ABSTRACT
Cutting is a frequently used model to study the process of adventitious root formation, and excision of cuttings leads to rapid wound response signaling. We recently showed that as a wound signal, reactive oxygen species (ROS, mainly hydrogen peroxide) participate in adventitious root induction of hypocotyl cuttings through regulation of auxin biosynthesis and transport. Here, superoxide anion (O2−•), an early type of ROS, exhibited rapid burst at the cutting site immediately in response to wounding in Arabidopsis hypocotyl cuttings. Diphenylene iodonium chloride (DPI, inhibitor of NADPH oxidase) overwhelmingly suppressed O2−• propagation through the hypocotyl. Compared to wild type, O2−• burst only occur in cut base, and upward transduction were inhibited completely in NADPH oxidase mutant AtRbohD. These results indicate O2−• generation and propagation in response to wound and via NADPH oxidase in adventitious root induction of hypocotyl cuttings.
KEYWORDS: Superoxide anion, reactive oxygen species, adventitious root, wound, signaling
The formation of adventitious roots is a multifactorial process in cuttings, which is very important for asexual propagation of plant, but the understanding of its regulation mechanism is not entirely clear. The origin of adventitious root rooting in stem cuttings is the removal of primary root system, which is mainly caused by two primary stimuli: the isolation of the cut part from the whole plant and wounding at the cutting site.1,2 Jasmonates, ethylene, and reactive oxygen species (ROS) are widely produced in response to wound.3–5
ROS are reactive forms of molecular oxygen, and mainly include superoxide anion (O2−•), singlet oxygen, hydrogen peroxide (H2O2), and hydroxyl radical.6 In recent years, beyond the traditional belief of ROS as toxic by-products of metabolic processes, more and more evidences show that ROS can be an important signal molecule in plant growth, development, and stress response.7,8 Our recent study indicates that wound-induced ROS participate in AR induction through regulation of auxin biosynthesis and transport.9
O2−• is a free radical formed by molecular oxygen metabolism in organism, and it can be further converted into H2O2.10 NADPH oxidase (NOX) is an oxidase located in the plasma membrane. It can obtain electrons from NADPH on the inner side of the plasma membrane and transfer them to molecular oxygen, so that O2 can be excited to form O2−• at the outer side of plasma membrane through single-electron reduction reaction. Subsequently, O2−• is converted to H2O2 by SOD catalysis or natural disproportionation. Studies have shown that NOX widely exists in plants, and it can respond to growth, development, and abiotic stress.11,12
Here, we detected the changes of intracellular O2−·level in hypocotyl after cuttings by using O2−·–sensitive fluorophore DHE.13 It was observed that intracellular O2−· are rapidly generated at the cutting site as a wound-induced signal, then propagated upward along the hypocotyl and mainly distributed in the stele of hypocotyl (Figure 1). To further investigate the spatial–temporal variation of wound-induced O2−· in whole hypocotyl cuttings, 6-mm-long hypocotyls were conceptually divided into three sections (base, middle, and upper) (Figure 2). DHE fluorescence intensity of basal part increased quickly and reached strongest at 8 min after cutting, and the fluorescence in the middle part was the strongest at 16 min and that in upper at 22 min (Figure 2). The result indicates that the more to the upper part of hypocotyl, the less O2−· level increased.
Figure 1.
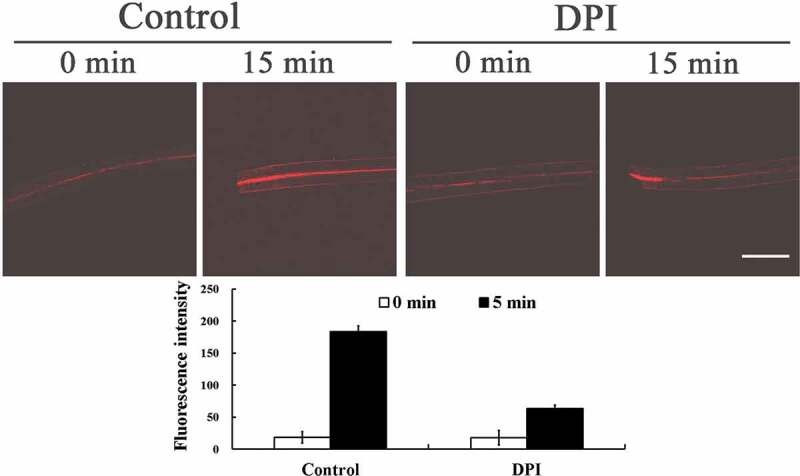
Detection of intracellular O2−· generation response to excision of primary roots in Arabidopsis wild type (Col-0) hypocotyl. Wild-type seedlings were preloaded with 250 μM DHE or combined with 3 μM DPI, then fluorescence images were detected at the base of the hypocotyl at the indicated time after excision. A Leica TCS-SP5 confocal laser scanning microscope was used for visualization and imaging (excitation at 543 nm and emission at 580–600 nm). Data of fluorescence pixel intensities are displayed as means ±SE of three replicates, each replicate with 3 seedlings. Bar = 250 µm
Figure 2.
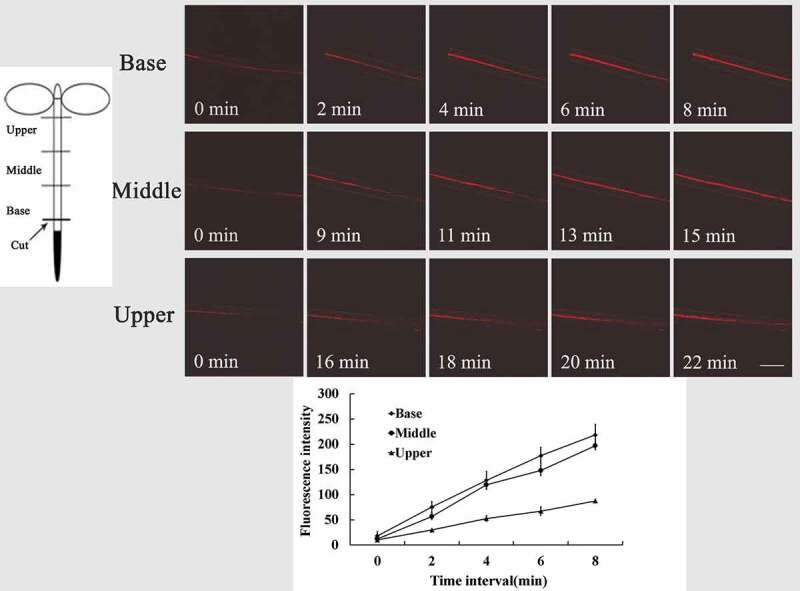
Spatial–temporal variation of intracellular O2−· fluorescence in different parts of Arabidopsis wild-type (Col-0) hypocotyls cuttings. Wild-type seedlings were preloaded with DHE, then fluorescence images of intracellular O2−· were detected at the base, middle, and upper hypocotyl at different times after excision of primary roots. Data of fluorescence pixel intensities are displayed as means ±SE of three replicates, each replicate with 3 seedlings. Bar = 250 µm
Diphenylene iodonium chloride (DPI), an inhibitor of NADPH oxidase, completely inhibited upward O2−· propagation, although it had no obvious effect on the O2−· generation at the cutting base (Figure 1), suggesting that NADPH oxidase is involved in O2−· upward propagation, but not in the basal O2−· burst. We further examined the changes of O2−· in NADPH oxidase mutant AtRbohD. Compared to wild-type plants (Figure 2), the O2−· burst occurred only at the cutting base and O2−· upward propagation was completely inhibited in AtRbohD (Figure 3). These results unequivocally indicate that RBOHD mediates O2−· upward propagation. Combined with our previous results,9 the consistency and similarity of O2−· and H2O2 signal changes confirms that the wound-induced ROS is NADPH oxidase-dependent. We conclude that O2−· is generated and propagated through the hypocotyl of the cuttings, similarly to H2O2, consistent with the role of these ROS in long distance signaling of adventitious root induction.
Figure 3.
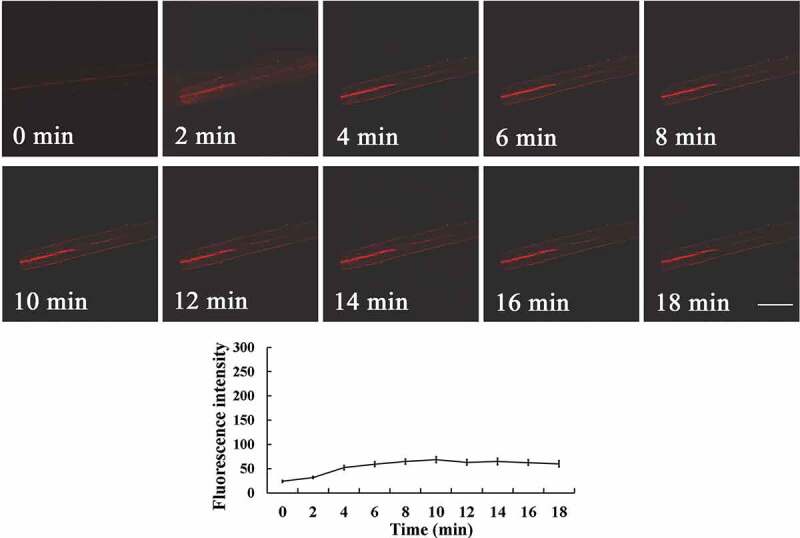
Changes in O2−· fluorescence in hypocotyls of Arabidopsis NADPH oxidase mutants AtRbohD. Seedlings of mutant AtRbohD were preloaded with DHE, and then fluorescence images were obtained at the hypocotyl base at different times after excision. Data of fluorescence pixel intensities are displayed as means ±SE of three replicates, each replicate with 3 seedlings. Bar = 250 µm
Acknowledgments
I would like to thank Dr Frantisek Baluska for this invitation.
Funding Statement
This work has been supported by National Natural Science Foundation of China [31000130], by Shaanxi Provincial Natural Science Foundation of China [2020JM-284], and by the Fundamental Research Funds for the Central Universities [GK201903067].
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author.
References
- 1.Da Costa CT, de Almeida MR, Ruedell CM, Schwambach J, Maraschin FS, Fett-Neto AG.. When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci. 2013;4:1. doi: 10.3389/fpls.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steffens B, Rasmussen A. The physiology of adventitious roots. Plant Physiol. 2016;170:603–3. doi: 10.1104/pp.15.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.León J, Rojo E, Sánchez-Serrano JJ. Wound signalling in plants. J Exp Bot. 2001;52:1–9. doi: 10.1093/jexbot/52.354.1. [DOI] [PubMed] [Google Scholar]
- 4.Schilmiller AL, Howe GA. Systemic signaling in the wound response. Curr Opin Plant Biol. 2005;8:369–377. doi: 10.1016/j.pbi.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal. 2009;2:ra45. doi: 10.1126/scisignal.2000448. [DOI] [PubMed] [Google Scholar]
- 6.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 7.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Breusegem FV. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Li P, Cai Q, Wang H, Li S, Cheng J, Li H, Yu Q, Wu S. Hydrogen peroxide homeostasis provides beneficial micro-environment for SHR-mediated periclinal division in Arabidopsis root. New Phytol. 2020. doi: 10.1111/nph.16824. [DOI] [PubMed] [Google Scholar]
- 9.Huang AX, Wang YS, Liu YY, Wang GD, She XP. Reactive oxygen species regulate auxin levels to mediate adventitious root induction in Arabidopsis hypocotyl cuttings. J Integr Plant Biol. 2020;62:912–926. doi: 10.1111/jipb.12870. [DOI] [PubMed] [Google Scholar]
- 10.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8(4):397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Neill S, Desikan R, Hancock J. Hydrogen peroxide signaling. Curr Opin Plant Biol. 2002;5:388–395. doi: 10.1016/S1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 12.Jiang M, Zhang J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot. 2002;53:2401–2410. doi: 10.1093/jxb/erf090. [DOI] [PubMed] [Google Scholar]
- 13.Zielonka J, Vasquez- Vivar J, Kalyanaraman B. Detection of 2- hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3(1):8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]


