Abstract
A symptomatic patient with atrial fibrillation and Cheyne-Stokes respiration (CSR) was implanted with a transvenous phrenic nerve stimulation (TPNS) device—the remedē System—that is indicated for adult patients with moderate to severe central sleep apnea. Sleep recordings demonstrated that TPNS eliminated periodic breathing by activating the diaphragm and stabilizing respiratory patterns. These recordings of preprogrammed periods on versus off TPNS illustrate prompt (1) stabilization of tidal airflow, respiratory effort, and oxygenation as stimulation amplitude increased stepwise and (2) recurrence of CSR immediately after TPNS deactivated. Despite differences in respiratory patterns, minute ventilation was comparable during periods on and off TPNS. These findings suggest that diaphragmatic pacing entrains ventilation without disrupting sleep, accounting for observed improvements in periodic breathing, gas exchange, sleep architecture, and quality of life. Effective means to relieve CSR could potentially mitigate nocturnal cardiovascular stress and disease progression.
Citation:
Schwartz AR, Sgambati FP, James KJ, et al. Novel phrenic nerve stimulator treats Cheyne-Stokes respiration: polysomnographic insights. J Clin Sleep Med. 2020;16(5):817–820.
INTRODUCTION
Cheyne-Stokes respiration (CSR) and central sleep apnea (CSA; CSR/CSA) are characterized by a periodic loss of respiratory pump muscle activity. Distinct CSR/CSA patterns are common in patients with underlying cardiovascular and neurologic disease1 and with exposure to hypoxia at altitude.2 In patients with cardiovascular disease, CSR/CSA has been associated with marked increases in heart failure readmissions,3 mortality,4 and clinical morbidity from sleep disturbance, fatigue, and impaired quality of life.1 Abundant physiologic, clinical, and epidemiologic evidence suggests that CSR/CSA with attendant intermittent hypoxemia, recurrent arousals, and surges in sympathetic activity can accelerate progression of cardiovascular disease.1 Nonetheless, effective options to treat this spectrum of disorders remain limited and have been restricted even further by findings that adaptive servo-ventilation (ASV) is associated with excess mortality in patients with heart failure due to systolic dysfunction.5 A recent randomized clinical trial offers an alternative and novel approach to treating CSR/CSA with transvenous phrenic nerve stimulation (TPNS).6 TPNS led to substantial improvements in CSR/CSA, sleep architecture, and quality of life in patients with and without heart failure, leading to Food and Drug Administration approval. The current case report describes the physiologic mechanisms of action for TPNS, based on detailed analysis of sleep recordings demonstrating CSR improvement.
REPORT OF CASE
A 78-year-old man presented to an outside sleep center with loud snoring, sleep maintenance, insomnia, and daytime fatigue. His body mass index was 29 kg/m2 and his apnea-hypopnea index (AHI) was 57 events/h with predominantly CSR/CSA events (central, mixed, and obstructive apnea indices: 17, 7, and 14 events/h; hypopnea index: 19 events/h). He had chronic atrial fibrillation despite several left atrial ablation procedures. His vital signs were normal. His ventricular rate was well controlled with metoprolol and digoxin. He was clinically euvolemic without rales or edema. His echocardiogram showed a left ventricular ejection fraction (LVEF) of 55–60% and a mildly enlarged left atrium. He was treated initially with ASV but continued to have residual sleep-disordered breathing events (14 events/h) and daytime somnolence, prompting consideration of alternative treatment modalities.
A baseline sleep study was repeated and showed an overall AHI of 32 events/h with predominantly CSA resembling CSR (central, mixed, and obstructive apnea indices: 14, 6, and 5 events/h; hypopnea index: 7 events/h), an oxygen desaturation index (≥4%) of 26 events/h, a low peripheral oxygen saturation (SpO2) of 85%, time spent with SpO2 below 90% (T-90%) of 1% and a prolonged lung-to-finger circulation time of 35–40 seconds. The patient was implanted with a TPNS device (remedē; Respicardia, Inc, Minnetonka, MN), and returned for follow-up every 6 months. A sleep study after 24 months of TPNS therapy showed an AHI of 3 events/h, an oxygen desaturation index (≥4%) of 2 events/h, a low SpO2 of 89% (T-90% of 0%), with a modest reduction in circulation time to 25–30 seconds.
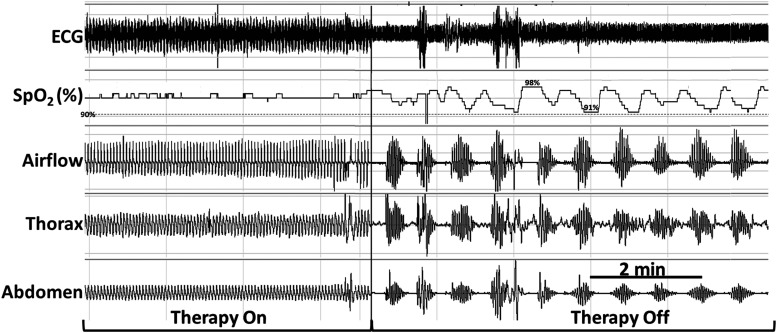
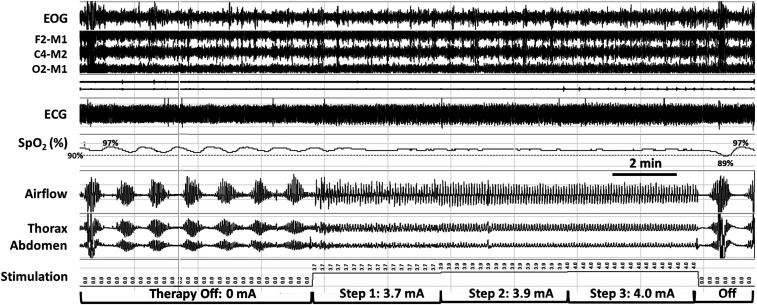
The remedē device applied TPNS automatically between 11:00 pm and 6:00 am whenever the patient was recumbent and motionless (Table 1). This TPNS delivery system resulted in high therapeutic adherence, as logged by the remedē system at 6.7 ± 0.2 hours/night. When the device automatically ceased stimulation at 6:00 am, CSR resumed (Figure 1). By design, TPNS paused during movement arousals and resumed therapy once the patient returned to a recumbent, motionless state (Figure 2). Stimulation began at an initial programmed amplitude of 3.7 mA and was automatically increased stepwise every 2 minutes until respiratory capture occurred or once TPNS reached a programmed maximum of 4.0 mA. TPNS rapidly stabilized respiratory patterns at 3.9 and 4.0 mA stimulation (Figure 2). To determine the impact of TPNS on minute ventilation, we assessed ventilation by computing the root mean square of the nasal airflow (pressure cannula) signal at 1-minute intervals.7 Compared with periods of stable breathing on TPNS, minute ventilation did not change significantly during adjacent periods off TPNS (on vs off; mean ± SE: 95.6% ± 1.4% vs 100% ± 4.1%, Figure 1; 100.0% ± 0.01% vs 100.0% ± 0.4%, P = .84; Figure 2).
Table 1.
Automated features of the phrenic nerve pacemaker.
| Automated Sensor Feature | Condition (Based on Programmed Thresholds) | Required for Therapy Delivery | Used for Stimulation Amplitude Modulation |
|---|---|---|---|
| Pitch | Recumbent | X | X |
| Activity | Inactive | X | X |
| Time of day | Time within typical sleep window | X | X |
| Lead impedance | Impedance within an acceptable range | X | |
| Respiration | Transthoracic impedance measurements within a preset range | X | |
| Body position | Position tracked within quadrant angles (supine, left, right, prone) | X | |
| Week number | Stimulation amplitude increased over several weeks allows for acclimatization | X |
Once required conditions are met, the device automatically increases or decreases stimulation current levels based on information about respiration, body pitch/position, activity, and/or time on therapy.
Figure 1. Recurrence of Cheyne-Stokes respiration with periodic oxyhemoglobin desaturations and transient arousals following the cessation of phrenic nerve stimulation.
The preprogrammed cessation of phrenic nerve stimulation at 6:00 am (see vertical line) resulted in the prompt recurrence of Cheyne-Stokes respiration with periodic oxyhemoglobin desaturations and transient arousals from sleep (not shown). Rhythmic stimulation bursts are seen in ECG signal during the Therapy On segment (left side) but not during the Therapy Off segment (right side). Occasional movement artifacts appear in the first several minutes of the Therapy On period. Signals include ECG, SpO2 (pulse oximetry, %), airflow (nasal pressure cannula), and thoracic and abdominal piezoelectric girth sensors. ECG = electrocardiogram; SpO2 = peripheral oxygen saturation.
Figure 2. Automatic stepwise increases in stimulation amplitude stabilize tidal airflow, respiratory effort, and oxyhemoglobin saturation as ventilation is captured progressively.
A period of stepwise increases in phrenic nerve stimulation amplitude (middle segment) is bracketed by periods off stimulation that began with sudden movement arousals (just before the start of recording segment; far left) and at end of stable breathing period (see movement artifact in thorax and abdomen; right). Automatic stepwise increases in stimulation amplitude stabilize tidal airflow, respiratory effort, and oxyhemoglobin saturation as ventilation is captured progressively. Signals include EOG, EEG derivations as shown, ECG, SpO2 (pulse oximetry, %), airflow (nasal pressure cannula), and thoracic and abdominal piezoelectric girth sensors. ECG = electrocardiogram; EOG = electrooculogram; SpO2 = peripheral oxygen saturation.
Comparing changes from the baseline and 24-month sleep studies, the patient’s sleep efficiency increased from 66% to 84%, rapid eye movement sleep increased from 10% to 18% of total sleep time (TST), and mean SpO2 increased from 93.1% to 95.2% during sleep (with reductions in the amount of time spent at SpO2 levels <94% from 65% to 8%, <92% from 12% to 1%, and <90% from 1% to 0% of TST). The patient remained in chronic atrial fibrillation without any change in his cardiac regimen or rhythm intervention over the course of the study period.
On clinical follow-up, the patient reported sound sleep on TPNS therapy with resolution of insomnia and daytime hypersomnolence; he reported moderate improvement on his Patient Global Assessment scale, reductions in Fatigue Severity Scale from 3.4 to 2, and marked improvement in his European Quality of Life visual analog scale from 40 to 75 (EQ-5D-5L scale of 0 to 100).
DISCUSSION
In this patient with typical CSR, we demonstrate that a novel TPNS device automatically captures ventilation and eliminates periodic breathing. Progressive increases in TPNS amplitude stabilized tidal airflow, respiratory effort, and nocturnal oxygenation, whereas preprogrammed cessation of TPNS was associated with an immediate recurrence of CSR during sleep. The findings suggest that diaphragmatic stimulation stabilizes respiratory patterns, thereby eliminating periodic breathing and improving gas exchange, sleep quality, and quality of life in symptomatic patients with CSR/CSA.6
Findings in our patient are most consistent with a mechano- rather than chemoreceptor mechanism of ventilatory capture in both the time course and ventilatory dynamics during on/off transitions in TPNS. CSR rapidly extinguished as TPNS increased automatically to therapeutic levels (Figure 2) and recurred instantaneously upon cessation of TPNS (Figure 1 and Figure 2). Such immediate on/off responses can be best explained by inhibitory responses to lung inflation, once TPNS amplitude generated sufficient tidal volumes. In fact, we saw graded responses in tidal volume and increasing evidence of diaphragmatic capture with stepwise increases in stimulation amplitude (Figure 2). As automated increases in stimulation stabilized ventilation, our patient transitioned comfortably from lighter to deeper stages of continuous sleep. In contrast, we would have suspected delayed rather than immediate ventilatory capture if CO2 chemoreceptors at the ventral medullary surface mediated this response. If TPNS were to overdrive ventilation and extinguish ventilatory drive by making our patient hypocapneic, we would have also expected a marked increase in ventilation on compared with off TPNS, which was not the case. Thus, both the time course and ventilatory dynamics during TPNS transitions suggest a mechano- rather than chemoreceptor mechanism for stabilizing respiratory and sleep patterns.
To date, options for treating CSR/CSA have been hampered by limitations in therapeutic efficacy and/or safety concerns. While studies have documented some degree of therapeutic efficacy with oxygen, theophylline, and acetazolamide, concerns remain about proarrhythmic effects of these agents, particularly in patients with underlying cardiac disease.8 ASV, a form of noninvasive positive airway pressure (PAP) ventilation that was initially viewed as effective in treating CSR/CSA, has since been contraindicated in patients with heart failure with reduced ejection fraction after a large randomized controlled trial (RCT) showed excess cardiovascular and all-cause mortality.5 Continuous PAP (CPAP) therapy is often used in practice, although a single multicenter randomized study failed to meet its primary mortality endpoint despite improvements in sleep-disordered breathing indices.9 In contrast, a recent RCT studying safety and efficacy of TPNS demonstrated substantial, durable improvements in sleep-disordered breathing, sleep architecture, and quality of life with no apparent increase in cardiovascular events.10 Unlike PAP treatment strategies, TPNS also holds a potential advantage of mimicking normal respiratory activity with negative rather than positive inspiratory intrathoracic pressure excursions, which may be less likely to alter cardiovascular hemodynamics.
Clinicians should consider distinct advantages and potential disadvantages of TPNS in treating patients with CSR/CSA. Advantages include the following: (1) TPNS has been found to be highly efficacious in treating CSR/CSA by restoring inspiratory negative pressure swings in intrathoracic pressure; (2) TPNS can be used for moderate to severe CSA regardless of etiology (including reduced LVEF patients) or the presence of another implanted cardiovascular device; (3) the current TPNS system obviates the perennial issues with PAP nonadherence by activating automatically when patients are most likely to be asleep; and (4) the preprogrammed ramp in stimulation allows patients to fall asleep, even after a spontaneous arousal. Nonetheless, this ramp may allow for some residual apneic/hypopneic events to occur during periods in which the stimulation amplitude is progressively increasing. The system can be individually programmed with amplitudes increased as tolerated to minimize ramp-up time and residual periodic breathing events. Additional disadvantages could also include the fact that (1) the TPNS device requires a minimally invasive implant procedure and several follow-up visits to initialize and titrate therapy and that (2) TPNS can uncover upper airway obstruction during CSR/CSA, which impedes ventilatory entrainment and limits therapeutic efficacy. For this reason, patients are selected with a predominance of central rather than obstructive sleep-disordered breathing events. Under these circumstances, our findings suggest that the respiratory pattern can be stabilized without altering overall levels of ventilation during adjacent periods on and off TPNS. Nevertheless, we acknowledge that our estimates of minute ventilation might vary from night to night across sleep stages, and that our method for measuring relative differences in ventilation during TPNS have not been validated.
This case demonstrates that prompt improvements in both sleep and breathing patterns can be achieved and sustained for the duration of the night, even without patient involvement. These improvements produce documented reductions in recurrent arousals and surges in sympathetic activity and blood pressure, thereby mitigating nocturnal hemodynamic stress. The time course and ventilatory dynamics during on/off transitions in phrenic nerve stimulation illustrate that ventilation can be captured when sufficient stimulation amplitude is applied in a patient with a patent upper airway during sleep. This therapeutic option has emerged against a backdrop of known limitations of CPAP and safety concerns of ASV in patients with CSR and a low ejection fraction (<45%), making it one of the few treatment options available to patients, especially those with patients with a reduced LVEF. This report outlines a process for treating CSA/CSR with TPNS, potentially harnessing the power of sleep to mitigate cardiovascular stress and long-term cardiovascular outcomes.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Johns Hopkins Center for Interdisciplinary Sleep Research and Education. This study was funded by National Institutes of Health HL R01HL144859 and Respicardia, Inc. A.R.S. and R.D.B. have been paid consultants to Respicardia, Inc. Their arrangements were reviewed and managed by the Johns Hopkins Office of Policy Coordination. K.J.J., T.P.G., R.E.G., and S.E.J. are employees and have equity in Respicardia, Inc, the manufacturer of the TPNS device. N.S. reports no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the support from the Johns Hopkins Center for Interdisciplinary Sleep Research and Education (www.cisre.jhu.edu) in the conduct of the clinical trial that included this patient.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ASV
adaptive servo-ventilation
- CPAP
continuous positive airway pressure
- CSA
central sleep apnea
- CSR
Cheyne-Stokes respiration
- LVEF
left ventricular ejection fraction
- PAP
positive airway pressure
- RCT
randomized controlled trial
- SpO2
peripheral oxygen saturation
- TPNS
transvenous phrenic nerve stimulation
- TST
total sleep time
REFERENCES
- 1.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. 10.1016/j.jacc.2016.11.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pham LV, Meinzen C, Arias RS, et al. Cross-sectional comparison of sleep-disordered breathing in native peruvian highlanders and lowlanders. High Alt Med Biol. 2017;18(1):11–19. 10.1089/ham.2016.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18(7):534–540. 10.1016/j.cardfail.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36(23):1463–1469. 10.1093/eurheartj/ehu522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. 10.1056/NEJMoa1506459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974–982. 10.1016/S0140-6736(16)30961-8 [DOI] [PubMed] [Google Scholar]
- 7.Thurnheer R, Xie X, Bloch KE. Accuracy of nasal cannula pressure recordings for assessment of ventilation during sleep. Am J Respir Crit Care Med. 2001;164(10):1914–1919. 10.1164/ajrccm.164.10.2102104 [DOI] [PubMed] [Google Scholar]
- 8.Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. 10.5665/sleep.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025–2033. 10.1056/NEJMoa051001 [DOI] [PubMed] [Google Scholar]
- 10.Costanzo MR, Ponikowski P, Javaheri S, et al. Sustained 12 month benefit of phrenic nerve stimulation for central sleep apnea. Am J Cardiol. 2018;121(11):1400–1408. 10.1016/j.amjcard.2018.02.022 [DOI] [PubMed] [Google Scholar]