Abstract
Spouses share common risks for cardiometabolic diseases: a person’s diabetes or hypertension raises the partner’s odds of developing the same condition. The mechanisms responsible for this disease concordance remain poorly understood. To examine three factors that may modulate partners’ cardiometabolic similarity—closeness, hostile marital behavior, and age— and to explore whether health behavior concordance plays a role, on two separate occasions 43 healthy couples ages 24 to 61 provided fasting glucose, metabolic data (fat and carbohydrate oxidation), and resting blood pressure before discussing one of their most severe marital disagreements. Accounting for the fixed effects of sex, age, study visit, and abdominal fat on cardiometabolic levels, we found that aspects of health behavior concordance were associated with greater similarity in glucose, diastolic blood pressure (DBP), and carbohydrate and fat metabolism. Independent of health behavior concordance, partners who felt closer and behaved in a less hostile way had more similar rates of fat oxidation; less hostile partners also shared greater overlap in carbohydrate oxidation. Likewise, fasting glucose and DBP were more similar within older couples compared to younger pairs, beyond the effects of health behavior concordance. In sum, our data captured preclinical similarities in cardiometabolic health among disease-free couples, which may form the basis for their long-term overlapping disease risks. Closer, less hostile, and older couples shared more similar fasting glucose, metabolic data, and blood pressure; importantly, health behavior concordance did not explain all associations. These novel data suggest that multiple paths may lead to couples’ shared disease risks.
Keywords: couple concordance, health, cardiometabolic function, closeness, age
1. Introduction
Spouses share common disease risks. Across multiple meta-analyses and large epidemiological studies, data from more than 100,000 couples have revealed the sizable hazard of being diagnosed with a health condition that the partner also has (Di Castelnuovo et al., 2008; Hippisley-Cox et al., 2002; Jurj et al., 2006; Leong et al., 2014). The evidence is especially decisive for couples’ overlapping risks in cardiometabolic diseases (Meyler et al., 2007): for example, a person’s type 2 diabetes or heart disease almost doubles the partner’s risk for the same condition (Hippisley-Cox et al., 2002; Jurj et al., 2006).
The mechanisms that drive this disease concordance remain understudied and poorly understood. One explanation for couples’ shared health fates is assortative mating, the tendency for people to choose a partner with similar characteristics, which may include the same health conditions, risk factors, and lifestyles (Meyler et al., 2007). However, empirical work has shown that disease incidence overlaps even after accounting for individuals’ own health risk factors (e.g., Hippisley-Cox and Pringle, 1998), suggesting that the effect of shared lives supersedes that of partners’ preexisting similarity. The finding that partners’ blood pressure and lipid metabolism converge over time also cannot be attributed to assortative mating (Di Castelnuovo et al., 2008).
Beyond choosing already-similar counterparts, partners share resources: they often cohabitate, pool finances, and overlap in their social networks. To varying degrees, spouses’ daily activities are intertwined, and each partner’s stressors and moods can affect both spouses (Kiecolt-Glaser and Wilson, 2017). Partners also transmit and converge on health behaviors such as physical activity, sleep, and diet (Berli et al., 2018; Bove et al., 2003; Gunn et al., 2015; Martire et al., 2013b). For example, knee osteoarthritis patients were more physically active when spouses took more steps (Martire et al., 2013b). Over the first few years of marriage, newlyweds’ diets became more similar (Bove et al., 2003). In an actigraphy study that compared couples’ sleep minute-by-minute, more than half the time, when a person was awake at night, the partner was also (Gunn et al., 2015). Surprisingly, prior work has not linked health behavior concordance to partners’ overlapping disease risks.
If couples’ shared lives promote their common disease risks, then couples whose routines, emotions, and identities are particularly intertwined should also share stronger health resemblance than their less interdependent counterparts (Kiecolt-Glaser and Wilson, 2017). For this reason, from a social-developmental perspective, we argue that closeness, marital quality, and age should be associated with couples’ health similarity (Kiecolt-Glaser and Wilson, 2017). According to prior work, couples who reported being closer also spent more time together (Iida et al., 2018), were more committed to their joint routines and rituals (Crespo et al., 2008), and felt more interconnected (Aron et al., 1992). In addition, a diary study revealed that spouses’ sleep quality suffered when osteoarthritis patients’ pain was higher than usual; the effect was most pronounced among the closest couples (Martire et al., 2013a). High marital satisfaction (and low marital discord) may have similar effects on couples’ health overlap—a prediction prior work has not tested. Marital quality and closeness are distinct yet related concepts, with moderate (Martire et al., 2013a) to high (Aron et al., 1992) correlations. Indeed, marital satisfaction explains considerable variance in the time partners spend together, and experimental work suggests that satisfaction likely drives the association (Reissman et al., 1993).
Older couples may also share greater health similarities than their younger counterparts. In addition to the likelihood of a longer shared history, social networks shrink in older age (Carstensen, 1995), increasing the centrality of the marital relationship for everyday routines, decisions, and emotions. According to social-emotional aging theories, as people perceive less time to live, they seek to maximize emotional well-being and meaningful experiences with loved ones (Carstensen, 1995). To serve this goal, people increasingly shift away from confrontation, avoiding and reframing stressors and social tensions (Charles, 2010). Consistent with theory, some studies have shown that older couples feel closer and more satisfied in their marriages than younger couples (Rook and Charles, 2017). Although older adults report fewer stressors on average (Rook and Charles, 2017), the increasing intertwinement of partners’ goals and routines with older age may boost shared stress. We posit that the overarching process of social-emotional development is likely more important than any single component (e.g., changes in social networks, emotion regulation strategies, attentional processes, goal selection and motivation), and age is the best proxy for this broad developmental process.
Most prior work has examined the effects of longer marriage duration, not older age, on greater health similarity (Meyler et al., 2007), with mixed results (Di Castelnuovo et al., 2008). Although marriage length and age are correlated, we posit that age more strongly predicts health similarity given the additional relevance of social-emotional factors. Indeed, in a rare study of more than 1700 couples that compared the effects of age and marriage duration, it was older age, not longer marriage duration, that explained partners’ greater blood pressure concordance (Suarez et al., 1983). Likewise, in another study, older couples’ blood glucose levels were more similar compared to younger counterparts’ (Cheraskin et al., 1968).
To identify pathways to couples’ shared disease risks before the onset of cardiometabolic diseases, we evaluated the associations of closeness, hostility (a behavioral indicator of marital discord), and age with partners’ similarity in cardiometabolic health. On two separate occasions, partners provided measures of five key cardiometabolic indicators: fasting blood glucose, systolic blood pressure (SBP), diastolic blood pressure (DBP), as well as fat and carbohydrate oxidation—all critical precursors to the development of cardiometabolic conditions (Lee et al., 2018; Petrie et al., 2018; Pujia et al., 2019). We hypothesized that closer, less hostile, and older couples would have more similar cardiometabolic values than their counterparts. We predicted that couples with more similar health behaviors—self-reported physical activity, diet, and sleep— would also share closer resemblance in their cardiometabolic function than those with less concordant health behaviors. Finally, we explored whether associations with closeness, marital behavior, and age held above and beyond health behavior concordance.
2. Method
2.1. Participants
Couples were recruited for a parent study of immune responses to high-fat meals (Kiecolt-Glaser et al., 2015). An initial online screen and follow-up in-person screen determined eligibility. Couples married fewer than 3 years and those who had sensory impairments that would interfere with study completion were excluded. Couples were not considered if either partner had a chronic health problem, e.g., anemia or diabetes (hemoglobin A1c, HbA1c > 6.5), smoked, abused substances, or used prescription medication other than birth control (n = 5) or levothyroxine (n = 3).
In the online screen, potential participants completed the 16-item version of the Couples Satisfaction Index (CSI); the full version was given at the end of the first visit (Funk and Rogge, 2007). Happier couples were overrepresented among applicants, a general challenge for marital research. Accordingly, in terms of both inclusion and scheduling, we prioritized dissatisfied couples to represent the full range of marital discord. We also spent considerable time and effort to recruit people who were healthy but overweight or obese to address aims relevant to the parent study’s meal component. A total of 350 interested individuals were excluded because either they or their spouse did not meet our stringent health criteria.
The sample consisted of 86 participants (43 couples). Participants were 38 years old on average (SD = 8.2, range = 24–61) and primarily White (81%). All couples were married, and the average length of marriage was 11.5 years (SD = 6.7, range = 3 – 27). With a clinical cutoff of 104.5 on the CSI, 15 (17.4%) of the 86 partners met criteria for clinically significant relationship distress. Most were employed full-time (70%).
2.2. Procedure
This research was approved by the Ohio State University (OSU) Institutional Review Board; participants provided written informed consent before participating. Participants completed two full-day study visits at the Clinical Research Center (CRC), a hospital research unit. During this double-blind, randomized crossover study, couples ate a high saturated fat meal at one visit and a high oleic sunflower oil meal at the other (in random order to test the parent study’s key aims). Couples were told to avoid alcohol and caffeine use within 1 day prior and strenuous physical activity within 2 days prior to both study visits. Participants were also instructed to stop taking aspirin, vitamins (except multivitamins), antioxidants, and any other dietary supplements for 7 days before each admission. On the day before each visit, participants received three standardized meals from the CRC’s metabolic kitchen, reducing any variability in physiology associated with recent food intake. They began a 12-h fast at 7:30 p.m. the evening before each visit.
At each admission, both members of the couple arrived at 7:30 a.m., after which a catheter was inserted into each person’s arm. After 10 min, the partners were fitted with a face mask used for indirect calorimetry and reclined to a 30-degree angle in their beds, lying still but awake for 25 min to assess resting metabolism. At the beginning of the metabolic assessment, three blood pressure measurements were taken in 2-min intervals. Next, fasting baseline blood samples were drawn, after which each member of the couple ate either the high saturated fat or high oleic sunflower oil meal; the husband and wife received the same meal and both were required to eat the entire meal.
Couples also engaged in a marital problem discussion on the morning of each visit, about 2 h following the meal. To initiate the discussion, an experimenter conducted a 10- to 20-min interview to identify the most contentious topics within the marriage for both partners. These topics were selected from an inventory of potential relationship problems each spouse had completed. Couples were then asked to discuss and try to resolve one or more marital issues that the experimenter judged to be the most conflict-producing (e.g., money, communication, or in-laws). The research team remained out of sight while videotaping the subsequent 20-min problem discussion.
The two study visits occurred 1–25 weeks apart (M = 4.45, SD = 4.76). Although 55% of visits occurred within 3 weeks, some were more widely spaced as a consequence of participants’ work schedules.
2.3. Problem Discussion Behavior
Marital disagreement discussions were coded using the Rapid Marital Interaction Coding System (RMICS), which discriminates well between distressed and nondistressed couples (Heyman, 2004). Consistent with prior work (Kiecolt-Glaser et al., 2015), a couple-level composite score summed the four negative RMICS codes: psychological abuse (e.g., disgust, contempt, belligerence, as well as nonverbal behaviors like glowering), distress-maintaining attributions (e.g., “You’re only being nice so that I’ll have sex with you tonight” or “You were being mean on purpose”), hostility (e.g., criticism, hostile voice tone, or rolling the eyes dramatically), and withdrawal (behaviors that suggest pulling back from the interaction or not listening). Hostile behavior was strongly correlated between partners (r=.88, p<.0001) and across visits (r=.75, p<.0001). Also, there were no differences in degree of hostility between husbands and wives (p = .166) or between visits (p = .980). Thus, the sum of both partners’ hostile behaviors were averaged across visits to create a couple’s hostility score (Kiecolt-Glaser et al., 2015). Holley and Guilford’s G (Xu and Lorber, 2014) was used to quantify inter-rater agreement for the RMICS hostility composite. Interrater agreement was high, with a G index of 0.88. Hostile behavior was used as the primary measure of marital quality because objectively rated marital interaction behaviors are more sensitive predictors of health and physiology than are self-report measures (Kiecolt-Glaser and Newton, 2001).
2.4. Relationship closeness
Before the marital disagreement discussion at the first visit, each partner completed the 1-item Inclusion of Other in Self (IOS) Scale—seven pairs of circles with varying overlap, meant to represent oneself and one’s partner (Aron et al., 1992). The individual is asked to choose the pair of circles that best describes the relationship. This is a well-established, reliable measure of partners’ subjective feelings of interpersonal closeness (Aron et al., 1992). To capture couple-level closeness, we averaged the two partners’ scores, which were correlated (r = 0.56, p = .0001) with no significant mean differences (p = .834).
2.5. Marital satisfaction
Administered at the first full-day visit before the marital disagreement discussion, the 32-item Couples Satisfaction Index (CSI) assessed marital satisfaction (Funk and Rogge, 2007). Developed using item response theory, the CSI can discriminate between satisfied and dissatisfied couples with greater precision than other commonly used marital scales (Funk and Rogge, 2007). Internal consistency of the scale was high (Cronbach’s α = .98). Marital satisfaction was strongly correlated between partners (r=.83, p<.0001); partners’ scores were averaged to create couple-level values. In ancillary analyses, we substituted couples’ hostile behavior for marital satisfaction scores to explore whether associations with cardiometabolic similarity would replicate with self-report data.
2.6. Health behavior concordance
We calculated each couple’s concordance on three health behaviors by creating a difference score between the partners: physical activity, diet quality, and sleep duration. In the screening questionnaire, both partners reported how many hours of vigorous physical activity participants had done in the past week (running, brisk walking, aerobics, jogging, etc.) long enough to break a sweat (Blair et al., 1985; Taylor et al., 1984). Energy expenditure estimates from the seven-day activity recall did not differ from those using accelerometer data (Taylor et al., 1984). Changes in energy expenditure over one year as calculated from the seven-day recall predicted changes in cardiorespiratory fitness (VO2 max), adiposity, and triglycerides (Blair et al., 1985). The absolute value of the difference score provided their level of activity concordance (with smaller values reflecting greater concordance).
The gold standard for assessing recent dietary intake, three 24-hour dietary recall interviews, was administered with the USDA Multiple Pass Approach (Conway et al., 2003). These data, comprised of two weekdays and one weekend day, were averaged across the interviews to maximize reliability. The Alternative Healthy Eating Index (aHEI) was calculated from the food recalls to estimate each partner’s diet quality (McCullough and Willett, 2006). The absolute value of the partners’ differences resulted in a dietary concordance score.
At each visit, participants were asked to report the number of hours they had spent asleep the night before. We took the absolute value of the difference between partners’ sleep duration, and averaged the sleep concordance scores across the two visits. Past-night sleep duration was correlated between the two visits (r=0.61, p<.0001), and did not change between visits (p=.831). The same was true for sleep concordance, with significant correlation between visits (r=.43, p=.005) and no change between visits (p=.164).
2.7. Cardiometabolic Indices
2.7.1. Fasting glucose.
Glucose serum samples were analyzed using the Dimension Xpand Clinical Chemistry System (Siemens MedicalDiagnostics, Decatur, GA). The analytical sensitivity is 1 mg/dL. The intra-assay coefficient of variation (CV) is 0.43%, indicating sufficient within-sample precision. Of these samples, 22% exceeded the healthy threshold of 100 mg/dL (M = 94.59, SD = 7.48), indicating meaningful variability.
2.7.2. Carbohydrate and fat oxidation.
Indirect calorimetry was used to estimate carbohydrate and fat oxidation (g/min, MCarbohydrate Oxidation = 0.36, SD = 0.20; MFat Oxidation = 0.32, SD = 0.19). This method calculated the amounts of oxygen inhaled and carbon dioxide exhaled from VO2 and VCO2 using the Weir formulas (Weir, 1949). Adjustments for the protein respiratory quotient were based on estimations of urinary ureanitrogen using the protein consumption from the previous day’s standardized meals (Simonson and DeFronzo, 1990).
2.7.3. Blood pressure.
Baseline systolic and diastolic blood pressure were measured with the Dinamap/Critikon 1846SX/P (GE Healthcare, Milwaukee, WI) three times at two-minute intervals (Muntner et al., 2019). The three measurements were then averaged for each person at each visit. Among all assessments, 46% exceeded the recommended healthy threshold of 120 mmHg for SBP (M = 119.50, SD = 15.66), and 22% fell above the recommended limit of 80 mmHg for DBP (M = 71.89, SD = 9.25), again demonstrating meaningful variability.
2.8. Analytic Plan
2.8.1. Model parameters and sequence.
We fit heterogeneous-variance multilevel models (MLMs) (Hedeker and Mermelstein, 2007) in SAS PROC MIXED to evaluate our research questions. These models, an extension of more traditional MLMs, allow us to test predictors of the random variance, in this case the variance within couples. Thus, we were able to evaluate the role of age, marital behavior, and closeness on within-couple variance (i.e., partners’ similarity) in the five cardiometabolic outcomes—fasting baseline levels of glucose, SBP, DBP, and carbohydrate and fat oxidation. See Supplemental Material for an example equation, syntax, and detailed explanation. To predict the variance within couples, we first covaried for the fixed effects of visit, sex, and grand-mean-centered age and abdominal fat given their systematic influences on the average levels of cardiometabolic indices. Models included separate random intercepts for husbands and wives to account for within-person correlation. Importantly, all predictors of within-couple variance (i.e., partner similarity) in cardiometabolic factors had to be at the same level as the variance, meaning it had to be at the couple level. Thus, the predictors of the random variance collected from each individual were differenced (for health behaviors) or averaged between partners (for age, hostility, and closeness), and those taken at both study visits were averaged across the two occasions. Age, closeness, hostile marital behavior, and indices of health behavior concordance were entered into the LOCAL=EXP() command of the REPEATED statement separately in univariate models, allowing us to model each as a predictor of within-couple variance. Log likelihood ratio tests then evaluated whether the inclusion of each predictor of the within-couple variance significantly improved fit according to −2 log likelihood, relative to the model without predictors of that same variance. In a second step, health behavior concordance variables were added as predictors of the within-couple variance to the univariate models that included age, closeness, and marital behavior. This allowed us to evaluate whether they accounted for the effects of these respective predictors on cardiometabolic similarity. In ancillary models, hostile behavior was substituted with couples’ self-reported marital satisfaction scores and age, with relationship length, to explore whether effects were similar.
2.8.2. Interpretation of the within-couple variance estimates.
In these models, a positive variance coefficient indicates greater within-couple variability (i.e., less similarity), whereas a negative coefficient reflects smaller within-couple variance (i.e., greater similarity). Thus, age and closeness were expected to negatively predict within-couple variability, such that older age and greater closeness would be associated with smaller variance, i.e., greater similarity. The opposite was predicted for hostile marital behavior and for the health behavior concordance measures, wherein less hostility and greater concordance would relate to smaller variance, i.e., greater similarity, in the cardiometabolic data.
3. Results
3.1. Correlations among age, closeness, marital behavior, and health behavior concordance
As shown in Table 1, couples who engaged in more hostility during their disagreement discussions also reported feeling less close (r = −.41, p = .006). A nonsignificant trend arose in the expected direction between older age and less hostile behavior (r = −.29, p = .060). Surprisingly, none of the health behavior concordance variables were significantly correlated with each other or with age. A nonsignificant trend emerged in the expected direction between greater closeness and stronger sleep concordance (r = −.29, p = .062). A nonsignificant trend also arose between greater hostility and higher dietary concordance (r = −.30, p = .057). Likewise, at a trend level, greater self-reported marital satisfaction was associated with more concordant physical activity (r = −.27, p = .084) and sleep (r = −.26, p = .097).
Table 1.
Description and intercorrelations among predictor variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | M (SD) | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age | -- | 38.2 (7.9) | |||||||
| 2. Closeness | −0.08 | -- | 4.9 (1.3) | ||||||
| 3. Hostile Behavior | −0.29‡ | −0.41* | -- | 22.7 (29.1) | |||||
| 4. Years Married | 0.78* | 0.11 | −0.33* | -- | 11.5 (6.7) | ||||
| 5. Marital Satisfaction | −0.09 | 0.81* | −0.50* | 0.04 | -- | 124.2 (31.6) | |||
| 6. Activity Concordance | 0.004 | −0.10 | 0.16 | 0.06 | −0.27‡ | -- | 1.8 (2.3) | ||
| 7. Diet Concordance | 0.18 | 0.05 | −0.30‡ | 0.16 | 0.10 | 0.17 | -- | 9.1 (7.4) | |
| 8. Sleep Concordance | −0.05 | −0.29‡ | 0.11 | −0.05 | −0.26‡ | 0.02 | 0.04 | -- | 1.0 (0.8) |
Note. All variables reflect couple-level averages, consistent with the study’s couple-level analysis.
, p < .05,
, p < .10
3.2. Are age, closeness, and marital behavior associated with cardiometabolic similarity?
3.2.1. Age.
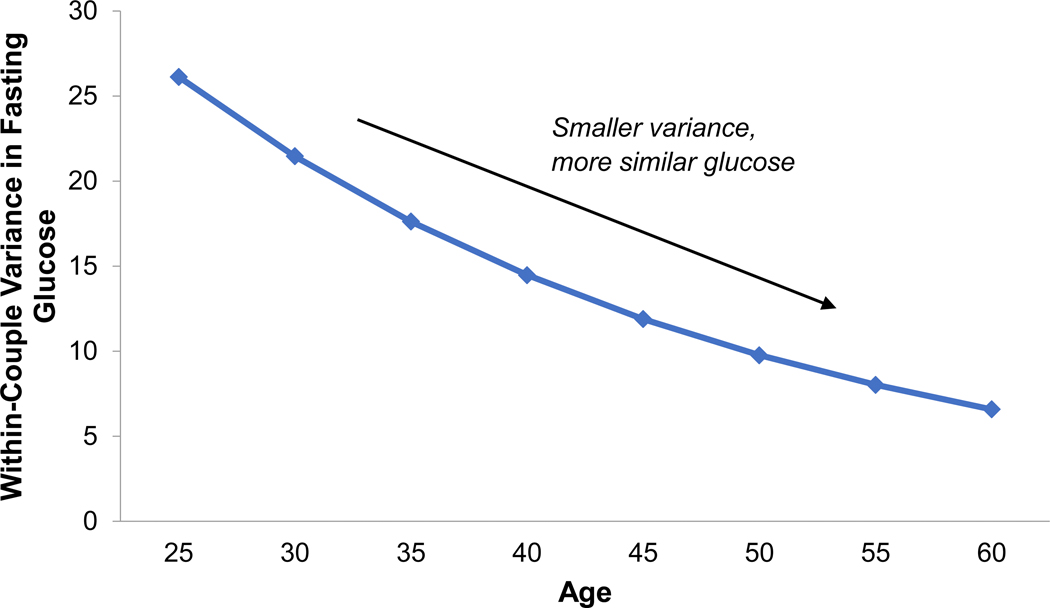
Older adults’ fasting glucose levels were more similar to their partner’s compared to the glucose similarity of younger couples (Table 2 and Figure 1, Estimate = −0.039, SE = 0.019, p = .034; Χ2(1) = 4.2, p = .040). Older husbands and wives also had more similar DBP compared to younger counterparts (Estimate = −0.062, SE = 0.020, p = .002; Χ2(1) = 9.2, p = .002). Age did not predict partners’ similarity in carbohydrate oxidation, fat oxidation, or SBP (ps > .250).
Table 2.
Heterogeneous variance estimates with age predicting spouses’ cardiometabolic similarity
| Glucose | Carbohydrate Oxidation | Fat Oxidation | SBP | DBP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Est. | SE | p | Est. | SE | p | Est. | SE | P | Est. | SE | p | Est. | SE | p |
| Age | −0.039 | 0.019 | 0.034 | −0.012 | 0.021 | 0.543 | −0.020 | 0.020 | 0.303 | −0.024 | 0.023 | 0.300 | −0.062 | 0.020 | 0.002 |
| −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | P | −2 LL | Χ2 | p | −2 LL | Χ2 | p | |
| Model fit | 1087.5 | 4.2 | 0.040 | −69.4 | 0.4 | 0.527 | −69.3 | 1.1 | 0.294 | 1320.8 | 1 | 0.317 | 1130.1 | 9.2 | 0.002 |
| Adjusted | Est. | SE | p | Est. | SE | p | Est. | SE | P | Est. | SE | p | Est. | SE | p |
| Age | −0.073 | 0.023 | 0.007 | −0.021 | 0.020 | 0.293 | −0.036 | 0.021 | 0.088 | −0.042 | 0.028 | 0.130 | −0.071 | 0.025 | 0.005 |
| Activity Concordance | 0.209 | 0.068 | 0.002 | 0.186 | 0.068 | 0.007 | 0.043 | 0.063 | 0.499 | 0.100 | 0.084 | 0.130 | 0.054 | 0.074 | 0.462 |
| Diet Concordance | −0.017 | 0.024 | 0.475 | 0.031 | 0.020 | 0.121 | 0.048 | 0.021 | 0.023 | −0.026 | 0.021 | 0.234 | 0.016 | 0.022 | 0.453 |
| Sleep Concordance | −0.325 | 0.220 | 0.140 | 0.097 | 0.232 | 0.676 | -0.150 | 0.237 | 0.526 | 0.267 | 0.195 | 0.172 | 0.248 | 0.172 | 0.148 |
| −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | P | −2 LL | Χ2 | p | −2 LL | Χ2 | p | |
| Model fit | 1019.5 | 68.0 | <.0001 | −70.6 | 1.2 | 0.753 | −66.3 | 3.0 | 0.392 | 1255 | 65.8 | <.0001 | 1067.5 | 62.6 | <.0001 |
Note. All results presented in the table are variance estimates. All models included the fixed effects of the individual’s age, abdominal fat, sex, and study visit, but these effects are not depicted. The couple’s age averaged between partners was examined as a predictor of within-couple variance in each cardiometabolic outcome—first in a univariate model, and next in a model including activity, diet, and sleep concordance. In univariate models, log likelihood ratio tests (df=1) evaluated whether the inclusion of age significantly improved model fit. Fully adjusted models examined whether the effects of age held controlling for health behavior concordance. Log likelihood ratio tests (df=3) investigated whether the class of health behavior concordance variables improved model fit, above and beyond age.
Figure 1.
Partners’ similarity in fasting glucose values increased with age (univariate effect shown, Estimate = −0.039, SE = 0.019, p = .034).
3.2.2. Closeness.
Closer couples had more similar carbohydrate oxidation compared to their less close counterparts (Table 3, Estimate = −0.213, SE = 0.106, p = .044; Χ2(1) = 4.2, p = .040), and more similar DBP (Estimate = −0.248, SE = 0.106, p = .020; Χ2(1) = 5.8, p = .016). Closeness did not predict similarity in fat oxidation, SBP, or glucose (ps > .119).
Table 3.
Heterogeneous variance estimates with closeness predicting spouses’ cardiometabolic similarity
| Glucose | Carbohydrate Oxidation | Fat Oxidation | SBP | DBP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p |
| Closeness | 0.119 | 0.124 | 0.335 | -0.213 | 0.106 | 0.044 | −0.130 | 0.110 | 0.239 | −0.165 | 0.106 | 0.120 | −0.248 | 0.106 | 0.020 |
| −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | |
| Model fit | 1090.8 | 0.9 | 0.343 | −73.2 | 4.2 | 0.040 | −69.6 | 1.4 | 0.237 | 1319.3 | 2.5 | 0.114 | 1133.5 | 5.8 | 0.016 |
| Adjusted | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p |
| Closeness | -- | -- | -- | −0.195 | 0.126 | 0.121 | −0.270 | 0.123 | 0.028 | −0.096 | 0.123 | 0.435 | −0.100 | 0.147 | 0.496 |
| Activity Concordance | -- | -- | -- | 0.154 | 0.073 | 0.035 | 0.013 | 0.060 | 0.028 | 0.054 | 0.079 | 0.497 | 0.022 | 0.076 | 0.774 |
| Diet Concordance | -- | -- | -- | 0.034 | 0.021 | 0.098 | 0.049 | 0.022 | 0.023 | −0.023 | 0.023 | 0.326 | 0.0064 | 0.024 | 0.794 |
| Sleep Concordance | -- | -- | -- | −0.065 | 0.257 | 0.801 | −0.529 | 0.276 | 0.055 | 0.195 | 0.223 | 0.382 | 0.301 | 0.214 | 0.160 |
| −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | |
| Model fit | -- | -- | -- | −71.9 | 1.3 | 0.729 | −68.1 | 1.5 | 0.682 | 1256.8 | 62.5 | <.0001 | 1074.9 | 58.6 | <.0001 |
Note. All results presented in the table are variance estimates. All models included the fixed effects of the individual’s age, abdominal fat, sex, and study visit, but these effects are not depicted. Couple closeness was examined as a predictor of within-couple variance in each cardiometabolic outcome—first in a univariate model, and next in a model including activity, diet, and sleep concordance. In univariate models, log likelihood ratio tests (df=1) evaluated whether the inclusion of closeness significantly improved model fit. Fully adjusted models examined whether the effects of closeness held controlling for health behavior concordance. The fully adjusted model predicting glucose failed to converge. Log likelihood ratio tests (df=3) investigated whether the class of health behavior concordance variables improved model fit, above and beyond closeness.
3.2.3. Hostile behavior.
Couples who behaved in a less hostile way during their marital disagreement discussion had more similar carbohydrate oxidation rates than did more hostile couples (Table 4, Estimate = 0.012, SE = 0.0049, p = .018; Χ2(1) = 6.4, p = .011). In the univariate models, hostile behavior did not predict similarity in fat oxidation, SBP, DBP, or glucose (ps > .181).
Table 4.
Heterogeneous variance estimates with hostile behavior predicting spouses’ cardiometabolic similarity
| Glucose | Carbohydrate Oxidation | Fat Oxidation | SBP | DBP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p |
| Hostility | 0.0048 | 0.0050 | 0.338 | 0.012 | 0.0049 | 0.018 | 0.0059 | 0.0044 | 0.181 | 0.0074 | 0.0063 | 0.239 | 0.0036 | 0.0062 | 0.564 |
| −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | |
| Model fit | 1090.7 | 1.0 | 0.317 | −75.4 | 6.4 | 0.011 | −70.1 | 1.9 | 0.168 | 1320.4 | 1.4 | 0.237 | 1139 | 0.30 | 0.584 |
| Adjusted | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p |
| Hostility | 0.0029 | 0.0056 | 0.604 | 0.014 | 0.0054 | 0.009 | 0.012 | 0.0051 | 0.017 | 0.0044 | 0.0072 | 0.539 | 0.0039 | 0.010 | 0.513 |
| Activity Concordance | 0.203 | 0.065 | 0.002 | 0.141 | 0.073 | 0.054 | −0.0091 | 0.062 | 0.884 | 0.061 | 0.080 | 0.444 | 0.014 | 0.076 | 0.858 |
| Diet Concordance | −0.036 | 0.024 | 0.122 | 0.055 | 0.022 | 0.011 | 0.064 | 0.023 | 0.005 | −0.019 | 0.025 | 0.449 | 0.012 | 0.025 | 0.622 |
| Sleep Concordance | −0.237 | 0.212 | 0.265 | 0.0073 | 0.238 | 0.976 | −0.292 | 0.243 | 0.231 | 0.251 | 0.202 | 0.213 | 0.390 | 0.160 | 0.015 |
| −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | −2 LL | Χ2 | p | |
| Model fit | 1026.2 | 64.5 | <.0001 | −77.2 | 1.8 | 0.180 | −69.8 | 0.3 | 0.960 | 1257 | 63.4 | <.0001 | 1074.9 | 64.1 | <.0001 |
Note. All results presented in the table are variance estimates. All models included the fixed effects of the individual’s age, abdominal fat, sex, and study visit, but these effects are not depicted. Couple hostile behavior was examined as a predictor of within-couple variance in each cardiometabolic outcome—first in a univariate model, and next in a model including activity, diet, and sleep concordance. In univariate models, log likelihood ratio tests (df=1) evaluated whether the inclusion of hostile behavior significantly improved model fit. Fully adjusted models examined whether the effects of hostile behavior held controlling for health behavior concordance. Log likelihood ratio tests (df=3) investigated whether the class of health behavior concordance variables improved model fit, above and beyond hostile behavior.
3.3. Is health behavior concordance associated with cardiometabolic similarity?
3.3.1. Activity.
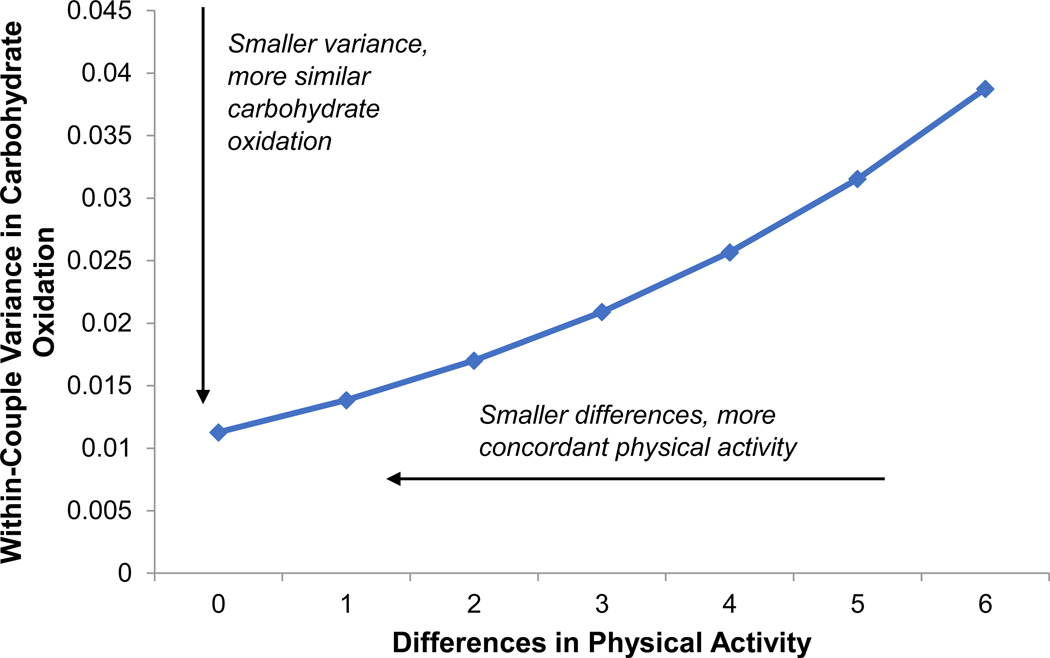
Partners with more similar activity levels also had more similar fasting glucose (Estimate = 0.188, SE = 0.059, p = .002; Χ2(1) = 36.8, p < .0001) and carbohydrate oxidation (Estimate = 0.206, SE = 0.067, p = .002; Χ2(1) = 4.9, p = .027). Activity concordance did not predict similarity in fat oxidation, SBP, or DBP (ps > .250).
3.3.2. Diet.
Partners with more concordant diets also had more similar rates of fat oxidation (Estimate = 0.041, SE = 0.020, p = .041), though the addition of diet similarity to the base model did not significantly improve model fit (Χ2(1) = 2.1, p = .147). A nonsignificant trend linking diet concordance to similarity in carbohydrate oxidation arose in the expected direction (Estimate = 0.036, SE = 0.021, p = .084; Χ2(1) = 2.9, p = .089).
3.3.3. Sleep.
Partners with more concordant sleep duration had more similar resting DBP compared to partners who slept disparate amounts (Estimate = 0.393, SE = 0.152, p = .010; Χ2(1) = 7.2, p = .007). Sleep concordance did not predict similarity in SBP, glucose, or carbohydrate or fat oxidation (ps > .250).
3.4. Does health behavior concordance account for associations of age, closeness, and marital behavior with cardiometabolic similarity?
3.4.1. Age.
Older age remained a significant predictor of greater glucose similarity (Table 2 and Figure 2, Estimate = −0.063, SE = 0.023, p = .007) and DBP similarity (Estimate = −0.071, SE = 0.025, p = .005), above and beyond the effects of activity, diet, and sleep concordance. Further, a nonsignificant trend between older age and more similar fat oxidation rates arose in the expected direction (Estimate = −0.036, SE = 0.021, p = .088). Effects on carbohydrate oxidation and SBP remained nonsignificant (ps > .130).
Figure 2.
In univariate models, partners’ activity concordance was associated with more similar rates of carbohydrate oxidation (Estimate = 0.206, SE = 0.067, p = .002).
3.4.2. Closeness.
After the addition of health behavior concordance covariates, couple closeness no longer predicted greater carbohydrate oxidation (Table 3, Estimate = −0.195, SE = 0.126, p = .121) or DBP similarity (Estimate = −0.100, SE = 0.147, p = .496) as it had in univariate models. However, greater closeness was associated with more similar fat oxidation rates after adjusting for health behavior concordance (Estimate = −0.270, SE = 0.123, p = .028). Closeness remained nonsignificant in its associations with SBP similarity (p > .250). The model predicting glucose similarity failed to converge.
3.4.3. Hostile behavior.
The association between less hostile marital behavior and more similar carbohydrate oxidation remained significant after including the effects of activity, diet, and sleep concordance (Table 4, Estimate = 0.014, SE = 0.0054, p = .009). Additionally, with activity, diet, and sleep concordance as covariates in the model, less hostility also predicted more similar fat oxidation rates between partners (Estimate = 0.012, SE = 0.0051, p = .017). The links between hostility and glucose, DBP, and SBP similarity remained nonsignificant (ps > .250).
3.5. Ancillary analyses exploring relationship length and self-reported marital satisfaction
Longer-married couples had more similar DBP compared to couples in shorter marriages (Supplemental Table 1, Estimate = −0.051, SE = 0.021, p = .014; Χ2(1) = 5.5, p = .019). This effect was reduced to non-significance when health behavior concordance variables were included (p = .099). Relationship length did not predict similarity in glucose, carbohydrate oxidation, fat oxidation, or SBP.
In univariate models, more satisfied partners had more similar SBP (Supplemental Table 2, Estimate = −0.0096, SE = 0.0049, p = .048; Χ2(1) = 81.3, p < .0001), DBP (Estimate = −0.011, SE = 0.0051, p = .033; Χ2(1) = 5.0, p = .025), and carbohydrate oxidation at a trend level (Estimate = −0.0082, SE = 0.0044, p = .061; Χ2(1) = 3.9, p = .048). However, these associations were reduced to non-significance when health behavior concordance variables were included (ps > .182). In one exception, a nonsignificant trend emerged with the inclusion of health behavior concordance variables, such that happier couples showed greater similarity in fat oxidation (Estimate = −0.0095, SE = 0.0050, p = .058). The model predicting glucose similarity failed to converge.
4. Discussion
Among healthy married couples, partners who felt closer, treated each other with less hostility, and who were older showed stronger similarity in at least one of four fasting baseline cardiometabolic measures—glucose, DBP, and carbohydrate and fat metabolism—compared to partners who were less close, more hostile, and younger. The same was true for health behavior concordance: partners who had more similar activity levels, sleep duration, and diet quality also shared greater resemblance in one or more of the same four health indices; none predicted SBP similarity. Associations with hostile behavior, closeness, and age remained significant or emerged after accounting for concordance in self-reported activity, sleep, and diet. Together, these findings provide novel evidence for the roles of shared environment and mutual influences in partners’ overlapping risks for cardiometabolic conditions.
4.1. Understanding the Roots of Spousal Health Concordance
The weight of evidence for spousal concordance in cardiometabolic diseases and their risk factors is undeniable. One meta-analysis found significant spousal overlap across 13 risk factors (e.g., hypertension, obesity) in data from over 100,000 couples in more than 200 samples (Di Castelnuovo et al., 2008). However, the mechanisms that drive partners’ overlap have received much less empirical attention. Indeed, a conceptual review of the health concordance literature found that authors primarily attributed the effects to one of three theoretical explanations: choosing a similar mate, sharing an environment with common resources, and directly influencing the partner’s behaviors and emotions (Meyler et al., 2007). Most studies did not empirically evaluate their corresponding theories.
The literature implicitly assumes that parallel health risks emerge for all couples, despite that meta-analyses have documented considerable variance in these links (e.g., Di Castelnuovo et al., 2008). The current study leveraged this variability, moving beyond the explanation of similar mate selection by comparing couples with varying behavioral and emotional interdependence (Kiecolt-Glaser and Wilson, 2017). Indeed, the selection hypothesis should apply equally to couples and, therefore, does not explain variable health overlap across couples. Further, assortative mating does not necessarily lead to happier, closer marriages; in fact, newlyweds who shared more similar personality traits declined more sharply in their relationship satisfaction across the marriage (Shiota and Levenson, 2007).
4.2. The Role of Age in Couples’ Cardiometabolic Similarity
Most prior studies that have acknowledged possible variation in couples’ shared risks tested whether partners’ health and well-being linearly converged over time; in cross-sectional data, they have examined whether within-couple correlations are stronger among longer-married couples, or more rarely, among older couples (Di Castelnuovo et al., 2008; Meyler et al., 2007). Although age and relationship length are correlated (r=.78, Table 1), less than 10% of studies that examined relationship length found that longer-married couples had stronger health correlations than those in shorter marriages, whereas 44% of studies focusing on age found larger correlations among older couples compared to younger counterparts (Di Castelnuovo et al., 2008). We replicated the effects of older age on greater similarity in glucose values (Cheraskin et al., 1968) and DBP (Di Castelnuovo et al., 2008). These associations persisted after accounting for health behavior concordance, suggesting the possible roles of shared emotions, stressors, and other routines. Also mirroring prior results, our ancillary analyses revealed that relationship length was a weaker predictor than age: longer-married couples had greater DBP similarity, but including health behavior concordance reduced this association to non-significance. Bolstering past work, the present findings underscore the unique importance of life stage and developmental period for shaping couples’ shared risks. Indeed, with increasing age, the social roles and responsibilities that organize couples’ lives shift from work to retirement, from childrearing to empty nests (Elder, 1998). Combined with shrinking networks (Carstensen, 1995), the marital relationship may play an increasingly central role in health and well-being.
4.3. The Importance of Closeness and Marital Behavior in Relation to Health Behavior Concordance
To our knowledge, this study is the first to show that both closeness and marital behavior predict aspects of cardiometabolic similarity. Consistent with our theoretical framework (Kiecolt-Glaser and Wilson, 2017), some of these associations held controlling for activity, diet, and sleep concordance. Other associations with hostility and closeness emerged after controlling for health behavior concordance—an example of statistical suppression (MacKinnon et al., 2000) that may point to distinct mechanistic pathways for partners’ health overlap. Our study also provided some of the first evidence linking health behavior concordance to dimensions of health similarity: in univariate models, couples with more similar activity levels also had closer glucose and carbohydrate oxidation values; those whose diets overlapped more had more similar fat oxidation; and couples who slept similar amounts also had greater DBP similarity. As a class, the three types of health behavior concordance explained significant variance in glucose, SBP, and DBP. However, except for a few marginal associations, marital behavior, closeness, and self-reported marital satisfaction were largely unrelated to health behavior concordance. Thus, health behavior concordance cannot be said to mediate or explain links between relationship factors and cardiometabolic similarity. This may be due to unique external influences on each partner’s health behaviors, e.g., personal work schedules and demands, as well as separate work and home routines. Alternatively, our self-report measures of activity and sleep may have limited the associations with relationship factors and cardiometabolic similarity.
The fact that some associations with hostile behavior and closeness arose adjusting for health behavior concordance may point to the importance of other aspects of life with a partner, e.g., shared stressors and emotional spillover. Closer partners identify more as a single unit, spend more time together, and experience more sleep disruption on days when a partner is in pain (Aron et al., 1992; Iida et al., 2018; Martire et al., 2013a). Further, prior work has shown that partners’ negative emotions fluctuate together, a pattern that is magnified on days when partners are at home together (Saxbe and Repetti, 2010), when they report the same stressful event (Berg et al., 2011b), and among couples who work through challenges more closely as a team (Berg et al., 2011b). Insofar as a satisfying marriage encourages partners to function as a team and to spend more time together (e.g., Reissman et al., 1993), satisfied couples should mirror the trends seen in close spousal pairs. Indeed, among adults with a painful musculoskeletal condition and their partners, happier wives’ emotional distress was more closely tied to their partner’s distress on days when they perceived the spouse was suffering more than usual—reflecting greater emotional attunement among the most satisfied couples (Monin et al., 2017). In parallel to these emotional patterns, our findings show that stronger closeness and less negative marital behavior also translate to stronger overlap in their cardiometabolic health, a novel result that helps to explain couples’ shared disease risks.
Notably, hostile behavior predicted lower carbohydrate oxidation independent of health behavior concordance—unlike closeness or self-reported marital satisfaction, whose effects on carbohydrate oxidation, DBP, and SBP similarity were superseded by behavioral concordance. This is consistent with a long history of studies showing that directly observed marital behavior predicts health and physiological outcomes more strongly than self-report measures (e.g., Kiecolt-Glaser and Newton, 2001). Indeed, behavioral observation is less susceptible to self-presentation biases than are self-report questionnaires, and coded patterns of hostility may fall outside of couples’ awareness (Heyman, 2001). Further, coded observation of the two marital disagreement discussions may capture a key behavioral mechanism—partners’ demonstrated ability to work together through an impasse in their marriage without personal attacks and hostile gestures. This skill would enable more frequent and more effective collaboration (Berg et al., 2011a), a clear path to health convergence.
In the current study, hostile behavior was moderately correlated with couples’ self-reported closeness (r = −0.41) and marital satisfaction (r = −0.50), which were strongly interrelated (r = 0.81). In samples and contexts where closeness and marital satisfaction are more weakly correlated (e.g., Martire et al., 2013a), the implications of satisfaction for shared cardiometabolic health may be distinct from those of closeness. For example, in one prior study, negative emotions were more contagious among unhappily married couples compared to their satisfied counterparts (Saxbe and Repetti, 2010), perhaps because satisfied couples more successfully shielded partners from their own bad moods or more effectively regulated their own emotions when the other felt low. That is, when happy couples manage to practice emotional independence, a blissful marriage may not spread negative emotions and health risks in the same way as when partners are emotionally enmeshed. The interplay of marital satisfaction and closeness and their joint health consequences must be teased apart in future studies.
Although results aligned with our hypotheses at a rate that far exceeded chance levels, some cardiometabolic measures were more consistently explained by our predictors than others. For instance, in contrast to DBP results, similarity in partners’ SBP was only predicted by self-reported marital satisfaction in ancillary analyses, and this effect was reduced to non-significance when health behavior concordance was included. This pattern parallels prior work showing stronger white coat effects on SBP compared to DBP (Jumabay et al., 2005; Vinyoles et al., 2008), which may have introduced greater variance in resting SBP unrelated to couple-level factors. Likewise, in univariate models, associations with closeness, marital quality, age, and health behavior concordance were more apparent between partners’ carbohydrate oxidation than fat oxidation. Although these two metabolic rates are inherently related (r = −0.71), they are not perfectly coupled: carbohydrate oxidation fluctuates more readily with nutrient availability (Wolfe, 1998), exercise (Potteiger et al., 2008), and sleep changes (Zitting et al., 2018). Diet concordance explained significant variance in fat oxidation similarity; only after accounting for this effect did links with closeness and hostile behavior become apparent. Partners’ glucose similarity was explained by their age and activity concordance, but not by closeness or marital behavior. In the general population, fasting plasma glucose can reliably predict greater incidence of diabetes, stroke, myocardial infarction, and earlier all-cause mortality (Lee et al., 2018); it is also highly correlated with HbA1c, a diagnostic marker of diabetes (Kam-On Chung et al., 2017). However, fasting glucose levels are also sensitive to recent physical activity and alcohol intake (Moebus et al., 2011), and glucose can change as markedly before a meal as it does after eating (Dunn et al., 2004). Such factors may have introduced excessive noise, challenging our ability to detect all hypothesized associations.
4.4. Strengths, Limitations, and Conclusions
As the first to assess the relevance of closeness and observed marital behavior for partners’ cardiometabolic similarity, the present study had notable strengths. The couples were free from preexisting health conditions, eliminating the role of disease-related assortative mating and providing a preclinical window into how partners may come to share common cardiovascular and metabolic disease risks. Whereas many prior studies assessed health once, partners’ health in this study was assessed on two separate occasions. To build on this work, future studies should gather accelerometer-based measures of activity and sleep, and explore whether specific features of activity (sedentary behavior, moderate-to-vigorous physical activity) and sleep (bedtime, wake time, efficiency) independently affect partners’ similarity; our self-report measures represent a limitation. A larger sample size would benefit the heterogeneous-variance models; when we attempted to examine four predictors of variance simultaneously, some of the models predicting glucose did not converge. Larger samples would also allow researchers to test whether average levels of cardiometabolic function moderate the effects of relationship factors and age on couples’ health similarity, or to categorize couples by their mean levels and similarity using latent class analysis. Future studies should recruit more racially diverse samples; most couples in our study were of White race.
To our knowledge, these data are the first to link aspects of the marital relationship, as well as health behavior concordance, to partners’ shared cardiometabolic health. Indeed, findings suggest that partners’ closeness and marital behavior may augment the overlap in their cardiometabolic profiles, apart from the contributions of parallel health behaviors. In this way, the results help to illuminate the mechanisms that lead to partners’ shared disease risks, and thus provide ways to leverage the couple’s relationship to maximize the benefits of health promotion.
Supplementary Material
Highlights.
Spouses share common risks for cardiometabolic diseases, according to prior work.
Nevertheless, mechanisms of disease concordance remain poorly understood.
In our study closer, happier, older couples had greater cardiometabolic similarity.
Associations were independent from the effects of health behavior concordance.
Acknowledgments
This work was supported in part by NIH grants K99/R00 AG056667, R21 CA158868, UL1TR001070, K05 CA172296, T32 DE014320, L30 AG06025; a Pelotonia Postdoctoral Fellowship from Ohio State University’s Comprehensive Cancer Center; and American Cancer Society Postdoctoral Fellowship Grant 121911-PF-12–040-01-CPPB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron A, Aron EN, Smollan D, 1992. Inclusion of other in the self scale and the structure of interpersonal closeness. J Pers Soc Psychol 63, 596–612. [Google Scholar]
- Berg CA, Schindler I, Smith TW, Skinner M, Beveridge RM, 2011a. Perceptions of the cognitive compensation and interpersonal enjoyment functions of collaboration among middle-aged and older married couples. Psychol Aging 26, 167–173. [DOI] [PubMed] [Google Scholar]
- Berg CA, Wiebe DJ, Butner J, 2011b. Affect covariation in marital couples dealing with stressors surrounding prostate cancer. Gerontology 57, 167–172. [DOI] [PubMed] [Google Scholar]
- Berli C, Lüscher J, Luszczynska A, Schwarzer R, Scholz U, 2018. Couples’ daily self-regulation: The health action process approach at the dyadic level. PLoS ONE 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SN, Haskell WL, Ho P, Paffenbarger RS Jr., Vranizan KM, Farquhar JW, Wood PD, 1985. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 122, 794–804. [DOI] [PubMed] [Google Scholar]
- Bove CF, Sobal J, Rauschenbach BS, 2003. Food choices among newly married couples: Convergence, conflict, individualism, and projects. Appetite 40, 25–41. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, 1995. Evidence for a life-span theory of socioemotional selectivity. Curr Dir Psychol Sci 4, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, 2010. Strength and vulnerability integration: A model of emotional well-being across adulthood. Psychol Bull 136, 1068–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheraskin E, Ringsdorf WM JR., Setyaadmadja ATSH, Barrett RA, Sibley GT, Reid RW, 1968. Environmental factors in blood glucose regulation. J Am Geriatr Soc 16, 823–825. [DOI] [PubMed] [Google Scholar]
- Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ, 2003. Effectiveness of the us department of agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr 77, 1171–1178. [DOI] [PubMed] [Google Scholar]
- Crespo C, Davide IN, Costa ME, Fletcher GJO, 2008. Family rituals in married couples: Links with attachment, relationship quality, and closeness. Pers Relationship 15, 191–203. [Google Scholar]
- Di Castelnuovo A, Quacquaruccio G, Donati MB, de Gaetano G, Iacoviello L, 2008. Spousal concordance for major coronary risk factors: A systematic review and meta-analysis. Am. J. Epidemiol. 169, 1–8. [DOI] [PubMed] [Google Scholar]
- Dunn TC, Eastman RC, Tamada JA, 2004. Rates of glucose change measured by blood glucose meter and the glucowatch biographer during day, night, and around mealtimes. Diabetes Care 27, 2161–2165. [DOI] [PubMed] [Google Scholar]
- Elder GH, 1998. The life course as developmental theory. Child Dev 69, 1–12. [PubMed] [Google Scholar]
- Funk JL, Rogge RD, 2007. Testing the ruler with item response theory: Increasing precision of measurement for relationship satisfaction with the couples satisfaction index. J Fam Psychol 21, 572–583. [DOI] [PubMed] [Google Scholar]
- Gunn HE, Buysse DJ, Hasler BP, Begley A, Troxel WM, 2015. Sleep concordance in couples is associated with relationship characteristics. Sleep 38, 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein RJ, 2007. Mixed-effects regression models with heterogeneous variance: Analyzing ecological momentary assessment (EMA) data of smoking, Modeling contextual effects in longitudinal studies. Lawrence Erlbaum Associates Publishers, Mahwah, NJ, US, pp. 183–206. [Google Scholar]
- Heyman RE, 2001. Observation of couple conflicts: Clinical assessment applications, stubborn truths, and shaky foundations. Psychol Assess 13, 5–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman RE, 2004. Rapid marital interaction coding system (RMICS), in: Kerig PK, Baucom DH (Eds.), Couple observational coding systems. Lawrence Erlbaum Associates, Nahwah, New Jersey, pp. 67–94. [Google Scholar]
- Hippisley-Cox J, Coupland C, Pringle M, Crown N, Hammersley V, 2002. Married couples’ risk of same disease: Cross sectional study. BMJ 325, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J, Pringle M, 1998. Are spouses of patients with hypertension at increased risk of having hypertension? A population-based case-control study. Brit J Gen Pract 48, 1580–1583. [PMC free article] [PubMed] [Google Scholar]
- Iida M, Seidman G, Shrout PE, 2018. Models of interdependent individuals versus dyadic processes in relationship research. J Soc Pers Relat 35, 59–88. [Google Scholar]
- Jumabay M, Ozawa Y, Kawamura H, Saito S, Izumi Y, Mitsubayashi H, Kasamaki Y, Nakayama T, Cheng Z, Ma Y, Mahumut M, 2005. White coat hypertension in centenarians. Am J Hypertens 18, 1040–1045. [DOI] [PubMed] [Google Scholar]
- Jurj AL, Wen W, Li HL, Zheng W, Yang G, Xiang YB, Gao YT, Shu XO, 2006. Spousal correlations for lifestyle factors and selected diseases in chinese couples. Ann Epidemiol 16, 285–291. [DOI] [PubMed] [Google Scholar]
- Kam-On Chung J, Xue H, Wing-Hang Pang E, Chuen-Chu Tam D, 2017. Accuracy of fasting plasma glucose and hemoglobin a1c testing for the early detection of diabetes: A pilot study. Frontiers in Laboratory Medicine 1, 76–81. [Google Scholar]
- Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Malarkey WB, Belury MA, 2015. Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology 52, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL, 2001. Marriage and health: His and hers. Psychol Bull 127, 472–503. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ, 2017. Lovesick: How couples’ relationships influence health. Annu Rev Clin Psycho 17, 421–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Kim SM, Choi S, Kim K, Jeong S-M, Son JS, Yun J-M, Park SM, 2018. The effect of change in fasting glucose on the risk of myocardial infarction, stroke, and all-cause mortality: A nationwide cohort study. Cardiovasc Diabetol 17, 51–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong A, Rahme E, Dasgupta K, 2014. Spousal diabetes as a diabetes risk factor: A systematic review and meta-analysis. BMC Med 12, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM, 2000. Equivalence of the mediation, confounding and suppression effect. Prev Sci 1, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire LM, Keefe FJ, Schulz R, Stephens MAP, Mogle JA, 2013a. The impact of daily arthritis pain on spouse sleep. Pain 154, 1725–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire LM, Stephens MAP, Mogle J, Schulz R, Brach J, Keefe FJ, 2013b. Daily spousal influence on physical activity in knee osteoarthritis. Ann Behav Med 45, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Willett WC, 2006. Evaluating adherence to recommended diets in adults: The alternate healthy eating index. Public Health Nutr 9, 152–157. [DOI] [PubMed] [Google Scholar]
- Meyler D, Stimpson JP, Peek MK, 2007. Health concordance within couples: A systematic review. Soc Sci Med 64, 2297–2310. [DOI] [PubMed] [Google Scholar]
- Moebus S, Göres L, Lösch C, Jöckel K-H, 2011. Impact of time since last caloric intake on blood glucose levels. Eur J Epidemiol 26, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin JK, Levy BR, Kane HS, 2017. To love is to suffer: Older adults’ daily emotional contagion to perceived spousal suffering. J Gerontol B-Psychol 72, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB, Wright JT, 2019. Measurement of blood pressure in humans: A scientific statement from the American Heart Association. Hypertension 73, e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie JR, Guzik TJ, Touyz RM, 2018. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can J Cardiol 34, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potteiger JA, Kirk EP, Jacobsen DJ, Donnelly JE, 2008. Changes in resting metabolic rate and substrate oxidation after 16 months of exercise training in overweight adults. Int J Sport Nutr Exerc Metab 18, 79–95. [DOI] [PubMed] [Google Scholar]
- Pujia A, Mazza E, Ferro Y, Gazzaruso C, Coppola A, Doldo P, Grembiale RD, Pujia R, Romeo S, Montalcini T, 2019. Lipid oxidation assessed by indirect calorimetry predicts metabolic syndrome and type 2 diabetes. Front Endocrinol 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissman C, Aron A, Bergen MR, 1993. Shared activities and marital satisfaction: Causal direction and self-expansion versus boredom. J Soc Pers Relat 10, 243–254. [Google Scholar]
- Rook KS, Charles ST, 2017. Close social ties and health in later life: Strengths and vulnerabilities. Am Psychol 72, 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D, Repetti RL, 2010. For better or worse? Coregulation of couples’ cortisol levels and mood states. J Pers Soc Psychol 98, 92–103. [DOI] [PubMed] [Google Scholar]
- Shiota MN, Levenson RW, 2007. Birds of a feather don’t always fly farthest: Similarity in big five personality predicts more negative marital satisfaction trajectories in long-term marriages. Psychol Aging 22, 666–675. [DOI] [PubMed] [Google Scholar]
- Simonson DC, DeFronzo RA, 1990. Indirect calorimetry: Methodological and interpretative problems. Am J Physiol 258, E399–412. [DOI] [PubMed] [Google Scholar]
- Suarez L, Criqui MH, Barrett-Connor E, 1983. Spouse concordance for systolic and diastolic blood pressure. Am J Epidemiol 118, 345–351. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Coffey T, Berra K, Iaffaldano R, Casey K, Haskell WL, 1984. Seven-day activity and self-report compared to a direct measure of physical activity. Am J Epidemiol 120, 818–824. [DOI] [PubMed] [Google Scholar]
- Vinyoles E, Felip À, Pujol E, de la Sierra A, Durà R, del Rey RH, Sobrino J, Gorostidi M, de la Figuera M, Segura J, Banegas JR, Ruilope LM, 2008. Clinical characteristics of isolated clinic hypertension. Journal of Hypertension 26, 438–445. [DOI] [PubMed] [Google Scholar]
- Weir JBDB, 1949. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR, 1998. Fat metabolism in exercise. Adv Exp Med Biol 441, 147–156. [DOI] [PubMed] [Google Scholar]
- Xu S, Lorber MF, 2014. Interrater agreement statistics with skewed data: Evaluation of alternatives to cohen’s kappa. J Consult Clin Psychol 82, 1219–1227. [DOI] [PubMed] [Google Scholar]
- Zitting K-M, Vujovic N, Yuan RK, Isherwood CM, Medina JE, Wang W, Buxton OM, Williams JS, Czeisler CA, Duffy JF, 2018. Human resting energy expenditure varies with circadian phase. Curr Biol 28, 3685–3690.e3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.