ABSTRACT
Epithelial and endothelial cell-cell contacts are established and maintained by several intercellular junctional complexes. These structurally and biochemically differentiated regions on the plasma membrane primarily include tight junctions (TJs), and anchoring junctions. While the adherens junctions (AJs) provide essential adhesive and mechanical properties, TJs hold the cells together and form a near leak-proof intercellular seal by the fusion of adjacent cell membranes. AJs and TJs play essential roles in vascular permeability. Considering their involvement in several key cellular functions such as barrier formation, proliferation, migration, survival, and differentiation, further research is warranted on the composition and signaling pathways regulating cell-cell junctions to develop novel therapeutics for diseases such as organ injuries. The current review article presents our current state of knowledge on various cell-cell junctions, their molecular composition, and mechanisms regulating their expression and function in endothelial and epithelial cells.
KEYWORDS: Adherens junctions, cell junctions, claudins, endothelium, epithelium, tight junctions
1. Introduction
Cells encompass the fluid matrix, and organelles containing various chemical and biological components wrapped in a limiting membrane.1 These are the basic units of structure and function in life.2 Prokaryotes or eukaryotes are two kinds of life forms on earth3 composed of a single cell or multiple cells, respectively.4 The latter share a common ancestor, which has evolved from the former just once in four billion years. Eukaryotic organisms, with much larger genomes and proteomes, are generally larger and more highly organized than prokaryotic cells.5 Cells make up tissues, tissues comprise organs, organs form organ systems, and organ systems work collectively to generate an organism and keep it alive.6 The increased organization within the eukaryotic cells has allowed the evolution of thousands of different types of dedicated eukaryotic cells that perform specialized functions such as protection, movement, energy storage, or reproduction.4 Epithelium and mesenchyme are the most important tissue types in the complex metazoan body with epithelium arising first in embryonic development and mesenchyme evolving from it by a switch-off mechanism of differentiation and maintenance of epithelial cells (EpCs). These tissues produce all the organs of the body through mutual inductions and interactions.7 The differentiation of blastomeres into an epithelial state is essentially the first coordinated activity an animal embryo undertakes. As in all sexually reproducing metazoans, the entirety of human cell type diversity begins from a single newly formed cell, the totipotent zygote which undergoes a series of repeated mitotic divisions – cleavage – dividing into progressively smaller daughter cells or blastomeres (numbering two, then four, eight, etc.).1 Furthermore, cells of the 8-cell embryo in mammals and the blastula stage of animals, in general, are composed entirely of epithelial cells that are held together by cell-cell junctions.7
2. Epithelial and endothelial cell-cell junctions
EpCs form the epithelia, one of the fundamental tissue units of the human body8 whereas the endothelial cells (ECs), derived from the mesoderm9 are a thin layer of squamous cells10 that form the inner-most structures covering the interior walls of arteries, capillaries and veins.11–13 While the basal side of these cells is attached to the basement membrane, the apical surface confronts the lumen14 toward space or blood in EpCs and ECs, respectively.7,11 These cells can collectively form the cellular sheets that act as a physical barrier15,16 playing a protective role against the external microorganisms in animal and human life forms.17–19 Cell-cell barriers are established and maintained by the presence of several types of intercellular junctions.16 Cell-cell junctions help with the attachment and communication between the cells,8 separation of the intravascular and extravascular compartments, and the maintenance of the cell polarity.20 Experimental evidence has suggested that protein-protein interactions in the cytosol of such transmembrane junctional proteins modulate the extracellular action of the protein, which achieves homophilic or heterophilic binding to extracellular domains of junctional proteins of neighboring cells. In this way, a controllable intercellular seal is created.21 The best and longest known distinct junctional domains are adherens junctions (AJs) and tight junctions (TJs).22,23 Cell-cell adhesion is especially important in maintaining tissue integrity and resisting the mechanical challenges experienced by EpCs and ECs.24 In addition to cell-to-cell adhesion, another type of junction, the gap junction (GJ), mediates cell-to-cell communication. EpCs, but not ECs, also form desmosomes.22
TJs are characterized as a set of continuous and anastomosing strands at the apical-most regions of the lateral cell membranes seal the paracellular spaces.25 AJs play an important role in contact inhibition of EC22 and EpC growth,26 a phenomenon wherein cells stop growing further when bordered by other cells thereby forming a confluent ‘cobblestone’ monolayer of polygonal cells.27 Contact inhibition in confluent cell cultures is a dramatic decrease in cell mobility and mitotic rate with increasing cell density. The stationary post-confluent layer established is insensitive to nutrient renewal.28 In EpCs, junctions are better organized, with TJ and AJ following a well-defined spatial distribution along the intercellular cleft. While the TJs (or zonula occludens) are concentrated at the apical side of the rim, the AJs (or zonula adherens) are located below the TJ. Unlike EpCs, ECs reveal a less defined junctional architecture wherein AJs are intermixed with TJs along the cleft.22 Moreover, TJs of endothelial sheets in vivo are leaky in general, since a wide variety of substances must be exchanged between the blood and organs through the paracellular as well as transcellular routes.25 Even though ECs and EpCs share numerous TJ components, the same molecules might be differentially assembled and regulated in the two cell types. Further, there is a substantial variability among different segments of the vascular tree. Particularly, in large vessels, TJs are well developed in arteries and less sophisticated in veins.22 When it comes to small vessels, these junctions are well organized in arterioles, but loosely organized (even with some gaps) in venules, a preferential site for the extravasation of plasma proteins and circulating leukocytes. Finally, brain vessels that contribute to the blood-brain barrier (BBB) have well developed TJs compared to other organs characterized by high rate trafficking.22
Intercellular junctions are structurally and biochemically differentiated regions of the plasma membrane through which adjacent cells interact in a specific manner. These structures were originally identified and named according to their morphology and purported function.29 To retain barrier function and to prevent the invasion of pathogens and their rapid systemic spread, cell junctions need to be kept tight and repaired quickly after vessel rupture.30 There are three functional categories of cell junction: anchoring junctions; tight, or occluding, junctions, and gap (GJ), or permeable, junctions Figure 1.17,31,32 The AJs and desmosomes provide essential adhesive and mechanical properties that contribute to barrier function but do not seal the paracellular space,33 the TJs hold cells together and form a near leakproof intercellular seal by fusion of adjacent cell membranes34 since interactions between cells are important for the assembly and maintenance of three-dimensional tissues.35 The latter is a selectively permeable barrier that generally represents the rate-limiting step of paracellular transport.33 Many cell types also possess GJs, which allow small molecules to pass from one cell to the next through channels.34
Figure 1.
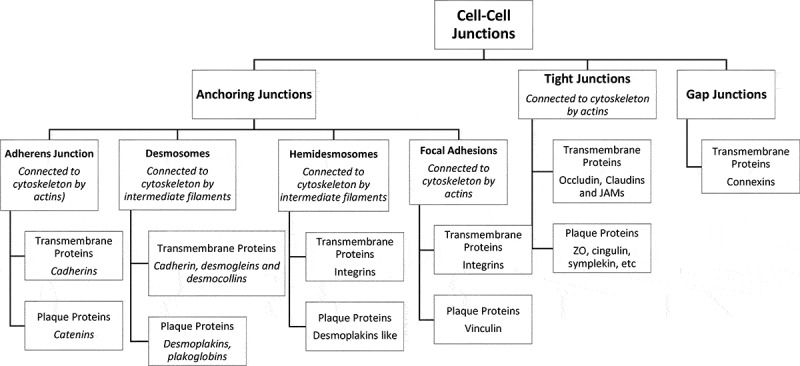
Types of cell junctions
3. Anchoring junctions and proteins present within
Epithelial and endothelial sheets are held together by anchoring junctions present near the apical portion of two adjacent cells, just behind the TJs36 Figure 2. AJs, the primary anchoring junction protein complexes are formed by transmembrane adhesion proteins, cadherins.37 Cadherins are important in mammalian embryogenesis31 in the direct anchoring of the stem cells to niche cells, organizing the niche, controlling cell division orientation, regulating signaling pathways, and affecting the mechanics of the cells.37 AJs are positioned at a site where the opposing membranes of adjacent cells are near to 20 nM distance and they associate with the circumferential belt of actin.8 The extracellular regions of these proteins mediate adhesion of cells to their neighbors while the intracellular regions interact with an array of proteins.35 Interestingly, the formation of AJs is a prerequisite for TJ assembly.38,39
Figure 2.
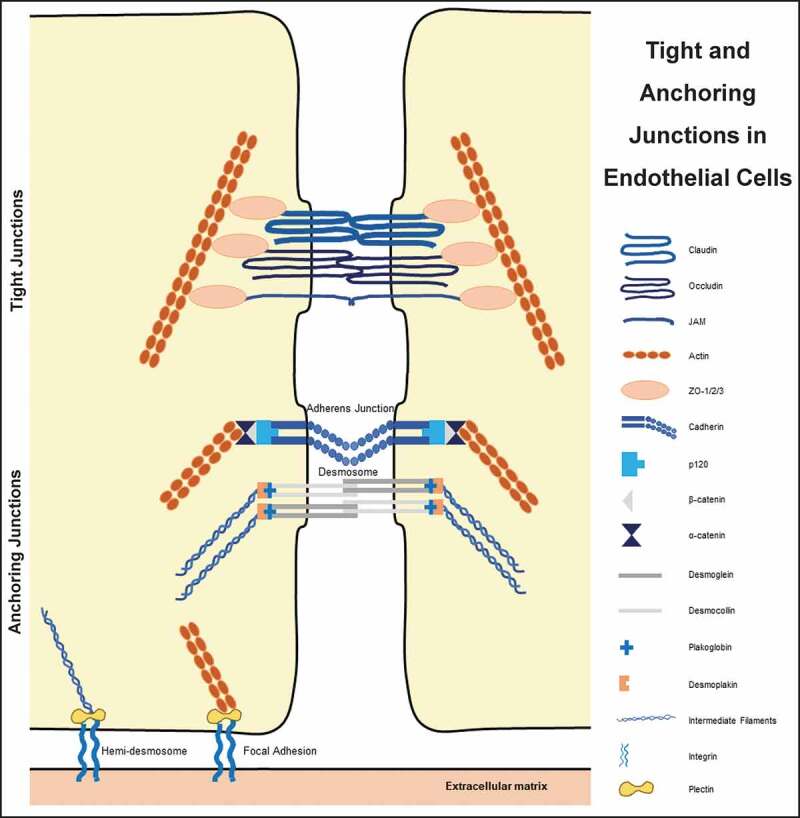
Connection of various tight and anchoring junctions to the cytoskeleton
AJs are primarily made up of cadherin–catenin protein complexes, which are linked to the actin cytoskeleton.40 While cadherins make the transmembrane protein part, plaque proteins are made up of catenins, plakoglobin, p120, and others.31 Over 170 proteins have been reported to colocalize with cadherin or catenins in AJs, and either directly interact with them or affect AJ dynamics.41 Cadherins are transmembrane, calcium-dependent membrane proteins8,41–43 that have an ectodomain consisting of five cadherin motifs and a cytoplasmic domain with two conserved motifs.42 They form antiparallel homotypic adhesive complexes with adjacent cells after dimerization and clustering.40,41,44 They are essential proteins for morphogenesis and tissue homeostasis.45 Cadherins regulate the plasticity and control the passage of solutes, water, and lymphoid cells across the cell layer through epithelial and endothelial cell junctions.46 The superfamily of transmembrane cadherin proteins is comprised of more than 100 members in humans, including other classical cadherins, numerous proto-cadherins, and cadherin-related proteins.47 Several tissue-specific cadherins have been identified, including epithelial (E)-cadherin, neuronal (N)-cadherin, placental (P)-cadherin, vascular endothelial (VE)- cadherin, and others.8 Disruption in the expression or function of such individual cadherins results in abnormal development of the respective organs.41
E-cadherin, also known as classical cadherin,8 is expressed primarily in epithelial cells,35,46 and is associated with the AJs of the epithelial junctional complex. These structures altogether help the cells form a tight, polarized cell layer that can perform barrier and transport functions.46 It promotes the polarized epithelial phenotype which is essential to the stabilization of cell-to-cell adhesion. Loss of E-cadherin function is associated with the gain of invasiveness and metastatic potential of cells and, consequently, malignant transformation.8 VE-cadherin, like E-cadherin, is associated with the AJ junctions that help these cells to form transport barriers.46 These are the major transmembrane components of endothelial AJs,41,46,48 and are expressed in vivo in any type of vessels, arterial, venous and lymphatic. Its expression represents an early step in the differentiation of the endothelial phenotype.48 VE-cadherin has emerged as an adhesion molecule that plays essential roles in microvascular permeability and the morphogenic and proliferative events associated with angiogenesis.49 Therefore, the VE-cadherin knockout mouse died during embryonic development in past studies.41 N-cadherin and retinal (R)-cadherin are widely expressed in the nervous system and are associated with small adherens-type junctions at synapses, as well as at growth cones and other parts of the neuron.46
With regards to cadherin structure, two domains can be distinguished: the ectodomain and the cytoplasmic domain. Whereas the ectodomain mediates both the homophilic binding and the adhesion recognition, the highly conserved cytoplasmic domain interacts with other proteins that mediate the binding of cadherin to the actin cytoskeleton, promoting signal transduction. When activated, cadherin ectodomain dimerizes and clusters to promote the association between the cadherin cytoplasmic tail and the cytoskeletal network. This association also involves the recruitment of α-catenin and β-catenin to stabilize the adhesion junction. Subsequently, β-catenin recruits α-catenin, which mediates the binding of cadherin–catenin complex to the actin molecules via other cytoplasmic proteins. At last, p120ctn binds to this complex to regulate its function.8,40 Catenins contribute to the indirect association of cadherins with the underlying actin cytoskeleton, as indicated for β-catenin (and probably γ-catenin/plakoglobin) at AJs.46,50 All catenins, except for the three α-catenin gene products [αE-catenin (epithelial), αN-catenin (neuronal) and αT-catenin (testes and heart)] possess a central Armadillo domain consisting of repeating coiled-coils of α-helices that allow varied interactions in distinct intracellular compartment.51 β-catenin and plakoglobin (or γ-catenin) form the two-member β-catenin subfamily and associate competitively with the distal cytoplasmic tails of classic cadherins, which are single-pass transmembrane proteins that enable cell-cell adhesion, motility, and communication at regions including the AJs of epithelia. Interestingly, the three members of the α-catenin family bind to cadherins indirectly through β-catenin or plakoglobin.51
β-Catenin is also an intracellular signal transduction molecule that mediates signaling in the WNT growth factor pathway.40,46,51 Generally, in the absence of an extracellular Wnt ligand, the cytosolic (non-cadherin bound) levels of β-catenin are low since it is targeted for degradation by a complex of proteins. Wnt signaling through its Frizzled-LRP receptor inhibits the targeting of β-catenin for degradation thereby allowing it to accumulate in the cytosol.46 It enters the nucleus, interacts with the transcription factor T-cell factor (TCF) or leukocyte enhancing factor (LEF), and activates the expression of target genes.46,50 α-Catenin is a cytoskeletal protein that generates widespread flattening between neighboring cell surfaces. It binds to actin and several other actin-binding proteins in addition to the N-terminal region of β-catenin but does not interact directly with cadherins. It binds to signaling proteins, such as formin-1, which regulate the actin cytoskeleton. It also seems to have a signaling role in the regulation of cell proliferation.46 p120-Catenin is another armadillo-repeat-containing protein that was earlier discovered as a substrate for the Src protein kinase. It binds to a different region of the cadherin cytoplasmic domain from β-catenin, and both proteins can bind cadherin concurrently.46 In addition to cell-cell adhesion, it plays integral roles in embryonic development, polarity, cell proliferation, and tumor cell migration. However, recent reports have generated an entirely new standpoint, suggesting that p120-catenin is involved in the anti-inflammatory responses in the absence and presence of infection.52
Similar to AJs, desmosomes are adhesive intercellular junctions that bind intermediate filaments to the cytoskeleton. It plays a critical role in tissues subjected to mechanical stress including the myocardium, bladder, and skin.53 When it comes to the interaction of a cell with the extracellular matrix, hemidesmosomes and focal adhesion come into play. Although both junctions utilize the integrins,54 hemidesmosomes deal with intermediate filaments55 whereas focal adhesions connect to the actin.56
3. Tight junctions (TJs) and proteins present within
TJs (a.k.a. occluding junctions and zonula occludens) are multi-molecular composites found at the apical side of the junctional complexes that connect EpCs.57,58 Their principal role is to generate cell polarity (or fence function), control paracellular transport, provide signaling input for a wide variety of cellular events,59 and maintain differences in the lipid and protein composition between the apical and basolateral domains of the plasma membrane.60,61 The TJ is the site where the plasma membranes of adjacent EpCs interact with one another to form a paracellular pathway29,34 that plays a major role in transepithelial ionic flows.62 Studies of junction formation in early development disclose the contribution of TJs to the early differentiation course and are largely accompanied by barrier formation.63 Cancer cells irreversibly and progressively lose TJs with dedifferentiation through genetic and epigenetic variations.25 TJs are a significant barrier to micro-organisms since these cross the mucosal epithelium, circulate through the bloodstream, and spread to other hosts.64 Although vascular permeability (VP) depends on both the paracellular pathway and the transcellular pathway of endothelial sheets, edema develops mainly because of the dysfunction of TJs between cells.25
TJs are composed of the transmembrane proteins called occludin, the claudins (CLDNs), and the junctional adhesion molecules (JAMs) Figure 2.16,32,65 These proteins must be properly targeted and positioned at the TJ to provide a paracellular seal. Assembly, scaffolding, and regulation of the paracellular seal are attained by the cytosolic plaque of proteins65 that also integrates ‘outside-in’ and ‘inside-out’ signaling.16,66 These are comprised of ZO-1, ZO-2, and ZO-3 that form a scaffold for transmembrane proteins, cingulin30,66–68 polarity complex proteins, guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs),66 MUPP-1, MAGI-1, AF-6,16,67 symplekin,69 7H665 and MPDZ.70
TJs differ in the degree of tightness in a tissue-dependent manner. Their tightness can be directly measured as transepithelial electrical resistance (TER). The number of TJ strands was found to correlate well with the TER values of TJs in various tissues.71 Two models have been proposed to elucidate the chemical nature of TJ strands. In the ‘protein model’, tight-junction strands signify units of integral membrane proteins that are polymerized linearly within lipid bilayers, whereas in the ‘lipid model’, lipids organized in inverted cylindrical micelles are proposed to constitute TJ strands.71 TJs are not evident by light microscopy and are inconspicuous when viewed by transmission electron microscopy. In contrast, freeze-fracture electron microscopy displays an intricate three-dimensional structure composed of paired intramembranous strands within adjacent cells.33
Two mechanisms have been proposed for the paracellular transport of solutes or ions at TJs. Whereas these molecules pass through a paracellular channel formed by the TJ strands in the ‘pore’ pathway, they supposedly pass through breaks in the TJ strands in the ‘leak’ pathway. The pore pathway can be sensed by the diffusion potential when it is selectively permeable to charged ions. The pore pathway is also permeable to uncharged solutes such as small polyethylene glycols, in a size-selective manner. On the contrary, the leak pathway is permeable to small and larger solutes with >8 Å diameter which includes both ions and uncharged solutes, and is not very size-selective, although there is a size limit for permeation. This mechanism presumably relies on the breaking and reorganization of the TJ strands.72
Occludin is the first transmembrane protein of the TJ discovered in 199333 exclusively localized in EpCs and ECs.22 It has four transmembrane domains, a long carboxy-terminal cytoplasmic domain, and a short amino-terminal cytoplasmic domain. Two isoforms of occludin are generated by alternative splicing.71 While the occludin knockout mice formed intact TJs, these animals presented a variety of abnormal phenotypes including postnatal growth retardation, thinning of compact bone, calcification in the brain, loss of cytoplasmic granules in salivary epithelial cells, testicular atrophy, male infertility, females not suckling their young, and gastric inflammation and hyperplasia.30
Junctional adhesion molecules are a family of adhesion molecules localized at the TJs of polarized EpCs and ECs.16,67 JAMs are composed of single-pass membrane proteins with two IgG-like motifs30 and are capable of forming homophilic and heterophilic interactions at the adhesion contact.16 They are ~40kDa,30 composed of seven members that include three classical JAMs (JAM-A, -B, -C) and four related proteins (JAM-4, JAM-L, CAR, ESAM).16 In contrast to CLDNs, the transfection of JAMs into fibroblasts did not induce TJ strand formation. However, in ECs, they interact with polarity complex proteins through their PDZ binding domains and are thought to regulate cell polarity and leukocytes migration.16 Because of their transmembrane topology, JAMs are poised both for receiving inputs from the cell interior and for translating extracellular adhesive events into functional responses.73
Besides, to play a role in mediating barrier formation and function, JAM-A is crucial for polarity, perhaps through interactions with the polarity protein PAR-3. Further, JAM-A knockout mice exhibit increased polymorphonuclear leukocyte infiltration and, consistent with the in vitro study, increased mucosal permeability.30 JAM-A and JAM-C are highly homologous, and both are involved in the regulation of VP. Despite these similarities, they appear to have contrasting roles in regulating the barrier function of ECs. Whilst genetic deletion and/or blockade of JAM-A largely results in increased EC permeability, knocking down JAM-C decreases EC permeability in vitro.74
The zona occludens (ZO) family includes ZO-1, ZO-2, and ZO-3, which contain three PDZ domains (PDZ1, PDZ2, PDZ3) belong to the membrane-associated guanylate kinase (MAGUK) family.75 ZO-1 was the first identified TJ protein in the year 1986.33 It is a scaffolding protein that provides the structural basis for the assembly of multiprotein complexes at the cytoplasmic surface of intercellular junctions.76 Besides, it connects the integral membrane proteins with the filamentous cytoskeleton.21 The knockout of ZO-1 was shown to be lethal for mouse embryos around the mid-gestation period.21,76 ZO-2 is another ZO protein that is reported to be involved in cell growth and proliferation.21 The indirect interaction of ZO proteins with the cytoskeleton involves several actin-binding proteins including cortactin, alpha-catenin, protein 4.1 R, the Ras target AF6/afadin as well as the actin- and myosin-binding proteins cingulin and Shroom.21
CLDNs are a family of transmembrane proteins with at least 24 members in mouse and human. CLDNs are the most vital structural and functional components of the TJs and the principal regulators in defining the properties of paracellular ion permeability of the EpCs.58 They are found in epithelia as well as endothelia and its expression correlates with integrity, proliferation, or cell death.29,59,77,78 They are localized in the apical lateral membranes of EpCs and ECs, connect adjacent cells,79 and compose size-, charge- and water-selective paracellular channels.80
4. CLDN family of TJ proteins and their diverse function
CLDNs appear to be major structural components of the TJs79 and hence regarded as its backbone.30 These key proteins of TJs,81 on one hand, tighten the paracellular cleft of epi- and endothelia against the unwanted passage of solutes, but on the other hand, also allow and regulate tissue-specific paracellular permeation.82 Different epithelial and endothelial tissues express different CLDNs with varying physicochemical properties.67 Twenty-seven human CLDN genes have been recognized, but it is not clear that all of these are expressed as proteins. Mouse and human orthologs mostly clustered together with an exception to CLDN13, which is absent in humans.33,59 Interestingly, multiple CLDN family members can co-exist in the same TJ strand while other combinations of CLDNs fail to do so.30 As the EpCs within the segments of the respiratory tree vary, the composition of CLDNs found in these cells also differs. Among these differences is CLDN18 which is uniquely expressed by the alveolar epithelial cells whereas other CLDNs, CLDN-4 and −7, are more ubiquitously expressed throughout the respiratory epithelium. Another CLDN which is expressed by both pulmonary EpCs and ECs is CLDN5.83
4.1. Significance of CLDNs
TJs were developed in fibroblasts, which usually lack these junctions when expressed with CLDNs thereby reflecting the striking ability of these proteins.33,77 The importance of CLDNs is becoming apparent in the pathophysiology of several diseases, including viral infections. Noteworthy is the discovery of CLDN1 as an essential host factor for hepatitis C virus (HCV) entry, which led to the detailed characterization of CLDN1 and its association with tetraspanin CD81 for the initiation of HCV infection. CLDN1 has also been shown to facilitate dengue virus entry.84 Moreover, its knockout in mice led to severe barrier defects33 and death within 1 day of birth owing to massive trans-epidermal water loss.79 The CLDN5 knockout mouse has reportedly displayed severe brain hemorrhage and died within 10 hours after birth.30 The CLDN11 knockout is viable; however, the mouse has hind limb weakness, slowed conductive velocities of the central nervous system, and male sterility. Mutations in CLDN-16 and −19 in humans were found to be associated with hypomagnesemia.30 CLDN-3 and −4 are receptors for the enterotoxin of Clostridium perfringens (CPE), which is a common cause of food poisoning.25 Further, TNF and IL-13 were reported to induce TJ barrier loss.33
4.2. Structure of CLDNs
CLDN15 crystal structure enabled investigators to construct a model that delineates development of channels by CLDNs. The model states that two cells partner to form CLDN channels: one cell contributes two CLDN molecules, whose extracellular portions join together to form one half of a barrel-shaped pore, and the adjacent cell mirrors this arrangement to contribute the other half.85 Human CLDNs possess between 207 and 305 amino acids and have calculated molecular masses of 21–34 kDa. Hydropathy plots show four transmembrane helices (TM1–4) and the general structure of all CLDNs consists of an intracellular NH2 terminus that, with the possible exception of CLDN-5, −16, and −25, is very short, together with a longer intracellular COOH terminus, two extracellular loops (ECL1, which is larger, and a smaller ECL2), and one short intracellular loop as represented in Figure 3. CLDN-5, −16, and −25 appear to be unusual, due to their long NH2 terminus and, therefore, high molecular mass. However, human mRNAs encoding these three CLDNs possess two start codons that potentially give rise to a short and long protein version each. The variations lie exclusively within the intracellular NH2 termini of these proteins and it is still a subject of debate which version is the physiologically related form. Further typical features of the CLDN family include a signature sequence within ECL1 and a COOH-terminal PDZ-binding motif, through which most of the human CLDNs except CLDN-12, −19a, −21, and −24 to −27 can interact with PDZ domains of TJ associated scaffolding/adapter proteins. ZO-1, −2, and −3, MUPP1, and MAGI-1 to −3 are the TJ associated PDZ domain proteins that bind actin directly or indirectly to anchor the TJ within the cytoskeleton.77
Figure 3.
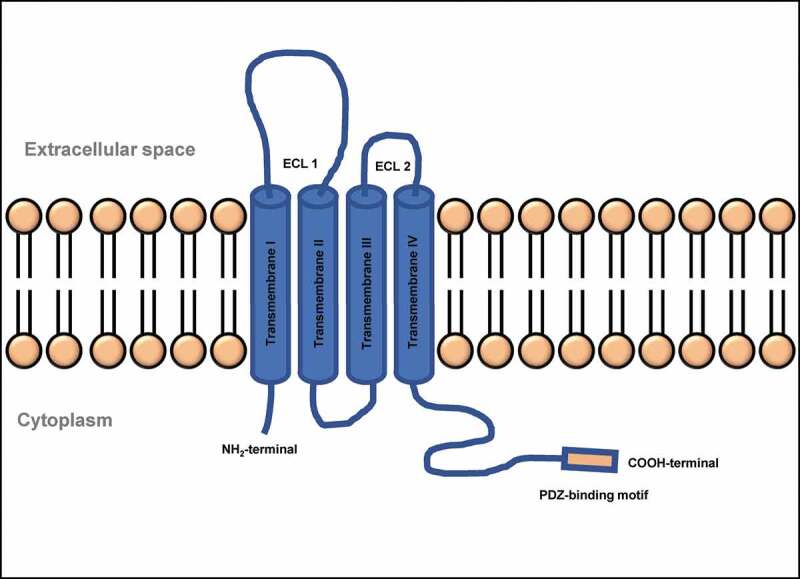
Structure of a typical TJ claudin. ECL, extra-cellular loop; NH2, amino group; COOH, carboxyl group
ECL1 in CLDNs is made up of 42–56 residues that lie between the first and second transmembrane helices and contains the highly conserved signature motif of CLDNs. Reports suggest that it likely has a well-defined structure and serves a vital conserved function of CLDNs. Further, it forms the lining of the paracellular pore and contains a specific ion-binding site.77 ECL2 has a highly conserved amino acid sequence and a previous chimera study suggests that ECL2 is not involved in defining paracellular permeability. When the ECL2 of CLDN5 was fused to maltose-binding protein and expressed in bacteria, it was found to dimerize suggesting that it mediates CLDN-CLDN interactions. The COOH-terminal end of CLDNs is long, extremely divergent in sequence between various CLDNs, and is predicted to be intracellular and mainly disordered. It plays a role in trafficking to TJ, PDZ-binding domain, protein degradation, phosphorylation, and palmitoylation.77
4.3. Types of CLDNs and their tissue distribution
CLDNs determine the barrier properties of the TJs. While many CLDNs seal this barrier, others form paracellular channels Figure 4.86 Based on permeability properties, members of the CLDN family can be categorized into sealing and channel-forming proteins.59 CLDN-2, −10b, and −15 are cationic pore-forming whereas CLDN-7, −10a and −17 are anionic pore-forming.77,79 CLDNs are also grouped as classic and non-classic; the former have high sequence similarities.84 Further, a phylogenetic tree sorts human CLDNs into eight subgroups, which form four major clusters: cluster I (subgroups A/B) CLDN-3, −4, −5, −6, −9/-8, −17; cluster II (subgroups D/E) CLDN-1, −7, −19/-2, −14, −20; cluster III (subgroups F) CLDN-10, −11, −15, −18; and cluster IV (subgroups C/G/H) CLDN-21, −22, −24/-12, −16, −25/-23, −26, −27.77
Figure 4.
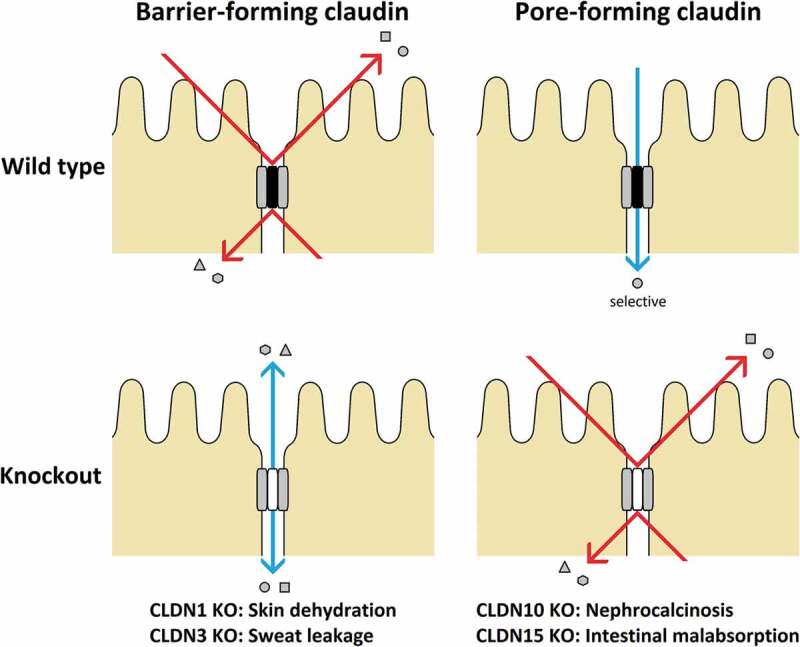
Types of CLDNs and their mechanisms
In the EpCs, CLDNs are expressed in all known epithelial tissues with multiple types expressed simultaneously.77 Vascular ECs also have TJs that express multiple CLDNs. The most predominantly found CLDN is CLDN5 but CLDN-1, −3, and −12 are also expressed in these tissues. CLDN-10 and −22 were reportedly expressed at a significant level in a purified preparation of brain capillary ECs where CLDN5 expression was almost 600-fold higher than CLDN-3. Overall, the number of CLDN isoforms identified so far in endothelia is far less than those in epithelia, suggesting that these are possibly going to turn out to be several epithelium-specific isoforms, including most of the pore-forming CLDNs.77 In addition to epithelia and endothelia, CLDNs are also found in a variety of other cell types. CLDN-11 and −19 are known to be expressed in interlamellar strands of myelin sheaths in the central and peripheral nervous system, respectively, to deal with insulation of myelinated nerves and facilitate nerve conduction. Pancreatic islet cells with TJ-like strands on their cell surface reportedly express CLDN-4. In mouse, CLDN-13 is expressed in hematopoietic tissues, including the bone marrow, spleen, and thymus. CLDNs have also been depicted in lymphocytes and monocytes, thymocytes, dendritic cells, osteoblasts and osteoclasts, astrocytes, and even neurons under certain conditions.77 CLDN-1, −3 to −5, −7, −8, and −18 are expressed in human bronchi and bronchioles but CLDN2 expression is debatable.60
4.4. Properties of CLDNs
Masking is an important property of CLDNs. For instance, incorporation of a pore-forming CLDN into an originally permeable TJ, or of a barrier-forming CLDN into an originally sealed TJ may prevent recognition of specific properties.77 Overexpression of one CLDN may result in the displacement of an endogenous CLDN from the TJ.87 If the dislocated CLDN has a robust ion permeability effect, the observed alterations may be (erroneously) believed to be a result of the overexpressed CLDN.77 Recent evidence reveals that certain CLDNs specifically need another (partner) CLDN to translocate to and insert into the TJ to be fully functional.77 These interactions within a single TJ can be of several types: homo- and heteromeric interactions that may occur in cis or trans.88 While cis-interaction occurs when two CLDNs interact from the same cell with their N-terminal extracellular loops, trans-interaction results when CLDNs from two adjacent cells interact through their C-terminal extracellular loops.29 These cis- and trans-interactions leads to the formation of a “zipper”-like structure, thus describing the CLDN-driven barrier.30
In addition to the aforesaid interactions, CLDNs may interact with other transmembrane TJ proteins, such as members of the TJ-associated marvel protein (TAMP) family.89 PDZ binding motifs at the COOH terminus of CLDNs help them bind to various scaffolding/adapter proteins which in turn links them to the cytoskeleton. These include ZO-1, −2, and −3; MAGI-1, −2, and −3; MUPP-1; Par3 and Par6; PALS1; and PATJ.77 Irrespective of the barrier- or pore-forming type, CLDNs have the potential to modulate the charge selectivity of the paracellular conductance. This selectivity is believed to be conferred by charged sites within the paracellular pathway formed by the CLDN.77 CLDN2 is the only CLDN that has been demonstrated to enhance paracellular water permeability when overexpressed in Madin-Darby Canine Kidney (MDCK) C7 cells.90 Neither overexpression of CLDN-10 nor −17 altered water permeability in this cell line. Intriguingly, not only were osmotic gradients capable of driving water across CLDN2 transfected cell layers, but also NaCl gradients that were osmotically compensated by the addition of mannitol.77 TJ permeability studies have found the presence of two populations of pores based on the size. Whereas small pore or restrictive pathway is permeable to small ions and neutral solutes, larger pore or nonrestrictive pathway (also referred to as the “leak” pathway) is permeable to macromolecules.77,91
It is a fundamental property of transmembrane channels at the molecular level. This establishes a mechanism by which channel permeability can be regulated by voltage, extracellular ligands, and intracellular or intramembrane signals. It is still unknown whether paracellular CLDN pores also exhibit gating.77 The initial hypothesis is that TJ strands seldom exhibit breaks through which macromolecules can diffuse from TJ mesh to TJ mesh and then reseal, without the compulsion to permanently open up a large gap across the entire TJ system. Another hypothesis is that the leak takes place at the tricellular junction. This is centered on the observation that moderate overexpression of tricellulin specifically lowers paracellular permeability to macromolecules, but not to small inorganic ions. Under these conditions, tricellulin exhibits a strictly tricellular distribution.77
5. Regulation of CLDNs
Regulation of CLDNs and thus of TJ properties occurs on various levels, such as transcription and posttranslational modification. In addition to these, the interaction of CLDNs with other CLDNs and scaffolding proteins (discussed earlier in Sections 4.2 and 4.4) determine TJ assembly, remodeling/modulation, and degradation.77 TNF-α/NF-ƙB and TGF-β-Smad/Snail pathways are the major regulators of CLDN expression. Other pathways reported to be involved in the regulation are related to PPARγ, SP1, HNF-1α, HNF-4α, CDX1, CDX2, GATA-4, and Grhl2.77 Apart from these, FoxO1 and ELF-3 are reported to regulate CLDN5 and CLDN7, respectively.92 Endocytosis is another essential regulator of CLDNs in paracellular barriers and can follow different pathways, depending on the CLDN subtype and stimulus.93 CLDN5 is removed from the TJ in a caveolin-dependent manner in ECs of the blood-brain barrier during the stroke, as well as in cultured ECs post cytokine CC-chemokine ligand 2 treatment.94 IFNγ causes CLDN1 to be dislocated from the plasma membrane to early and recycling endosomes after 48 h, a process similar to macropinocytosis. Calcium depletion in epithelial T84 cells results in clathrin-dependent internalization of CLDN-1 and −4 into subapical ring-like organelles that are positive for syntaxin-4, but not for markers of lysosomes, the Golgi apparatus, or late or recycling endosomes.93
The abovementioned data suggest there may be a weakly characterized compartment involved in the internalization of CLDNs. Although there is plenty of information regarding stimuli leading to TJ disruption, there is only restricted knowledge on constitutive internalization pathways of these proteins. After internalization, CLDNs can either be targeted for degradation or for recycling to the plasma membrane. In MDCK-II, CLDN-1 and −2 are continuously recycled, and this depends on a functioning ESCRT complex (endosomal sorting complex required for transport). Degradation of internalized CLDNs has been shown to depend on both lysosomal and proteasomal pathways. In MDCK-I cells, CLDN1 is ubiquitinated and subsequently degraded via the lysosome whereas human CLDN5 is disrupted by the proteasome after poly-ubiquitination at K199, and also by a ubiquitin-independent lysosomal mechanism in ECs. Although the details of this remodeling are unclear, this indicates the continuous turnover of TJs.93 Reports reveal that CLDNs undergo posttranslational modifications comprised of phosphorylation, ubiquitinylation, and palmitoylation. Computational data also predicts O-linked N-acetylglucosamine modification (O-glycosylation) sites, N-linked glycosylation sites, and further phosphorylation sites.77
6. CLDNs in human diseases
Disruption of the TJ barrier increases the back diffusion of ions, solutes, and water across transporting epithelia thereby contributing to diseases.95 Mutations of CLDNs are known to cause four Mendelian inherited disorders, neonatal sclerosing cholangitis with ichthyosis (CLDN1), autosomal recessive, nonsyndromic deafness (CLDN14), familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC, due to CLDN16), and FHHNC with ocular involvement (CLDN19). Polymorphisms in CLDN genes are associated with polygenic diseases, including CLDN1 with atopic dermatitis, CLDN5 with schizophrenia, and CLDN14 in kidney stone disease.77 CLDN dysregulation is reportedly associated with enhanced intestinal permeability, prolonged activation of inflammation, epithelial-to-mesenchymal transition (EMT), and tumor progression in inflammatory bowel disease as well as consequent colitis-associated colorectal cancer.96 CLDN1 was demonstrated to be overexpressed in colorectal cancer (CRC) compared with the level in the normal mucosa.81 CLDN-3 and −4 are among the most frequently deregulated CLDNs in cancer and their expression is typically found upregulated in cancers of the ovaries, breast, prostate cancer, and pancreas.97 Changes in the expression of several CLDNs have also been reported in ocular diseases, particularly in diabetic retinopathy.98
CLDNs represent promising targets for detection, diagnosis, and therapy of various cancers.99 Reports from recent studies reveal a role for CLDNs as key prognosis factors in cancers. Lechpammer et al. demonstrated the potential of CLDNs in renal cell carcinoma as a diagnostic and prognostic factor.100 While low expression of CLDN1 has been linked with a poor prognosis in stage II colon cancer, CLDN10 expression is an independent prognostic factor for hepatocellular carcinoma recurrence after curative hepatectomy.99 Further, the distal nephron proteins CLDN-7 and −8 were found to have the potential to be used as immunohistochemical biomarkers in the differential diagnosis of chromophobe renal cell carcinoma and oncocytoma.101
Monoclonal antibodies against CLDN1 was found to be promising in preventing HCV entry. These antibodies could prevent viral infection post-liver transplantation, and virus spread in chronically infected patients. Offner et al. investigated CLDNs as a potential target in antibody-based therapies for carcinomas by generating antibodies against the extracellular domains of CLDN-1, −3, and −4. Fujiwara et al. targeted CLDN4 in colorectal cancer using an anti-CLDN-4 extracellular domain antibody which was reported to be promising and observed to enhance the anti-tumorigenic potential of 5-fluorouracil and anti-EGFR antibodies. Some of the monoclonal antibodies such as anti-CLDN18.2 (IMAB362-CLDN-18.2) and the anti-CLDN6 (IMAB027-CLDN-6) have also found their way into clinical trials.100 Ideal monoclonal antibody 362 (IMAB362), also referred to as Claudiximab, is a first-in-class chimeric antibody for the treatment of gastric cancer. These molecularly targeted therapies bind to cancer-selective targets that are predominantly expressed in tumor cells and show minimal or no expression in healthy tissues. This unique cancer-cell selectivity of IMABs permits for maximal anticancer potency while reducing toxicity. They also have a broader therapeutic window allowing optimal dosing.99
Another opportunity to utilize CLDNs lies in their potential to behave as receptors for microbes. Clostridium perfringens enterotoxin (CPE) has the potential to bind with CLDN receptors leading to the formation of a Calcium influx pore causing host cell death. This interaction between CLDN and CPE is gaining significance in receptor decoy therapeutics for potential applications in gastrointestinal disease, cancer therapy/diagnoses, and drug delivery. CLDN-3 and −4 have been widely demonstrated to function as CPE receptors thereby raising a great opportunity to target cancers with dysregulated CLDN-3 and −4 cancers, especially breast, ovarian, and pancreatic cancers.99,100
7. Cell-cell junctions and regulation of vascular permeability
VP is a complex process by which molecules cross the endothelial barrier102 through transcellular or paracellular routes.103 Several mechanisms including the breakdown of VE-cadherin junctional contacts, activation of other Src-family protein-tyrosine kinases, focal adhesion kinase (FAK), and Akt pathway104–111 contributing reductions in several claudins, either zona occludens 1 and occludin reportedly decreases TJs and AJs thereby causing increased permeability.112
Akt1, a serine-threonine kinase104 has a substantial effect on the expression of the CLDN family of TJ proteins Figure 5.109 Intriguingly, VP inducing (VEGF) as well as inhibiting (Ang-1) agents are reported to activate Akt. However, its activation by VEGF and Ang-1 results in reciprocal regulation causing disruption and strengthening of the barrier, respectively.109 Stimulation of Akt by shear stress or Ang-1 signaling induces endothelial quiescence by phosphorylation of FoxO1 which causes its nuclear exclusion to prevent FoxO1-β-catenin interaction. On the contrary, FoxO1, when interacts with β-catenin and TCF, activates Ang-2 and other genes important for matrix remodeling and migration. It also reportedly results in the repression of CLDN5 expression. Therefore, inhibition of FoxO1 is vital for restricting the expression of barrier destabilizing proteins113 and Akt1-FoxO signaling plays a crucial role in the transcriptional up-regulation of several CLDNs.109,110
Figure 5.

Regulation of adherens and tight junction by Src and Akt
In addition to the regulation of TJs, Akt is also involved in the modulation of AJ elements such as VE-cadherin. Studies from our laboratory based on HMECs reveal that long-term stimulation with VEGF and Ang-1 increases Akt and normalizes Src activities leading to endothelial-barrier protection.110 Reports also indicate that VEGF-A stimulates VEGF-R2 dimerization and disrupts the endothelial barrier function through activation of Src. The process involves phosphorylation of a guanine-nucleotide-exchange factor (VAV2) for the GTPase Rac which induces phosphorylation of VE-cadherin followed by its recruitment to β-arrestin-2 eventually causing its internalization.114,115 However, Ang-1 is believed to avert the VEGF-A-induced barrier disruption by activating RhoA which promotes interaction between mDia1 and Src, thereby preventing the binding of Src to VEGFR2 and the subsequent phosphorylation and internalization of VE-cadherin.116 Another study reveals the time-variant effects of Src activation. While immediate activation of Src causes activation of endothelial barrier function via phosphorylation of VE-cadherin at Y731, prolonged activation phosphorylates the same AJ molecule at Y685 thereby enhancing the permeability.117
Long-term treatment with TGFβ1 was also associated with an endothelial- and epithelial-barrier breakdown due to inhibition of Akt and activation of Src activities.104,107,108,110,118–120 Further, direct inhibition of Akt activity through pharmacological (Triciribine) and genetic (shRNA for Akt1) approaches caused Src activation in the long-term suggesting that Akt and Src are reciprocally regulated in the growth factor-induced long-term endothelial-barrier regulation.110
Reports reveal the involvement of p38 MAP-kinases in the regulation of dynamic changes related to GJs and TJs during regeneration hepatocytes in rats.121 These were also found to regulate cavitation and TJ function in the mouse blastocyst.122 PKC is another pathway associated with the regulation of TJ assembly in the pre-implantation mouse embryo.123 Interestingly, it was reportedly associated with enhancement of the barrier function of human nasal epithelial cells via transcriptional up-regulation of TJ proteins.124
8. Conclusions and future directions
Considering their involvement in several key functions of the cells including barrier regulation, proliferation, migration, survival, and differentiation, cell-cell junctions need to be studied in more detail. Their aberrant expressions are not only a cause of several diseases but also reliable markers for diagnosing these illnesses. The current review portrays the reciprocal regulation of Akt and Src in the maintenance of AJs and TJs. However, these mechanisms have been studied in isolation and, therefore, it is not clear how distinct signaling mechanisms cross-talk with one another. Much work remains to define the contribution of individual signaling molecules which can then be targeted by pharmacological or genetic modulation to treat VP-related diseases. Hence, further research is warranted on the signaling pathways involved in the regulation of cell-cell junction. Finally, the engineering of monoclonal antibodies against substantial cell junction elements can pave the way for targeted therapies.
Acknowledgments
Funding provided by the NHLBI grant R01HL103952 and NCATS grant UL1TR002378 to PRS and NEI grant R01EY028569 provided to SPN are acknowledged.
Funding Statement
This work was supported by the National Center for Advancing Translational Sciences [UL1TR002378]; National Eye Institute [R01EY028569]; National Heart, Lung, and Blood Institute [R01HL103952].
Disclosure statement
Authors declare that there are no financial or other conflicts of interest exist.
References
- 1.Vickaryous MK, Hall BK.. Human cell type diversity, evolution, development, and classification with special reference to cells derived from the neural crest. Biol Rev Camb Philos Soc. 2006;81:1–18. doi: 10.1017/S1464793106007068. [DOI] [PubMed] [Google Scholar]
- 2.Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, Bodenmiller B, Campbell P, Carninci P, Clatworthy M, et al. The human cell atlas. Elife. 2017;6:e27041. doi: 10.7554/eLife.27041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archibald JM. Endosymbiosis and eukaryotic cell evolution. Curr Biol. 2015;25:R911–21. doi: 10.1016/j.cub.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 4.Purcell A. Basic Biology: an Introduction. of New Zealand: Knowledge on Life National Library; 2018. New Zealand ISBN Agency, National Library of New Zealand. [Google Scholar]
- 5.Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- 6.Bailey R. Types of cells in the human body. ThoughtCo, Aug. 27, 2020, thoughtco.com/types-of-cells-in-the-body-373388. [Google Scholar]
- 7.Tyler S. Epithelium–the primary building block for metazoan complexity. Integr Comp Biol. 2003;43:55–63. doi: 10.1093/icb/43.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira AR, Felgueiras J, Fardilha M. Signaling pathways in anchoring junctions of epithelial cells: cell-to-cell and cell-to-extracellular matrix interactions. J Recept Signal Transduct Res. 2015;35:67–75. doi: 10.3109/10799893.2014.931426. [DOI] [PubMed] [Google Scholar]
- 9.Dyer LA, Patterson C. Development of the endothelium: an emphasis on heterogeneity. Semin Thromb Hemost. 2010;36:227–235. doi: 10.1055/s-0030-1253446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adil MS, Somanath PR. Endothelial permeability assays in vitro. In: . Methods in Molecular Biology. Springer, New York, NY. doi: 10.1007/7651_2020_309. [DOI] [PubMed] [Google Scholar]
- 11.Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci. Sep 7;20(18):4411. doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minami T, Aird WC. Endothelial cell gene regulation. Trends Cardiovasc Med. 2005;15:174–184. doi: 10.1016/j.tcm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Sahni SK. Endothelial cell infection and hemostasis. Thromb Res. 2007;119:531–549. doi: 10.1016/j.thromres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Overeem Aw, Bryant Dm, Van Ijzendoorn Sc. Mechanisms of Apical-Aug. doi: 10.1016/j.tcb.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Charras G, Yap AS. Tensile forces and mechanotransduction at cell-cell junctions. Curr Biol. 2018;28:R445–r57. doi: 10.1016/j.cub.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Garrido-Urbani S, Bradfield PF, Imhof BA. Tight junction dynamics: the role of junctional adhesion molecules (JAMs). Cell Tissue Res. 2014;355:701–715. doi: 10.1007/s00441-014-1820-1. [DOI] [PubMed] [Google Scholar]
- 17.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dicks LMT, Dreyer L, Smith C, van Staden AD, Review: A. The fate of bacteriocins in the human gastro-intestinal tract: do they cross the gut-blood barrier? Front Microbiol. 2018;9:2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnittler HJ. Structural and functional aspects of intercellular junctions in vascular endothelium. Basic Res Cardiol. 1998;93 Suppl 3:30–39. doi: 10.1007/s003950050205. [DOI] [PubMed] [Google Scholar]
- 21.Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol. 2010;2010:402593. doi: 10.1155/2010/402593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 23.Lampugnani MG. Endothelial cell-to-cell junctions: adhesion and signaling in physiology and pathology. Cold Spring Harb Perspect Med. 2012 Oct 1;2(10):a006528. doi: 10.1101/cshperspect.a006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tehrani M, Sarvestani AS. Force-driven growth of intercellular junctions. J Theor Biol. 2017;421:101–111. doi: 10.1016/j.jtbi.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Sawada N. Tight junction-related human diseases. Pathol Int. 2013;63:1–12. doi: 10.1111/pin.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendonsa AM, Na TY, Gumbiner BM. E-cadherin in contact inhibition and cancer. Oncogene. 2018;37:4769–4780. doi: 10.1038/s41388-018-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krüger-Genge A, Dietze S, Yan W, Liu Y, Fang L, Kratz K, Lendlein A, Jung F. Endothelial cell migration, adhesion and proliferation on different polymeric substrates. Clin Hemorheol Microcirc. 2018;70:511–529. doi: 10.3233/CH-189317. [DOI] [PubMed] [Google Scholar]
- 28.Puliafito A, Hufnagel L, Neveu P, Streichan S, Sigal A, Fygenson DK, Shraiman BI. Collective and single cell behavior in epithelial contact inhibition. Proc Natl Acad Sci U S A. 2012;109:739–744. doi: 10.1073/pnas.1007809109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevenson BR, Paul DL. The molecular constituents of intercellular junctions. Curr Opin Cell Biol. 1989;1:884–891. doi: 10.1016/0955-0674(89)90054-9. [DOI] [PubMed] [Google Scholar]
- 30.Runkle EA, Mu D. Tight junction proteins: from barrier to tumorigenesis. Cancer Lett. 2013;337:41–48. doi: 10.1016/j.canlet.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sturtzel C. Endothelial Cells. Adv Exp Med Biol. 2017;1003:71–91. [DOI] [PubMed] [Google Scholar]
- 32.Firestone GL, Kapadia BJ. Minireview: steroid/nuclear receptor-regulated dynamics of occluding and anchoring junctions. Mol Endocrinol. 2014;28:1769–1784. doi: 10.1210/me.2014-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018 Jan 2;10(1):a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alberts BM. Cell. Encyclopaedia Britannica. 2020. https://www.britannica.com/science/cell-biology [Google Scholar]
- 35.Campbell HK, Maiers JL, DeMali KA. Interplay between tight junctions & adherens junctions. Exp Cell Res. 2017;358:39–44. doi: 10.1016/j.yexcr.2017.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lechler T. Adherens junctions and stem cells. Subcell Biochem. 2012;60:359–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francis H, Kennedy L, Alpini G. Dual ablation of β- and γ-catenin: critical regulators of junctions and their functions. Hepatology. 2018;67:2079–2081. doi: 10.1002/hep.29761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollard TD, Earnshaw WC, Lippincott-Schwartz J, Johnson GT, editors. Chapter 31 - Intercellular junctions. Cell biology. Third ed. Elsevier; 2017. p. 543–553. [Google Scholar]
- 40.Lien WH, Klezovitch O, Vasioukhin V. Cadherin-catenin proteins in vertebrate development. Curr Opin Cell Biol. 2006;18:499–506. doi: 10.1016/j.ceb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Zaidel-Bar R. Cadherin adhesome at a glance. J Cell Sci. 2013;126:373–378. doi: 10.1242/jcs.111559. [DOI] [PubMed] [Google Scholar]
- 42.Hulpiau P, van Roy F. Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol. 2009;41:349–369. doi: 10.1016/j.biocel.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Brigidi GS, Bamji SX. Cadherin-catenin adhesion complexes at the synapse. Curr Opin Neurobiol. 2011;21:208–214. doi: 10.1016/j.conb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 45.Leckband D, Sivasankar S. Biophysics of cadherin adhesion. Subcell Biochem. 2012;60:63–88. [DOI] [PubMed] [Google Scholar]
- 46.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 47.van Roy F. Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer. 2014;14:121–134. doi: 10.1038/nrc3647. [DOI] [PubMed] [Google Scholar]
- 48.Bravi L, Dejana E, Lampugnani MG. VE-cadherin at a glance. Cell Tissue Res. 2014;355:515–522. doi: 10.1007/s00441-014-1843-7. [DOI] [PubMed] [Google Scholar]
- 49.Vincent PA, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: adhesion at arm’s length. Am J Physiol Cell Physiol. 2004;286:C987–97. [DOI] [PubMed] [Google Scholar]
- 50.McCrea PD, Gu D. The catenin family at a glance. J Cell Sci. 2010;123:637–642. doi: 10.1242/jcs.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCrea PD, Gottardi CJ. Beyond β-catenin: prospects for a larger catenin network in the nucleus. Nat Rev Mol Cell Biol. 2016;17:55–64. doi: 10.1038/nrm.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu G. p120-Catenin: a novel regulator of innate immunity and inflammation. Crit Rev Immunol. 2012;32:127–138. doi: 10.1615/CritRevImmunol.v32.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delva E, Tucker DK, Kowalczyk AP. The desmosome. Cold Spring Harb Perspect Biol. 2009;1:a002543. doi: 10.1101/cshperspect.a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Pascalis C, Etienne-Manneville S, Weaver VM. Single and collective cell migration: the mechanics of adhesions. Mol Biol Cell. 2017;28:1833–1846. doi: 10.1091/mbc.e17-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H, Labouesse M. The making of hemidesmosome structures in vivo. Dev Dyn. 2010;239:1465–1476. [DOI] [PubMed] [Google Scholar]
- 56.Uchil PD, Pawliczek T, Reynolds TD, Ding S, Hinz A, Munro JB, Huang F, Floyd RW, Yang H, Hamilton WL, et al. TRIM15 is a focal adhesion protein that regulates focal adhesion disassembly. J Cell Sci. 2014;127:3928–3942. doi: 10.1242/jcs.143537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 58.Fan J, Tatum R, Hoggard J, Chen YH. Claudin-7 modulates Cl(-) and Na(+) homeostasis and WNK4 expression in renal collecting duct cells. Int J Mol Sci. 2019 Aug 3;20(15):3798. doi: 10.3390/ijms20153798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venugopal S, Anwer S, Szászi K. Claudin-2: roles beyond permeability functions. Int J Mol Sci. 2019;20(22):5655. doi: 10.3390/ijms20225655.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soini Y. Claudins in lung diseases. Respir Res. 2011;12:70. doi: 10.1186/1465-9921-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Citi S, Sabanay H, Kendrick-Jones J, Geiger B. Cingulin: characterization and localization. J Cell Sci. 1989;93:107–122. [DOI] [PubMed] [Google Scholar]
- 62.Negri AL. Role of claudins in renal calcium management and handling. Vol. 35. Nefrología (Madr): Cantabria; 2015. no.4. [DOI] [PubMed] [Google Scholar]
- 63.Díaz-Coránguez M, Liu X, Antonetti DA. Tight junctions in cell proliferation. Int J Mol Sci. 2019, 20(23), 5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergelson JM. Intercellular junctional proteins as receptors and barriers to virus infection and spread. Cell Host Microbe. 2009;5:517–521. doi: 10.1016/j.chom.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. [DOI] [PubMed] [Google Scholar]
- 66.Vasileva E, Sluysmans S, Bochaton-Piallat ML, Citi S. Cell-specific diversity in the expression and organization of cytoplasmic plaque proteins of apical junctions. Ann N Y Acad Sci. 2017;1405:160–176. doi: 10.1111/nyas.13391. [DOI] [PubMed] [Google Scholar]
- 67.Zheng K, Trivedi M, Siahaan TJ. Structure and function of the intercellular junctions: barrier of paracellular drug delivery. Curr Pharm Des. 2006;12:2813–2824. doi: 10.2174/138161206777947722. [DOI] [PubMed] [Google Scholar]
- 68.Givens C, Tzima E. Vessels with cingulin are leakproof. Arterioscler Thromb Vasc Biol. 2016;36:584–585. doi: 10.1161/ATVBAHA.116.307238. [DOI] [PubMed] [Google Scholar]
- 69.Keon BH, Schäfer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157–165. doi: 10.1016/j.semcdb.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 72.Tsukita S, Tanaka H, Tamura A. The Claudins: from Tight Junctions to Biological Systems. Trends Biochem Sci. 2019;44:141–152. doi: 10.1016/j.tibs.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Bazzoni G. Pathobiology of junctional adhesion molecules. Antioxid Redox Signal. 2011;15:1221–1234. doi: 10.1089/ars.2010.3867. [DOI] [PubMed] [Google Scholar]
- 74.Reglero-Real N, Colom B, Bodkin JV, Nourshargh S. Endothelial cell junctional adhesion molecules: role and regulation of expression in inflammation. Arterioscler Thromb Vasc Biol. 2016;36:2048–2057. doi: 10.1161/ATVBAHA.116.307610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X, Lu S, Nagy JI. Direct association of connexin36 with zonula occludens-2 and zonula occludens-3. Neurochem Int. 2009;54:393–402. doi: 10.1016/j.neuint.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bleich M, Günzel D. Physiology, pathophysiology, and clinical impact of claudins. Pflugers Arch. 2017;469:1–2. doi: 10.1007/s00424-016-1918-2. [DOI] [PubMed] [Google Scholar]
- 79.Rosenthal R, Günzel D, Theune D, Czichos C, Schulzke JD, Fromm M. Water channels and barriers formed by claudins. Ann N Y Acad Sci. 2017;1397:100–109. doi: 10.1111/nyas.13383. [DOI] [PubMed] [Google Scholar]
- 80.Milatz S. A novel claudinopathy based on claudin-10 mutations. Int J Mol Sci. 2019 Oct 30;20(21):5396. doi: 10.3390/ijms20215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun L, Feng L, Cui J. Increased expression of claudin-17 promotes a malignant phenotype in hepatocyte via Tyk2/Stat3 signaling and is associated with poor prognosis in patients with hepatocellular carcinoma. Diagn Pathol. 2018;13:72. doi: 10.1186/s13000-018-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conrad MP, Piontek J, Günzel D, Fromm M, Krug SM. Molecular basis of claudin-17 anion selectivity. Cell Mol Life Sci. 2016;73:185–200. doi: 10.1007/s00018-015-1987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schlingmann B, Molina SA, Koval M. Claudins: gatekeepers of lung epithelial function. Semin Cell Dev Biol. 2015;42:47–57. doi: 10.1016/j.semcdb.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tawar RG, Colpitts CC, Lupberger J, El-Saghire H, Zeisel MB, Baumert TF. Claudins and pathogenesis of viral infection. Semin Cell Dev Biol. 2015;42:39–46. doi: 10.1016/j.semcdb.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 85.Sedwick C. Claudins get a closer look. J Gen Physiol. 2018;150:893. doi: 10.1085/jgp.201812136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krug SM, Günzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell Mol Life Sci. 2012;69:2765–2778. doi: 10.1007/s00018-012-0949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turksen K, Troy TC. Permeability barrier dysfunction in transgenic mice overexpressing claudin 6. Development. 2002;129:1775–1784. [DOI] [PubMed] [Google Scholar]
- 88.Van Itallie CM, Anderson JM. Claudin interactions in and out of the tight junction. Tissue Barriers. 2013;1:e25247. doi: 10.4161/tisb.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cording J, Berg J, Käding N, Bellmann C, Tscheik C, Westphal JK, Milatz S, Gunzel D, Wolburg H, Piontek J, et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci. 2013;126:554–564. doi: 10.1242/jcs.114306. [DOI] [PubMed] [Google Scholar]
- 90.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 91.Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol. 2001;281:C388–97. doi: 10.1152/ajpcell.2001.281.2.C388. [DOI] [PubMed] [Google Scholar]
- 92.Khan N, Asif AR. Transcriptional regulators of claudins in epithelial tight junctions. Mediators Inflamm. 2015;2015:219843. doi: 10.1155/2015/219843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gehne N, Lamik A, Lehmann M, Haseloff RF, Andjelkovic AV, Blasig IE. Cross-over endocytosis of claudins is mediated by interactions via their extracellular loops. PLoS One. 2017;12:e0182106. doi: 10.1371/journal.pone.0182106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 95.Marcelino Cereijido JMA. Tight junctions. 2nd ed. CRC Press; CRC Press, Florida, USA: 2001. [Google Scholar]
- 96.Zhu L, Han J, Li L, Wang Y, Li Y, Zhang S. Claudin family participates in the pathogenesis of inflammatory bowel diseases and colitis-associated colorectal cancer. Front Immunol. 2019;10:1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kwon MJ. Emerging roles of claudins in human cancer. Int J Mol Sci. 2013;14:18148–18180. doi: 10.3390/ijms140918148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rudraraju M, Narayanan SP, Somanath PR. Regulation of blood-retinal barrier cell-junctions in diabetic retinopathy. Pharmacol Res. 2020;161:105115. doi: 10.1016/j.phrs.2020.105115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 100.Gowrikumar S, Singh AB, Dhawan P. Role of claudin proteins in regulating cancer stem cells and chemoresistance-potential implication in disease prognosis and therapy. Int J Mol Sci. 2019 Dec 20;21(1):53. doi: 10.3390/ijms21010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Osunkoya AO, Cohen C, Lawson D, Picken MM, Amin MB, Young AN. Claudin-7 and claudin-8: immunohistochemical markers for the differential diagnosis of chromophobe renal cell carcinoma and renal oncocytoma. Hum Pathol. 2009;40:206–210. doi: 10.1016/j.humpath.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 102.Adil MS, Somanath PR. Vascular permeability assays in vivo. Methods Mol Biol. 2020. Aug 18. doi: 10.1007/7651_2020_310. [DOI] [PubMed] [Google Scholar]
- 103.Park-Windhol C, D’Amore PA. Disorders of vascular permeability. Annu Rev Pathol. 2016;11:251–281. doi: 10.1146/annurev-pathol-012615-044506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alwhaibi A, Verma A, Adil MS, Somanath PR. The unconventional role of Akt1 in the advanced cancers and in diabetes-promoted carcinogenesis. Pharmacol Res. 2019;145:104270. doi: 10.1016/j.phrs.2019.104270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Artham S, Gao F, Verma A, Alwhaibi A, Sabbineni H, Hafez S, Ergul A, Somanath PR. Endothelial stromelysin1 regulation by the forkhead box-O transcription factors is crucial in the exudative phase of acute lung injury. Pharmacol Res. 2019;141:249–263. doi: 10.1016/j.phrs.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Artham S, Verma A, Newsome AS, Somanath PR. Patients with acute respiratory distress syndrome exhibit increased stromelysin1 activity in the blood samples. Cytokine. 2020;131:155086. doi: 10.1016/j.cyto.2020.155086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao F, Alwhaibi A, Artham S, Verma A, Somanath PR. Endothelial Akt1 loss promotes prostate cancer metastasis via beta-catenin-regulated tight-junction protein turnover. Br J Cancer. 2018;118:1464–1475. doi: 10.1038/s41416-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao F, Alwhaibi A, Sabbineni H, Verma A, Eldahshan W, Somanath PR. Suppression of Akt1-beta-catenin pathway in advanced prostate cancer promotes TGFbeta1-mediated epithelial to mesenchymal transition and metastasis. Cancer Lett. 2017;402:177–189. doi: 10.1016/j.canlet.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao F, Artham S, Sabbineni H, Al-Azayzih A, Peng XD, Hay N, Adams RH, Byzova TV, Somanath PR. Akt1 promotes stimuli-induced endothelial-barrier protection through FoxO-mediated tight-junction protein turnover. Cell Mol Life Sci. 2016;73:3917–3933. doi: 10.1007/s00018-016-2232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao F, Sabbineni H, Artham S, Somanath PR. Modulation of long-term endothelial-barrier integrity is conditional to the cross-talk between Akt and Src signaling. J Cell Physiol. 2017;232:2599–2609. doi: 10.1002/jcp.25791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kottke MA, Walters TJ. Where’s the leak in vascular barriers? A review. Shock. 2016;46:20–36. doi: 10.1097/SHK.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 113.Goddard LM, Iruela-Arispe ML. Cellular and molecular regulation of vascular permeability. Thromb Haemost. 2013;109:407–415. doi: 10.1160/TH12-09-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol. 2013;3:211. doi: 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 116.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 117.Klomp JE, Shaaya M, Matsche J, Rebiai R, Aaron JS, Collins KB, Huyot V, Gonzalez AM, Muller WA, Chew T-L, et al. Time-variant SRC kinase activation determines endothelial permeability response. Cell Chem Biol. 2019;26:1081–94.e6. doi: 10.1016/j.chembiol.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao F, Al-Azayzih A, Somanath PR. Discrete functions of GSK3alpha and GSK3beta isoforms in prostate tumor growth and micrometastasis. Oncotarget. 2015;6:5947–5962. doi: 10.18632/oncotarget.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sabbineni H, Verma A, Artham S, Anderson D, Amaka O, Liu F, Narayanan SP, Somanath PR. Pharmacological inhibition of beta-catenin prevents EndMT in vitro and vascular remodeling in vivo resulting from endothelial Akt1 suppression. Biochem Pharmacol. 2019;164:205–215. doi: 10.1016/j.bcp.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sabbineni H, Verma A, Somanath PR. Isoform-specific effects of transforming growth factor beta on endothelial-to-mesenchymal transition. J Cell Physiol. 2018;233:8418–8428. doi: 10.1002/jcp.26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamamoto T, Kojima T, Murata M, Takano K, Go M, Hatakeyama N, Chiba H, Sawada N. p38 MAP-kinase regulates function of gap and tight junctions during regeneration of rat hepatocytes. J Hepatol. 2005;42:707–718. doi: 10.1016/j.jhep.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 122.Bell CE, Watson AJ, Schubert M. p38 MAPK regulates cavitation and tight junction function in the mouse blastocyst. PLoS One. 2013;8:e59528. doi: 10.1371/journal.pone.0059528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eckert JJ, McCallum A, Mears A, Rumsby MG, Cameron IT, Fleming TP. PKC signalling regulates tight junction membrane assembly in the pre-implantation mouse embryo. Reproduction. 2004;127:653–667. doi: 10.1530/rep.1.00150. [DOI] [PubMed] [Google Scholar]
- 124.Koizumi J, Kojima T, Ogasawara N, Kamekura R, Kurose M, Go M, Harimaya A, Murata M, Osanai M, Chiba H, et al. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol Pharmacol. 2008;74:432–442. doi: 10.1124/mol.107.043711. [DOI] [PubMed] [Google Scholar]


