Abstract
Study Objectives:
Children with Down syndrome (DS) have a high prevalence of obstructive sleep apnea (OSA). Anti-inflammatory medications have been shown to be an effective treatment for mild OSA in otherwise healthy children. However, the efficacy in children with DS and mild OSA has not been investigated. Our aim was to examine the polysomnographic changes of children with DS and mild OSA treated with medication.
Methods:
A retrospective chart review was performed in children with DS (< 18 years) and mild OSA (obstructive apnea-hypopnea index ≤ 5 events/h) diagnosed by polysomnography (PSG) between 2006 and 2018. Patients were included if they were treated with medications (intranasal corticosteroids and/or montelukast) or by observation with a duration of at least 3 months and had baseline and follow-up PSGs. Demographic data, comorbid diagnoses, and PSG data were collected and analyzed.
Results:
Forty-five children met inclusion criteria. In the medication group, 29 children were identified. The median age was 7.4 years (interquartile range [IQR] 4.9–9.3). In the observation group, 16 children were identified. The median age was 4.0 years (IQR 3.2–5.3). The median time from baseline to follow-up PSG was 14.0 months (IQR 10.0–22.9) for the medication group and 10.5 months (IQR 6.5–33.5) for the observation group. There were no significant changes in the median obstructive apnea-hypopnea index from the baseline to follow-up PSG in either the medication group (2.8 [IQR 2.2–3.6) versus 3.5 [IQR 1.4–4.8) events/h; P = .25) or the observation group (2.3 [IQR 1.3–3.1] versus 2.9 [IQR 1.9–6.8] events/h; P = .12). Similarly, there were no significant differences in apnea-hypopnea index, oxygen nadir or end-tidal carbon dioxide between the groups (P = .07-1).
Conclusions:
In our cohort, medication therapy did not significantly improve polysomnographic measures in children with DS and mild OSA. Several factors such as hypotonia and relative macroglossia may explain the ineffectiveness of medical therapy for OSA in this population. Further prospective studies are necessary to confirm these results and to evaluate if a subgroup of DS children may benefit from medical therapy.
Citation:
Yu W, Sarber KM, Howard JJM, et al. Children with Down syndrome and mild OSA: treatment with medication versus observation. J Clin Sleep Med. 2020;16(6):899–906.
Keywords: Down syndrome, medication, observation, obstructive sleep apnea, pediatric
BRIEF SUMMARY
Current Knowledge/Study Rationale: Anti-inflammatory medications have been shown to be an effective treatment for mild obstructive sleep apnea (OSA) in otherwise healthy children. Although children with Down syndrome (DS) have a high prevalence of OSA, the efficacy of medication therapy in children with DS and mild OSA has not been investigated.
Study Impact: Our data suggest that medication therapy did not significantly improve polysomnographic measures in children with DS and mild OSA, and several factors such as hypotonia and relative macroglossia may explain the ineffectiveness of medical therapy for OSA in this population. Further prospective studies are necessary to confirm these results and to evaluate a subgroup of DS children who may benefit from medical therapy.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common condition in childhood and can result in significant cardiovascular, metabolic, cognitive, and behavioral consequences if left untreated.1 Down syndrome (DS) is a common genetic disorder with certain characteristics of intellectual disability, hypotonia, macroglossia, craniofacial abnormalities, and congenital cardiac defects.2 It is reported that 50–75% of children with DS have OSA and the severity is moderate-to-severe in nearly half.3,4 Adenotonsillectomy is recommended as the first-line treatment for OSA in children with adenotonsillar hypertrophy.1,5 However, this is not always curative, especially in children with DS.6,7 For children with DS and moderate-to-severe OSA, it was shown that adenotonsillectomy could result in an AHI reduction into the mild range (defined as obstructive apnea-hypopnea index [oAHI] < 5 events/h) in 48% of those children.8 Thus, a majority of these cases continue to have residual OSA after adenotonsillectomy.7,9 However, investigation into the treatment of mild OSA in children with DS is still lacking.
The American Academy of Pediatrics clinical practice guideline for the diagnosis and management of childhood OSA recommends continuous positive airway pressure (CPAP) for children with contraindications to surgery or persistent OSA postoperatively.5 However, due to many factors, including poor adherence and possible midface remodeling risk with long-term use, CPAP may not be the best option for children with mild OSA.5,10
Anti-inflammatory medications have been shown to be an effective treatment for mild OSA in otherwise healthy children, and intranasal corticosteroids are usually recommended as an option for children with mild OSA postoperatively.5 In a double-blind randomized crossover trial with budesonide nasal spray versus placebo, Kheirandish-Gozal and Gozal11 showed a significant reduction in respiratory disturbances with the use of intranasal budesonide in children with mild OSA aged 6 to 12 years. The same group later reported on oral montelukast to treat moderate-to-severe OSA in children aged 2–10 years, showing significant reduction in the severity of OSA during the treatment period.12 Montelukast, a leukotriene inhibitor, is now widely used in the treatment of pediatric OSA.12,13 Goldbart et al13 reported that montelukast could effectively improve polysomnographic measures and symptoms for mild OSA in children aged 2 to 10 years. A retrospective study conducted by Kheirandish-Gozal et al14 showed beneficial effects using a combination of intranasal corticosteroids and oral montelukast in more than 80% of children aged 2 to 14 years with mild OSA after 3 months of treatment.
These studies all exclude children with genetic disorders such as DS.12,15 Studies regarding the efficacy of medication therapies to treat mild OSA in the specific pediatric population of DS are scarce. Thus, the main goal of our study is to retrospectively explore the efficacy of intranasal corticosteroids (eg, fluticasone propionate, budesonide, or mometasone furoate) and/or oral montelukast versus observation in the management of mild OSA in children and adolescents with DS.
METHODS
A retrospective chart review was performed to identify children of aged less than 18 years with DS and mild OSA (oAHI < 5 events/h) diagnosed by polysomnography (PSG) between 2006 and 2018. Patients were included if they had treatment with medications or observation and underwent follow-up PSG no sooner than 3 months after baseline PSG. The study was approved by the institutional review board of Cincinnati Children’s Hospital Medical Center (Study ID:2018-3952).
Inclusion in the medication group required initiation of medication within 6 months following baseline PSG. Medication treatment included intranasal corticosteroids (fluticasone propionate, budesonide, or mometasone furoate) or oral montelukast alone or a combination of intranasal corticosteroid with oral montelukast. Patients with prior remote surgery were included if the surgeries were performed more than 2 years prior to medication initiation. Upper airway surgeries included adenotonsillectomy, tonsillectomy, adenoidectomy, and revision adenoidectomy. Patients were also excluded if their surgeries were performed within 2 years following medication initiation. Rigorous medical chart review was performed to validate medication prescription refill until their follow-up PSG date, which was considered as the treatment endpoint in the study. If prescriptions were not filled appropriately, the patients were excluded. All medications in the treatment group were prescribed by sleep physicians for mild OSA (the prescriber is unclear in one patient). Demographic data, comorbid diagnoses, baseline, and follow-up PSG data were collected. Baseline and follow-up sleep and respiratory parameters were analyzed within and between the 2 groups.
Within the medication group, further analysis was performed to compare patients with or without remote surgery to evaluate for differences in response to medication treatment (subgroup 1= with remote surgery, subgroup 2 = without remote surgery). Subgroup analysis was also performed to compare patients who were given different types of medication (subgroup 1= intranasal corticosteroids alone, subgroup 2 = montelukast alone, subgroup 3 = a combination of intranasal corticosteroids and montelukast).
Polysomnography
All patients underwent overnight PSG (Grass Telefactor, West Warwick, RI) at Cincinnati Children’s Hospital Medical Center. The standard pediatric montage was used, and the following parameters were recorded simultaneously: bilateral electrooculogram, electroencephalography (C3A2, C4A1, O1A2, O2A1), submental, tibial and intercostal electromyography, tracheal microphone, electrocardiography, pulse oximetry and pulse waveform, thoracic and abdominal inductance plethysmography, nasal thermistor, nasal pressure transducer (Protech, Mukilteo, WA), end-tidal carbon dioxide (ETco2) (BCI Capnoguard, Dublin, OH), and video monitoring using an infrared video camera.
PSG was performed in accordance with the American Academy of Sleep Medicine guidelines,16 results were scored by registered sleep technologists and reviewed and interpreted by a board-certified pediatric sleep specialist. An obstructive sleep apnea was scored when there was a drop in the peak flow signal excursions ≥ 90% from nasal thermistor for ≥ 2 breaths associated with the presence of respiratory effort. Hypopnea was scored when the peak signal excursions dropped by ≥ 30% from nasal pressure transducer for ≥ 2 breaths and was associated with ≥ 3% oxygen desaturation or with an arousal. Central apnea was defined as absent respiratory effort for at least 2 breath cycles, associated with an arousal or awakening or 3% desaturation. Sleep-related hypoventilation was scored if ETco2 persisted > 50 mm Hg for 25% of total sleep time.
The AHI was calculated as the total number of apneas and hypopneas per hour of sleep. oAHI was calculated as the number of obstructive and mixed apneas and hypopneas per hour of sleep. Oxygen desaturation nadir was defined as the lowest oxygen saturation during a respiratory event. The severity of OSA was defined using the oAHI. Mild OSA was defined as oAHI ≥ 1 and < 5 events/h. Moderate OSA was defined as oAHI ≥ 5 and < 10 events/h and severe OSA as oAHI ≥ 10 events/h.
Statistical analysis
Data were reported as median with interquartile range (IQR) for continuous variables and as percentages for categorical variables. Wilcoxon rank sum test was used to compare continuous variables such as age, body mass index (BMI), and PSG parameters. Chi-squared test or Fisher exact test was applied to compare categorical variables such as sex, race, and ethnicity. Similar statistical tests were used for comparison within the group and between the 2 groups. All statistical analyses were conducted using SAS v9.4 (Cary, NC). P < .05 was considered as statistically significant.
RESULTS
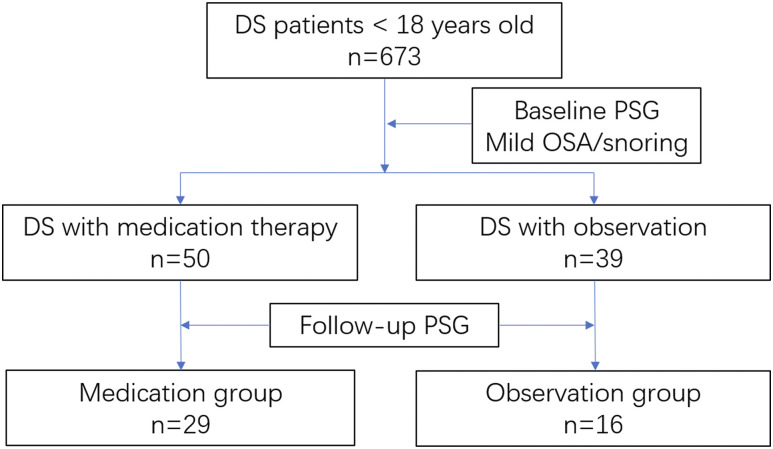
In this retrospective study, we reviewed a total number of 673 charts of children with DS and at least one PSG in our center. Fifty patients were identified with mild OSA who were treated with medication therapy (intranasal corticosteroids and/or oral montelukast). Thirty-nine patients were identified with mild OSA who underwent observation. However, only 29 of 50 patients in the medication group and 16 of 39 patients in the observation group had follow-up PSGs. Thus, a total number of 45 patients with DS was included in our study (Figure 1 and Figure 2).
Figure 1. Flow diagram illustrating inclusion criteria of study cohort into the medication and observation groups.
DS = Down syndrome, OSA = obstructive sleep apnea, PSG = polysomnography.
Baseline characteristics
Demographic and clinical information of study cohort including age, sex, race, ethnicity, BMI, follow-up duration, and comorbidities are summarized in Table 1 and Table 2. There were significantly more boys in the observation group than in the medication group (75% vs 31%, P = .006). Patients in the observation group were significantly younger than those in the medication group (median age 4.0 years [IQR 3.2–5.3] vs 7.4 years [IQR 4.9–9.3), P = .003). Baseline oAHI was 2.8 (IQR 2.2–3.6) events/h in the medication group and 2.3 (IQR 1.3–3.1) events/h in the observation group (P = .84). The median time between baseline and follow-up PSG was 14.0 (IQR 10.0–22.9) months in the medication group and 10.5 (IQR 6.5–33.5) months in the observation group (P = .85). The majority of patients in both groups had no tonsillar hypertrophy (tonsil 0 or 1+) and there was no significant difference between medication and observation groups (72% vs 75%; P = .39). There were no significant differences in race, ethnicity, baseline BMI, or baseline BMI percentile between the 2 groups (P = .09–.85). There were no significant differences in follow-up BMI (BMI at the time of follow-up PSG) or follow-up BMI percentile between the 2 groups (P = .68 and P = .81). In addition, there were no significant differences between baseline and follow-up BMI (P = .73) or baseline and follow-up BMI percentile (P = .31) in the observation group. In the medication group, although there was a significant difference between baseline and follow-up BMI (P = .014), there was no significant difference between baseline and follow-up BMI percentile (P = .67). Comorbidities between the medication and observation groups were similar in regard to other sleep disorders such as insomnia (21% vs 25%, P = .73) and hypersomnia (3% vs 19%, P = .12) (Table 2). Other medical comorbidities including thyroid diseases and eye and ear problems were also similar (P = .08-1) between the 2 groups and details can be found in Table 2.
Table 1.
Demographic and clinical characteristics for the medication and observation groups.
| Characteristics | Medication (n = 29) | Observation (n = 16) | P Value |
|---|---|---|---|
| Start age, y | 7.4 (4.9, 9.3) | 4.0 (3.2, 5.3) | .003* |
| Male sex, n (%) | 9 (31%) | 12 (75%) | .006* |
| Race, n (%) | |||
| White | 24 (83%) | 15 (94%) | .40 |
| Nonwhite | 5 (17%) | 1 (6%) | |
| Hispanic ethnicity, n (%) | 2 (7%) | 0 (0%) | .78 |
| No tonsillar hypertrophy | 21 (72%) | 12 (75%) | .39 |
| Baseline BMI, kg/m2 | 18.7 (17.1, 20.4) | 18.1 (17.6, 20.0) | .83 |
| Baseline BMI percentile, % | 78.5 (41.0, 89.8) | 91.5 (79.1, 96.3) | .09 |
| oAHI, events/h | 2.8 (2.2, 3.6) | 2.3 (1.3, 3.1) | .84 |
| Duration, months | 14.0 (10.0, 22.9) | 10.5 (6.5, 33.5) | .85 |
| Follow-up BMI, kg/m2 | 18.1 (16.9, 22.2) | 18.1 (16.8, 20.6) | .68 |
| Follow-up BMI percentile, % | 81.3 (63.9, 95.0) | 85.8 (71.6, 93.7) | .81 |
Data reported as median with interquartile range (IQR) for continuous data and frequency with percentage (%) for categorical data.*Statistically significant value. BMI = body mass index, oAHI = obstructive apnea-hypopnea index.
Table 2.
Comorbidities for the medication and observation groups reported as frequency with percentage.
| Comorbidities | Medication (n = 29) | Observation (n = 16) | P Value |
|---|---|---|---|
| Sleep disorders, n (%) | |||
| Insomnia | 6 (21%) | 4 (25%) | .73 |
| Parasomnia | 1 (3%) | 1 (6%) | > .99 |
| Hypersomnia | 1 (3%) | 3 (19%) | .12 |
| PLMD/RLS | 4 (14%) | 0 (0%) | .28 |
| Common disorders, n (%) | |||
| Allergy | 11 (38%) | 6 (38%) | > .99 |
| Asthma | 3 (10%) | 4 (25%) | .23 |
| Thyroid diseases | 15 (52%) | 8 (50%) | > .99 |
| Cardiovascular diseases | 14 (48%) | 7 (44%) | > .99 |
| Language impairment | 23 (79%) | 9 (56%) | .17 |
| Eye problems | 22 (76%) | 9 (56%) | .20 |
| Ear issues | 27 (93%) | 11 (69%) | .08 |
PLMD = periodic leg movement disorder, RLS = restless legs syndrome.
Objective measurements for sleep and respiratory parameters
Table 3 provides the baseline and follow-up PSG measurements for each group. There were no significant changes in the oAHI from the baseline to follow-up PSG in either the medication group (from a median of 2.8 events/h [IQR 2.2–3.6] to a median of 3.5 events/h [IQR 1.4–4.8], P = .25) or the observation group (from a median of 2.3 events/h [IQR 1.3–3.1] to 2.9 events/h [IQR 1.9–6.8], P = .12). Similarly, there were no significant changes in the AHI, oxygen nadir, or ETco2 pressure more than 50 mm Hg in either group (P = .07-1).
Table 3.
Median baseline and follow-up polysomnography values.
| PSG Results | Medication (n = 29) | Observation (n = 16) | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | P Value | Baseline | Follow-up | P Value | |
| Sleep parameters | ||||||
| TST, min | 433.5 (403.0, 490.5) | 448.7 (409.5, 484.5) | .29 | 420.0 (379.5, 459.5) | 418.8 (361.0, 448.8) | .54 |
| SE, % | 84.1 (75.0, 88.4) | 86.1 (81.5, 90.9) | .02* | 83.5 (73.8, 88.4) | 80.3 (66.7, 88.8) | .27 |
| SL, min | 18.0 (5.5, 29.8) | 16.1 (5.5, 36.5) | .51 | 17.8 (4.8, 64.0) | 16.3 (3.8, 29.0) | .44 |
| REM, % | 22.2 (20.5, 26.2) | 23.3 (20.0, 26.4) | .98 | 18.9 (14.6, 25.2) | 19.9 (12.9, 25.4) | .41 |
| Arousal index, events/h | 12.8 (9.5, 16.3) | 13.2 (11.5, 15.1) | .85 | 9.7 (7.3, 13.1) | 11.1 (9.6, 16.1) | .20 |
| Respiratory parameters | ||||||
| oAHI,-events/h | 2.8 (2.2, 3.6) | 3.5 (1.4, 4.8) | .25 | 2.3 (1.3, 3.1) | 2.9 (1.9, 6.8) | .12 |
| AHI, events/h | 3.5 (2.5, 3.9) | 3.8 (2.2, 5.7) | .14 | 3.5 (2.4, 4.7) | 4.3 (3.1, 7.2) | .14 |
| SpO2 nadir, % | 93.0 (92.0,93.6) | 91.9 (89.2, 94.8) | .07 | 90.5 (88.0, 92.5) | 87.4 (86.3, 92.0) | .47 |
| ETco2_50, % | 0.1 (0, 1.0) | 0.1 (0, 2.0) | .25 | 23.1 (2.8, 38.7) | 6.9 (.1, 71.7) | > .99 |
Data show interquartile range (IQR) for children with DS and mild OSA in the medication and observation groups.*Statistically significant change. AHI = apnea-hypopnea index, ETtco2_50 = end-tidal carbon dioxide pressure more than 50 millimeter of mercury, oAHI = obstructive apnea-hypopnea index, REM = rapid eye movement sleep, SE = sleep efficiency, SL = sleep latency, SpO2 nadir = nadir oxygen saturation, TST = total sleep time.
The medication group exhibited a significant improvement in sleep efficiency from a median of 84.1% (IQR 75.0–88.4%) to 86.1% (IQR 81.5–90.9%) (P = .02). There were no significant changes in total sleep time, sleep latency, arousal index, or percentage of time spent in rapid eye movement sleep between baseline and follow-up PSG for this group (Table 3). There were no significant changes in sleep efficiency, total sleep time, sleep latency, arousal index, or percentage of time spent in rapid eye movement sleep seen in the observation group.
Table 4 shows the changes in sleep and respiratory parameters between the medication and the observation groups. The symbol Δ defines the change from baseline to follow-up for each sleep and respiratory parameter. No significant changes were found in ΔoAHI between the 2 groups (P = .34). Similarly, there were no significant changes in ΔAHI, Δoxygen nadir, or ΔETco2 more than 50 mm Hg between the 2 groups (P = .64-1).
Table 4.
Comparison of changes in sleep and respiratory parameters between the medication and the observation groups.
| PSG Results | Medication (n = 29) | Observation (n = 16) | P Value |
|---|---|---|---|
| Sleep parameters | |||
| ΔTST, min | −21.0 (−51.0, 37.0) | 36.5 (−65.3, 95.0) | .20 |
| ΔSE, % | -4.1 (−8.3, 2.3) | 3.9 (−5.1, 18.0) | .03* |
| ΔSL, min | 3.5 (−11.0, 17.7) | 1.0 (−9.8, 32.3) | .87 |
| ΔREM sleep, % | −0.5 (−3.5, 5.3) | 2.9 (−2.3, 5.7) | .50 |
| ΔArousal index, events/h | −1.0 (−3.6, 3.0) | −2.3 (−5.0, 1.7) | .36 |
| Respiratory parameters | |||
| ΔoAHI, events/h | −0.5 (−2.0, 1.0) | −0.9 (−6.5, .8) | .34 |
| ΔAHI, events/h | −0.5 (−2.3,1.1) | −0.3 (−4.2, .5) | .64 |
| ΔSpO2 nadir, % | 1.7 (.2, 2.5) | 0.6 (−2.0, 6.7) | .90 |
| ΔETco2_50, % | 0.0 (−.1, 1.1) | 0.4 (−16.5, 14.3) | > .99 |
Data show interquartile range (IQR). *Statistically significant change. Δ = Baseline follow-up, AHI = apnea-hypopnea index, ETco2_50 = end-tidal carbon dioxide pressure more than 50 millimeter of mercury, oAHI = obstructive apnea-hypopnea index, REM = rapid eye movement, SE = sleep efficiency, SL = sleep latency, SpO2 nadir = nadir oxygen saturation, TST = total sleep time.
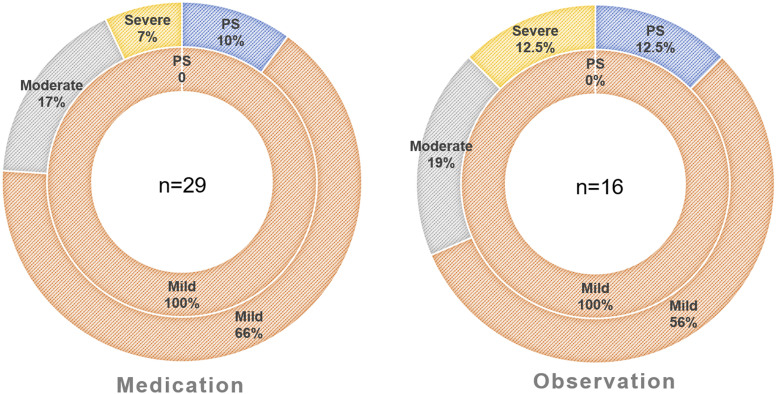
Among children in the medication group (n = 29), 3 of them (10%) resolved on follow-up PSG; 19 of them (66%) continued to exhibit mild OSA, 5 of them (17%) progressed to moderate OSA, and 2 of them (7%) progressed to severe OSA (Figure 2). Among children in the observation group (n = 16), 2 of them (12.5%) resolved on follow-up PSG, 9 of them (56%) continued to exhibit mild OSA, 3 of them (19%) progressed to moderate OSA, and 2 of them (12.5%) progressed to severe OSA (Figure 2).
Figure 2. Donut charts showing the severity distribution of obstructive sleep apnea.
Left: medication group (n = 29), right: observation group (n = 16). Inner rings represent baseline PSG results, and outer rings represent follow-up PSG results. PS = primary snoring, PSG = polysomnography.
Subgroup analyses
Within the medication group, 59% of children (n = 17) had a remote surgery history. None of the patients in the observation group had previous history of upper airway surgeries. There were no significant changes in the oAHI from the baseline to follow-up PSG in either subgroup with history of remote surgery (3.1 events/h [IQR 2.3–3.6] [baseline] vs 3.8 events/h [IQR 2.4–5.7] [follow-up]; P = .07) or without history of surgery (2.6 events/h [IQR 2.2–3.6] [baseline] vs 2.7 events/h [IQR 1.3–3.9] [follow-up]; P = .73).
Subgroup analyses revealed that neither type of medication nor combination of medications led to a significant change in the oAHI. Four patients were treated with nasal corticosteroids alone (oAHI 1.8 events/h [IQR 1.4–2.1] [baseline] vs 1.3 events/h [IQR 0.8–5.3] [follow-up]; P = 1.0). Seven patient were treated with montelukast alone (oAHI 2.7 events/h [IQR 2.1–3.6] [baseline] vs 3.5 events/h [IQR 1.1–5.0] [follow-up]; P = .81). Eighteen patients were treated with a combination of nasal corticosteroids and oral montelukast (oAHI 3.3 events/h [IQR 2.4–3.8] [baseline] vs 3.8 events/h [IQR 1.8–4.8] [follow-up]; P = .33).
DISCUSSION
Our retrospective review of children with DS demonstrated that medication therapies (intranasal corticosteroids and/or oral montelukast) did not improve mild OSA after a median follow-up of 14.0 months (IQR 10.0–22.9). Upon follow-up, 7 (24%) children in the medication group progressed to moderate or severe OSA. Only 3 (10%) children resolved after medication treatment. Due to the lack of self-reported outcome data, such as daytime sleepiness or quality of life, we are unable to assess whether medication therapies would lead to self-reported improvement.
DS is a common genetic disorder caused by an extra copy of chromosome 21, with a prevalence of approximately 1 in 691 infants in the United States.17,18 Patients with DS commonly have multiple medical problems, such as hearing loss (75%), vision problems (60%), OSA (50–75%), and congenital heart disease (40–50%).3 Sleep disorders in DS have received increasing attention due to negative sequelae on behavior, cognition, and cardiovascular systems.19–22 A recent meta-analysis revealed that OSA prevalence in children with DS was 69–76% based on AHI by PSG, which is significantly higher than the 1–6% prevalence in the general population of children.4,23 In addition, OSA in children with DS tends to be difficult to treat due to the multiple factors that contribute to OSA in this population.4
It is generally accepted that the most commonly identified risk factor for pediatric OSA is adenotonsillar hypertrophy.1,6 For children with DS, anatomical abnormalities such as maxillary and mandibular hypoplasia, narrow nasopharynx, shortened palate, and relative macroglossia (due to crowding of the oropharynx) also greatly contribute to their OSA, in addition to adenotonsillar hypertrophy.24 Laryngomalacia and subglottic and tracheal stenosis also play a role in the development of airway obstruction in some children with DS.25,26 Other factors such as generalized hypotonia, an immature immune system (leading to more respiratory infections), a propensity for obesity, and mucopolysaccharide deposition due to hypothyroidism are all risk factors in the pathogenesis and evolution of sleep apnea in patients with DS.24,27–29 This might explain why we could not obtain as promising results as in otherwise healthy children when treating mild OSA in children with DS using anti-inflammatory medication therapies in the current study.
Anti-inflammatory medication therapies are good candidate options for treating mild OSA in children for whom adenotonsillectomy is contraindicated or with residual mild OSA postoperatively.5 Several clinical studies have shown that these medications ameliorate mild OSA or prevent OSA recurrence postoperatively in otherwise healthy children11–15,30 as low-grade systemic and local upper airway inflammation is present.31 Basic research studies demonstrate that corticosteroids-sensitive glucocorticoid receptors are abundantly expressed in adenotonsillar tissues as well as leukotrienes and their receptors, which gave evidence that corticosteroids and leukotriene modifiers could be useful in the treatment of pediatric OSA especially for those with adenotonsillar hypertrophy.32,33 In a subgroup analysis in our study, we looked at the effect of medication therapies on children with DS with and without a remote history of adenotonsillectomy (> 2 years away from medication initiation). Results showed that a remote history of adenotonsillectomy did not affect the outcome as neither improved in oAHI.
Additionally, we explored the efficacy of antileukotriene and intranasal steroid medications alone or in combination to treat mild OSA. Subgroup analysis indicated neither medication alone nor in combination had a significant impact on PSG measurements in our DS cohort. However, due to our low numbers in each subgroup and the night-to-night variability of PSG, these results for subgroup analysis might be underpowered. It is not the goal of our study to evaluate effectiveness of each medication, but to assess the effectiveness of medication treatment as a group. Although this would limit our ability to draw conclusions, our data would represent real clinical settings. The unpromising results of medication therapies on treating mild OSA in children with DS in our study might also simply reflect a floor effect as we included only patients with mild OSA. However, those patients with moderate to severe OSA are often managed with CPAP or surgery. Therefore, assessment of medication effectiveness in moderate to severe OSA is likely to be confounded by intermittent attempts at CPAP use or other surgical options, especially in children with DS. Prospective studies with a large sample size and various severity of OSA are needed to confirm our results and to evaluate a subgroup of DS who may benefit from medical therapies.
In the American Academy of Pediatrics guidelines for diagnosis and management of childhood OSA, it is mentioned that the long-term effect of intranasal corticosteroids should be emphasized as well as their adverse effects.5 In our retrospective review, no severe adverse effects were reported with use of intranasal corticosteroids after a median of 14.0 months (IQR 10.0–22.9).
Within the observation group, no significant polysomnographic changes were found for children at a median age of 4.0 years (IQR 3.2–5.3) after an observation period of 10.5 months (IQR 6.5–33.5). Upon follow-up, 3 (19%) children developed moderate OSA and 2 (12.5%) progressed to severe OSA. Two children (12.5%) in our cohort had spontaneous resolution after a period of observation compared with the CHAT (Childhood Adenotonsillectomy Trial) study, where 46% of children exhibited PSG normalization.6
An important limitation of our study is that 21 patients in the medication group (42%) and 23 patients in the observation group (59%), who otherwise met all inclusion criteria, did not undergo a follow-up PSG. Chart review showed that for those who did have a follow-up, most exhibited resolution of presenting symptoms. Follow-up PSG was not recommended either due to economic cost or patient willingness. Our results are therefore limited and prospective studies are necessary to confirm our findings.
Our study has other limitations. First, it is a retrospective study that is susceptible to selection bias. To eliminate confounding factors, rigorous chart review was performed to ensure that patients met all inclusion criteria. Both baseline and follow-up PSGs should be valid with intact recording data. The interval between baseline PSG and medication/observation initiation should be no more than 6 months. And in the medication group, surgical history should be limited to at least 2 years away from baseline PSG date if the children had previous surgery to avoid the overlap effect of upper airway surgery and medication on OSA. The exclusion of patients may inadvertently lead to selection bias. Second, a rigorous chart review was performed to validate medication prescription refill until their follow-up PSG; however, we cannot completely exclude medication nonadherences as patient may not take medication even with proper refills. In addition, the duration of medication treatment varied from 10 to 22.9 months. Both medication adherence and variation of treatment duration would limit our ability to assess the real effectiveness of medical therapy. On the other hand, our results are more generalizable in an intention-to-treat manner and are more reflective of real clinical settings. Third, our study is a retrospective observational study and the time period of the study is determined based on the feasibility of data extraction. No priori power analysis was performed to determine the required number of patients to be included. Fourth, there was a significant difference in age and sex between the medication and observation groups. In the medication group, children were older and predominately female, while in the observation group, patients were younger and predominately male. In the population with DS, the correlation between age, sex, and pediatric OSA is controversial. Some observed that OSA was associated with older age, whereas others reported an inverse correlation between age and OSA in children with DS.4,24,34–36 Similarly, some reported male patients with DS were more likely to have OSA, whereas others showed that male sex did not have independent predictive power in the presence of the other factors or was not significantly correlated to the oAHI.24,34,37 It is unclear why there is an age difference between the 2 groups in our cohort. One potential explanation is that medications are often prescribed for residual mild OSA after surgery as the majority of patients in the medication group (59%) had remote surgical history (adenotonsillectomy). Therefore, the age of patients in the medication group is likely to be older than those in the observation group who were considered for conservative watchful waiting at an earlier age. Another potential explanation is that both physicians and parents may be more hesitant to pursue medications, such as nasal steroid and/or montelukast, at a very young age. Both potential explanations would lead to selection bias. Finally, our small sample size does not allow us to derive significant meaning from our subgroup analysis of surgical history and the medication type. Thus, future multicenter prospective studies with a larger patient cohort are necessary to confirm our results and to control for these confounding factors.
CONCLUSIONS
To our knowledge, this is the first study that investigates the efficacy of medication therapies on mild OSA in children with DS with a follow up of PSG measurements. Based on PSG parameters, our study suggests that medication therapies (intranasal corticosteroids and/or oral montelukast) are not effective in treating mild OSA in children with DS. Other factors in addition to adenotonsillar hypertrophy, such as hypotonia and relative macroglossia, may explain the lack of efficacy of medical therapy for OSA in this population. Further prospective studies with multi-institutional collaborations are necessary to confirm these results and to identify and evaluate a subgroup of DS children who may benefit from medical therapy.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was funded by the Cincinnati Children’s Research Foundation and Shanghai Ninth People’s Foundation for Overseas Scholarship. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- DS
Down syndrome
- ETco2
end-tidal carbon dioxide
- IQR
interquartile range
- oAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PS
primary snoring
- PSG
polysomnography
REFERENCES
- 1.Section on Pediatric Pulmonology SoOSAS, American Academy of Pediatrics . Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):704–712. 10.1542/peds.109.4.704 [DOI] [PubMed] [Google Scholar]
- 2.Cielo CM, Konstantinopoulou S, Hoque R. OSAS in specific pediatric populations. Curr Probl Pediatr Adolesc Health Care. 2016;46(1):11–18. 10.1016/j.cppeds.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 3.Bull MJ. Health supervision for children with Down syndrome. Pediatrics. 2011;128(2):393–406. 10.1542/peds.2011-1605 [DOI] [PubMed] [Google Scholar]
- 4.Lee CF, Lee CH, Hsueh WY, Lin MT, Kang KT. Prevalence of obstructive sleep apnea in children with down syndrome: a meta-analysis. J Clin Sleep Med. 2018;14(5):867–875. 10.5664/jcsm.7126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. 10.1542/peds.2012-1671 [DOI] [PubMed] [Google Scholar]
- 6.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merrell JA, Shott OSAS, Sr. OSAS in Down syndrome: T&A versus T&A plus lateral pharyngoplasty. Int J Pediatr Otorhinolaryngol. 2007;71(8):1197–1203. 10.1016/j.ijporl.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 8.Ingram DG, Ruiz AG, Gao D, Friedman NR. Success of tonsillectomy for obstructive sleep apnea in children with down syndrome. J Clin Sleep Med. 2017;13(8):975–980. 10.5664/jcsm.6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farhood Z, Isley JW, Ong AA, et al. Adenotonsillectomy outcomes in patients with Down syndrome and obstructive sleep apnea. Laryngoscope. 2017;127(6):1465–1470. 10.1002/lary.26398 [DOI] [PubMed] [Google Scholar]
- 10.Roberts SD, Kapadia H, Greenlee G, Chen ML. Midfacial and dental changes associated with nasal positive airway pressure in children with obstructive sleep apnea and craniofacial conditions. J Clin Sleep Med. 2016;12(4):469–475. 10.5664/jcsm.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics. 2008;122(1):e149–e155. 10.1542/peds.2007-3398 [DOI] [PubMed] [Google Scholar]
- 12.Kheirandish-Gozal L, Bandla HP, Gozal D. Montelukast for children with obstructive sleep apnea: results of a double-blind, randomized, placebo-controlled trial. Ann Am Thorac Soc. 2016;13(10):1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldbart AD, Greenberg-Dotan S, Tal A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130(3):e575–e580. 10.1542/peds.2012-0310 [DOI] [PubMed] [Google Scholar]
- 14.Kheirandish-Gozal L, Bhattacharjee R, Bandla HPR, Gozal D. Antiinflammatory therapy outcomes for mild OSA in children. Chest. 2014;146(1):88–95. 10.1378/chest.13-2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan CC, Au CT, Lam HS, Lee DL, Wing YK, Li AM. Intranasal corticosteroids for mild childhood obstructive sleep apnea–a randomized, placebo-controlled study. Sleep Med. 2015;16(3):358–363. 10.1016/j.sleep.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 16.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5(10):725–738. 10.1038/nrg1448 [DOI] [PubMed] [Google Scholar]
- 18.Parker SE, Mai CT, Canfield MA, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016. 10.1002/bdra.20735 [DOI] [PubMed] [Google Scholar]
- 19.Churchill SS, Kieckhefer GM, Landis CA, Ward TM. Sleep measurement and monitoring in children with Down syndrome: a review of the literature, 1960-2010. Sleep Med Rev. 2012;16(5):477–488. 10.1016/j.smrv.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lal C, White DR, Joseph JE, van Bakergem K, LaRosa A. Sleep-disordered breathing in Down syndrome. Chest. 2015;147(2):570–579. 10.1378/chest.14-0266 [DOI] [PubMed] [Google Scholar]
- 21.Horne RS, Wijayaratne P, Nixon GM, Walter LM. Sleep and sleep disordered breathing in children with down syndrome: Effects on behaviour, neurocognition and the cardiovascular system. Sleep Med Rev. 2019;44:1–11. 10.1016/j.smrv.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 22.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1991;88(1):132–139. [PubMed] [Google Scholar]
- 23.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. 10.1513/pats.200708-135MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. Sleep. 2016;39(3):699–704. 10.5665/sleep.5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertrand P, Navarro H, Caussade S, Holmgren N, Sanchez I. Airway anomalies in children with Down syndrome: endoscopic findings. Pediatr Pulmonol. 2003;36(2):137–141. 10.1002/ppul.10332 [DOI] [PubMed] [Google Scholar]
- 26.Bravo MN, Kaul A, Rutter MJ, Elluru RG. Down syndrome and complete tracheal rings. J Pediatr. 2006;148(3):392–395. 10.1016/j.jpeds.2005.10.023 [DOI] [PubMed] [Google Scholar]
- 27.Shott SR. Down syndrome: common otolaryngologic manifestations. Am J Med Genet C Semin Med Genet. 2006;142C(3):131–140. 10.1002/ajmg.c.30095 [DOI] [PubMed] [Google Scholar]
- 28.van Gameren-Oosterom HB, van Dommelen P, Schonbeck Y, Oudesluys-Murphy AM, van Wouwe JP, Buitendijk SE. Prevalence of overweight in Dutch children with Down syndrome. Pediatrics. 2012;130(6):e1520–e1526. 10.1542/peds.2012-0886 [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Zhang W, Tan J, Zhao M, Zhang Q, Lei P. Role of hypothyroidism in obstructive sleep apnea: a meta-analysis. Curr Med Res Opin. 2016;32(6):1059–1064. 10.1185/03007995.2016.1157461 [DOI] [PubMed] [Google Scholar]
- 30.Brouillette RT, Manoukian JJ, Ducharme FM, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. 2001;138(6):838–844. 10.1067/mpd.2001.114474 [DOI] [PubMed] [Google Scholar]
- 31.Sheldon SH. Principles and Practice of Pediatric Sleep Medicine. 2nd edition. London: Saunders; 2014. [Google Scholar]
- 32.Kheirandish-Gozal L, Serpero LD, Dayyat E, et al. Corticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoea. Eur Respir J. 2009;33(5):1077–1084. 10.1183/09031936.00130608 [DOI] [PubMed] [Google Scholar]
- 33.Dayyat E, Serpero LD, Kheirandish-Gozal L, et al. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest. 2009;135(5):1142–1149. 10.1378/chest.08-2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Miguel-Díez J, Villa-Asensi JR, Alvarez-Sala JL. Prevalence of sleep-disordered breathing in children with Down syndrome: polygraphic findings in 108 children. Sleep. 2003;26(8):1006–1009. 10.1093/sleep/26.8.1006 [DOI] [PubMed] [Google Scholar]
- 35.Ng DK, Hui HN, Chan CH, et al. Obstructive sleep apnoea in children with Down syndrome. Singapore Med J. 2006;47(9):774–779. [PubMed] [Google Scholar]
- 36.Shires CB, Anold SL, Schoumacher RA, Dehoff GW, Donepudi SK, Stocks RM. Body mass index as an indicator of obstructive sleep apnea in pediatric Down syndrome. Int J Pediatr Otorhinolaryngol. 2010;74(7):768–772. 10.1016/j.ijporl.2010.03.050 [DOI] [PubMed] [Google Scholar]
- 37.Hill CM, Evans HJ, Elphick H, et al. Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome. Sleep Med. 2016;27-28:99–106. 10.1016/j.sleep.2016.10.001 [DOI] [PubMed] [Google Scholar]