ABSTRACT
Small GTPases, together with their regulatory and effector molecules, are key intermediaries in the complex signalling pathways that control almost all cellular processes, working as molecular switches to transduce extracellular cues into cellular responses that drive vital functions, such as intracellular transport, biomolecule synthesis, gene activation and cell survival. How all of these networks are linked to metabolic pathways is a subject of intensive study. Because any response to cellular action requires some form of energy input, elucidating how cells coordinate the signals that lead to a tangible response involving metabolism is central to understand cellular activities. In this review, we summarize recent advances in our understanding of the molecular basis of the crosstalk between small GTPases of the Ras superfamily, specifically Rac1 and Ras/Rap1, and glycogen phosphorylase in T lymphocytes.
Abbreviations: ADCY: adenylyl cyclase; ADCY6: adenylyl cyclase 6; BCR: B cell receptor; cAMP: 3ʹ,5ʹ-cyclic adenosine monophosphate; CRIB: Cdc42/Rac binding domain; DLPFC: dysfunction of the dorsolateral prefrontal cortex; EGFR: epidermal growth factor receptor; Epac2: exchange protein directly activated by cAMP; GDP: guanodine-5ʹ-diphosphate; GPCRs: G protein-coupled receptors; GTP: guanodin-5ʹ-triphosphate; IL2: interleukin 2; IL2-R: interleukin 2 receptor; JAK: janus kinases; MAPK: mitogen-activated protein kinase; O-GlcNAc: O-glycosylation; PAK1: p21 activated kinase 1; PI3K: phosphatidylinositol 3-kinase; PK: phosphorylase kinase; PKA: cAMP-dependent protein kinase A; PKCθ: protein kinase Cθ; PLCγ: phospholipase Cγ; Src: proto-oncogene tyrosine-protein kinase c; STAT: signal transducer and activator of transcription proteins.
KEYWORDS: IL-2R, EGFR, small GTPases, glycogen phosphorylase, T lymphocytes
Introduction
Small GTPases of the Ras superfamily are key mediators in the complex signalling networks that shape cellular activity [1]. These proteins are classified into five large families, with each specialized in the regulation of one or more functions: (i) the Ras family is involved in cellular growth control and metabolism; (ii) the Rho family is involved in actin cytoskeleton reorganization, and cooperates with Ras family in cell cycle regulation, gene expression and cellular transformation; (iii) the Rab family is specialized in vesicular and membrane trafficking; (iv) the Arf family participates in vesicular biogenesis, intracellular transport and actin remodelling; and lastly (v) the Ran family regulates nucleocytoplasmic transport and microtubule organization during the cell cycle [1]. Small GTPases of the Ras superfamily function as molecular switches that cycle between an inactive guanosine-5ʹ-diphosphate (GDP)-bound and an active guanosine-5ʹ-triphosphate (GTP)-bound state. The transition from inactive to active states is regulated by guanine nucleotide exchange factors (GEFs), whereas the reverse process is performed by GTPase-activating proteins (GAPs), which stimulate the intrinsic GTPase activity to generate GDP and inorganic phosphate (Pi) [2]. Finally, the GDP-dissociation inhibitors (GDIs) act to prevent GDP dissociation from GTPases, and to keep the latter sequestered and inactive in the cytosol [3].
In their active configuration, small GTPases of the Ras superfamily interact with downstream effector molecules to elicit a variety of biological responses [2]. Functional specificity of these proteins depends mainly on the cellular system, the type of stimulus, and their intracellular localization. Signals emanating from membrane receptors, such as G protein-coupled receptors [4,5], growth factor receptors [6–9], or tyrosine kinase-linked receptors [10,11] frequently lead to the activation of one or more GTPase, ensuring a rapid and efficient pleiotropic response [12]. However, the kinetics of activation as well as the effector molecules engaged often differ strongly between the different GTPase family members.1
Small GTPases in the immune system
Small GTPases participate in essential biological functions in the immune system. The best characterized GTPases – both structurally and functionally – are Ras proteins, which play critical roles in numerous cellular processes, including lymphocyte proliferation [3,13], maturation and positive selection of T lymphocytes, and programmed cell death during the immune response [14]. The three classical Ras genes (H-Ras, N-Ras and K-Ras (which is expressed as the splice variants K-Ras4A and K-Ras4B)) are differently expressed in T lymphocytes, being N-Ras protein the most important isoform expressed in this cell lineage. N-Ras protein is crucial to reach T-cell activation and a proper downstream Ras signalling [15]. However, both H-Ras and K-Ras 4B variant are also involved in important functions, such as T-cell differentiation in the periphery and thymocyte development respectively [16].
These Ras isoforms are identical in aminoacid sequence at the N-terminal region, which mediate interactions with common downstream targets [17]. Yet, they differ in the amino acid sequence of the C-terminal region, which results in a distinct spatial localization of each isoform within the plasma membrane and might thus explain their non-redundant functionality of each isoform [17,18].
In addition to Ras proteins, small GTPases of the Rho family also play important roles in immune system functions, including the development and activation of lymphocytes, the formation of immunological synapses, dendritic cell function [19,20], phagocytosis processes, and in polarized exocytosis in cytotoxic lymphocytes [21].
The motility of lymphocytes and dendritic cells in response to chemokines is a process inherent to small GTPases of the Rho family, including RhoA, Rac1 and Cdc42 [19,20]. Indeed, the deregulation of the control of the active/inactive states of RhoA, Rac1 or Cdc42, is responsible for several immune pathologies and cancer development. For instance, Wiskott-Aldrich syndrome is an immunodeficiency disease caused by mutations in the gene encoding WASP, an effector molecule for Cdc42 [22]. Also, Rac1 has been implicated in p210-BCR-ABL-mediated transformation in acute myeloid leukaemia [23,24].
The GTPases of the Rac subfamily, Rac1, Rac2, Rac3 and RhoG [1,25] are gaining increasing relevance in T cell biology [6,26–29]. These Rac subfamily members have different expression patterns: Rac1 and RhoG are ubiquitously expressed whereas Rac2 expression is circumscribed to cells of haematopoietic origin and that of Rac3 predominantly occurs in the brain [25]. From a functional point of view, the importance of Rac1 expression is reflected by the fact that Rac1-knockout mice are embryonic lethal [30]. By contrast, Rac2−/–, Rac3−/–, or RhoG−/ – mice show no apparent altered phenotype [31–33] even when they do have cell-type specific functional deficiencies–notably, the macrophages of Rac2−/-mice show a reduced M1 to M2 differentiation potential [32] and those of RhoG−/- animals have a slightly increased antigen receptor cross-linking ability [31].
Rac1 and Rac2 proteins are involved in T cell activation, division, and migration, as well as in B cell development and receptor (BCR) signalling [34,35]. On the other hand, Rac1 and Rac2 have redundant functions, with Rac2 usually playing a more prominent role as reflected by the fact that T cells express more Rac2 than Rac1 [36]. Nevertheless, both Rac1 and Rac2 show similar affinity in their interaction with the Rac binding domains of several effector molecules, including p67phox. However, the involvement of one GTPase or another in a given cellular function depends on the cell lineage [37].
T cell receptor (TCR) engagement by antigens promotes the rapid activation of GTPases and other signalling molecules [38]. Activated Rac transduce signals through several intermediate molecules, including PI3K [39,40], phospholipase C (PLC)γ1 and protein kinase C (PKC)ϴ [28,29]. Importantly, Rac1 controls the translocation of RAS guanyl-releasing protein 1 (RasGRP1, a GEF for Ras) to the actin juxtamembrane structure to facilitate Ras/ERK pathway activation [41]. The integration of these signalling events culminates in the nucleus via the transcription factors AP-1, NFAT and NF-κB, which actively participate in the transcription of the genes coding for IL-2 and IL-2 receptor (IL-2R) α chain, thereby contributing to the clonal expansion of T cells [21,42–45].
In contrast to its well established role in the TCR-mediated activation programme, how Rac1 functions in IL-2 signalling is not entirely clear. IL-2 is a cytokine that is key to stimulate T lymphocyte clonal expansion [46]. The binding of IL-2 to its high affinity receptor (IL-2R) triggers multiple signalling pathways, of which the three most important for cell cycle progression and inhibition of apoptosis are the janus kinases (JAK)/signal transducer and activator of transcription proteins (STAT), and the PI3K and Ras/Raf/MAPK pathways, respectively [47–49]. JAKs initiate signalling from activated IL-2R. According to the current IL-2R signalling model, IL-2-activated JAKs recruit critical Src homology 2 (SH2)-containing signalling mediators, leading to signal propagation in the cytoplasm. Tyrosine phosphorylation of STAT3 and STAT5 is mediated by JAK1 and JAK3, causing STAT dimerization and subsequent nuclear translocation and DNA binding [50,51]. Additionally, IL-2 mediates activation of the PI3K/AKT pathway, which regulates downstream signalling molecules such as p70S6K and mTOR, both of which are required for activation of the cell cycle regulator E2F and cell cycle progression [52–56].
The role of Ras in the signalling cascades initiated by IL-2 is well established. Upon IL-2/IL-2R engagement, the adapter protein SHC is anchored to the phosphorylated IL-2Rβ chain [57]. Subsequently, SHC is tyrosine phosphorylated, allowing the recruitment of the Grb2/SOS complex, which controls the activation of the Ras/Raf/MAPKinase pathway. Activation of this cascade results in phosphorylation and activation of transcription factors such as AP-1, ELK1 and Myc, which regulate the expression of genes involved in cell proliferation [58–60]. To accomplish this transcription factor regulation, T cells possibly require not only the Ras/MAPK pathway, but also a complex cooperation with other signalling networks, including some GTPases of the Rho family. Indeed, RhoA cooperates with ERK-dependent signalling pathways to transcribe c-fos in response to IL-2 [61]. Moreover, Rac1 participates in IL-2-induced actin cytoskeleton-rearrangement in a murine T cell line [62]. However, the relevance of the latter Rac1-mediated response in T cell proliferation is still unclear, as there are many signalling pathways still to be discovered that might depend on this GTPase. In this regard, we previously reported that active Rac1 binds to and activates glycogen phosphorylase, which seems to be key to control IL-2-mediated T lymphocyte proliferation [63].
Relationship between glycogen phosphorylase and small GTPases in the immune system
The functional specificity of GTPases is believed to be determined by the interactions that they establish with specific GEFs and other effector molecules [64]. Because of their pleiotropic functions, there has been a long-standing interest in identifying the protein partners that GTPases associate with, and some have been identified by non-hypothesis-driven proteomic approaches [65]. Indeed, proteomics has emerged as a powerful platform to screen putative protein-protein interactions owing to its sensitivity [66]. Using this technology, we identified the muscle isoform of glycogen phosphorylase (GPM) as a protein that binds to the active form of the GTPase Rac1, revealing that GPM is in fact a Rac1 specific effector molecule and linking Rac1 activity to glycogen metabolism [63].
Glycogen phosphorylase uses Pi to release glucose as glucose 1-phosphate from intracellular glycogen stores. Glycogen is a branched polysaccharide of D-glucose (Glc; 1–4) and is the main form of carbohydrate storage in liver, skeletal muscle and brain [67–70]. Glycogen phosphorylase is biologically active as a homodimer associated with its cofactor pyridoxal phosphate (vitamin B6). Three different glycogen phosphorylase isoenzymes are known: brain (GPB), liver (GPL) and muscle (GPM) [67,68]. Although these isoforms share 70% of sequence homology, with the muscle and brain isoforms being evolutionarily closer [70], there are substantial functional differences between them. In combination with glucose-6-phosphatase, GPL releases glucose from liver glycogen stores into the bloodstream, thereby making this sugar available to all tissues. By contrast, GPB and GPM release glucose into the cytoplasm, to provide intracellular energy and to regulate cellular responses [71].
Activation mechanisms also differ among glycogen phosphorylase isoforms. GPL is solely activated by reversible phosphorylation on serine at position 14. In the 1950s Sutherland and Cori established that both adrenaline and glucagon mediate glycogenolysis in the liver [72]. Both hormones bind to their cognate receptors, which are members of the G protein-coupled receptor (GPCR) superfamily. The engagement of these receptors triggers a signalling cascade mediated by the Gαs protein subunit in a GTP-dependent manner. Specifically, GTP-bound Gαs activates adenylyl cyclase (ADCY) [73] to hydrolyse ATP, generating 3ʹ,5ʹ-cyclic adenosine monophosphate (cAMP), which associates and activates cAMP-dependent protein kinase A (PKA), which in turn phosphorylates and activates phosphorylase kinase (PK) that ultimately activates glycogen phosphorylase through phosphorylation at Ser-14 (Figure 1) [74]. Additionally, GPB and GPM can also be regulated by allosteric modification through the binding of energy availability sensors, such as AMP or glucose [68].
Figure 1.
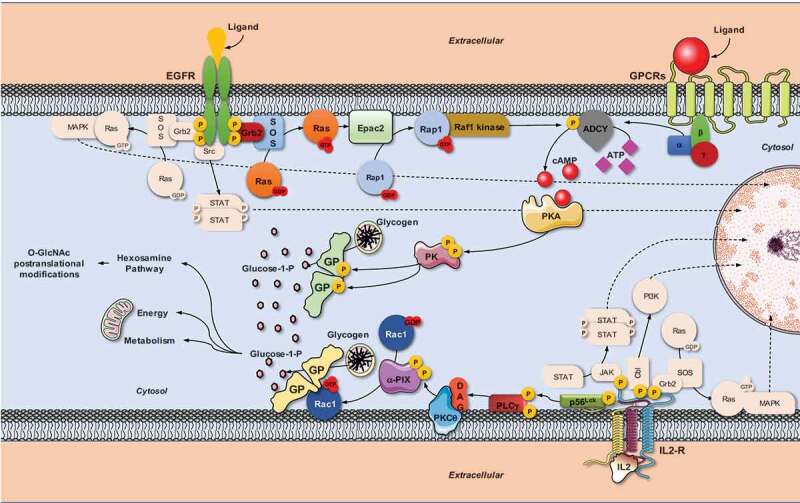
Scheme summarizing the involvement of the small GTPases of the Ras superfamily in glycogen phosphorylase regulation. The intracellular signalling networks that trigger the activation of glycogen phosphorylase (GP) and glycogen breakdown varies according to the receptors involved. G protein-coupled receptors (GPCRs) transduce signal through the canonical pathway in which the αs subunit of the heterotrimeric G protein activates adenylyl cyclase (ADCY) by allosteric mechanisms generating 3ʹ,5ʹ-cyclic adenosine monophosphate (cAMP) to activate GP by reversible phosphorylation via the PKA/PK pathway. EGFR transduces signals using part of the canonical pathway; in this case, ADCY is activated by serine phosphorylation and the communication between EGFR to ADCY is established by the participation of the Ras/Epac2/Rap1/Raf1 pathway. On the other hand, IL-2 receptor does not require the involvement of ADCY to activate GP. The IL-2 receptor controls the Lck/PLCγ/PKCθ/αPix pathway, which turns on Rac1 GTPase, which then binds and directly activates GP by an allosteric mechanism
Rac1 does not appear to control GPM activity through phosphorylation, as IL-2 stimulation does not seem to modify the GPM phosphorylation state at Ser-14 [63]. Rather, the interaction between the Rac1 and GPM occurs through a GPM region that is homologous to the Cdc42/Rac binding domain (CRIB) of p21 activated kinase 1 (PAK1), suggesting that Rac1 regulates GPM activity by allosteric modification [63].
Since the discovery of the association between Rac1 and GPM [63], it has been identified the mechanism through which the IL-2/IL-2R complex stimulates Rac1 in T lymphocytes. IL-2R, a tyrosine kinase-linked receptor, recruits and activates lymphocyte-specific protein tyrosine kinase (Lck), a member of the src tyrosine kinase family, to phosphorylate and activate PLC1γ and consequently generate diacylglycerol, which activates PKCϴ. The latter phosphorylates the Rac1 GEFαPix, to activate Rac1, which binds and activates glycogen phosphorylase [28,29]. The overall evidence indicates that this pathway participates in the control of T cell migration and proliferation (Figure 1). The signalling pathway that links IL2-R to GPM via Rac1 may be as functionally important as the three canonical pathways that were previously described for IL-2-stimulated T lymphocytes: JAK/STAT, Ras/MAPK and PI3K. Thus, GPM can regulate its activity independently of both ADCY and AMP generated by metabolic sensors. Accordingly, we established a new function of small GTPases of the Rho family in glycogen regulation in T lymphocytes [28,29,63].
The relationship between Rac1 GTPase protein and GPM does not seem to be exclusive for lymphoid cells: at the brain tissue level, GPM is mainly expressed in astrocytes whereas GPB is also expressed in neurons [75]. Astrocytes are key players in numerous brain functions, including energy supply to neurons [75,76]. In this regard, Pinacho et al. have suggested that the alteration of glycogen degradation in the dysfunction of the dorsolateral prefrontal cortex (DLPFC) associated with schizophrenia could be related to a deficient GPM and Rac1 expression in astrocytes [75]. Additionally, astrocytes respond to numerous types of central nervous system injuries that cause severe and persistent locomotor and sensory dysfunction [77] through a process known as astrogliosis. Thus, during the acute phase of the injury, astrocytes increase in number and migrate to the site of the injury to isolate the inflammatory region from neighbouring tissue. Besides its function in the haematopoietic system, the Rac GTPase/GPM axis could therefore be involved in the physiology of astrocytes by controlling migration as well as by participating in other brain functions such as control of the blood-brain barrier, regulation of blood flow or synaptic functions [76].
That IL-2R mediates glycogen phosphorylase activation via Rac 1 in an ADCY-independent manner does not preclude the possibility that other GTPases of the Ras superfamily might also activate glycogen phosphorylase through the canonical, ADCY-dependent pathway [28,29]. In fact, it has recently reported that epidermal growth factor receptor (EGFR) activation in T lymphocytes allows Ras to cooperate with Rap1 to control glycogen phosphorylase activation [6]. This signalling pathway requires, firstly that exchange protein directly activated by cAMP (EPAC2) acts as the bridge between Ras and Rap1 and, secondly Rap1 in its active configuration associates with and activates Raf1, allowing it to phosphorylate ADCY type 6, in order to modulate glycogen phosphorylase activation through the canonical pathway (Figure 1) [6].
Regarding the exchange proteins activated by cAMP, Epac2 – but not Epac1 – binds N-Ras and K-Ras with more affinity than H-Ras [78]. Llavero et al have recently reported that the overexpression of the constitutively active form of H-Ras leads to the activation of Rap1 and GP through Epac2 in T-cells [6]. In this respect, although it might appear counterintuitive that H-Ras isoform with low affinity for Epac2 activates Rap1 in T-cells, the massive expression of the H-Ras constitutively active form could facilitate Epac2 translocation from the cytosol to the plasma membrane to activate Rap1 at the plasma membrane as suggested by Li et al. [78]. Given this scenario and considering that N-Ras protein is the most abundant isoform and has important functions in the immune system [15], it could be postulated that N-Ras protein would be controlling the Rap1/GP pathway in T-cells.
In this review, we present two new regulatory mechanisms of glycogen phosphorylase activation, both controlled by GTPases of the Ras superfamily, but that can be both ADCY dependent and independent. The use of one mechanism over the other is determined by the stimulus, with IL-2 requiring Rac1 and modulating glycogen phosphorylase via allosteric activation, whereas EGFR used Ras and Rap to activate glycogen phosphorylase through phosphorylation via Raf 1 (Figure 1). From a functional perspective, whereas IL-2R controls T lymphocyte migration and proliferation through the Rac1/glycogen phosphorylase pathway, EGFR signalling through the ADCY6/glycogen phosphorylase pathway could be a mechanism to deactivate IL-2R and return T lymphocytes to their inactive state.
Future perspectives
One of the enigmas of T lymphocyte clonal expansion concerns how these cells coordinate all the signals they are exposed to in order to gain energy and proliferate, and ultimately produce an efficient immune response [6]. In resting conditions, T lymphocytes consume glucose at a low rate to maintain basal cellular processes, whereas their activation produces metabolic changes to deal with the increasing demands for energy. Glucose metabolism in activated lymphocytes is primarily dependent on glycolysis [79], with glycolysis-derived ATP ensuring energy supply as well as basic molecules in excess, thereby fuelling the cellular growth necessary for a prolonged immune response [80–82]. Mitogenic stimulation of T lymphocytes increases the rate not only of glucose uptake, but also of glutamine consumption, with the rate of glutamine utilization by lymphocytes comparable to if not greater than glucose consumption [83]. T lymphocytes also have the possibility to use glycogen, fatty acids and ketone bodies as energy substrates, and accordingly also possess the necessary enzymes of gluconeogenesis, pentose phosphate pathway, and ketone body synthesis [84]. The activity of hexokinase in T lymphocytes is much more important than glycogen phosphorylase, suggesting that endogenous glycogen is a less essential energy substrate than exogenous glucose [85,86].
Our working hypothesis is that the biological significance of the Rac1/glycogen phosphorylase and Ras/Epac2/Rap1/Raf1/ADCY6/glycogen phosphorylase pathways could be attributed to the functioning of early intracellular signalling pathways that regulate reversible O-glycosylation by serine and threonine residues in proteins, rather than energy. The equilibrium between O-glycosylation/phosphorylation of proteins constitutes a nutrient sensor that modulates intracellular signalling transcription and actin cytoskeleton modifications [87]. The imbalance between the levels of phosphorylation and glycosylation underlies pathologies such as human neurodegenerative diseases, type 2 diabetes, cancer, infectious diseases [88,89] and perhaps even some rare diseases such as McArdle’s disease, a condition caused by inherited deficiency of GPM. Future studies should address the functional importance of these signalling cascades to ensure an efficient immune response.
Acknowledgments
We thank Dr. Kenneth McCreath for editorial assistance during manuscript preparation.
Funding Statement
This work was supported by the Instituto de Salud Carlos III [PI15/00431];Instituto de Salud Carlos III [PI18/00207];Instituto de Salud Carlos III [PI15/00558 and PI18/00139];Instituto de Salud Carlos III [PI17/02052].
Note
MLM is recipient of a fellowship from Foundation ‘Jesús de Gangoiti y Barrera.’ AAS is recipient of Basque Government Predoctoral Fellowship PRE-2017-0016. MAM is supported by Spanish Ministry of Economy and Competitiveness (Fondo de Investigaciones Sanitarias (FIS)) Grants PI15/00431, JA is supported by Spanish Ministry of Economy and Competitiveness (Fondo de Investigaciones Sanitarias (FIS)) Grants PI17/02052, ALM is supported by Spanish Ministry of Economy and Competitiveness (Fondo de Investigaciones Sanitarias (FIS)) Grants PI15/00558 and PI18/00139 and JLZ is supported by Spanish Ministry of Economy and Competitiveness (Fondo de Investigaciones Sanitarias (FIS)) Grant PI18/00207.
Author contributions
All authors wrote and revised manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Wennerberg K, Rossman KL, Der CJ.. The Ras superfamily at a glance. J Cell Sci. 2005;118(Pt 5):843–846. [DOI] [PubMed] [Google Scholar]
- [2].Johnson DS, Chen YH. Ras family of small GTPases in immunity and inflammation. Curr Opin Pharmacol. 2012;12(4):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Simanshu DK, Nissley DV, McCormick F, et al. Their regulators in human disease. Cell. 2017;170(1):17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lambert NA, Johnston CA, Cappell SD, et al. Regulators of G-protein signaling accelerate GPCR signaling kinetics and govern sensitivity solely by accelerating GTPase activity. Proc Natl Acad Sci USA. 2010;107(15):7066–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pavlos NJ, Friedman PA. GPCR signaling and trafficking: the long and short of it. Trends Endocrinol Metab. 2017;28(3):213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Llavero F, Luque Montoro M, Arrazola Sastre A, et al. Epidermal growth factor receptor controls glycogen phosphorylase in T cells through small GTPases of the RAS family. J Biol Chem. 2019;294(12):4345–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Di Domenico M, Giordano A. Signal transduction growth factors: the effective governance of transcription and cellular adhesion in cancer invasion. Oncotarget. 2017;8(22):36869–36884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hanna S, El-Sibai M. Signaling networks of Rho GTPases in cell motility. Cell Signal. 2013;25(10):1955–1961. [DOI] [PubMed] [Google Scholar]
- [9].Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bresnick AR, Backer JM. PI3Kbeta-A versatile transducer for GPCR, RTK, and small GTPase signaling. Endocrinology. 2019;160(3):536–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schlessinger J. Receptor tyrosine kinases: legacy of the first two decades. Cold Spring Harb Perspect Biol. 2014;6(3):a008912-a008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cai A, Zhou Y, Li L. Rho-GTPase and atherosclerosis: pleiotropic effects of statins. J Am Heart Assoc. 2015;4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fischer AM, Katayama CD, Pages G, et al. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23(4):431–443. [DOI] [PubMed] [Google Scholar]
- [15].Perez de Castro I, Bivona TG, Philips MR, et al. Ras activation in Jurkat T cells following low-grade stimulation of the T-cell receptor is specific to N-Ras and occurs only on the Golgi apparatus. Mol Cell Biol. 2004. April;24(8):3485–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Iborra S, Soto M, Stark-Aroeira L, et al. H-ras and N-ras are dispensable for T-cell development and activation but critical for protective Th1 immunity. Blood. 2011. May 12;117(19):5102–5111. [DOI] [PubMed] [Google Scholar]
- [17].Lapinski PE, King PD. Regulation of Ras signal transduction during T cell development and activation. Am J Clin Exp Immunol. 2012;1(2):147–153. [PMC free article] [PubMed] [Google Scholar]
- [18].Olson MF, Marais R. Ras protein signalling. Semin Immunol. 2000;12(1):63–73. [DOI] [PubMed] [Google Scholar]
- [19].Benvenuti F, Hugues S, Walmsley M, et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305(5687):1150–1153. [DOI] [PubMed] [Google Scholar]
- [20].Miletic AV, Swat M, Fujikawa K, et al. Cytoskeletal remodeling in lymphocyte activation. Curr Opin Immunol. 2003;15(3):261–268. [DOI] [PubMed] [Google Scholar]
- [21].Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. 2007;7(2):131–143. [DOI] [PubMed] [Google Scholar]
- [22].Haddad E, Zugaza JL, Louache F, et al. The interaction between Cdc42 and WASP is required for SDF-1-induced T-lymphocyte chemotaxis. Blood. 2001;97(1):33–38. [DOI] [PubMed] [Google Scholar]
- [23].Kharas MG, Fruman DA. ABL oncogenes and phosphoinositide 3-kinase: mechanism of activation and downstream effectors. Cancer Res. 2005;65(6):2047–2053. [DOI] [PubMed] [Google Scholar]
- [24].Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6(2):167–180. [DOI] [PubMed] [Google Scholar]
- [25].Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9(9):690–701. [DOI] [PubMed] [Google Scholar]
- [26].Durand-Onayli V, Haslauer T, Harzschel A, et al. Rac GTPases in hematological malignancies. Int J Mol Sci. 2018;19(12):4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kumari S, Depoil D, Martinelli R, et al. Actin foci facilitate activation of the phospholipase C-gamma in primary T lymphocytes via the WASP pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Llavero F, Artaso A, Lacerda HM, et al. Lck/PLCgamma control migration and proliferation of interleukin (IL)-2-stimulated T cells via the Rac1 GTPase/glycogen phosphorylase pathway. Cell Signal. 2016;28(11):1713–1724. [DOI] [PubMed] [Google Scholar]
- [29].Llavero F, Urzelai B, Osinalde N, et al. Guanine nucleotide exchange factor alphaPIX leads to activation of the Rac 1 GTPase/glycogen phosphorylase pathway in interleukin (IL)-2-stimulated T cells. J Biol Chem. 2015;290(14):9171–9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sugihara K, Nakatsuji N, Nakamura K, et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17(26):3427–3433. [DOI] [PubMed] [Google Scholar]
- [31].Vigorito E, Bell S, Hebeis BJ, et al. Immunological function in mice lacking the Rac-related GTPase RhoG. Mol Cell Biol. 2004;24(2):719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Joshi S, Singh AR, Zulcic M, et al. Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. PLoS One. 2014;9(4):e95893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Corbetta S, Gualdoni S, Albertinazzi C, et al. Generation and characterization of Rac3 knockout mice. Mol Cell Biol. 2005;25(13):5763–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Walmsley MJ, Ooi SK, Reynolds LF, et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302(5644):459–462. [DOI] [PubMed] [Google Scholar]
- [35].Saoudi A, Kassem S, Dejean A, et al. Rho-GTPases as key regulators of T lymphocyte biology. Small GTPases. 2014;5:e983862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Faroudi M, Hons M, Zachacz A, et al. Critical roles for Rac GTPases in T-cell migration to and within lymph nodes. Blood. 2010;116(25):5536–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ming W, Li S, Billadeau DD, et al. The Rac effector p67phox regulates phagocyte NADPH oxidase by stimulating Vav1 guanine nucleotide exchange activity. Mol Cell Biol. 2007;27(1):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Morillon YM 2nd, Lessey-Morillon EC, Clark M, et al. Antibody binding to CD4 induces Rac GTPase activation and alters T cell migration. J Immunol. 2016;197(9):3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Campa CC, Ciraolo E, Ghigo A, et al. Crossroads of PI3K and Rac pathways. Small GTPases. 2015;6(2):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ebi H, Costa C, Faber AC, et al. PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P-Rex1. Proc Natl Acad Sci USA. 2013;110(52):21124–21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Caloca MJ, Zugaza JL, Matallanas D, et al. Vav mediates Ras stimulation by direct activation of the GDP/GTP exchange factor Ras GRP1. Embo J. 2003;22(13):3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Behrens A, Sabapathy K, Graef I, et al. Jun N-terminal kinase 2 modulates thymocyte apoptosis and T cell activation through c-Jun and nuclear factor of activated T cell (NF-AT). Proc Natl Acad Sci USA. 2001;98(4):1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Allison KA, Sajti E, Collier JG, et al. Affinity and dose of TCR engagement yield proportional enhancer and gene activity in CD4+ T cells. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Paul S, Schaefer BC. A new look at T cell receptor signaling to nuclear factor-kappaB. Trends Immunol. 2013;34(6):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yan Y, Zhang GX, Williams MS, et al. TCR stimulation upregulates MS4a4B expression through induction of AP-1 transcription factor during T cell activation. Mol Immunol. 2012;52(2):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gordon J, MacLean LD. A lymphocyte-stimulating factor produced in vitro. Nature. 1965;208(5012):795–796. [DOI] [PubMed] [Google Scholar]
- [47].Crawley JB, Rawlinson L, Lali FV, et al. T cell proliferation in response to interleukins 2 and 7 requires p38MAP kinase activation. J Biol Chem. 1997;272(23):15023–15027. [DOI] [PubMed] [Google Scholar]
- [48].Damjanovich S, Bene L, Matko J, et al. Preassembly of interleukin 2 (IL-2) receptor subunits on resting Kit 225 K6 T cells and their modulation by IL-2, IL-7, and IL-15: a fluorescence resonance energy transfer study. Proc Natl Acad Sci USA. 1997;94(24):13134–13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Graves JD, Krebs EG. Protein phosphorylation and signal transduction. Pharmacol Ther. 1999;82(2–3):111–121. [DOI] [PubMed] [Google Scholar]
- [50].Gaffen SL. Signaling domains of the interleukin 2 receptor. Cytokine. 2001;14(2):63–77. [DOI] [PubMed] [Google Scholar]
- [51].Lockyer HM, Tran E, Nelson BH. STAT5 is essential for Akt/p70S6 kinase activity during IL-2-induced lymphocyte proliferation. J Immunol. 2007;179(8):5301–5308. [DOI] [PubMed] [Google Scholar]
- [52].Brennan P, Babbage JW, Burgering BM, et al. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7(5):679–689. [DOI] [PubMed] [Google Scholar]
- [53].Brennan P, Babbage JW, Thomas G, et al. p70(s6k) integrates phosphatidylinositol 3-kinase and rapamycin-regulated signals for E2F regulation in T lymphocytes. Mol Cell Biol. 1999;19(7):4729–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gala S, Marreiros A, Stewart GJ, et al. Overexpression of E2F-1 leads to cytokine-independent proliferation and survival in the hematopoietic cell line BaF-B03. Blood. 2001;97(1):227–234. [DOI] [PubMed] [Google Scholar]
- [55].Gao N, Flynn DC, Zhang Z, et al. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004;287(2):C281–91. [DOI] [PubMed] [Google Scholar]
- [56].Kirkham PA, Lam EW, Takamatsu HH, et al. Transcription factor E2F controls the reversible gamma delta T cell growth arrest mediated through WC1. J Immunol. 1998;161(4):1630–1636. [PubMed] [Google Scholar]
- [57].Yu A, Zhu L, Altman NH, et al. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30(2):204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cianferoni A, Massaad M, Feske S, et al. Defective nuclear translocation of nuclear factor of activated T cells and extracellular signal-regulated kinase underlies deficient IL-2 gene expression in Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2005;116(6):1364–1371. [DOI] [PubMed] [Google Scholar]
- [59].Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. [DOI] [PubMed] [Google Scholar]
- [60].Mortellaro A, Songia S, Gnocchi P, et al. New immunosuppressive drug PNU156804 blocks IL-2-dependent proliferation and NF-kappa B and AP-1 activation. J Immunol. 1999;162(12):7102–7109. [PubMed] [Google Scholar]
- [61].Arnaud M, Mzali R, Gesbert F, et al. Interaction of the tyrosine phosphatase SHP-2 with Gab2 regulates Rho-dependent activation of the c-fos serum response element by interleukin-2. Biochem J. 2004;382(Pt 2):545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Arrieumerlou C, Donnadieu E, Brennan P, et al. Involvement of phosphoinositide 3-kinase and Rac in membrane ruffling induced by IL-2 in T cells. Eur J Immunol. 1998;28(6):1877–1885. [DOI] [PubMed] [Google Scholar]
- [63].Arrizabalaga O, Lacerda HM, Zubiaga AM, et al. Rac1 protein regulates glycogen phosphorylase activation and controls interleukin (IL)-2-dependent T cell proliferation. J Biol Chem. 2012;287(15):11878–11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29(4):356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lin Q, Yang W, Baird D, et al. Identification of a DOCK180-related guanine nucleotide exchange factor that is capable of mediating a positive feedback activation of Cdc42. J Biol Chem. 2006;281(46):35253–35262. [DOI] [PubMed] [Google Scholar]
- [66].Altelaar AF, Heck AJ. Trends in ultrasensitive proteomics. Curr Opin Chem Biol. 2012;16(1–2):206–213. [DOI] [PubMed] [Google Scholar]
- [67].Fukui T, Shimomura S, Nakano K. Potato and rabbit muscle phosphorylases: comparative studies on the structure, function and regulation of regulatory and nonregulatory enzymes. Mol Cell Biochem. 1982;42(3):129–144. [DOI] [PubMed] [Google Scholar]
- [68].Johnson LN. Glycogen phosphorylase: control by phosphorylation and allosteric effectors. Faseb J. 1992;6(6):2274–2282. [DOI] [PubMed] [Google Scholar]
- [69].Richter F, Bohme HJ, Hofmann E. Developmental changes of glycogen phosphorylase b isozymes in rat tissues. Biomed Biochim Acta. 1983;42(10):1229–1235. [PubMed] [Google Scholar]
- [70].Sato K, Satoh K, Sato T, et al. Isozyme patterns of glycogen phosphorylase in rat tissues and transplantable hepatomas. Cancer Res. 1976;36(2 Pt 1):487–495. [PubMed] [Google Scholar]
- [71].Greenberg CC, Jurczak MJ, Danos AM, et al. Glycogen branches out: new perspectives on the role of glycogen metabolism in the integration of metabolic pathways. Am J Physiol Endocrinol Metab. 2006;291(1):E1–E8. [DOI] [PubMed] [Google Scholar]
- [72].Sutherland EW, Cori CF. Effect of hyperglycemic-glycogenolytic factor and epinephrine on liver phosphorylase. J Biol Chem. 1951;188(2):531–543. [PubMed] [Google Scholar]
- [73].Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213(3):589–602. [DOI] [PubMed] [Google Scholar]
- [74].Cohen P. The role of protein phosphorylation in the hormonal control of enzyme activity. Eur J Biochem. 1985;151(3):439–448. [DOI] [PubMed] [Google Scholar]
- [75].Pinacho R, Vila E, Prades R, et al. The glial phosphorylase of glycogen isoform is reduced in the dorsolateral prefrontal cortex in chronic schizophrenia. Schizophr Res. 2016;177(1–3):37–43. [DOI] [PubMed] [Google Scholar]
- [76].Pekny M, Pekna M, Messing A, et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131:323–345. [DOI] [PubMed] [Google Scholar]
- [77].Ishii T, Ueyama T, Shigyo M, et al. A novel Rac1-GSPT1 signaling pathway controls astrogliosis following central nervous system injury. J Biol Chem. 2017;292(4):1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li Y, Asuri S, Rebhun JF, et al. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J Biol Chem. 2006;281(5):2506–2514. [DOI] [PubMed] [Google Scholar]
- [79].Kumari A. Sweet Biochemistry: Remembering Structures, Cycles, and Pathways by Mnamonics. Academic Press: eBook ISBN: 978012814454. 2018. p. 1–5. [Google Scholar]
- [80].Boothby M, Rickert RC. Metabolic regulation of the immune humoral response. Immunity. 2017;46(5):743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Araujo L, Khim P, Mkhikian H, et al. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev. 2012;249(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mehta MM, Weinberg SE, Steinert EM, et al. Hexokinase 2 is dispensable for T cell-dependent immunity. Cancer Metab. 2018;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Roberts DJ, Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22(2):248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wang Z, Udeshi ND, Slawson C, et al. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal. 2010;3(104):ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chatterjee B, Thakur SS. Investigation of post-translational modifications in type 2 diabetes. Clin Proteomics. 2018;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zachara NE. Critical observations that shaped our understanding of the function(s) of intracellular glycosylation (O-GlcNAc). FEBS Lett. 2018;592(23):3950–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]


