Abstract
Study Objectives:
In-laboratory titration polysomnography (PSG) is standard to determine optimal therapeutic continuous positive airway pressure (CPAP) in children with obstructive sleep apnea (OSA). The use of auto-titrating CPAP devices (autoCPAP) as an alternative to CPAP titration has not been well studied in children. We hypothesized that autoCPAP-derived pressures (PMEAN, PPEAKMEAN, P90) would be similar to titration PSG pressure (PPSG).
Methods:
This is a retrospective study of children with OSAS initiated on autoCPAP between 2007 and 2017, who used autoCPAP for at least 2 h/night and who had adequate titration PSG were included in the analysis. AutoCPAP-derived pressures were obtained from use downloads and compared with PPSG. PPSG predictive factors were analyzed by median regression. Nonparametric methods were used for analysis.
Results:
Of 110 children initiated on autoCPAP, 44 satisfied the inclusion criteria. Age (median (interquartile range)) was 13.01 (9.98–16.72) years, and 63.6% were obese. PPSG median (interquartile range) was 8 (7–11) cmH2O, mean autoCPAP-derived pressure (PMEAN) was 6.2 (5.6–7.6) cmH2O, peak mean pressure (PPEAKMEAN) was 9.4 (7.7–11.1) cmH2O, and average device pressure ≤ 90% of the time (P90) was 8.1 (7.2–9.7) cmH2O. AutoCPAP-derived pressures correlated with PPSG (P < .05). PMEAN was lower than the other 3 pressures (P < .0002). Median regression analysis demonstrated that after adjusting for patient characteristics such as age, sex, and obesity status, autoCPAP-derived pressures remained significant predictors of PPSG (P < .05). There were no significant interactions between these patient characteristics and autoCPAP-derived pressures.
Conclusions:
This study demonstrates that autoCPAP-derived pressures correlate with the titration PSG-derived pressures. These results indicate that autoCPAP can be used in the pediatric population and can determine pressures that are close to the titration pressures.
Citation:
Khaytin I, Tapia IE, Xanthopoulos MS, et al. Auto-titrating CPAP for the treatment of obstructive sleep apnea in children. J Clin Sleep Med. 2020;16(6):871–878.
Keywords: auto-titrating CPAP, children, continuous positive airway pressure, device pressure, obstructive sleep apnea, pediatrics, titration polysomnography, treatment pressure
BRIEF SUMMARY
Current Knowledge/Study Rationale: In-laboratory titration polysomnography is standard to determine optimal therapeutic continuous positive airway pressure (CPAP) in children with obstructive sleep apnea treated with CPAP. The use of auto-titrating CPAP devices as an alternative is not well studied in children.
Study Impact: This study demonstrates that auto-titrating CPAP–derived pressures correlate with the titration polysomnography-derived pressures. These results indicate that auto-titrating CPAP can be used in the pediatric population and can determine pressures that are close to the titration pressures.
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep breathing disorder associated with multiple neurobehavioral and medical problems in children.1–3 Its prevalence in the pediatric population is estimated to be as high as 5.7%.4 The first-line modality of treating OSA in children is adenotonsillectomy. Adenotonsillectomy has been shown to improve behavior, quality of life, and symptoms of OSA in children.5 However, studies have shown that 134,6 to 73%4,7 of children continue to have residual symptoms of OSA postoperatively.
The secondary modality of treating OSA in children, as recommended by the American Academy of Pediatrics, is continuous positive airway pressure (CPAP).4 It is used for children with residual symptoms and polysomnographic evidence of OSA after adenotonsillectomy or for children who are not appropriate candidates for surgery. Currently, recommendations for starting CPAP treatment in children include manual titration of pressure during an in-laboratory titration polysomnography (PSG).8 Titration PSG has been the standard of care to find precise therapeutic CPAP pressures in children; however, disadvantages include the patient spending a night in the sleep laboratory, cost, and possibly delays in achieving therapeutic pressures.
One alternative to manual titration in a sleep laboratory is the use of auto-titrating CPAP devices (autoCPAP). Such devices, using proprietary algorithms, adjust the delivered pressure based on several parameters that the device detects, such as airway resistance and inspiratory flow contours. In adults, autoCPAP is widely used in place of in-laboratory titration and is similar to CPAP in reducing symptoms of OSA.9 However, it is not known whether autoCPAP provides similar pressure to that of in-laboratory-derived CPAP pressures in children as in adults. Because of smaller airway sizes, faster respiratory rates, and differences in pulmonary mechanics, airflow and other respiratory parameters are different in children.10 Moreover, each manufacturer uses its own proprietary algorithm to determine the appropriate pressure, which may not be optimized for children. In addition, autoCPAP has been understudied in children.11–14
The present study was aimed to evaluate the relationship between pressures determined by autoCPAP and manual titration in children treated with CPAP and to determine which patient parameters are clinically relevant in selecting appropriate candidates for autoCPAP. We hypothesized that autoCPAP-derived pressures would be similar to in-laboratory derived pressures.
METHODS
Study design and participants
This study is a retrospective review of children with OSA who underwent an autoCPAP initiation at the Children’s Hospital of Philadelphia (CHOP) Sleep Center between January 2007 and December 2017. The study was approved by the CHOP institutional review board. All participants were diagnosed with OSA based on diagnostic PSG. PSG was performed according to the American Academy of Sleep Medicine standards. They were scored by registered polysomnographic technicians using The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (AASM Scoring Manual)15 rules and interpreted by board-certified sleep medicine physicians. Most children underwent evaluation for adenotonsillectomy and if necessary, another polysomnographic study establishing residual postsurgical OSA. During the study period, approximately 900 children were deemed appropriate to be initiated on CPAP by their sleep medicine physicians, and 110 of these children were initiated on autoCPAP at the discretion of their sleep medicine physician with consideration of the advantages and disadvantages of autoCPAP. The lower and upper limits of autoCPAP pressures were chosen empirically by the treating physician based on the clinical experience and any prior titration data if available. All patients were followed in the CHOP Sleep Center interdisciplinary CPAP program, where patients were routinely assessed for medical and technical support, side effects, and adherence.
Patients initiated on autoCPAP were identified by review of our center’s CPAP database, which includes every patient treated with CPAP as an outpatient in the Sleep Center during the study period. Only participants who had a baseline PSG study, an adequate titration PSG, and who had autoCPAP use data within 90 days from the titration study were included in the analyses. In this study, a cutoff of at least 75% reduction of obstructive apnea-hypopnea index (OAHI) on the optimal pressure compared with the baseline OAHI was chosen to define adequate titration PSG. Considering the time limitations associated with an overnight sleep study, it is common for the study to end before the highest pressure needed to eliminate all events is achieved, as evidenced by commonly nonzero residual OAHI. Additionally, to be included in the study, the autoCPAP use data download had to demonstrate that the participant used autoCPAP for at least 120 minutes on the nights used to assess autoCPAP mean pressure (PMEAN), which is the mean pressure maintained by the autoCPAP device during the time it was used; autoCPAP peak average pressure (PPEAKMEAN), which is the mean of all peak pressures across the nights the machine was used; and average device pressure ≤ 90% of the time (P90), which is the average pressure the patient spent not more than 90% of the treatment time. The children that were not tolerating autoCPAP were thus not included in this study.
Assessments
All baseline and titration PSG studies were performed at the CHOP sleep laboratory by registered polysomnographic technologists experienced with children. Each technologist was responsible for monitoring study parameters and correcting problems with sensors throughout the night. Each technologist supervised the studies of 1–2 children per night. During titration studies, the technologist adjusted CPAP pressure to eliminate all obstructive events and snoring. Titration PSGs were performed using Remstar Omnilab (Philips Respironics, Murrysville, PA) equipment in accordance with the AASM Scoring Manual technical and digital specifications.15 The following polysomnographic variables were extracted from the baseline and titration studies: age, race, sex, weight, height, baseline OAHI, optimal CPAP titration pressure as recommended by the physician interpreting the study or highest pressure reached during the study, and OAHI at that pressure. Several of the children had more than 1 titration after a single baseline polysomnography. In that case, only the first titration PSG closest to the baseline date was included in this study.
AutoCPAP use data were downloaded using the Encore Anywhere (Philips Respironics, Murrysville, PA) web-based interface for 90 days preceding the titration study. If during that period, no use was found, use during 90 days after the titration study was downloaded. If during that time the patient also did not have autoCPAP use satisfying the above criteria, the patient was excluded from the study. The following parameters were extracted from EncoreAnywhere: days downloaded (up to 90 days), days used, average number of minutes autoCPAP was used on the nights used, maximum and minimum set pressure, and minutes in large leak. Additionally, the following machine-calculated pressures were recorded: PMEAN, PPEAKMEAN, and P90.
Data analysis
Data were analyzed using Matlab version 2017a (Mathworks) software and R version 3.4.4. The data were summarized using standard descriptive statistics. Continuous variables were summarized by the mean and standard deviation or median and interquartile range (IQR), and categorical variables were summarized by the count and percentage. Spearman correlation was used to evaluate the correlations between autoCPAP pressures and PSG pressure. Quantile regression at median was used to evaluate how autoCPAP pressures predict PSG pressures, adjusting for age, obesity, and baseline OAHI. The participants were defined as obese if their body mass index (BMI) was equal or more than 95th percentile for the age.16 The interactive effects between autoCPAP pressures and age, obesity, and OAHI on PSG pressures were also evaluated. We also evaluated Bland-Altman plots and Lin’s concordance correlation coefficients to visualize and quantify the degree of agreement between each of the autoCPAP determined pressures (PMEAN, PPEAKMEAN, and P90) and titration pressures. P ≤ .05 was considered significant.
RESULTS
Over the study period, 110 children were started on autoCPAP. Of these, 66 children did not meet all the inclusion criteria of having had a titration, having used autoCPAP within 90 days of titration, or having used autoCPAP for more than 120 minutes on the nights used. Forty-four children met all the inclusion criteria. Table 1 describes the characteristics of the diverse patient population. Most of the study participants were preteens and teens at the time of the titration study. There were no children with failure to thrive or respiratory failure in the study population. Most children also had 1 or several behavioral problems (Table 2).
Table 1.
Demographic and clinical characteristics of the study group.
| Characteristics | Median (IQR) or Number (%) |
|---|---|
| Age at baseline (years) | 13.01 (9.98, 16.72) |
| Age at titration (years) | 13.62 (10.36, 17.44) |
| Male, n (%) | 25 (56.82%) |
| Race, n (%) | |
| African American | 22 (50.00%) |
| White | 19 (43.18%) |
| Other | 3 (6.82%) |
| BMI-z | 2.78 (0.54, 4.50) |
| Obese, n (%) | 28 (63.64%) |
| Baseline OAHI (events/h) | 18.35 (9.76, 29.92) |
| Baseline CAHI (events/h) | 0.40 (0, 1.10) |
| Baseline total sleep time (minutes) | 399 (355, 434) |
| Baseline SpO2 min (%) | 89 (88, 92) |
| Baseline EtCO2 max (mm Hg) | 50 (47, 54) |
| Titration OAHI (events/h) | 1.00 (0.40, 1.65) |
| AutoCPAP machine used, n (%) | |
| DreamStation AutoCPAP (500 × 110) | 27 (61%) |
| DreamStation AutoBiPAP (700 × 110) | 1 (2%) |
| REMstar Auto (System One 60 series) | 16 (36%) |
Total number of participants (N) was 44 children. Age, BMI-z, and PSG parameters were not distributed normally, and therefore median and interquartile range are provided. Data in the table are presented as median (IQR) or N (%). AutoCPAP, auto-titrated continuous positive airway pressure, BMI-z = modified body mass index z-score (obese, BMI ≥ 95%), CAHI = central apnea-hypopnea index, OAHI = obstructive apnea-hypopnea index, PSG = polysomnogram.
Table 2.
Clinical diagnoses of the study population.
| Diagnoses | Number (%) |
|---|---|
| Behavioral problems | 40 (90.9%) |
| Asthma | 36 (81.8%) |
| Cerebral palsy and congenital anomalies | 6 (13.6%) |
| Musculoskeletal problems | 4 (8.7%) |
| Other neurologic problems | 18 (39.1%) |
| Chromosomal abnormalities including trisomy 21 | 7 (15.9%) |
| Status after adenotonsillectomy | 29 (65.9%) |
Number of children and percent of the total study population (44 children) with a given diagnosis. Most children had more than 1 diagnosis.
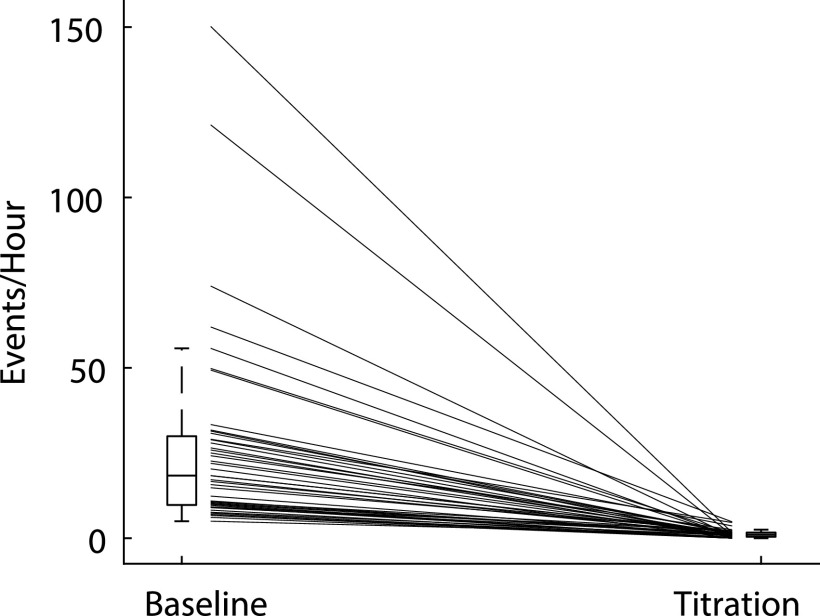
All children showed improvement of OSA after titration of CPAP, with OAHI decreasing from 18.4 [9.8–29.9] (median [IQR]) events/h (range, 5–150 events/h) on the baseline PSG to 1.0 [0.4–1.7] (median [IQR]) events/h (range, 0–4.8 events/h) at optimal pressure during the titration (Figure 1). There was no correlation between age and OAHI (r = .003, P = .98) at the time of the baseline PSG. Additionally, there was no correlation between modified BMI z-score17 and OAHI (r = .13, P = .4).
Figure 1. The obstructive apnea-hypopnea index (OAHI) during baseline study and at the optimal pressure during a titration study.
Box and whiskers plot of the OAHI during baseline and titration study. The box represents the 25th and 75th percentile with the median line. Whiskers extend approximately 1.5 times the interquartile range. The lines between 2 boxes connect baseline and titration OAHI for individual participants. Only participants whose OAHI decreased by at least 75% were included in the study.
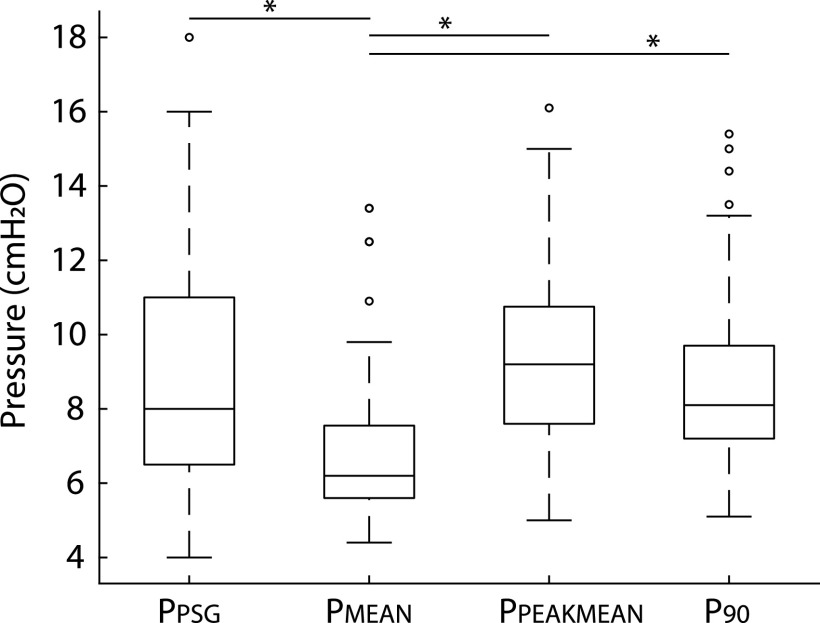
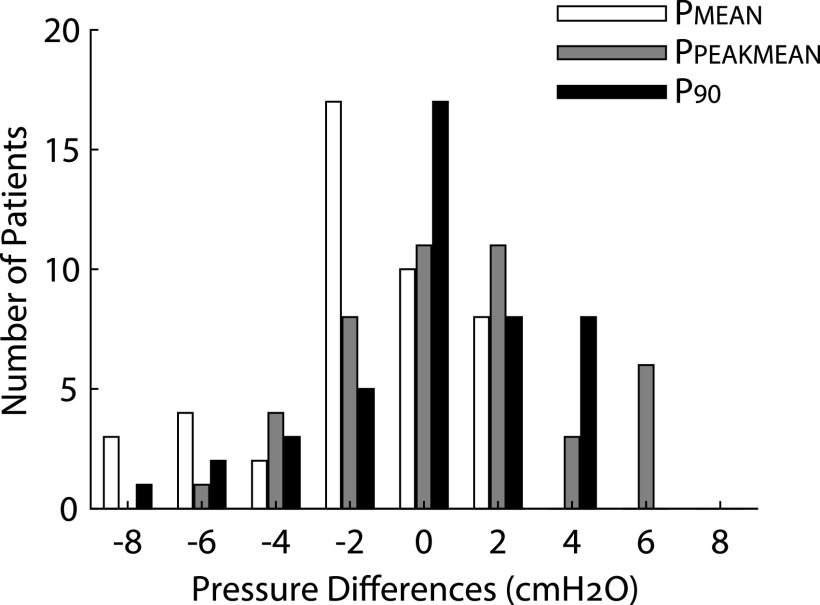
The lower autoCPAP limit varied between 4 and 12 cmH2O (median [IQR]: 4 [4–6]), and the upper limit varied between 8 and 20 cmH2O (median [IQR]: 12 [12–15]). Average autoCPAP use was 80 ± 24.9% (SD) of nights, with average use of 359.7 ± 148.7 minutes (SD) on these nights. Thirty-two of the participants (73%) used autoCPAP at least 4 hours on the nights used. As shown in Figure 2, there was no difference between titration pressure, PPEAKMEAN, and P90, but PMEAN was significantly lower than the other 3 pressures (P < .0002). However, all 3 autoCPAP pressures were correlated with titration pressure (Figure 3). Bland-Altman plots of the pressures corroborated these results (Figure 4). The Lin’s concordance coefficients for the 3 autoCPAP pressures and titration pressure were 0.44 (0.11, 0.68), 0.48 (0.15 0.71), and 0.50 (0.15, 0.74), respectively. This is considered a moderate concordance. When examining the pressure differences (Figure 5), P90 pressure was within 1 cmH2O of titration pressure for 17 (38%) participants and within 3 cmH2O of the titration pressure for 30 (68%) participants. PPEAKMEAN and PMEAN were within 1 cmH2O of titration pressure for 11 (25%) and 10 (22%) participants, respectively. PPEAKMEAN and PMEAN were within 3 cmH2O of titration pressure for 30 (68%) and 35 (79%) participants, respectively.
Figure 2. Comparison of titration pressure (PPSG) and autoCPAP PMEAN, PPEAKMEAN, and P90 pressures.
Box and whisker plot of the titration (PPSG) and 3 autoCPAP pressures. The box represents the 25th and 75th percentile with the median line. Whiskers extend approximately 1.5 times the interquartile range. The only pressure that was statistically significantly different from the other 3 pressures was PMEAN (P < .0002). AutoCPAP, auto-titrated continuous positive airway pressure, PMEAN = autoCPAP mean pressure, PPEAKMEAN = autoCPAP peak mean pressure, PPSG = optimal titration pressure, P90 = average pressure ≤90% of the time.
Figure 3. Median regression analysis of correlation between the titration pressure and 3 autoCPAP pressures.
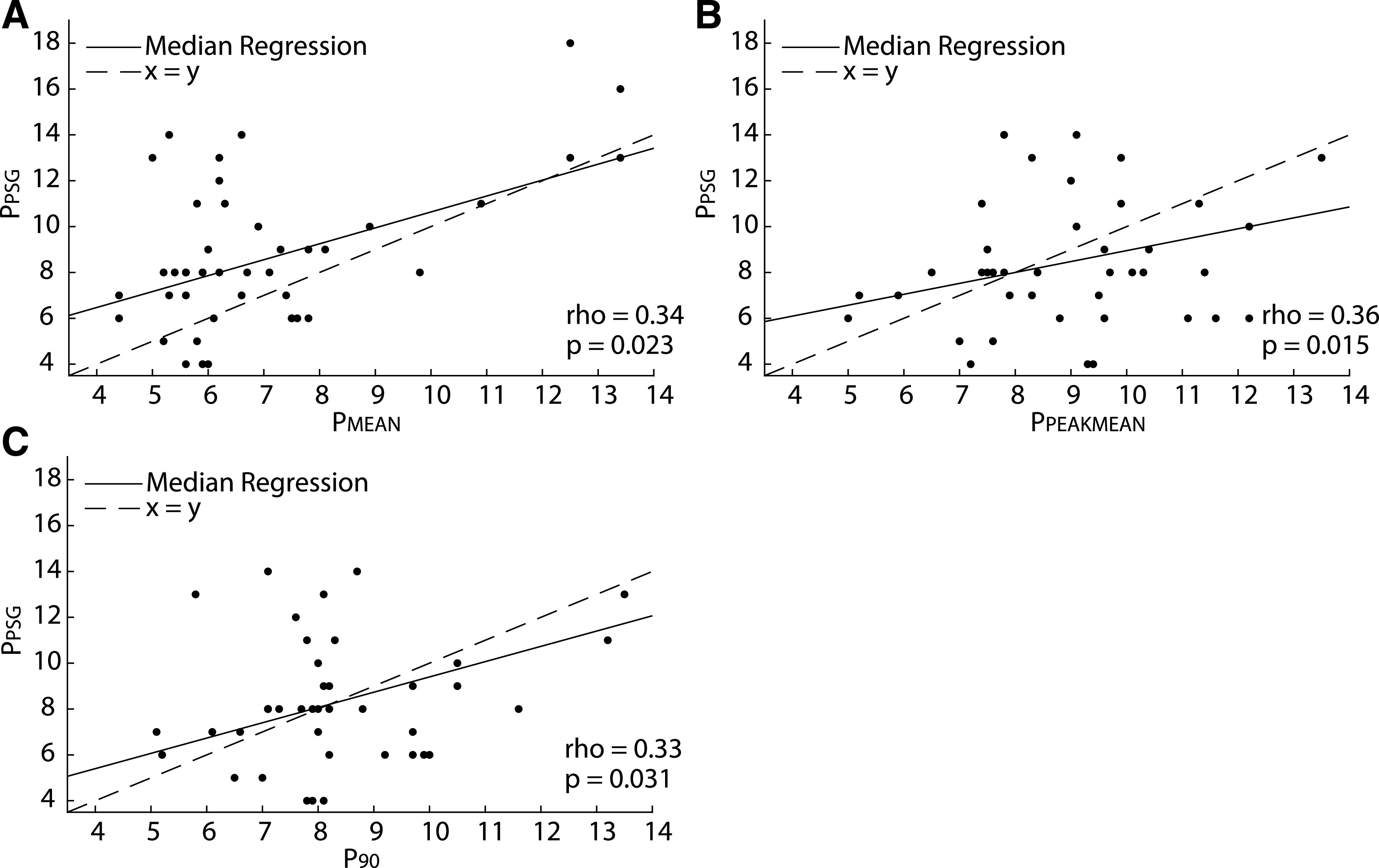
Correlation calculation between titration pressure (PPSG) and (A) autoCPAP mean pressure (PMEAN), (B) peak mean pressure (PPEAKMEAN), and (C) average pressure ≤ 90% of the time (P90) using Spearman rank correlation. There was significant correlation between all three autoCPAP pressures and the titration pressure. AutoCPAP, auto-titrated continuous positive airway pressure,
Figure 4. Bland-Altman plots of comparison of the titration pressure and 3 autoCPAP pressures.
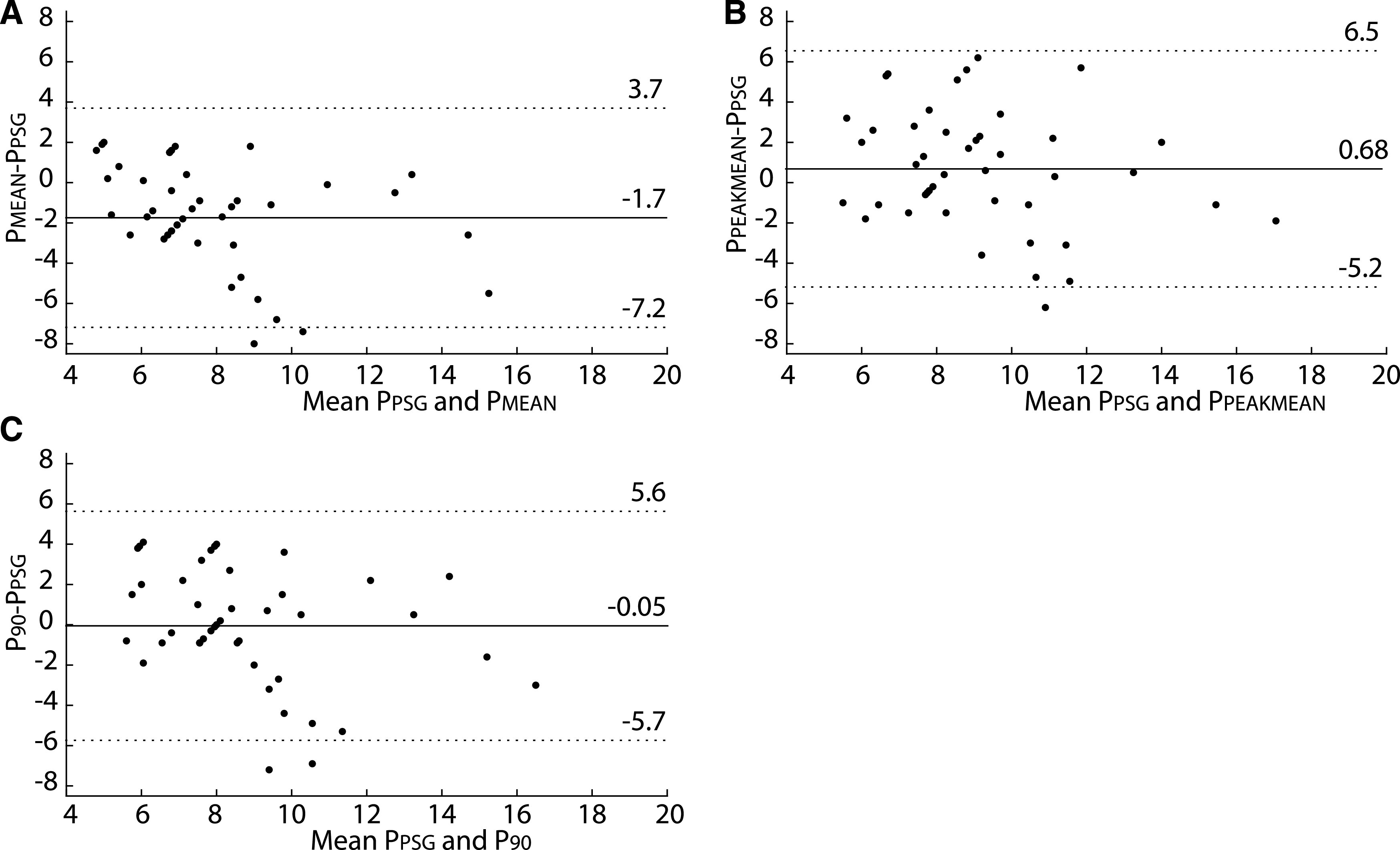
Bland-Altman plots of titration pressure (PPSG) compared with 3 autoCPAP pressures. (A) PPSG compared with PMEAN. (B) PPSG compared with PPEAKMEAN. (C) PPSG compared with P90. Means of PPEAKMEAN and P90 were similar to the titration pressure. PMEAN was smaller than the titration pressure. Each dot shows the average of 2 pressures on the x-axis vs difference of 2 pressures on the y-axis for a single participant. AutoCPAP, auto-titrated continuous positive airway pressure, interrupted horizontal lines = ±1.96 times the standard deviation of the differences between pressures, solid horizontal line = mean of the differences.
Figure 5. Distribution of the pressure difference between PPSG and 3 autoCPAP pressures.
Histogram of the distribution of differences between PMEAN, PPEAKMEAN, and P90 and the titration pressure (PPSG). AutoCPAP, auto-titrated continuous positive airway pressure,
Median regression analysis demonstrated that even after adjusting for patient characteristics such as age, modified BMI z-score, baseline OAHI, race, and sex, the autoCPAP pressures remained statistically significant predictors of the titration pressure with P < .05. However, there was no statistically significant interaction between autoCPAP pressures and patient characteristics, such as age, race, obesity status, baseline OAHI, or minutes in large leak.
DISCUSSION
This study shows that autoCPAP is a useful approach to initiating CPAP for the treatment of OSA in an older population of children with a wide variety of clinical disorders excluding respiratory failure followed in an interdisciplinary pediatric sleep center. AutoCPAP can deliver treatment pressures that reasonably agree with the gold standard of manual titration pressure in our study population.
The autoCPAP algorithms and its application in children to determine changes in pressure (eg, when an increase in pressure is needed) are based on inspiratory airflow contours. In brief, if the algorithm determines the inspiratory airflow is completely obstructed or flow limited, pressures will be increased to alleviate obstruction. Obstructed inspiratory flow contours of children during sleep can differ greatly from adults, and some children exhibit unique inspiratory flow contours that could lead to inappropriate pressure adjustments. Taken together, autoCPAP might work well for some children and not for others. The exact algorithm used to determine the pressure is proprietary and varies from manufacturer to manufacturer. In this study, all of the patients used 1 of 3 Respironics (Philips Respironics, Murrysville, PA) autoCPAP machines. This provides both an advantage of consistency across the whole study population but is also a limitation of this study because, from the adult literature,18 it is known that autoCPAP machines from different manufacturers have different response to the same respiratory events. Respironics autoCPAP machines provide statistics on the pressures used over the course of the treatment using 3 average pressures. The mean pressure (PMEAN) is simply the average pressure that the machine delivered over the whole time it was used. For instance, assuming the pressure can only be increased in discrete 1-cmH2O steps, if on a given night the patient spent 4 hours with pressure of 6 cmH2O, 4 hours with a pressure of 7 cmH2O, and 2 hours a with pressure of 8 cmH2O, the PMEAN pressure for that night is 6.8 cmH2O. The peak mean pressure (PPEAKMEAN) is an average of all the highest pressures that the unit was delivering over the all nights it was used. In the above example, the PPEAKMEAN pressure is 8 cmH2O for that night. Finally, the average pressure ≤90% of the time (P90) is the average device pressure not more than 90% of the night19 was spent. In the above example, the P90 pressure for that night is 7 cmH2O. Figure 6 is an illustration of a single night pressure changes with corresponding autoCPAP pressures.
Figure 6. Example of the autoCPAP-reported pressures.
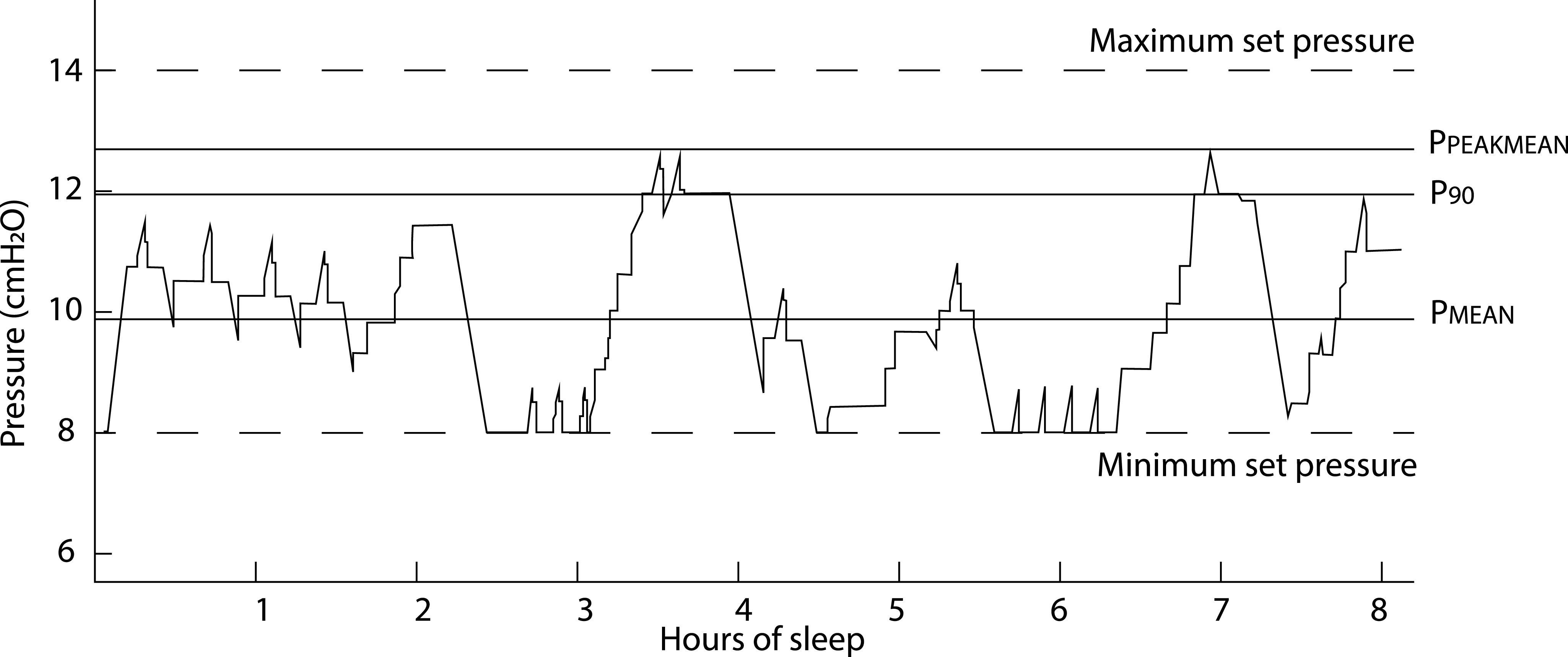
This is a recording of autoCPAP pressure during a single night of recording. Maximum and minimum set pressures are pressure limits set by a physician. AutoCPAP, auto-titrated continuous positive airway pressure, PMEAN = autoCPAP mean pressure, PPEAKMEAN = peak mean pressure, and P90 = average pressure ≤90% of the time.
In this study, we compared the 3 autoCPAP-derived pressures with a pressure obtained using manual titration in the sleep laboratory. The titration pressure is considered the gold standard but has its own limitations. During manual titration, polysomnography technologists are trained to increase pressure to eliminate obstructive events and snoring. Therefore, ideal titration yields the highest CPAP pressure that is needed to eliminate all obstructive events and snoring. However, the actual pressure required to eliminate these events may vary throughout the night or from one night to the next based on a variety of factors including position or sleep stage. Additionally, clinical practice shows that a single night titration may not be sufficient to find the pressure that eliminates all obstructive events.20
All these factors are necessary to consider when comparing the 3 autoCPAP pressures and the titration pressure. Because, under ideal conditions, the titration pressure is high enough to eliminate all of the obstructive events, and the patient may not need that much pressure during the whole night, the PMEAN pressure is expected to be lower than the titration pressure. On the other hand, the PPEAKMEAN pressure represents the average of the highest pressures used over the course of the treatment. Therefore, it is expected to be very similar or higher than the titration pressure. The P90 pressure is expected to be somewhere in between. It does not include the outliers but is a good indicator of the pressure that the autoCPAP delivered most of the time.
Our results confirm these considerations. We found that all 3 autoCPAP pressures correlated well with in-laboratory titration pressures. Furthermore, the PPEAKMEAN and P90 pressures were no different from the titration pressure. The difference between PMEAN pressure and the titration pressure was statistically significant but explainable by the autoCPAP reducing pressure when possible. These findings confirm that the autoCPAP algorithm is able to determine appropriate treatment pressure for most children in this study. However, we also found that there is a wide distribution of the differences between titration pressure and PPEAKMEAN and P90 pressures. We found that, although 68% of children had PPEAKMEAN and P90 within 3 cmH2O of the titration pressure, the maximum difference between either pressure and the titration pressure was 6 cmH2O. There can be several reasons for such a spread. It is possible that for some children, autoCPAP use was insufficient to achieve the optimal treatment pressure. Alternatively, it is possible, that because of time limitation, a single titration study may not be sufficient to determine the optimal treatment pressure. Finally, autoCPAP algorithms have been developed for adults and may not be optimal for children. Transparency of proprietary algorithms would be helpful to determine the appropriateness of autoCPAP for the treatment of OSA in pediatric patients. However, most children initiated on autoCPAP in our Sleep Center received treatment pressures that were close to the titration pressures. Moreover, the differences in pressures that we observed were not excessively different than the differences that were observed in a similar study in the adult population, where the difference between titration and calculated pressure was also found to be between 0 and 1 cmH2O on average.21
In an attempt to predict the ideal pediatric patient for autoCPAP, we looked at whether any demographic parameters would predict a better agreement between the titration and autoCPAP pressures. Theoretically, autoCPAP may be able to determine optimal pressure for children who are older and more similar in their physiology to adults22 However, median regression analysis did not demonstrate significant effect by age, sex, race, baseline OAHI, modified BMI z-score, or obesity status on how well autoCPAP pressures correlate with the titration pressure. Further studies with a less diverse population and larger cohort may be needed to determine the effects of the above factors on autoCPAP ability to determine optimal pressure.
The results of this study are similar to those of previous pediatric research on this topic. Palombini et al12 found that autoCPAP can resolve OSA in most of their pediatric patients; however, some of the children continued to have mild to moderate OSA using the Rechtschaffen and Kales criteria.23,24 Marshall et al11 demonstrated that in the home environment autoCPAP can be used to improve sleep-disordered breathing in children with sickle cell disease. However, neither of these 2 studies directly compare autoCPAP effectiveness in finding treatment pressure to the titration sleep study. Mihai et al14 looked at 26 patients initiated on autoCPAP between 2013 and 2015. They found that for most of the studied children (69%), autoCPAP-determined treatment pressures were similar to the titration-determined pressures.
Overall, this study shows that autoCPAP can be used in a pediatric population and agrees with the previously published study by Mihai et al.14 Our results demonstrate that for most children in our study, autoCPAP-delivered pressures were close to the optimal pressure determined by overnight titration study. The distribution of the pressure differences suggests that some children may benefit from the manual titration or frequent assessment of autoCPAP-derived pressures in the outpatient setting. This study does support that in children initiated on autoCPAP, PMEAN pressure may be a more appropriate starting pressure for a titration PSG than the arbitrary initial pressure of 4–5 cmH2O typically used in the CHOP sleep laboratory, because PMEAN is closer to the optimal pressure. This may allow time to reach therapeutic pressures during the in-laboratory titration night. It should be noted that the children in our study were followed for adherence, medical and technical concerns, and side effects while on autoCPAP by our clinical CPAP team in the context of an interdisciplinary sleep center.25 Patients that were not tolerating autoCPAP or not able to use it were not included in this study, so these results should be applied in the appropriate context.
There are several limitations of this study, starting with the retrospective design. As discussed above, the children included in this study had a wide range of ages, BMI z-scores, and medical conditions. Therefore, the study group included children with very different pathologies. A larger prospective study may have better power to determine patient parameters that affect the agreement between the pressures. Additionally, considering the titration pressure itself has limitations, as discussed above, the clinical significance of the differences between the titration pressure and the autoCPAP pressures will need to be explored in future studies.
In summary, every patient requires careful clinical consideration when deciding the utility of titration PSG. AutoCPAP is a promising approach when initiating CPAP for the treatment of OSA in older children who are followed in a pediatric sleep center. Future studies might help to identify children in whom autoCPAP can effectively replace traditional titration polysomnography, as well as those in whom it does not. Future prospective studies of autoCPAP in children are necessary to establish appropriate clinical parameters, adherence, and long-term outcomes of autoCPAP use in children with OSA.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Children’s Hospital of Philadelphia. This study was funded by National Institutes of Health Grants NIH UL1RR024134 and K01HL130719, K23HL135346, and Research Electronic Data Capture (REDCap). The authors report no conflicts of interest.
ABBREVIATIONS
- autoCPAP
auto-titrating continuous positive airway pressure devices
- CHOP
Children’s Hospital of Philadelphia
- CPAP
continuous positive airway pressure
- IQR
interquartile range
- OSA
obstructive sleep apnea
- PMEAN
autoCPAP mean pressure
- PPEAKMEAN
autoCPAP peak average pressure
- PPSG
titration PSG pressure
- P90
average device pressure ≤90% of the time
REFERENCES
- 1.Chan J, Edman JC, Koltai PJ. Obstructive sleep apnea in children. Am Fam Physician. 2004;69(5):1147–1154. [PubMed] [Google Scholar]
- 2.Li Z, Celestin J, Lockey RF. Pediatric sleep apnea syndrome: an update. J Allergy Clin Immunol Pract. 2016;4(5):852–861. 10.1016/j.jaip.2016.02.022 [DOI] [PubMed] [Google Scholar]
- 3.Ehsan Z, Ishman SL. Pediatric obstructive sleep apnea. Otolaryngol Clin North Am. 2016;49(6):1449–1464. 10.1016/j.otc.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 4.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. 10.1542/peds.2012-1672 [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–683. 10.1164/rccm.200912-1930OC [DOI] [PubMed] [Google Scholar]
- 7.Ye J, Liu H, Zhang GH, et al. Outcome of adenotonsillectomy for obstructive sleep apnea syndrome in children. Ann Otol Rhinol Laryngol. 2010;119(8):506–513. 10.1177/000348941011900802 [DOI] [PubMed] [Google Scholar]
- 8.Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34(3):379–388. 10.1093/sleep/34.3.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ip S, D’Ambrosio C, Patel K, et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Rev. 2012;1(1):20. 10.1186/2046-4053-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsubie HS. BaHammam AS. Obstructive sleep apnoea: children are not little adults. Paediatr Respir Rev. 2017;21:72–79. [DOI] [PubMed] [Google Scholar]
- 11.Marshall MJ, Bucks RS, Hogan AM, et al. Auto-adjusting positive airway pressure in children with sickle cell anemia: results of a phase I randomized controlled trial. Haematologica. 2009;94(7):1006–1010. 10.3324/haematol.2008.005215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palombini L, Pelayo R, Guilleminault C. Efficacy of automated continuous positive airway pressure in children with sleep-related breathing disorders in an attended setting. Pediatrics. 2004;113(5):e412–e417. 10.1542/peds.113.5.e412 [DOI] [PubMed] [Google Scholar]
- 13.Amin R, Al-Saleh S, Narang I. Domiciliary noninvasive positive airway pressure therapy in children. Pediatr Pulmonol. 2016;51(4):335–348. 10.1002/ppul.23353 [DOI] [PubMed] [Google Scholar]
- 14.Mihai R, Vandeleur M, Pecoraro S, Davey MJ, Nixon GM. Autotitrating CPAP as a tool for CPAP initiation for children. J Clin Sleep Med. 2017;13(5):713–719. 10.5664/jcsm.6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry RB, Albertario CL, Harding SM, et al. ; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.4. Darien, IL: American Academy of Sleep Medicine; 2018. [Google Scholar]
- 16.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention . A SAS program for the 2000 CDC growth charts (ages 0 to <20 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. Accessed January 10, 2020.
- 18.Isetta V, Navajas D, Montserrat JM, Farre R. Comparative assessment of several automatic CPAP devices’ responses: a bench test study. ERJ Open Res. 2015;1(1):00031–2015. 10.1183/23120541.00031-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Philips-Respironics. Frequently asked questions. https://www.mysleepmapper.com/Help/Faq#. Accessed January 10, 2020.
- 20.Callahan CY, Norman RG, Taxin Z, Mooney AM, Rapoport DM, Ayappa I. Multinight recording and analysis of continuous positive airway pressure airflow in the home for titration and management of sleep disordered breathing. Sleep. 2013;36(4):535–545. 10.5665/sleep.2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noseda A, Andre S, Potmans V, Kentos M, de Maertelaer V, Hoffmann G. CPAP with algorithm-based versus titrated pressure: a randomized study. Sleep Med. 2009;10(9):988–992. 10.1016/j.sleep.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 22.Bakker J, Campbell A, Neill A. Randomised controlled trial of auto-adjusting positive airway pressure in morbidly obese patients requiring high therapeutic pressure delivery. J Sleep Res. 2011;20(1 Pt 2):233–240. 10.1111/j.1365-2869.2010.00846.x [DOI] [PubMed] [Google Scholar]
- 23.Novelli L, Ferri R, Bruni O. Sleep classification according to AASM and Rechtschaffen and Kales: effects on sleep scoring parameters of children and adolescents. J Sleep Res. 2010;19(1 Pt 2):238–247. 10.1111/j.1365-2869.2009.00785.x [DOI] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales AR. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: United States Government Printing Office; 1968. [Google Scholar]
- 25.Riley EB, Fieldston ES, Xanthopoulos MS, et al. Financial analysis of an intensive pediatric continuous positive airway pressure program. Sleep. 2017;40(2):zsw051. [DOI] [PubMed] [Google Scholar]