ABSTRACT
Not much information is available to substantiate the possible role of γ -aminobutyric acid (GABA) signaling in mitigating water-deficit stress in snap bean (Phaseolus vulgaris L.) plants under semiarid conditions. Present work aims to investigate the role of exogenous GABA (foliar application; 0.5, 1 and 2 mM) in amelioration of drought stress and improvement of field performance on snap bean plants raised under two drip irrigation regimes (100% and 70% of water requirements). Water stress led to significant reduction in plant growth, leaf relative water content (RWC), cell membrane stability index (CMSI), nutrient uptake (N, P, K, Ca, Fe and Zn), pod yield and its content from protein and total soluble solids (TSS). Meanwhile, lipid peroxidation (malondialdehyde content- MDA), osmolyte content (free amino acids- FAA, proline, soluble sugars) antioxidative defense (activity of superoxide dismutase- SOD, catalase- CAT, peroxidase- POX and ascorbate peroxidase- APX) and the pod fiber content exhibited significantly increase due to water stress. Exogenous GABA application (especially at 2 mM) revealed partial normalization of the effects of drought stress in snap bean plants. GABA-induced mitigation of drought stress was manifested by improvement in growth, water status, membrane integrity, osmotic adjustment, antioxidant defense and nutrient acquisition. Furthermore, GABA application during water stress in snap bean plants resulted in improvement of field performance being manifested by increased pod yield and its quality attributes. To sum up, exogenous GABA appears to function as an effective priming molecule to alleviate drought stress in snap bean plants under semiarid conditions.
KEYWORDS: Crop yield, drip irrigation, gamma-aminobutyric acid, osmolytes, phaseolus vulgaris, drought
1. Introduction
The availability of freshwater sources for field irrigation appears as the major constraint for sustainable agriculture and for maintaining food security worldwide. This constrains may, however, be exacerbated in the semiarid regions with low annual precipitation (200 to 750 mm/year).1,2 In plants, drought stress or water deficit can cause deleterious effects at morphological, biochemical and molecular levels.3–6 Among the various physiological responses, the excessive release of reactive oxygen species (ROS) induced the oxidative damage to plant cell components, i.e. protein, lipids and nucleic acids7–9 and the development of several efficient non-enzymatic and enzymatic antioxidant systems.10–12 Plant resilience to drought stress is mostly attained by the accumulation of compatible solutes, ion homeostasis and redox management.3,13–15 Furthermore, drought stress interferes with hormonal balance, gene expression, signaling pathways, photosynthetic efficiency and reduces crop yield.6,16–20 Drought stress signaling during field irrigation is associated with a plethora of physiological changes associated with ROS, phytohormone and calcium crosstalk in plants.6,16 Calcium sensor proteins and ROS outburst have been reported to be the early signaling events associated with drought stress signaling.16 Plasma membrane plays a pivotal role in the perception and transmission of drought signaling in plants.16–20 Both ABA-dependent and ABA-independent signaling pathways are operative during drought stress tolerance in plants. ABA-dependent signaling events are associated with the activation of several transcription factors thus regulating gene expression during drought tolerance.16 In this context, it is important to access the role of priming compounds in amelioration of the effects of drought stress in plants. Various physiological and biochemical changes associated with priming molecules are likely to decipher the mechanisms of drought stress regulation in plants subjected to deficit irrigation.
Gamma-aminobutyric acid (GABA) is a neurotransmitter non-proteinogenic amino acid which widely presented in all living organisms like microorganisms, animals, insects, worms and plants.21–23 Following its first report in Solanum tuberosum, 24 investigations in the last decades have deciphered the role of GABA in the signaling process and regulation of plant growth, primary metabolism, gene expression, ion homeostasis and inducing of antioxidative defense systems during abiotic stress.25–27 Moreover, it can act as a temporary nitrogen pool and as a regulator to the cytoplasmic pH during stress conditions.21,28 Recent investigations have deciphered the involvement of GABA signaling in the expression of stress-related transcription factors (WRKY, MYB and bZIP), regulation of calcium signaling and redox homeostasis.29,30 Several previous studies confirmed that GABA can alleviate the deleterious effects of different abiotic stresses including drought, 29,31 chilling, 32 heat stress, 30 salinity33 and heavy metals.34 Under water deficiency, GABA can act as a protective agent in plants by maintaining the relative water content (RWC)29 and cell membrane stability.35
Snap bean (Phaseolus vulgaris L.) is an annual legume crop commonly grown in the subtropical and temperate regions of the world. The crop is widely consumed for its nutritive value of high protein content and minerals.11,36,37 However, several previous studies reported that snap bean plants are very sensitive to any oscillation in the soil moisture.38–40 Since water deficit can cause a disturbance of fertility and reducing the eventual yield of green pods or seeds.41 Furthermore, the tolerance of snap bean to water stress depends on the duration and severity of the exposure to water shortage and the stage of development %. In this respect, it has been found that snap bean plants are more susceptible to water stress at the flowering and pod-filling stages than the other developmental stages. The balance between the rational use of freshwater and the productivity of snap bean is still an important research question under semiarid conditions. Reports by Doğan and Akinci42 revealed that water-stress-induced alteration in nutrient acquisition (Na, K, Ca, Mg and Mn) in snap bean leaves. To date, insufficient information exists on the role of GABA in mitigation of drought stress in snap bean plants. Moreover, improved field performance of snap bean plants during water-deficit irrigation shall be a beneficial outcome of their optimum water use efficiency in semi-arid cultivable lands. Soil texture, moisture content and climatic variations are likely to cause less adverse effects in the presence of bio-priming molecules being applied to snap bean cultivars. In the present work, we hypothesized the efficacy of exogenous GABA in improving field performance, pod yield, plant growth, osmotic tolerance and redox regulation in water-stressed snap bean plants. In the present work, water stress-induced snap bean plants were subjected to GABA treatment (foliar spray) in three variable concentrations (0.5, 1 and 2 mM) and its ameliorative role was analyzed with respect to plant biomass, osmotic tolerance, antioxidative defense and nutrient acquisition. Furthermore, GABA application was found to be effective in improving yield attributes (pod yield, protein content, total soluble solids) in snap bean plants raised under water-deficit irrigation. The work was conducted under monitored conditions of solar radiation, precipitation, wind speed, air temperature and relative humidity (Table 1) in two growing seasons of 2018 and 2019. Two levels of irrigation water (70% and 100%) were applied to compare the effects of water-deficit irrigation. GABA application was provided in regular intervals of pre- and post-irrigational stages of snap bean cultivation. Thus, GABA was found to be an effective stress-priming molecule to manage to snap bean cultivation under water-deficit irrigation in semi-arid regions.
Table 1.
Monthly averages of soil temperature, solar radiation, precipitation, wind speed, air temperature and relative humidity during the period of cultivation (May–September) in the season 2018 and 2019
| Soil temperature |
Solar radiation Dgt |
Wind direction dig |
Precipitation |
Wind speed |
HC Air temperature |
HC Relative humidity |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [°C] |
[MJ/m2] |
[deg] |
[mm] |
[m/sec] |
[°C] |
[%] |
|||||
| Date | aver | min | max | aver | aver | sum | aver | aver | min | max | aver |
| 03/01/2018 | 23 | 17.3 | 28.9 | 547.45 | 99.5 | 11.2 | 0.9 | 17.8 | 3.6 | 37.6 | 60 |
| 04/01/2018 | 23.7 | 19.7 | 29.2 | 407.94 | 110.05 | 5.2 | 0.1 | 19.2 | 7.3 | 37.5 | 59 |
| 05/01/2018 | 27.2 | 23.9 | 30.5 | 367.02 | 100 | 0 | 0 | 24.9 | 11.6 | 40.9 | 57 |
| 03/01/2019 | 19.8 | 15.3 | 23.7 | 1007.34 | 111.26 | 0 | 1.2 | 19.1 | 7.5 | 36.9 | 58 |
| 04/01/2019 | 22.9 | 18.5 | 28.1 | 1318.77 | 100.07 | 0.8 | 1.7 | 21.7 | 6.8 | 38.5 | 46 |
| 05/01/2019 | 27.5 | 24.2 | 31.6 | 1368.46 | 97.2 | 0.2 | 1.4 | 26 | 10.2 | 45.6 | 47 |
2. Materials and Methods
The field experiment was conducted in a private farm called “De Bruin” (Wadi El-Natroun, Beheira Governorate, Egypt) during the growing seasons of 2018 and 2019. Table 1 summarizes the climatic data which were recorded by a close agrometeorological station, Cairo-Alex desert road to know the environmental conditions surrounding the plants in the experimental site while, the texture of the experimental soil and its physical and chemical properties are shown in Table (2).
Table 2.
Physical and chemical analysis of the experimental soil before cultivation in the seasons of 2018 and 2019
| Analyzed fraction | 2018 | 2019 |
|---|---|---|
| Physical properties | ||
| Clay % | 5.0 | 5.8 |
| Silt % | 16 | 14 |
| Sand | 79 | 80.2 |
| Soil type | Loamy sand | Loamy sand |
| Chemical properties | ||
| pH | 8 | 7.9 |
| Available nitrogen (ppm) | 23.4 | 27.8 |
| Available phosphorus (ppm) | 17.5 | 19.00 |
| Available potassium (ppm) | 44.0 | 41.8 |
| CaCO3 (mg/100 g soil. | 3.80 | 3.40 |
2.1. Experimental layout
Snap bean (Phaseolus vulgaris, L.) cv. Pulista seeds were purchased from Royal Sluis Company (Netherlands) and sown in March of 2018 and 2019 seasons. The experimental setup was prepared in a split-plot layout with three replicates. Drip irrigation was applied (100% and 70% of water requirements) in the surface of the main plots and GABA treatments (foliar applications) were distributed randomly in the sub-plots. The experimental unit area measured 45 m2 (15 m length x 3 m width) and was comprised of 5 rows with a separation of 0.6 m between each row. The plant distance was kept to 7 cm apart on one side. Snap bean plants were irrigated using drippers of 4 L.h−1 capacity and 0.3 m distance between drippers. Plants present between the drippers received adequate water obtained from lateral wetting of the soil. A flow-meter was used to monitor the follow the flow rate for each irrigation level treatment. Three rows without irrigation served as a border between both irrigation levels.
2.2. Fertilization and agricultural management
Experimental soil was comprised of 25 m3 compost, 100 kg P as calcium superphosphate (15.5% P2O5) and 50 kg N as ammonium sulfate (20.5% N) which were dressed in the soil per hectare. Chemical fertilizers (125 Kg N and 125 kg K per ha as ammonium sulfate and potassium sulfate, respectively) were injected regularly within the irrigation system. The application of other nutrients and all cultural processes including disease and pest management strategies were followed according to the guidelines of the Egyptian Ministry of Agriculture.
2.3. Calculation of water requirements
According to the water requirements of snap bean plants, two levels of irrigation water (100% and 70%) were applied 20 d after sowing (DAS). The plots were irrigated via manual valves for each experimental plot. The total amount of irrigation water was measured according to the Food and Agricultural Organization (FAO) Penman–Monteith (PM) procedure, FAO 56.43 The second step involved the analysis of the values of crop water consumptive use (ETcrop) as described by Doorenbos and Pruitt.44
2.4. Measurement of water usage
The total amount of water applied through the drip irrigation system was measured for each water regime treatment and appears in Table 3
Table 3.
Average amounts of applied water in the seasons of 2018 and 2019
| Date | Irrigation (m3. ha−1) | Average of daily requirement (m3. ha−1. Day−1) |
||
|---|---|---|---|---|
| 1–15 Mar (15 d) | 142.6 | 9.6 | ||
| 16–19 (4 d) | 53.8 | 13.2 | ||
| Starting date of both different irrigation treatments | ||||
| Date |
70% of water requirements |
Average of daily requirements |
100% of water requirements |
Average of daily requirement |
| 20–31 Mar (12 d) | 111.8 | 9.4 | 159.8 | 13.2 |
| 1–15 April (15 d) | 276.7 | 18.5 | 395.5 | 26.4 |
| 16–30 April (15 d) | 307.7 | 20.4 | 439.4 | 29.3 |
| 1–15 May (15 d) | 364.3 | 24.2 | 520.6 | 34.8 |
| 16–29 May (14 d) | 264.5 | 19.0 | 377.8 | 26.9 |
| Total (m3. ha−1) | 1520.9 | 2089.0 | ||
2.5. Foliar application of GABA
Gamma-aminobutyric acid (GABA) (Sigma) treatment was applied twice in the growing phase of the plants and four variable concentrations (0, 0.5, 1 or 2 mM). The first set of treatment was applied after full emergence and 3 d before the start of irrigation (17 DAS). Thus, plants could assimilate GABA in their tissues before the exposure to water deficit. The second set of treatment was applied at the flowering stage (35 DAS). Distilled water was sprayed as a control (0 mM GABA) and tween 20 at 0.05 ml L−1 was used as a wetting agent for different foliar treatments. Leaf samples were collected randomly at 60 DAS from the inner rows to determine plant vegetative growth and analyze various biochemical constitutes.
2.6. Analysis of vegetative growth
Followed by sampling (60 DAS), shoot fresh weight (FW) was recorded by digital balance. Following by recording of fresh weight, the samples were cleaned by washing with tap water and completely dried in an air-forced ventilated oven at 105°C. Followed by drying the shoot dry weight was recorded. Average leaf area.plant−1 was calculated as a function of unit area and leaf fresh weight using the following equation.45
Leaf area (cm2) = (Disk area* No. Disks* Leaf f.wt)/Disk f.wt
2.7. Measurement of leaf relative water content (RWC)
Relative water content was determined according to Ünyayar, et al. .46 Leaf discs were obtained from 10 leaves and the fresh weight was recorded. Followed by measurement the leaf discs were placed immediately in distilled water for 2 h at 25 ◦C and their turgid weights (TW) were recorded. The samples were then dried in an oven at 110 ◦C for 24 h (DW). Relative water content (RWC) was calculated by using the following formula: RWC = (FW-DW)/(TW-DW)*100
2.8. Determination of cell membrane stability index (CMSI)
Cell membrane stability was measured by the electrolyte leakage technique as described by Singh, et al.47 with certain modifications. Samples (10 leaf discs −1.8 cm diameter) from each treatment were selected randomly from fully expanded leaves, cleaned and incubated in 10 ml deionized water for 24 h on a shaker. Followed by incubation in water EC1 values were measured by EC meters (DOH-SD1, TC-OMEGA, USA/Canada). Followed by these samples were autoclaved at 120°C for 20 min to determine the values of EC2. Cell membrane stability index was calculated using the following equation: CMSI = 1 - (EC1/EC2) X 100.
2.9. Estimation of lipid peroxidation
The extent of lipid peroxidation was measured by estimating malondialdehyde (MDA) content according to Heath and Packer.48 Frozen tissues (1 gm) were homogenized in 10 ml 0.1% (w/v) trichloroacetic acid (TCA). Followed by homogenization the samples were centrifuged at 4,500 rpm for 15 min. The reaction mixture thus prepared contained 1 ml supernatant and 4 ml 0.5% (w/v) thiobarbituric acid (TBA) dissolved in 20% (w/v) TCA. The mixture was heated in boiling water for 30 min. Followed by heating the mixture was cooled at room temperature and further centrifuged at 4500 rpm for 15 min. The absorbance of the supernatant was recorded spectrophotometrically (Chrom Tech CT-2200) at 535 nm and rectified for nonspecific turbidity at 600 nm The MDA concentration (nmol.g−1 FW) was calculated using Δ OD (A532-A600) and the extinction coefficient (ε = 155 mM−1cm−1).
2.10. Quantification of osmolytes
Proline concentration was determined with ninhydrin reagent as described by Bates, et al. .49 Total soluble sugars (TSS) were estimated by phenol-sulfuric acid method as described by Chow and Landhäusser.50 Free amino acids were determined by ninhydrin reagent as glycine according to the method of Hamilton, et al.51
2.11. Elemental analysis
Dry leaves were ground to powder and digested using sulfuric acid and hydrogen peroxide. Leaf mineral concentrations of N, P, K, Ca, Fe and Zn were determined according to Cottenie, et al. .52 Nitrogen (N) was determined by the usual Kjeldahl method (Velp Scientifica, Europe). The colorimetric method by UV/VIS spectrophotometer was adopted to determine phosphorous content. Potassium content was determined by the method of flame spectrophotometry. Calcium, iron and zinc were determined by atomic absorption spectrophotometry (AAS-Hitachi, Tokyo, Japan).
2.12. Estimation of antioxidant enzymes
Total soluble protein content was determined according to Bradford.53 Superoxide dismutase (SOD, EC 1.15.1.1) assay was based on the method described by Beyer and Fridovich.54 Catalase (CAT) (EC 1.11.1.6) activity was determined according to the method of Cakmak, et al. .55 Ascorbate peroxidase (APX) (EC 1.11.1.11) activity was measured according to the method of Nakano and Asada56 by monitoring the decrease of absorbance at 290 nm following the ascorbate oxidation for 3 min using a spectrophotometer (Chrom Tech CT-2200). Peroxidase (EC1.11.1.7) activity was quantified by the method of Hammerschmidt, et al.57
2.13. Analysis of green pod yield and quality
Green pods were collected several times after fruit setting until the end of the experiment to determine the total yield. In order to analyze pod yield quality; total soluble solids were determined using the hand refractometer (OPTIKA, HR-190).58 Fiber percentage in pods was determined according to Rai and Mudgal.59 The total protein in pods was estimated using the conversion factor of 6.25X nitrogen percentage content.58
Statistical analysis
All experimental data were statistically analyzed using SAS.60 The standard deviation of the means was calculated and post hoc analysis for significant differences was analyzed using LSD test (P ≤ 0.05).
3. Results
According to the data presented in Tables 1&2, the various climatic factors at the site of experiment varied in the range of monthly averages for soil temperature (19.8–27.5°C), solar radiation (367.02–1368.46 MJ/m2), wind direction (97.2°-111.26°), precipitation (0–11.8 mm), wind speed (0–1.7 m/sec), leaf wetness (1615–19,545 min), air temperature (3.6–11.2) and relative humidity (46–60%) in the months of the two growing seasons of 2018 and 2019. Analysis of edaphic factors (physicochemical properties) revealed the presence of loamy-sandy soil to be present in the experimental site. In both the growing seasons, the soil was comprised of the prevalent sandy part (79–80.2%), followed by silt (14–16%) and clay (5–5.8%). The analysis of chemical properties of soil revealed a higher amount of K levels followed by N, P and Ca. Thus, lower percentages of organic matter and prevalence of sand followed by low annual precipitation reduce the water-holding capacity of the soil in this semi-arid cultivation site. Thus, drought conditions in the experimental site may cause an additive adverse effect with the climate changes, leading eventually to poor plant growth, reduced water use efficiency and crop productivity.
To analyze the effect of GABA in water-stressed plants, findings were compared to the respective control sets (plants raised in well-watered conditions and in the absence of GABA). The mean comparison of all parameters in response to the two variables and their interaction (well-watered condition, water deficit and GABA treatment) are summarized in Table 4. ANOVA followed by post hoc LSD analysis revealed that both variables- irrigation (70% and 100%) and GABA treatments (0.5 mM, 1 mM and 2 mM) imposed significant effects to most of the analyzed parameters in snap bean plants. Water-deficit irrigation (70%) had significant negative effects on all parameters at P ≤ 0.001 level and changes in K+ (%) were significant at P ≤ 0.05. GABA treatment led to the mitigation of water stress by positively upregulating the value of most of the parameters. GABA-treatment exhibited significant differences for almost all parameters except for MDA and Zn content. All other parameters were significant at P ≤ 0.001 level, while changes in fiber content and Fe content were significant at P ≤ 0.01 and P ≤ 0.05 levels, respectively. The interactions between irrigation and GABA treatment were significant for MDA content (P ≤ 0.001), proline content (P ≤ 0.001), free amino acid content (P ≤ 0.05), catalase activity (P ≤ 0.01), ascorbate peroxidase activity (P ≤ 0.05) and total soluble solid (P ≤ 0.05). Thus, irrigation and GABA treatments imposed significant effects to most of the biochemical and physiological attributes in snap bean plants.
Table 4.
Mean comparison shows the main effects of the irrigation levels (Well-watered and water-deficit) and the foliar applications of gamma-aminobutyric acid (GABA) at 0, 0.5, 1 and 2 mM on the vegetative growth, lipid peroxidation, water status, cell membrane stability index, osmolytes, antioxidant enzymes, mineral nutrients, pod yield and some pod quality attributes of snap bean plants
| Irrigation |
GABA |
Significance |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Well-watered | Water-deficit | LSD≤0.05 | 0 mM | 0.5 mM | 1 mM | 2 mM | LSD≤0.05 | Irrigation | GABA | Interaction |
| Shoot fresh weight (g. plant −1) | 156.3 A | 117.9 B | 3.95 | 124.3 B | 141.0 A | 140.2 A | 142.8 A | 5.59 | *** | *** | ns |
| Shoot dry weight (g. plant −1) | 29.2 A | 21.9 B | 0.64 | 23.7 C | 26.3 AB | 25.5 B | 26.6 A | 0.91 | *** | *** | ns |
| Leaf area (cm2.plant−1) | 1639 A | 1310 B | 39.3 | 1382 B | 1504 A | 1507 A | 1503 A | 55.6 | *** | *** | ns |
| RWC (%) | 85.7 A | 71.5 B | 0.77 | 76.7 B | 78.9 A | 78.8 A | 79.9 A | 1.09 | *** | *** | ns |
| CMSI (%) | 81.9 A | 72.8 B | 0.83 | 74.9 C | 76.9 B | 78.3 A | 79.1 A | 1.17 | *** | *** | ns |
| MDA (nmol.g−1 FW) | 7.38 B | 8.58 A | 0.30 | 8.26 A | 7.96 AB | 7.87 AB | 7.83 B | 0.42 | *** | ns | *** |
| Proline (µg.g−1 FW) | 193.8 B | 600.1 A | 22.8 | 335.5 C | 388.6 B | 428.7 A | 435.1 A | 32.2 | *** | *** | *** |
| FAA (µg.g−1 FW) | 552 B | 932 A | 46.3 | 622.4 C | 737.7 B | 818.7 A | 790.1 AB | 65.5 | *** | *** | * |
| Soluble sugars (mg.g−1 FW) | 2.80 B | 3.51 A | 0.08 | 2.88 B | 3.21 A | 3.24 A | 3.30 A | 0.11 | *** | *** | ns |
| SOD (∆ O.Dmin −1. mg −1 protein) | 6.59 B | 8.74 A | 0.37 | 6.80 C | 7.45 B | 8.09 A | 8.32 A | 0.52 | *** | *** | ns |
| CAT (∆ O.Dmin −1. mg −1 protein) | 1.89 B | 3.63 A | 0.20 | 2.49 C | 2.58 BC | 2.83 AB | 3.12 A | 0.29 | *** | *** | ** |
| POX (∆ O.Dmin −1. mg −1 protein) | 26.07 B | 42.39 A | 1.59 | 31.29 C | 33.87 B | 35.45 AB | 36.31 A | 2.26 | *** | *** | ns |
| APX (∆ O.Dmin −1. mg −1 protein) | 1.32 B | 2.28 A | 0.07 | 1.67 C | 1.77 BC | 1.85 AB | 1.89 A | 0.107 | *** | *** | * |
| N (%) | 3.38 A | 2.99 B | 0.12 | 3.05 B | 3.01 B | 3.18 B | 3.48 A | 0.18 | *** | *** | ns |
| P (%) | 0.537 A | 0.493 B | 0.017 | 0.504 B | 0.491 B | 0.514 B | 0.553 A | 0.024 | *** | *** | ns |
| K (%) | 3.09 A | 2.94 B | 0.125 | 2.90 BC | 2.84 C | 3.03 B | 3.29 A | 0.176 | * | *** | ns |
| Ca (%) | 1.71 A | 1.32 B | 0.07 | 1.26 B | 1.61 A | 1.58 A | 1.59 A | 0.104 | *** | *** | ns |
| Fe (ppm) | 185.2 A | 135.3 B | 10.7 | 155.3 B | 150.4 B | 162.3 AB | 173.1 A | 15.09 | *** | * | ns |
| Zn (ppm) | 64.9 A | 53.4 B | 5.1 | 58.3 AB | 55.3 B | 60.1 AB | 62.9 A | 7.23 | *** | ns | ns |
| Pod yield (ton.ha−1) | 11.98 A | 7.68 B | 0.28 | 8.74 C | 9.52 B | 10.39 A | 10.65 A | 0.40 | *** | *** | ns |
| Protein (g. 100 g−1 DW) | 18.96 A | 17.19 B | 0.81 | 16.26 C | 17.53 B | 19.08 A | 19.43 A | 1.15 | *** | *** | ns |
| Fibers (g. 100 g−1 DW) | 3.42 B | 4.18 A | 0.11 | 3.97 A | 3.81 B | 3.75 B | 3.67 B | 0.157 | *** | ** | ns |
| TSS | 4.41 A | 3.43 B | 0.14 | 3.61 C | 3.65 C | 4.09 B | 4.35 A | 0.20 | *** | *** | * |
| Data of the two seasons of 2018 and 2019 were subjected to combined analysis with 3 replicates in each season. The different capital letters within a row indicate significantly different values according to LSD’s multiple range tests (P ≤ 0.05).; RWC, leaf relative water content; MDA, malondialdehyde; CMSI, cell membrane stability index; FAA, free amino acids; SOD, superoxide dismutase; APX, ascorbate peroxidase; POX, peroxidase; CAT, catalase; TSS, total soluble solids. ns, not significant, * P ≤ 0.05, ** P ≤ 0.01 and *** P ≤ 0.001. | |||||||||||
3.1 GABA application improves plant biomass and reduces osmotic stress in water-deficient snap bean plants
In order to investigate the role of exogenous GABA in mitigation of water stress foliar spray was applied in variable concentrations (0.5, 1 and 2 mM). Plant biomass (shoot DW and FW), leaf area, relative water content and lipid peroxidation (MDA content) were analyzed as plant growth factors and osmotic parameters in the absence and presence of water stress and GABA treatment. Furthermore, the cellular membrane stability index (CMSI) was also estimated to analyze the extent of drought or water stress (Figure 1).
Figure 1.
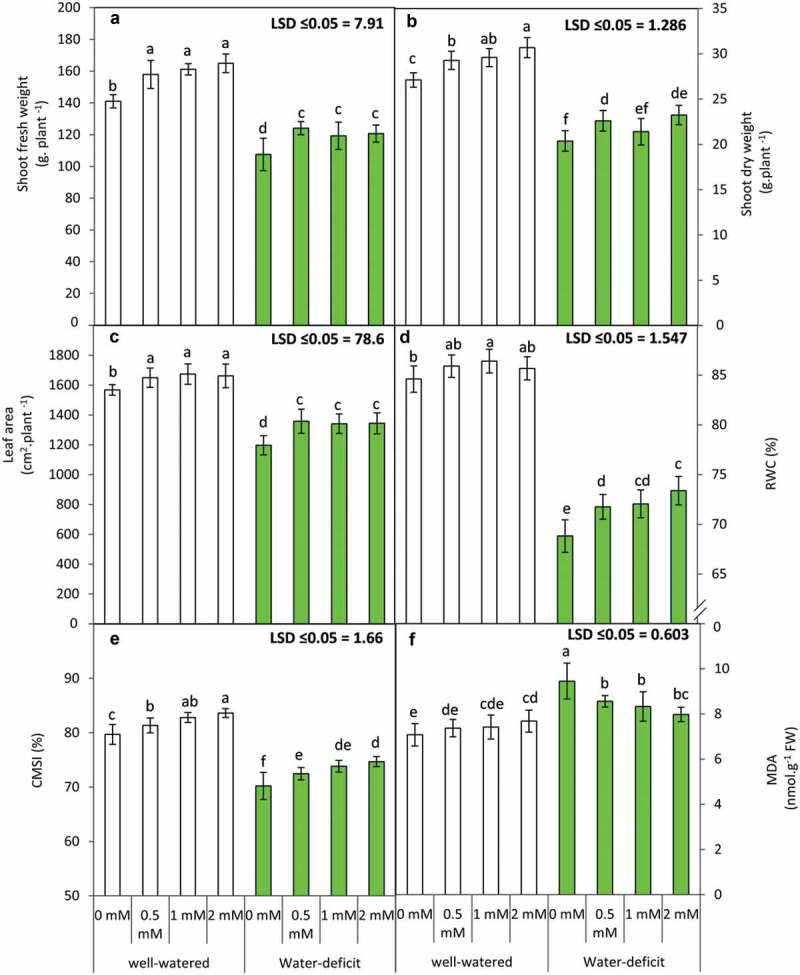
Shoot fresh weight (a), shoot dry weight (b), leaf area (c), leaf relative water content “RWC” (d), cell membrane stability index “CMSI’ (e) and lipid peroxidation as indicated by malondialdehyde ”MDA” (f) of the snap bean plants at 60 DAS as influenced by the foliar application of GABA (0, 0.5, 1 and 2 mM) under two irrigation regime. Means were presented ± SD. Different letters are significant differences, according to LSD’s multiple range tests (P < .05)
Water deficit in snap bean plants results in significant (p ≤ 0.05) reduction in shoot biomass (dry weight and fresh weight) and leaf area in comparison with plants raised in well-watered conditions. GABA application in variable concentrations (0.5, 1 and 2 mM) results in partial recovery of biomass and leaf area in well-watered state which is all the higher at a concentration of 2 mM. However, all three concentrations of GABA application in water-stressed plants exhibit similar effects for recovery of shoot biomass and leaf area in comparison with plants raised in the absence of GABA treatment. Water-stressed snap bean plants exhibit a significant decrease in relative water content which, interestingly, shows higher recovery in the presence of 2 mM exogenous GABA. Water stress-induced membrane lipid peroxidation in snap bean plants is evident by an increase in malondialdehyde (MDA) content in comparison with that in well-watered plants. GABA application (0.5, 1 and 2 mM) results in decreased MDA content in water-stressed plants in comparison to control (plants raised in absence of GABA). Cellular membrane stability index (CMSI) analysis exhibits marked effects of water deficit (drought stress) which is evident in the form of a decrease in CMSI % in water-stressed plants. GABA-induced increase in CMSI (%) exhibits a positive correlation with increasing GABA concentrations both in well-watered and water-stressed plants. Thus, exogenous GABA application (in variable concentrations) in water-stressed plants results in increased shoot biomass, higher leaf area and alleviates osmotic stress. Relative water content (%) and CMSI% exhibit higher recovery in the presence of 2 mM GABA in water-stressed plants. Furthermore, exogenous GABA reduces the extent of lipid peroxidation (MDS content) in water-stressed plants. Present findings reveal significant differences being observed for the effect of irrigation and GABA application on plant biomass, MDA content and CMSI% (Table 4)
3.2 Foliar application of GABA promotes FAA, proline and soluble sugar content in water-stressed plants
Plants that were exposed to water stress exhibited a significant (p ≤ 0.05) increase in FAA, proline and soluble sugar content (Figure 2). Furthermore, the foliar application of GABA exhibited a significant (concentration-dependent) increase in all the three parameters (FAA, proline and soluble sugar). Treatment with 2 mM GABA was most efficient in all three cases, while the more conspicuous increase was observed for FAA and proline content. Plants raised in well-watered conditions, however, exhibited marginal changes in FAA, proline and soluble sugar content upon GABA application. Present findings reveal significant differences being observed for the effect of irrigation and GABA application on FAA, proline and soluble sugar content. Furthermore, the interaction of irrigation and GABA was observed to impart significant differences to proline and FAA content (Table 4).
Figure 2.
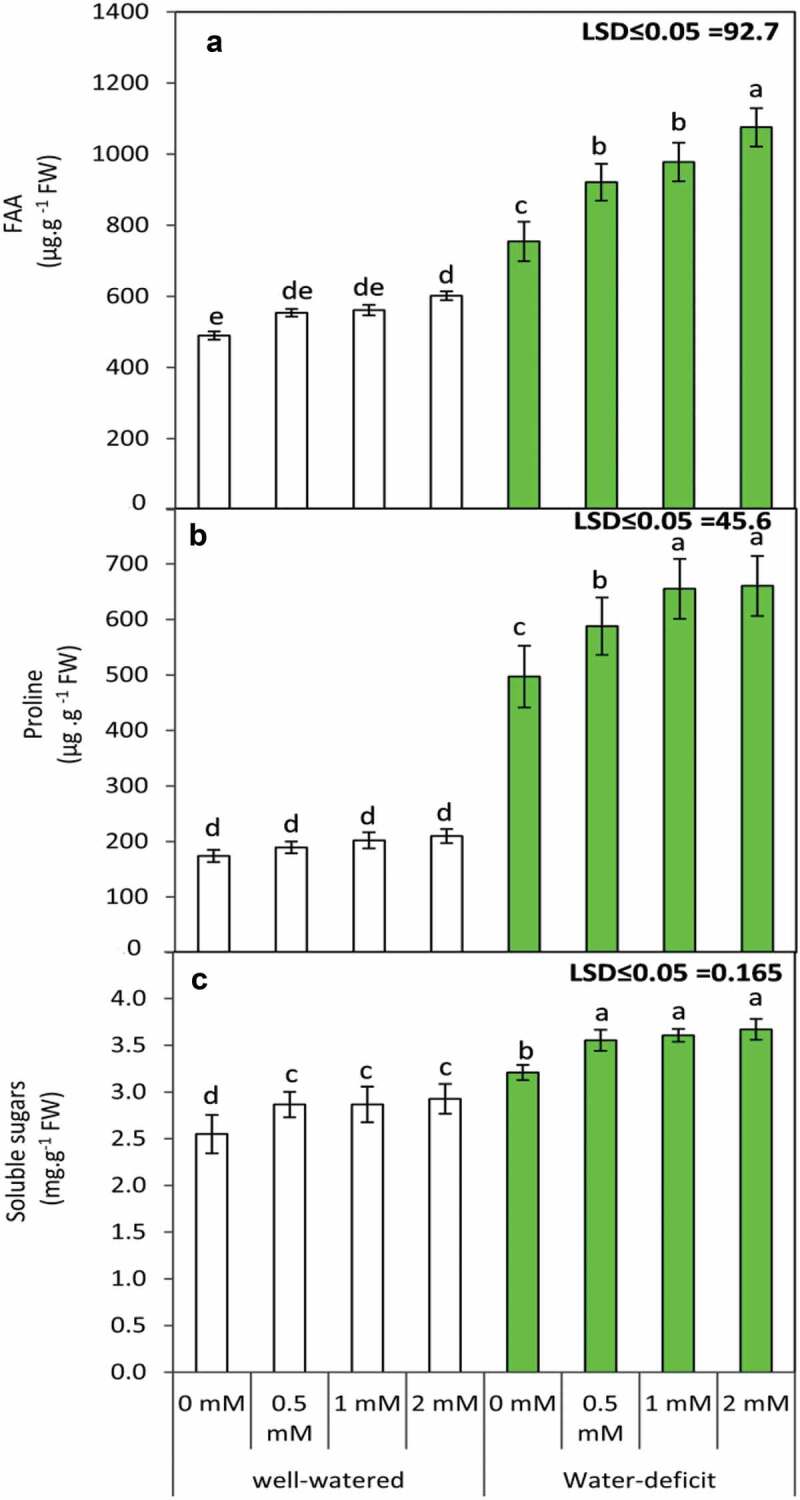
Free amino acids ”FAA” (a), Proline (b) and total soluble sugars (c) in the leaves of the snap bean plants at 60 DAS as influenced by the foliar application of GABA (0, 0.5, 1 and 2 mM) under two irrigation regimes Means were presented ± SD. Different letters are significant differences, according to LSD’s multiple range tests (P < .05)
3.3. GABA application upregulates antioxidant enzyme activity in water-stressed plants
In the present investigation water deficit in snap bean plants lead to significant (p ≤ 0.05) upregulation in the activity of SOD, POX, CAT and APX (Figure 3) in comparison with plants raised in well-watered conditions. The upregulation of enzyme activity was further prominent in the presence of GABA application which was all the more efficient in the presence of 2 mM GABA concentration. Contrastingly, GABA treatment in plants of well-watered condition exhibited a marginal increase in the activity of SOD, POX, CAT and APX. Unlike the water-stressed plants, treatment with 2 mM GABA did not bring about any significant change in the activity of CAT, POX and APX in plants raised in well-watered condition. Present findings reveal significant differences being observed for the effect of irrigation and GABA application on SOD, POD and CAT activity (Table 4). The interaction of irrigation and GABA application was significant for CAT activity (Table 4).
Figure 3.
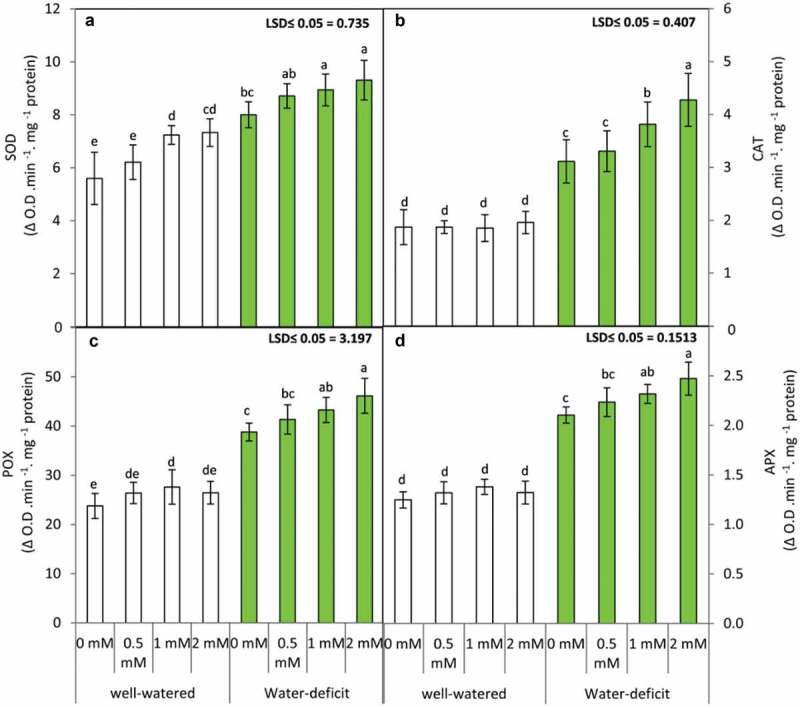
Superoxide dismutase ”SOD” (a), catalase “CAT” (b) peroxidase “POX” (c) and ascorbate peroxidase ”APX” (d) in the leaves of the snap bean plants at 60 DAS as influenced by the foliar application of GABA (0, 0.5, 1 and 2 mM) under two irrigation regimes. Means were presented ± SD. Different letters are significant differences, according to LSD’s multiple range tests (P < .05)
3.4. GABA application improves nutrient acquisition in plants subjected to water stress
In order to observe the nutritional status of the plants subjected to water deficit, N, P, K, Ca, Fe and Zn content (Figure 4) were analyzed in the absence and presence of water stress and GABA treatment. Water stress adversely affected the nutritional level of plants which was evident by a significant decrease in N, P, K, Ca, Fe and Zn content. GABA application specifically at 2 mM significantly increased N, P, K, Fe and Zn content in water-stressed plants. Contrastingly, 0.5 mM GABA was effective to bring about a higher increase in Ca content in water stressed-plants. GABA treatment exerted a marginal increase in nutrient content in plants raised in well-watered condition (control). The effect of irrigation and GABA application resulted in significant differences in the content of most of the nutrients analyzed in the present work (Table 4).
Figure 4.
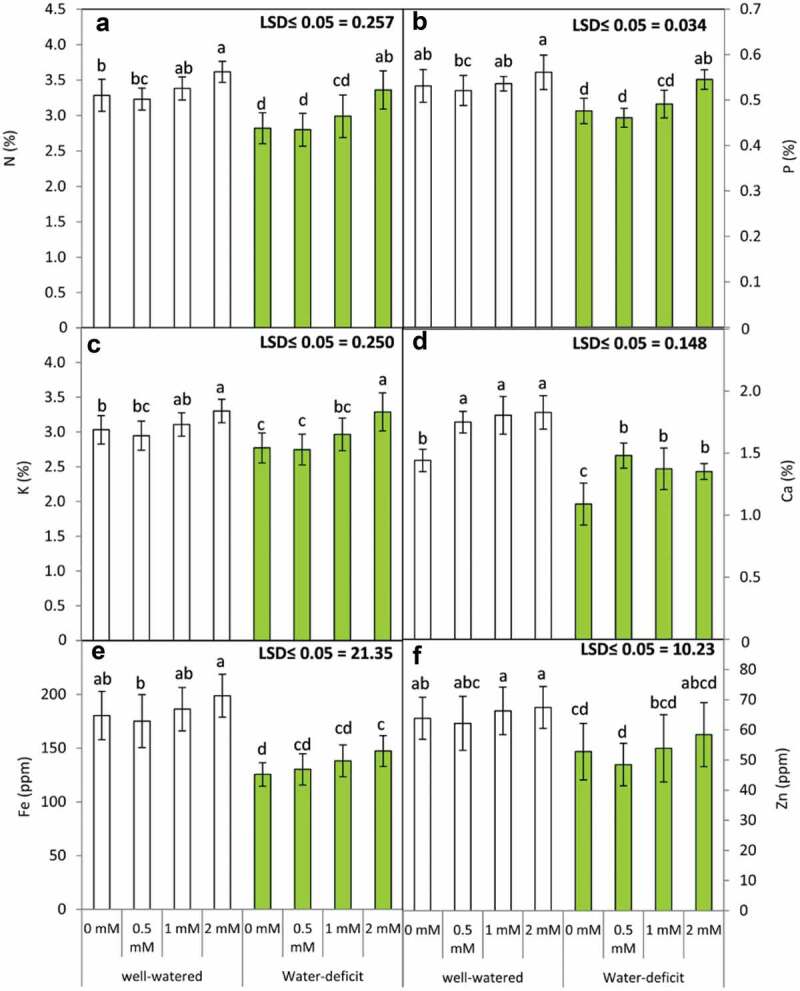
Leaf mineral content including N (a), P (b), K (c) Ca (d), Fe (e) and Zn (f)in the leaves of the snap bean plants at 60 DAS as influenced by the foliar application of GABA (0, 0.5, 1 and 2 mM) under two irrigation regimes. means were presented ± SD. Different letters are significant differences, according to LSD’s multiple range tests (P < .05)
3.5. GABA application modulates yield parameters in water-stressed plants
Yield parameters including pod yield, protein, fiber and TSS content (Figure 5) were assessed to observe the effect of water stress and GABA application on crop productivity. Water stress led to a significant reduction in pod yield, protein and TSS content, while fiber content exhibited a marked increase in water-stressed plants. GABA application (specifically 2 mM) tends to partially normalize the effects of water stress on yield parameters. GABA treatment significantly increases pod yield, protein content and TSS content while it tends to decrease fiber content in water-stressed plants. Interestingly, a similar trend was also observed for GABA-mediated improvement of yield parameters in plants raised in well-watered conditions. The yield attributes exhibited significant differences in the presence of deficit irrigation and GABA application (Table 4).
Figure 5.
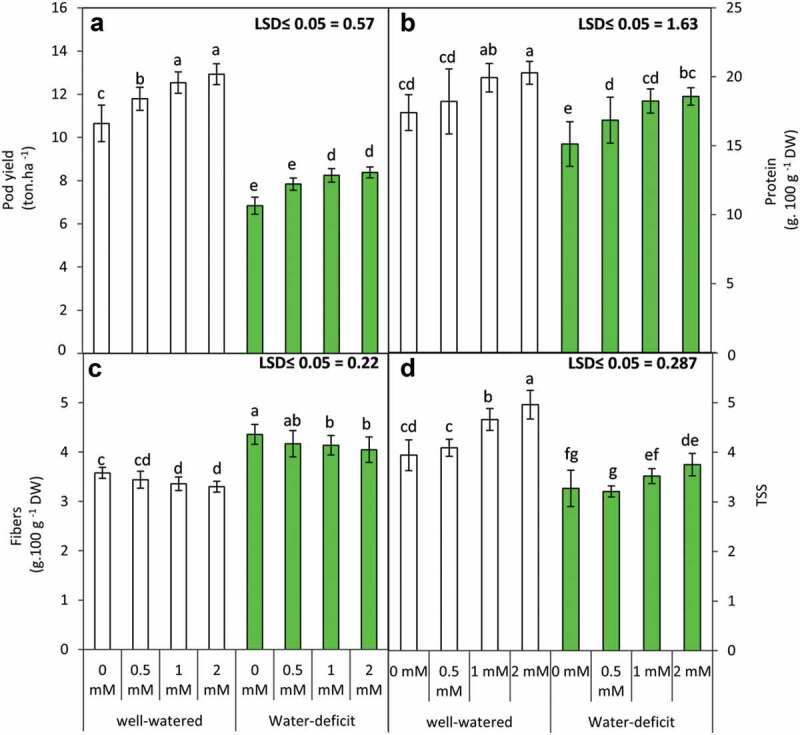
Pod yield (a) and its content from protein (b), fibers (c) and total soluble solids “TSS” (d) of the snap bean plants as influenced by the foliar application of GABA (0, 0.5, 1 and 2 mM) under two irrigation regimes. Means were presented ± SD. Different letters are significant differences, according to LSD’s multiple range tests (P < .05)
4. Discussion
Water-stress in the semi-arid cultivation soils imposes serious hurdles to crop sustainability and fruit yield.61,62 Furthermore, solar radiation, temperature and soil composition appear to play pivotal roles in regulating plant growth (shoot–root ratio), crop moisture stress index (CMSI) and various other physiological parameters in plant organs.62 Earlier investigations substantiate the fact that water-stressed Phaseolus vulgaris plants undergo alteration in osmotic status, plant growth, bean yield and proline content.40,63,64 In line with the earlier known evidence, the present work aims to investigate the role of gamma-aminobutyric acid (GABA) in mitigation of water-stress in snap bean plants. In this study, the drip irrigation method was applied in two sets of plants raised in well-watered and water-deficit conditions and grown over two seasons of 2018 and 2019. Followed by sowing, plants were subjected to water deficit for 40 d in each growing season. Following water-stress investigations were performed in leaves and pods obtained from 60 d old plants.
GABA has been known to function as an effective stress-priming molecule in plants. Recent investigations suggest the involvement of GABA in water-stress response, osmotic regulation and antioxidative defense.25,27,30,65 Earlier known evidences reveal the role of GABA in inducing tolerance to drought stress in Perennial ryegrass (Lolium perenne), bentgrass (Agrostis stolonifera) and bread wheat (Triticum aestivum).29–31 GABA application results in the modulation of antioxidative defense, relative water content, plant growth and field performance during drought stress.29–31 Furthermore, GABA application has been observed to upregulate the expression of CDPK26, MAPK1, ABF3, WRKY75, MYB13, HSP70, MT1, 14-3-3 and genes (SOD, CAT, POD, APX, MDHAR, DHAR and GR) in drought-stressed Agrostis stolonifera.30 Our current understandings of the mechanisms of GABA-induced drought tolerance in snap bean plants, however, require further investigations at the molecular level.
In the present work, GABA treatment (foliar application) was applied in variable concentrations (0.5, 1 and 2 mM). A total of 2 Mm GABA was observed to be most efficient in regulating shoot biomass (dry and fresh weight), leaf area, RWC, osmolyte accumulation and antioxidant enzyme activity. Furthermore, GABA treatment resulted in improved nutrient acquisition (N, P, K, Ca, Fe and Zn) in water-stressed plants. A comparison of the mean values obtained by statistical analysis revealed a significant interaction between the variables (well-watered condition, water deficit and GABA treatment). The detailed analysis of the mean values and their significant differences in comparison with control has been summarized in Table 4.
In the present work, GABA treatment improves shoot dry weight and fresh weight, relative water content and decreases the extent of lipid peroxidation (MDA content) in water-stressed bean plants (Figure 1). Earlier reports on the role of GABA in modulating water content and lipid peroxidation during heavy metal stress have been reported in Brassica and Zea sp.34,66 Leaf area has also been observed to be increased during GABA supplementation during water stress. Thus, GABA application (specifically 2 mM) is likely to improve shoot biomass and osmotic balance (osmolyte accumulation) in water-stressed snap bean plants (Figures 1 & 2). GABA-induced alleviation of membrane lipid peroxidation has earlier been reported in salinity and alkalinity-stressed musk melon plants.67,68 Earlier investigations in white clover plants reveal the drought-ameliorating role of exogenous GABA which normalizes the effects of PEG-induced drought stress.69 GABA application brought about an increase in tissue water content reduced electrolytic leakage, lipid peroxidation and leaf wilt in white clover plants.
In the present work, GABA application seems to exert marginal influence on these plant growth parameters in well-watered conditions (Figure 1 and Figure 2). Water stress in snap bean triggers the accumulation of the three osmolytes (free amino acids, proline and soluble sugars) (Figure 2). Present evidence is in congruence with earlier reports on the role of GABA in the regulation of osmolytes, namely, proline and soluble sugars.34,66,70 Moreover, GABA application further enhances the accumulation of osmolytes thus suggesting its role osmotic tolerance in water-stressed plants (present work). In the control set of plants (well-watered) GABA exerts marginal changes in osmolyte levels. Thus, GABA application (especially 2 mM) is effective in imparting osmotic tolerance in water-stressed plants. GABA application in drought-stressed white clover plants resulted in higher accumulation of endogenous GABA which was accompanied by a positive upregulation in proline metabolism and enzymes of GABA-shunt pathway.69 Thus, earlier findings on GABA-induced positive regulation of proline metabolism provide supporting evidence to our present findings. GABA application (0.5, 1 and 2 mM) results in a surge in proline accumulation in water-stressed snap bean plants (present work). GABA is also known to be an effective regulator of carbon metabolism and modulates the activity of NADP-dependent isocitrate dehydrogenase (NADP-ICDH) and phosphoenolpyruvate carboxylase (PEPCase).25 Upregulation of GABA shunt has been attributed to GABA-induced accumulation of TCA cycle intermediates.71 The present findings reveal a marginal increase in the accumulation of soluble sugars during GABA supplementation in water-stressed snap bean plants. Thus, in the present work, GABA signaling during water-stress is expected to regulate carbohydrate metabolism.
Water deficit during drought stress is known to trigger both enzymatic and non-enzymatic antioxidative defense thus leading to redox homeostasis.72 In the present study, our results depict that water-stress resulted in a marked increase in the activity of SOD, CAT. POX and APX (Figure 3). A similar trend was observed for all the four enzymes thus suggesting their integrative role in regulating water stress tolerance in snap bean. Exogenous application of GABA positively upregulates the activity of all the four antioxidant enzymes. Previous reports have established the role of GABA in the modulation of antioxidative defense in Brassica and Zea sp. .34,66 GABA-mediated surge in the activity of superoxide dismutase (SOD), ascorbate peroxidase (APX) and dehydroascorbate reductase (DHAR) is accompanied by reduced lipid peroxidation in salinity and alkalinity-stressed musk-melon plants.67 In support to earlier findings, present investigation in snap bean plants reveals GABA-induced upregulation of antioxidative enzymes to be accompanied by reduced lipid peroxidation.
Phaseolus vulgaris is sensitive to water-stress and is known to suffer in shoot growth and yield attributes.73–77 The reduction in leaf area in water-stressed snap bean has been reported to cause a reduction in photosynthesis rate and dry matter yield.75 Our observation of water-stress-induced reduction in leaf area and dip in dry matter yield in snap bean plants (present work) are in congruence with the earlier observations. Although various aspects of water-stress-induced changes in yield attributes of Phaseolus vulgaris have been documented, 73–77 GABA-mediated priming of water stress does not have sufficient reports.
Drought stress is known to adversely affect nutrient uptake and restricts nutrient translocation in plant organs.78 Water use–efficiency during drought stress has been known to be intimately associated with N uptake (N, ammonium and nitrate) in plants.79,80 The activity of high-affinity nitrate transporters is in turn known to regulate aquaporin activity thus modulating water transport. In the present work, water stress resulted in a significant decrease in N, P, K, Ca, Fe and Zn content in snap bean leaves. In congruence to the present findings, earlier reports suggest water stress associated-decrease in the activity of N-uptake proteins (NRT1 and NRT2).81 GABA application improves N uptake in the leaves of water-stressed snap bean plants (present work) (Figure 4). Endogenous GABA levels are known to trigger nitrate uptake and nitrogen metabolism in Arabidopsis roots subjected to nutrient stress.25 Furthermore, Sulieman82 discusses the role of GABA in enhancing the nitrogen-fixing efficiency in legumes. According to investigations by Barbosa, Singh, Cherry and Locy, 25 GABA functions as an important modulator of nitrate uptake in roots of Arabidopsis. Additionally, GABA application results in upregulation of enzymes associated with N-metabolism, namely – nitrate reductase (NR), glutamine synthetase (GS), glutamate synthase (NADH-GOGAT). GABA appears to function as an important sensor of N-deficiency, which in turn triggers nitrate uptake through plant roots. Similar to the findings in Arabidopsis, GABA application has been known to upregulate nitrate reductase and glutamine synthetase activity in cultivars of maize.83 In our study during water stress, N levels in leaves exhibit a significant decrease which in turn is partially recovered by GABA application. Thus, GABA-induced increase in N content in leaves is possibly operated through increased nitrate uptake through roots and remobilization of N from other plant organs. Although persuasive at this moment, further investigations are necessary to reveal the routes of N uptake and its mobilization induced by GABA treatments. Similar to earlier reports, in the present work water-stress induced a decrease in P, K, content is likely attributed to their reduced absorption by roots and impairment of nutrient transport through xylem.84 During drought stress, P is likely to be converted from the immobile to insoluble form84 and a reduction is observed for P-uptake proteins (PHT1).81 Interesting reports by Su, et al.85 reveal GABA-induced upregulation of H+-ATPase activity, followed by improved membrane potential and decrease in stress-induced K+ leakage from salinity-stressed Arabidopsis roots. In support with these investigations, our study reveals improved K+ content in leaves of water-stressed snap bean plants subjected to GABA treatment.
Calcium is an immobile element and requires sufficient water supply for its optimum uptake.86 GABA application is known to exert positive effects in increasing Ca2+ and calmodulin content in NaCl-stressed barley plants.87 The increase in calcium content resulted due to higher calcium uptake through roots accompanied by altered distribution of calcium in tissues. The present findings reveal increased calcium content in leaves of water-stressed snap bean plants subjected to GABA (0.5 mM) application. Increased endogenous calcium levels have been reported to trigger glutamate decarboxylase (GAD) activity thus causing higher accumulation of GABA in heat-stressed Arabidopsis.88 Thus, GABA-Ca2+ crosstalk appears to be a crucial route of stress tolerance in plants. GABA signaling is essentially mediated by negative regulation of aluminum-activated malate transporter (ALMT) activity which in turn regulates anion flux in the cell.89 The authors state the role of GABA as a legitimate signaling molecule in plants. Thus, improvement in nutrient acquisition and uptake of essential elements (N, Ca, P, K, Fe and Zn) in water-stressed snap bean plants (present work) is likely to be regulated by the control of ion influx in plant organs. GABA application has been known to down-regulate the expression of 14-3-3 gene family proteins in Arabidopsis.90 While 14-3-3 proteins are considered as important players in the regulation of enzymes of carbon and nitrogen metabolism, GABA-induced regulation of these enzymes might be mediated by the changes in the expression of 14-3-3 proteins.
In the present work, GABA treatment improves the uptake of all the nutrients which also correlate with improved relative water content (RWC), increased cellular membrane stability index in leaves of snap bean plants. Fe and Zn are the major micronutrients transported through xylem stream. Thus, GABA application collectively improves nutrient acquisition in leaves of water-stressed snap beans. Improvement in membrane stability is also reflected by a lower extent of lipid peroxidation (decreased MDA content) which is accompanied by an elevation in antioxidant enzyme activity.
Water stress is known to affect crop yield in snap bean.73–77 According to Manjeru, Madonzi, Makeredza, Nciizah and Sithole, 40 water stress adversely affects flower size and grain yield. Furthermore, water stress largely affects the flowering and seed filling stage of the plant thus affecting pod size and seeds per pod. Irrigation with an 80% reduction in water availability causes 70% bud abortion and results in 53% decrease in pod number in Phaseolus vulgaris plants.91 The shallow root system of Phaseolus vulgaris has been attributed to its sensitivity to water stress.92 Seed quality and dry matter yield have been reported to be affected by water stress.73,77,93 In our present study pod yield, total protein content and total soluble solids (TSS) were observed to be decreased due to water stress. However, the reverse effect was observed for fiber content which increased significantly. GABA treatment (up to 2 mM) was effective in alleviating the effect of water stress on the yield attributes. Investigations have priorly reported the role of GABA in drought stress alleviation and improvement of various physiological and agronomic attributes.29,30,70
In the present study, snap bean plants responded to the exposure of water-deficit irrigation in two growing seasons. The three variables in the experiment, well-watered condition, water deficit and GABA treatment exhibit interactions, where GABA treatment mitigates water stress and tends to normalize its effects. Importantly, GABA exerts prospective role as a stress-priming molecule in snap bean plants exposed to semi-arid irrigation system. A total of 2 mM GABA concentration appears to be more efficient to bring about ameliorative changes.
5. Conclusions
Present findings reveal the role of GABA as an effective stress-priming neurotransmitter which exerts beneficial effects in water-stressed bean plants. Among the various neurotransmitters associated with abiotic stress tolerance in plants, fewer reports are available to substantiate the role of GABA in mitigation of drought stress in snap bean crop. GABA-supplementation is effective in bringing about improvement in yield attributes and osmotic tolerance in bean plants. GABA application during irrigation in arid zone is likely to possess agronomic importance for crop improvement. Future investigations are necessary to elucidate the mechanistic role of GABA in the regulation of various other biomolecules during water stress. Investigations on leaf area index, stomatal conductivity and root architecture regulation by GABA are likely to provide promising agronomic benefits in near future. Furthermore, GABA application to roots and foliage are expected to provide stress-priming responses during drought stress in crops raised under deficit irrigation. Reconstruction of bio-engineered plants for GABA metabolic pathway shall serve as a better approach toward crop sustainability in arid zones. Further investigations are necessary to decipher the molecular mechanisms of GABA signaling in drought-stressed snap bean plants which are likely to be associated with gene expression, regulation of transcription factors and hormonal metabolism.
Acknowledgements
We thank Taif University Researchers Supporting Project number (TURSP-2020/120), Taif University, Taif, Saudi Arabia.
Funding Statement
This research was funded by Taif University, grant number (TURSP-2020/120)
Author contributions
Conceptualization, H.E, S.M, R.F, O.A, M.H, A.Ab, S.A, N.H, S.M, N.E, E.A and M.I.; methodology, H.E, S.M, R.F, O.A, M.H, A.Ab, S.A, N.H and M.I.; software, H.E, S.M, E.A and M.I.; validation, H.E, S.M, R.F, O.A, M.H, A.Ab, S.A, N.H and M.I.; formal analysis, S.M, N.E, A.G and E.A.; investigation, H.E, S.M, A.E, S.M, N.E, A.G, E.A and M.I.; resources, H.E, A.E, H.I, N.M, E.A and M.I.; data curation, H.E, S.M and M.I.; writing—original draft preparation, H.E, S.M, A.E, E.A and M.I.; writing—review and editing, H.E, S.M, R.F, O.A, M.H, A.Ab, S.A, N.H, A.E, H.I, N.M, N.E, A.G, E.A and M.I.; visualization, H.E, S.M and M.I.; supervision, A.E, A.G, E.A and M.I.; project administration, H.E, S.M, E.A and M.I.; funding acquisition, H.E, A.E, A.G, E.A and M.I. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Gallart F, Solé A, Puigdefábregas J, Lázaro R.. Badland systems in the mediterranean. Chichester: John Wiley & Sons, Ltd; 2002. p. 1–15. [Google Scholar]
- 2.Lal R. Soil carbon sequestration impacts on global climate change and food security. science. 1623-1627;2004(304). doi: 10.1126/SCIENCE.1097396. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad P, Jamsheed S, Hameed A, Rasool S, Sharma I, Azooz M, Hasanuzzaman M. Drought stress induced oxidative damage and antioxidants in plants. Oxidative damage to plants. Academic Press: Elsevier; 2014. pp. 345–367. doi: 10.1016/B978-0-12-799963-0.00011-3. [DOI] [Google Scholar]
- 4.Alhaithloul HA, Soliman MH, Ameta KL, El-Esawi MA, Elkelish A. Changes in Ecophysiology, Osmolytes, and Secondary metabolites of the medicinal plants of mentha piperita and catharanthus roseus subjected to drought and heat stress. Biomolecules. 2020;10:43. doi: 10.3390/biom10010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habib N, Ali Q, Ali S, Javed MT, Zulqurnain Haider M, Perveen R, Shahid MR, Rizwan M, Abdel-Daim MM, Elkelish A. Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit conditions: growth, osmoregulation, and antioxidative defense mechanism. Plants. 2020;9(2):285. doi: 10.3390/plants9020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harb A, Krishnan A, Ambavaram MM, Pereira A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010:pp. 110.161752. doi: 10.1104/pp.110.161752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and stress combination. The Plant Journal. 2017;90:856–867. doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- 8.Miller G, Suzuki N, Ciftci‐Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 9.Noctor G, Mhamdi A, Foyer CH. The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol. 2014;164(4):1636–1648. doi: 10.1104/pp.113.233478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkeilsh A, Awad YM, Soliman MH, Abu-Elsaoud A, Abdelhamid MT, El-Metwally IM. Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J Plant Res. 2019;132:881–901. doi: 10.1007/s10265-019-01143-5. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim M, Ibrahim HA, Abd El-Gawad H. Folic acid as a protective agent in snap bean plants under water deficit conditions. The Journal of Horticultural Science and Biotechnology. 2020:1–16. doi: 10.1080/14620316.2020.1793691.. [DOI] [Google Scholar]
- 12.Ibrahim MF, Elbar OHA, Farag R, Hikal M, El-Kelish A, El-Yazied AA, Alkahtani J, El-Gawad HGA. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants. 2020;9(10):1276. doi: 10.3390/plants9101276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017;40(1):4–10. doi: 10.1111/pce.12800. [DOI] [PubMed] [Google Scholar]
- 14.Nawaz F, Shehzad MA, Majeed S, Ahmad KS, Aqib M, Usmani MM, Shabbir RN. Role of mineral nutrition in improving drought and salinity tolerance in field Crops. Agronomic crops. Singapore: Springer; 2020. pp. 129–147. doi: 10.1007/978-981-15-0025-1_8. [DOI] [Google Scholar]
- 15.Waraich EA, Ahmad R, Ashraf M. Role of mineral nutrition in alleviation of drought stress in plants. Australian Journal of Crop Science. 2011;5:764. [Google Scholar]
- 16.Huang G-T, Ma S-L, Bai L-P, Zhang L, Ma H, Jia P, Liu J, Zhong M, Guo Z-F. Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Rep. 2012;39(2):969–987. doi: 10.1007/s11033-011-0823-1.. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim M, Ibrahim HA. Assessment of selenium role in promoting or inhibiting potato plants under water stress. Journal of Horticultural Science & Ornamental Plants. 2016;8:125–139. [Google Scholar]
- 18.Ibrahim MFM, Abd El-Samad G, Ashour H, El-Sawy AM, Hikal M, Elkelish A, El-Gawad HA, El-Yazied AA, Hozzein WN, Farag R. Regulation of agronomic traits, nutrient uptake, osmolytes and antioxidants of maize as influenced by exogenous potassium silicate under deficit irrigation and semiarid conditions. Agronomy. 2020;10(8):1212. doi: 10.3390/agronomy10081212.. [DOI] [Google Scholar]
- 19.Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161(11):1189–1202. doi: 10.1016/J.JPLPH.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Zou -J-J, Wei F-J, Wang C, Wu -J-J, Ratnasekera D, Liu W-X, Wu W-H. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid-and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010;154:1232–1243. doi: 10.1104/pp.110.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouche N, Fromm H. GABA in plants: just a metabolite? Trends Plant Sci. 2004;9(3):110–115. doi: 10.1016/J.TPLANTS.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Dhakal R, Bajpai VK, Baek K-H. Production of GABA (γ-aminobutyric acid) by microorganisms: a review. Brazilian Journal of Microbiology. 2012;43:1230–1241. doi: 10.1590/S1517-83822012000400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gou Z, Wang X, Wang W. Evolution of neurotransmitter gamma-aminobutyric acid, glutamate and their receptors. Zoological Research. 2012;33:E76–81. doi: 10.3724/SP.J.1141.2012.E05-06E75. [DOI] [PubMed] [Google Scholar]
- 24.Steward F. γ-Aminobutyric acid: a constituent of the potato tuber? Science. 1949;110:439–440. [Google Scholar]
- 25.Barbosa JM, Singh NK, Cherry JH, Locy RD. Nitrate uptake and utilization is modulated by exogenous γ-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiology and Biochemistry. 2010;48(6):443–450. doi: 10.1016/j.plaphy.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ. Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Science. 2012;193:130–135. doi: 10.1016/j.plantsci.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Signorelli S, Dans PD, Coitiño EL, Borsani O, Monza J. Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLoS One. 2015;10:e0115349. doi: 10.1371/journal.pone.0115349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaeli S, Fromm H. Closing the loop on the GABA shunt in plants: are GABA metabolism and signaling entwined? Front Plant Sci. 2015;6:419. doi: 10.3389/fpls.2015.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan S, Laskowski K, Shukla V, Merewitz EB. Mitigation of drought stress damage by exogenous application of a non-protein amino acid γ–aminobutyric acid on perennial ryegrass. Journal of the American Society for Horticultural Science. 2013;138:358–366. doi: 10.21273/JASHS.138.5.358. [DOI] [Google Scholar]
- 30.Li Z, Peng Y, Huang B. Alteration of transcripts of stress-protective genes and transcriptional factors by γ-aminobutyric acid (GABA) associated with improved heat and drought tolerance in creeping bentgrass (Agrostis stolonifera). Int J Mol Sci. 1623;2018(19). doi: 10.3390/ijms19061623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farooq M, Nawaz A, Chaudhry M, Indrasti R, Rehman A. Improving resistance against terminal drought in bread wheat by exogenous application of proline and gamma‐aminobutyric acid. Journal of Agronomy and Crop Science. 2017;203:464–472. doi: 10.1111/JAC.12222. [DOI] [Google Scholar]
- 32.Wang Y, Luo Z, Huang X, Yang K, Gao S, Du R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci Hortic (Amsterdam). 2014;168:132–137. doi: 10.1016/J.SCIENTA.2014.01.022. [DOI] [Google Scholar]
- 33.Wu X, Jia Q, Ji S, Gong B, Li J, Lü G, Gao H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020;20(1):1–21. doi: 10.1186/s12870-020-02669-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seifikalhor M, Aliniaeifard S, Bernard F, Seif M, Latifi M, Hassani B, Didaran F, Bosacchi M, Rezadoost H, Li T. γ-Aminobutyric acid confers cadmium tolerance in maize plants by concerted regulation of polyamine metabolism and antioxidant defense systems. Sci Rep. 2020;10(1):1–18. doi: 10.1038/s41598-020-59592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Xie B, An W, Li J, Zhai Z, Duan L. Effects of exogenous GABA on yield, quality and high temperature tolerance of winter wheat at the anthesis stage. Journal of Triticeae Crops. 2009;29:623–626. [Google Scholar]
- 36.Graham PH, Vance CP. Legumes: importance and constraints to greater use. Plant Physiol. 2003;131(3):872–877. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell DC, Lawrence FR, Hartman TJ, Curran JM. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J Am Diet Assoc. 2009;109(5):909–913. doi: 10.1016/j.jada.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Darkwa K, Ambachew D, Mohammed H, Asfaw A, Blair MW. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. The Crop Journal. 2016;4(5):367–376. doi: 10.1016/J.CJ.2016.06.007. [DOI] [Google Scholar]
- 39.Hinkossa A, Gebeyehu S, Zelleke H. Differential effects of post-flowering drought stress on growth and yield of the basic generations of two common bean (phaseolus vulgaris l.) populations Science. Technology and Arts Research Journal. 2013;2(1):22–31. doi: 10.4314/STAR.V2I1.98839. [DOI] [Google Scholar]
- 40.Manjeru P, Madonzi T, Makeredza B, Nciizah A, Sithole M. Effects of water stress at different growth stages on yield and yield components of common bean (Phaseolus vulgaris). In Proceedings of African Crop Science Conference Proceedings. Egypt; pp. 299–303. [Google Scholar]
- 41.Nemeskéri E, Helyes L. Physiological responses of selected vegetable crop species to water stress. Agronomy. 2019;9(8):447. doi: 10.3390/AGRONOMY9080447. [DOI] [Google Scholar]
- 42.Doğan N, Akinci Ş. Effects of water stress on the uptake of nutrients by bean seedlings (Phaseolus vulgaris L. Fresenius Environmental Bulletin. 2011;20:2163–2170. [Google Scholar]
- 43.Allen RG, Pereira LS, Raes D, Smith M. Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. Fao, Rome. 1998;300:D05109. [Google Scholar]
- 44.Doorenbos J, Pruitt W. Crop water requirements . FAO irrigation and drainage paper 24. Land and Water Development Division, FAO, Rome 1977, 144. [Google Scholar]
- 45.Leaf Area-Leaf KH. Weight Relationships in the Soybean Canopy 1. Crop Sci. 1972;12:180–183. doi: 10.2135/CROPSCI1972.0011183X001200020007X. [DOI] [Google Scholar]
- 46.Ünyayar S, Keleþ Y, Ünal E. Proline and ABA levels in two sunflower genotypes subjected to water stress. In Proceedings of Bulg. J. Plant Physiol. Turkey. 2004;30(3-4):34–47. [Google Scholar]
- 47.Singh A, Kumar J, Kumar P. Effects of plant growth regulators and sucrose on post harvest physiology, membrane stability and vase life of cut spikes of gladiolus. Plant Growth Regul. 2008;55(3):221. doi: 10.1007/s10725-008-9278-3. [DOI] [Google Scholar]
- 48.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 49.Bates L, Waldren R, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 50.Chow PS, Landhäusser SM. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004;24(10):1129–1136. doi: 10.1093/TREEPHYS/24.10.1129. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton PB, Van Slyke DD, Lemish S. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. Journal of Biological Chemistry. 1943;150:231–250. [Google Scholar]
- 52.Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R. Chemical analysis of plants and soils. IWONL, Brussels. 1982;63. [Google Scholar]
- 53.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 54.Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161(2):559–566. doi: 10.1016/0003-2697(87)90489-1.. [DOI] [PubMed] [Google Scholar]
- 55.Cakmak I, Strbac D, Marschner H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J Exp Bot. 1993;44(1):127–132. doi: 10.1093/JXB/44.1.127. [DOI] [Google Scholar]
- 56.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- 57.Hammerschmidt R, Nuckles E, Kuć J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiological Plant Pathology. 1982;20(1):73–82. doi: 10.1016/0048-4059(82)90025-X. [DOI] [Google Scholar]
- 58.Officia AOAC. Methods of Analysis of association of official agricultural chemists. Washengton D.C, USA: Wiley;1990, 15. 1045–1106 [Google Scholar]
- 59.Rai S, Mudgal V. Synergistic effect of sodium hydroxide and steam pressure treatment on composition changes and fibre utilization of wheat straw. Biological Wastes. 1988;24(2):105–113. doi: 10.1016/0269-7483(88)90053-5. [DOI] [Google Scholar]
- 60.SAS. SAS/STAT User’s Guide: Release . 6.03 ed.Cary (NC): SAS Inst. Inc; Cary,NC. 1988. [Google Scholar]
- 61.Paul K, Pauk J, Kondic-Spika A, Grausgruber H, Allahverdiyev T, Sass L, Vass I. Co-occurrence of mild salinity and drought synergistically enhances biomass and grain retardation in wheat. Front Plant Sci. 2019;10:501. doi: 10.3389/fpls.2019.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zlatev Z, Lidon FC. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emirates Journal of Food and Agriculture. 2012:57–72. doi: 10.9755/EJFA.V24I1.10599. [DOI] [Google Scholar]
- 63.Ghanbari AA, Mousavi SH, Gorji AM, İdupulapati R. Effects of water stress on leaves and seeds of bean (Phaseolus vulgaris L.). Turkish Journal of Field Crops. 2013;18:73–77. doi: 10.17557/TJFC.54612. [DOI] [Google Scholar]
- 64.Ghanbari AA, Shakiba MR, Toorchi M, Choukan R. Nitrogen changes in the leaves and accumulation of some minerals in the seeds of red, white and chitti beans (‘Phaseolus vulgaris’) under water deficit conditions. Australian Journal of Crop Science. 2013;7:706. [Google Scholar]
- 65.Li Y, Fan Y, Ma Y, Zhang Z, Yue H, Wang L, Li J, Jiao Y. Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J Plant Growth Regul. 2017;36:436–449. doi: 10.1007/s00344-016-9652-8. [DOI] [Google Scholar]
- 66.Mahmud JA, Hasanuzzaman M, Nahar K, Rahman A, Hossain MS, Fujita M. γ-aminobutyric acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicology. 2017;26(5):675–690. doi: 10.1007/s10646-017-1800-9. [DOI] [PubMed] [Google Scholar]
- 67.Jin X, Liu T, Xu J, Gao Z, Hu X. Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol. 2019;19(1):1–15. doi: 10.1186/s12870-019-1660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J, Liu T, Yang S, Jin X, Qu F, Huang N, Hu X. Polyamines are involved in GABA-regulated salinity-alkalinity stress tolerance in muskmelon. Environ Exp Bot. 2019;164:181–189. doi: 10.1016/J.ENVEXPBOT.2019.05.011. [DOI] [Google Scholar]
- 69.Yong B, Xie H, Li Z, Li Y-P, Zhang Y, Nie G, Zhang X-Q, Ma X, Huang L-K, Yan Y-H. Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front Physiol. 2017;8:1107. doi: 10.3389/fphys.2017.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vijayakumari K, Puthur JT. γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2016;78(1):57–67. doi: 10.1007/s10725-015-0074-6. [DOI] [Google Scholar]
- 71.Araújo WL, Ishizaki K, Nunes-Nesi A, Larson TR, Tohge T, Krahnert I, Witt S, Obata T, Schauer N, Graham IA. Identification of the 2-hydroxyglutarate and isovaleryl-coa dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of arabidopsis mitochondria. Plant Cell. 2010;22(5):1549–1563. doi: 10.1105/tpc.110.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laxa M, Liebthal M, Telman W, Chibani K, Dietz K-J. The role of the plant antioxidant system in drought tolerance. Antioxidants. 2019;8(4):94. doi: 10.3390/antiox8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boutraa T, Sanders F. Effects of interactions of moisture regime and nutrient addition on nodulation and carbon partitioning in two cultivars of bean (Phaseolus vulgaris L.). Journal of Agronomy and Crop Science. 2001;186:229–237. doi: 10.1046/J.1439-037X.2001.00474.X. [DOI] [Google Scholar]
- 74.Durigon A, Evers J, Metselaar K, van Lier, Q. DJ. Water stress permanently alters shoot architecture in common bean plants. Agronomy. 2019;9:160. doi: 10.3390/AGRONOMY9030160. [DOI] [Google Scholar]
- 75.França MGC, Thi ATP, Pimentel C, Rossiello ROP, Zuily-Fodil Y, Laffray D. Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environ Exp Bot. 2000;43(3):227–237. doi: 10.1016/S0098-8472(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 76.Ramirez-Vallejo P, Kelly JD. Traits related to drought resistance in common bean. Euphytica. 1998;99(2):127–136. doi: 10.1023/A:1018353200015. [DOI] [Google Scholar]
- 77.Rosales-Serna R, Kohashi-Shibata J, Acosta-Gallegos JA, Trejo-López C, Ortiz-Cereceres JN, Kelly JD. Biomass distribution, maturity acceleration and yield in drought-stressed common bean cultivars. Field Crops Res. 2004;85(2–3):203–211. doi: 10.1016/S0378-4290(03)00161-8. [DOI] [Google Scholar]
- 78.Hu Y, Schmidhalter U. Drought and salinity: a comparison of their effects on mineral nutrition of plants. Journal of Plant Nutrition and Soil Science. 2005;168:541–549. doi: 10.1002/JPLN.200420516. [DOI] [Google Scholar]
- 79.Ding L, Lu Z, Gao L, Guo S, Shen SQ. Is Nitrogen a Key Determinant of Water Transport and Photosynthesis in Higher Plants Upon Drought Stress? Front Plant Sci. 2018;9:1143. doi: 10.3389/fpls.2018.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Plett DC, Ranathunge K, Melino VJ, Kuya N, Uga Y, Kronzucker HJ. The intersection of nitrogen nutrition and water use in plants: new paths toward improved crop productivity. J Exp Bot. 2020. doi: 10.1093/jxb/eraa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bista DR, Heckathorn SA, Jayawardena DM, Mishra S, Boldt JK. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and-tolerant grasses. Plants. 2018;7:28. doi: 10.3390/plants7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Does SS. GABA increase the efficiency of symbiotic N2 fixation in legumes? Plant Signal Behav. 2011;6:32–36. doi: 10.4161/psb.6.1.14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li W, Liu J, Ashraf U, Li G, Li Y, Lu W, Gao L, Han F, Exogenous HJ. γ-aminobutyric acid (GABA) application improved early growth, net photosynthesis, and associated physio-biochemical events in maize. Front Plant Sci. 2016;7:919. doi: 10.3389/fpls.2016.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ge T-D, Sun N-B, Bai L-P, Tong C-L, Sui F-G. Effects of drought stress on phosphorus and potassium uptake dynamics in summer maize (Zea mays) throughout the growth cycle. Acta Physiologiae Plantarum. 2012;34(6):2179–2186. doi: 10.1007/s11738-012-1018-7.. [DOI] [Google Scholar]
- 85.Su N, Wu Q, Chen J, Shabala L, Mithöfer A, Wang H, Qu M, Yu M, Cui J, Shabala S. GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J Exp Bot. 2019;70(21):6349–6361. doi: 10.1093/jxb/erz367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naeem M, Naeem MS, Ahmad R, Ihsan MZ, Ashraf MY, Hussain Y, Fahad S. Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Archives of Agronomy and Soil Science. 2018;64(1):116–131. doi: 10.1080/03650340.2017.1327713.. [DOI] [Google Scholar]
- 87.Ma Y, Wang P, Gu Z, Tao Y, Shen C, Zhou Y, Han Y, Yang R. Ca2+ involved in GABA signal transduction for phenolics accumulation in germinated hulless barley under NaCl stress. Food Chemistry: X. 2019;2:100023. doi: 10.1016/j.fochx.2019.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Locy R, Wu S, Bisnette J, Barger T, McNabb D, Zik M, Fromm H, Singh N, Cherry J. The regulation of GABA accumulation by heat stress in Arabidopsis. Plant Tolerance to Abiotic Stresses in Agriculture: role of Genetic Engineering. Springer, Dordrecht, 2000. pp. 39–52. doi: 10.1007/978-94-011-4323-3_3. [DOI] [Google Scholar]
- 89.Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun. 2015;6(1):7879. doi: 10.1038/ncomms8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lancien M, Roberts MR. Regulation of Arabidopsis thaliana 14‐3‐3 gene expression by γ‐aminobutyric acid. Plant Cell Environ. 2006;29:1430–1436. doi: 10.1111/J.1365-3040.2006.01526.X. [DOI] [PubMed] [Google Scholar]
- 91.Mouhouche B, Ruget F, Delécolle R. Effects of Water Stress Applied at Different Phenological Phases on Yield Components of Dwarf Bean (Phaseolus Vulgaris L). 1998;18:197–205. doi: 10.1051/AGRO:19980303. [DOI] [Google Scholar]
- 92.Strock CF, Burridge J, Massas AS, Beaver J, Beebe S, Camilo SA, Fourie D, Jochua C, Miguel M, Miklas PN. Seedling root architecture and its relationship with seed yield across diverse environments in Phaseolus vulgaris. Field Crops Res. 2019;237:53–64. doi: 10.1016/j.fcr.2019.04.012. [DOI] [Google Scholar]
- 93.Wallace D. Adaptation of Phaseolus to different environments. In: R.J. Summerfield & A.H. Bunting (Eds.), Advances in Legume Sciencepp. 349–357. Royal Botanic Garden, Kew, England. [Google Scholar]


