ABSTRACT
The blood-brain barrier (BBB), a dynamic interface between blood and brain constituted mainly by endothelial cells of brain microvessels, robustly restricts the entry of potentially harmful blood-sourced substances and cells into the brain, however, many therapeutically active agents concurrently cannot gain access into the brain at effective doses in the presence of an intact barrier. On the other hand, breakdown of BBB integrity may involve in the pathogenesis of various neurodegenerative diseases. Besides, certain diseases/disorders such as Alzheimer’s disease, hypertension, and epilepsy are associated with varying degrees of BBB disruption. In this review, we aim to highlight the current knowledge on the cellular and molecular composition of the BBB with special emphasis on the major transport pathways across the barrier type endothelial cells. We further provide a discussion on the innovative brain drug delivery strategies in which the obstacle formed by BBB interferes with effective pharmacological treatment of neurodegenerative diseases/disorders.
KEYWORDS: Blood-brain barrier, endothelial cells, tight junctions, paracellular permeability, transcellular permeability
Introduction
The brain is the most critical organ that controls body systems in humans. Oxygen and nutrients, mainly glucose and amino acids, are supplied to the cells in the brain parenchyma by an elaborate network of blood capillaries. The estimated total length of brain microvessels is about 600–700 km and the total area of the endothelial surface in brain vasculature including capillaries, venules, arterioles, veins, and arteries approximates 20 m2.1,2 The brain is extremely sensitive to a wide range of potentially toxic substances in circulation, and the proper neuronal function necessitates an optimal microenvironment that is controlled and regulated by three different barrier systems; the blood-brain barrier (BBB) formed by brain microvessel endothelial cells (Figure 1), the blood-cerebrospinal fluid barrier (BCSFB) formed by choroid plexus epithelial cells (Figure 2Figure 3), and the meningeal barrier formed by arachnoid epithelial cells.3–5 It is suggested that neuronal homeostasis within the brain parenchyma is mainly regulated by the BBB since the total area of the luminal surface with BBB activity is estimated to be about 1000 times larger than that with BCSFB.6
Figure 1.
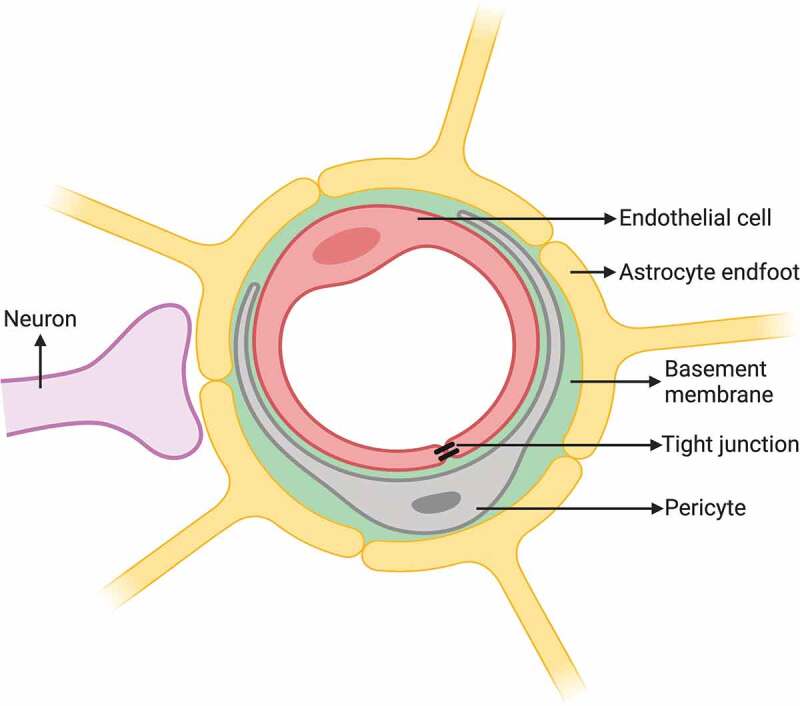
Schematic drawing of the BBB constituted by barrier type endothelial cell with TJ sharing the basement membrane with pericyte and the surrounding astrocyte endfeet. Created with BioRender.com
Figure 2.
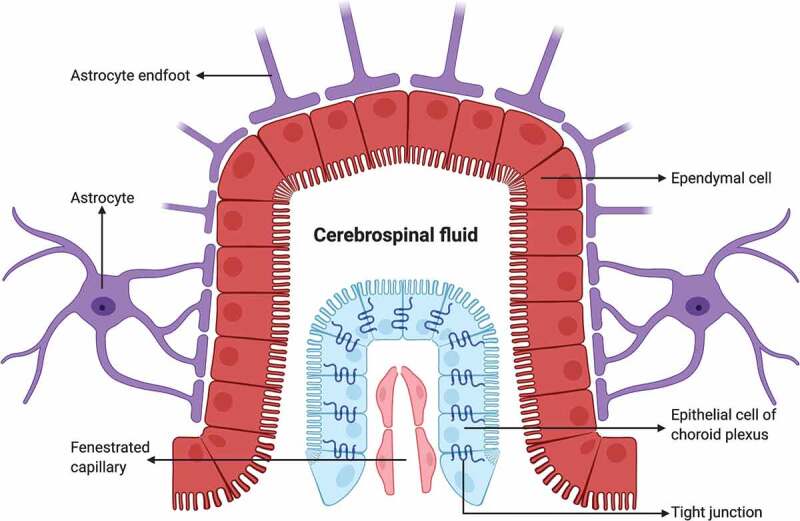
Schematic drawing of the BCSFB constituted by epithelial cells of choroid plexus with TJs, which secrete CSF derived from plasma in blood capillary without barrier properties into the ventricular space lined with ependymal cells. Created with BioRender.com
Figure 3.
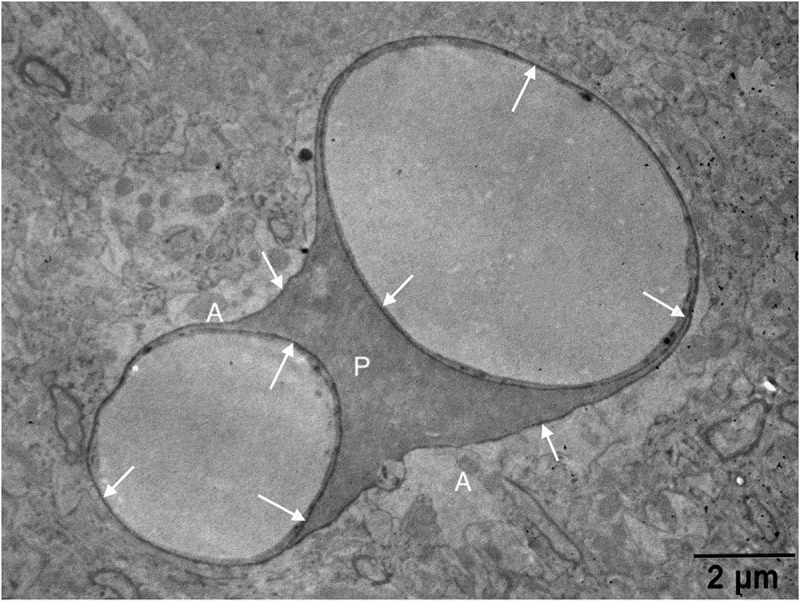
An electron micrograph from our image archive showing a pericyte (p) partly investing the endothelial cells of blood capillaries. Note that both endothelial cells and the pericyte are embedded in the basement membrane marked by extravasated electron-dense horseradish peroxidase tracer accumulation (arrows) owing to BBB disruption. A: astrocyte endfoot
The concept of the BBB was first established in the late 19th century by Paul Ehrlich, who observed that trypan blue dye injected into the rat circulation resulted in the staining of peripheral organs but not the brain and spinal cord. In the following years, Goldman injected trypan blue into the cerebrospinal fluid (CSF), and he demonstrated that the staining was only restricted to the central nervous system (CNS), but not to the other body tissues.7 By the advent of electron microscopy and its widespread use in the evaluation of biological tissues in the 1960s, the presence of the BBB was confirmed using an electron-dense tracer, horseradish peroxidase (40 kD), which had been observed to pass through the vascular endothelium in peripheral tissues in contrast to that in the brain.8
The BBB, as a dynamic regulatory interface between blood and brain, protects neuronal microenvironment required for the proper functioning of neuronal circuits, synaptic transmission and remodeling, angiogenesis, and neurogenesis, by constantly controlling trafficking of molecules and preventing circulatory immune cell entry into the brain via paracellular and transcellular pathways.3,9–12 The access of certain blood-borne neuroactive solutes, such as glutamate, glycine, norepinephrine, epinephrine, and peptide hormones into the brain is also significantly limited by the action BBB.13–15 A healthy BBB not only protects the neurons but also is crucial for the physiologic functions of glial cells and pericytes.
The afore-mentioned protective activities of BBB brings with a concomitant obstacle for the access of therapeutic agents into the brain at effective doses for the treatment of neurodegenerative diseases. The presence of an intact BBB excludes approximately 98% of small molecule drugs and nearly all large therapeutics, such as recombinant peptides, proteins, anti-sense-agents, and genetic vectors from the brain.16 Moreover, the fraction of therapeutic antibodies such as immunoglobulin G that reaches into the brain parenchyma following intravenous administration is estimated to be as low as 0.1%.17 On the other hand, certain systemic diseases or CNS disorders are likely to evoke alterations in BBB integrity, leading to BBB disruption and loss of neuronal homeostasis.18–21
In this review, we present an overview of the structure and function of the BBB in both healthy and pathological conditions, the alterations in BBB integrity associated with neurodegenerative disorders/diseases and novel strategies to enhance targeted drug delivery into the brain.
Neurovascular unit
In the last 2 decades, the term “neurovascular unit” has started to be used to define a well-structured complex that is involved in the development and maintenance of BBB integrity and in the regulation of cerebral blood flow. The major constituents of the unit are barrier type endothelial cells interacting with the basement membrane, pericytes, vascular smooth muscle cells, astrocytes, microglia, oligodendroglia, and neurons.22–26
Barrier type endothelial cells
The cellular structures that lie in the interface between the blood and the brain are endothelial cells, pericytes, and astrocyte endfeet, which along with a cellular product, basement membrane, collectively compose the major constituents of the BBB and orchestrate the trafficking of molecules and cells between the two compartments. The primary part of the BBB is made up of capillaries that have luminal diameters of less than 10 µm.27,28 In contrast to the various types of peripheral capillaries with different capacities of permeability found in other organs, brain capillaries display barrier type endothelial cells with a variety of distinguished properties, which render them the chief element of BBB structure and the main actor in the maintenance of neuronal homeostasis. Accumulated data show that brain capillary endothelial cells are sealed with tight junctions (TJs), which restrict the paracellular transport, and lack endothelial fenestrations and possess relatively few caveolar vesicles which limit the transcellular transport.3,29,30 In addition, barrier type endothelial cells exhibit specific transport and carrier proteins that are located both in the luminal and abluminal plasma membranes.31–33 Moreover, these cells are also considered to form an endocrine secretory tissue that produces a variety of local hormones such as nitric oxide, prostaglandins, and cytokines.34–36 A continuous basement membrane, pericytes and astrocyte endfeet surrounding the endothelium provide anatomical support to the BBB.3,37,38
Physical barrier (Tight junctions)
Barrier type endothelial cells are normally adjoined to each other by TJs and adherens junctions, which collectively constitute a physical barrier that limits the paracellular pathway.22 The exchange of polar substances between the blood and the brain is strictly controlled and significantly reduced by the TJ proteins.3,39
Metabolic barrier
The brain capillary endothelial cells express a variety of enzymes including γ-glutamyl transpeptidase, alkaline phosphatase, aromatic acid decarboxylase, monoamine oxidase, and cytochrome P450, which metabolize and inactivate molecules such as neuroactive and neurotoxic compounds, peptides, and ATP.6,36,40–43 Barrier type endothelial cells also possess certain crucial transporters such as Na+-K+ ATPase, and glucose transporter (Glut)-1 which take part in the regulation of the composition of neuronal microenvironment.44,45
Efflux barrier
The barrier type endothelial cells of the brain capillaries express certain efflux transporters, designated as multidrug-resistance transporters localized predominantly on the luminal plasma membrane.46,47 These transporters include P-gp, also called mdr-1, and breast cancer resistance protein which extrude the administered xenobiotics, compounds that include lipophilic and cationic drugs, from the brain capillary endothelial cells back to the circulation and hence reduce the delivery of drugs into the brain parenchyma at effective doses thereby posing an obstacle to the treatment of neurodegenerative disorders/diseases. A number of chemical compounds and chemotherapeutic agents used in daily clinical practice have been described as the substrates of these transporters.48,49 Successful management of tumors and refractory epilepsy is reported to be overwhelmed by the activity of P-gp.50,51 Although inhibitors of P-gp and breast cancer resistance protein have been used effectively to overcome the drug resistance in experimental animals, human data are still lacking.52–54
Basement membrane
The basement membrane of the capillary wall in the brain is structurally an organized protein sheet with a thickness of 50–100 nm and surrounds both endothelial cells and pericytes. It is a highly dynamic constituent of the BBB and plays an essential role in the maintenance of BBB integrity.55–57 The contents of the basement membrane are secreted by endothelial cells and pericytes and are mainly composed of laminins, collagen type IV isoforms, fibrillins, vitronectin, fibronectin, elastin, nidogens, and heparan sulfate.56,58 In addition, soluble factors (e.g., growth factors and cytokines), enzymes responsible for matrix degradation, and proteins such as lectins and semaphorins are also present in the basement membrane structure.56,59,60 Laminins primarily play a role in the organization and scaffolding, and collagen type IV is essential for the stability of the basement membrane.59,61 Matrix metalloproteinases that are activated by certain pathological insults may disrupt the integrity of the basement membrane resulting in BBB breakdown through an impairment in the functional activity of TJ proteins.19,22
Pericytes
Pericytes, one of the components of the neurovascular unit, are located between endothelial cells and astrocyte endfeet (Figure 1). They are embedded in the same basement membrane surrounding the endothelial cells and thus are physically separated from both endothelial cells and astrocyte endfeet.3,62,63 The available data on the percentage of the surface area of the abluminal plasma membrane of endothelial cells covered by the pericytes are contradictory with reported values ranging from 22 to 99% .64–68 Pericytes are contractile cells, which provide physical support to and determine the vasodynamic properties of brain capillaries and hence contribute to the regulation of cerebral blood flow by controlling the luminal diameter.69–71 A recent study suggested that pericytes can construct tunneling nanotubes that regulate neurovascular coupling and control capillary blood flow.72 Pericytes are also essential for the induction of barrier characteristics in the endothelial cells including the formation of TJs and are involved in the regulation of BBB integrity and the transport of substances into the brain parenchyma.62,63,73–76 Moreover, pericytes produce extracellular matrix proteins and play a crucial role in the regulation of endothelial cell proliferation, migration, and differentiation.77–80 Besides, they also take part in the clearance of toxic cellular byproducts.66,73,74,81 In experimental animals, pericyte deficiency induced by a platelet-derived growth factor mutation has been shown to cause a reduction in the expression of certain TJ proteins leading to a substantial BBB disruption.63,73
Astrocytes
Astrocytes, the most abundant cell type in the brain, are characterized by their expression of the intermediate filament glial fibrillary acidic protein (GFAP) and their numerous cellular processes extending from the cell body. Most of these processes terminate as endfeet, which contact with the abluminal side of the basement membrane of brain capillaries to interact with endothelial cells and pericytes. Astrocytic perivascular endfeet are estimated to cover over 99% of the brain microvasculature wall (Figures 1 and 4).23,42,65
Figure 4.
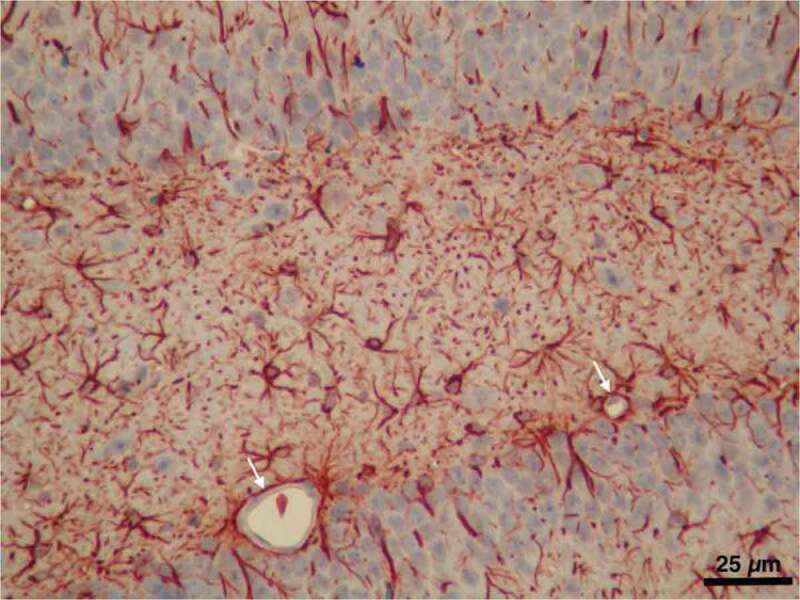
A light micrograph from our image archive showing astrocytes labeled by immunostaining for GFAP in the hippocampal region of the brain. Note the microvessels almost entirely surrounded with astrocyte endfeet (arrows)
As a component of the neurovascular unit, astrocytes contribute to the regulation of vascular tone and local blood flow into the brain parenchyma and hence play an important role in the transport of oxygen and nutrients to neurons to maintain brain homeostasis. Astrocytes are the main actors that determine neuronal activity by regulating ion concentration and extracellular pH within the interstitial space and the uptake of glutamate and GABA in the synaptic region.82–84 They express aquaporin-4 water channel proteins that promote perivascular clearance of waste material and hence form the newly characterized “glymphatic system” (CNS waste clearance system). Astrocytes also express certain transporters such as P-gp and Glut-1 along with Kir4.1 K+ channel proteins that aid in the maintenance of the neuronal resting membrane potential by removing extracellular K +.85–89
Astrocytes orchestrate the development of BBB properties and barrier maturation by releasing specific factors .19,42,90–92 They are involved in the maintenance of BBB integrity by providing functional and anatomical support.93,94 The alterations in astrocyte characteristics are reported to be associated with impairment in BBB integrity.95 Data from the studies of our research group have shown BBB disruption along with alteration in GFAP immunoreactivity in astrocytes in experimental models of hypertension, febrile seizures, and irradiation.96–98 On the contrary, there are some reports in the literature that oppose the necessity of glial cells for the maintenance of BBB integrity.99,100 Furthermore, reactive astrocytes were found to disrupt the BBB integrity by releasing vascular endothelial growth factor.101
Microglia
Microglia are long-living resident immune cells of the CNS and account for around 12–16% of the total cell population in the brain. They constitute the major cell type that acts in the protection of the brain against immunologic insults and thus contribute to the maintenance of neuronal homeostasis.102,103 The breakdown of BBB may alter microglial activity through interaction with activated endothelial cells even in the absence of neurodegeneration, and hence brain regions with hypertrophied/activated microglial-like cells associated with vasculature may potentially display vascular damage and BBB compromise.104 The activated microglia produce pro-inflammatory cytokines, including IL-1β and TNF-α, that further enhance the degree of BBB disruption.105,106 Activation of microglia by lipopolysaccharide has been shown to decrease trans-endothelial electrical resistance (TEER) by disrupting TJ proteins, including claudin-5 and zonula occludens (ZO)-1 in an in vitro model of BBB.107 Although microglia are defined as the cells that form the first line of defense against immunologic compromise, a putative role of these cells with regard to the maintenance of BBB integrity in physiologic conditions or restoration of disrupted BBB in the course of CNS diseases/disorders remains unclear.
Neuron
Almost every neuron in the human brain is estimated to be nourished by a capillary microvessel positioned within an average distance of 15 μm.108,109 Accumulated data have demonstrated that there is a direct interaction between neurons and barrier type endothelial cells, pericytes, as well as astrocytes.22,23,110,111 The soma, axon, or dendrite of a neuron in close proximity to a brain capillary may contact pericytes and endothelial cells via the basement membrane.6,112 Communication of neurons with glial and endothelial cells is essential for their survival and functions. Depending on metabolic requirements, neurons not only modulate various endothelial cell functions, including permeability, by activating specific enzymes expressed by the endothelial cells but also release growth factors to stimulate angiogenesis.19,113,114 In the meantime, alterations in neuronal activity have been reported to affect BBB integrity.115 Trans-endothelial electrical resistance and expression of TJ proteins have been found to be positively influenced when endothelial cells were co-cultured with neurons in vitro.116
Functions of the blood-brain barrier
Brain capillaries, like their peripheral counterparts, essentially provide oxygen and nutrients to parenchymal cells, including neurons, and remove the produced waste materials. Importantly, endothelial cells of the microvessels in the brain display strong barrier properties which enable strict control of ionic and fluid movements between the circulation and the brain parenchyma to regulate the neuronal microenvironment. The transfer of substances from the blood to the brain parenchyma across the BBB is accomplished through a transcellular route in which receptor and carrier-mediated transporters are critical for transcytosis, and paracellular route in which TJs are the chief determinants of permeability.
Characteristics of the blood-brain barrier
The endothelial cells of the brain capillaries are the fundamental anatomical structures of the BBB. These cells express not only a variety of specific transport and carrier proteins which enable tightly controlled trafficking of molecules, but also TJ proteins which account for one of the barrier characteristics reflected by high electrical resistance (approximately 1800 Ω cm2) compared with that in peripheral capillaries (2–20 ohm cm2).117,118
Tight junctions
The intercellular cleft between adjacent endothelial cells of brain capillaries houses two major types of junctional complex; TJs and adherence junctions (Figure 5Figure 6Figure 7). Tight junctions are highly dynamic structures that effectively limit the movement of water and solutes and regulate lateral diffusion through the paracellular pathway.22,23,119,120 These structures are formed by transmembrane proteins claudins, occludin, and junctional adhesion molecules (JAMs) which interact with the actin cytoskeleton of the endothelial cells by a number of cytoplasmic accessory proteins including ZO proteins, cingulin, AF-6, and 7H6.22,121–124 The interaction between ZO proteins and transmembrane proteins, including claudins and occludin have been shown to determine the stability and function of TJs.22,125–127 In contrast to the epithelial cells which exhibit intercellular gaps sealed by TJs localized at the most apical point of cellular attachment immediately above the clearly distinguishable adherens junctions, barrier type endothelial cells in the brain are joined together by TJs and adherens junctions showing more variable localizations and intermingled appearances.128
Figure 5.
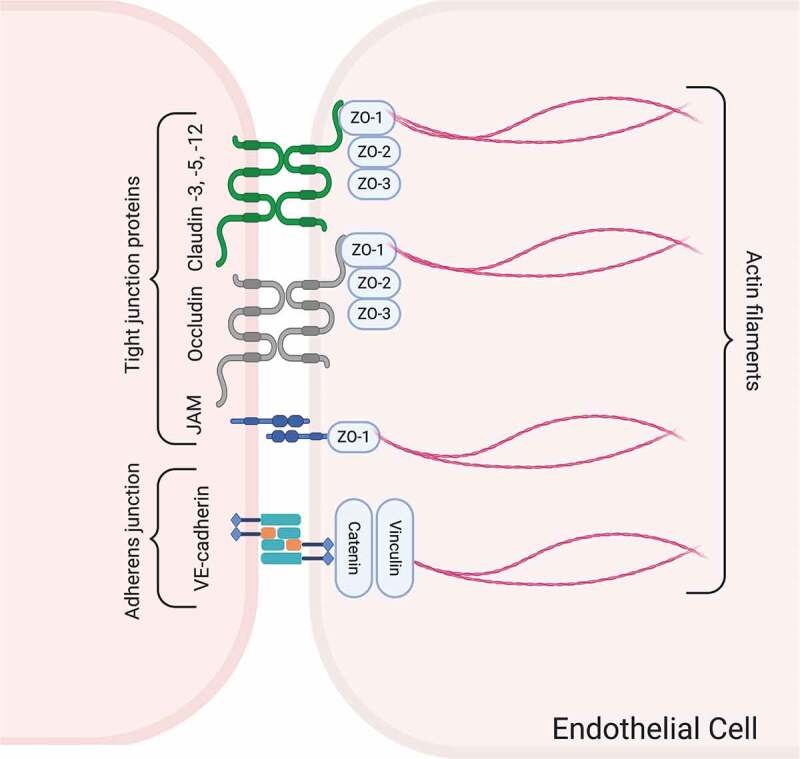
Schematic drawing of the junctional complex between barrier type endothelial cells of the brain, which controls the trafficking of substances through the paracellular pathway. The major proteins comprising TJs and adherens junctions and their linkage to the actin cytoskeleton are illustrated. Created with BioRender.com
Figure 6.
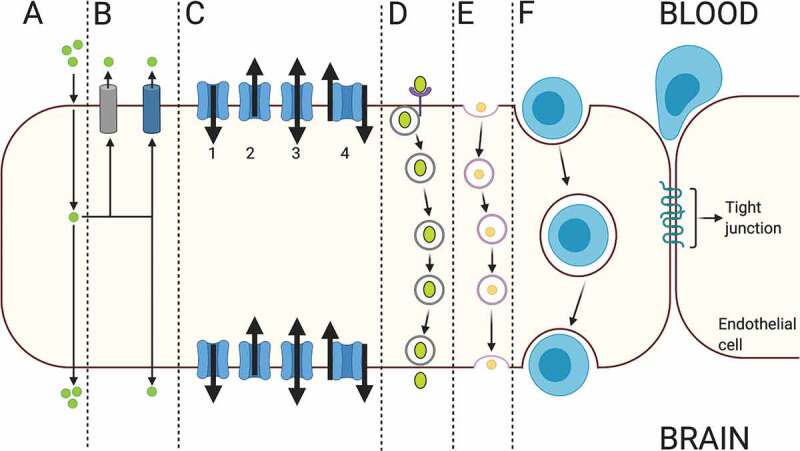
The schematic drawing of various routes of transcellular transport across barrier type endothelial cells in the brain. A: passive diffusion, B: efflux transport, C: carrier-mediated transport, D: receptor-mediated transport, E: adsorptive-mediated transport, F; cellular transport. Created with BioRender.com
Figure 7.
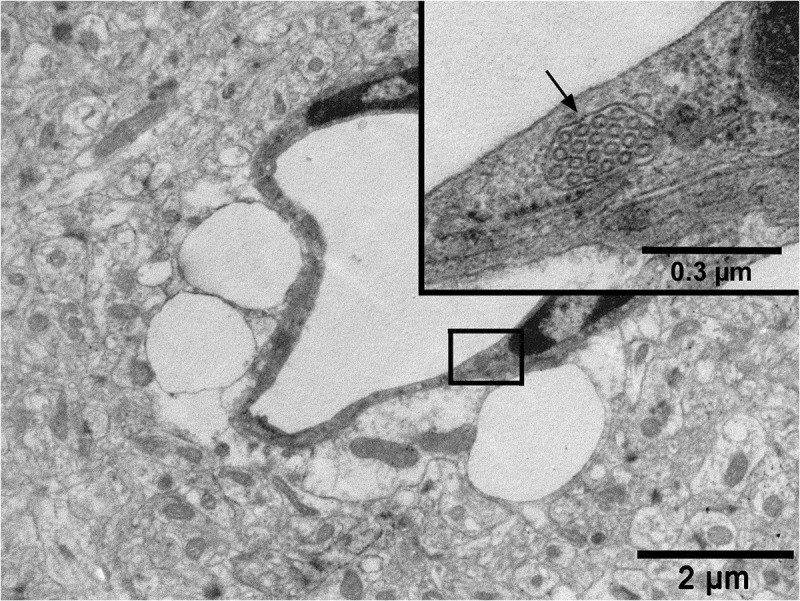
An electron micrograph from a previous study from our research group (reproduced from Ref. # 96 with permission from Elsevier Science) showing a capillary from the hippocampus region of the brain of a rat with cortical dysplasia exposed to febrile seizures. Note a conglomerate of caveolar vesicles within a cargo (arrow in the inset) in the cytoplasm of a brain capillary endothelial cell and the intensive pericapillary edema with swollen astrocyte endfeet
There are currently defined 27 members of claudins, and a number of them including claudin-1, −3, −5, −11, and −12 have been identified in the TJs between barrier type endothelial cells.19,129–132 However, only claudin-1, −3, and −5 are most likely responsible for controlling the paracellular pathway while the roles of claudin-11 and 12 with regard to barrier function are yet to be elucidated.23,130,133–135 Claudin-5 is the most enriched isoform in the brain endothelium and determines the sealing properties of TJs of the BBB.134 Claudin-5 knockout mice display a selective increase in paracellular permeability for small molecules.130 On the other hand, it is suggested that claudin-12 is not directly involved in the establishment or maintenance of BBB integrity.136
Occludin, the first TJ transmembrane protein described, has been defined as one of the major TJ proteins that controls the paracellular pathway of BBB.125,137 Early studies showed an enhancement in TEER and reduction in paracellular diffusion by an increase in the expression of the occludin protein.138,139 Moreover, dephosphorylation of occludin caused BBB failure in an experimental model of multiple sclerosis.140 An autopsy series of fatal human septic cases showed that occludin expression in the barrier type endothelial cells was lost in the brain.141 In diabetic rats, occludin and ZO-1 expression were decreased concomitant with an increase in BBB permeability to 14 C-sucrose.142 Data from the studies of our research group have shown BBB disruption along with a reduction of occludin immunoreactivity in barrier type endothelial cells in in vivo experimental models of sepsis, hypertension, and irradiation.97,98,143,144 In contrast, it is also suggested that occludin does not have the ability to establish TJ structure by itself but rather exerts a regulatory function on the barrier properties.145 Accordingly, occludin-deficient mice display well-developed TJ complexes without any evidence of BBB hyperpermeability.139
Among the three isoforms of JAMs, JAM-1, JAM-2, and JAM-3, an interaction with occludin and claudins in TJs of brain capillaries to provide cell-to-cell adhesion has been described for JAM-1.123,133,146 The members of JAM family also play an important role in leukocyte adhesion and transmigration to the brain parenchyma across BBB.147
Zonula occludens proteins (ZO-1, ZO-2, and ZO-3) are membrane-associated guanylate kinase homologs located in the cytoplasmic domain of TJs between endothelial cells. Among the members of ZO protein family, ZO-1 plays a central role in the assembly and organization of claudins, occludin, and JAMs, and links these TJ proteins to the cortical actin cytoskeleton.133,148–150 ZO-1 is also a central regulator of vascular endothelial-cadherin–dependent adherens junctions that orchestrate the tuning of cell-cell tension, migration, angiogenesis, and barrier formation.150 Data from our lab demonstrated decreased immunoreactivity for ZO-1 in brain capillary endothelial cells along with increased BBB permeability in an in vivo model of seizure and by radiation therapy in rats.97,151 Besides, the loss or dissociation of ZO-1 and occludin from the junctional complex associated with BBB disruption have been shown in a variety of pathological conditions such as hypoxia, subarachnoidal hemorrhage, and Parkinson’s disease.97,151–154
Adherens junctions
Adherens junctions are constituted by Ca1+-dependent transmembrane cadherin proteins that form homotypic adhesive complexes between neighboring endothelial cells and bind to the actin cytoskeleton via cytoplasmic anchoring proteins called catenins.3,155,156 Although the primary function of adherens junctions is the attachment of adjacent endothelial cells, they may also involve in the formation and maintenance of tightness of the TJs.119 However, the role of these junctional complexes in the development and normal physiology of BBB remains to be elucidated. Certain pathologic conditions causing disruption of adherens junctional proteins are associated with a loss of BBB integrity.123
Transport pathways across the blood-brain barrier
Under physiological conditions, the BBB exhibits low permeability compared to peripheral blood vessels.157 Lipid solubility, electrical charge, molecular size, and hydrogen bonding capacity are the main determinants of the ability of a molecule in circulation to enter the brain across an intact functioning BBB. The paracellular passage of molecules is considerably minimized, while ions and solutes can diffuse between adjacent cells according to their concentration gradients. On the other hand, most of the nonpolar lipid-soluble molecules of small molecular weight (<400-500 Da) such as carbon dioxide, nitric oxide, ethanol, and oxygen, are readily transported into the brain parenchyma by the process of the passive diffusion mainly via transcellular route through the lipid bilayer of the endothelial cell membrane.3,12,16,42,158,159
Hydrophilic and charged molecules can only penetrate the BBB by active transport systems, including receptor-mediated transport, carrier-mediated transport, and adsorptive-mediated transport to enter the brain.42 Molecules such as glucose, transferrin, and amino acids and ions, including potassium, sodium, calcium, and bicarbonate, utilize these active transport systems; however, growth factors and cytokines have limited ability to permeate across the BBB.160,161 On the other hand, efflux transporters such as P-gp pump their substrates such as drugs and metabolites back to the circulation.162–164
The paracellular pathway
The discovery of the ultrastructure of BBB enabled a clear understanding of the trafficking of substances across the barrier, and the paracellular pathway strictly controlled and regulated by the TJs localized along the interendothelial space was described.8 The specialized TJ proteins effectively prevent undesirable passive diffusion of lipophilic or low molecular weight substances and passage of immune cells through the gaps between the endothelial cells of brain capillaries and provide an obstacle for the bulk flow of water and plasma-sourced solutes by the paracellular route.
The transendothelial pathway
The trafficking of substances across the barrier type brain capillary endothelial cells is primarily mediated by the transcellular route. As a prominent characteristic of the barrier type endothelial cells, this pathway utilizes various types of influx transporters collectively called nutrient transporters, while efflux transporters pump their specific substrates back to the bloodstream .44,165,166 The transport of molecules by the transcellular pathway is bidirectional using receptor and carrier proteins located on both luminal and abluminal membranes of barrier type endothelial cells by energy-dependent or -independent processes.167 In addition, fluid-phase and adsorptive endocytosis is used to transport some non-lipid-soluble molecules of small molecular weight and macromolecules like albumin, immunoglobulins, and other proteins.168,169
Receptor-mediated transport
The main pathway in the trafficking of molecules across the BBB is receptor-mediated transcytosis which mediates the transport of the circulatory substances including transferrin, low-density lipoproteins, leptin, insulin, and insulin-like growth factor into the brain; however, a shift to ligand-nonspecific caveolar transcytosis is observed by aging.33,41,170–173 Receptor-mediated transcytosis has recently been the focus of interest in targeted drug delivery studies in which molecular Trojan horses, vectors that can bind specific receptors of the pathway, are used to enable the transport of drugs into the brain at effective doses in certain CNS diseases/disorder resistant to pharmacological treatment.174,175
Carrier-mediated transport
The circulatory-sourced vital substances that are essential for the energy and neurotransmitter metabolism in the brain, including nutrients such as glucose, vitamins, and hormones require the carrier-mediated transporters located both on luminal and abluminal plasma membranes of barrier type of endothelial cells to reach into the brain parenchyma through a saturable transport process.2,19,31,176–180 Glut-1 mediates the uptake of D-glucose, the main energy source of the brain, by barrier type endothelial cells and delivery to astrocytes and neurons.181–183 In the opposite direction, the uptake of D-glucose from the brain interstitium into the circulation is accomplished by the Na+-D-glucose cotransporter Sglt1, expressed in the brain capillary endothelial cells, which further contributes to the adjustment of glucose concentration in the brain interstitium.183 Glut-1 is also reported to be crucial for angiogenesis during brain development.184 The transport of certain ions in exchange of or simultaneously with other ions is exerted by exchanger pumps including sodium/potassium pump and sodium-hydrogen, chloride-bicarbonate and sodium-calcium exchangers, and cotransporters such as sodium-potassium-two chloride cotransporter localized on the abluminal and/or luminal side of the barrier type endothelial cells.185–187
Caveolae-mediated endocytosis
Caveolae are characteristic flask-shaped membrane invaginations with a diameter of 50–80 nm, which are mainly responsible for endothelial transcytosis in barrier type of brain capillary endothelial cells.188,189 The caveolar membranes contain caveolin-1/2 and vesicle-associated membrane protein-2 as well as receptors for certain essential enzymes, hormones, plasma carrier proteins, and cytokines.190,191 Caveolin 1, which is the principal component of caveolae, can also influence the expression of TJ proteins.192 The expression of the caveolin-1 is significantly increased in barrier type endothelial cells under several pathological conditions and by aging.193–195 Major Facilitator Superfamily Domain containing 2a (Mfsd2a), a lipid transporter highly expressed in the endothelial cells of brain microvessels, inhibits caveolae production and hence plays an important role in barrier characteristics.196–198 Therefore, brain capillary endothelial cells exhibit few caveolae, whereas arteriolar endothelial cells in which Mfsd2a transcript levels are low display abundant caveolae.199,200 Knock-out of Mfsd2a in mice caused increased caveolae production and transcellular permeability in the brain microvasculature.195,197
Caveolae are characteristic flask-shaped membrane invaginations with a diameter of 50–80 nm, which contain caveolin-1/2 and vesicle-associated membrane protein-2 as well as receptors for certain essential enzymes, hormones, plasma carrier proteins, and cytokines.190,191 Caveolin 1, which is the principal component of caveolae, can also influence the expression of TJ proteins.192 Major Facilitator Superfamily Domain containing 2a (Mfsd2a), a lipid transporter highly expressed in the endothelial cells of brain microvessels, inhibits caveolae production and hence plays an important role in barrier characteristics.196–198 Therefore, brain capillary endothelial cells exhibit few caveolae, whereas arteriolar endothelial cells in which Mfsd2a transcript levels are low display abundant caveolae.199,200 Knock-out of Mfsd2a in mice caused increased caveolae production and transcellular permeability in the brain microvasculature.195,197 On the other hand, the expression of the caveolin-1 is significantly increased in barrier type endothelial cells under several pathological conditions and by aging.193–195 Increase of caveolar vesicles in brain capillary endothelium has been documented in experimental animals with hypertension and during febrile seizures in experimental models of cortical dysplasia, a malformation of the cerebral cortex .96,201
Efflux transport
The multidrug-resistance proteins primarily consist of multidrug-resistance protein-1 or P-gp, multidrug resistance-associated protein, and breast cancer resistance protein.202 The P-gp, as an efflux pump, is located on the luminal side of the BBB and restricts the permeation of a large number of toxins into the brain parenchyma, providing neuroprotection and detoxification.203–205 On the other hand, the efflux pumps also restrict the entry of therapeutic agents, including antibiotics, chemotherapeutics, and antiepileptic drugs into the brain parenchyma at effective doses and thus severely contribute to the development of pharmacoresistance in the treatment of certain types of brain tumors and epilepsy.44,202,206
Cell movement across the BBB
Under inflammatory conditions, circulatory-sourced immune cells and neutrophils can penetrate into the brain via paracellular pathway which requires opening and rearrangement of TJ complexes and transendothelial pathways which involves the dynamic organization of cellular processes of leukocytes called invadosomes and various vesicles and vesiculo-vacuolar organelles forming a transcellular pore through the endothelial cells.207–210 The cell surface levels of endothelial intercellular adhesion molecule-1 and caveolin-1 also play a crucial role in transcellular immune cell entry into the brain.211,212
Circumventricular organs
In contrast to the capillaries located in the brain parenchyma, blood microvessels in the circumventricular organs do not display barrier properties. The endothelial cells of these microvessels have fenestrae, which allow the free diffusion of substances between the blood and CNS.37,213,214 These organs consist of secretory structures like pineal gland, subcommisural organ, median eminence, and choroid plexuses and sensory regions, including area postrema, subfornical organ, and organum vasculosum of the lamina terminalis.215 The exchange of hormones and other molecules between the circulation and CNS is accomplished mainly in circumventricular organs in which increased vascularization facilitates the sensory and secretory roles to mediate the communication between the brain and the periphery.215,216
BBB disruption in pathological conditions
While the integrity of the BBB is crucial for the maintenance of neuronal homeostasis, alterations in functional and structural properties of the barrier are closely interrelated with the occurrence of certain brain pathologies. A variety of CNS diseases/disorders including epilepsy,175,217 ischemic stroke,218 multiple sclerosis,219 traumatic brain injury,220 and Alzheimer’s disease221 are characterized by BBB disruption. On the other hand, CNS manifestations associated with BBB breakdown may develop in systemic diseases such as sepsis222,223 and hypertension.201
Drug delivery into the brain for clinic implications
Innovative strategies have been developed for overcoming BBB to enable the access of therapeutic drugs at effective doses in CNS diseases/disorders in experimental settings; however, their current use in clinical practice is still limited. Circumventing BBB by temporarily disrupting the TJs between brain capillary endothelial cells has been reported to allow the access of pharmacologic agents into the CNS in both experimental animals and humans. The intravenous administration of bradykinin and histamine, intraarterial infusion of hyperosmolar solutions like mannitol and application of transcranial focused ultrasound together with microbubbles temporarily open the TJs between barrier type endothelial cells in the brain.224–226 Moreover, conjugating the pharmacological agents with nanocarriers such as liposomes, nanopolymers, nanoparticles, viruses, and exosomes which normally have access into the brain using the endothelial transcellular transport mechanisms is being extensively studied in the last decade as an alternative approach to the drug delivery into the brain43,227–229
Future challenges
Our understanding of the transport dynamics across BBB in both physiological and pathological conditions has advanced considerably in recent years by the rapid development of advanced molecular techniques, imaging modalities, and nanotechnology. In this context, the accumulated data on the behavior of BBB will pave the way to elucidate the mechanisms underlying the response of the neurovascular unit in neurodegenerative disorders/diseases and to develop novel therapeutic strategies. On the other hand, exploiting ways for the targeted delivery of pharmacologically active substances into the brain through transendothelial transport pathways using nanocarriers or by the reversible opening of both transcellular and paracellular routes will allow BBB permeation of therapeutic agents at effective doses. We believe that an elaborate network of expertise with the collaboration of researchers from various disciplines, including medicine, chemistry, bioengineering, and electronics may enable us to overcome future challenges.
Acknowledgments
The authors gratefully acknowledge use of the services and facilities of the Koç University Research Center for Translational Medicine (KUTTAM), funded by the Presidency of Turkey, Presidency of Strategy and Budget. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Presidency of Strategy and Budget. The authors would also like to thank Ph.D. student Uğur Akcan for his contribution to the preparation of schematic illustrations of the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):1–20. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardridge WM. Blood–brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets. 2015;19(8):1059–1072. doi: 10.1517/14728222.2015.1042364. [DOI] [PubMed] [Google Scholar]
- 3.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Daneman R, Engelhardt B. Brain barriers in health and disease. Neurobiol Dis. 2017;107:1–3. doi: 10.1016/j.nbd.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt B, Sorokin L. The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31(4):497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WM. Introduction to the blood–brain barrier: methodology, biology and pathology. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 7.Saunders NR, Dreifuss -J-J, Dziegielewska KM, Johansson PA, Habgood MD, Mã¸llgã¥rd K, Bauer H-C. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front Neurosci. 2014;8:404. doi: 10.3389/fnins.2014.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyrna F, Hanske S, Krueger M, Bechmann I. The blood-brain barrier. J Neuroimmune Pharmacol. 2013;8(4):763–773. doi: 10.1007/s11481-013-9473-5. [DOI] [PubMed] [Google Scholar]
- 10.Keaney J, Campbell M. The dynamic blood-brain barrier. Febs J. 2015;282(21):4067–4079. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- 11.Yazdani S, Jaldin‐Fincati JR, Pereira RVS, Klip A. Endothelial cell barriers: transport of molecules between blood and tissues. Traffic. 2019;20(6):390–403. doi: 10.1111/tra.12645. [DOI] [PubMed] [Google Scholar]
- 12.Harilal S, Jose J, Parambi DGT, Kumar R, Unnikrishnan MK, Uddin MS, Mathew GE, Pratap R, Marathakam A, Mathew B. Revisiting the blood-brain barrier: a hard nut to crack in the transportation of drug molecules. Brain Res Bull. 2020;160:121–140. doi: 10.1016/j.brainresbull.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr. 2000:130:1016. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- 14.Smith QR. Carrier-mediated transport to enhance drug delivery to brain. International Congress Series. 2005;1277:63–74. doi: 10.1016/j.ics.2005.02.012. [DOI] [Google Scholar]
- 15.Strazielle N, Ghersi-Egea JF. Physiology of blood–brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol Pharm. 2013;10(5):1473–1491. doi: 10.1021/mp300518e. [DOI] [PubMed] [Google Scholar]
- 16.Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Golde TE. Open questions for Alzheimer’s disease immunotherapy. Alzheimers Res Ther. 2014;6(1):3. doi: 10.1186/alzrt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49:143–155. [PubMed] [Google Scholar]
- 19.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5):1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharif Y, Jumah F, Coplan L, Krosser A, Sharif K, Tubbs RS. Blood brain barrier: a review of its anatomy and physiology in health and disease. Clin Anat. 2018;31(6):812–823. doi: 10.1002/ca.23083. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 23.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 24.Choi Y-K, Kim K-W. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41(5):345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- 25.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L-R, Liu J-C, Bao J-S, Bai -Q-Q, Wang G-Q. Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol. 2020;11:1024. doi: 10.3389/fimmu.2020.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenstermacher J, Gross P, Sposito N, Acuff V, Pettersen S, Gruber K. Structural and functional variations in capillary systems within the brain. Ann N Y Acad Sci. 1988;529(1 Fourth Colloq):21–30. doi: 10.1111/j.1749-6632.1988.tb51416.x. [DOI] [PubMed] [Google Scholar]
- 28.Anzabi M, Angleys H, Aamand R, Ardalan M, Mouridsen K, Rasmussen PM, Sørensen JCH, Plesnila N, Østergaard L, Iversen NK. Capillary flow disturbances after experimental subarachnoid hemorrhage: a contributor to delayed cerebral ischemia? Microcirculation. 2019;26(3):e12516. doi: 10.1111/micc.12516. [DOI] [PubMed] [Google Scholar]
- 29.Sedlakova R, Shivers RR, Del Maestro RF. Ultrastructure of the blood-brain barrier in the rabbit. J Submicrosc Cytol Pathol. 1999;31:149–161. [PubMed] [Google Scholar]
- 30.Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20(1):57–76. doi: 10.1023/a:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for cns drug discovery and development. . Pharmaceutical Research. 2007;24(9):1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 32.Barar J, Rafi MA, Pourseif MM, Omidi Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. Bioimpacts. 2016;6(4):225–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulgar VM. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front Neurosci. 2019;12:1019. doi: 10.3389/fnins.2018.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mándi Y, Ocsovszki I, Szabo D, Nagy Z, Nelson J, Molnar J. Nitric oxide production and MDR expression by human brain endothelial cells. Anticancer Res. 1998;18:3049–3052. [PubMed] [Google Scholar]
- 35.Reyes TM, Fabry Z, Coe CL. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Res. 1999;851(1–2):215–220. doi: 10.1016/s0006-8993(99)02189-7. [DOI] [PubMed] [Google Scholar]
- 36.Banks WA. The blood–brain barrier as an endocrine tissue. Nat Rev Endocrinol. 2019;15(8):444–455. doi: 10.1038/s41574-019-0213-7. [DOI] [PubMed] [Google Scholar]
- 37.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Sekiguchi R, Yamada KM. Basement membranes in development and disease. Curr Top Dev Biol. 2018;130:143–191. doi: 10.1016/bs.ctdb.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berndt P, Winkler L, Cording J, Breitkreuz-Korff O, Rex A, Dithmer S, Rausch V, Blasig R, Richter M, Sporbert A, et al. Tight junction proteins at the blood–brain barrier: far more than claudin-5. Cell Mol Life Sci. 2019;76(10):1987–2002. doi: 10.1007/s00018-019-03030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.el-Bacha RS, Minn A. Drug metabolizing enzymes in cerebrovascular endothelial cells afford a metabolic protection to the brain. Cell Mol Biol (Noisy-le-grand). 1999;45:15–23. [PubMed] [Google Scholar]
- 41.Pardridge WM. Molecular biology of the blood–brain barrier. Mol Biotechnol. 2005;30(1):57–70. doi: 10.1385/MB:30:1:057. [DOI] [PubMed] [Google Scholar]
- 42.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 43.Pandit R, Chen L, Götz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 2019;S0169-409X(19). [DOI] [PubMed] [Google Scholar]
- 44.Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76(1):22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Löscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 47.Soontornmalai A, Vlaming ML, Fritschy J-M. Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood–brain barrier. Neuroscience. 2006;138(1):159–169. doi: 10.1016/j.neuroscience.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Lee JS, Paull K, Alvarez M, Hose C, Monks A, Grever M, Fojo AT, Bates SE. Rhodamine efflux patterns predict P-glycoprotein substrates in the national cancer institute drug screen. Mol Pharmacol. 1994;46:627–638. [PubMed] [Google Scholar]
- 49.Alvarez M, Paull K, Monks A, Hose C, Lee JS, Weinstein J, Grever M, Bates S, Fojo T. Generation of a drug resistance profile by quantitation of mdr-1/P-glycoprotein in the cell lines of the national cancer institute anticancer drug screen. J Clin Invest. 1995;95(5):2205–2214. doi: 10.1172/JCI117910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaudhary PM, Roninson IB. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J Natl Cancer Inst. 1993;85(8):632–639. doi: 10.1093/jnci/85.8.632. [DOI] [PubMed] [Google Scholar]
- 51.Löscher W, Luna-Tortós C, Römermann K, Fedrowitz FM. Do ATP-binding cassette transporters cause pharmacoresistance in epilepsy? problems and approaches in determining which antiepileptic drugs are affected. Curr Pharm Des. 2011;17(26):2808–2828. doi: 10.2174/138161211797440212. [DOI] [PubMed] [Google Scholar]
- 52.O’Brien FE, Dinan TG, Griffin BT, Cryan JF. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: clinical significance of in vitro and in vivo findings. Br J Pharmacol. 2012;165(2):289–312. doi: 10.1111/j.1476-5381.2011.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iorio AL, Ros M, Fantappiè O, Lucchesi M, Facchini L, Stival A, Becciani S, Guidi M, Favre C, Martino M, et al. Blood-brain barrier and breast cancer resistance protein: a limit to the therapy of cns tumors and neurodegenerative diseases. Anticancer Agents Med Chem. 2016;16(7):810–815. doi: 10.2174/1871520616666151120121928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saaby L, Trasborg J, Rasmussen MA, Holst B, Brodin B. IPEC-J2 rMdr1a, a new cell line with functional expression of rat P-glycoprotein encoded by rat mdr1a for drug screening purposes. Pharmaceutics. 2020;12(7):E673. doi: 10.3390/pharmaceutics12070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vracko R, Benditt EP. Capillary basal lamina thickening. Its relationship to endothelial cell death and replacement. J Cell Biol. 1970;47(1):281–285. doi: 10.1083/jcb.47.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab. 2017;37(10):3300–3317. doi: 10.1177/0271678X17722436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu L, Nirwane A, Yao Y. Basement membrane and blood–brain barrier. Stroke Vasc Neurol. 2019;4(2):78–82. doi: 10.1136/svn-2018-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989;180(3):487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- 59.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85(3):979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 60.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10(10):712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 61.McKee KK, Harrison D, Capizzi S, Yurchenco PD. Role of laminin terminal globular domains in basement membrane assembly. J Biol Chem. 2007;282(29):21437–21447. doi: 10.1074/jbc.M702963200. [DOI] [PubMed] [Google Scholar]
- 62.Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53(6):637–644. [DOI] [PubMed] [Google Scholar]
- 63.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood–brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 64.Frank RN, Dutta S, Mancini MA. Pericyte coverage is greater in the retinal than in the cerebral capillaries of the rat. Invest Ophthalmol Vis Sci. 1987;28:1086–1091. [PubMed] [Google Scholar]
- 65.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 66.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011;14(11):1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dalkara T, Alarcon-Martinez L. Cerebral microvascular pericytes and neurogliovascular signaling in health and disease. Brain Res. 2015;1623:3–17. doi: 10.1016/j.brainres.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 68.Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14(16):1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- 70.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kisler K, Nikolakopoulou AM, Sweeney MD, Lazic D, Zhao Z, Zlokovic BV. Acute ablation of cortical pericytes leads to rapid neurovascular uncoupling. Front Cell Neurosci. 2020;14:27. doi: 10.3389/fncel.2020.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alarcon-Martinez L, Villafranca-Baughman D, Quintero H, Kacerovsky JB, Dotigny F, Murai KK, Prat A, Drapeau P, Di Polo A. Interpericyte tunnelling nanotubes regulate neurovascular coupling [published online ahead of print, 2020 Aug 12]. Nature. 2020. doi: 10.1038/s41586-020-2589-x. [DOI] [PubMed] [Google Scholar]
- 73.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic B. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown LS, Foster CG, Courtney J-M, King NE, Howells DW, Sutherland BA. Pericytes and neurovascular function in the healthy and diseased brain. Front Cell Neurosci. 2019;13:282. doi: 10.3389/fncel.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heymans M, Figueiredo R, Dehouck L, Francisco D, Sano Y, Shimizu F, Kanda T, Bruggmann R, Engelhardt B, Winter P, et al. Contribution of brain pericytes in blood–brain barrier formation and maintenance: a transcriptomic study of cocultured human endothelial cells derived from hematopoietic stem cells. Fluids Barriers CNS. 2020;17(1):48. doi: 10.1186/s12987-020-00208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 78.van Dijk CG, Nieuweboer FE, Pei JY, Xu YJ, Burgisser P, van Mulligen E, El Azzouzi H, Duncker DJ, Verhaar MC, Cheng C. The complex mural cell: pericyte function in health and disease. Int J Cardiol. 2015;190:75–89. doi: 10.1016/j.ijcard.2015.03.258. [DOI] [PubMed] [Google Scholar]
- 79.Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartmann DA, Underly RG, Grant RI, Watson AN, Lindner V, Shih AY. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics. 2015;2(4):041402. doi: 10.1117/1.NPh.2.4.041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berthiaume AA, Hartmann DA, Majesky MW, Bhat NR, Shih AY. Pericyte structural remodeling in cerebrovascular health and homeostasis. Front Aging Neurosci. 2018;10:210. doi: 10.3389/fnagi.2018.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 83.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton J-Y, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37(2):275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- 85.Leino RL, Gerhart DZ, van Bueren AM, McCall AL, Drewes LR. Ultrastructural localization of GLUT 1 and GLUT 3 glucose transporters in rat brain. J Neurosci Res. 1997;49(5):617–626. [DOI] [PubMed] [Google Scholar]
- 86.Mercier C, Masseguin C, Roux F, Gabrion J, Scherrmann JM. Expression of P-glycoprotein (ABCB1) and Mrp1 (ABCC1) in adult rat brain: focus on astrocytes. Brain Res. 2004;1021(1):32–40. doi: 10.1016/j.brainres.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 87.Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 2008;107(3):589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol. 2018;13(1):379–394. doi: 10.1146/annurev-pathol-051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parra-Abarca J, Rivera-Ramírez N, Villa-Maldonado LF, García-Hernández U, Aguilera P, Arias-Montaño JA. Histamine H1 and H3 receptor activation increases the expression of glucose transporter 1 (GLUT-1) in rat cerebro-cortical astrocytes in primary culture. Neurochem Int. 2019;131:104565. doi: 10.1016/j.neuint.2019.104565. [DOI] [PubMed] [Google Scholar]
- 90.Davson H, Oldendorf WH. Symposium on membrane transport. Transport in the central nervous system. Proc R Soc Med. 1967;60:326–329. [PMC free article] [PubMed] [Google Scholar]
- 91.Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 92.Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood–brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25(1):25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheslow L, Alvarez JI. Glial-endothelial crosstalk regulates blood–brain barrier function. Curr Opin Pharmacol. 2016;26:39–46. doi: 10.1016/j.coph.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 94.Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles [published correction appears in Nat Neurosci. 2017;20(8):1189][published correction appears in nat neurosci. 2020]. Nat Neurosci. 2016;19(12):1619–1627. doi: 10.1038/nn.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Willis CL, Nolan CC, Reith SN, Lister T, Prior MJ, Guerin CJ, Mavroudis G, Ray DE. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004;45(4):325–337. doi: 10.1002/glia.10333. [DOI] [PubMed] [Google Scholar]
- 96.Ahishali B, Kaya M, Orhan N, Arican N, Ekizoglu O, Elmas I, Kucuk M, Kemikler G, Kalayci R, Gurses C. Effects of levetiracetam on blood-brain barrier disturbances following hyperthermia-induced seizures in rats with cortical dysplasia. Life Sci. 2010;87(19–22):609–619. doi: 10.1016/j.lfs.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 97.Kaya M, Palanduz A, Kalayci R, Kemikler G, Simsek G, Bilgic B, Ahishali B, Arican N, Kocyildiz ZC, Elmas I, et al. Effects of lipopolysaccharide on the radiation-induced changes in the blood–brain barrier and the astrocytes. Brain Res. 2004;1019(1–2):105–112. doi: 10.1016/j.brainres.2004.05.102. [DOI] [PubMed] [Google Scholar]
- 98.Kalayci R, Kaya M, Uzun H, Bilgic B, Ahishali B, Arican N, Elmas İ, Küçük M. Influence of hypercholesterolemia and hypertension on the integrity of the blood–brain barrier in rats. Int J Neurosci. 2009;119(10):1881–1904. doi: 10.1080/14647270802336650. [DOI] [PubMed] [Google Scholar]
- 99.Krum JM, Kenyon KL, Rosenstein JM. Expression of blood–brain barrier characteristics following neuronal loss and astroglial damage after administration of anti-thy-1 immunotoxin. Exp Neurol. 1997;146(1):33–45. doi: 10.1006/exnr.1997.6528. [DOI] [PubMed] [Google Scholar]
- 100.Kubotera H, Ikeshima-Kataoka H, Hatashita Y, Allegra Mascaro AL, Pavone FS, Inoue T. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci Rep. 2019;9(1):1263. doi: 10.1038/s41598-018-37419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122(7):2454–2468. doi: 10.1172/jci60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29(11):1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 103.Kabba JA, Xu Y, Christian H, Ruan W, Chenai K, Xiang Y, Zhang L, Saavedra JM, Microglia: PT. Housekeeper of the central nervous system. Cell Mol Neurobiol. 2018;38(1):53–71. doi: 10.1007/s10571-017-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bowyer JF, Sarkar S, Tranter KM, Hanig JP, Miller DB, O’Callaghan JP. Vascular-directed responses of microglia produced by methamphetamine exposure: indirect evidence that microglia are involved in vascular repair? J Neuroinflammation. 2016;13(1):64. doi: 10.1186/s12974-016-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MH. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 2016;131(3):347–363. doi: 10.1007/s00401-015-1524-y. [DOI] [PubMed] [Google Scholar]
- 106.Thurgur H, Pinteaux E. Microglia in the neurovascular unit: blood–brain barrier–microglia interactions after central nervous system disorders. Neuroscience. 2019;405:55–67. doi: 10.1016/j.neuroscience.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 107.Sumi N, Nishioku T, Takata F, Matsumoto J, Watanabe T, Shuto H, Yamauchi A, Dohgu S, Kataoka Y. Lipopolysaccharide-activated microglia induce dysfunction of the blood–brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell Mol Neurobiol. 2010;30(2):247–253. doi: 10.1007/s10571-009-9446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28(4):202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 109.Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, Karten HJ, Lyden PD, Kleinfeld D. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J Neurosci. 2009;29(46):14553–14570. doi: 10.1523/JNEUROSCI.3287-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tong XK, Hamel E. Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer’s disease. Neuroscience. 1999;92(1):163–175. doi: 10.1016/s0306-4522(98)00750-7. [DOI] [PubMed] [Google Scholar]
- 111.Vaucher E, Tong X-K, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol. 2000;421(2):161–171. [PubMed] [Google Scholar]
- 112.Klein B, Kuschinsky W, Schröck H, Vetterlein F. Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am J Physiol. 1986;251(6):H1333–H1340. doi: 10.1152/ajpheart.1986.251.6.H1333. [DOI] [PubMed] [Google Scholar]
- 113.Tontsch U, Bauer H-C. Glial cells and neurons induce blood-brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res. 1991;539(2):247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- 114.Kaplan L, Chow BW, Gu C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci. 2020;21(8):416–432. doi: 10.1038/s41583-020-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lacoste B, Comin CH, Ben-Zvi A, Kaeser PS, Xu X, Costa Lda L, Gu C. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron. 2014;83(5):1117–1130. doi: 10.1016/j.neuron.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Canfield SG, Stebbins MJ, Faubion MG, Gastfriend BD, Palecek SP, Shusta EV. An isogenic neurovascular unit model comprised of human induced pluripotent stem cell-derived brain microvascular endothelial cells, pericytes, astrocytes, and neurons. Fluids Barriers CNS. 2019;16(1):25. doi: 10.1186/s12987-019-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429(1):47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 119.Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta. 2009;1788(4):892–910. doi: 10.1016/j.bbamem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 120.Lochhead JJ, Yang J, Ronaldson PT, Davis TP, Structure F. Regulation of the blood-brain barrier tight junction in central nervous system disorders. Front. Physiol. 2020;11:914. doi: 10.3389/fphys.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147(1):185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wolburg H, Lippoldt A. Tight junctions of the blood–brain barrier. Vascul Pharmacol. 2002;38(6):323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 123.Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98(13):3699–3707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- 124.Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers. 2016;4(1):e1154641. doi: 10.1080/21688370.2016.1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6):1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57(6):883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 127.Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol. 2010;2010:402593. doi: 10.1155/2010/402593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209(4):493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bélanger M, Asashima T, Ohtsuki S, Yamaguchi H, Ito S, Terasaki T. Hyperammonemia induces transport of taurine and creatine and suppresses claudin-12 gene expression in brain capillary endothelial cells in vitro. Neurochem Int. 2007;50(1):95–101. doi: 10.1016/j.neuint.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 130.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. J Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mineta KY, Yamamoto YY, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K. Predicted expansion of the claudin multigene family. FEBS. 2011;585(4):606–612. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 132.Winkler L, Blasig R, Breitkreuz-Korff O, Berndt P, Dithmer S, Helms HC, Puchkov D, Devraj K, Kaya M, Qin Z, et al. Tight junctions in the blood–brain barrier promote edema formation and infarct size in stroke – ambivalent effects of sealing proteins. J Cereb Blood Flow Metab. 2020:0271678X2090468. 271678 × 20904687. doi: 10.1177/0271678X20904687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist’s view. Brain Res Brain Res Rev. 2003;42(3):221–242. doi: 10.1016/s0165-0173(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 134.Greene C, Campbell M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers. 2016;4(1):e1138017. doi: 10.1080/21688370.2015.1138017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Greene C, Hanley N, Campbell M. Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS. 2019;16(1):3. doi: 10.1186/s12987-019-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Castro Dias M, Coisne C, Baden P, Enzmann G, Garrett L, Becker L, Hölter SM, Angelis MH, Deutsch U, Engelhardt B. Claudin-12 is not required for blood–brain barrier tight junction function. Fluids Barriers CNS. 2019;16(1):30. doi: 10.1186/s12987-019-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110:1603–1613. [DOI] [PubMed] [Google Scholar]
- 138.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136(2):399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saitou M, Furuse M, Sasaki H, Schulzke J-D, Fromm M, Takano H, Noda T, Tsukita S, Nelson WJ. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11(12):4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morgan L, Shah B, Rivers LE, Barden L, Groom AJ, Chung R, Higazi D, Desmond H, Smith T, Staddon JM. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience. 2007;147(3):664–673. doi: 10.1016/j.neuroscience.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 141.Erikson K, Tuominen H, Vakkala M, Liisanantti JH, Karttunen T, Syrjälä H, Ala-Kokko TI. Brain tight junction protein expression in sepsis in an autopsy series. Crit Care. 2020;24(1):385. doi: 10.1186/s13054-020-03101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood–brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2006;50(1):202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 143.Esen F, Senturk E, Ozcan PE, Ahishali B, Arican N, Orhan N, Ekizoglu O, Kucuk M, Kaya M. Intravenous immunoglobulins prevent the breakdown of the blood-brain barrier in experimentally induced sepsis. Crit Care Med. 2012;40(4):1214–1220. doi: 10.1097/CCM.0b013e31823779ca. [DOI] [PubMed] [Google Scholar]
- 144.Avtan SM, Kaya M, Orhan N, Arslan A, Arican N, Toklu AS, Gürses C, Elmas I, Kucuk M, Ahishali B. The effects of hyperbaric oxygen therapy on blood–brain barrier permeability in septic rats. Brain Res. 2011;1412:63–72. doi: 10.1016/j.brainres.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 145.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172(2):521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-A expression during blood–brain barrier breakdown. Acta Neuropathol. 2008;115(6):635–642. doi: 10.1007/s00401-008-0364-4. [DOI] [PubMed] [Google Scholar]
- 147.Kummer D, Ebnet K. Junctional adhesion molecules (JAMs): the JAM-integrin connection. Cells. 2018;7(4):25. doi: 10.3390/cells7040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147(6):1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fanning AS, Anderson JM. Zonula occludens-1 and −2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann NY Acad Sci. 2009;1165(1):113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015;208(6):821–838. doi: 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arican N, Kaya M, Kalayci R, Uzun H, Ahishali B, Bilgic B, Elmas I, Kucuk M, Gurses C, Uzun M. Effects of lipopolysaccharide on blood–brain barrier permeability during pentylenetetrazole-induced epileptic seizures in rats. Life Sci. 2006;79(1):1–7. doi: 10.1016/j.lfs.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 152.Fujii M, Duris K, Altay O, Soejima Y, Sherchan P, Zhang JH. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem Int. 2012;60(3):327–333. doi: 10.1016/j.neuint.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu J, Wang F, Liu S, Du J, Hu X, Xiong J, Fang R, Chen W, Sun J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci. 2017;381:176–181. doi: 10.1016/j.jns.2017.08.3235. [DOI] [PubMed] [Google Scholar]
- 154.Tanaka K, Matsumoto S, Yamada T, Yamasaki R, Suzuki M, Kido MA, Kira J-I. Reduced post-ischemic brain injury in transient receptor potential vanilloid 4 knockout mice. Front Neurosci. 2020;14:453. Published 2020 May 12. doi: 10.3389/fnins.2020.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11(5):554–560. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 156.Dejana E, Giampietro C. Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol. 2012;19(3):218–233. doi: 10.1097/moh.0b013e3283523e1c. [DOI] [PubMed] [Google Scholar]
- 157.Banks WA, Greig NH. Small molecules as central nervous system therapeutics: old challenges, new directions, and a philosophic divide. Future Med Chem. 2019;11(6):489–493. doi: 10.4155/fmc-2018-0436. [DOI] [PubMed] [Google Scholar]
- 158.Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90–105. [DOI] [PubMed] [Google Scholar]
- 159.Pardridge WM. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McLay RN. Granulocyte-macrophage colony-stimulating factor crosses the blood-- brain and blood--spinal cord barriers. Brain. 1997;120(11):2083–2091. doi: 10.1093/brain/120.11.2083. [DOI] [PubMed] [Google Scholar]
- 161.Lajoie JM, Shusta EV. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu Rev Pharmacol Toxicol. 2015;55(1):613–631. doi: 10.1146/annurev-pharmtox-010814-124852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kusuhara H, Sugiyama Y. Efflux transport systems for drugs at the blood–brain barrier and blood–cerebrospinal fluid barrier (part 1). Drug Discov Today. 2001;6(3):150–156. doi: 10.1016/s1359-6446(00)01632-9. [DOI] [PubMed] [Google Scholar]
- 163.Kusuhara H, Sugiyama Y. Efflux transport systems for drugs at the blood–brain barrier and blood–cerebrospinal fluid barrier (part 2). Drug Discov Today. 2001;6(4):206–212. doi: 10.1016/s1359-6446(00)01643-3. [DOI] [PubMed] [Google Scholar]
- 164.Hoosain FG, Choonara YE, Tomar LK, Kumar P, Tyagi C, Du Toit LC, Pillay V. bypassing p-glycoprotein drug efflux mechanisms: possible applications in pharmacoresistant schizophrenia therapy. BioMed Research International. 2015;2015:484963. doi: 10.1155/2015/484963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mittapalli RK, Manda VK, Adkins CE, Geldenhuys WJ, Lockman PR. Exploiting nutrient transporters at the blood–brain barrier to improve brain distribution of small molecules. Ther Deliv. 2010;1(6):775–784. doi: 10.4155/tde.10.76. [DOI] [PubMed] [Google Scholar]
- 166.Moura RP, Martins C, Pinto S, Sousa F, Sarmento B. Blood-brain barrier receptors and transporters: an insight on their function and how to exploit them through nanotechnology. Expert Opin Drug Deliv. 2019;16(3):271–285. doi: 10.1080/17425247.2019.1583205. [DOI] [PubMed] [Google Scholar]
- 167.Villaseñor R, Lampe J, Schwaninger M, Collin L. Intracellular transport and regulation of transcytosis across the blood–brain barrier. Cell Mol Life Sci. 2019;76(6):1081–1092. doi: 10.1007/s00018-018-2982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kang Y-S, Pardridge WM. Brain delivery of biotin bound to a conjugate of neutral avidin and cationized human albumin. Pharm Res. 1994;11(9):1257–1264. doi: 10.1023/a:1018982125649. [DOI] [PubMed] [Google Scholar]
- 169.Fong CW. Permeability of the blood–brain barrier: molecular mechanism of transport of drugs and physiologically important compounds. J Membr Biol. 2015;248(4):651–669. doi: 10.1007/s00232-015-9778-9. [DOI] [PubMed] [Google Scholar]
- 170.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312(5990):162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 171.Méresse S, Delbart C, Fruchart J-C, Cecchelli R. Low-density lipoprotein receptor on endothelium of brain capillaries. J Neurochem. 1989;53(2):340–345. doi: 10.1111/j.1471-4159.1989.tb07340.x. [DOI] [PubMed] [Google Scholar]
- 172.Deane R. IgG-assisted age-dependent clearance of alzheimer’s amyloid peptide by the blood-brain barrier neonatal fc receptor. J Neurosci. 2005;25(50):11495–11503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang AC, Stevens MY, Chen MB, Lee DP, Stähli D, Gate D, Contrepois K, Chen W, Iram T, Zhang L, et al. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583(7816):425–430. doi: 10.1038/s41586-020-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kulakova A, Indrakumar S, Sønderby P, Gentiluomo L, Streicher W, Roessner D, Frieß W, Peters G, Harris P. Small angle X-ray scattering and molecular dynamic simulations provide molecular insight for stability of recombinant human transferrin. J Struct Biol X. 2019;4:100017. doi: 10.1016/j.yjsbx.2019.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ugur Yilmaz C, Emik S, Orhan N, Temizyurek A, Atis M, Akcan U, Khodadust R, Arican N, Kucuk M, Gurses C, et al. Targeted delivery of lacosamide-conjugated gold nanoparticles into the brain in temporal lobe epilepsy in rats. Life Sci. 2020;257:118081. doi: 10.1016/j.lfs.2020.118081. [DOI] [PubMed] [Google Scholar]
- 176.Farrell CL, Pardridge WM. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci U S A. 1991;88(13):5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tsuji A, Tamai II. Carrier-mediated or specialized transport of drugs across the blood–brain barrier. Adv Drug Deliv Rev. 1999;36(2–3):277–290. doi: 10.1016/s0169-409x(98)00084-2. [DOI] [PubMed] [Google Scholar]
- 178.Hawkins RA, O’Kane RL, Simpson IA, Viña JR. Structure of the blood–brain barrier and its role in the transport of amino acids. . The Journal of Nutrition. 2006;136(1):218S–226S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 179.Brasnjevic I, Steinbusch HW, Schmitz C, Martinez-Martinez P. Delivery of peptide and protein drugs over the blood–brain barrier. Prog Neurobiol. 2009;87(4):212–251. doi: 10.1016/j.pneurobio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 180.Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Betz AL, Bowman PD, Goldstein GW. Hexose transport in microvascular endothelial cells cultured from bovine retina. Exp Eye Res. 1983;36(2):269–277. doi: 10.1016/0014-4835(83)90011-8. [DOI] [PubMed] [Google Scholar]
- 182.Qutub AA, Hunt CA. Glucose transport to the brain: a systems model. Brain Res Brain Res Rev. 2005;49(3):595–617. doi: 10.1016/j.brainresrev.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 183.Koepsell H. Glucose transporters in brain in health and disease. Pflugers Arch. 2020. doi: 10.1007/s00424-020-02441-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Veys K, Fan Z, Ghobrial M, Bouché A, Garcia-Caballero M, Vriens K, Conchinha NV, Seuwen A, Schlegel F, Gorski T, et al. Role of the GLUT1 glucose transporter in postnatal cns angiogenesis and blood-brain barrier integrity. Circ Res. 2020;127(4):466–482. doi: 10.1161/CIRCRESAHA.119.316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vorbrodt AW. Ultrastructural cytochemistry of blood-brain barrier endothelia. Prog Histochem Cytochem. 1988;18(3):1–99. doi: 10.1016/s0079-6336(88)80001-9. [DOI] [PubMed] [Google Scholar]
- 186.O’Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood–brain barrier Na–K–Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2006;26(10):1234–1249. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- 187.Taylor CJ, Nicola PA, Wang S, Barrand MA, Hladky SB. Transporters involved in regulation of intracellular pH in primary cultured rat brain endothelial cells. J Physiol. 2006;576(3):769–785. doi: 10.1113/jphysiol.2006.117374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Virgintino D, Robertson D, Errede M, Benagiano V, Tauer U, Roncali L, Bertossi M. Expression of caveolin-1 in human brain microvessels. Neuroscience. 2002;115(1):145–152. doi: 10.1016/s0306-4522(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 189.Parton RG. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J Cell Sci. 2006;119(5):787–796. doi: 10.1242/jcs.02853. [DOI] [PubMed] [Google Scholar]
- 190.Sánchez FA, Rana R, Kim DD, Iwahashi T, Zheng R, Lal BK, Gordon DM, Meininger CJ, Durán WN. Internalization of eNOS and NO delivery to subcellular targets determine agonist-induced hyperpermeability. Proc Natl Acad Sci U S A. 2009;106(16):6849–6853. doi: 10.1073/pnas.0812694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chidlow JH, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovascular Research. 2010;86(2):219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood. 2007;109(4):1515–1523. doi: 10.1182/blood-2006-07-034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao LN, Yang ZH, Liu YH, Ying HQ, Zhang H, Xue YX. Vascular endothelial growth factor increases permeability of the blood–tumor barrier via caveolae-mediated transcellular pathway. J Mol Neurosci. 2011;44(2):122–129. doi: 10.1007/s12031-010-9487-x. [DOI] [PubMed] [Google Scholar]
- 194.Gu Y, Zheng G, Xu M, Li Y, Chen X, Zhu W, Tong Y, Chung SK, Liu KJ, Shen J. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J Neurochem. 2012;120(1):147–156. doi: 10.1111/j.1471-4159.2011.07542.x. [DOI] [PubMed] [Google Scholar]
- 195.Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish C, Gu C. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94(3):581–594.e5. doi: 10.1016/j.neuron.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 197.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature. 2014;509(7501):507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang Z, Zheng Y, Wang F, Zhong J, Zhao T, Xie Q, Zhu T, Ma F, Tang Q, Zhou B, et al. Mfsd2a and Spns2 are essential for sphingosine-1-phosphate transport in the formation and maintenance of the blood-brain barrier. Sci Adv. 2020;6(22):eaay8627. doi: 10.1126/sciadv.aay8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 200.Chow BW, Nuñez V, Kaplan L, Granger AJ, Bistrong K, Zucker HL, Kumar P, Sabatini BL, Gu C. Caveolae in CNS arterioles mediate neurovascular coupling. Nature. 2020;579(7797):106–110. doi: 10.1038/s41586-020-2026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Atış M, Akcan U, Uğur Yılmaz C, Orhan N, Düzgün P, Deniz Ceylan U, Arıcan N, Karahüseyinoğlu S, Nur Şahin G, Ahıshalı B, et al. Effects of methyl-beta-cyclodextrin on blood-brain barrier permeability in angiotensin II-induced hypertensive rats. Brain Res. 2019;1715:148–155. doi: 10.1016/j.brainres.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 202.Miller DS. Regulation of ABC transporters at the blood-brain barrier. Clin Pharmacol Ther. 2015;97(4):395–403. doi: 10.1002/cpt.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, Scherrmann JM, De Waziers I, Declèves X. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem. 2008;107(6):1518–1528. doi: 10.1111/j.1471-4159.2008.05720.x. [DOI] [PubMed] [Google Scholar]
- 204.Abdullahi W, Davis TP, Ronaldson PT. Functional expression of p-glycoprotein and organic anion transporting polypeptides at the blood-brain barrier: understanding transport mechanisms for improved cns drug delivery? AAPS. 2017;19(4):931–939. doi: 10.1208/s12248-017-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Morris ME, Rodriguez-Cruz V, Felmlee MA. SLC and ABC Transporters: expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. Aaps J. 2017;19(5):1317–1331. doi: 10.1208/s12248-017-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kaya M, Orhan N, Karabacak E, Bahceci MB, Arican N, Ahishali B, Kemikler G, Uslu A, Cevik A, Yilmaz CU, et al. Vagus nerve stimulation inhibits seizure activity and protects blood–brain barrier integrity in kindled rats with cortical dysplasia. Life Sci. 2013;92(4–5):289–297. doi: 10.1016/j.lfs.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 207.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109(2):181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 208.Reijerkerk A, Kooij G, van der Pol SM, Leyen T, van Het Hof B, Couraud PO, Vivien D, Dijkstra CD, de Vries HE. Tissue-type plasminogen activator is a regulator of monocyte diapedesis through the brain endothelial barrier. J Immunol. 2008;181(5):3567–3574. doi: 10.4049/jimmunol.181.5.3567. [DOI] [PubMed] [Google Scholar]
- 209.Carman CV. Mechanisms for transcellular diapedesis: probing and pathfinding by `invadosome-like protrusions’. J Cell Sci. 2009;122(17):3025–3035. doi: 10.1242/jcs.047522. [DOI] [PubMed] [Google Scholar]
- 210.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol. 2012;33(12):579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 211.Abadier M, Haghayegh Jahromi N, Cardoso Alves L, Boscacci R, Vestweber D, Barnum S, Deutsch U, Engelhardt B, Lyck R. Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood-brain barrier. Eur J Immunol. 2015;45(4):1043–1058. doi: 10.1002/eji.201445125. [DOI] [PubMed] [Google Scholar]
- 212.Lutz SE, Smith JR, Kim DH, Olson CVL, Ellefsen K, Bates JM, Gandhi SP, Agalliu D. Caveolin1 Is required for th1 cell infiltration, but not tight junction remodeling, at the blood-brain barrier in autoimmune neuroinflammation. Cell Rep. 2017;21(8):2104–2117. doi: 10.1016/j.celrep.2017.10.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bombardi C, Grandis A, Chiocchetti R, Lucchi ML, Callegari E, Bortolami R. Membrane-transport systems in the fenestrated capillaries of the area postrema in rat and calf. Anat Rec A Discov Mol Cell Evol Biol. 2004;279A(1):664–670. doi: 10.1002/ar.a.20041. [DOI] [PubMed] [Google Scholar]
- 214.Miyata S. New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front Neurosci. 2015;9:390. doi: 10.3389/fnins.2015.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kiecker C. The origins of the circumventricular organs. J Anat. 2018;232(4):540–553. doi: 10.1111/joa.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mimee A, Smith PM, Ferguson AV. Circumventricular organs: targets for integration of circulating fluid and energy balance signals? Physiol Behav. 2013;121:96–102. doi: 10.1016/j.physbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 217.van Vliet EA, Aronica E, Gorter JA. Blood–brain barrier dysfunction, seizures and epilepsy. Semin Cell Dev Biol. 2015;38:26–34. doi: 10.1016/j.semcdb.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 218.Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 2018;315(3):C343–C356. doi: 10.1152/ajpcell.00095.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bell L, Koeniger T, Tacke S, Kuerten S. Characterization of blood–brain barrier integrity in a B-cell-dependent mouse model of multiple sclerosis. Histochem Cell Biol. 2019;151(6):489–499. doi: 10.1007/s00418-019-01768-6. [DOI] [PubMed] [Google Scholar]
- 220.Cash A, Theus MH. Mechanisms of blood–brain barrier dysfunction in traumatic brain injury. Int J Mol Sci. 2020;21(9):3344. doi: 10.3390/ijms21093344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Esen F, Erdem T, Aktan D, Orhan M, Kaya M, Eraksoy H, Cakar N, Telci L. Effect of magnesium sulfate administration on blood–brain barrier in a rat model of intraperitoneal sepsis: a randomized controlled experimental study. Crit Care. 2005;9(1):R18–23. doi: 10.1186/cc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Danielski LG, Giustina AD, Badawy M, Barichello T, Quevedo J, Dal-Pizzol F, Petronilho F. Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol Neurobiol. 2018;55(2):1045–1053. doi: 10.1007/s12035-016-0356-7. [DOI] [PubMed] [Google Scholar]
- 224.Kaya M, Gulturk S, Elmas I, Kalayci R, Arican N, Kocyildiz ZC, Kucuk M, Yorulmaz H, Sivas A. The effects of magnesium sulfate on blood-brain barrier disruption caused by intracarotid injection of hyperosmolar mannitol in rats. Life Sci. 2004;76(2):201–212. doi: 10.1016/j.lfs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 225.Sedeyn JC, Wu H, Hobbs RD, Levin EC, Nagele RG, Venkataraman V. Histamine induces Alzheimer’s disease-like blood brain barrier breach and local cellular responses in mouse brain organotypic cultures. Biomed Res Int. 2015;2015:937148. doi: 10.1155/2015/937148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rich M, Whitsitt Q, Lubin F, Bolding M. A benchtop approach to the location specific blood brain barrier opening using focused ultrasound in a rat model. J Vis Exp. 2020;(160). doi: 10.3791/61113. [DOI] [PubMed] [Google Scholar]
- 227.Colarusso A, Maroccia Z, Parrilli E, Germinario EAP, Fortuna Loizzo A, Loizzo S, Ricceri ML, Tutino ML, Fiorentin C, Fabbri A. Cnf1 variants endowed with the ability to cross the blood–brain barrier: a new potential therapeutic strategy for glioblastoma. Toxins (Basel). 2020;12(5):291. doi: 10.3390/toxins12050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Naqvi S, Panghal A, Flora SJS. Nanotechnology: a promising approach for delivery of neuroprotective drugs. Front Neurosci. 2020;14:494. doi: 10.3389/fnins.2020.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ogawa K, Kato N, Kawakami S. Recent strategies for targeted brain drug delivery. Chem Pharm Bull (Tokyo). 2020;68(7):567–582. doi: 10.1248/cpb.c20-00041. [DOI] [PubMed] [Google Scholar]


