Abstract
Study Objectives:
The objectives of this study were to evaluate the independent association between sleep-disordered breathing (SDB) using overnight polysomnography and left ventricular (LV) scar using cardiac magnetic resonance (CMR) with late-gadolinium enhancement in a community-based cohort of the Multi-Ethnic Study of Atherosclerosis.
Methods:
Our analytical sample includes 934 participants from the fifth examination of the Multiethnic Study of Atherosclerosis who underwent both polysomnography and CMR. SDB was categorized as follows: no-SDB (apnea-hypopnea index [AHI] < 5 events/h), mild SDB (5 events/h ≤ AHI < 15 events/h), and moderate-severe SDB (AHI ≥ 15 events/h). LV scar was considered present if there was presence of scar on CMR (late-gadolinium enhancement > 0%). Logistic regression with multivariable adjustment for confounders (age, sex, race/ethnicity, body mass index, and cardiometabolic risk factors) was used to examine the independent association of SDB with LV scar. Confounders were identified using directed acyclic graphs.
Results:
The mean age of our sample was 67.0 ± 8.5 years (SD), with 49% (n = 461) females and a prevalence of SDB (AHI ≥ 5 events/h) of 63% (n = 590). LV scar was more prevalent in individuals with SDB (9.5%) versus those without SDB (3.8%; P < .01), and 88% of all LV scars were clinically unrecognized. After multivariable adjustment, both mild SDB and moderate-severe SDB were independently associated with LV scar (odds ratio, 2.53; 95% confidence interval, 1.13–5.64 and odds ratio, 2.31; 95% confidence interval, 1.01–5.24, respectively).
Conclusions:
In a community-based cohort, SDB (including mild) is independently associated with a more than 2-fold increase in the odds of LV scar presence measured using CMR with late-gadolinium enhancement. Most LV scars were clinically unrecognized. The impact of SDB treatment on subclinical myocardial infarction needs to be investigated in future studies.
Citation:
Shah NA, Reid M, Kizer JR, et al. Sleep-disordered breathing and left ventricular scar on cardiac magnetic resonance: results of the Multi-Ethnic Study of Atherosclerosis. J Clin Sleep Med. 2020;16(6):855–862.
Keywords: cardiac magnetic resonance, late-gadolinium enhancement, left ventricular scar, myocardial injury, obstructive sleep apnea, sleep apnea, sleep-disordered breathing
BRIEF SUMMARY
Current Knowledge/Study Rationale: Unrecognized myocardial scars are associated with increased mortality, and atypical myocardial scars are found in nearly 70% of individuals who have suffered sudden cardiac death.
Study Impact: Our study demonstrates that sleep-disordered breathing is independently associated with a more than 2-fold increase in the odds of left ventricular scar presence measured using cardiac magnetic resonance; most were atypical and clinically unrecognized. Although this study was limited by its observational design and cannot demonstrate causality, the results of this study underscore the importance of the evaluation of sleep-disordered breathing in the community and the need to investigate the impact of sleep-disordered breathing treatment on subclinical myocardial injury in future studies.
INTRODUCTION
Sleep-disordered breathing (SDB) affects more than 18 million American adults1 and is characterized by repeated collapse of the upper airway during sleep, resulting in intermittent hypoxemia and frequent arousals from sleep. SDB is linked with cardiovascular disease (CVD) via its adverse effects such as sympathetic activation,2 endothelial dysfunction,3 and inflammation.4
SDB has also been associated with myocardial injury among community dwelling individuals. However, available evidence on this is nonconclusive.5,6 Most studies have measured myocardial injury using circulating troponin levels, which although a highly specific biomarker of myocardial injury, can also be elevated in chronic conditions such as heart failure and kidney disease. Therefore, subtle elevations in circulating troponin levels can be nonspecific.
The advent of cardiac magnetic resonance (CMR) imaging techniques, including late-gadolinium enhancement (LGE), permits accurate evaluation of myocardial injury and left ventricular (LV) scar with excellent histopathologic correlation.7 To the best of our knowledge, no study has investigated the association between SDB and myocardial injury among community-dwelling individuals using CMR with LGE to quantify LV scar.
We therefore leveraged the availability of overnight polysomnography (PSG) and contrast-enhanced CMR with LGE in the community-based Multi-Ethnic Study of Atherosclerosis (MESA) to assess whether SDB is independently associated with focal LV scar using CMR with LGE in a community dwelling cohort of individuals. Demonstration of such an association could unveil the potential impact of SDB on subclinical myocardial injury, from both mild and moderate-severe SDB. This could allow refinement of the mechanisms and consequences through which SDB impacts cardiovascular health, permitting improved interventions.
METHODS
Study sample
MESA is a longitudinal study that recruited participants from 6 US communities from 2000 to 2002 to assess risk factors for the incidence and progression of CVD. The cohort consisted of 6,814 men and women 45–84 years of age who were either non-Hispanic white, Chinese American, Hispanic, or black and were free of clinically recognized CVD at baseline. Participants were studied every 2 years with in-clinic examinations, starting in July 2000. The current analyses used data from MESA exam 5 (2010–2012) and an associated MESA sleep ancillary study (2010–2013). Ethical standards were used in conduction of all research, and was approved by institutional review boards (approvals: Wake Forest University, IRB00008492; Columbia University, IRB00002973; Johns Hopkins University, IRB00001656; University of Minnesota, IRB00000438; Northwestern University, IRB00005003; University of California, Los Angeles, IRB00000172; University of Washington, IRB00005647).
Sleep data
Participants who reported not regularly using treatment for SDB were invited to participate in the MESA Sleep study (n = 4,077), where 2,237 participants were enrolled, of which 2,057 participants had acceptable unattended overnight in-home polysomnograms as previously described.8 The Compumedics Somte devices (Compumedics Ltd, Abbotsville, Australia) were used to conduct the polysomnograms using procedures previously described.9–11 An apnea was defined as 90% reduction in airflow lasting for 10 seconds or longer and further distinguished as central or obstructive based on respiratory effort detected using inductance plethysmography. Hypopneas were defined as a 30% or more reduction in airflow for 10 seconds or longer in association with at least a 4% desaturation. The apnea-hypopnea index (AHI) was defined as the sum of all apneas plus hypopneas divided by total sleep time. SDB was categorized according to the following clinical cutoffs: no-SDB when AHI < 5 events/h, mild SDB when 5 events/h ≤ AHI < 15 events/h, and moderate-severe SDB when AHI ≥ 15 events/h.
Cardiac imaging and image analysis
Participants attending exam 5 (2010–2012) underwent CMR imaging on 1.5-T scanners. All centers followed the same imaging protocol, and all studies were analyzed by trained, blinded, image data analysts at a centralized imaging reading center (Johns Hopkins University). Using QMass (version 7.2; Medis, Leiden, Netherlands), myocardial scar was defined as focal LGE either in 1 short axis and 1 long axis image at a matching location or 2 neighboring short axis slices.12 An ischemic pattern scar, or typical scar, was defined as a myocardial scar comprising the subendocardium in a distribution of the coronary artery. A nonischemic pattern scar, or atypical scar, was defined as a myocardial scar that primarily affected the midwall or subepicardium exclusive of subendocardial involvement and one that did not follow coronary artery distribution.13 Silent myocardial infarctions (MIs) by electrocardiogram (ECG), classified using Minnesota code,14 were identified based on major Q-wave abnormalities representing old MIs. The mean duration in days (SD) between the PSG studies and CMR was 350.2 ± 205.7.
Covariates
Height and weight were measured using standardized approaches, and sociodemographic and comorbidity data were obtained from questionnaires.15 Age was assessed as a continuous variable. Race/ethnicity was categorized into 4 groups: non-Hispanic white (reference), black, Hispanic, and Chinese American. Smoking use (self-reported) was categorized into 3 groups: never smoker (reference), former smoker, and current smoker. Body mass index (BMI) was derived from measured height and weight (kg/m2). Presence of hypertension was a yes/no variable based on the sixth report of the Joint National Committee16 criteria. Dyslipidemia was categorized as a yes/no variable based on a low-density lipoprotein level ≥ 160 mg/dl, high-density lipoprotein level < 40 mg/dl in men and < 50 mg/dl in women, triglycerides ≥ 150 mg/dl, or use of statins. Coronary heart disease (CHD) was a yes/no variable based on the presence of CHD (MI) resuscitated cardiac arrest, definite angina, or probable angina (if followed by revascularization).15 Diabetes was categorized into a yes/no variable defined as untreated or treated diabetes based on the 2003 American Diabetes Association fasting criteria. Physical activity was derived from the Typical Week Physical Activity Survey17 and was grouped into 3 levels of moderate intensity physical activity (low, moderate, high; classified using the metabolic equivalent minutes per day energy expenditure).18 Alcohol use was categorized as a dichotomous yes/no variable based on self-reported data regarding “presently drinks alcohol.” Hypoxic burden19 was assessed by measuring the total area under the desaturation curve associated with all respiratory events during the sleep period on the PSG. Sleep duration was measured using 7-day actigraphy (MESA exam 5; Philips Respironics, Murrysville, PA), and we defined short sleep duration as <6 hours and long sleep duration as >8 hours. The arousal index was defined as the average number of EEG arousals lasting 3 or more seconds per hour of sleep.
Statistical analysis
Baseline characteristics of the study participants are reported as mean with standard deviation (continuous variables) or as numbers with percentages (categorical variables). For continuous variables, the analysis of variance test or Kruskal–Wallis test evaluated the variation of continuously measured imaging indices by AHI categories. Chi-squared tests were performed to examine whether the distributions of categorical variables differed by SDB categories. Logistic regression with multivariable adjustment (age, sex, race/ethnicity, BMI, diabetes, smoking, dyslipidemia, and prior history of CHD) was used to examine the independent association of SDB (categorical: mild and moderate-severe) with presence of LV scar. Confounders were identified using directed acyclic graphs. Hypertension is considered to be on the intermediate pathway. We therefore did not consider it as a confounder. However, we provide a separate model with hypertension as a confounder. We also present a model with adjustment for alcohol use because it is unclear whether it is a true confounder. We assess the influence of physical activity and hypoxic burden to our final models. Finally, we assess for effect modification by sex in our final model.
RESULTS
Baseline characteristics
A total of 934 individuals had both acceptable PSG studies and LGE imaging. The prevalence of SDB (AHI ≥ 5 events/h) was 63% (n = 590/934). Table 1 describes the baseline characteristics of the overall cohort by SDB status. The overall cohort was predominantly non-Hispanic white (42%), with 11% Chinese American, 26% non-Hispanic black, and 22% Hispanic. Increasing SDB severity was associated with older age and greater proportion of men, as well as higher BMI, systolic and diastolic blood pressure, and diabetes. SDB was also associated with less or low physical activity. The demographic distribution of our study was similar to the overall MESA and the sleep ancillary cohorts (data not shown).
Table 1.
Baseline characteristics of the cohort by SDB severity, MESA study 2010–2013.
| Overall Sample (n = 934) | No SDB (n = 344) | Mild SDB (n = 293) | Moderate-Severe SDB (n = 297) | P | |
|---|---|---|---|---|---|
| Age (y) | 67.0 (8.5) | 65.7 (8.2) | 67.4 (8.4) | 68.0 (8.6) | .0018 |
| Height (cm) | 166.5 (9.9) | 165.7 (9.7) | 165.8 (10.3) | 168.0 (9.6) | .0036 |
| Weights (lb) | 173.4 (36.4) | 162.2 (33.6) | 172.3 (33.0) | 187.5 (38.0) | <.0001 |
| BMI (kg/m2) | 28.3 (4.9) | 26.7 (4.5) | 28.4 (4.4) | 30.0 (5.2) | <.0001 |
| Sex | <.0001 | ||||
| Female | 461, 49.4% | 210, 61.0% | 152, 51.9% | 99, 33.3% | |
| Male | 473, 50.6% | 134, 39.0% | 141, 48.1% | 198, 66.7% | |
| Race | .0441 | ||||
| Non-Hispanic white | 388, 41.5% | 157, 45.6% | 124, 42.3% | 107, 36% | |
| Chinese American | 99, 10.6% | 37, 10.8% | 26, 8.9% | 36, 12.1% | |
| Black | 242, 25.9% | 93, 27.0% | 73, 24.9% | 76, 25.6% | |
| Hispanic | 205, 21.9% | 57, 16.6% | 70, 23.9% | 78, 26.3% | |
| Education | .2276 | ||||
| Less than high school | 249, 26.7% | 84, 24.5% | 75, 25.7% | 90, 30.3% | |
| Less than bachelor degree | 268, 28.8% | 102, 29.7% | 75, 25.7% | 91, 30.6% | |
| Bachelor degree | 190, 20.4% | 71, 20.7% | 70, 20.4% | 49, 16.5% | |
| Graduate or professional school | 225, 24.1% | 86, 25.1% | 72, 24.7% | 67, 22.6% | |
| Smoking status | .2306 | ||||
| Never smoker | 420, 45.0% | 164, 47.8% | 127, 43.3% | 129, 43.4% | |
| Former smoker | 446, 47.8% | 151, 44.0% | 142, 48.5% | 153, 51.5% | |
| Current smoker | 67, 7.2% | 28, 8.2% | 24, 8.2% | 15, 5.1% | |
| Hypertension | 514, 55.0% | 167, 48.6% | 171, 58.4% | 176, 59.3% | .0095 |
| Use of statins | 325, 34.8% | 106, 30.8% | 109, 37.2% | 110, 37.0% | .1489 |
| Systolic blood pressure (mm Hg) | 120.6 (18.5) | 118.0 (18.6) | 121.6 (18.5) | 122.6 (18.2) | .0043 |
| Diastolic blood pressure (mm Hg) | 68.5 (9.4) | 67.4 (9.9) | 68.6 (9.3) | 69.7 (8.7) | .0054 |
| Coronary heart disease | 57, 6.5% | 13, 4.2% | 19, 6.8% | 25, 8.7% | .0763 |
| LDL cholesterol (mg/dl) | 107.7 (31.5) | 108.5 (31.7) | 107.7 (31.1) | 107.0 (31.6) | .8356 |
| HDL cholesterol (mg/dl) | 54.5 (15.9) | 58.6 (17.0) | 54.0 (15.2) | 50.3 (13.9) | <.0001 |
| Triglycerides (mg/dl) | 110.2 (64.8) | 103.0 (61.3) | 110.0 (69.2) | 119.0 (63.3) | .0081 |
| Diabetes | 144, 15.5% | 37, 4.0% | 40, 4.3% | 67, 7.2% | .0001 |
| Alcohol use (currently drink alcohol) | 444, 47.6% | 159, 17.0% | 136, 14.6% | 149, 16.0% | .5591 |
| Physical activity (MET-min/d) | .0414 | ||||
| Low | 309, 33.1% | 101, 10.8% | 89, 9.5% | 119, 12.7% | |
| Moderate | 317, 33.9% | 123, 13.2% | 101, 10.8% | 93, 10.0% | |
| High | 308, 33.0% | 120, 12.9% | 103, 11.0% | 85, 9.1% |
Values are mean (SD) or number, %. BMI = body mass index, HDL = high density lipoprotein, LDL = low density lipoprotein, MET = metabolic equivalent, SDB = sleep-disordered breathing.
Sleep characteristics
The overall prevalence of SDB was 63% (n = 590), with mild SDB present in 31% (n = 293) and moderate-severe SDB present in 32% (n = 297) of the cohort. Table 2 provides the sleep characteristics based on SDB status. Individuals with moderate-severe SDB had the shortest total sleep duration on their PSGs (5.8 hours or 352 minutes). Furthermore, participants in the moderate-to-severe SDB category (ie, AHI ≥ 15 events/h) spent a longer duration with oxygen saturation less than 90% compared with participants with no or mild SDB. Similarly, individuals in the moderate-severe SDB group had the highest hypoxic burden compared with those with no or mild SDB.
Table 2.
Sleep characteristics of the cohort by SDB severity, MESA study 2010–2013.
| Overall Sample (n = 934) | No SDB (n = 344) | Mild SDB (n = 293) | Moderate-Severe SDB (n = 297) | P | |
|---|---|---|---|---|---|
| Time REM (min) | 68.0 (30.3) | 74.0 (30.6) | 70.8 (28.2) | 58.1 (29.6) | <.0001 |
| TST (min) | 364.4 (79.5) | 373.7 (77.9) | 366.1 (76.1) | 352.0 (83.1) | .0023 |
| Average SAO2 in sleep | 94.3 (1.7) | 95.2 (1.3) | 94.2 (1.5) | 93.4 (1.8) | <.0001 |
| Minimum SAO2 in sleep | 83.3 (7.7) | 88.9 (3.1) | 82.8 (5.6) | 77.5 (8.7) | <.0001 |
| Percent time <90% desaturation (%) | 3.6 (8.8) | 0.6 (3.9) | 2.8 (8.9) | 7.8 (11.1) | <.0001 |
| Hypoxic burden | 29.5 (34.9) | 10.3 (7.6) | 23.1 (13.5) | 57.9 (48.1) | <.0001 |
Values are mean (SD). REM = rapid eye movement, SAO2 = oxygen saturation, SDB = sleep-disordered breathing, TST = total sleep time.
LV scar distribution
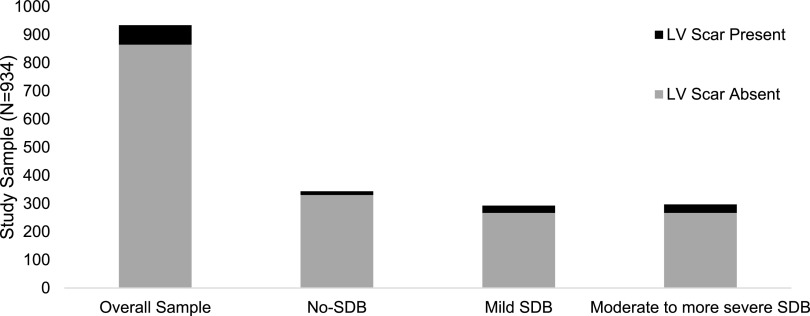
The prevalence of myocardial scar was 7.4% (69 of 934). Furthermore, LV scar was more prevalent in individuals with SDB (9.5%) versus those without SDB (3.8%; P < .01). The prevalence of LV scar increased as SDB severity increased (P = .0048). Furthermore, the percent of LV scar increased with increasing SDB severity, where the mean LV scar in individuals without SDB was 3.8%, in those with mild SDB was 4.0%, and among those with moderate-severe SDB was 7.3%. Figure 1 shows the distribution of presence and absence of LV scar by SDB category.
Figure 1. LV scar presence and absence by SDB group.
This figure shows the distribution of the presence and absence of LV scar by SDB category. Prevalence of myocardial scar in the study sample was 7.4% (69 of 934). LV scar was more prevalent in individuals with SDB (9.5%) versus those without SDB (3.8%; P < .01). The prevalence of LV scar increased with SDB severity (P = .0048). LV = left ventricular, SDB = sleep-disordered breathing.
Most LV scars (88.4%; 61 of 69) were unrecognized by clinical history or ECG adjudication. Furthermore, most LV scars were small, with a mean of 5.41% of the left ventricle (SD, 6.81). Among the clinically unrecognized scars, 37.7% were typical (23 of 61), and 62.3% were atypical (38/61). Among the clinically recognized myocardial scars, 62.5% were typical (5 of 8), and 37.5% were atypical (3 of 8). The distribution of the type of LV scar was similar across the SDB categories—no SDB: typical = 5 (38.5%) and atypical = 8 (61.5%); mild SDB: typical = 11 (42.3%) and atypical = 15 (57.7%); moderate to more severe SDB: typical = 11 (36.7%) and atypical = 63.3% (P = .91). The distribution of LV scar types in our sample was similar to that in the overall MESA cohort (data not shown).12
Regression analyses
In an unadjusted analysis (model 0, Table 3), mild (5 events/h ≤ AHI < 15 events/h) and moderate-severe SDB (AHI ≥ 5 events/h) were associated with LV scar (odds ratio [OR], 2.48; 95% confidence interval [CI], 1.25–4.92 and OR, 2.86; 95% CI, 1.46–5.59), respectively. After adjusting for age, sex, race/ethnicity, BMI, smoking status, diabetes, dyslipidemia, and prior history of CHD (model 2, Table 3), both mild SDB (5 events/h ≤ AHI < 15 events/h) and moderate-severe SDB were independently associated with LV scar (OR, 2.53; 95% CI, 1.13–5.64 and OR, 2.31; 95% CI, 1.01–5.24, respectively). Further adjustment for alcohol use (model 4, Table 3) resulted in minimal attenuation of the OR for both mild SDB (OR, 2.51; 95% CI, 1.13–5.61) and moderate-severe SDB (OR, 2.27; 95% CI, 1.00–5.16). Because hypertension is on the intermediate pathway for the association between SDB and LV scar, we did not adjust for it in our primary model. We, however, provide a separate model with hypertension adjustment (model 3, Table 3) that demonstrated similar ORs from model 2. We further assessed the influence of adding hypoxic burden to our fully adjusted model and found that the OR for mild SDB (2.54; 95% CI, 1.13–5.68) was similar to that of model 2, whereas the OR for moderate-severe SDB was attenuated (2.37; 95% CI, 0.97–5.77; data not shown). Of note, hypoxic burden is moderately correlated with AHI (r = .56). Adjustment for physical activity did not change our findings (data not shown). Finally, sex stratification did not reveal sex-specific associations between SDB and LV scar (P interaction = .7864; data not shown). We also assessed the independent association between each of the following variables: (1) objectively measured sleep duration, (2) hypoxic burden, and (3) sleep fragmentation (measured as arousal index) and LV scar. Both hypoxic burden and arousal index were categorized into quartiles.
Table 3.
Differences in presence of LV scar according to SDB using multivariable logistic regression, MESA study 2010–2013.
| Model 0 | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|
| Mild SDB (5 ≤ AHI < 15 events/h), n = 293 | 2.48 (1.25–4.92) | 2.23 (1.10–4.52) | 2.53 (1.13–5.64) | 2.51 (1.13–5.61) | 2.51 (1.13–5.61) |
| Moderate-severe SDB (AHI ≥15 events/h), n = 297 | 2.86 (1.46–5.59) | 2.02 (1.01–4.06) | 2.31 (1.01–5.24) | 2.32 (1.02–5.26) | 2.27 (1.00–5.16) |
Presence of LV scar (n = 934). Values are odds ratio (95% confidence interval). Model 0, unadjusted; model 1, age, sex, race/ethnicity; model 2, model 1 + BMI, smoking status, diabetes, dyslipidemia, CHD; model 3, model 2 + hypertension; model 4, model 2 + alcohol use. AHI = apnea-hypopnea index, BMI = body mass index, CHD = coronary heart disease, LV = left ventricular, SDB = sleep-disordered breathing.
Neither short sleep (<6 hours, n = 308) nor long sleep duration (>8 hours, n = 82) was independently associated with LV scar in fully adjusted models. Similarly, there was no significant association between hypoxic burden and LV scar after adjustment for confounding variables. Finally, there was no significant association between arousal index and LV scar. All of these models were adjusted for the same covariates listed in model 3, Table 3 (data not shown).
To assess the association between SDB and LV scar among those without clinically adjudicated coronary events, we restricted our analytical sample to exclude individuals with adjudicated MIs (n = 29), resulting in a sample size of n = 905. In this restricted cohort, both mild and moderate-severe SDB were independently associated with LV scar, with ORs of 2.71 (95% CI, 1.13–6.50) and 2.71 (95% CI, 1.12–6.58), respectively, after adjusting for variables in model 2.
DISCUSSION
Our study demonstrates an independent association between SDB and LV scar using CMR with LGE among community-dwelling individuals in MESA. After adjusting for confounding variables, SDB was associated with more than a 2-fold increase in the odds of having clinically unrecognized LV scar. This association was evident even in individuals with milder forms of SDB. Our findings suggest that SDB (even in its mildest form) is linked with myocardial injury that is clinically unrecognized.
SDB is characterized by intermittent hypoxemia, arousal from sleep, and intrathoracic pressure swings. These characteristics of SDB are associated with sympathetic activation,20,21 systemic inflammation,22 and increased afterload,23 which are intermediary mechanisms for the development of hypertension,24 atherosclerosis,25 and myocardial ischemia,26 all of which can result in myocardial injury.6 Prior studies investigating whether SDB is associated with myocardial injury used ECG tracings to assess myocardial injury.26,27 These studies, although supportive of a potential association between SDB and myocardial injury, were limited by use of ECG changes as a surrogate measure for myocardial injury. ECG changes can be nonspecific and may represent artifacts during sleep and variations from respiratory events or ischemia that may not necessarily result in myocardial damage leading to scar formation.
Similar to the studies that used ECG, the studies28,29 that use cardiac troponin levels (including hs-cTnT), as a surrogate for myocardial injury also have several limitations. Chronic conditions such as heart failure, kidney disease, diabetes, hypertension, and stroke often result in elevation of circulating troponin levels. Although specific for myocardial damage, troponin levels are often nonspecific for LV scar. Increased circulating cardiac troponin levels may reflect increased myocardial cell wall permeability, decreased clearance (in the case of chronic kidney disease), and release of membranous blebs from cardiomyocytes as opposed to myocardial necrosis and LV scar formation.30 Furthermore, and specific to SDB, myocardial strain and myocardial ischemia (reduced myocardial oxygen supply) from intermittent hypoxemia and increased mechanical load can also result in transient cardiac troponin elevations that may not reflect true myocardial necrosis (which would result in myocardial scar on CMR with LGE).
Our study addressed the aforementioned limitations by leveraging advanced cardiac imaging to quantify LV scar among community-dwelling individuals.7,31 Myocardial scar captured via CMR and LGE has also been shown to be associated with future cardiac event risk.12,31,32 A major strength of our study is the accurate assessment of LV scar, which is readily detected by CMR with LGE as opposed to ECG or other cardiac biomarkers.12,32 The CMRs in MESA were analyzed by trained image data analysts who were blinded to all clinical data. It is important to acknowledge other studies that have used CMR in individuals with SDB. However, in these studies, CMR was either done without LGE and therefore myocardial scar was not assessed33 or, when LGE was obtained, it was in the post-MI period.34 Our current study is distinctive from this prior work as our primary objective was to examine the association between SDB and myocardial scar using CMR with LGE in a community-dwelling cohort of individuals. Myocardial scar assessed via CMR with LGE has been shown to have higher sensitivity and excellent histopathologic correlation to myocardial injury.32,35,36 To the best of our knowledge, no other study has assessed subclinical myocardial injury associated with SDB using CMR and LGE in a community-based cohort.
Most studies on SDB and CVD endpoints have focused on moderate-severe SDB, leaving out mild SDB, which is often not considered an independent risk factor for CVD outcomes. Our study addresses this limitation by not only including mild SDB but also by demonstrating that mild SDB increases the odds of LV scar presence by more than 2.5-fold. This is a particularly novel and clinically meaningful finding. Furthermore, our study is strengthened by multivariable adjustment for potential confounders including age, BMI, history of CHD, hypertension, diabetes, dyslipidemia, and smoking. We also further adjusted for physical activity and alcohol use, and it did not alter our study findings.
Most (>80%) LV scars in our study were clinically unrecognized. It is important to emphasize that unrecognized myocardial scars are associated with increased mortality.32 Moreover, atypical myocardial scars are found in nearly 70% of individuals who had sudden cardiac death (SCD).37 In this study, one third of the unrecognized LV scars were typical and were likely unrecognized myocardial infarctions. The remaining two thirds of the unrecognized LV scars were atypical and were likely nonischemic in origin. Atypical scars represent fibrosis arising from nonischemic insults (eg, infectious, toxic, immune). Their presence in nonischemic dilated cardiomyopathy portends worse survival,38 but their clinical significance in healthy populations in the absence of structural heart disease or ventricular dysfunction remains uncertain. In summary, SDB, even in its mildest form, may lead to unrecognized myocardial injury, from both ischemic and nonischemic etiologies, which are associated with increased risk of mortality and SCD. Gami et al39 reported that moderate-severe SDB is associated with SCD. However, the specific mechanisms for this association remain unknown. Numerous nonspecific mechanisms for the association between SDB and SCD have been proposed, which include myocardial ischemia, cardiac autonomic dysfunction, QT prolongation, sympathetic overdrive, and LV failure.39 Our study may have identified another potentially direct mechanism for the association between SDB and SCD (ie, unrecognized small LV scars), which may serve as an arrhythmogenic focus for fatal ventricular arrhythmias, thereby increasing the risk for SCD.31,36 This theoretical and plausible mechanism needs to be investigated in future studies.
Limitations of our work must be considered. Because of the cross-sectional nature of our study and the lack of prospective study design, reverse causation cannot be fully addressed. However, given that most myocardial scars in our study were small, they are less likely to significantly impair cardiac function (ie, reduce LV ejection fraction or result in symptomatic heart failure, both of which can result in SDB). Therefore, it is less likely that reverse causation contributes to our study findings. Another limitation of our work is that we do not have simultaneous measures of cardiac troponin levels or other biomarkers of cardiac injury. This would have allowed us to compare myocardial scar quantification by LGE with circulating troponin levels. However, this limitation would not alter our study findings. Furthermore, we adjust for many covariates that we consider as important confounding variables, yet the likelihood of residual confounding from unknown variables is a concern. Additionally, our study is observational and cannot demonstrate causality. Finally, the number of participants with LV scar was modest, and larger studies will be necessary to more precisely evaluate this association.
CONCLUSIONS
In summary, our study demonstrates an independent association between SDB and LV scar using CMR with LGE among community-dwelling individuals in MESA. After adjusting for confounding variables, SDB was associated with more than a 2-fold increase in the odds of having clinically unrecognized LV scar on CMR. This association was evident even in individuals with milder forms of SDB. Our findings suggest that SDB (even in its mildest form) is linked with myocardial injury that is clinically unrecognized. Our work highlights the importance of assessing the entire spectrum of SDB, especially as it pertains to its impact on CVD outcomes. Our study findings also may have identified a possible explanation for the association between SDB and SCD. Future studies should assess whether subclinical myocardial fibrosis is reversible with SDB treatment, thereby reducing future risk of future CVD events including SCD.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Icahn School of Medicine at Mount Sinai. This study was funded by contracts with University of Washington Coordinating Center (HHSN268201500003I, N01-HC-95159), University of California, Los Angeles Field Center (N01-HC-95160), Columbia University Field Center (N01-HC-95161), Johns Hopkins University Field Center (N01-HC-95162), University of Minnesota Field Center (N01-HC-95163), Northwestern University Field Center (N01-HC-95164), Wake Forest University Field Center (N01-HC-95165),Central Laboratory (N01-HC-95166), Ultrasound Reading Center (N01-HC-95167), MRI Reading Center (N01-HC-95168) and CT Reading Center (N01-HC-95169) from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040 (Columbia Clinical and Translational Science Awards [CTSA]), UL1-TR-001079 (Johns Hopkins Institute for Clinical and Translational Research [ICTR]), and UL1-TR-001420 (Wake Forest University Clinical and Translational Science Awards [CTSA]) from National Center for Advancing Translational Sciences (NCATS). The MESA Sleep study was support by National Heart, Lung, and Blood Institute Grant HL56984. Dr. Susan Redline was partially supported by Grant R35 HL135818. Dr. Neomi A Shah has funding from the National Institute of Health/National Heart, Lung, and Blood Institute (Grants 5K23HL125923-03, 1R03HL140273-01, 1R01HL143221-01). Jorge Kizer reports stock ownership in AmGen, Gilead Sciences, Johnson & Johnson, and Pfizer. All other authors report no conflict of interest.
ACKNOWLEDGMENTS
The authors thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CHD
coronary heart disease
- CMR
cardiac magnetic resonance
- CVD
cardiovascular disease
- ECG
electrocardiogram
- LGE
late-gadolinium enhancement
- LV
left ventricular
- MESA
Multiethnic Study of Atherosclerosis
- MI
myocardial infarction
- PSG
polysomnography
- SCD
sudden cardiac death
- SDB
sleep-disordered breathing
REFERENCES
- 1.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130(3):780–786. 10.1378/chest.130.3.780 [DOI] [PubMed] [Google Scholar]
- 2.Bradley TD, Tkacova R, Hall MJ, Ando S, Floras JS. Augmented sympathetic neural response to simulated obstructive apnoea in human heart failure. Clin Sci (Lond). 2003;104(3):231–238. 10.1042/cs1040231 [DOI] [PubMed] [Google Scholar]
- 3.Patt BT, Jarjoura D, Haddad DN, et al. Endothelial dysfunction in the microcirculation of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182(12):1540–1545. 10.1164/rccm.201002-0162OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svensson M, Venge P, Janson C, Lindberg E. Relationship between sleep-disordered breathing and markers of systemic inflammation in women from the general population. J Sleep Res. 2012;21(2):147–154. 10.1111/j.1365-2869.2011.00946.x [DOI] [PubMed] [Google Scholar]
- 5.Einvik G, Røsjø H, Randby A, et al. Severity of obstructive sleep apnea is associated with cardiac troponin I concentrations in a community-based sample: data from the Akershus Sleep Apnea Project. Sleep. 2014;37(6):1111–1116. 10.5665/sleep.3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roca GQ, Redline S, Punjabi N, et al. Sleep apnea is associated with subclinical myocardial injury in the community: the ARIC-SHHS study. Am J Respir Crit Care Med. 2013;188(12):1460–1465. 10.1164/rccm.201309-1572OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon JCC, Reed E, Sheppard MN, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43(12):2260–2264. 10.1016/j.jacc.2004.03.035 [DOI] [PubMed] [Google Scholar]
- 8.Lutsey PL, Mcclelland RL, Duprez D, et al. Objectively measured sleep characteristics and prevalence of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis Sleep study. Thorax. 2015;70(9):880–887. 10.1136/thoraxjnl-2015-206871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. 10.1093/sleep/21.7.759 [DOI] [PubMed] [Google Scholar]
- 10.ASDA . EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. 10.1093/sleep/15.2.173 [DOI] [PubMed] [Google Scholar]
- 11.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3(2):169–200. 10.5664/jcsm.26818 [DOI] [PubMed] [Google Scholar]
- 12.Turkbey EB, Nacif MS, Guo M, et al. Prevalence and correlates of myocardial scar in a US cohort. J Am Med Assoc. 2015;314(18):1945. 10.1001/jama.2015.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpe GJ, Sharma RK, Wu CO. Electrocardiographic impact of myocardial diffuse fibrosis and scar: MESA. Radiology. 2017;282(3):690–698. 10.1148/radiol.2016160816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prineas RJ, Crow RS, Zhang Z-MThe Minnesota Code Manual of Electrocardiographic Findings. New York: Springer; 2010. 10.1007/978-1-84882-778-3 [DOI] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 16.Kaplan NM. The 6th Joint National Committee report (JNC-6): new guidelines for hypertension therapy from the USA. Keio J Med. 1998;47(2):99–105. 10.2302/kjm.47.99 [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–813. 10.1089/152460999319129 [DOI] [PubMed] [Google Scholar]
- 18.LaMonte MJ, Durstine JL, Addy CL, Irwin ML, Ainsworth BE. Physical activity, physical fitness, and framingham 10-year risk score: the cross-cultural activity participation study. J Cardiopulm Rehabil. 2001;21(2):63–70. 10.1097/00008483-200103000-00001 [DOI] [PubMed] [Google Scholar]
- 19.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2018;40(14):1149–1157. 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaye DM, Mansfield D, Aggarwal A, Naughton MT, Esler MD. Acute effects of continuous positive airway pressure on cardiac sympathetic tone in congestive heart failure. Circulation. 2001;103(19):2336–2338. 10.1161/01.CIR.103.19.2336 [DOI] [PubMed] [Google Scholar]
- 21.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. 10.1172/JCI118235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5(8):e12065. 10.1371/journal.pone.0012065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall MJ, Ando S, Floras JS, Bradley TD. Magnitude and time course of hemodynamic responses to Mueller maneuvers in patients with congestive heart failure. J Appl Physiol. 1998;85(4):1476–1484. 10.1152/jappl.1998.85.4.1476 [DOI] [PubMed] [Google Scholar]
- 24.Peppard PE, Young T, Palta M, Skatrud JB. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 25.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(5):613–618. 10.1164/rccm.200503-340OC [DOI] [PubMed] [Google Scholar]
- 26.Schafer H, Koehler U, Ploch T, Peter JH. Sleep-related myocardial ischemia and sleep structure in patients with obstructive sleep apnea and coronary heart disease. Chest. 1997;111(2):387–393. 10.1378/chest.111.2.387 [DOI] [PubMed] [Google Scholar]
- 27.Hanly P, Sasson Z, Zuberi N, Lunn K. ST-segment depression during sleep in obstructive sleep apnea. Am J Cardiol. 1993;71(15):1341–1345. 10.1016/0002-9149(93)90552-N [DOI] [PubMed] [Google Scholar]
- 28.Gami AS, Svatikova A, Wolk R, et al. Cardiac troponin T in obstructive sleep apnea. Chest. 2004;125(6):2097–2100. 10.1378/chest.125.6.2097 [DOI] [PubMed] [Google Scholar]
- 29.Zhang XB, Zeng HQ, Du YP, Lyu Z, Zhan FF. High-sensitivity cardiac troponin T in obstructive sleep apnea patients without cardiovascular diseases: efficacy of CPAP treatment. Chron Respir Dis. 2018;15(2):157–164. 10.1177/1479972317740127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017;113(14):1708–1718. 10.1093/cvr/cvx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113(23):2733–2743. 10.1161/CIRCULATIONAHA.105.570648 [DOI] [PubMed] [Google Scholar]
- 32.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. J Am Med Assoc. 2012;308(9):890. 10.1001/2012.jama.11089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141(3):674–681. 10.1378/chest.11-0615 [DOI] [PubMed] [Google Scholar]
- 34.Buchner S, Satzl A, Debl K, et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur Heart J. 2014;35(3):192–199. 10.1093/eurheartj/eht450 [DOI] [PubMed] [Google Scholar]
- 35.Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114(1):32–39. 10.1161/CIRCULATIONAHA.106.613414 [DOI] [PubMed] [Google Scholar]
- 36.Kwong RY, Sattar H, Wu H, et al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118(10):1011–1020. 10.1161/CIRCULATIONAHA.107.727826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman WP, Tracy RE, Strong JP, Johnson WD, Oalmann MC. Pathology of sudden coronary death. Ann N Y Acad Sci. 1982;382(1):39–49. 10.1111/j.1749-6632.1982.tb55205.x [DOI] [PubMed] [Google Scholar]
- 38.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51(25):2414–2421. 10.1016/j.jacc.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mavrogeni S, Petrou E, Kolovou G, Theodorakis G, Iliodromitis E. Prediction of ventricular arrhythmias using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2013;14(6):518–525. 10.1093/ehjci/jes302 [DOI] [PubMed] [Google Scholar]