Abstract
Study Objectives:
Our aim was to evaluate the effect of lemborexant versus zolpidem tartrate extended release 6.25 mg (ZOL) or placebo (PBO) on postural stability, auditory awakening threshold (AAT), and cognitive performance (cognitive performance assessment battery [CPAB]).
Methods:
Healthy women (≥ 55 years) and men (≥ 65 years) were randomized, double-blind, to 1 of 4-period, single-dose crossover sequences, starting with lemborexant 5 mg (LEM5), 10 mg (LEM10), ZOL, or PBO. A ≥ 14-day washout followed all 4 treatments. Assessments were middle-of-the-night (MOTN) change from baseline in postural stability (primary prespecified comparison: LEM vs ZOL), AAT, absolute AAT, and CPAB for LEM5 and LEM10 versus ZOL and PBO; and morning change from baseline in postural stability and CPAB for LEM5 and LEM10 versus ZOL and PBO. Change from baseline measures were time-matched to a baseline night/morning when no study drug was administered.
Results:
MOTN: Mean MOTN change from baseline in body sway was significantly higher for ZOL versus both lemborexant doses. There were no differences among the treatments regarding decibels required to awaken a participant. LEM5 was not statistically different from PBO on any CPAB domain; LEM10 and ZOL showed poorer performance on some tests of attention and/or memory. Morning: Body sway and cognitive performance following LEM5 or LEM10 did not differ from PBO; body sway was significantly higher for ZOL than PBO. Rates of treatment-emergent adverse events were low; there were no serious adverse events.
Conclusions:
Lemborexant causes less postural instability than a commonly used sedative-hypnotic and does not impair the ability to awaken to auditory signals.
Clinical Trials Registration:
Registry: ClinicalTrials.gov; Name: Crossover Study to Evaluate the Effect of Lemborexant Versus Placebo and Zolpidem on Postural Stability, Auditory Awakening Threshold, and Cognitive Performance in Healthy Subjects 55 Years and Older; URL: https://clinicaltrials.gov/ct2/show/NCT03008447; Identifier: NCT03008447.
Citation:
Murphy P, Kumar D, Zammit G, Rosenberg R, Moline M. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765–773.
Keywords: attention, auditory awakening, lemborexant, memory, older adults, postural stability, zolpidem
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep-promoting drugs should facilitate sleep onset and maintenance without hindering the ability to awaken to salient stimuli, cause postural instability upon getting out of bed, or negatively impact performance on cognitive tasks. Lemborexant (E2006, DayvigoTM, Eisai Inc., Woodcliff Lake, NJ), an orally active dual orexin receptor antagonist recently approved by the Food and Drug Administration to treat insomnia,1 has demonstrated efficacy for treatment of insomnia while minimizing next-morning residual effects.
Study Impact: In this study of older adults, lemborexant did not interfere with awakening to an external stimulus, was associated with less body sway than zolpidem tartrate extended release upon middle-of-the-night awakening, and facilitated the return to sleep. There were no next-morning residual effects of lemborexant on postural stability or cognitive performance.
INTRODUCTION
Although many treatments for insomnia improve sleep onset,2 a need remains for agents that more effectively promote sleep throughout the night.3 New therapies should ideally be without the safety issues related to depression of the central nervous system, such as those seen with benzodiazepines and γ-aminobutyric acid (GABA)-ergic hypnotic agents.4 This is of particular importance in older adults, who may spend more time awake in the middle of the night (MOTN) than younger adults5 but who are also vulnerable to treatment-related cognitive and psychomotor impairments that can result in serious injury.6,7 New pharmacological treatments should effectively reduce wakefulness without hindering the ability to awaken to salient stimuli. In addition, once awakened during the night, there should ideally be minimal disruption to important cognitive functions, and the return to sleep after an awakening should be facilitated.3,7
Lemborexant (E2006), an orally active dual orexin receptor antagonist (DORA) recently approved by the Food and Drug Administration to treat insomnia,1 has demonstrated efficacy for treatment of insomnia while minimizing next-morning residual effects.8 In a recent phase 3 trial (SUNRISE-1; NCT02783729), lemborexant was superior to placebo (PBO) and zolpidem tartrate extended release 6.25 mg (ZOL) on measures of both sleep onset and maintenance and was effective in the latter half of the night in older individuals with insomnia.9
Here we present results of a clinical study that assessed important safety aspects of treatment with sleep-promoting drugs in the MOTN. These aspects include the ability to awaken to noises in the environment, stability upon getting out of bed (eg, to use the bathroom), and performance on tasks of memory and attention. Performing tests of postural stability and cognitive performance in the MOTN and repeating them upon morning awakening helps determine the extent to which the sleep-promoting drugs have residual morning effects on postural stability, memory, and attention.
METHODS
Design overview
This was a randomized, double-blind, double-dummy, placebo-controlled and active comparator, 4-period crossover study. The trial was conducted between November 21, 2016 and October 30, 2017 at 4 clinical sites in the United States. In the pre-randomization phase (up to 21 days), participants were screened for eligibility, including an adaptation/screening night spent in the sleep laboratory to identify symptoms of sleep apnea and periodic limb movement disorder (Figure 1). Each eligible participant had a baseline night, during which procedures identical to treatment nights were followed. This allowed for time-matched baselines of the auditory awakening threshold (AAT), postural stability, and cognitive performance assessments. The randomization phase (approximately 60 days) comprised four 1-day treatment periods, followed by a minimum 14-day washout between treatment periods 1 and 3, and a 14-day follow-up interval before the end-of-study visit.
Figure 1. Study design.
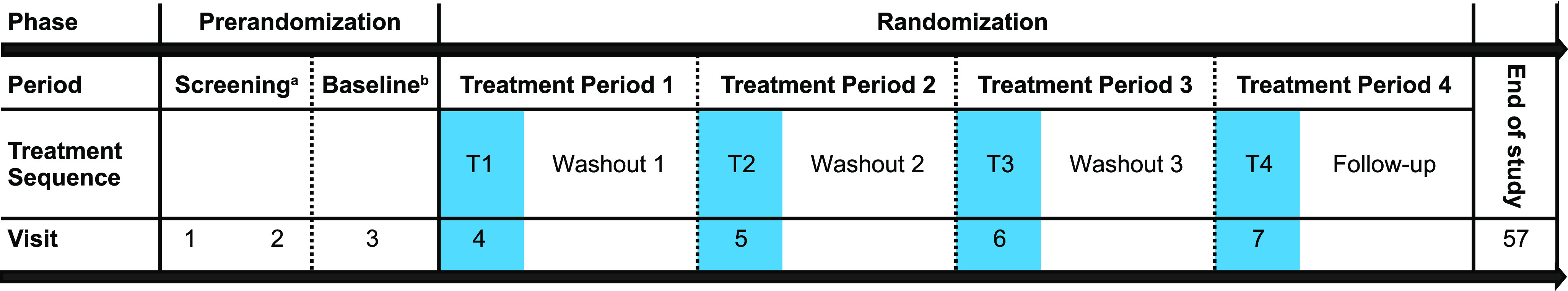
aAt screening visit 1, participants received a sleep diary, in which they provided information about the timing, quantity, and quality of the previous night’s sleep. Participants completed the sleep diary for at least 7 consecutive mornings within the first hour after morning waketime to confirm that the participant was a good sleeper without insomnia. At screening visit 2, an 8-hour PSG was performed at the participant’s mean habitual baseline to screen for symptoms of sleep apnea and/or periodic limb movement disorder. Participants were also introduced to the computerized CPAB for familiarization purposes. bBaseline assessments: 8-hour PSG with auditory awakening threshold, postural stability and CPAB at 4 hours postbedtime and postural stability and CPAB at 8 hours post bedtime. CPAB = cognitive performance test battery, PSG = polysomnography.
The trial protocol was approved by relevant institutional review boards and was conducted in accordance with principles of Good Clinical Practice and the Declaration of Helsinki and any applicable local regulations. Written informed consent was obtained from all participants after they received an explanation of study procedures, risks, and benefits. The trial was registered at ClinicalTrials.gov (identifier: NCT03008447). Prespecified primary and secondary outcomes were listed on the ClinicalTrials.gov website prior to study initiation.
Objectives
The primary objective of the study was to demonstrate that the mean change from baseline in postural stability when participants were awakened in the MOTN (at approximately 4 hours postdose) is significantly less after lemborexant 5 mg (LEM5) or lemborexant 10 mg (LEM10) than after ZOL. The secondary objectives of this study were to evaluate the effect of both doses of lemborexant compared with ZOL on postural stability within 5 minutes of morning waketime (at 8 hours postdose) and versus PBO when participants were awakened from sleep in the MOTN and upon morning awakening. Other secondary objectives were to evaluate the effect of LEM5 and LEM10 compared with ZOL and PBO on AAT in the MOTN; the effect of LEM5 and LEM10 compared with ZOL and PBO on cognitive performance when participants were awakened from sleep at approximately 4 hours postdose and within 15 minutes of morning waketime at 8 hours postdose; and the safety and tolerability of lemborexant. Exploratory objectives included return-to-sleep latency (RSL) for LEM5, LEM10, ZOL, and PBO after assessments were completed following awakening at approximately 4 hours postdose and comparison of the effect of ZOL versus PBO on postural stability at approximately 4 hours postdose for evaluation of assay sensitivity.
Participants
Healthy, nonsmoking older volunteers (women ≥ 55 years of age and men ≥ 65 years) were eligible to enroll in this study. Dose administration was based on the prescribing information for ZOL (6.25 mg zolpidem tartrate extended release is the approved dose for women and men > 65 years of age).10 The prevalence of insomnia is higher in women who are perimenopausal, late-menopausal, or postmenopausal compared with those who are premenopausal.11 The inclusion of women ≥ 55 years was intended, as this is an important demographic. The same age criteria were used in the pivotal phase 3 SUNRISE-1 trial (NCT02783729). Participants were required to have regular sleep timing and duration. Eligible participants must also have been able to detect a 1,000 Hz tone at 20 dB. Participants with an apnea-hypopnea index > 15 events/h of sleep or periodic limb movement with arousal index > 15 events/h of sleep as measured on the polysomnography (PSG) adaptation night were excluded. Additional details on enrollment criteria and concomitant drug/therapy are available in Supplementary Appendices S1 and S2.
Randomization and interventions
Each participant was randomized (1:1:1:1) to 1 of 4 treatment sequences, starting with LEM5, LEM10, ZOL, or PBO, using a Williams Latin square design.12 The dose of ZOL selected in this study was appropriate for the age and sex of the participants included in the study.10 Participants were administered a single oral dose of the assigned study drug within 5 minutes of mean habitual bedtime as determined by a sleep log completed prior to randomization. Information on concomitant drugs/therapy is available in Supplementary Appendix S2.
Measures
Participants who were asleep were awakened by an AAT test (secondary endpoint) at approximately 4 hours postdose/bedtime. This was followed by the assessment of postural stability (primary objective) and the computerized performance assessment battery (CPAB; secondary endpoint). If the participant was awake 4–4.5 hours postdose, the AAT was not administered, but subsequent assessments were conducted.
Postural stability
Postural stability assessments were conducted at the bedside within 5 minutes of being awakened in MOTN at approximately 4 hours postdose/bedtime (after the AAT) and upon morning awakening (or after an 8-hour time-in-bed period for those already awake 8 hours postbedtime). Body sway was measured using an ataxiameter connected to a cable around the participant’s waist.13 The participant was instructed to stand with feet shoulder-width apart and with eyes closed. The amount of body sway in 60 seconds was determined. A unit of body sway was defined as 1/3 degree angle of arc movement of the ataxiameter, with a higher number indicating more body sway (less postural stability). The participant’s foot positioning (distance between heels in centimeters) was documented at baseline, and the same foot positioning was maintained for all subsequent postural stability assessment trials. During the time that the participants were awake, ambient light levels were maintained at 80–100 lux using lighting fixtures or goggles (eg, if participants were escorted to use a bathroom facility).
Auditory awakening threshold
The AAT was initiated if the participant was in nonrapid eye movement stage 2 (N2). The technician allowed 5 minutes of N2, then initiated AAT. If there were not 5 minutes of N2-stage sleep by 4.5 hours postdose/postbedtime, the AAT was initiated regardless of sleep stage. If the participant was awake 4–4.5 hours postdose/postbedtime, the AAT was not administered, but subsequent assessments were conducted. Computer-generated tones of 1,000 Hz were delivered through earbuds with an intensity (loudness) starting at 15 dB, increasing every 15 seconds by 5 dB. When participants verbally responded, “I’m awake,” the dB level preceding that response was used as the threshold for awakening. If not awakened by the maximum tone of 105 dB, the technician awakened the participant.
Computerized cognitive performance assessment battery
The CPAB14 (secondary outcome) was administered after the postural stability test, starting within 15 minutes of the MOTN awakening (or after the postural stability test if the participant was already awake). Participants were trained on the CPAB tests before baseline. The CPAB comprised 9 tasks assessing various aspects of memory and attention. Output variables from the 9 tasks were combined to derive scores for 4 cognitive domains: power of attention, continuity of attention, quality of memory, and speed of memory retrieval. For each participant, on each trial and each task of the CPAB, the stimuli were presented in a different order, minimizing practice effects. Additional details are available in Supplementary Appendix S3.
Return-to-sleep latency
RSL was measured after participants completed the MOTN assessments. Overhead lights were turned off, and the time to return to sleep was measured by PSG. The RSL was defined as the duration in minutes (with 30-s precision) from the lights off after MOTN assessments (“second lights off”) to the first epoch of N2, nonrapid eye movement stage 3 (N3), or rapid eye movement (REM) sleep.
Adverse events
Safety was assessed over the study duration based on incidence of adverse events (AEs) and laboratory assessments, including blood chemistry, hematology, urinalysis, vital signs, and electrocardiograms. All AEs were graded for severity and relatedness to treatment.
Statistical analyses
Data for efficacy outcomes are presented for all participants who completed all 4 treatment periods (per protocol completers analysis set). Additional analyses with imputation assuming that missing values were missing at random (MAR) and, secondly, that missing values were missing not at random (MNAR) utilizing the complete case missing value pattern were performed. Safety outcomes are presented in the safety analysis set, defined as all participants who received at least 1 dose of lemborexant (5 or 10 mg) and had at least 1 postdose safety assessment.
For the primary outcome of postural stability, the effect of alcohol on body sway was used as a benchmark.15 This study had 97% power to detect a 7-unit difference between ZOL and lemborexant, with 48 participants, a 2-sided 0.05 significance level, and standard deviation (SD) of 12. A sequential gate-keeping procedure was used for the 2 doses of lemborexant compared with ZOL for body sway at approximately 4 hours postdose, where 5 mg was tested first. These calculations also ensured 97% power to detect a 7-unit treatment difference between lemborexant and PBO and that the assay sensitivity of the comparison of ZOL with PBO would be detectable (assumed to be at least a 14-unit change from baseline).
Statistical analyses included a repeated mixed-effects model for a crossover study. The model was adjusted for treatment, sequence (the order in which treatments were received), period (ie, of the 4 treatment periods), baseline (time-matched, ie, 4 and 8 hours postdose), time (hours postdose, ie, 4 and 8 hours), treatment × time, and baseline × time as fixed effects, and time with the participant as a random effect and a repeated effect for time, with the participant within sequence. The treatment-by-time interaction was used to construct the treatment comparisons at a specific time.
Because of potential effects of sleep inertia (defined as a physiological state of impaired cognitive and sensorimotor performance that is present immediately after awakening), during which an individual experiences feelings of drowsiness, disorientation, and a decline in motor dexterity,16 additional sensitivity analyses were conducted. These included analyses of AAT, excluding those who were awake or awakened from a sleep stage other than N2 and postural stability excluding those already awake at 4–4.5 hours postdose.
Additional statistical methods are presented in Supplementary Appendix S4.
RESULTS
Participant flow and baseline demographics
Of 163 participants who consented to participate, 100 (61.3%) failed the screening process and 63 (38.7%) were randomized to 1 of the 4 treatment sequences (Figure S1 in the supplemental material). In total, 53 (84.1%) participants completed the study (all 4 treatment periods and end-of-study follow-up) and 10 (15.9%) participants discontinued from the study. Three participants completed the 4 treatment periods but discontinued in the follow-up period; thus, a total of 56 of 63 (88.9%) participants were included in the analyses. Most participants were women (77.8%) and white (61.9%) (Table 1). Baseline demographics by treatment sequence are provided in Table S1.
Table 1.
Baseline demographics and characteristics.
| All Randomized Participants (n = 63) | |
|---|---|
| Age, years | |
| Mean | 63.5 (6.2) |
| Median | 63.0 |
| Range | 55, 80 |
| 55–64 years, n (%) | 35 (55.6) |
| ≥ 65 years, n (%) | 28 (44.4) |
| Sex, n (%) | |
| Men | 14 (22.2) |
| Women | 49 (77.8) |
| Race, n (%) | |
| White | 39 (61.9) |
| Black or African American | 18 (28.6) |
| Other | 6 (9.5) |
| Auditory awakening threshold (4 h), dB | 48.3 (23.1) |
| Body swaya | |
| 4 hours | 23.2 (16.4) |
| 8 hours | 25.4 (19.1) |
| Power of Attention, ms | |
| 4 hours | 1,400.9 (146.1) |
| 8 hours | 1,445.3 (232.8) |
| Continuity of Attention, unit | |
| 4 hours | 92.1 (3.1) |
| 8 hours | 91.9 (2.7) |
| Quality of Memory, unit | |
| 4 hours | 361.2 (63.7) |
| 8 hours | 334.3 (65.2) |
| Speed of Memory Retrieval, ms | |
| 4 hours | 4,586.9 (917.0) |
| 8 hours | 4,570.8 (902.0) |
Data are mean (standard deviation) unless stated otherwise.
A unit of body sway is defined as 1/3 degree angle of arc movement of the ataxiameter.
As there were so few dropouts, data are presented as observed cases/completers. Results were similar based on all randomized participants using both MAR and MNAR methods. Results based on the MNAR method are presented in Supplementary Appendix S5.
Safety outcome analyses
Postural stability as measured by body sway
Least squares mean change from time-matched baseline in body sway was statistically significantly larger (worse) for all active treatments in the MOTN (Figure 2) compared with PBO (P ≤ .05). Least squares mean differences (95% confidence interval [CI]) to PBO were 21.4 (15.8, 26.9), 6.8 (1.2, 12.3), and 9.3 (3.7, 14.8) for ZOL, LEM5, and LEM10, respectively. However, body sway was significantly worse with ZOL (19.6) compared with either dose of lemborexant (primary endpoint; LEM5 5.0, LEM10 7.5; P < .0001). Least squares mean differences (95% CI) to ZOL were 14.6 (9.0, 20.1) and 12.1 (6.5, 17.6) for LEM5 and LEM10, respectively. When assessed in the morning, neither dose of lemborexant was associated with significant change from baseline body sway, but body sway in the ZOL group (5.9) remained significantly greater than in PBO participants (−1.1; P < .05). Least squares mean differences (95% CI) to PBO in the morning were 7.0 (1.5, 12.6), 2.4 (−3.1, 8.0), and 1.8 (−3.8, 7.3) for ZOL, LEM5, and LEM10, respectively.
Figure 2. Change from baseline body sway.
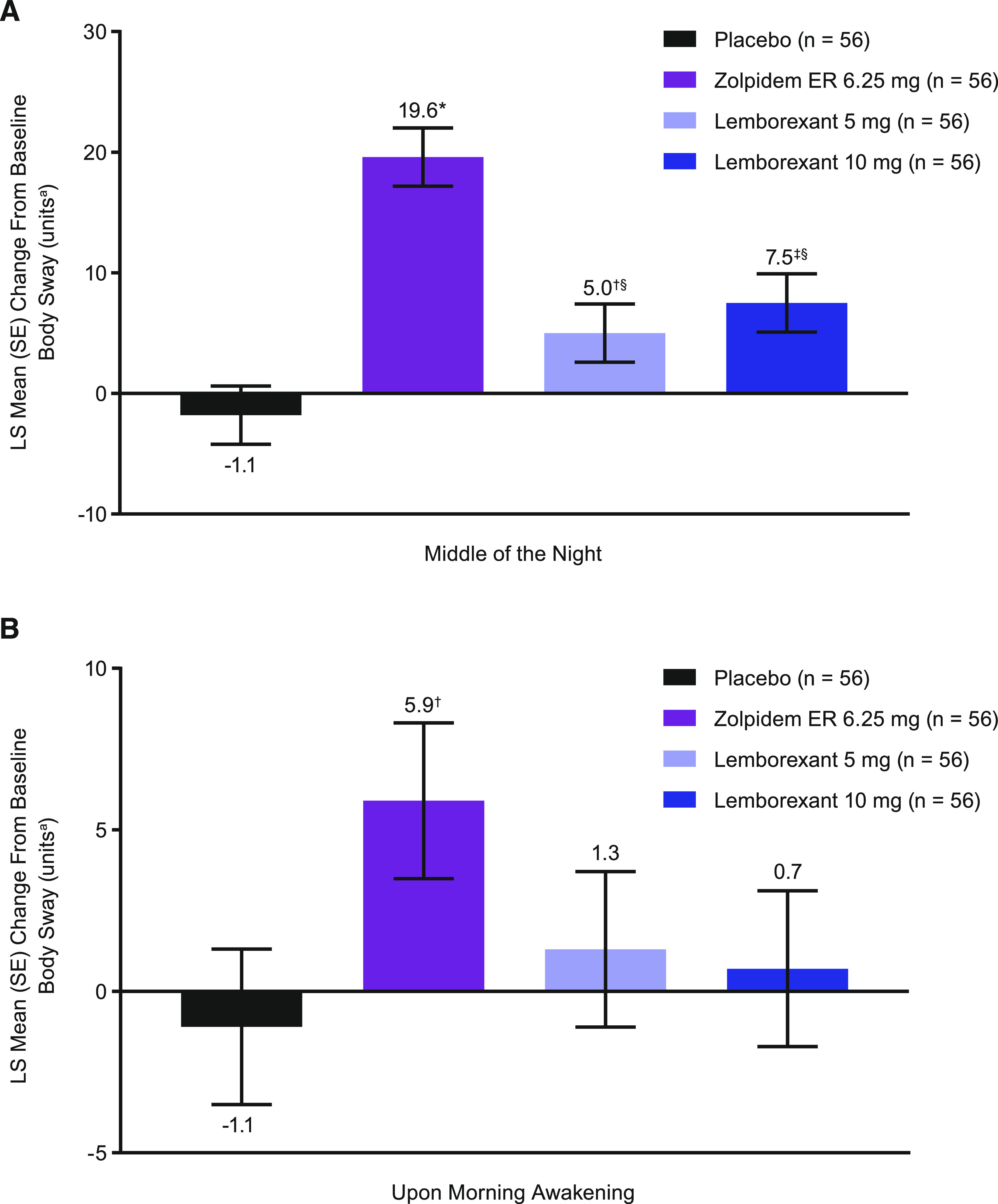
Change from baseline body sway (A) in the middle of the night and (B) upon morning awakening. *P ≤ .0001, †P < .05, ‡P ≤ .001 vs placebo; §P ≤ .0001 vs zolpidem based on mixed-effect model repeated measurement analysis with treatment, sequence, period, baseline (time-matched), time (hours postdose), treatment × time, and baseline × time as fixed effects, and time with participant as a random effect and a repeated effect for time, with participant within sequence. aA unit of body sway is defined as 1/3 degree angle of arc movement of the ataxiameter. ER = extended release, LS = least squares, SE = standard error.
In the sleep inertia-related sensitivity analysis of participants who were asleep across all treatment periods at the 4-hour time point (n = 27; Table S2), mean change from time-matched baseline in body sway was higher than PBO for all active treatments. However, the magnitude of change was lower than in the full analysis set (n = 56), except during the ZOL condition, for which the increase from baseline was larger (when excluding participants who were already awake at the 4-hour time point).
Auditory awakening threshold
At baseline, for all participants that completed, the mean (SD) observed values for AAT were similar across treatments (overall mean [SD]: 55.4 dB [29.6]). At approximately 4 hours postdose, neither dose of lemborexant nor ZOL interfered with the ability to be awakened by the auditory stimulus; the mean changes from the time-matched baseline were −3.2, −1.7, −3.8, and −3.5 dB for PBO, ZOL, LEM5, and LEM10, respectively. There were also no significant differences among the treatments in the decibel intensity required to awaken participants (Figure 3).
Figure 3. Percentage of participants in N2, other sleep stages, already awake, or not awakened by maximum decibel tone (awakened by technician).
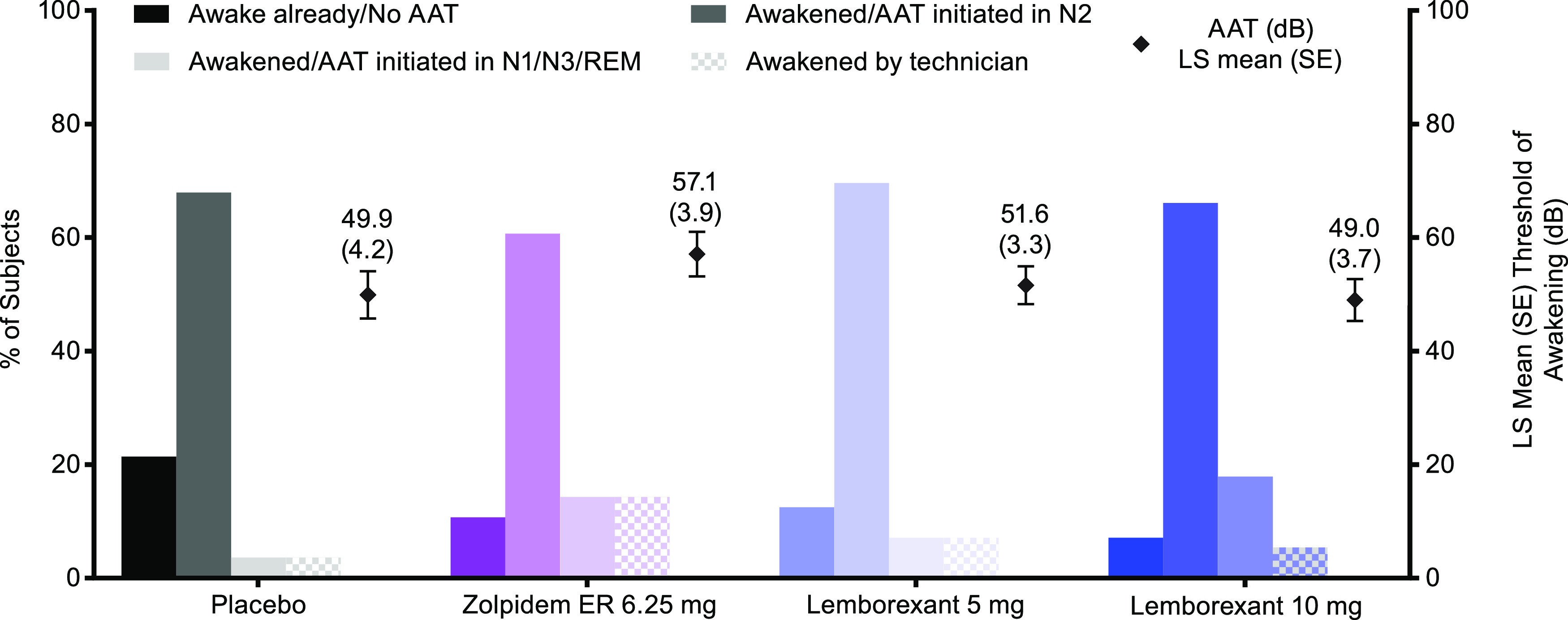
AAT = auditory awakening threshold, ER = extended release, LS = least squares, N1/N2/N3 = nonrapid eye movement stage 1/2/3, REM = rapid eye movement, SE = standard error.
Most participants (> 60% in all treatment groups) were in sleep stage N2 when the AAT was initiated (Figure 3). The AAT was initiated in N1/N3/REM sleep in < 20% of patients in all groups. In the ZOL group, 8 (14%) participants did not awaken to the maximum tone, compared with 2 (4%) participants in the PBO group, 4 (7%) participants in the LEM5 group, and 3 (5%) participants in the LEM10 group. Additional information regarding participants already awake during the window from 4 to 4.5 hours postdose are available in Supplementary Appendix S5.
Cognitive performance test battery
At approximately 4 hours postdose, LEM5 did not differ significantly from PBO on any of the CPAB domains (Table S3, Figure S2). Power of attention in the MOTN was similar (P > .05) for LEM5 versus ZOL, but significantly worse for LEM10 versus PBO and ZOL. Continuity of attention in the MOTN was significantly worse for ZOL versus LEM5, and performance after LEM10 and ZOL was worse than PBO. The same treatment patterns were observed for quality of memory in the MOTN. Speed of memory retrieval in the MOTN was worse with ZOL and LEM10 than PBO, but both doses of lemborexant performed significantly better than ZOL. Upon morning awakening (8 hours postdose), there was no evidence for residual effects of either dose of lemborexant or ZOL on any of the CPAB domains (P > .05 vs PBO for all comparisons).
Return to sleep latency
Median (range) RSL at baseline was 35.0 (4–122) minutes, respectively, in all treatment groups. Median RSL at approximately 4 hours postdose was 27.5, 12.5, 8.0, and 4.5 minutes for PBO, ZOL, LEM5, and LEM10, respectively. Least squares mean (standard error) change from baseline in RSL was −0.1 (4.1), −21.1 (2.3), −22.5 (2.7), −28.8 (2.7) for PBO, ZOL, LEM5, and LEM10, respectively. At approximately 4 hours postdose, least squares mean RSL for LEM5 and LEM10 was significantly shorter than for PBO (P < .0001 for both), indicating that both doses facilitated the return to sleep following an awakening in the MOTN (Figure 4). ZOL facilitated the return to sleep significantly faster than PBO (P < .0001), and LEM10 facilitated the return to sleep significantly faster than ZOL (P < .05). LEM5 was not statistically different from ZOL.
Figure 4. Return to sleep latency.
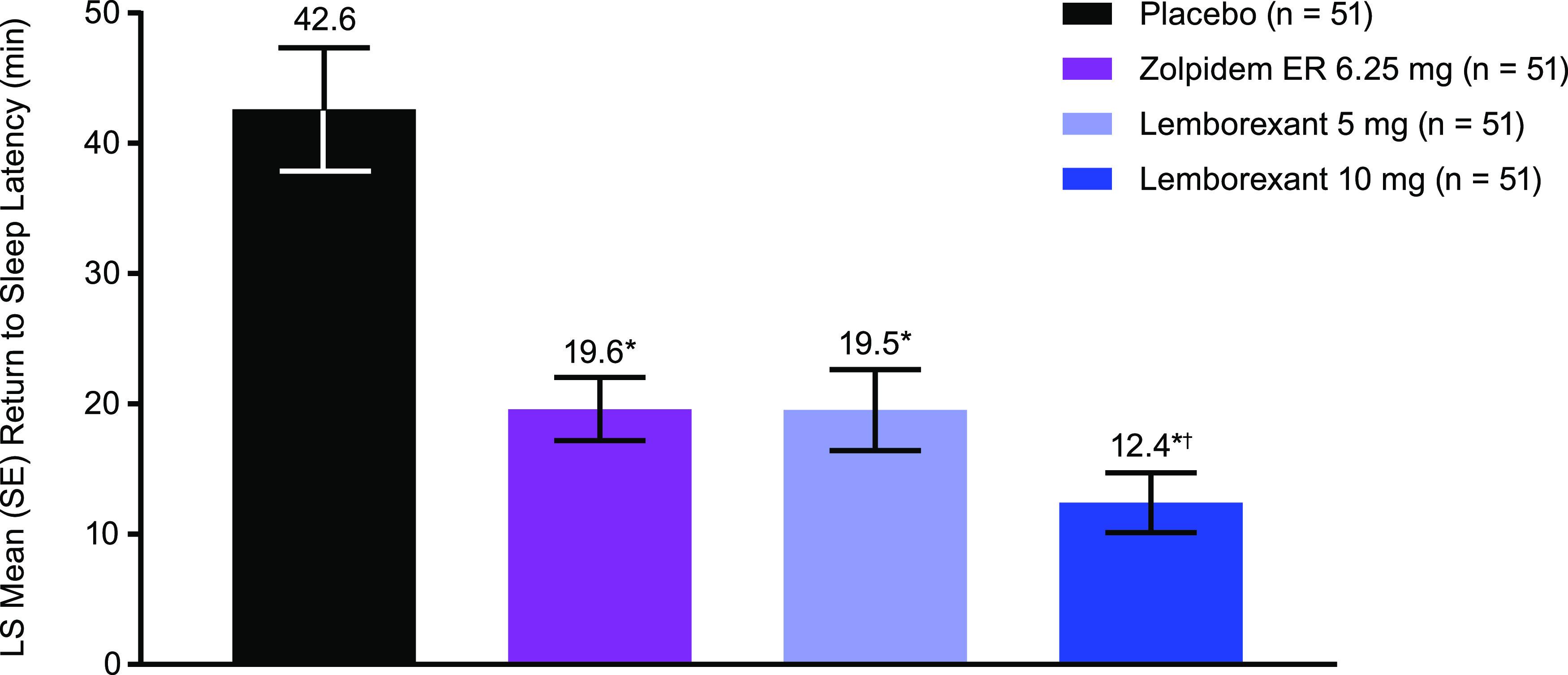
n = 51 for all groups owing to technical difficulties with polysomnography in 4 participants and 1 participant never awakened during the night in 1 treatment condition. *P ≤ .0001 vs. placebo; †P < .05 vs zolpidem based on an unstructured covariance matrix; model adjusted for treatment, sequence, and period as fixed effects, and a repeated effect of participant within sequence. ER = extended release, LS = least squares, SE = standard error.
Adverse events
There were no serious AEs or deaths during the study (Table 2). Rates of treatment-emergent AEs were low and were mainly mild or moderate in severity, regardless of treatment group. There were no dose-related trends with regard to the incidence of treatment-emergent AEs in the lemborexant treatment groups. There were no AEs of somnolence. Two participants in the LEM5 group discontinued because of an AE: 1 participant experienced a symptomatic severe low hemoglobin level, which was also low at screening, and 1 participant reported both sleep paralysis and hypnopompic hallucinations following LEM5 administration.
Table 2.
Safety summary (safety analysis set).
| Category, n (%) | PBO (n = 57) | ZOL (n = 58) | LEM5 (n = 59) | LEM10 (n = 58) |
|---|---|---|---|---|
| Any TEAE | 4 (7.0) | 4 (6.9) | 5 (8.5) | 5 (8.6) |
| Treatment-related TEAE | 1 (1.8) | 1 (1.7) | 2 (3.4) | 1 (1.7) |
| Severe TEAE | 0 | 0 | 1 (1.7) | 0 |
| SAE | 0 | 0 | 0 | 0 |
| AE leading to study drug discontinuation | 0 | 0 | 2 (3.4) | 0 |
AE = adverse event, LEM5 = lemborexant 5 mg, LEM10 = lemborexant 10 mg, PBO = placebo, SAE = serious adverse event, TEAE = treatment-emergent adverse event, ZOL = zolpidem tartrate extended release 6.25 mg.
DISCUSSION
This phase 1 study of lemborexant in healthy participants aged ≥ 55 years demonstrated the relative safety of lemborexant compared with ZOL on several important aspects of safety with sleep-promoting drugs. These data demonstrate that ZOL had a larger negative impact on postural stability in the MOTN than both LEM and PBO. Further, lemborexant performed similarly to PBO regarding the ability to awaken in the MOTN.
Although postural stability is not a direct assessment of fall risk, it is associated with increased risk of falling.17 In the current study, the increase in MOTN body sway in the ZOL group was more than 2 times higher than that observed with the higher dose of lemborexant, and more than 3 times that observed with the lower dose of lemborexant or PBO. Body sway was higher for both doses of lemborexant versus PBO in the MOTN, but there was no evidence for residual morning effects of either dose of lemborexant. In contrast, body sway in the ZOL group was statistically higher compared with PBO upon morning awakening. This is an important finding because, although not every patient wakes up in the MOTN, every patient gets out of bed in the morning.
No statistical differences between treatment groups on the AAT were observed, suggesting that neither lemborexant nor ZOL interfered with awakening to an external noise. Relatively low decibels awakened participants at baseline, indicating that the AAT as implemented may be sensitive to changes in thresholds resulting from administration of sleep-promoting drugs. Decreased arousability and increased fall risk following forced awakenings around the time of maximum plasma drug concentration (tmax) have been previously reported for zolpidem 10 mg in healthy males.18 Although not statistically significant, at least twice as many participants in the ZOL group as in the other groups did not awaken to the maximum dB level and had to be awakened by the technician, which may indicate a trend toward decreased arousability for ZOL in this study. A recent phase 4 study of suvorexant, which belongs to the same drug class as lemborexant, found that suvorexant preserved the ability of nonelderly patients with insomnia to respond to nocturnal environmental stimuli.19
The lower dose of lemborexant did not differ significantly from PBO on change from baseline in any of the CPAB domains in the MOTN, but LEM10 did generally have a negative effect on memory and attention compared with PBO on all CPAB domains. ZOL performed statistically worse than PBO on both memory tests and Continuity of Attention, and statistically worse than LEM5 on Continuity of Attention, Quality of Memory, and Speed of Memory Retrieval in the MOTN. There were no morning residual effects of lemborexant or ZOL on tests of memory and attention in this study. Effects on cognitive function have been reported in other studies of ZOL,20 and negative effects on memory are consistent with the prescribing information for ZOL.10
Participants treated with LEM5 or LEM10 fell back to sleep significantly faster than participants treated with PBO, and LEM10 facilitated the return to sleep significantly faster than ZOL. These findings are consistent with the aforementioned phase 3 trial of lemborexant, in which lemborexant treatment resulted in a significantly shorter duration of long (> 5 minutes) awakenings, versus both PBO and ZOL, at both the beginning and end of 1 month of treatment, as demonstrated with PSG (Eisai Inc., data on file).
This study utilized a four-way complete crossover design, similar to that used by Drake et al.18 This design reduces the influence of confounding covariates and increases the power of the study to detect meaningful changes with fewer participants but has a potential for high dropout rate, particularly when lengthy washout periods are employed to avoid carryover effects. In our study, most participants completed the study. There was no carryover effect, and sequence and period effects were also not statistically significant (P > .23). The results were not influenced by the small proportion of missing data, as demonstrated by sensitivity analyses.
Unlike in the study design employed by Drake et al, herein, participants were assessed at 4 hours postdose rather than at the time (tmax).18,19 MOTN is approximately 2–3 hours after tmax for ZOL and lemborexant.21,22 Although studies have demonstrated that negative effects on postural stability for ZOL and other similarly acting hypnotics are of greatest magnitude around the tmax, statistically significant effects can still be observed at 4 hours postdose.23–27
In this study, each participant had a baseline night, without study drug administration, during which procedures identical to treatment nights were followed. This allowed for a time-matched baseline for all assessments. Similar studies have used predose/prebedtime values as baseline, which does not account for the influence of time-of-night or potential sleep inertia. This study also preferentially awakened participants for the AAT from sleep stage N2 to reduce the variability and control for the potential effect of sleep inertia upon the postawakening assessments. It is generally recognized that sleep inertia is worse upon awakening from slow-wave sleep stage N3 and potentially from REM sleep than from N2 sleep.28
The unexpectedly high number of participants who were not asleep at 4–4.5 hours postdose reduced the power of the study to detect meaningful differences in AAT. The fact that a participant was already awake in the MOTN also means that little or no effect of sleep inertia was operating on the participant’s subsequent postural stability assessment. Sleep inertia has been shown to have a major effect on balance and cognitive performance in the MOTN.29 Sensitivity analyses demonstrated that when only those participants asleep immediately before the assessments were included, mean change from baseline body sway was lower for those receiving lemborexant but higher in those receiving ZOL compared with when data were analyzed from the full cohort.
In conclusion, lemborexant was associated with less body sway than ZOL in the MOTN, was not significantly different to PBO in the ability of participants to awaken to an external stimulus, and facilitated the return to sleep. The LEM10 dose did impair some aspects of cognitive performance in the MOTN. Upon morning awakening, there was no evidence for residual effects of lemborexant on postural stability or cognitive performance, whereas there was evidence for postural instability in the morning with ZOL. A major concern for clinicians who prescribe sleeping pills is safety, and, therefore, these results suggest that lemborexant may be a useful potential option, especially in patients at risk for falls.
DISCLOSURE STATEMENT
All authors have seen and approved the final manuscript. This study was sponsored by Eisai Inc. Medical writing assistance was provided by Samantha Forster, PhD, CMPP, of ProScribe, part of the Envision Pharma Group. Envision Pharma’s services complied with international guidelines for Good Publication Practice (GPP3). Patricia Murphy is a former employee of Eisai Inc. Dinesh Kumar and Margaret. The authors contributed to this paper as follows: Patricia Murphy, as Study Director, had full access to all study data and takes responsibility for all aspects of the study, including the integrity of the data and the accuracy of the data analysis. Patricia Murphy and Margaret Moline supervised the study. Patricia Murphy, Margaret Moline, Gary Zammit, and Russell Rosenberg were involved in the study concept and design. All authors were involved in the analysis and interpretation of data, the drafting of the manuscript, and in critical revision of the manuscript for important intellectual content. Dinesh Kumar conducted the statistical analysis; Patricia Murphy and Margaret Moline obtained funding. All authors have seen and approved the final manuscript. Moline are current employees of Eisai Inc. Gary Zammit is an employee and shareholder of Clinilabs Drug Development Corporation; has ownership interest in the Sleep Disorders Institute and Home Sleep and Respiratory Care; has served as a consultant for Eisai Inc., Janssen Pharmaceutical, Purdue, and Takeda; and has served on the speakers bureau for Merck. Russell Rosenberg has received grant/research support from Actelion Pharmaceuticals, Eisai Inc., Flamel Pharmaceuticals, Jazz Pharmaceuticals, Merck, and Philips Respironics.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors to thank Kate Pinner, original statistician, Eisai Inc.; Gleb Filippov, Medical Monitor, Eisai Inc.; Louise Berkman, Clinical Operations Lead, Eisai Inc.; and Min-Kun Chang, Clinical Operations, Eisai Inc. for their contributions to this study.
ABBREVIATIONS
- AAT
auditory awakening threshold
- AE
adverse event
- CI
confidence interval
- CPAB
cognitive performance assessment battery
- DORA
dual orexin receptor antagonist
- GABA
gamma-aminobutyric acid
- LEM5
lemborexant 5 mg
- LEM10
lemborexant 10 mg
- MAR
missing at random
- MOTN
middle of the night
- N2
nonrapid eye movement stage 2
- N3
nonrapid eye movement stage 3
- MNAR
missing not at random
- PBO
placebo
- PSG
polysomnography
- REM
rapid eye movement
- RSL
return-to-sleep latency
- SD
standard deviation
- tmax
time to peak plasma concentration
- ZOL
zolpidem tartrate extended release 6.25 mg
REFERENCES
- 1. Dayvigo [prescribing information] (lemborexant), Woodcliff Lake, NJ: Eisai Inc. US. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212028s000lbl.pdf. Accessed January 08, 2020.
- 2.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(2):307–349. 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18(1):49–56. 10.1080/10401230500464711 [DOI] [PubMed] [Google Scholar]
- 4.Lie JD, Tu KN, Shen DD, Wong BM. Pharmacological treatment of insomnia. P T. 2015;40(11):759–771. [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke JR, Ancoli-Israel S. Normal and abnormal sleep in the elderly. Handb Clin Neurol. 2011;98653–665. 10.1016/B978-0-444-52006-7.00041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. 10.1136/bmj.38623.768588.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340–2372. 10.1016/j.clinthera.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 8.Murphy P, Moline M, Mayleben D, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. 10.5664/jcsm.6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg R, Murphy P, Chou C, Dhadda S, Zammit G, Moline M. Comparison of lemborexant with zolpidem extended release and placebo: topline results from a phase 3 study in subjects 55 years and older with insomnia (P163). J Sleep Res. 2018;27S1165–165.28880425 [Google Scholar]
- 10.Ambien CR [prescribing information] (zolpidem tartrate extended-release). Bridgewater, NJ: Sanofi-Aventis US. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021774s018lbl.pdf. Accessed January 08, 2020.
- 11.Jehan S, Masters-Isarilov A, Salifu I, et al. Sleep disorders in postmenopausal women. J Sleep Disord. Ther. 2015;4(5):1000212. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B-S, Wang X-J, Gong L-K. The construction of a Williams design and randomization in cross-over clinical trials using SAS. J Stat Softw. 2009;29Code Snippet 11–10. 10.18637/jss.v029.c01 [DOI] [Google Scholar]
- 13.Wright BM. A simple mechanical ataxia-meter. J Physiol. 1971;218Suppl27–28. [PubMed] [Google Scholar]
- 14.Wesnes KA. The value of assessing cognitive function in drug development. Dialogues Clin Neurosci. 2000;2(3):183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wesnes KA, Garratt C, Wickens M, Gudgeon A, Oliver S. Effects of sibutramine alone and with alcohol on cognitive function in healthy volunteers. Br J Clin Pharmacol. 2000;49(2):110–117. 10.1046/j.1365-2125.2000.00131.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk DJ, Czeisler CA. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8(1):1–8. 10.1111/j.1365-2869.1999.00128.x [DOI] [PubMed] [Google Scholar]
- 17.Fernie GR, Gryfe CI, Holliday PJ, Llewellyn A. The relationship of postural sway in standing to the incidence of falls in geriatric subjects. Age Ageing. 1982;11(1):11–16. 10.1093/ageing/11.1.11 [DOI] [PubMed] [Google Scholar]
- 18.Drake CL, Durrence H, Cheng P, et al. Arousability and fall risk during forced awakenings from nocturnal sleep among healthy males following administration of zolpidem 10 mg and doxepin 6 mg: a randomized, placebo-controlled, four-way crossover trial. Sleep. 2017;40(7. 10.1093/sleep/zsx086. [DOI] [PubMed] [Google Scholar]
- 19.Drake CL, Kalmbach DA, Cheng P, et al. Can the orexin antagonist suvorexant preserve the ability to awaken to auditory stimuli while improving sleep?. J Clin Sleep Med. 2019;15(09):1285–1291. 10.5664/jcsm.7920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsunaga Y, Tagaya H, Fukase Y, et al. Effects of zolpidem/triazolam on cognitive performance 12 hours after acute administration. Sleep Med. 2018;52213–218. 10.1016/j.sleep.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 21.Kirkwood C, Neill J, Breden E. Zolpidem modified-release in insomnia. Neuropsychiatr Dis Treat. 2007;3(5):521–526. [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno T, Ishida T, Kusano K. Disposition and metabolism of [(14)C]lemborexant, a novel dual orexin receptor antagonist, in rats and monkeys. Xenobiotica. 2018;49(6):1–10. [DOI] [PubMed] [Google Scholar]
- 23.Allain H, Bentué-Ferrer D, Polard E, Akwa Y, Patat A. Postural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly: a comparative review. Drugs Aging. 2005;22(9):749–765. 10.2165/00002512-200522090-00004 [DOI] [PubMed] [Google Scholar]
- 24.Boyle J, Danjou P, Alexander R, et al. Tolerability, pharmacokinetics and night-time effects on postural sway and critical flicker fusion of gaboxadol and zolpidem in elderly subjects. Br J Clin Pharmacol. 2009;67(2):180–190. 10.1111/j.1365-2125.2008.03331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattila MJ, Vanakoski J, Kalska H, Seppälä T. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacol Biochem Behav. 1998;59(4):917–923. 10.1016/S0091-3057(97)00506-6 [DOI] [PubMed] [Google Scholar]
- 26.Mets MA, Volkerts ER, Olivier B, Verster JC. Effect of hypnotic drugs on body balance and standing steadiness. Sleep Med Rev. 2010;14(4):259–267. 10.1016/j.smrv.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 27.Zammit G, Wang-Weigand S, Rosenthal M, Peng X. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34–40. 10.5664/jcsm.27390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tassi P, Muzet A. Sleep inertia. Sleep Med Rev. 2000;4(4):341–353. 10.1053/smrv.2000.0098 [DOI] [PubMed] [Google Scholar]
- 29.Frey DJ, Ortega JD, Wiseman C, Farley CT, Wright KP Jr. Influence of zolpidem and sleep inertia on balance and cognition during nighttime awakening: a randomized placebo-controlled trial. J Am Geriatr Soc. 2011;59(1):73–81. 10.1111/j.1532-5415.2010.03229.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


