Abstract
Study Objectives:
Herbal medicines are frequently used by adults with sleep difficulties. However, evidence of their efficacy is limited. Therefore, the goal of this study was to examine the sleep-enhancing effects of a standardized saffron extract (affron).
Methods:
This was a 28-day, parallel-group, double-blind, randomized controlled trial. Sixty-three healthy adults aged 18–70 with self-reported sleep problems were recruited and randomized to receive either saffron extract (affron; 14 mg twice daily) or a placebo. Outcome measures included the Insomnia Severity Index (ISI; primary outcome measure) collected at baseline and days 7, 14, 21, and 28 and the Restorative Sleep Questionnaire (RSQ) and the Pittsburgh Sleep Diary (PSD) collected on days −1, 0, 3, 7, 14, 27, and 28.
Results:
Based on data collected from 55 participants, saffron was associated with greater improvements in ISI total score (P = .017), RSQ total score (P = .029), and PSD sleep quality ratings (P = .014) than the placebo. Saffron intake was well tolerated with no reported adverse effects.
Conclusions:
Saffron intake was associated with improvements in sleep quality in adults with self-reported sleep complaints. Further studies using larger samples sizes, treatment periods, objective outcome measures, and volunteers with varying demographic and psychographic characteristics are required to replicate and extend these findings.
Clinical Trial Registration:
Registry: Australian New Zealand Clinical Trials Registry; Name: Effects of Saffron on Sleep Quality in Healthy Adults with Self-Reported Unsatisfactory Sleep; URL: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=377781; Identifier: ACTRN12619000863134.
Citation:
Lopresti AL, Smith SJ, Metse AP, Drummond PD. Effects of saffron on sleep quality in healthy adults with self-reported poor sleep: a randomized, double-blind, placebo-controlled trial. J Clin Sleep Med. 2020;16(6):937–947.
Keywords: sleep, insomnia, saffron, herbal
BRIEF SUMMARY
Current Knowledge/Study Rationale: Research into the efficacy of herbal medicines as natural sleep-enhancing agents is limited. In this 8-week, randomized, double-blind, placebo-controlled study, the sleep-enhancing effects of a standardized saffron extract (affron) were investigated in adults with self-reported poor sleep.
Study Impact: Saffron supplementation for 8 weeks was associated with improvements in insomnia, sleep quality, and restorative sleep. Further research is required to replicate and extend these findings in other populations.
INTRODUCTION
Results from population-based surveys in Australia and other developed countries indicate that 10–45% of adults report regular difficulty either falling or staying asleep (hereafter, “poor sleep”).1–3 This has significant health implications as poor sleep quality can have a negative impact on both mental and physical health and can interfere with daily function.4 In a cohort of almost 5,000 adults, poor sleep was associated with a 29% increased risk of cardiovascular disease.5 Sleep problems, such as insomnia, are also associated with a 2.6 times greater risk of developing depression.6 Insomnia is also associated with reduced work productivity and increased work absenteeism.7,8 In 2010, diagnosed sleep disorders in Australia were estimated to cost $5.1 billion (Australian dollars), comprising 5% from direct health care costs, 60% from productivity losses, and 13% from informal care and other indirect costs, resulting from motor vehicle and workplace accidents.9
Herbal medicines are one of the most frequently used complementary and alternative treatments for insomnia. In community-dwelling older adults with self-reported sleep problems, use of a sleep product was reported by 35% of respondents, with 22% using over-the-counter sleep aids and 12.5% using herbal or natural aids.10 In a randomly selected sample of almost 1,000 adults, 18.5% of participants reported using a natural sleep aid over the past 12 months.11 However, the safety and efficacy of many herbal medicines for the treatment of sleep problems are uncertain. In a systematic review of 14 randomized controlled trials of herbal medicines (comprising valerian, kava, and chamomile) and involving 1,600 participants with insomnia, it was concluded that, although these treatments were generally safe and well tolerated, there was insufficient evidence that they provided any benefit to adults with insomnia.12 This indicates that, despite their widespread use, there is a paucity of data on the efficacy of natural herbal products.
Saffron, a spice derived from the stigmas of the Crocus sativus flower, has been confirmed in several systematic reviews and meta-analyses to be an effective natural agent for the treatment of mild-to-moderate depression.13–15 As a sleep-enhancing agent, there is preliminary evidence to suggest it may also be an effective natural sleep aid. In a small pilot study, the 4-week administration of a saffron extract (affron) resulted in greater improvements in sleep quality, sleep latency, and daytime dysfunction in a subset of poor sleepers compared with placebo.16 As an adjunct to antidepressants in adults with unremitted depression, symptomatic improvements in sleep quality were also noted after saffron administration.17 In a randomized, double-blind, placebo-controlled, crossover study, the 14-day administration of crocetin (an active constituent in saffron) in adults with mild sleep complaints was associated with increases in electroencephalography (EEG) delta activity and self-reported improvements in sleepiness and refreshment on rising. However, there were no significant changes in other sleep parameters.18 Improvements in sleep quality have also been reported in adults with type 2 diabetes with comorbid depression and anxiety after an 8-week intake of saffron.19 Although promising, the robustness of these findings is hindered by small sample sizes, short treatment periods, use of nonstandardized saffron extracts, and investigations in populations where sleep is not the primary presenting complaint. Therefore, the goal of this study was to investigate the sleep-enhancing effects and safety of a standardized saffron extract (affron) in adults with self-reported poor sleep.
METHODS
Study design
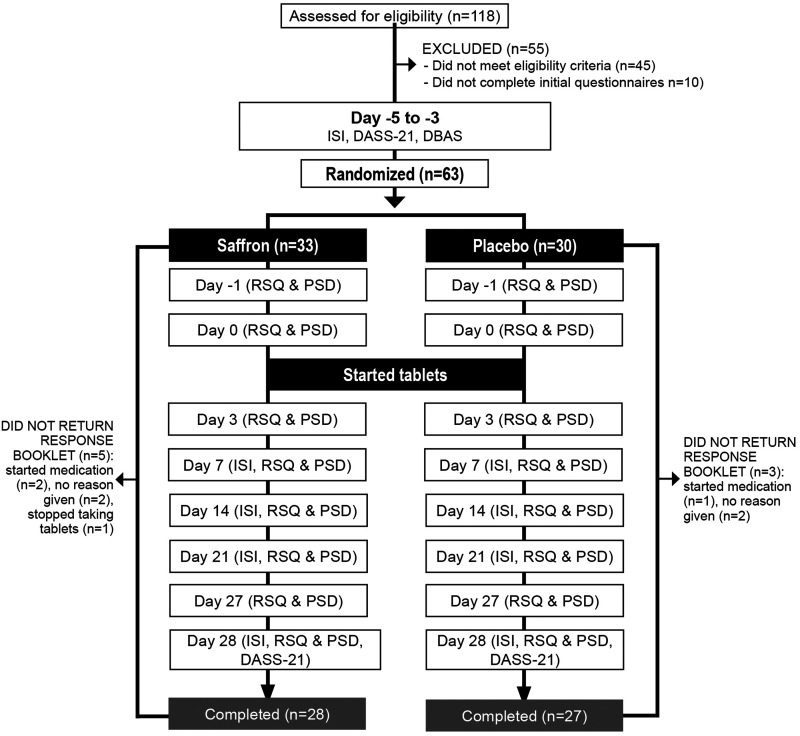
This was a 2-arm, parallel-group, 28-day, randomized, double-blind, placebo-controlled trial (Figure 1). The trial protocol was approved by the Human Research Ethics Committee at the National Institute of Integrative Medicine (approval number 0054E_2019) and was prospectively registered with the Australian New Zealand Clinical Trials Registry (Trial ID: ACTRN12619000863134).
Figure 1. Systematic illustration of the study design.
DASS-21 = Depression, Anxiety, Stress Scale–21; DBAS = Dysfunctional Beliefs Associated with Sleep Questionnaire; ISI = Insomnia Severity Index; PSD = Pittsburgh Sleep Diary; RSQ = Restorative Sleep Questionnaire.
An a priori power analysis was undertaken to estimate the required sample size (based on a single outcome variable). In a recent randomized controlled pilot study, saffron had an effect size of 1.1 in adults with poor sleep compared with the placebo.16 Sample size calculations were based on a more conservative effect size of 0.8. Assuming a power of 80% and a type 1 error rate (α) of 5%, the number of participants required per group to find an effect for the Insomnia Severity Index (ISI) was estimated as 21. After allowing for a 15% drop-out rate, we aimed to recruit at least 25 participants per group.20
Recruitment and randomization
Participants were recruited across Perth, Western Australia, through social media advertisements between July and August 2019. As interest to participate in the study was high, we were able to recruit more than our minimum projected sample size of 50 prior to our projected timeline (n = 63). Interested participants were directed to a website landing page providing details about the study and a link to complete an initial online screening questionnaire. This online questionnaire screened for current depressive and/or anxiety symptoms; any medication use; history of medical/psychiatric disorders; alcohol, nicotine, and other drug use; supplement and vitamin intake; and pregnancy/breastfeeding status. If assessed as likely eligible, volunteers participated in a phone interview with an investigator. The phone interview comprised a structured series of questions to further clarify details pertaining to the eligibility criteria (eg, current and past mental health history, alcohol and other drug use, supplement intake, current medical conditions, and medication intake) and to obtain further demographic details. Suitable participants were then required to complete online versions of the ISI, Depression, Anxiety, and Stress Scale–21 (DASS-21), Dysfunctional Beliefs Associated with Sleep Questionnaire (DBAS), and an informed consent form. Eligible and consenting participants were randomly assigned to 1 of 2 groups (saffron or placebo) using a randomization calculator (http://www.randomization.com). The randomization calculator ensured sequence concealment. The randomization structure comprised 10 randomly permuted blocks, containing 6 participants per block. The participant identification number was allocated according to the order of participant enrollment in the study. All tablets were packed in identical bottles labeled with 2 intervention codes (held by the study sponsor until final data collection). Participants and study investigators were blinded to treatment group allocation until all outcome data were collected. No financial compensation was provided to participants for volunteering in this study, although at the completion of the study, participants allocated to the placebo condition were offered a free 4-week supply of saffron tablets.
Participants
Inclusion criteria
Physically healthy male and female participants aged 18–70 years, with self-reported symptoms of poor sleep lasting more than 4 weeks, were recruited for this study. Participants had an ISI score of between 8 (subthreshold insomnia) and 21 (moderate-severity insomnia). All participants were medication free for at least 4 weeks apart from contraceptive pills and no more than once per week use of pain-relieving medications. Volunteers had a body mass index between 18 and 30 kg/m2 and typical bedtime between 9 pm and 12 am. Participants were also required to be fluent in English and to have consented (via an online consent form) to all pertinent aspects of the trial.
Exclusion criteria
Participants employed in night-shift work or rotational-shift work were ineligible to participate in the study. Individuals experiencing a sleep disorder other than moderate insomnia (eg, sleep apnea, restless legs syndrome, periodic limb movement disorder), chronic, severe sleep disturbance for more than 1 year, diagnosis of a mental health disorder (other than mild depressive or anxiety symptoms as measured by the DASS-21), coffee intake greater than 3 cups per day (or equivalent caffeine intake from other caffeinated drinks, eg, tea, energy drinks), and alcohol consumption greater than 14 standard drinks per week were also ineligible for the study. Participants were also ineligible for the study if they were experiencing external factors that may affect sleep patterns (eg, infants/children regularly awakening, excessive noise, snoring partner), were currently receiving nonpharmacological treatment for sleep disorders (eg, cognitive behavioral therapy [CBT], relaxation therapy), had a current or 12-month history of illicit drug abuse, were currently taking supplements that may affect sleep, were taking saffron supplements, or had a diagnosed medical condition including but not limited to diabetes, hyper-/hypotension, cardiovascular disease, gastrointestinal disease requiring regular use of medications, gallbladder disease/gallstones/biliary disease, endocrine disease, psychiatric disorder, neurological disease (Parkinson disease, Alzheimer disease, intracranial hemorrhage, head or brain injury), or acute or chronic pain affecting sleep. Pregnant women, women who were breastfeeding, or women who intended to become pregnant were also ineligible to participate in the study.
Interventions
Placebo and saffron tablets were identical in appearance, being matched for color coating, shape, and size. The active treatment, supplied by Pharmactive Biotech Products SL, contained 14 mg of a standardized saffron extract (affron), derived from the stigmas of Crocus sativus L. and standardized to contain more than 3.5% Lepticrosalides, a measure of bioactive compounds present in saffron, including safranal and crocin isomers. The saffron stigmas were cultivated in Alborea (Albacete, Spain) and extracted in the factory of Pharmactive Biotech Products SL in Madrid (Spain) to produce affron 3.5% Lepticrosalides. The placebo tablets contained the same excipients as the active tablet (microcrystalline cellulose and calcium hydrogen phosphate). All tablets were manufactured and packed in an Australian Therapeutic Goods Administration–registered plant. All participants were instructed to take 1 tablet, twice daily, with or without food for 28 days. Medication adherence was measured by tablet count by the participant on day 28. Efficacy of participant treatment blinding was examined by asking participants to predict group allocation (placebo, saffron, or uncertain) at the completion of the study. Saffron and placebo tablets were mailed to participants with directions for use provided on tablet bottles. Participants were also provided with an information sheet about tablet intake and what to do if they missed a dose. This information was also verbally conveyed to participants during their initial telephone interview.
Outcome measures
Primary outcome measures
Insomnia Severity Index:
The ISI is a self-report instrument measuring respondents’ perception of both nocturnal and diurnal symptoms of insomnia. The ISI comprises 7 items assessing the perceived severity of difficulties initiating sleep, staying asleep, early morning awakenings, satisfaction with current sleep pattern, interference with daily functioning, noticeability of impairment attributed to the sleep problem, and degree of distress or concern caused by the sleep problem. The ISI has good psychometric properties and is sensitive to treatment in clinical trials.21 The ISI was completed at baseline (from days −5 to −3) and days 7, 14, 21, and 28.
Secondary outcome measures
Restorative Sleep Questionnaire:
The Restorative Sleep Questionnaire (RSQ) is a validated 11-item questionnaire that assesses restorative sleep by asking respondents to rate on a 5-point scale feelings of tiredness, mood, and energy. The RSQ has good psychometric properties and is able to distinguish between healthy controls, patients with primary insomnia, and insomnia patients with isolated nonrestorative sleep complaints.22 The RSQ was completed at days −1, 0, 3, 7, 14, 21, 27, and 28.
Pittsburgh Sleep Diary:
The Pittsburgh Sleep Diary (PSD) is a 14-item sleep diary that respondents complete upon awakening. The PSD shows good retest reliability over a mean inter-test interval of 22 months. Scores also correlate with circadian type, self-reported sleep quality, and objective actigraphy measurements.23 Scores are calculated for total sleep time (hours), sleep latency (minutes), number of awakenings after sleep onset, sleep quality rating (5-point Likert rating ranging from very bad [1] to very good [5]), mood rating at final awakening (5-point Likert rating ranging from very calm [1] to very tense [5]), and alertness rating at final awakening (5-point Likert rating ranging from very sleepy [1] to very alert [5]). The PSD was completed at days −1, 0, 3, 7, 14, 21, 27, and 28.
Depression, Anxiety, and Stress Scale–21:
The DASS-21 is a validated self-report measure assessing symptoms of stress, anxiety, and depression.24 Twenty-one questions are rated on a 4-point scale (0–3), ranging from never to almost always (lower scores indicate a reduction in symptoms). Subscale scores for depression, anxiety, and stress are calculated. The DASS-21 was completed at baseline (days −5 to −3) and day 28.
Process measures
Dysfunctional Beliefs Associated with Sleep Questionnaire:
The DBAS is a 16-item questionnaire that assesses beliefs and attitudes about sleep. The DBAS has good reliability as evidenced by adequate internal consistency and temporal stability over a 2-week period. Scores also correlate with other self-report measures of insomnia severity, anxiety, and depression.25 The DBAS was completed at baseline to examine the impact of dysfunctional beliefs about sleep on change in sleep quality over time. If significant dysfunctional beliefs about sleep present a potential barrier to successful change, this will help to inform further interventional trials.
Adverse events
The tolerability and safety of supplement intake by participants was assessed at day 28 through an online question querying adverse effects that were believed to be associated with supplement intake. Participants were also requested to contact researchers immediately if any adverse effects were experienced.
Data collection procedures
Initial screening questionnaires comprising the ISI, DASS-21, and DBAS were completed online. A response booklet containing copies of the required questionnaires and sleep diaries was then mailed to all participants. The dates for completion of each questionnaire and diary were recorded in the booklet. Participants were also advised to keep their response booklet near their bed and to complete it within 30 minutes after awakening.
Statistical analysis
An independent-samples t test was used to compare demographic variables across the 2 treatment groups for continuous variables, and Pearson’s chi-square was used to compare categorical data. To evaluate primary study objectives, a repeated-measures analysis of variance (ANOVA) was used to compare within-group changes over time and group (saffron versus placebo) × time interaction effects. The ISI total score was analyzed for baseline and mean score across days 7, 14, 21, and 28. Total scores on the RSQ and item scores on the PSD were analyzed for mean baseline (days −1 and day 0) and mean score across days 3, 7, 14, 21, 27, and 28. Eta-squared (η2) was calculated to examine effect sizes.
The Shapiro-Wilk normality test was conducted to examine the normality of group data. This demonstrated that data were not normally distributed, and this was not corrected by data transformations. However, a repeated-measures ANOVA was considered the most appropriate option for statistical analyses as it is relatively robust to violations of normality.26 Where necessary, degrees of freedom were adjusted using the Greenhouse-Geisser approach to correct for violations of the sphericity assumption. To examine the influence of dysfunctional beliefs about sleep on changes in sleep quality, a Pearson’s correlation coefficient was calculated between the baseline DBAS total score and percentage change in ISI score (baseline to average ISI score from days 7 to 28). Data from all participants who returned their response booklets were included in analyses. All data were analyzed using SPSS (version 24; IBM Corporation, Armonk, NY).
RESULTS
Study population
Baseline questionnaire and demographic information
From 118 individuals who completed the initial online screening questionnaire, 55 individuals were either ineligible (n = 45) or did not complete the initial questionnaires (n = 10). Sixty-three volunteers participated in the study and data from 55 participants who completed all questionnaires over the 28-day period were used for statistical analyses. Baseline data of these 55 participants are detailed in Table 1 and baseline demographic details of the total recruited sample are detailed in Table S1 in the supplemental material. Eight participants withdrew or did not return the response booklets. There were no significant differences in drop-out rates across groups. Reasons for withdrawal included no reason given (n = 4), the commencement of new medication (n = 3), and deciding to stop taking tablets (n = 1). No participant reported withdrawing from the study due to reported adverse events associated with supplement intake.
Table 1.
Baseline demographic characteristics of participants.
| Saffron (n = 28) | Placebo (n = 27) | P | |
|---|---|---|---|
| Age, years | |||
| Mean | 47.86 | 52.63 | .058a |
| SE | 2.05 | 1.33 | |
| BMI, kg/m2 | |||
| Mean | 25.24 | 25.64 | .684a |
| SE | 0.73 | 0.65 | |
| Sex, n (%) | |||
| Male | 4 (86) | 5 (19) | .671b |
| Female | 24 (14) | 22 (81) | |
| Marital status, n (%) | |||
| Single | 25 (89) | 23 (85) | .684b |
| Married | 3 (11) | 4 (15) | |
| Educational level, n (%) | |||
| Secondary | 39 (32) | 8 (30) | .659b |
| Tertiary | 15 (54) | 17 (63) | |
| Postgraduate | 4 (14) | 2 (7) | |
| Exercise level, n (%) | |||
| Never/rarely | 9 (32) | 15 (56) | .131b |
| 1–2 times a week | 13 (46) | 6 (22) | |
| 3–5 times a week | 6 (21) | 6 (22) | |
| Duration of sleep problems, n (%) | |||
| <6 months | 5 (18) | 1 (4) | .394b |
| 6–12 months | 3 (11) | 3 (11) | |
| 1–2 years | 8 (29) | 8 (30) | |
| ≥2 years | 12 (43) | 15 (56) | |
| ISI | |||
| Mean | 15.75 | 14.74 | .290a |
| SE | 0.65 | 0.69 | |
| DBAS | |||
| Mean | 74.50 | 78.59 | .550a |
| SE | 3.95 | 5.58 | |
| DASS-21 | |||
| Depression | |||
| Mean | 4.29 | 3.26 | .527a |
| SE | 1.22 | 1.05 | |
| Anxiety | |||
| Mean | 3.07 | 3.19 | .917a |
| SE | 0.69 | 0.84 | |
| Stress | |||
| Mean | 8.64 | 8.15 | .746a |
| SE | 1.04 | 1.11 | |
| Total | |||
| Mean | 16.00 | 14.59 | .679a |
| SE | 2.39 | 2.40 | |
| PSD | |||
| Total sleep time, hours | |||
| Mean | 7.33 | 6.92 | .307a |
| SE | 0.28 | 0.28 | |
| Sleep latency, minutes | |||
| Mean | 39.38 | 32.61 | .444a |
| SE | 7.16 | 4.97 | |
| Number of wakings after sleep onset | |||
| Mean | 3.23 | 3.15 | .826a |
| SE | 0.26 | 0.28 | |
| Sleep quality | |||
| Mean | 2.46 | 2.78 | .120a |
| SE | 0.11 | 0.16 | |
| Mood on awakening | |||
| Mean | 2.88 | 2.72 | .435a |
| SE | 0.11 | 0.16 | |
| Alertness on awakening | |||
| Mean | 2.77 | 3.02 | .254a |
| SE | 0.14 | 0.17 | |
| RSQ | |||
| Mean | 46.73 | 52.93 | .154a |
| SE | 2.46 | 3.54 |
BMI = body mass index; DASS-21 = Depression, Anxiety, Stress Scale–21; DBAS = Dysfunctional Beliefs Associated with Sleep Questionnaire; ISI = Insomnia Severity Index; PSD = Pittsburgh Sleep Diary; RSQ = Restorative Sleep Questionnaire. aIndependent-samples t test. bChi-square test.
Outcome measures
Insomnia Severity Index (primary outcome measure)
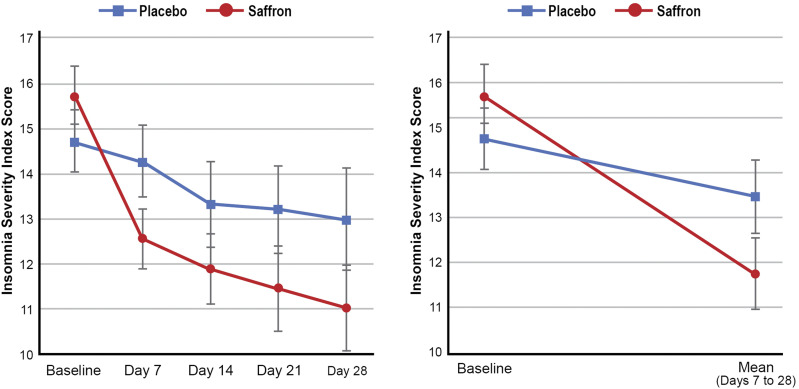
Changes in ISI total score across the 2 treatment groups and repeated-measures ANOVA significance levels are detailed in Table 2 and Figure 2. Reductions in the ISI score were greater in the saffron group than in the placebo group (F1,53 = 6.07, P = .017, η2 = 10.3%). Saffron was associated with a statistically significant reduction in ISI score over time (F1,27 = 26.32, P < .001). However, no significant change was observed in the placebo group (F1,26 = 2.65, P = .116). Within-group contrasts suggest that the majority of changes in ISI scores occurred in the first 7 days of saffron treatment as there were statistically significant improvements from days 0 to 7 (F1,27 = 21.94, P < .001) but no statistically significant changes from days 7 to 14 (F1,27 = 12.89, P = .185), days 14 to 21 (F1,27 = 5.14, P = .352), or days 21 to 28 (F1,27 = 5.14, P = .422).
Table 2.
Change in sleep measures.
| Baseline | Follow-up | Cohen’s d Effect Size | Repeated-Measures ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Time Effects, P | Between-Group Main Effects, P | Time × Group Interaction, P | η2, % | ||||
| ISI (lower scores indicate improved sleep) | |||||||
| Saffron (n = 28) | |||||||
| Meana | 15.75 | 11.74b | 1.07 | .000 | .694 | .017 | 10.3 |
| SE | 0.66 | 0.81 | |||||
| Placebo (n = 27) | |||||||
| Meana | 14.74 | 13.46b | 0.31 | .116 | |||
| SE | 0.67 | 0.83 | |||||
| PSD | |||||||
| Total sleep time, hours | |||||||
| Saffron (n = 28) | |||||||
| Meanb | 7.33a | 7.49c | 0.13 | .477 | .147 | .763 | 0.2 |
| SE | 0.28 | 0.20 | |||||
| Placebo (n = 27) | |||||||
| Meanb | 6.92a | 6.99c | 0.05 | .767 | |||
| SE | 0.29 | 0.20 | |||||
| Sleep latency, minutes | |||||||
| Saffron (n = 28) | |||||||
| Meanb | 39.38a | 29.49c | 0.32 | .149 | .702 | .298 | 2.0 |
| SE | 6.15 | 4.73 | |||||
| Placebo (n = 27) | |||||||
| Meanb | 32.61a | 31.06c | 0.06 | .711 | |||
| SE | 6.26 | 4.81 | |||||
| Number of awakenings after sleep onset | |||||||
| Saffron (n = 28) | |||||||
| Meanb | 3.23a | 2.51c | 0.52 | .001 | .573 | .053 | 6.9 |
| SE | 0.27 | 0.25 | |||||
| Placebo (n = 27) | |||||||
| Meanb | 3.15a | 2.98c | 0.13 | .443 | |||
| SE | 0.27 | 0.25 | |||||
| Sleep quality (higher scores indicate greater quality) | |||||||
| Saffron (n = 28) | |||||||
| Meanb | 2.46a | 2.99c | 0.88 | .000 | .679 | .014 | 10.9 |
| SE | 0.14 | 0.12 | |||||
| Placebo (n = 27) | |||||||
| Meanb | 2.78a | 2.81c | 0.04 | .848 | |||
| SE | 0.14 | 0.12 | |||||
| Mood on awakening (lower scores indicate greater calmness) | |||||||
| Saffron (n = 28) | |||||||
| Meanb | 2.88a | 2.84c | 0.06 | .746 | .757 | .270 | 2.3 |
| SE | 0.14 | 0.14 | |||||
| Placebo (n = 27) | |||||||
| Meanb | 2.72a | 2.88c | 0.20 | .257 | |||
| SE | 0.14 | 0.14 | |||||
| Alertness on awakening (higher scores indicate greater alertness) | |||||||
| Saffron (n = 28) | |||||||
| Meanb | 2.77a | 3.07c | 0.46 | .037 | .763 | .061 | 6.5 |
| SE | 0.15 | 0.12 | |||||
| Placebo (n = 27) | |||||||
| Meanb | 3.02a | 2.92c | 0.13 | .539 | |||
| SE | 0.16 | 0.12 | |||||
| RSQ (high scores indicate greater restorative sleep) | |||||||
| Saffron (n = 28) | |||||||
| Meanb | 46.73a | 56.12c | 0.72 | .000 | .526 | .029 | 8.6 |
| SE | 3.00 | 2.69 | |||||
| Placebo (n = 27) | |||||||
| Meanb | 52.93a | 54.62c | 0.10 | .569 | |||
| SE | 3.06 | 2.74 | |||||
ANOVA = analysis of variance; ISI = Insomnia Severity Index; PSD = Pittsburgh Sleep Diary; RSQ = Restorative Sleep Questionnaire. aMean baseline score from days −1 and 0. bMean score from days 7 to 28. cMean score from days 3 to 28.
Figure 2. Change in Insomnia Severity Index scores (error bars depict SEs).
Restorative Sleep Questionnaire (secondary outcome measure 1)
Changes in RSQ total score across the 2 treatment groups and repeated-measures ANOVA significance levels are detailed in Table 2. Reductions in the RSQ score were greater in the saffron group than in the placebo group (F1,53 = 5.01, P = .029, η2 = 8.6%). Saffron was associated with a statistically significant reduction in RSQ score over time (F1,27 = 25.25, P < .001). However, no significant change was observed in the placebo group (F1,26 = 0.33, P = .569).
Pittsburgh Sleep Diary (secondary outcome measure 2)
Changes in PSD scores across the 2 treatment groups and repeated-measures ANOVA significance levels are detailed in Table 2. Improvements in sleep quality were greater in the saffron group than in the placebo group (F1,53 = 6.51, P = .014, η2 = 10.9%), and there were near-significant effects in number of awakenings after sleep onset (F1,53 = 3.92, P = .053, η2 = 6.9%) and alertness upon awakening (F1,53 = 3.66, P = .061, η2 = 6.5%). Saffron was associated with statistically significant improvements in the number of awakenings after sleep onset (F1,27 = 14.65, P = .001), sleep quality (F1,27 = 24.58, P < .001), and alertness upon awakening (F1,27 = 4.82, P = .037). No other significant changes were observed in other PSD sleep parameters. In the placebo group, there were no statistically significant changes in any PSD sleep parameters over time.
Depression, Anxiety, and Stress Scale–21 (secondary outcome measure 3)
Changes in DASS-21 subscores across the 2 treatment groups and repeated-measures ANOVA significance levels are detailed in Table 3. All baseline scores were within normal levels and repeated-measures ANOVAs revealed no statistically significant changes in DASS-21 depression, anxiety, or stress scores over time in either the saffron or placebo group.
Table 3.
Change in DASS-21 scores.
| Baseline | Day 28 | Repeated-Measures ANOVA, P Value | |||
|---|---|---|---|---|---|
| Time Effects | Between-Group Main Effects | Time × Group Interaction | |||
| Depression | |||||
| Saffron | |||||
| Mean | 4.21 | 4.41 | .852 | .305 | .467 |
| SE | 1.11 | 0.88 | |||
| Placebo | |||||
| Mean | 3.31 | 2.38 | .397 | ||
| SE | 1.17 | 0.93 | |||
| Anxiety | |||||
| Saffron | |||||
| Mean | 2.97 | 3.59 | .286 | .643 | .102 |
| SE | 0.74 | 0.69 | |||
| Placebo | |||||
| Mean | 3.31 | 2.38 | .228 | ||
| SE | 0.79 | 0.72 | |||
| Stress | |||||
| Saffron | |||||
| Mean | 8.41 | 9.52 | .411 | .949 | .995 |
| SE | 1.05 | 1.31 | |||
| Placebo | |||||
| Mean | 8.38 | 9.50 | .405 | ||
| SE | 1.11 | 1.38 | |||
ANOVA = analysis of variance; DASS-21 = Depression, Anxiety, Stress Scale–21.
Dysfunctional Beliefs Associated with Sleep Questionnaire (process measure)
A statistically significant correlation between baseline DBAS score and percentage change in ISI score was observed (r = −.325, P = .015), indicating higher DBAS scores were associated with lower change scores.
Intake of supplements
At day 28, participants recorded their quantity of remaining supplements. All participants reported taking more than 70% of their tablets, although consistency of use over the 28-day period could not be ascertained.
Efficacy of participant blinding
To evaluate the efficacy of condition concealment over the study, participants were asked at the completion of the study to predict condition allocation (ie, placebo, saffron, or uncertain). Efficacy of group concealment was high as 88% in the saffron group, and 71% in the placebo group either incorrectly guessed treatment allocation or were unsure.
Adverse events
No significant adverse events were reported by participants and no participant withdrew from the study due to concerns associated with supplement intake. Further confirmation of the tolerability and safety of saffron intake is provided by an examination of satisfaction ratings at the end of the study, which indicated that 4% of participants in the saffron group (compared with 15% in the placebo group) were dissatisfied with their tablet intake.
DISCUSSION
In this parallel, randomized, double-blind, placebo-controlled trial, the 28-day intake of a standardized saffron extract (affron) was associated with a significant improvement in sleep quality in adults with self-reported poor sleep. Compared with placebo, affron taken at 14 mg twice daily was associated with greater reductions in insomnia severity, as measured by the ISI, and nonrestorative sleep, as measured by the RSQ. Based on sleep diary recordings, saffron also significantly increased ratings of sleep quality, and there were strong trends suggesting improvements in ratings of alertness upon awakening and reductions in the number of awakenings after sleep onset. An examination of changes in ISI scores over time suggests that saffron was associated with relatively rapid improvements in sleep quality as the bulk of sleep improvements occurred in the first 7 days of treatment, with continued, albeit less pronounced, improvements thereafter. It should be noted that, even though saffron was associated with greater improvements in sleep quality compared with the placebo, subthreshold (borderline) insomnia (mean ISI score of 11.7) persisted at the end of the 28-day intervention. An absence of insomnia on the ISI is a score of 7 or less, whereas a score of 15 or more represents clinical (moderate or severe) insomnia.21
Saffron intake was well tolerated with no reported adverse events and positive satisfaction ratings. Mood ratings as measured by the DASS-21 also remained constant over the 28-day period, with pre- and postintervention levels remaining within the normal ranges on all these subscales. Interestingly, in this study, dysfunctional beliefs about sleep as measured by the DBAS negatively impacted treatment outcomes in all participants. This suggests that dysfunctional sleep beliefs present as a potential barrier to successful treatment.
The positive results of this study are congruent with previous research examining the sleep-enhancing effects of saffron16,18,19; however, this study adds to the body of evidence by using an Australian population with self-reported sleep disturbances and no comorbid medical or psychiatric conditions. Moreover, validated outcome measures were used and the average efficacy of saffron intake over a 28-day period was examined. Unlike many of the previous studies, a standardized saffron extract (affron) was also investigated. Saffron is subject to adulteration,27 so the quality of extracts can vary significantly. By investigating a standardized extract, the quality and active constituents of saffron used in this study (which undergo chromatographic profiling by the raw ingredient manufacturer prior to commercial release) can be re-examined in future studies.
The mechanisms associated with saffron’s potential sleep-enhancing qualities are uncertain. The serotonergic, glutaminergic, and γ-aminobutyric acid (GABA)–ergic systems that are implicated in sleep and insomnia28–30 are influenced by saffron administration.31–34 Saffron’s anti-inflammatory effects35 may also be associated with its sleep-enhancing and sleep-restorative effects as insomnia is associated with increased inflammatory markers.36 In animal models, the individual saffron constituents, comprising safranal, crocin, and crocetin, are associated with increases in non–rapid eye movement sleep.37,38 Saffron also modifies EEG activity by increasing delta power.18 As saffron has positive effects on depressive and anxiety symptoms, its sleep-enhancing effects may also result from its impact on affective symptoms. However, in this study, the recruited population had either no or very mild affective distress and no change was noted in mood-related symptoms, as measured by the DASS-21. While the sleep-enhancing effects of saffron were unlikely to be due to mood-related improvements, they might have been due to distress-related perceptions associated with sleep, which according to the DBAS were associated with reduced treatment efficacy. As participants did not complete a postevaluation of the DBAS, changes in dysfunctional beliefs about sleep associated with saffron intervention could not be determined. Further research is required to elucidate these findings and the primary mechanisms associated with saffron’s sleep-enhancing effects.
Limitations and directions for future research
Despite positive improvements in sleep quality associated with saffron intake, this study had several limitations. The recruited sample comprised a population with a mild severity of sleep problems (ISI score <21) so the efficacy of saffron in individuals with a greater severity of insomnia is unknown. Most participants also were female (87%) and peri- or postmenopausal. Therefore, the applicability of these findings in males, and younger or older females, requires examination in future trials. Outcome measures comprised validated, self-reported sleep measures; however, no objective measure of sleep change was included. Polysomnography and actigraphy assessments will be important to include in future studies to help validate the results from self-reported assessments. However, it is important to be aware that while self-reported sleep measures do not always closely correspond with objective measures of sleep,39 they are equally predictive of sleep-related morbidity and mortality.40,41
A single dose of 14 mg of affron twice daily was used in the study. The efficacy and safety of using differing doses require further investigation. Dose-escalation studies may also be helpful to examine the impact of dose on the magnitude of treatment effects. As saffron is subject to adulteration and the quality of extracts can vary significantly, the generalizability of these findings to other saffron extracts should also be made cautiously. Therefore, replication using other saffron extracts is essential to assess the generalizability of findings.
In this study, dysfunctional sleep beliefs presented as a barrier to successful treatment. Therefore, modifying dysfunctional beliefs via interventions such as CBT may be associated with greater treatment efficacy. It may be prudent to investigate saffron as an adjunct to CBT compared with a stand-alone intervention in future studies. Moreover, adjunct interventions may increase the likelihood of complete symptom remission as, on average, participants continued to present with borderline insomnia. Finally, the sleep-enhancing effects of saffron in differing populations require further investigation. This includes individuals with comorbid medical and/or psychiatric conditions, chronic or severe sleep disturbances, and in participants with a range of demographic and psychographic characteristics.
The results from this study indicate that a standardized saffron extract (affron) at a dose of 14 mg twice daily for 28 days improved sleep quality in adults with self-reported poor sleep, with most of these changes occurring in the first 7 days of treatment. Saffron was well tolerated with no reported adverse effects. While positive, these findings require replication using a larger sample size and differing populations.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was funded by Pharmactive Biotech Products SL. Pharmactive Biotech Products was not involved in the design of the research, analysis of data, or in the writing of the report. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors gratefully acknowledge Pharmactive Biotech Products SL for funding the project and supplying affron and LIPA Pharmaceuticals for the preparation of the tablets.
ABBREVIATIONS
- ANOVA
analysis of variance
- CBT
cognitive behavioral therapy
- DASS-21
Depression, Anxiety, and Stress Scale–21
- DBAS
Dysfunctional Beliefs Associated with Sleep Questionnaire
- EEG
electroencephalography
- ISI
Insomnia Severity Index
- PSD
Pittsburgh Sleep Diary
- RSQ
Restorative Sleep Questionnaire
REFERENCES
- 1.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. 10.1093/sleep/31.4.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. 10.5664/jcsm.27286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams RJ, Appleton SL, Taylor AW, et al. Sleep health of Australian adults in 2016: results of the 2016 Sleep Health Foundation national survey. Sleep Health. 2017;3(1):35–42. 10.1016/j.sleh.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 4.Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. 10.2147/NSS.S134864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertisch SM, Pollock BD, Mittleman MA, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep. 2018;41(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1-3):10–19. 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 7.Espie CA, Pawlecki B, Waterfield D, Fitton K, Radocchia M, Luik AI. Insomnia symptoms and their association with workplace productivity: cross-sectional and pre-post intervention analyses from a large multinational manufacturing company. Sleep Health. 2018;4(3):307–312. 10.1016/j.sleh.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 8.Daley M, Morin CM, LeBlanc M, Gregoire JP, Savard J, Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427–438. 10.1016/j.sleep.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 9.Hillman DR, Lack LC. Public health implications of sleep loss: the community burden. Med J Aust. 2013;199(8):S7–S10. [DOI] [PubMed] [Google Scholar]
- 10.Maust DT, Solway E, Clark SJ, Kirch M, Singer DC, Malani P. Prescription and nonprescription sleep product use among older adults in the United States. Am J Geriatr Psychiatry. 2019;27(1):32–41. 10.1016/j.jagp.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Ortuño MM, Belanger L, Ivers H, LeBlanc M, Morin CM. The use of natural products for sleep: a common practice? Sleep Med. 2009;10(9):982–987. 10.1016/j.sleep.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 12.Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1–12. 10.1016/j.smrv.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Marx W, Lane M, Rocks T, et al. The effect of saffron supplementation on symptoms of depression and anxiety: a systematic review and meta-analysis. Nutr Rev. 2019;77(8):557–571. 10.1093/nutrit/nuz023 [DOI] [PubMed] [Google Scholar]
- 14.Lopresti AL, Drummond PD. Saffron (Crocus sativus) for depression: a systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Hum Psychopharmacol. 2014;29(6):517–527. 10.1002/hup.2434 [DOI] [PubMed] [Google Scholar]
- 15.Toth B, Hegyi P, Lantos T, et al. The efficacy of saffron in the treatment of mild to moderate depression: a meta-analysis. Planta Med. 2019;85(1):24–31. [DOI] [PubMed] [Google Scholar]
- 16.Nishide A, Fujita T, Nagaregawa Y, et al. Sleep enhancement by saffron extract affron® in randomized control trial. Jpn Pharmacol Ther. 2018;46(8):1407–1415. [Google Scholar]
- 17.Lopresti AL, Smith SJ, Hood SD, Drummond PD. Efficacy of a standardised saffron extract (affron(R)) as an add-on to antidepressant medication for the treatment of persistent depressive symptoms in adults: a randomised, double-blind, placebo-controlled study. J Psychopharmacol. 2019;33(11):1415–1427. [DOI] [PubMed] [Google Scholar]
- 18.Umigai N, Takeda R, Mori A. Effect of crocetin on quality of sleep: a randomized, double-blind, placebo-controlled, crossover study. Complement Ther Med. 2018;41:47–51. 10.1016/j.ctim.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 19.Milajerdi A, Jazayeri S, Shirzadi E, et al. The effects of alcoholic extract of saffron (Crocus satious L.) on mild to moderate comorbid depression-anxiety, sleep quality, and life satisfaction in type 2 diabetes mellitus: a double-blind, randomized and placebo-controlled clinical trial. Complement Ther Med. 2018;41:196–202. 10.1016/j.ctim.2018.09.023 [DOI] [PubMed] [Google Scholar]
- 20.Soper DS. A-priori sample size calculator for Student t-tests [software]. Available at: http://www.danielsoper.com/statcalc. Accessed February 11, 2019.
- 21.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drake CL, Hays RD, Morlock R, et al. Development and evaluation of a measure to assess restorative sleep. J Clin Sleep Med. 2014;10(7):733–741. 10.5664/jcsm.3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monk TH, Reynolds CF 3rd, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3(2):111–120. 10.1111/j.1365-2869.1994.tb00114.x [DOI] [PubMed] [Google Scholar]
- 24.Brown TA, Chorpita BF, Korotitsch W, Barlow DH. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav Res Ther. 1997;35(1):79–89. 10.1016/S0005-7967(96)00068-X [DOI] [PubMed] [Google Scholar]
- 25.Morin CM, Vallieres A, Ivers H. Dysfunctional Beliefs and Attitudes about Sleep (DBAS): validation of a brief version (DBAS-16). Sleep. 2007;30(11):1547–1554. 10.1093/sleep/30.11.1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabachnick BG, Fidell LS. Using multivariate statistics. Boston: Allyn; 2007. [Google Scholar]
- 27.Khilare V, Tiknaik A, Prakash B, et al. Multiple tests on saffron find new adulterant materials and reveal that Ist grade saffron is rare in the market. Food Chem. 2019;272:635–642. 10.1016/j.foodchem.2018.08.089 [DOI] [PubMed] [Google Scholar]
- 28.van Dalfsen JH, Markus CR. The involvement of sleep in the relationship between the serotonin transporter gene-linked polymorphic region (5-HTTLPR) and depression: a systematic review. J Affect Disord. 2019;256:205–212. 10.1016/j.jad.2019.05.047 [DOI] [PubMed] [Google Scholar]
- 29.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111(2):231–239. 10.1016/S0306-4522(02)00034-9 [DOI] [PubMed] [Google Scholar]
- 30.Meyerhoff DJ, Mon A, Metzler T, Neylan TC. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep. 2014;37(5):893–900. 10.5665/sleep.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Georgiadou G, Tarantilis PA, Pitsikas N. Effects of the active constituents of Crocus sativus L., crocins, in an animal model of obsessive-compulsive disorder. Neurosci Lett. 2012;528(1):27–30. 10.1016/j.neulet.2012.08.081 [DOI] [PubMed] [Google Scholar]
- 32.Hosseinzadeh H, Sadeghnia HR. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: involvement of GABAergic and opioids systems. Phytomedicine. 2007;14(4):256–262. 10.1016/j.phymed.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 33.Sadeghnia HR, Cortez MA, Liu D, Hosseinzadeh H, Snead OC 3rd. Antiabsence effects of safranal in acute experimental seizure models: EEG and autoradiography. J Pharm Sci. 2008;11(3):1–14. 10.18433/J38G6J [DOI] [PubMed] [Google Scholar]
- 34.Berger F, Hensel A, Nieber K. Saffron extract and trans-crocetin inhibit glutamatergic synaptic transmission in rat cortical brain slices. Neuroscience. 2011;180:238–247. 10.1016/j.neuroscience.2011.02.037 [DOI] [PubMed] [Google Scholar]
- 35.Zeinali M, Zirak MR, Rezaee SA, Karimi G, Hosseinzadeh H. Immunoregulatory and anti-inflammatory properties of Crocus sativus (Saffron) and its main active constituents: a review. Iran J Basic Med Sci. 2019;22(4):334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slavish DC, Graham-Engeland JE, Engeland CG, Taylor DJ, Buxton OM. Insomnia symptoms are associated with elevated C-reactive protein in young adults. Psychol Health. 2018;33(11):1396–1415. 10.1080/08870446.2018.1500577 [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Xu XH, Liu TY, et al. Safranal enhances non-rapid eye movement sleep in pentobarbital-treated mice. CNS Neurosci Ther. 2012;18(8):623–630. 10.1111/j.1755-5949.2012.00334.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masaki M, Aritake K, Tanaka H, Shoyama Y, Huang ZL, Urade Y. Crocin promotes non-rapid eye movement sleep in mice. Mol Nutr Food Res. 2012;56(2):304–308. 10.1002/mnfr.201100181 [DOI] [PubMed] [Google Scholar]
- 39.Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22(5):462–468. 10.2188/jea.JE20120012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33(4):531–538. 10.1093/sleep/33.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011;12(3):215–221. 10.1016/j.sleep.2010.07.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.