Interferon gamma (IFNγ)-targeted immunotherapy with emapalumab, a fully human monoclonal antibody, was recently approved by the US Food and Drug Administration for the treatment of adult and pediatric patients with primary hemophagocytic lymphohistiocytosis (HLH) who have refractory, recurrent or progressive disease or intolerance with conventional HLH therapy.1,2 Moreover, emapalumab has shown promising efficacy in the treatment of patients with graft failure (GF) requiring a second allogeneic hematopoietic stem cell transplantation (HSCT).3 Interestingly, there is growing evidence to support common pathophysiolological mechanisms between HLH and immune-mediated GF, highlighting the key role of IFNγ in both conditions.3
IFNγ is a cytokine produced by macrophages and lymphocytes that plays a critical role in both innate and adaptive immune responses. In patients with complete IFNγ receptor deficiency or developing anti-IFNγ autoantibodies, the absence of IFNγ biological activity leads to an increased susceptibility to specific infections, such as mycobacterial infections.4,5 For this reason, latent tubercolosis (TB) infection represented an exclusion criterion in clinical trials investigating the therapeutic role of emapalumab in primary (clinicaltrials.gov identifiers: NCT03312751; NCT01818492) and secondary (clinicaltrials. gov identifiers: NCT03311854) HLH.
Here we report a case of secondary HLH-related GF in the context of HLA-haploidentical HSCT treated with emapalumab in the presence of concomitant life-threatening infections including disseminated bacille Calmette- Guérin disease (BCGitis).
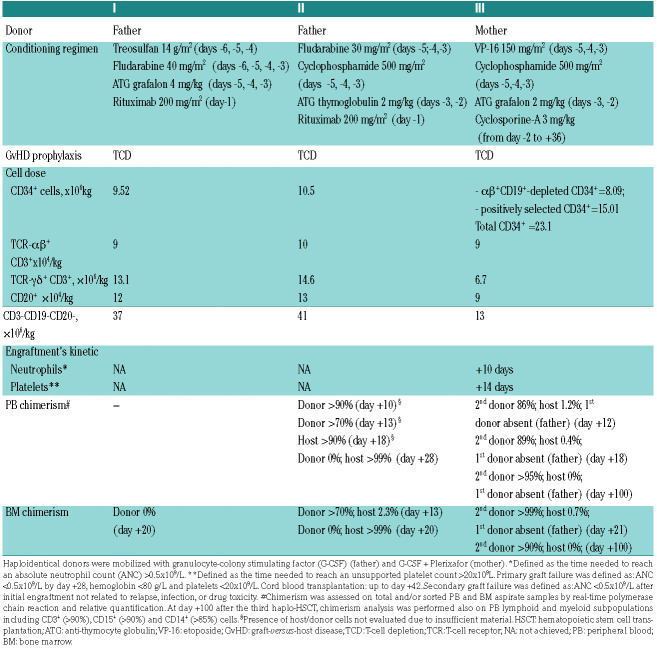
Table 1.
Characteristics of the three HLA-haploidentical (haplo)-hematopoietic stem cell transplantations performed.
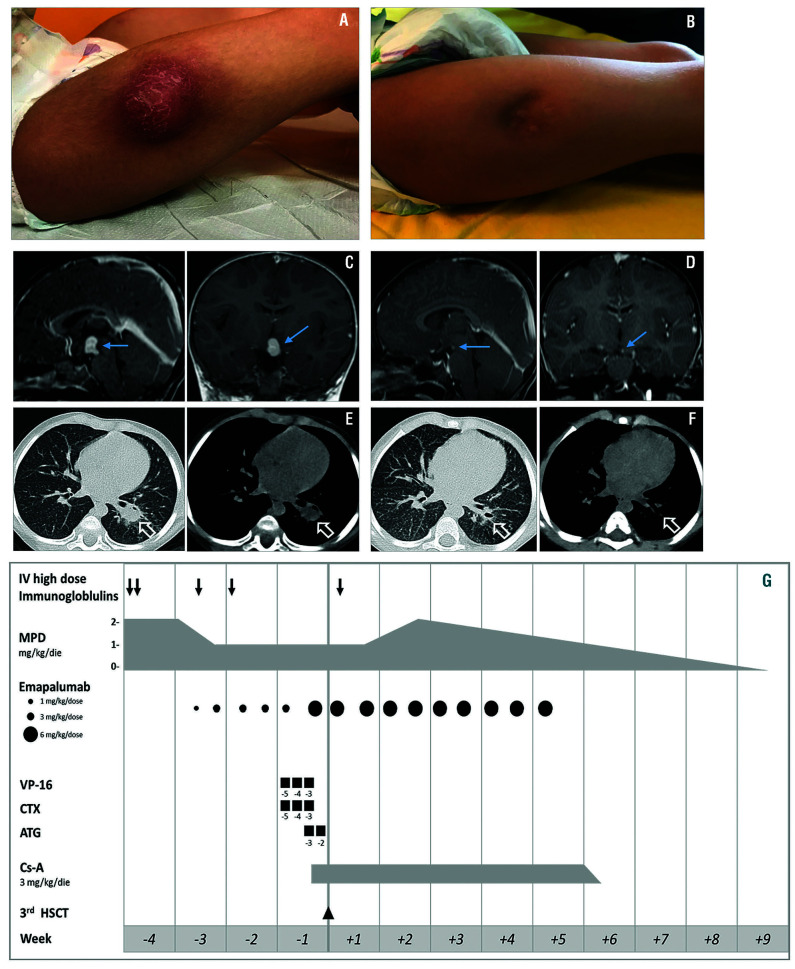
Figure 1.
Clinical, brain magnetic resonance imaging (MRI) and chest computed tomography (CT) images of tubercolosis (TB) and fungal infections, and schematic summary of treatments. (A) Localized abscesses on lower limbs at the sites of polyethylene glycol-modified adenosine deaminase injection before surgical incision. (B) Localized abscesses on lower limbs at day +100 after the third haplo-HSCT. Surgical incision was performed before HSC-gene therapy (GT). (C) Brain MRI at diagnosis of intracerebral TB granuloma. Sagittal and coronal post-contrast T1W brain MRI images show a hypothalamic contrast enhancing lesion suggestive for tuberculous granuloma. (D) Brain MRI at day +100 after the third haplo-HSCT showing marked reduction of the tuberculous granuloma. (E) CT images of the lungs at time of aspergillosis diagnosis after the second haplo-HSCT. Axial chest CT images with lung window (left) and mediastinal window (right) show a left lower lobe pulmonary mass compatible with pulmonary aspergillosis. (F) CT images of the lungs at day +100 after the third haplo-HSCT showing marked improvement. (G) Schematic representation of the treatment given before and during the third haplo-HSCT to control secondary HLH and prevent graft failure. MPD: methylprednisolone; VP-16: etoposide; CTX: cyclophosphamide; ATG: anti-thymocyte globulin; Cs-A: cyclosporine-A.
The patient is a 4-year old girl with severe combined immunodeficiency caused by adenosine deaminase deficiency (ADA-SCID) referred to our institution for ex vivo hematopoietic stem cell (HSC)-gene therapy (GT) with Strimvelis® in the absence of a human leukocyte antigen (HLA)-identical sibling donor.6 She was in poor clinical condition at presentation with bilateral abscesses on lower limbs, corresponding to the sites of polyethylene glycol-modified adenosine deaminase (PEG-ADA) injections (Figure 1A). Presence of Mycobacterium bovis was identified by direct next-generation sequencing performed on the material drained from the limb abscesses using the Deeplex®Myc-TB, an all-in-one test for specieslevel identification, genotyping and prediction of antibiotic resistance in Mycobacterium tuberculosis complex. Results were further confirmed by standard mycobacteriology procedure and whole genome sequencing of the strain. Moreover, magnetic resonance imaging (MRI) documented an intracerebral granuloma (Figure 1C) and vertebral osteolytic lesions, that, along with the limb abscesses, led to the diagnosis of disseminated BCGitis, as reactivation of the BCG vaccine strain received at birth. She was treated with surgical incision of the abscesses and anti-TB treatment (four-drug regimen [isoniazid, rifampicin, ethambutol, moxifloxacin] for 12 months as intensive phase; two-drug regimen [isoniazid, rifampicin] for 6 months as continuation phase). After 7 months of anti-TB treatment, at resolution of cutaneous abscesses and with residual encephalic mycobacterial lesions, the patient was considered eligible and treated with Strimvelis®. Failure of engraftment of gene-corrected HSC was declared at day +90 and enzyme replacement therapy (ERT) was resumed.
Subsequently, due to the lack of a matched unrelated donor and after ERT withdrawal, the patient received a first HLA-haploidentical HSCT after αβ+ T-cell and CD19+ B-cell depletion (TCD) from the father after reduced toxicity conditioning regimen (Table 1).7 However, HSCT failed due to primary GF, likely related to concomitant adenovirus reactivation in the periengraftment phase. A second paternal haplo-HSCT was performed after reduced intensity conditioning employing exceeding HSC cryopreserved from the first transplant and infused on d+31 post first HSCT (Table 1).
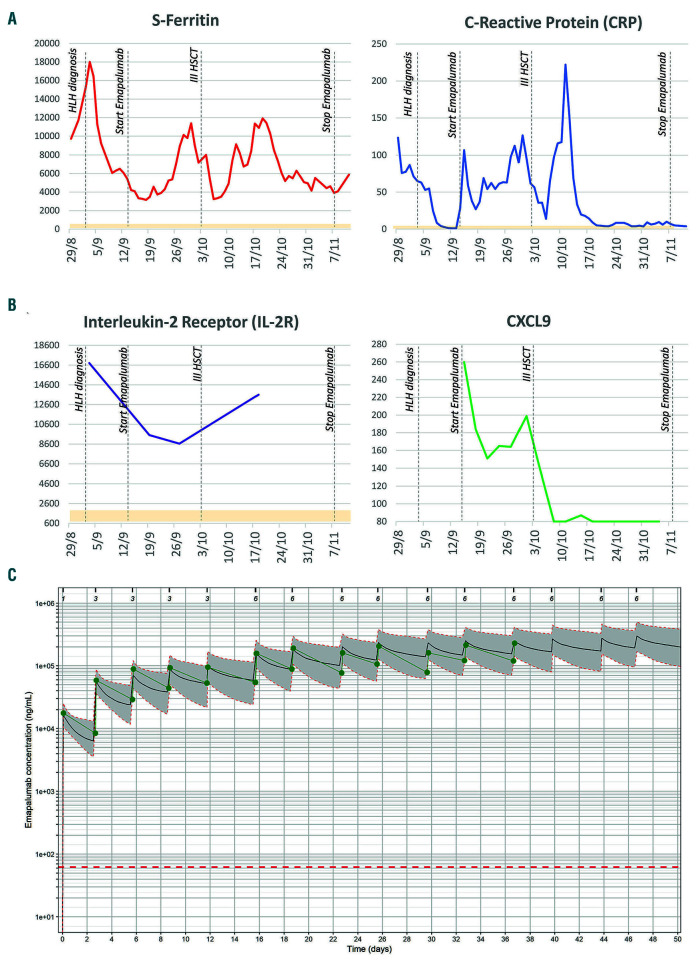
On day +13 after the second haplo-HSCT, the patient showed persistent fever, hepatosplenomegaly, high levels of triglycerides (383 mg/dL) and markedly elevated inflammatory markers such as ferritin (18,000 mg/dL) and soluble IL2 receptor (16,809 pg/mL; reference values 600-2,000) (Figure 2A and B). Donor chimerism on both peripheral blood (PB) and bone marrow (BM) was documented on days +10 and +13, respectively; however, it was followed by secondary GF with complete loss of donor engraftment (day +18). BM morphology showed hypocellularity with features of active hemophagocytosis. A secondary HLH was diagnosed based on 6 out of 8 HLH-2004 criteria,8 likely triggered by concurrent infections, including Stenotrophomonas maltophilia bacteremia, invasive pulmonary aspergillosis (Figure 1E) and adenovirus reactivation. Treatment with methylprednisolone (2 mg/kg/day) and high-dose immunoglobulins was started.
In order to control HLH and reduce the possibility of GF after a third HSCT, compassionate use of emapalumab was requested and approved for this severely immunocompromised patient unable to tolerate standard HLH immunochemotherapy.
At time of emapalumab initiation, adenovirus reactivation and invasive pulmonary aspergillosis were active: adenovirus was detected both in plasma and stool with 1,940 copies/mL and >1,000,000 copies/mL, respectively; while galactomannan levels were above the upper limit of detection (index >6). Intensive antimicrobial treatment included antivirals (intravenous cidofovir, later switched to oral brincidofovir) and antifungals (voriconazole plus anidulafungin, later switched to liposomial amphotericine- B plus anidulafungin to minimize drugs interactions). Conversely, rifampicin and isoniazid were continued as secondary prophylaxis to avoid the risk of reactivation of TB, which was regularly monitored through blood cultures, fecal polymerase chain reaction (PCR) for Mycobacterium bovis and brain MRI. Emapalumab was administered intravenously twice a week for a total of 15 infusions with the objective of prompt tapering of glucocorticoids. After the first dose at 1 mg/kg, emapalumab dose was increased to 3 mg/kg: the laboratory parameters, while not worsening, did not show any satisfactory improvement. Thereafter, emapalumab dose was increased to 6 mg/kg, based on deterioration of inflammatory parameters (e.g., ferritin, C-reactive protein) (Figure 2A). IFNγ levels were not particularly elevated in this patient, as documented by CXCL9 values around 260 pg/mL at start of emapalumab treatment (Figure 2B). Nonetheless, the pharmacokinetics (PK) of emapalumab (Figure 2C) was affected by target-mediated drug disposition, documenting high IFNγ production and requiring emapalumab dose increase. CXCL9 progressively decreased to levels below 80 pg/mL, documenting complete neutralization of IFNγ. By the time of the third haplo-HSCT, glucocorticoids dose was reduced to approximately 50% of the starting dose, while maintaining a good clinical control of HLH. The patient, despite the occasional temporary worsening of a few HLH laboratory parameters, did not progress into overt HLH, likely due to the neutralization of IFNγ.
The patient received the third TCD haplo-HSCT from the mother after a total of six emapalumab doses. Conditioning regimen included chemotherapeutic agents active against HLH,8 while cyclosporine-A (Cs-A) was added for graft-rejection prevention (Table 1). Anti-HLA antibodies were undetectable before and after HSCT. Neutrophil and platelet engraftment occurred on days +10 and +14, respectively. BM aspirate at day +21 was normo-cellulated with no evidence of hemophagocytosis and showed full donor chimerism. Emapalumab was administered until achievement of sustained donor engraftment (day +28) (Figure 1G). No adverse events occurred. HLH clinical and laboratory parameters progressively improved (Figure 2A and B) allowing Cs-A and steroids tapering and ultimately discontinuation (days +36 and +59, respectively) (Figure 1G).
Remarkably, during blockade of IFN-γ with emapalumab, infections remained stable or improved with antimicrobial medications. At the end of treatment, no sign of reactivation of cutaneous TB lesions was observed (Figure 1B) and the brain imaging showed improvement of the lesions documented prior to emapalumab (Figure 1D). Bacteremia resolved and invasive pulmonary Aspergillosis improved with favorable radiological evolution (Figure 1F) and reduction of galactomannan up to negativity at day +132 post HSCT. After 8-week treatment with emapalumab, at day +39 post HSCT, adenovirus became undetectable in plasma. Treatment with brincidofovir was continued until negativity also in stool and therefore withdrawn one month later. At day +100 post HSCT the patient was clinically well with full donor chimerism on total BM and PB, as well as on lymphoid and myeloid subpopulations (Table 1). She is currently nine months after the third haplo HSCT and the patient remains in good clinical conditions and is infection-free. Based on emapalumab half-life, the patient has remained on anti-TB prophylaxis to mitigate the risk of reactivation until measurable levels of the drug were present.
Figure 2.
Significant inflammatory markers from the hemophagocytic lymphohistiocytosis (HLH) diagnosis up to the end of treatment with emapalumab and pharmacokinetics (PK) of the drug. (A) Serum ferritin (normal values [nv]: 15-150 ng/mL) and C-reactive protein (CRP) (nv <6 mg/L); trends are reported in red and blue lines, respectively. (B) IL2 receptor (nv 600-2000 pg/mL) and CXCL9 levels are reported in purple and green, respectively. Normal ranges are reported in light yellow. (C) Concentration-time profile of emapalumab in the patient. Green dots and solid lines represent observed emapalumab concentrations. Black solid lines represent simulated concentrations for the specific patient (i.e., taking into consideration the dosage schedule and measured total interferon gamma (IFNγ) concentrations) based on the population pharmacokinetic model of emapalumab in HLH patients. Gray area surrounded by orange dotted lines represents the 90% prediction interval of the simulated concentrations. Dotted red line represents the limit of quantification of the bioanalytical assay (62.5 ng/mL). Ticks and numbers on the top line represent times of administration and dose in mg/kg. IL2: interleukin 2; CXCL9: chemokine (C-X-C motif) ligand 9; HSCT: hematopoietic stem cell transplantation.
In conclusion, we report the case of a very fragile, heavily immunosuppressed patient affected by ADASCID who experienced GF after multiple HSCT in the presence of life-threatening infections including disseminated TB, who was safely treated with emapalumab.
The activation of the IFNγ pathway has a well-documented double role both in controlling mycobacterial infections and, also, in sustaining HLH hyperinflammatory response.10,11 In our patient, we had to face both challenges: on the one hand to prevent TB reactivation and on the other to inhibit the hyperinflammation responsible for both HLH and GF.
Upon review of emapalumab safety and efficacy data reported in primary2,12,13 and secondary14 HLH, and despite the potential risk of TB reactivation, the benefit/risk ratio of treating with emapalumab was deemed favorable. Interestingly, during emapalumab treatment, the initial TB abscesses remained inactive and brain TB findings improved. In this context, neutralization of IFNγ might have contributed to control HLH without the prolonged use of additional myelosuppressive drugs. Moreover, since a third GF was not observed, our findings suggest that, in association with other lines of immunosuppressive/chemotherapic agents, emapalumab might have played a role in reducing the risk of graft rejection, as already shown in both murine models and humans.3,15,16 In addition, while an intrinsic defect of the mesenchymal/osteblast compartment in ADA-SCID patients with reduced capacity to support in vitro and in vivo hematopoiesis9 may have contributed to the repeated GF, the concomitant use of Cs-A in the peri-transplant phase and the mega-dose of CD34+ cells infused after the third haplo-HSCT may have played a role in preventing rejection.
This seminal case suggests the feasibility and safety of emapalumab administration to manage secondary HLH and repeated GF also in patients bearing multiple active infections, including TB.
Supplementary Material
Acknowledgments
We are grateful to Ambra Corti for support in regulatory aspects of the study, and Manuela Gavina, Francesca Dionisio, Stefania Giannelli and Claudia Sartirana for technical support. We thank Emanuele Borroni for support with the mycobacteriology investigations.
References
- 1.Al-Salama ZT. Emapalumab: first global approval. Drugs. 2019; 79(1):99-103. [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020; 382(19):1811-1822. [DOI] [PubMed] [Google Scholar]
- 3.Merli P, Caruana I, De Vito R, et al. Role of interferon-γ in immunemediated graft failure after allogeneic hematopoietic stem cell transplantation. Haematologica. 2019;104(11):2314-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorman SE, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364(9451):2113-2121. [DOI] [PubMed] [Google Scholar]
- 5.Lammas DA, Casanova JL, Kumararatne DS. Clinical consequences of defects in the IL-12-dependent interferon-gamma (IFN-γ) pathway. Clin Exp Immunol. 2000;121(3):417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohn DB, Hershfield MS, Puck JM, et al. Consensus approach for the management of severe combined immune deficiency caused by adenosine deaminase deficiency. J Allergy Clin Immunol. 2019; 143(3):852-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822-826. [DOI] [PubMed] [Google Scholar]
- 8.Henter JI, Horne A, Aricò M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. [DOI] [PubMed] [Google Scholar]
- 9.Sauer AV, Mrak E, Jofra Hernandez R, et al. ADA-deficient SCID is associated with a specific microenvironment and bone phenotype characterized by RANKL/OPG imbalance and osteoblast insufficiency. Blood. 2009;114(15):3216-3226. [DOI] [PubMed] [Google Scholar]
- 10.Zhang SY, Boisson-Dupuis S, Chapgier A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-α/β, IFN-γ, and IFN-l in host defense. Immunol Rev. 2008;226:29-40. [DOI] [PubMed] [Google Scholar]
- 11.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004; 104(3):735-743. [DOI] [PubMed] [Google Scholar]
- 12.Locatelli F, Jordan MB, Allen CE, et al. Safety and efficacy of emapalumab in pediatric patients with primary hemophagocytic lymphohistiocytosis. Blood. 2018;132(Suppl 1):LBA-6. [Google Scholar]
- 13.De Benedetti F, Brogan P, Grom P, et al. Emapalumab, an interferon gamma (IFNγ) blocking monoclonal antibody, in patients with macrophage activation syndrome (MAS) complicating systemic juvenile idiopathic arthritis (sJIA). Ann Rheum Dis. 2019;78 (Suppl 2):178. [Google Scholar]
- 14.Lounder DT, Bin Q, de Min C, Jordan MB. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv. 2019;3(1):47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prencipe G, Caiello I, Pascarella A, et al. Neutralization of IFN-γ reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol. 2018;141(4):1439-1449. [DOI] [PubMed] [Google Scholar]
- 16.Buatois V, Chatel L, Cons L, et al. Use of a mouse model to identify a blood biomarker for IFNγ activity in pediatric secondary hemophagocytic lymphohistiocytosis. Transl Res. 2017;180:37-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.