves online via the SKIP study website. Surveys included the PSQI, CSHQ, and SRBD/PSQ.28–30 Adults were screened as eligible if PSQI was greater than 5.30 Children were eligible if CSHQ was greater than 41 and SRBD/PSQ was less than 0.33.28,29 Through automated survey scoring, parents were notified if they (both parent and child) were eligible for the study; those who were eligible were asked to indicate whether they were interested in learning more about the study. Among those who expressed interest in the study, a research team member called the parent and used an approved script and answered questions about the study. For those who agreed to participate in the study, the baseline data collection session was scheduled (T0) at which time written informed consent from the parent and assent from the child were completed. Figure 1 depicts the study protocol beginning with baseline.
Figure 1. SKIP study protocol.
Enrollment
After consent and assent were obtained, each child and parent were given an actiwatch to wear on the nondominant wrist and a sleep diary to complete each day. Participants were instructed on use of the watch and how to complete the diary each morning and each evening for 10 days. At the end of 10 days, the actiwatches and diaries were returned to investigators by mail. Study personnel scored the actigraphy data and then scheduled a home visit (T1) within 2 weeks.
Intervention protocol
During the T1 visit, dyads were given written and verbal feedback on their baseline actigraphy data by the principal investigator, a licensed pediatric nurse practitioner, including total sleep time, nighttime awakenings, sleep efficiency, and bedtime range. Based on this assessment, 1 or more of the SKIP modules (bedtime routine, sleep environment, sleep quality) were recommended. To begin the intervention, participants were provided a link to the SKIP intervention website and prompted to select 1 module. Assessments immediately after the intervention and at 12-week follow-up included both actigraphy and sleep diaries. Additionally, at the 12-week follow-up assessment, participants completed a brief online survey (individually) and semistructured interview (parent and child together) to assess the acceptability of the intervention and to identify areas for future intervention refinement.
Intervention
Theoretical underpinning
SKIP was theoretically derived from both the CSM-PC and social cognitive theory.26,31 The CSM-PC emphasizes that the management of a child’s health is not assumed by the individual but rather shared by parent and child.26 Parent-child shared management is a dynamic process, given that parent and child health management responsibilities constantly evolve as the child matures. A key feature of the CSM-PC is appraisal of health management actions. The parent and child’s appraisal of their actions will lead to either reinforced or revised actions in the future.26 As such, SKIP engaged both the parent and the child as equal participants who worked together to select sleep improvement activities as a team and later appraise their activities together. Social cognitive theory stresses that goal setting, action planning, and self-monitoring are important motivational processes.31 To support sleep improvement, participants were prompted to select weekly evidence-based sleep improvement goals. This approach allowed each parent-child dyad to tailor the intervention to their own needs and priorities. Consistent with social cognitive theory, after selecting goals, participants were prompted to anticipate barriers and problem solve those barriers together (action planning). Each subsequent week, participants were also prompted to evaluate their progress together (self-monitoring).
SKIP development
SKIP was developed iteratively in consultation with experts in clinical sleep medicine (MLC), sleep science (TMW, CAL), family-centered behavior change (MMG), and informatics (HT). SKIP drew on study team expertise and prior work, national guidelines, and extant literature related to behavioral interventions for insomnia in children. Collectively, these resources highlighted the importance of bedtime routines, optimizing the sleep environment through decreased light and media exposure, and sleep hygiene.8,9,24,32 We also incorporated elements of Cognitive Behavioral Therapy for Insomnia (CBT-I), including relaxation techniques (breathing and progressive muscle relaxation) and stimulus control (bed only for sleeping, regular wake time, avoiding naps).19,22,33
SKIP module process and content
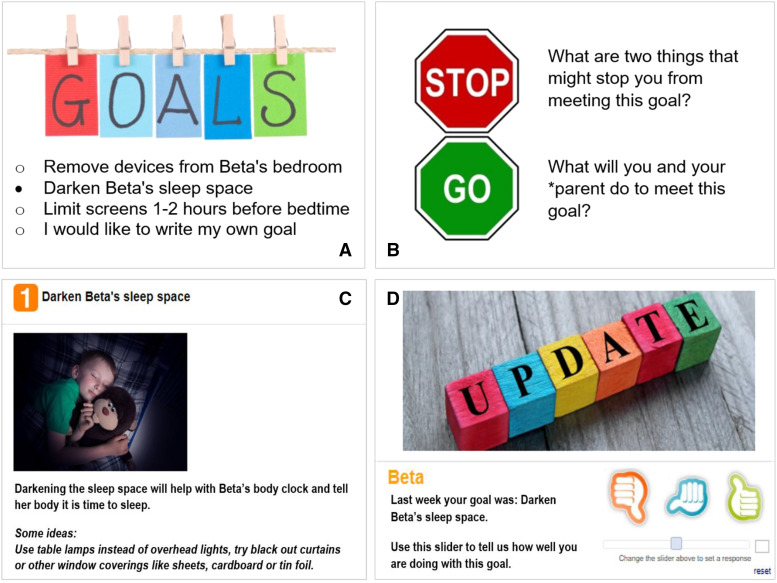
SKIP was organized into 3 online modules with a focus on the sleep environment, bedtime,routine and sleep quality. Each 30-minute module began with approximately 5–7 minutes of educational content and guided dyads through submitting module-related goals. In each module, upon selection of a specific goal, the SKIP website would provide short tips for success. For example, if darkening the sleep space was selected, the website recommended use of table lamps instead of overhead lights, as well as darkening the room with window coverings such as blackout curtains, sheets, cardboard, or foil (Figure 2). Next, participants were asked to anticipate barriers to achieving their selected goals and to problem solve how to address these anticipated barriers together. During intervention weeks 2–8, participants also completed progress reports on their selected goals (Figure 2).
Figure 2. SKIP module progression.
SKIP sleep environment module exemplar: (A) goal setting; (B) anticipating barriers and problem solving; (C) goal selection summary and tips; (D) progress report on prior week’s goal.
The sleep environment module focused on preventing melatonin disruption by limiting media and light exposure before bed and creating a darkened sleep space.22,24,32 Evidence-based goals included removing electronic devices from the bedroom, reducing electronic media use before bed, and dimming lights before bedtime.22,32 The bedtime routine module focused on establishing and implementing a consistent bedtime routine.6,8,9,12,18 A template was provided for participants to build their personalized bedtime routine, including activities they must do to prepare for bed (ie brush teeth, put on pajamas) and activities that help them relax (ie read, quiet music). Bedtime routine goals focused on the implementation process of the bedtime routine, ranging from trialing (and possibly revising) a new bedtime routine, increasing the number of nights the bedtime routine is used, and choosing consistent time to start the routine. The sleep quality module focused on both sleep hygiene and sleep quantity. Sleep quality goals included establishing a consistent bedtime and/or wake time, use of bed for sleep only, and bedtime relaxation (progressive relaxation, belly breathing).12,19,22,23,34,35 Goals related to sleep quantity were based on the National Sleep Foundation’s age-specific sleep duration recommendations.36
SKIP delivery procedures
REDCap, a secure, web-based software platform designed to support data capture and management for research studies, was used both for intervention delivery and data collection.37 To access SKIP, REDCap generated personalized weblinks that were emailed to parents, which ensured that children did not access the internet without parental supervision. Parent-child dyads were instructed to access SKIP weblink together. SKIP module content and activities were organized into a series of REDCap surveys assigned to intervention-specific events (intervention weeks 1–8). Each week, through a series of branching and if/then syntax, the website would display the content for whichever module the participants selected. After participants submitted their weekly activities, REDCap would generate an automated email reminder 7 days later with a link to complete the following week’s activities. Should the dyad not complete the SKIP activities, daily email reminders were automatically sent to parents. Additional REDCap features used to promote dyad engagement included “cross-event piping” to build a printable summary of each week’s goals and tips for success, display goals from the prior week within the progress report surveys, and personalize SKIP content with participant names.35
Measures
Demographics
Parents completed a survey including information both about parent and child demographics. Parent characteristics on the survey included sex, education, race, and ethnicity. Child characteristics on the survey included age, sex, grade level, race, ethnicity, insurance coverage, and age at asthma diagnosis.
Childhood Asthma Control Test38
The children in this study all had an established asthma diagnosis and had a prescription for daily controller medication. As such, asthma control (not severity) was assessed, which aligns with the National Heart, Lung, and Blood Institute asthma guidelines.3 The Childhood Asthma Control Test is a 7-item questionnaire using both child and parent report to classify asthma control.38 Children complete 4 items using a 4-point scale while parents independently complete 3 items using a 6-point scale. The summed scores range from 0 to 27, with higher scores indicating better asthma control. A score of ≥20 indicates well-controlled asthma, 13–19 indicates not well-controlled, and ≤12 indicates poorly controlled.38 The Childhood Asthma Control Test has established validity and reliability in children ages 4–11 years and their parent.38 Cronbach’s α for the present study was α = 0.79.
SKIP feasibility and acceptability
Feasibility was measured by the number of parent-child dyads who were eligible, enrolled, and retained. Acceptability was measured by questionnaire and semistructured interview at the 12-week follow-up visit. Participants individually completed a 9-item investigator-developed SKIP Acceptability Questionnaire to rate the ease of completing electronic surveys, sleep diaries, actigraphy, weekly SKIP intervention activities, and overall study acceptability. Survey items were rated on a 1–5 point scale, with 1 = strongly disagree and 5 = strongly agree. Next, parent-child dyads participated in a semistructured interview related to SKIP acceptability. Nine semistructured interview questions were used to guide the interview (Figure 3). Specifically, participants were asked about what they liked about the SKIP intervention, what they would like to change, and for any additional feedback.
Figure 3. SKIP study postintervention semistructured interview questions.
Sleep diary and actigraphy
Participants were asked to keep a bedtime and waketime paper and pencil sleep diary during each time point. Sleep diaries were used to record time to bed, sleep onset, awakenings, wake time, and time out of bed. Paper, rather than electronic, sleep diaries were used to avoid requiring study participants to use electronic devices to complete sleep diaries before bed. Concurrently, participants wore an actiwatch Spectrum or Pro (Philips Respironics, Inc, Bend, OR) on their nondominant wrist for 10 days during each time point (baseline T0, immediately after intervention T2, and 12 weeks after intervention T3). Data were collected in 1-minute epochs and scored using Actiware software version 6.0.9 (Philips Respironics) with each minute scored as either wake or sleep using the medium sensitivity threshold.39
Actigraphy data were visually inspected in conjunction with the sleep diary and screened for artifacts before scoring. All actigrams were independently scored by the principal investigator (JS) and a research assistant following a standard protocol.39 Actigraphy data were excluded if the participant did not have a minimum of 4 days of data at each time point. The actigraphy parameters of interest included the following: TST (number of minutes asleep from sleep onset to sleep offset), WASO (the total time spent awake during the sleep interval), SE (ratio of time asleep divided by time in bed), and bedtime range (range of bedtimes in minutes during the 10 days the actiwatches were worn).
Analysis
Deidentified quantitative data were exported from REDCap and Philips Actiware. Descriptive statistics were used to summarize participant baseline characteristics (demographics and child asthma control) and weekly module selections. Study feasibility (aim 1) was measured by examining the number of parents (and children) who were screened for eligibility, enrolled, and completed the intervention (Figure 4). SKIP acceptability (aim 1) was measured by the percent of participants who rated acceptability questionnaire items as agree or strongly agree and thematic analysis of semistructured interviews. Thematic analysis involved the following phases: familiarization, initial code generation, searching for themes, reviewing themes, defining and naming themes, and reporting themes.40,41 Data trustworthiness (credibility, dependability, confirmability) was ensured through the use of member checking throughout semistructured interviews, data triangulation, researcher triangulation, and audit trails. Following immersion in the data, the principal investigator generated initial codes that were then triangulated with another study team member.40,41 Next, a list of codes was generated and grouped into preliminary themes, which were then reviewed and refined with the study team. Finally, themes were defined and named.
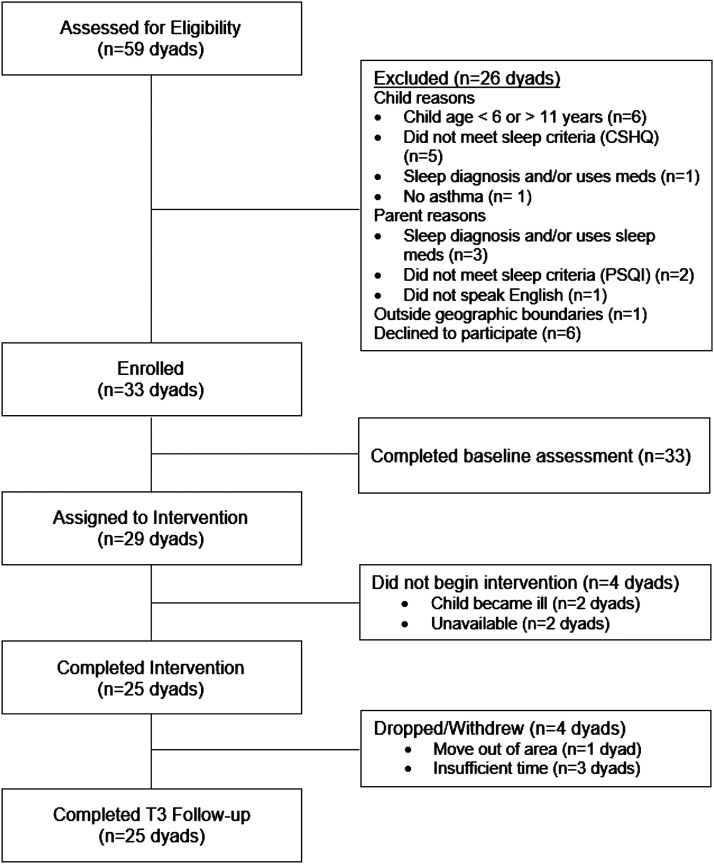
Figure 4. SKIP study flow.
Preliminary efficacy of SKIP (aim 2) on parent and child actigraphic sleep outcomes (TST, WASO, SE, and bedtime range) were assessed using longitudinal mixed-effects regression models. Additionally, we calculated the coefficient of variance, calculated as the standard deviation of bedtime divided by mean bedtime.42 All models included time as a fixed effect (in weeks), treated as a categorical variable. The models also included random intercepts for each participant to account for the dependence of sleep outcome scores across time. Conclusions about preliminary efficacy were based on comparisons of outcomes estimated with appropriate contrasts from the longitudinal model. All hypothesis tests were two-tailed with P < .05 considered significant. No adjustment was made for multiple comparisons. The Statistical Package for the Social Sciences (SPSS) software version 19 (IBM Corp, Armonk, NY) was used for descriptive analyses. Stata statistical software version 15 (StataCorp, College Station, TX) was used for all mixed effects regression analyses.
RESULTS
Table 1 shows the baseline characteristics of the sample (n = 25 parents and children). In brief, the mean age of the children was 8.3 ± 1.7 years; 52% had not well-controlled or poorly controlled asthma. All children had insurance for routine and emergency care, and 96% had insurance for prescription medications. Most of the participant parents were mothers. Most dyads (n = 12) engaged with all 3 modules, 8 dyads selected just 2 modules, and 5 spent the entire 8 weeks on 1 module. Across the study sample, the most time was spent on sleep quality (mean = 2.9 ± 2.1), followed by bedtime routine (mean = 2.6 ± 2.3) and sleep environment (mean = 2.4 ± 2.2).
Table 1.
Demographic characteristics of the sample.
| Parent | n (%) | Child | n (%) |
|---|---|---|---|
| Sex | Sex | ||
| Female | 24 (96%) | Female | 12 (48%) |
| Male | 1 (4%) | Male | 13 (52%) |
| Race | Race | ||
| American Indian or Alaska Native | 1 (4%) | Asian | 1 (4%) |
| Asian | 2 (8%) | Black or African American | 4 (16%) |
| Black or African American | 2 (8%) | Caucasian or white | 19 (76%) |
| Caucasian or white | 19 (76%) | Unknown | 1 (4%) |
| Prefer not to say | 1 (4%) | ||
| Ethnicity | Ethnicity | ||
| Hispanic or Latino | 2 (8%) | Hispanic or Latino | 5 (20%) |
| Not Hispanic or Latino | 21 (84%) | Not Hispanic or Latino | 19 (76%) |
| Prefer not to say | 2 (8%) | Prefer not to say | 1 (4%) |
| Education (highest completed) | Education (current grade) | ||
| High school or GED | 3 (12%) | Kindergarten | 7 (28%) |
| Some college, no degree | 4 (16%) | First grade | 4 (16%) |
| Associate degree or vocational | 2 (8%) | Second grade | 5 (20%) |
| Bachelor’s degree | 8 (32%) | Third grade | 2 (8%) |
| Master’s degree | 7 (28%) | Fourth grade | 5 (20%) |
| Doctoral degree | 1 (4%) | Fifth grade | 2 (8%) |
| Screening PSQI | M = 7.0 ± 2.0 | ||
| Screening CSHQ | M = 53.4 ± 8.2 | ||
| Screening SRBD/PSQ | M = 0.23 ± 0.09 |
CSHQ = Child Sleep Habits Questionnaire, M = mean, PSQI = Pittsburgh Sleep Quality Index, SRBD/PSQ = Sleep Related Breathing Disorder Subscale of the Pediatric Sleep Questionnaire.
Feasibility
Fifty-nine parents completed the online SKIP eligibility screening on behalf of themselves and their child (Figure 2), with 66% of dyads eligible. Of the 20 dyads who were excluded, 13 were excluded for child reasons (child outside 6–11 years, did not meet sleep criteria, had a sleep diagnosis and/or current use of sleep medication, or did not have asthma), 6 for parent reasons (sleep diagnosis and/or current use of sleep medication, did not meet sleep criteria, or did not speak English), and 1 for being outside of the geographic boundaries of the study. Thirty-three of 39 eligible dyads enrolled in the study and completed baseline measures (85% participation rate). Of 29 dyads that started the intervention, 25 (86%) completed the intervention and all study visits.
Acceptability
Sixty-one percent of children and 92% of parents reported overall satisfaction (agree, strongly agree) with the intervention (Table 2). Thematic analysis of the SKIP semistructured interviews resulted in 3 overall themes: user engagement, accessibility and usability, and burdensome experiences.
Table 2.
SKIP acceptability questionnaire scores.
| Item | Child (n = 23), n (%) | Parent (n = 25), n (%) |
|---|---|---|
| SKIP intervention satisfaction | 14 (61) | 23 (92) |
| SKIP website ease of use | 16 (70) | 21 (84) |
| SKIP activities were helpful and made sense | 14 (61) | 22 (88) |
| SKIP activities were long enough | 16 (70) | 23 (92) |
| There were enough SKIP activities | 16 (70) | 22 (88) |
| I completed all SKIP activities | 15 (65) | 20 (80) |
| Made changes to improve my sleep | 16 (70) | 22 (88) |
| I am confident these changes will lasta | 14 (61) | 20 (87)a |
| Recommend SKIP to others | 13 (57) | 24 (96) |
Number of participants rating acceptability items as agree or strongly agree. aAdditional item response option of either “does not apply to me or not needed”; such scores were excluded percentage calculations (n = 23).
User engagement and success
User engagement and success reflects that users found SKIP valuable and a worthwhile use of their time that resulted in improved sleep. User engagement included the following subthemes: teamwork, flexibility to focus on priorities, and achievable goals and results. Working together as a team was beneficial. “[it is] hard to change a kid’s behavior. Working as a team was a good strategy for us. I just didn’t expect to get better too!” (parent). Dyads also liked having the flexibility to focus on their own priorities. “We liked picking our own goals; focus on what we want to.” (child). Similarly, selecting from achievable goals was motivating, “Yeah, and I liked the specific goals and how [the website] broke it down so that it was easy to do. Made us want to keep going.” (parent). Not only were the goals achievable, but they led to improved sleep. “It was pretty easy to learn to sleep better” (child). All of the participants reported that they would continue with the changes they made to their sleep.
Accessibility and usability
Although participants reported that SKIP was accessible and easy to use, they also had recommendations for improvement. One child shared that “The emails helped my mom, she didn’t need to find the website on her own, she just clicked the link or I helped her,” whereas his mother added “Yeah, it really was simple, easy, and fast.” Although the website was accessible, many participants would prefer an app. One parent shared that “the website was ok, but text or app might be easier. It could send reminders instead of emails.” Overall, one third of participants reported liking the website format, whereas 14 suggested an app format might be easier to use and improve accessibility. Additionally, the child participants did like that SKIP was designed with them in mind, “I liked that there were videos and pictures instead of just words. It was colorful too.” (child). In fact, participants suggested more audio and video content be added to the modules.
Balancing routines and burden
The final theme reflects that participants appreciated that SKIP sought to build new sleep routines, yet they had suggestions for reducing some of burdensome aspects of SKIP. They found that SKIP’s approach of weekly check-ins to build routine was useful to stay focused and to do things that work. One child reported “the weekly questions were fun, not hard. I liked the pictures that would show up when I met my goal.” Similarly, the parent shared that checking in weekly “made me think about my goal, and made me stay on top of it. The approach worked for us—consistent and reporting back.” Some participants, however, found the weekly check-ins burdensome. “It would be nice to find [a] way to carry prior goals forward and add option to repeat same goals as last week. I didn’t love having to fill in the information again if I was sticking with the same goal” (parent). Also, “there was a lot of repetition with the website. I think it is because the modules were built as surveys, but it could be more streamlined.” (parent). Similarly, approximately half of the participants reported that the 8-week intervention duration felt “just right,” but others felt it was either too long or too short. One child summed this up perfectly by stating, “everybody is different, so why don’t we get to choose when we stop?”
Sleep outcomes
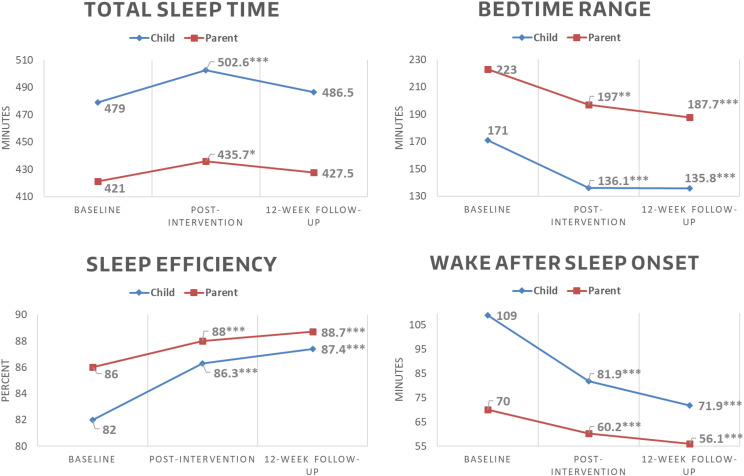
Compared with baseline, children in SKIP had significantly improved sleep outcomes (TST, WASO, SE, bedtime range) except for bedtime coefficient of variability immediately after the intervention (Table 3). These significant trends were maintained at the 12-week postintervention follow-up for WASO, SE and bedtime range, whereas TST was unchanged from baseline (Figure 5). Parents in SKIP had similar results, with significantly improved sleep outcomes immediately postintervention (TST, WASO, SE, bedtime range) except for bedtime coefficient of variation. These significant trends were maintained at the 12-week postintervention follow-up for SE, WASO, and bedtime range, but TST was unchanged from baseline. Interestingly, parent bedtime coefficient of variability was significantly improved at follow-up compared with baseline.
Table 3.
Change in outcome variables at each SKIP time point.
| Outcome | Baseline Mean (SD) | Postintervention Δ from Baseline (95% CI) | d | 12-Week Follow-Up Δ from Baseline (95% CI) | d | |
|---|---|---|---|---|---|---|
| Child | Sleep time (min) | 479 (74) | 26.6 (15.9 to 37.4)c | 0.31 | 7.5 (−3.5 to 18.5) | 0.12 |
| WASO (min) | 109 (64) | −27.1 (−34.2 to −19.8)c | 0.72 | −37.1 (−44.5 to −29.7)c | 1.01 | |
| Sleep efficiency (%) | 82 (10) | 4.3 (3.2 to 5.5)c | 0.69 | 5.4 (4.2 to 6.5)c | 0.94 | |
| Bedtime range (min) | 171 (104) | −34.9 (−42.5 to −27.4)c | 0.37 | −35.2 (−42.9 to −27.5)c | 0.47 | |
| Bedtime CV | 0.12 (0.18) | 0.01 (−0.02 to 0.03) | 0.12 | −0.02 (−0.05 to 0.00) | 0.12 | |
| Parent | Sleep time (min) | 421 (78) | 14.7 (1.3 to 28.1)a | 0.32 | 6.5 (−7.5 to 20.4) | 0.10 |
| WASO (min) | 70 (40) | −9.8 (−15.3 to −4.4)c | 0.45 | −13.9 (−19.5 to −8.2)c | 0.68 | |
| Sleep efficiency (%) | 86 (7) | 2.0 (1.0 to 7.9)c | 0.51 | 2.7 (1.7 to 3.7)c | 0.74 | |
| Bedtime range (min) | 223 (168) | −26.0 (−40.8 to −11.2)b | 0.07 | −35.3 (−51.0 to −19.7)c | 0.60 | |
| Bedtime CV | 0.33 (0.33) | 0.00 (−0.05 to 0.05) | 0.00 | −0.20 (−0.25 to −0.15)c | 0.61 |
Effect size magnitude: 0.2 = small, 0.5 = moderate, 0.8 = large. aP < .05; bP < .01, cP < .001. CI = confidence interval, CV = coefficient of variation, d = Cohen’s d, SD = standard deviation, WASO = wake after sleep onset, Δ = change.
Figure 5. SKIP intervention preliminary efficacy by outcome variable.
*P < .05; **P < .01; ***P < .001.
DISCUSSION
The present study evaluated the feasibility, acceptability, and preliminary efficacy of SKIP in school-age children with asthma and sleep disturbance and their parents also with sleep disturbance. Findings from this pilot study indicate that SKIP was feasible and acceptable, and we observed improvements in sleep outcomes for child and parent participants, with lasting effects in WASO, SE, and bedtime range at the 12-week follow-up.
Feasibility
Participant eligibility (66% of dyads screened), enrollment (85% of eligible dyads), and retention (86% of dyads who started intervention) provide evidence of SKIP feasibility. We used a variety of recruitment strategies, including online social media advertisements and flyers in pediatric offices and distributed by school nurses. Our most fruitful recruitment strategy was partnering with school nurses to distribute study flyers. Our online eligibility screening and automated scoring was simple and easy to use for both participants and study personnel. Four dyads withdrew from the intervention (14% attrition), which is consistent with other studies of children with chronic conditions.34
Acceptability
Postintervention interviews and surveys indicated that SKIP was acceptable, with 61% of children and 92% of parents indicating overall satisfaction with the intervention. Themes that emerged from the semistructured interviews (user engagement, accessibility and usability, and burdensome experiences) reflect high acceptability among parents and children. Users found SKIP to be engaging, with flexibility in setting sleep priorities, facilitating teamwork, and providing achievable goals. The most frequent suggestions for improvement were to make the SKIP intervention duration tailored, such that participants could choose when they were finished, and to transition SKIP to an app-based platform to improve accessibility and convenience. Although group and internet-delivered CBT-I has been effective in adults and children with insomnia, to our knowledge, SKIP was one of the first interventions to engage parent-child dyads in sleep shared management using a web-based platform.19
Preliminary efficacy
Child and parent participants demonstrated significant improvements in all objectively measured sleep outcomes immediately postintervention (TST, WASO, SE, and bedtime range). These significant improvements were retained at the 12-week follow-up for WASO, SE, and bedtime range. Effect sizes were large for child WASO and SE and moderate for parent WASO, SE, and bedtime range. In comparison, Schlarb et al23 evaluated a CBT-I intervention for school-age children (5–10 years) using actigraphic outcome measures. At the 12-month follow-up, effect sizes were small for nighttime awakenings and TST and large for SE. For the present study, the improvements in parent and child sleep outcomes provide evidence that a web-based delivery of a sleep intervention that combines sleep guidelines and selected CBT-I components may be a cost-effective alternative to more expensive and intensive in-person sessions. The web format may also be a scalable and sustainable alternative to CBT-I, particularly if the intervention procedures were to incorporate web conferencing study visits (rather than in person) and mailed actiwatches, a successful strategy that has been used by members of the study team.
We drew on the CSM-PC to engage both parent and child as equal participants who worked together as a team to improve sleep.26 Few behavioral interventions in respiratory conditions use theoretical underpinning, yet those that do often demonstrate efficacy.43,44 Given the co-occurrence of sleep disturbances in children with chronic conditions and their parents and increasing calls for family-based sleep interventions, a shared management intervention that showed preliminary efficacy, such as SKIP, holds considerable promise as a potential addition to the clinical care of children with asthma and their parents.2,9,45
Disentangling the relationship between sleep disturbance and asthma is complex. Sleep disturbance has long been considered an outcome of asthma control.2–4,11,46 Yet, there is growing evidence that there may be a bidirectional relationship between sleep disturbance and asthma.2,10,46,47 Prior studies suggest that sleep disturbance may worsen asthma status, although the exact mechanism is not clear. Examining a relationship between sleep disturbance and asthma control was beyond the scope of the present study, although future studies are warranted to explore a potential bidirectional relationship.
Limitations and strengths
This pilot study has limitations. First, the study did not have a comparison group, although there is evidence that behavioral sleep disturbances rarely spontaneously resolve.8 Second, the sample was quite small and predominantly white and college educated, although the sample demographics are largely representative of the western Washington area. Third, there is also the potential that self-report and social desirability biases may have exaggerated the effects observed. Despite these limitations, this study has numerous strengths. SKIP was a novel, theoretically based intervention. By engaging parent-child dyads in shared management, our study participants were able to tailor the intervention to their own needs and priorities. The use of objective sleep outcome measures (TST, WASO, SE, and bedtime range) is an important strength countering potential self-report bias. Additionally, SKIP was easily accessible and used a single web-based platform (REDCap) both to deliver the intervention and collect study data.
CONCLUSIONS
The present study has some important implications. A web-based, shared management intervention designed both for a child and a parent was feasible, acceptable, and potentially efficacious at improving both child and parent sleep outcomes (WASO, SE, bedtime range). Further testing the SKIP intervention, integrating participant suggestions, in a larger, controlled clinical trial is warranted. Parents and children often disagree on reports of sleep and asthma symptoms48–50; therefore, studies that use objective and self-report measures both from parents and from their children are recommended. Children eventually grow into adults; therefore, their inclusion in health interventions holds promise not only at improving their present health but also at establishing lifelong healthy sleep behaviors.
DISCLOSURE STATEMENT
All authors have seen and approved of this manuscript. This study was supported by University of Washington Center for Innovation in Sleep Self-Management Grant NIH/NINR P30 NR016585, National Center For Advancing Translational Sciences of the National Institutes of Health Award KL2 TR002317, and Health Resources and Services Administration of the US Department of Health and Human Services Grant T72MC00007 (University of Washington Pediatric Pulmonary Center). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Government. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Dr Ana Carolina Sauer Liberato for assistance with recruitment and data collection and the Seattle Children’s Research Institute Sleep Health in Preschoolers study team for assistance with participant recruitment.
ABBREVIATIONS
- CBT-I
Cognitive Behavioral Therapy for Insomnia
- CSHQ
Child Sleep Habits Questionnaire
- CSM-PC
Common Sense Model of Parent-Child Shared Regulation
- PSQI
Pittsburgh Sleep Quality Index
- SE
sleep efficiency
- SKIP
Sleep Intervention for Kids and Parents
- SRBD/PSQ
Sleep-Related Breathing Disorder Subscale of the Pediatric Sleep Questionnaire
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC). 2017 National Health Interview Survey (NHIS). Most recent asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed May 25, 2018.
- 2.Meltzer LJ, Pugliese CE. Sleep in young children with asthma and their parents. J Child Health Care. 2017;21(3):301–311. 10.1177/1367493517712064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Heart, Lung, and Blood Institute (NHLBI). National Asthma Education Prevention Program (NAEPP) Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health; 2007. [Google Scholar]
- 4.Li Z, Thompson LA, Gross HE, et al. Longitudinal associations among asthma control, sleep problems, and health-related quality of life in children with asthma: a report from the PROMIS((R)) Pediatric Asthma Study. Sleep Med. 2016;20:41–50. 10.1016/j.sleep.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banasiak NC. Understanding the relationship between asthma and sleep in the pediatric population. J Pediatr Health Care. 2016;30(6):546–550. 10.1016/j.pedhc.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 6.Dewald-Kaufmann J, de Bruin E, Michael G. Cognitive behavioral therapy for insomnia (CBT-i) in school-aged children and adolescents. Sleep Med Clin. 2019;14(2):155–165. 10.1016/j.jsmc.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol. 2014;39(8):932–948. 10.1093/jpepsy/jsu041 [DOI] [PubMed] [Google Scholar]
- 8.Meltzer LJ. Clinical management of behavioral insomnia of childhood: treatment of bedtime problems and night wakings in young children. Behav Sleep Med. 2010;8(3):172–189. 10.1080/15402002.2010.487464 [DOI] [PubMed] [Google Scholar]
- 9.Moore M, Meltzer LJ, Mindell JA. Bedtime problems and night wakings in children. Prim Care. 2008;35(3):569–581. 10.1016/j.pop.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Meltzer LJ, Ullrich M, Szefler SJ. Sleep duration, sleep hygiene, and insomnia in adolescents with asthma. J Allergy Clin Immunol Pract. 2014;2(5):562–569. 10.1016/j.jaip.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Huang IC, Thompson L, et al. The relationships between asthma control, daytime sleepiness, and quality of life among children with asthma: a path analysis. Sleep Med. 2013;14(7):641–647. 10.1016/j.sleep.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez FD, Chen S, Langan SM, et al. Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155(5):556. 10.1001/jamadermatol.2018.5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meltzer LJ, Booster GD. Sleep disturbance in caregivers of children with respiratory and atopic disease. J Pediatr Psychol. 2016;41(6):643–650. 10.1093/jpepsy/jsw016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheezum RR, Parker EA, Sampson NR, et al. Nightwatch: sleep disruption of caregivers of children with asthma in detroit. J Asthma Allergy Educ. 2013;4(5):217–225. 10.1177/2150129713478635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson DA, Meltzer LJ, Zhang T, et al. The influence of psychosocial stressors and socioeconomic status on sleep among caregivers of teenagers with asthma, the Puff City study. Sleep Health. 2018;4(2):141–146. 10.1016/j.sleh.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellin MH, Osteen P, Kub J, et al. Stress and quality of life in urban caregivers of children with poorly controlled asthma: a longitudinal analysis. J Pediatr Health Care. 2015;29(6):536–546. 10.1016/j.pedhc.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sampson NR, Parker EA, Cheezum RR, et al. A life course perspective on stress and health among caregivers of children with asthma in Detroit. Fam Community Health. 2013;36(1):51–62. 10.1097/FCH.0b013e31826d7620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Z, Shi L, Deng M. Efficacy of cognitive behavioral therapy in children and adolescents with insomnia: a systematic review and meta-analysis. Braz J Med Biol Res. 2018;51(6):1–8. 10.1590/1414-431x20187070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Bruin EJ, van Steensel FJ, Meijer AM. Cost-effectiveness of group and internet cognitive behavioral therapy for insomnia in adolescents: results from a randomized controlled trial. Sleep. 2016;39(8):1571–1581. 10.5665/sleep.6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aslund L, Arnberg F, Kanstrup M, Lekander M. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14(11):1937–1947. 10.5664/jcsm.7498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quach J, Hiscock H, Ukoumunne OC, Wake M. A brief sleep intervention improves outcomes in the school entry year: a randomized controlled trial. Pediatrics. 2011;128(4):692–701. 10.1542/peds.2011-0409 [DOI] [PubMed] [Google Scholar]
- 22.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlarb AA, Bihlmaier I, Velten-Schurian K, Poets CF, Hautzinger M. Short- and long-term effects of CBT-I in groups for school-age children suffering from chronic insomnia: the KiSS program. Behav Sleep Med. 2018;16(4):380–397. 10.1080/15402002.2016.1228642 [DOI] [PubMed] [Google Scholar]
- 24. Garrison MM. Sleep health in preschoolers: a randomized controlled trial (SHIP): identification no. NCT02255721. https://www.clinicaltrials.gov/ct2/. Accessed September 12, 2016.
- 25.Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. 10.1016/j.smrv.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 26.Sonney JT, Insel KC. Reformulating the common sense model of self-regulation: toward parent-child shared regulation. Nurs Sci Q. 2016;29(2):154–159. 10.1177/0894318416630091 [DOI] [PubMed] [Google Scholar]
- 27.Kieckhefer GM, Trahms CM. Supporting development of children with chronic conditions: from compliance toward shared management. Pediatr Nurs. 2000;26(4):354–363. [PubMed] [Google Scholar]
- 28.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. 10.1093/sleep/23.8.1d [DOI] [PubMed] [Google Scholar]
- 29.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. 10.1016/S1389-9457(99)00009-X [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 31.Bandura A. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52(1):1–26. 10.1146/annurev.psych.52.1.1 [DOI] [PubMed] [Google Scholar]
- 32.Garrison MM, Liekweg K, Christakis DA. Media use and child sleep: the impact of content, timing, and environment. Pediatrics. 2011;128(1):29–35. 10.1542/peds.2010-3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohayon M, Wickwire EM, Hirshkowitz M, et al. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health. 2017;3(1):6–19. 10.1016/j.sleh.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 34.Karlson CW, Rapoff MA. Attrition in randomized controlled trials for pediatric chronic conditions. J Pediatr Psychol. 2009;34(7):782–793. 10.1093/jpepsy/jsn122 [DOI] [PubMed] [Google Scholar]
- 35.Meischke H, Lozano P, Zhou C, Garrison MM, Christakis D. Engagement in “My Child’s Asthma”, an interactive web-based pediatric asthma management intervention. Int J Med Inform. 2011;80(11):765–774. 10.1016/j.ijmedinf.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Int J Med Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu AH, Zeiger RS, Sorkness CA, et al. The Childhood Asthma Control Test: retrospective determination and clinical validation of a cut point to identify children with very poorly controlled asthma. J Allergy Clin Immunol. 2010;126(2):267–273, e261. [DOI] [PubMed] [Google Scholar]
- 39.Ward TM, Lentz M, Kieckhefer GM, Landis CA. Polysomnography and actigraphy concordance in juvenile idiopathic arthritis, asthma and healthy children. J Sleep Res. 2012;21(1):113–121. 10.1111/j.1365-2869.2011.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lincoln Y, Guba E. Establishing trustworthiness. In: Lincoln Y, Guba E, eds. Naturalistic Inquiry. Beverly Hills, CA; SAGE; 1985:289-331. [Google Scholar]
- 41.Nowell LS, Norris JM, White DE, Moules NJ. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16(1):1–13. 10.1177/1609406917733847 [DOI] [Google Scholar]
- 42.Becker SP, Sidol CA, Van Dyk TR, et al. Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: a systematic review. Sleep Med Rev. 2017;34:94–121. 10.1016/j.smrv.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Durra M, Torio MB, Cafazzo JA. The use of behavior change theory in Internet-based asthma self-management interventions: a systematic review. J Med Internet Res. 2015;17(4):e89. 10.2196/jmir.4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCullough AR, Ryan C, Macindoe C, et al. Behavior change theory, content and delivery of interventions to enhance adherence in chronic respiratory disease: a systematic review. Respir Med. 2016;116:78–84. 10.1016/j.rmed.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 45.Pina LR, Sien SW, Ward T, et al. From personal informatics to family informatics: understanding family practices around health monitoring. Proceedings of the 2017 ACM Conference on Computer Supported Cooperative Work and Social Computing; 2017; Portland, Oregon, USA.. 10.1145/2998181.2998362 [DOI] [Google Scholar]
- 46.van Maanen A, Wijga AH, Gehring U, et al. Sleep in children with asthma: results of the PIAMA study. Eur Respir J. 2013;41(4):832–837. 10.1183/09031936.00019412 [DOI] [PubMed] [Google Scholar]
- 47.Hanson MD, Chen E. The temporal relationships between sleep, cortisol, and lung functioning in youth with asthma. J Pediatr Psychol. 2008;33(3):312–316. 10.1093/jpepsy/jsm120 [DOI] [PubMed] [Google Scholar]
- 48.Kieckhefer GM, Lentz MJ, Tsai SY, Ward TM. Parent-child agreement in report of nighttime respiratory symptoms and sleep disruptions and quality. J Pediatr Health Care. 2009;23(5):315–326. 10.1016/j.pedhc.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonney J, Insel KC, Segrin C, Gerald LB, Moore IM. Association of asthma illness representations and reported controller medication adherence among school-aged children and their parents. J Pediatr Health Care. 2017;31(6):703–712. 10.1016/j.pedhc.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 50.Sonney J, Segrin C, Kolstad T. Parent- and child-reported asthma responsibility in school-age children: examining agreement, disagreement, and family functioning. J Pediatr Health Care. 2019;33(4):386–393. 10.1016/j.pedhc.2018.11.005 [DOI] [PubMed] [Google Scholar]