Abstract
Objectives
To summarise the evidence on diagnostic issues in difficult-to-treat rheumatoid arthritis (D2T RA) informing the EULAR recommendations for the management of D2T RA.
Methods
A systematic literature review (SLR) was performed regarding the optimal confirmation of a diagnosis of rheumatoid arthritis (RA) and of mimicking diseases and the assessment of inflammatory disease activity. PubMed and Embase databases were searched up to December 2019. Relevant papers were selected and appraised.
Results
Eighty-two papers were selected for detailed assessment. The identified evidence had several limitations: (1) no studies were found including D2T RA patients specifically, and only the minority of studies included RA patients in whom there was explicit doubt about the diagnosis of RA or presence of inflammatory activity; (2) mostly only correlations were reported, not directly useful to evaluate the accuracy of detecting inflammatory activity in clinical practice; (3) heterogeneous, and often suboptimal, reference standards were used and (4) (thus) only very few studies had a low risk of bias.
To ascertain a diagnosis of RA or relevant mimicking disease, no diagnostic test with sufficient validity and accuracy was identified. To ascertain inflammatory activity in patients with RA in general and in those with obesity and fibromyalgia, ultrasonography (US) was studied most extensively and was found to be the most promising diagnostic test.
Conclusions
This SLR highlights the scarcity of high-quality studies regarding diagnostic issues in D2T RA. No diagnostic tests with sufficient validity and accuracy were found to confirm nor exclude the diagnosis of RA nor its mimicking diseases in D2T RA patients. Despite the lack of high-quality direct evidence, US may have an additional value to assess the presence of inflammatory activity in D2T RA patients, including those with concomitant obesity or fibromyalgia.
Keywords: arthritis, rheumatoid, ultrasonography, synovitis
Key messages.
Ascertaining the diagnosis of rheumatoid arthritis (RA) and the inflammatory origin of the complaints are important in the management of difficult-to-treat (D2T) RA.
This systematic literature review, conducted to inform the EULAR recommendations for the management of D2T RA, provides an extensive overview of the current literature regarding diagnostic issues in D2T RA.
The identified evidence had several limitations: (1) study population could not be considered as having D2T RA; (2) typically no appropriate diagnostic association measures were reported; (3) heterogeneous and suboptimal reference standards were used and (4) most studies (thus) had a moderate to high risk of bias.
No diagnostic tests with sufficient validity and accuracy were found to confirm nor exclude the diagnosis of RA nor its mimicking diseases in D2T RA patients.
Despite the lack of high-quality direct evidence, ultrasonography may have an additional value to traditional clinical assessment to assess the presence of inflammatory activity in D2T RA patients, including those with concomitant obesity or fibromyalgia.
INTRODUCTION
Treatment options for rheumatoid arthritis (RA) have largely expanded and treatment strategies have improved over the past decades. Nowadays, many patients reach remission or low disease activity when following the current EULAR recommendations and/or American College of Rheumatology (ACR) guideline for the management of RA.1 2 However, there is still a substantial proportion of RA patients that remains symptomatic even though they have been treated according to these recommendations. This patient group is referred to as having ‘difficult-to-treat (D2T) RA’. This disease state is expected to affect 5%–20% of all patients with RA, depending on the specific definition used.3–5 D2T RA has recently been defined as patients who failed at least two biological/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) with different mechanisms of action after failing conventional synthetic (cs)DMARD therapy. Additionally, patients should have signs and/or symptoms suggestive of active disease, which is perceived as problematic by the patient and/or rheumatologist.6 The unmet need for these patients was previously underlined by an international survey that was conducted among rheumatologists.7 Consequently, the importance has been acknowledged by EULAR with the approval of a Task Force on the development of management recommendations for D2T RA.
In D2T RA patients, DMARD therapy is frequently changed in routine daily practice in case of signs and/or symptoms suggestive of active disease.4 However, D2T RA is a heterogeneous disease state and various factors could contribute to the persistence of these signs and/or symptoms: factors related to inflammation (eg, having underlying immunological disease mechanisms driving ‘true’ refractory disease or treatment non-adherence), factors of non-inflammatory origin (eg, concomitant fibromyalgia) or both.4 7 8 All these contributing factors may require different pharmacological and non-pharmacological therapeutic strategies,4 which are reviewed in a separate systematic literature review (SLR).9
Importantly, intensification or other changes in DMARD therapy to reduce inflammation may only be appropriate in patients with insufficient response to therapy due to inflammatory RA activity.4 Symptoms of other diseases, for example, psoriatic arthritis and polyarticular gouty arthritis, may mimic RA possibly leading to misdiagnosis of the disease.4 8 10 Additionally, coexistence of certain circumstances, for example, obesity, pain syndromes and osteoarthritis, may hamper proper grading of disease activity by influencing diagnostic measures.4 8 Therefore, in D2T RA, it will be important to ascertain the diagnosis of RA and the presence of inflammatory RA activity before adjusting therapeutic strategies.
The aim of this SLR was first to explore and summarise how to optimally confirm the diagnosis of RA in a D2T RA patient and how to optimally diagnose and rule out alternative or coexisting mimicking diseases. In addition, this SLR focused on the assessment of the presence of inflammatory activity in D2T RA patients and in those with comorbidities that may influence this assessment. This SLR, together with the other SLR focusing on therapeutic strategies in D2T RA,9 was conducted to inform the EULAR recommendations for the management of D2T RA.
Methods
Research questions
This SLR was conducted following the EULAR standardised operating procedures.11 Three clinical questions on diagnostic issues in D2T RA patients were proposed by the fellow (NMTR), comethodologist (PMJW) and postdoctoral fellow (AH) and then approved by the steering committee (GN (convenor), JMvL (coconvenor), DvdH (methodologist) and MK (fellow)). At the first Task Force meeting, which was held in August 2018, the questions were discussed, amended and then approved by the whole Task Force.
The clinical questions were focused on diagnostic techniques for (1) the confirmation of the diagnosis of RA or a relevant differential diagnoses (either as alternative or coexisting mimicking disease), (2a) the assessment of inflammatory activity in RA patients and (2b) the assessment of inflammatory activity in patients with RA with comorbidities that might influence the assessment of inflammatory activity. Mimicking diseases deemed of interest were gouty arthritis, calcium pyrophosphate deposition disease, psoriatic arthritis, spondyloarthritis, polymyalgia rheumatica, systemic lupus erythematosus, reactive arthritis, paraneoplastic syndromes, osteoarthritis and fibromyalgia. Comorbidities of interest that might influence the assessment of inflammatory activity were infections, malignancies, obesity, pain syndromes (including fibromyalgia), osteoarthritis, subluxations and joint dislocations. The clinical questions were transformed into epidemiological questions using the ‘Patients, Indicator test, Comparison test (ie, reference standard), Outcome format’ (online supplemental file).12
rmdopen-2020-001511supp001.pdf (124.4KB, pdf)
Search strategy
The databases of PubMed and Embase were searched for papers in English until December 2018 for search 1 and December 2019 for search 2. Additionally, the conference abstracts of EULAR and ACR were screened, from 2017 to 2018 for search 1 and from 2017 until 2019 for search 2. Advice regarding the setup of the search strategy was provided by two experienced librarians of Utrecht University (FPW and PHW).
The first search focused on the diagnosis of RA and relevant differential diagnoses. In addition to terms for RA and terms related to diagnostic studies, terms for misdiagnosis and common alternative and coexisting mimicking diseases were included (online supplemental file for search details). During the Task Force meeting, it was agreed to perform a limited search on recent literature on this topic as not much research on (mis-)diagnosis relevant to our project was expected to be present and to have a more focused approach given the many clinical questions on D2T RA that were defined by the Task Force. Therefore, a search limit was set to the last ten years and reference screening was not performed.
The second search focused on the assessment of inflammatory activity. In addition to terms for RA and terms related to diagnostic studies, terms for D2T RA and comorbidities that might influence assessment of inflammatory activity, general terms for inflammation and specific tests to assess inflammatory activity were included (search details in online supplemental file). A search limit was set to the last ten years. In addition, the reference lists of selected papers were manually screened. References published in the year 2000 and later were eligible for inclusion. This cut-off was chosen because of the introduction of bDMARDs around this time and, herewith, the beginning of a new diagnostic and therapeutic landscape regarding acceptable disease activity in the field of RA.
Selection of studies
First, titles and abstracts were screened in duplicate by the fellows (NMTR and MK) according to a set list of selection criteria (online supplemental file) until the percentage of conflicts was below 5%. In case of conflicts or when in doubt, eligibility was discussed with the comethodologist (PMJW). Second, all full text versions of the selected papers were screened in duplicate by the fellows (NMTR and MK). Disagreements were discussed with the comethodologist (PMJW) until consensus was reached.
As specific evidence on D2T RA patients was expected to be scarce, we decided not to focus on D2T RA patients only, but on a broader population of RA patients. Regarding the diagnosis of RA and relevant differential diagnoses, papers were eligible when focusing on patients clinically diagnosed with RA and suspected of a mimicking disease, patients suspected of RA according to the classification criteria in whom a new diagnostic test was evaluated, or patients suspected of RA, but not satisfying classification criteria. Regarding the assessment of inflammatory activity, we decided to only exclude papers when the study population included treatment naïve RA patients. Additionally, in this population, diagnostic tests beyond currently used reference standards had to be evaluated.
Data extraction and quality assessment
Information on study design, patient characteristics, index test, reference standard and diagnostic outcomes were extracted from the included papers using a predetermined format (online supplemental file).
Risk of bias (RoB) and applicability of the included original papers were assessed using the Quality Assessment of Diagnostic Accuracy Studies tool V.2 13 and highest RoB as found among categories was reported here (low, moderate, high). For SLRs, RoB was assessed using ‘A MeaSurement Tool to Assess systematic Reviews’ V.2 and overall RoB was reported according to its scoring system (low, moderate, high, critically high).14
One important item included in RoB assessment is the reference standard used. For the diagnosis of RA and relevant differential diagnoses, we deemed a clinical diagnosis according to a rheumatologist as the appropriate reference standard. For the assessment of the presence of inflammatory activity, the preferred reference standard differed between study populations. For the general established RA population, we considered validated Disease Activity Score, Composite Disease Activity Index (eg, DAS28 or CDAI) as appropriate to assess the presence of inflammatory activity at patient level, and the clinical assessment of swelling in the joint at joint level (ie, in a specific joint). In patients in whom there is explicit doubt about the presence of inflammatory activity (including patients with mimicking diseases), the traditional measures are not trustworthy. Therefore, in studies assessing this population we considered (scores based on) established imaging measures as a more appropriate reference standard.
Data extraction and quality assessment were performed in duplicate by the fellows (NMTR and AH) until the number of conflicts was below 5%. Disagreements and remaining doubts were discussed with the comethodologist (PMJW) until consensus was reached.
Statistical analyses
Extracted data were summarised descriptively regarding study and patient characteristics and reported diagnostic association measures. Preferably, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratios (LRs) and ORs were reported. If these were not available, other association measures (typically (Pearson or Spearman) correlation coefficients) were reported, although these measures do not well reflect diagnostic accuracy of measures and thus provide lower quality of evidence.15 Pooling of results was considered based on clinical and statistical homogeneity.
Results
Study characteristics
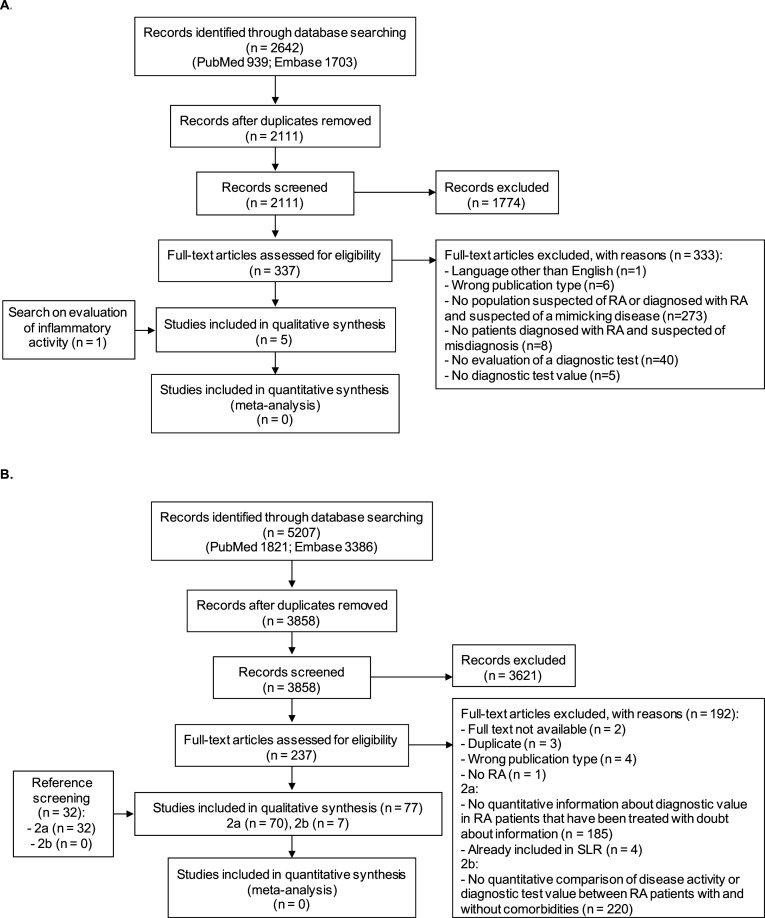
The first search regarding the diagnosis of RA and relevant differential diagnoses yielded 2111 unique papers. Title and abstract screening resulted in 337 papers, which were fully reviewed. Four of them fulfilled the selection criteria and were included for data extraction (figure 1A). One additional paper was included via the search on the assessment of inflammatory activity as this paper focused on the diagnosis of RA. Of these five papers, one paper regarded the optimal confirmation of a diagnosis of RA16 and four papers the confirmation of a coexisting mimicking disease in patients with RA.17–20
Figure 1.
Flow charts of search and selection of papers. (A) (Mis-)diagnosis of RA and relevant differential diagnoses. (B) The assessment of inflammatory activity in (2a) RA patients, and (2b) RA patients with comorbidities that might influence the assessment. RA, rheumatoid arthritis; SLR, systematic literature review.
The second search on the assessment of inflammatory disease activity resulted in 3858 unique papers. After title and abstract screening, 237 papers were selected for full-text screening and 45 papers were selected for inclusion. Additionally, 32 papers were selected via reference screening (figure 1B). Seventy of 77 papers were selected for the assessment of inflammatory activity in general,21–90 the seven remaining papers studied the assessment of inflammatory activity in patients with RA with specific comorbidities.91–97
Heterogeneity in diagnostic tests, diagnostic association measures and reference standards used prohibited pooling the data in an appropriate way. The majority of studies regarding the assessment of inflammatory activity reported correlations only, instead of the preferred diagnostic association measures (ie, sensitivity, specificity, PPV, NPV, LRs or ORs). All quantitative information on diagnostic tests is summarised in online supplemental tables 1–5.
rmdopen-2020-001511supp002.pdf (80.2KB, pdf)
rmdopen-2020-001511supp003.pdf (88.2KB, pdf)
rmdopen-2020-001511supp004.pdf (232.5KB, pdf)
rmdopen-2020-001511supp005.pdf (140.5KB, pdf)
rmdopen-2020-001511supp006.pdf (101.5KB, pdf)
Most studies were found to have a moderate or high RoB. For confirmation of the diagnosis of RA, predominantly because the cut-off for the optimal sensitivity and specificity was selected by using a receiver operating characteristic (ROC) curve analysis of the data of the same patient cohorts (ie, no predefined cut-off). For assessment of inflammatory activity, predominantly because the reference standard used was not optimal. The patient flow and timing of index test vs reference standard were generally described clearly and were appropriate in most studies.
Overall concerns about applicability for the majority of studies were moderate or high, mainly since the patient populations of included studies did not contain (D2T) RA patients in whom there was explicit doubt about the diagnosis of RA or the presence of inflammatory activity (RoB assessment and concerns regarding applicability per paper in online supplemental tables 1–5).
Optimal confirmation of the diagnosis of RA
One study (low RoB) was found assessing the confirmation of the diagnosis of RA, in patients with a self-reported diagnosis (table 1, online supplemental table 1).16 Index tests that were assessed in this study were the ACR 1987 classification criteria98 and adapted versions of these criteria by including synovitis on ultrasonography (US), erosions on US or X-rays and rheumatoid factor (RF) and anticitrullinated protein antibodies (ACPA) positivity in various combinations. Additionally, the RA MRI scoring system (RAMRIS) scale99 for synovitis was assessed as an alternative for the ACR 1987 classification criteria. The reference standard in this study was the clinical diagnosis made by one rheumatologist after a retrospectively conducted review of all available relevant evidence (except for outcomes of MRI, US and ACPA). The ACR 1987 criteria were found to have a sensitivity of 44% and specificity of 94%. Using the adapted ACR 1987 criteria including Grey scale (GS) synovitis on US, erosions on US and RF, the sensitivity increased to 72% at the expense of a minor decrease in specificity to 91%. Using the RAMRIS scale for the assessment of synovitis in metacarpophalangeal (MCP) joints 2–5, resulted in a sensitivity of 69% and a specificity of 100% (table 1).
Table 1.
Papers on the confirmation of the diagnosis of RA
| Paper | Design | Study population | Diagnostic test for RA | Reference standard* | Time interval | Sens, % (95% CI) |
Spec, % (95% CI) |
RoB |
| Pedersen, 201416 | CS | Patients with self-reported RA (n=51) | ACR 1987 criteria Adapted ACR 1987 criteria adding:
RAMRIS scale for synovitis (MCP joints 2–5) RAMRIS scale for synovitis (combined wrist and MCP joints 2–5) |
Clinical diagnosis according to a rheumatologist | NR | 44 (22 to 69) 72 (47 to 90) 72 (47 to 90) 56 (31 to 79) 39 (17 to 64) 69 (39 to 91) 62 (32 to 86) |
94 (81 to 99) 91 (77 to 83) 87 (73 to 97) 91 (77 to 98) 100 (90 to 100) 100 (73 to 100) 94 (73 to 100) |
L |
*A clinical diagnosis according to a rheumatologist was deemed as the appropriate reference standard.
ACPA, anti-citrullinated protein antibody; ACR, American College of Rheumatology; CS, cross-sectional; GS, Grey scale; L, low (green); MCP, metacarpophalangeal; NR, not reported; PD, power doppler; RA, rheumatoid arthritis; RAMRIS, Rheumatoid arthritis MRI scoring system; RF, rheumatoid factor; RoB, risk of bias; sens, sensitivity; spec, specificity; US, ultrasonography.
Diagnosis of alternative or coexisting mimicking diseases in patients with RA
Four papers were found on coexisting mimicking diseases in RA, papers on alternative mimicking diagnoses in patients with RA were not found. Three of four papers reported on fibromyalgia as a coexisting mimicking disease (table 2, online supplemental table 2). In all three papers, the cut-off for the optimal sensitivity and specificity was selected by using an ROC analysis of the data of the same patient cohorts, resulting in a high RoB. The first study assessed the Fibromyalgia Rapid Screening Tool as a diagnostic test for fibromyalgia in consecutive patients suspected of RA or a mimicking disease.17 Using the clinical diagnosis of fibromyalgia according to a rheumatologist as reference standard, a sensitivity of 83% and specificity of 88% were found. In the second study, a score, derived using the individual components of the DAS28 and rearranging the formula (table 1), was used to assess a diagnosis of fibromyalgia.20 Using the diagnosis of fibromyalgia according to the 2010 criteria,100 a sensitivity of 81% and specificity of 80% were found. The third study used a case–control design, in which microRNA let-7a, −21–5 p, −143 and −103a-3p were assessed to diagnose concomitant fibromyalgia in established patients with RA.18 MicroRNA-143 was found to be downregulated in patients with concomitant fibromyalgia. A sensitivity and specificity of 90% and 70% were found.
Table 2.
Papers on the diagnosis of mimicking disease in patients with RA
| Paper | Design | Study population | Diagnostic test for mimicking disease | Reference standard for mimicking disease* | Time interval | Sens, % (95% CI)† |
Spec, % (95% CI)† |
RoB |
| Fan, 201617 | CS | Established RA (n=279), with final diagnosis:
|
Fibromyalgia Rapid Screening Tool ≥5 (/6) | FM according to:
|
Concurrent | 71 (42 to 92) 83 (51 to 95) |
84 (79 to 89) 88 (83 to 91) |
H |
| Ghib, 2018‡18 | CC | Established RA (n=20):
|
MicroRNA-143 >1.0 | FM: NR | NR | 90 | 70 | H |
| Salaffi, 201820 | CS | Established RA (n=292), with final diagnosis:
|
Derived DAS28 patient-reported components (DAS28-P)* >0.6312 *(0.56×√TJC +0.014× GH)/DAS28 |
FM according to ACR 2010 criteria119 | NR | 81 (67 to 92) | 80 (75 to 85) | H |
| Sato, 201219 | CS | Established RA (n=118), with final diagnosis:
|
Procalcitonin ≥0.5 ng/mL Procalcitonin ≥0.2 ng/mL WCC >8500/m3 CRP ≥0.3 mg/dL ESR >15 mm/hour Procalcitonin ≥0.5 ng/mL+WCC >8500/m3 |
Bacterial Infection: symptoms, bacterial culture tests, imaging studies, and response to antibiotic therapy | Within period of hospital admission | 26 34 66 70 98 21 |
98 89 59 5 9 1 |
L |
*A clinical diagnosis according to a rheumatologist was deemed as the appropriate reference standard.
†95% CI, if reported.
‡Abstract.
ACR, American College of Rheumatology; CC, case control; CRP, C reactive protein; CS, cross-sectional; DAS28, Disease Activity Score Assessing 28 joints; ESR, erythrocyte sedimentation rate; FM, fibromyalgia; GH, global health; H, high (red); L, low (green); NR, not reported; RA, rheumatoid arthritis; RoB, risk of bias; sens, sensitivity; spec, specificity; TJC, tender joint count; VAS, Visual Analogue Scale; WCC, white cell count.;
The only study with a low RoB, was a cross-sectional study reporting on bacterial infections as a mimicking disease in patients with RA who presented with a flare (table 2, online supplemental table 2).19 The reference standard for the presence of bacterial infections was the agreed diagnosis by physicians based on symptoms, bacterial culture tests, imaging and response to antibiotic therapy. Erythrocyte sedimentation rate (ESR) >15 mm/hour was found to have the highest sensitivity with 98%. Procalcitonin≥0.5 ng/mL was found to have the highest specificity with 98%.
Assessment of inflammatory activity
Seventy papers evaluated the assessment of inflammatory activity in RA patients at patient and/or joint level.21–90 Fifty-eight different diagnostic tests were analysed: 51 biomarkers, 6 imaging measures and one used histology (online supplemental tables 3 and 4). Different reference standards were used for inflammatory activity: composite indices (DAS28, CDAI, Simplified Disease Activity Index), clinical assessment (swollen joint count (SJC (28/32/66)), tender joint count (TJC (28/32/66))) and imaging measures (US, MRI, folate scan). No studies with a low or moderate RoB were found that evaluated a diagnostic test in a population in whom there was explicit doubt about the presence of inflammatory activity and that also reported appropriate diagnostic association measures.
Patient level
In papers at patient level, 57 different diagnostic tests were assessed (online supplemental table 3). Seventeen biomarkers and two imaging measures (US sum scores and optical spectral transmission (OST) measures) were assessed in more than one study using the same reference standard used per diagnostic test (table 3). The majority of papers at patient level reported correlation measures only.
Table 3.
Concise summary of papers on the assessment of inflammatory activity at patient level
| Papers/design | Replicated diagnostic tests (*: n) | Reference standards (*: n)† | Results | RoB:* |
| Composite indices as reference standard | ||||
| 54 papers (4 SLRs (*14/22/7/14), 46 CS, 2CS‡, 2 CC)22–29 34 35 37–40 43–50 52–58 61–65 68–80 82–88 RA patients:
|
Biomarkers: MBDA score (2: 3936); miR-146a (1 SLR: 638); ACPA (8: 568); Neutrophile lymphocyte ratio (3: 523); Platelet lymphocyte ratio (2: 421); Leptin (6: 404); IL-6 (4: 373); VEGF (5: 344); MMP-3 (2: 173); IL-17 (2: 121+NR); TNF(a) (2: 185); RF (2: 165); Fibrinogen (2: 152); Resistin (2: 141); IL-2 (2: 111); IL-4 (2: 111); IL-10 (2: 111) Imaging (sum scores): US all types (9: 2060+NR): US GS (2: 57), US PD (5: 646); OST measures (3: 171) |
DAS28 (51: 8656+NR); CDAI (7: 4186); SDAI (6: 4140); Composite score, not further specified (1: 1307) | Following replicated diagnostic tests with DAS28 as reference standard:
Following replicated imaging measures with composite index as reference standard:
SLR concludes: ‘Ultrasonography can be regarded as a valuable tool for globally examining the extent of synovitis in RA. However, it is presently difficult to determine a minimal number of joints to be included in a global ultrasonography score. Further validation of proposed scores is needed.’ |
L: 5 |
| M: 43 | ||||
| H: 6 | ||||
| Clinical assessment as reference standard | ||||
| 20 papers (17 CS, 2 CS‡, 1 CC)24 26–30 35 43 47 52 57 63 65 66 68 72 74 75 82 88 RA patients:
|
Biomarkers: IL-6 (2: 205); VEGF (2: 205); ACPA (3: 181); Leptin (2: 87) Imaging (sum scores): US (5: 299); OST measure (2: 109) |
SJC28/32/66 (19: 1170); TJC 28/32/66 (20: 1207) | Following diagnostic tests with SJC as reference standard:
Following diagnostic tests with TJC as reference standard:
|
H: 20 |
| Imaging as reference standard | ||||
| 12 papers (1 SLR (*14), 8 CS, 2 CS‡, 1 CC)25 26 35 38 40 47 59 72 74 75 83 88 RA patients:
|
Biomarkers: IL-6 (2: 207); VEGF (3: 277) Imaging (sum scores): US (4: 110+NR); OST measures (2: 109) |
US (11: 1865); MRI (2: 1325) | Following diagnostic tests with US as reference standard:
SLR concludes: ‘Ultrasonography can be regarded as a valuable tool for globally examining the extent of synovitis in RA. However, it is presently difficult to determine a minimal number of joints to be included in a global ultrasonography score. Further validation of proposed scores is needed.’ |
M: 2 |
| H: 10 | ||||
*Number of studies.
†For the general established RA population, validated composite disease activity indices (eg, DAS28 or CDAI) were deemed as appropriate to assess the presence of inflammatory activity at patient level. In patients in whom there is explicit doubt about the presence of inflammatory activity, the traditional measures are not trustworthy. Therefore, in studies assessing this population we considered scores based on established imaging measures as a more appropriate reference standard.
‡Abstract.
ACPA, anticitrullinated protein antibody; CC, case control; CDAI, Clinical Disease Activity Index; CS, cross-sectional; DAS28, Disease Activity Score Assessing 28 joints; ESR, erythrocyte sedimentation rate; GS, Grey scale; H, high (red); IL, interleukin; L, low (green); M, moderate (yellow); MBDA, multi-biomarker disease activity; miRNA, micro RNA; MMP-3, matrix metalloproteinase-3; NR, not reported; ns, not significant; OST, optical spectral transmission; PD, power Doppler; RA, rheumatoid arthritis; RF, rheumatoid factor; RoB, risk of bias; SDAI, Simplified Disease Activity Index; SJC, swollen joint count; SLR, systematic literature review; TJC, tender joint count; TNF, tumour necrosis factor; US, ultrasonography; VEGF, vascular endothelial growth factor.
Only one study (moderate RoB) explicitly evaluated patients in whom there was doubt about the presence of inflammation, although this study did not report appropriate diagnostic association measures.25 In patients who had symptoms suggestive of inflammatory joint pain, weak or non-statistically significant correlations were found between DAS28 and US sum scores (US sum scores of hands and feet: r=0.14; US sum scores of MTP joints: r=0.03). In established patients with RA in whom there was not explicit doubt about the presence of inflammation, moderate to strong correlations between US sum scores and composite indices were found in eight other papers (range of r: 0.40–0.70, statistically significant (s) in six of eight papers (two low RoB, five moderate RoB, one high RoB)).24 27 35 40 74 78 80 83 One of these papers was an SLR, in which the authors concluded that US can be a valuable tool to globally assess the extent of synovitis, although it is presently difficult to determine a minimal number of joints to be included in an US sum score.40
Only four papers reported an appropriate diagnostic association measure and had a low or moderate RoB, although these papers assessed established patients with RA in whom there was not explicit doubt about the presence of inflammation.49 55 84 85 All four papers had a moderate RoB and assessed a different biomarker using DAS28 as a reference standard: high-sensitivity cardiac troponin (DAS28 >5.1: PPV 21.2%, NPV 94.6%), human neutrophil peptides 1–3 (DAS28 >2.6: sensitivity 72%, specificity 70.6%), ACPA (DAS28 not further specified: OR 2.0, 95% CI 1.004 to 3.983) and matrix metalloproteinase-3 (MMP-3, DAS28 >3.2: sensitivity 93.2%; specificity 82.8%). Of these biomarkers, ACPA and MMP-3 were assessed in more than one study, although only correlation coefficients were reported in the other papers (ACPA, r: −0.13–0.44, s in one of seven papers (six moderate RoB, one high RoB); MMP-3, r: 0.30 and 0.61, s in 2 of 2 papers (one low RoB, one moderate RoB)).22 35 52 53 62–65 73
Additionally, the SLR about the multi-biomarker disease activity (MBDA) score (including 22 studies, moderate RoB) reported that in three of four papers the MBDA score discriminated between low vs moderate/high disease activity (MBDA≥30).34 101–104 The appropriate diagnostic association measures were not reported in the SLR and could only be calculated in one of these three papers (DAS28-CRP≥2.7 (at the 6 months visit (ie, non-treatment naïve patients): sensitivity 69%, specificity 64%).101 Furthermore, moderate statistically significant correlations were reported between the MBDA score and DAS28-CRP (r: 0.41 (pooled r, SLR) and 0.52, both moderate RoB).34 56
Joint level
At joint level, 15 different diagnostic tests were assessed (table 4, onine supplemental table 4). Four diagnostic tests (clinically swollen joints, OST measures, US and MRI) were assessed in more than one study with the same reference standard used per diagnostic test. In none of the studies, there was explicit doubt about the presence of inflammatory activity.
Table 4.
Concise summary of papers on the assessment of inflammatory activity at joint level
| Papers/design | Diagnostic tests (*: n) | Reference standards (*: n)† | Results | RoB: * |
| Clinical assessment as reference standard | ||||
| 4 papers (3 CS, 1 CC)21 42 72 88 341 RA patients |
Imaging: US (1: 165); OST measure (3: 176) |
Clinical evaluation according to physician (2: 132); Clinically swollen joint (2: 109); Clinically tender joint (1: 50) |
OST measures as diagnostic test with following types of clinical assessment as reference standard:
Treatment influenced on the basis of US findings: 51.7% (*1) |
H: 4 |
| Imaging as reference standard | ||||
| 14 papers (1 SLR (*14), 9 CS, 2 CC, 1 CS)‡26 31–33 37 41 59 60 67 72 81 83 88 89 RA patients:
|
Clinical assessment: Clinically tender joints (2: 102) Clinically swollen joints (3: 163) Biomarkers: IL-2, IL-4, IL-6, IL-10, IL-17, TNF, IFN, VEGF (1: 64) Other imaging measure than reference standard: OST measures (5: 222); US (3: 456); MRI (1: 19); Contrast-enhanced MRI (1: 43); Fluorescence optical imaging (1: 18) |
US (8: 526); FolateScan (1: 40); MRI (4: 295) |
Clinically swollen joints (*2):
OST measure as diagnostic test with US as reference standard (*5):
US as diagnostic test with MRI as reference standard (*2 of which 1 SLR): sens 64%–91%, spec 60%–94%.
None of the other diagnostic tests were replicated using the same diagnostic accuracy measures. |
M: 1 |
| H: 13 | ||||
| Histology as reference standard | ||||
| 1 paper (CS)27 RA patients with at least 1 joint amenable to biopsy, n=15 |
Imaging: US (GS and PD; 1: 15) |
Krenn index of cellular inflammation (1: 15); Krenn lining layer score (1: 15); Inflammatory cell infiltrates (1: 15); |
US (GS) as diagnostic test with following histology measures as reference standard:
US (PD) as diagnostic test with following histology measures as reference standard:
|
H: 1 |
*Number of studies.
†For the general established RA population, the clinical assessment of swelling in the joint was deemed as appropriate to assess the presence of inflammatory activity at joint level (ie, in a specific joint). In patients in whom there is explicit doubt about the presence of inflammatory activity, the traditional measures are not trustworthy. Therefore, in studies assessing this population we considered established imaging measures as a more appropriate reference standard.
‡Abstract.
CC, case control; CS, cross-sectional; GS, Grey scale; H, high; IFN, interferon; IL, interleukin; L, low; MCP, metacarpophalangeal; NPV, negative predictive value; NR, not reported; OST, optical spectral transmission; PD, power Doppler; PIP, proximal interphalangeal; PPV, positive predictive value; RA, rheumatoid arthritis; RoB, risk of bias; s, significant; sens, sensitivity; SLR, systematic literature review; spec, specificity; TNF, tumour necrosis factor; US, ultrasonography; VEGF, vascular endothelial growth factor.
Almost all studies had a high RoB, predominantly because the cut-off for the optimal sensitivity and specificity was selected by using an ROC curve analysis of the data of the same patient cohort or because the reference standard was not appropriate. The only paper with a moderate RoB was an SLR (including 14 studies), which was performed without critical flaws.67 In this SLR, synovitis of different joints was assessed with US as diagnostic test and MRI as a reference standard. However, the reference standard used in this SLR (ie, MRI) was regarded as inappropriate to assess the presence of inflammatory activity in the general established RA population, which hampers its applicability.
Using the reference standard deemed appropriate to us (ie, clinical diagnosis of swelling of a joint), three papers were found assessing OST (high RoB).42 72 88 Each study used different diagnostic association measures to report the diagnostic value of OST measures in different joints (sensitivity 37%–59% and specificity 86%–93%; PPV 46% and NPV 86%; area under the ROC 0.88).
Assessment of inflammatory activity in patients with RA with comorbidities
Studies assessing diagnostic tests for the assessment of inflammatory activity in patients with RA with a specific comorbidity that may influence the assessment were found for obesity and fibromyalgia (table 5, online supplemental table 5).
Table 5.
Concise summary of papers on the assessment of inflammatory activity in RA patients with comorbidities that may influence the assessment
| Papers/design | Diagnostic tests (#: n) | Reference standards (*: n)† | Results | RoB: * |
|
Obesity At patient level |
||||
| 3 papers (3 CS)92 96 97 RA patients, n=756:
|
|
|
|
M: 2 |
| H: 1 | ||||
| At joint level | ||||
| 1 paper (1 CS)91 RA patients, n=43:
|
Clinically swollen joint (1: 43) | US (PD; 1: 43) | Per higher BMI category the chance of synovitis according to US decreased correcting for age, gender and clinically swollen joints (ie, the SJC overestimates disease activity in obese patients): OR BMI 0.52 (95%CI 0.30 to 0.93, p=0.03) | M: 1 |
| Fibromyalgia At patient level |
||||
| 3 papers (2 CS‡, 1 CC)93–95 RA patients, n=239
|
|
7-joint US score (GS/PD; 2: 111); DAS28 (1: 130); CDAI (1: 130); SDAI (1: 130) | Correlation coefficient in patients without versus with fibromyalgia with 7-joint US score (GS/PD) as reference standard:
In patients with fibromyalgia a discrepancy between traditional and modified composite scores originates, with higher traditional scores in these patients. Mean increment (95% CI, p value), adjusted for age, sex and nodular disease:
|
M: 2 |
| H: 1 | ||||
*Number of studies.
†In patients with comorbidities that may influence the assessment of inflammatory activity, the traditional measures may not be trustworthy. Therefore, in studies assessing this population we considered (scores based on) established imaging measures as a more appropriate reference standard.
‡Abstract.
ACR, American College of Rheumatology; BMI, body mass index; CC, case-control; CDAI, Clinical Disease Activity Index; CRP, C reactive protein; CS, cross-sectional; DAS28, disease activity score assessing 28 joints; ESR, erythrocyte sedimentation rate; GS, Grey scale; H, high (red); L, low (green); M, moderate (yellow); MBDA, multi-biomarker disease activity; NR, not reported; ns, not significant; PD, power Doppler; r, correlation coefficient; RA, rheumatoid arthritis; RoB, risk of bias; SDAI, simplified disease activity index; SJC, swollen joint count; TJC, tender joint count; US, ultrasonography.
Inflammatory activity in patients with RA with and without obesity was assessed in four papers (patient level: two moderate RoB, one high RoB; joint level: one moderate RoB).91 92 96 97 In the first study at patient level with moderate RoB, an US sum score of 28 joints and a DAS28 in which SJC was based on US assessment were compared with traditional SJC28 and DAS28.96 In patients with a body mass index (BMI) below 25, no significant differences were found between the US-based and traditional measures. In patients with a BMI above 25, the US28 sum score was significantly higher than SJC28 (mean difference in patients with BMI 25–30: 1.818, p=0.001; BMI >30: 1.600, p=0.049). While comparing US-DAS28 with DAS28, US-DAS28 was only statistically significantly higher than DAS28 in patients with a BMI between 25 and 30 (table 5). In the other study at patient level with moderate RoB, lower extremity SJC (only joints below the waist) was found to be increased in patients with a BMI above 30, corrected for patient and physician global disease activity, ESR and TJC (OR 1.633, p=0.005).97 This association was less clear for SJC44 (OR 1.765, p=0.090), suggesting that upper extremity assessment is not significantly influenced by obesity. In the study at joint level (moderate RoB), clinical assessment of a joint being swollen was found to be overestimated in patients with obesity.91 The probability of synovitis according to US decreased per higher BMI category (BMI <25, BMI 25–30, BMI >30), corrected for age, gender and clinical assessment of a joint being swollen (OR BMI 0.52 (95%CI 0.30 to 0.93, p=0.03)).
Three papers evaluated the assessment of inflammatory activity in RA patients with and without fibromyalgia at patient level (two moderate RoB, one high RoB).92–94 The first study with moderate RoB assessed the correlation of composite indices with 7-joint US scores.93 Statistically significant correlations were found with 7-joint US scores based on GS in patients with and without fibromyalgia (range of r: 0.36 to 0.43 and 0.39 to 0.57, respectively). Using 7-joint US scores based on power Doppler (PD), a significant correlation was found in patients without fibromyalgia (range of r: 0.35–0.38), although correlations were found not to be statistically significant in patients with fibromyalgia (range of r: 0.01–0.12). In the other study with moderate RoB, statistically significant correlations were found between SJCs and 7-point US scores for synovitis and for tenosynovitis based on GS and PD in patients without fibromyalgia (range of r: 0.44–0.57).95 Again, correlations were not statistically significant in patients with fibromyalgia (r: not given).
Discussion
In this SLR, evidence was sought regarding the optimal confirmation of RA and relevant differential diagnoses as well as the assessment of inflammatory activity in D2T RA patients in whom there was doubt about the diagnosis or the presence of inflammatory activity. Several limitations were found in the selected evidence. First, no studies were identified including D2T RA patients specifically and only the minority of studies included RA patients in whom there was explicit doubt about the diagnosis of RA or about the presence of inflammatory activity. Second, a heterogeneous collection of diagnostic tests was evaluated using different association measures, hampering pooling of results. Third, only very few studies with a low RoB were found. Additional limitations were found in the evidence regarding the assessment of inflammatory activity in D2T RA patients. Mostly, only correlation measures were reported, which are not directly appropriate to assess a test for indicating the presence or absence of inflammatory disease activity in clinical practice (although a strong correlation is likely a prerequisite). Furthermore, major heterogeneity was found in reference standards used in these studies, reflecting the lack of a true gold standard to assess inflammatory activity. Taking all the above-mentioned limitations into account, the identified evidence should be regarded as indirect for the population of D2T RA patients and the results should be interpreted carefully.
Limited evidence was found to consider specific diagnostic tests to confirm or rule out the diagnosis of RA or relevant differential diagnoses. None of the diagnostic tests in the studies regarding the diagnosis of RA or relevant differential diagnoses were replicated, limiting the validity of the results. The only study with a low RoB showed that adapted ACR 1987 criteria (including GS synovitis, US erosions, RF) and RAMRIS scale of MCP joints had an additional value above the traditional ACR 1987 criteria to rule out the diagnosis of RA (sensitivity 69% and 72%, respectively, compared with 42%), although probably still too low to rule out RA with sufficient certainty.16 Moreover, classification criteria, such as the ACR 1987 criteria, should only be applied after a diagnosis is made and are inappropriate to make a diagnosis, making these results not applicable to ascertain the diagnosis of RA in clinical practice.105 Furthermore, in the other study with a low RoB in RA patients who presented with a flare, ESR <15 mm/hour was shown to be able to rule out and procalcitonin ≥0.5 ng/mL to confirm bacterial infection as a mimicking disease.19 Some studies were found assessing the diagnosis of (concomitant) fibromyalgia, although all these studies had a high RoB.17 18 20
As ‘best available direct evidence’ to assess the presence of inflammatory activity in RA patients in whom there was explicit doubt about the presence of inflammatory activity, only one study was identified, having a moderate RoB. In this study, only weak and statistically non-significant correlations were reported between an US sum score and DAS28.25 In the general population of RA patients who are not treatment naïve, US was studied most extensively among all diagnostic tests in papers with low to moderate RoB.24 27 35 40 67 74 78 83 All papers reported moderate to strong correlations between DAS28 and US sum scores, although also here appropriate diagnostic association measures were not reported. These moderate to strong correlations in the general RA population together with the absence of at least a moderate correlation in patients in whom there is explicit doubt about the presence of inflammatory activity (and thus in in whom traditional measures may not be trusted), suggest that US may have an additional value in these patients. However, the optimal number of joints to include in an US sum score to assess inflammatory activity at patient level differed per study and is currently unclear.40 This limitation hampers the current use of an US sum score in clinical practice.
As the ‘best available indirect evidence’ to assess the presence of inflammatory activity in RA patients in whom there was not explicit doubt about the presence of inflammatory activity, MMP-3 and the MBDA score were studied most extensively in studies reporting the appropriate diagnostic association measures with low to moderate RoB.34 35 56 64 However, for MMP-3, no validated cut-off was found35 64 and, for the MBDA score, the cut-off could not be validated in all studies,101–104 106 limiting the applicability for use in daily practice. At joint level, studies with low to moderate RoB assessing US as well as other diagnostic tests with the preferred reference standard at joint level (ie, clinically swollen joints) were not found.
Presence of obesity and fibromyalgia in patients with RA was found to hamper proper grading of disease activity using traditional composite indices.91 94 96 97 Presence of fibromyalgia led to overestimation of disease activity compared with US and modified composite indices, while the influence of obesity on the assessment of disease activity was conflicting between studies. Two studies reported an overestimation of disease activity using traditional composite indices compared with US, at least in the joints of the lower extremities.91 97 On the contrary, the presence of obesity was found to lead to underestimation of inflammatory activity using SJC compared with a US-based SJC in another study.96 In obese patients, composite indices may not only be influenced by the SJC, but also by acute phase reactants. Acute phase reactants may be elevated through the production of inflammatory mediators from adipocytes, resulting in increased composite indices in obese patients.4
US was studied most extensively to assess the presence of inflammatory activity in patients with concomitant obesity or fibromyalgia, in studies having a moderate RoB. In these patients, correlations between US and composite indices were weaker or not statistically significant anymore compared with patients without these comorbidities. This suggests that US may have an additional value to traditional measures to assess inflammatory RA activity in patients with these comorbidities.91 93 95 No studies were found regarding other comorbidities that might influence assessment of inflammatory disease activity.
A previous EULAR project has focused on the development of the EULAR recommendations for the use of imaging of the joints in RA and there is some overlap with our SLR (recommendation 3: ‘ Ultrasound and MRI are superior to clinical examination in the detection of joint inflammation; these techniques should be considered for a more accurate assessment of inflammation’).107 The statement regarding US is consistent with the findings of our SLR. However, the results of our SLR do not clearly indicate the usefulness of MRI. Most studies on MRI were not included in our SLR, predominantly because they were focused on treatment naïve RA patients, were published before the year 2000 or assessed the change in inflammatory activity instead of the presence of inflammatory activity as relevant for our question.108–117
In addition to the limitations in the evidence that was found, this SLR has some limitations itself. Although an extensive literature search has been performed, relevant papers might have been missed. Regarding the diagnosis of RA and relevant differential diagnoses, it was chosen to perform a limited search focusing on the last ten years and not to perform reference screening because not much relevant evidence was presently expected before this time and to enable focusing more on the other clinical questions regarding D2T RA where more relevant literature was expected. After the second Task Force meeting was postponed due to the COVID-19 outbreak, it was decided not to update the search for this specific question because of the same above-mentioned reasons. Regarding the assessment of inflammatory activity, the search focused on the last 10 years, although references of selected papers were also screened and relevant papers published from the year 2000 were selected because of the introduction of bDMARDs around this time point. Additionally, for this search, we focused on non-treatment naïve RA patients resulting in the exclusion of papers focusing on RA patients in the early phase of the disease. However, we felt this was well-justifiable as D2T RA patients are by definition established RA patients and evidence on early RA was deemed too indirect for our present work. Although, as above decisions could be considered limitations, it should be stressed that choices were made by the Task Force, including experienced clinicians, researchers and methodologists and with input from experienced librarians. Therefore, we think the methodological stringency of this SLR and its focus on established RA patients in the present diagnostic and therapeutic era, have resulted in a comprehensive overview of the current literature.
Further guidance on the diagnostic issues in D2T RA, including the clinical implications of the results, will be provided by the EULAR Task Force on D2T RA in their recommendations for the management of D2T RA, which will be published soon.118 Additionally, a research agenda will be provided including topics that should be addressed in future studies.
In conclusion, this SLR highlights the scarcity of evidence on the optimal confirmation or ruling out of a diagnosis of RA and relevant differential diagnoses in D2T RA patients. Therefore, textbook knowledge on potential alternative and/or coexisting mimicking diseases remains highly relevant. When currently used clinical measures may not be trusted as in D2T RA patients, US may have some additional value to assess the presence of inflammatory activity in these patients as well as in those with concomitant obesity or fibromyalgia. However, more high-quality studies addressing D2T RA patients in whom there is reasonable doubt about the diagnosis and about the presence of inflammatory activity are required.
Acknowledgments
We would like to thank M.J.H. de Hair (MJHdH) for her valuable input in the initial phase of this project and F.P. Weijdema (FPW) and P.H. Wiersma (PHW) for their input to the search strategies.
Footnotes
Presented at: Parts of this manuscript have been presented at EULAR 2020 (Roodenrijs NMT, Kedves MH, Hamar A, et al. THU0110 Diagnostic issues in difficult-to-treat rheumatoid arthritis: preliminary results of a systematic literature review informing the 2020 EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis 2020;79:265-6).
Contributors: NMTR drafted the research questions, performed the systematic literature review including risk of bias assessment, contributed to data analysis and interpretation of data, and drafted the manuscript. MK and AH contributed to systematic literature review including risk of bias assessment. GN, JMvL and DvdH contributed to interpretation of data and manuscript preparation. PMJW drafted the research questions, supervised the systematic literature review including risk of bias assessment, contributed to interpretation of data and manuscript preparation. All authors reviewed and approved the final manuscript.
Funding: This project was funded by the European League Against Rheumatism.
Competing interests: NMTR, MK, AH and PMJW declare to have no competing interests. GN received fees from Amgen, AbbVie, BMS, Boehringer Ingelheim, Janssen, KRKA, Merck, MSD, Novartis, Pfizer, Roche, UCB; research grants from Pfizer, AbbVie. JMvL reports personal fees from Arxx Tx, Gesyntha, Magenta, Sanofi Genzyme, Leadiant, Boehringer-Ingelheim, Galapagos; grants and personal fees from Roche; grants from Astra Zeneca, MSD, Thermofisher. DvdH received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi, Eli-Lilly, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB. All competing interests are outside the submitted work.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available from the corresponding author on reasonable request.
References
- 1.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Saag KG, Bridges SL, et al. 2015 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 3.Buch MH Defining refractory rheumatoid arthritis. Ann Rheum Dis 2018;77:966–9. 10.1136/annrheumdis-2017-212862 [DOI] [PubMed] [Google Scholar]
- 4.de Hair MJH, Jacobs JWG, Schoneveld JLM, et al. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology 2018;57:1135–44. 10.1093/rheumatology/kex349 [DOI] [PubMed] [Google Scholar]
- 5.Kearsley-Fleet L, Davies R, De Cock D, et al. Biologic refractory disease in rheumatoid arthritis: results from the British Society for rheumatology biologics register for rheumatoid arthritis. Ann Rheum Dis 2018;77:1405–12. 10.1136/annrheumdis-2018-213378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy G, Roodenrijs NM, Welsing PM, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis 2021;80:31–5. 10.1136/annrheumdis-2020-217344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roodenrijs NMT, de Hair MJH, van der Goes MC, et al. Characteristics of difficult-to-treat rheumatoid arthritis: results of an international survey. Ann Rheum Dis 2018;77:1705–9. 10.1136/annrheumdis-2018-213687 [DOI] [PubMed] [Google Scholar]
- 8.Roodenrijs NMT, van der Goes MC, Welsing PMJ, et al. Difficult-to-treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology 2020:keaa860 10.1093/rheumatology/keaa860 [DOI] [PubMed] [Google Scholar]
- 9.Roodenrijs NMT, Hamar A, Kedves M, et al. Pharmacological and non-pharmacological therapeutic strategies in difficult-to-treat rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open 2021;7:e001512. 10.1136/rmdopen-2020-001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paalanen K, Puolakka K, Nikiphorou E. Is seronegative rheumatoid arthritis true rheumatoid arthritis? A nationwide cohort study. Rheumatology;37 10.1093/rheumatology/keaa623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Heijde D, Aletaha D, Carmona L, et al. 2014 update of the EULAR standardised operating procedures for EULAR-endorsed recommendations. Ann Rheum Dis 2015;74:8–13. 10.1136/annrheumdis-2014-206350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson M, Tiwari A, Fu R. A framework to facilitate the use of systematic reviews and meta-analyses in the design of primary research studies. Rockville, MD: Agency for Healthcare Research and Quality (US), 2012. https://www.ncbi.nlm.nih.gov/sites/books/NBK83621/ [PubMed] [Google Scholar]
- 13.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 14.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan CJ, Aban I. Methods for evaluating the agreement between diagnostic tests. J Nucl Cardiol 2016;23:511–3. 10.1007/s12350-015-0175-7 [DOI] [PubMed] [Google Scholar]
- 16.Pedersen JK, Lorenzen T, Ejbjerg B, et al. Low-Field magnetic resonance imaging or combined ultrasonography and anti-cyclic citrullinated peptide antibody improve correct classification of individuals as established rheumatoid arthritis: results of a population-based, cross-sectional study. BMC Musculoskelet Disord 2014;15:268. 10.1186/1471-2474-15-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan A, Tournadre A, Pereira B, et al. Performance of fibromyalgia rapid screening tool (first) to detect fibromyalgia syndrome in rheumatic diseases. Rheumatology 2016;55:1746–50. 10.1093/rheumatology/kew244 [DOI] [PubMed] [Google Scholar]
- 18.Ghib LJ, Cojocneanu-Petric R, Budisan L. THU0518 the diagnostic value of selected microRNAs in patients with fibromyalgia associated with rheumatoid arthritis: a pilot study. Ann Rheum Dis 2018;77:463–4. [Google Scholar]
- 19.Sato H, Tanabe N, Murasawa A, et al. Procalcitonin is a specific marker for detecting bacterial infection in patients with rheumatoid arthritis. J Rheumatol 2012;39:1517–23. 10.3899/jrheum.111601 [DOI] [PubMed] [Google Scholar]
- 20.Salaffi F, Di Carlo M, Carotti M, et al. The subjective components of the disease activity score 28-joints (DAS28) in rheumatoid arthritis patients and coexisting fibromyalgia. Rheumatol Int 2018;38:1911–8. 10.1007/s00296-018-4096-z [DOI] [PubMed] [Google Scholar]
- 21.Agrawal S, Bhagat SS, Dasgupta B. Improvement in diagnosis and management of musculoskeletal conditions with one-stop clinic-based ultrasonography. Mod Rheumatol 2009;19:53–6. 10.3109/s10165-008-0122-4 [DOI] [PubMed] [Google Scholar]
- 22.Algergawy SA, Abd El-Sabour M, Osman AS, et al. Early diagnostic and prognostic values of anti-cyclic citrullinated peptide antibody and cartilage oligomeric matrix protein in rheumatoid arthritis. Egypt J Immunol 2013;20:11–20. [PubMed] [Google Scholar]
- 23.Bustos Rivera-Bahena C, Xibillé-Friedmann D-X, González-Christen J, et al. Peripheral blood leptin and resistin levels as clinical activity biomarkers in Mexican rheumatoid arthritis patients. Reumatol Clin 2016;12:323–6. 10.1016/j.reuma.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 24.Ceponis A, Onishi M, Bluestein HG, et al. Utility of the ultrasound examination of the hand and wrist joints in the management of established rheumatoid arthritis. Arthritis Care Res 2014;66:236–44. 10.1002/acr.22119 [DOI] [PubMed] [Google Scholar]
- 25.Ciurtin C, Brown G, Cotton A. THU0138 Das 28 correlated poorly with the objective evidence of inflammation as detected by ultrasound (US) examination of hands and feet in patients with established rheumatoid arthritis (RA). Ann Rheum Dis 2017;76. [Google Scholar]
- 26.do Prado AD, Bisi MC, Piovesan DM, et al. Ultrasound power Doppler synovitis is associated with plasma IL-6 in established rheumatoid arthritis. Cytokine 2016;83:27–32. 10.1016/j.cyto.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 27.Filer A, Mandelin AI, DiCarlo E. Histological and clinical correlates of ultrasound measures of joint inflammation: analysis of RA tissue obtained by ultrasound guided biopsy in phase 1 of the accelerating medicines partnership RA network. Arthritis Rheumatol 2017;69. [Google Scholar]
- 28.Gunaydin R, Kaya T, Atay A, et al. Serum leptin levels in rheumatoid arthritis and relationship with disease activity. South Med J 2006;99:1078–83. 10.1097/01.smj.0000240625.27772.79 [DOI] [PubMed] [Google Scholar]
- 29.Ha YJ, Kang E-J, Lee S-W, et al. Usefulness of serum leucine-rich alpha-2 glycoprotein as a disease activity biomarker in patients with rheumatoid arthritis. J Korean Med Sci 2014;29:1199–204. 10.3346/jkms.2014.29.9.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammer HB, Sexton J, Michelsen B. FRI0042 Tender joints have low agreement with patient’s evaluation of spontaneous joint pain, joint swelling and ultrasound verified synovitis in patients with established rheumatoid arthritis. Ann Rheum Dis 2018;77:567–8. [Google Scholar]
- 31.Hermann K-GA, Backhaus M, Schneider U, et al. Rheumatoid arthritis of the shoulder joint: comparison of conventional radiography, ultrasound, and dynamic contrast-enhanced magnetic resonance imaging. Arthritis Rheum 2003;48:3338–49. 10.1002/art.11349 [DOI] [PubMed] [Google Scholar]
- 32.Inamo J, Kaneko Y, Sakata K. THU0162 residual synovitis in ankles and feet detected by ultrasonography in patients with rheumatoid arthritis. Ann Rheum Dis 2018;77:300–2. [DOI] [PubMed] [Google Scholar]
- 33.Amitai I, Werner S, Schicke B, et al. Comparison of photo optical imaging with musculoskeletal ultrasound and clinical examination in the assessment of inflammatory activity in proximal interphalangeal joints in rheumatoid arthritis and osteoarthritis. J Rheumatol 2015;42:1595–602. 10.3899/jrheum.150098 [DOI] [PubMed] [Google Scholar]
- 34.Johnson TM, Register KA, Schmidt CM, et al. Correlation of the Multi‐Biomarker disease activity score with rheumatoid arthritis disease activity measures: a systematic review and Meta‐Analysis. Arthritis Care Res 2019;71:1459–72. 10.1002/acr.23785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawashiri S-y, Kawakami A, Iwamoto N, et al. The power Doppler ultrasonography score from 24 synovial sites or 6 simplified synovial sites, including the metacarpophalangeal joints, reflects the clinical disease activity and level of serum biomarkers in patients with rheumatoid arthritis. Rheumatology 2011;50:962–5. 10.1093/rheumatology/keq415 [DOI] [PubMed] [Google Scholar]
- 36.Kawashiri S-ya, Suzuki T, Nishino A, et al. Automated breast volume scanner, a new automated ultrasonic device, is useful to examine joint injuries in patients with rheumatoid arthritis. Modern Rheumatology 2015;25:837–41. 10.3109/14397595.2015.1040226 [DOI] [PubMed] [Google Scholar]
- 37.Krabbe S, Ammitzbøll-Danielsen M, Østergaard M, et al. Sensitivity and specificity of optical spectral transmission imaging in detecting joint inflammation in rheumatoid arthritis. Ann Rheum Dis 2016;75:632–3. 10.1136/annrheumdis-2015-208399 [DOI] [PubMed] [Google Scholar]
- 38.Kurosaka D, Hirai K, Nishioka M, et al. Clinical significance of serum levels of vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in patients with rheumatoid arthritis. J Rheumatol 2010;37:1121–8. 10.3899/jrheum.090941 [DOI] [PubMed] [Google Scholar]
- 39.Lee S-W, Park M-C, Park Y-B, et al. Measurement of the serum leptin level could assist disease activity monitoring in rheumatoid arthritis. Rheumatol Int 2007;27:537–40. 10.1007/s00296-006-0253-x [DOI] [PubMed] [Google Scholar]
- 40.Mandl P, Naredo E, Wakefield RJ, et al. A systematic literature review analysis of ultrasound joint count and scoring systems to assess synovitis in rheumatoid arthritis according to the OMERACT filter. J Rheumatol 2011;38:2055–62. 10.3899/jrheum.110424 [DOI] [PubMed] [Google Scholar]
- 41.Matteson EL, Lowe VJ, Prendergast FG, et al. Assessment of disease activity in rheumatoid arthritis using a novel folate targeted radiopharmaceutical Folatescan. Clin Exp Rheumatol 2009;27:253–9. [PMC free article] [PubMed] [Google Scholar]
- 42.Meier AJL, Rensen WHJ, de Bokx PK, et al. Potential of optical spectral transmission measurements for joint inflammation measurements in rheumatoid arthritis patients. J Biomed Opt 2012;17:081420. 10.1117/1.JBO.17.8.081420 [DOI] [PubMed] [Google Scholar]
- 43.Metawi SA, Abbas D, Kamal MM, et al. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clin Rheumatol 2011;30:1201–7. 10.1007/s10067-011-1737-y [DOI] [PubMed] [Google Scholar]
- 44.Andrés Cerezo L, Šumová B, Prajzlerová K, et al. Calgizzarin (S100A11): a novel inflammatory mediator associated with disease activity of rheumatoid arthritis. Arthritis Res Ther 2017;19:79. 10.1186/s13075-017-1288-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milman N, Karsh J, Booth RA. Correlation of a multi-cytokine panel with clinical disease activity in patients with rheumatoid arthritis. Clin Biochem 2010;43:1309–14. 10.1016/j.clinbiochem.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 46.Ni M, Wei W, Wang Y, et al. Serum levels of calreticulin in correlation with disease activity in patients with rheumatoid arthritis. J Clin Immunol 2013;33:947–53. 10.1007/s10875-013-9885-2 [DOI] [PubMed] [Google Scholar]
- 47.Nordal HH, Brokstad KA, Solheim M, et al. Calprotectin (S100A8/A9) has the strongest association with ultrasound-detected synovitis and predicts response to biologic treatment: results from a longitudinal study of patients with established rheumatoid arthritis. Arthritis Res Ther 2017;19:3 10.1186/s13075-016-1201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olama SM, Senna MK, Elarman M. Synovial/Serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol Int 2012;32:683–90. 10.1007/s00296-010-1698-5 [DOI] [PubMed] [Google Scholar]
- 49.Önder B, Kurtaran A, Kimyon S, et al. Association of anti-CCP positivity with serum ferritin and DAS-28. Rheumatol Int 2009;30:223–7. 10.1007/s00296-009-0941-4 [DOI] [PubMed] [Google Scholar]
- 50.Oner SY, Volkan O, Oner C. Serum leptin levels do not correlate with disease activity in rheumatoid arthritis. Acta Reumatol Port 2015;2015:50–4. [PubMed] [Google Scholar]
- 51.Ostendorf B, Peters R, Dann P, et al. Magnetic resonance imaging and miniarthroscopy of metacarpophalangeal joints: sensitive detection of morphologic changes in rheumatoid arthritis. Arthritis Rheum 2001;44:2492–502. [DOI] [PubMed] [Google Scholar]
- 52.Papadopoulos NG, Tsiaousis GZ, Pavlitou-Tsiontsi A, et al. Does the presence of anti-CCP autoantibodies and their serum levels influence the severity and activity in rheumatoid arthritis patients? Clin Rev Allergy Immunol 2008;34:11–15. 10.1007/s12016-007-8018-1 [DOI] [PubMed] [Google Scholar]
- 53.Predeteanu D, Varzaru L, Balanescu A, et al. Anti-cyclic citrullinated peptide antibodies--activity markers in rheumatoid arthritis. J Med Life 2009;2:36–41. [PMC free article] [PubMed] [Google Scholar]
- 54.Rodríguez-Carrio J, Alperi-López M, López P. Red cell distribution width is associated with cardiovascular risk and disease parameters in rheumatoid arthritis. Rheumatol 2014;54:641–6. [DOI] [PubMed] [Google Scholar]
- 55.Avouac J, Meune C, Chenevier-Gobeaux C, et al. Inflammation and disease activity are associated with high circulating cardiac markers in rheumatoid arthritis independently of traditional cardiovascular risk factors. J Rheumatol 2014;41:248–55. 10.3899/jrheum.130713 [DOI] [PubMed] [Google Scholar]
- 56.Roodenrijs NMT, de Hair MJH, Wheater G, et al. The multi-biomarker disease activity score tracks response to rituximab treatment in rheumatoid arthritis patients: a post hoc analysis of three cohort studies. Arthritis Res Ther 2018;20:256 10.1186/s13075-018-1750-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rooney T, Scherzer R, Shigenaga JK, et al. Levels of plasma fibrinogen are elevated in well-controlled rheumatoid arthritis. Rheumatology 2011;50:1458–65. 10.1093/rheumatology/ker011 [DOI] [PubMed] [Google Scholar]
- 58.Sahebari M, Mirfeizi Z, Rezaieyazdi Z, et al. 25(OH) vitamin D serum values and rheumatoid arthritis disease activity (DA S28 ESR). Caspian J Intern Med 2014;5:148–855. [PMC free article] [PubMed] [Google Scholar]
- 59.Schäfer VS, Hartung W, Hoffstetter P, et al. Quantitative assessment of synovitis in patients with rheumatoid arthritis using fluorescence optical imaging. Arthritis Res Ther 2013;15:R124 10.1186/ar4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheel AK, et al. First clinical evaluation of sagittal laser optical tomography for detection of synovitis in arthritic finger joints. Ann Rheum Dis 2005;64:239–45. 10.1136/ard.2004.024224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senolt L, Housa D, Vernerová Z, et al. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis 2007;66:458–63. 10.1136/ard.2006.054734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serdaroğlu M, Çakırbay H, Değer O, et al. The association of anti-CCP antibodies with disease activity in rheumatoid arthritis. Rheumatol Int 2008;28:965–70. 10.1007/s00296-008-0570-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah BG, Quershi HJ, Zafar U. Serum anti-CCP antibody and its correlation with disease activity in local Pakistani rheumatoid arthritis patients. Pakistan J Med Heal Sci 2014;8:1041–4. [Google Scholar]
- 64.Skacelova M, Hermanova Z, Horak P, et al. Higher levels of matrix metalloproteinase-3 in patients with RA reflect disease activity and structural damage. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:296–302. 10.5507/bp.2017.015 [DOI] [PubMed] [Google Scholar]
- 65.Sockalingam S, Khuan CS, Sthaneshwar P. Prevalence of anti cyclic citrullinated peptide antibodies in Malaysian rheumatoid arthritis patients and its correlation with disease activity. Int J Rheum Dis 2009;12:211–5. 10.1111/j.1756-185X.2009.01412.x [DOI] [PubMed] [Google Scholar]
- 66.Axelsen MB, Eshed I, Duer-Jensen A, et al. Whole-Body MRI assessment of disease activity and structural damage in rheumatoid arthritis: first step towards an MRI joint count. Rheumatology 2014;53:845–53. 10.1093/rheumatology/ket425 [DOI] [PubMed] [Google Scholar]
- 67.Takase-Minegishi K, Horita N, Kobayashi K, et al. Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: systematic review and meta-analysis. Rheumatology 2018;57:49–58. 10.1093/rheumatology/kex036 [DOI] [PubMed] [Google Scholar]
- 68.Targońska-Stępniak B, Majdan M, Dryglewska M. Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol Int 2008;28:585–91. 10.1007/s00296-007-0480-9 [DOI] [PubMed] [Google Scholar]
- 69.Tekeoğlu İbrahim, Gürol G, Harman H, et al. Overlooked hematological markers of disease activity in rheumatoid arthritis. Int J Rheum Dis 2016;19:1078–82. 10.1111/1756-185X.12805 [DOI] [PubMed] [Google Scholar]
- 70.Uslu AU, Küçük A, Şahin A, et al. Two new inflammatory markers associated with disease activity Score-28 in patients with rheumatoid arthritis: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int J Rheum Dis 2015;18:731–5. 10.1111/1756-185X.12582 [DOI] [PubMed] [Google Scholar]
- 71.Valle Y, Ledezma-Lozano IY, Torres-Carrillo N, et al. Circulating TNFRI and TNFRII levels correlated with the disease activity score (DAS28) in rheumatoid arthritis. Scand J Rheumatol 2009;38:332–5. 10.1080/03009740902865456 [DOI] [PubMed] [Google Scholar]
- 72.van Onna M, Ten Cate DF, Tsoi KL, et al. Assessment of disease activity in patients with rheumatoid arthritis using optical spectral transmission measurements, a non-invasive imaging technique. Ann Rheum Dis 2016;75:511–8. 10.1136/annrheumdis-2015-207315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanichapuntu M, Phuekfon P, Suwannalai P, et al. Are anti-citrulline autoantibodies better serum markers for rheumatoid arthritis than rheumatoid factor in Thai population? Rheumatol Int 2010;30:755–9. 10.1007/s00296-009-1058-5 [DOI] [PubMed] [Google Scholar]
- 74.Vlad V, Berghea F, Libianu S, et al. Ultrasound in rheumatoid arthritis - Volar versus dorsal synovitis evaluation and scoring. BMC Musculoskelet Disord 2011;12:124 10.1186/1471-2474-12-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witt M, Frielinghausen J, Mueller R, et al. Evaluation of a novel semi-automated ultrasound system for the detection of synovitis: a prospective study involving 45 patients with rheumatoid arthritis. Ultrasound Int Open 2016;2:E117–23. 10.1055/s-0042-115774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yildirim K, Karatay S, Melikoglu MA, et al. Associations between acute phase reactant levels and disease activity score (DAS28) in patients with rheumatoid arthritis. Ann Clin Lab Sci 2004;34:423–6. [PubMed] [Google Scholar]
- 77.Bae S-C, Lee YH. MiR-146a levels in rheumatoid arthritis and their correlation with disease activity: a meta-analysis. Int J Rheum Dis 2018;21:1335–42. 10.1111/1756-185X.13338 [DOI] [PubMed] [Google Scholar]
- 78.Yokota K, Tsuzuki Wada T, Akiyama Y, et al. Detection of synovial inflammation in rheumatic diseases using superb microvascular imaging: comparison with conventional power Doppler imaging. Mod Rheumatol 2018;28:327–33. 10.1080/14397595.2017.1337288 [DOI] [PubMed] [Google Scholar]
- 79.Zengin O, Onder ME, Kalem A, et al. New inflammatory markers in early rheumatoid arthritis. Z Rheumatol 2018;77:144–50. 10.1007/s00393-016-0187-y [DOI] [PubMed] [Google Scholar]
- 80.Zufferey P, Brulhart L, Tamborrini G, et al. Ultrasound evaluation of synovitis in RA: correlation with clinical disease activity and sensitivity to change in an observational cohort study. Joint Bone Spine 2014;81:222–7. 10.1016/j.jbspin.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 81.Abdelzaher MG, Tharwat S, AbdElkhalek A, et al. Ultrasound versus magnetic resonance imaging in the evaluation of shoulder joint pathologies in a cohort of rheumatoid arthritis patients. Int J Rheum Dis 2019;22:2158–64. 10.1111/1756-185X.13728 [DOI] [PubMed] [Google Scholar]
- 82.Myngbay A, Bexeitov Y, Adilbayeva A, et al. CTHRC1: a new candidate biomarker for improved rheumatoid arthritis diagnosis. Front Immunol 2019;10:1353 10.3389/fimmu.2019.01353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ngai Ng S, Bjørndal Axelsen M, Ostergaard M. OP0135 how well does whole body magnetic resonance imaging agree with whole body ultrasound in the assessment of joint inflammation in rheumatoid arthritis patients. Ann Rheum Dis 2019;78:143. [Google Scholar]
- 84.Okcu M, Oktayoglu P, Mete N, et al. A useful marker in the assessment of remission and activation of disease in patients with rheumatoid arthritis: serum human neutrophil peptides 1-3. J Back Musculoskelet Rehabil 2018;31:1145–50. 10.3233/BMR-160743 [DOI] [PubMed] [Google Scholar]
- 85.Tuncer T, Kaya A, Gulkesen A, et al. Matrix metalloproteinase-3 levels in relation to disease activity and radiological progression in rheumatoid arthritis. Adv Clin Exp Med 2019;28:665–70. 10.17219/acem/94065 [DOI] [PubMed] [Google Scholar]
- 86.Yuan Z-C, Wang J-M, Huang A-F, et al. Elevated expression of interleukin-37 in patients with rheumatoid arthritis. Int J Rheum Dis 2019;22:1123–9. 10.1111/1756-185X.13539 [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Yuan Y, Pan Z, et al. Elevated circulating IL-17 level is associated with inflammatory arthritis and disease activity: a meta-analysis. Clinica Chimica Acta 2019;496:76–83. 10.1016/j.cca.2019.06.026 [DOI] [PubMed] [Google Scholar]
- 88.Besselink NJ, van der Meijde P, Rensen WHJ, et al. Optical spectral transmission to assess inflammation in hand and wrist joints of rheumatoid arthritis patients. Rheumatology 2018;57:865–72. 10.1093/rheumatology/kex531 [DOI] [PubMed] [Google Scholar]
- 89.Boesen M, Ellegaard K, Boesen L, et al. Ultrasound Doppler score correlates with OMERACT RAMRIS bone marrow oedema and synovitis score in the wrist joint of patients with rheumatoid arthritis. Ultraschall in Med 2012;33:E166–72. 10.1055/s-0029-1245922 [DOI] [PubMed] [Google Scholar]
- 90.Bonfiglioli KR, Chakr R, Lima R. SAT0632 ultrasound in the management of rheumatoid arthritis using a novel pragmatic algorithm: a multicentric observational study. Ann Rheum Dis 2018;77:1167–8. [Google Scholar]
- 91.Bauer EM, Ben-Artzi A, Duffy EL, et al. Joint-specific assessment of swelling and power Doppler in obese rheumatoid arthritis patients. BMC Musculoskelet Disord 2017;18:99 10.1186/s12891-017-1406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Curtis JR, Greenberg JD, Harrold LR, et al. Influence of obesity, age, and comorbidities on the multi-biomarker disease activity test in rheumatoid arthritis. Semin Arthritis Rheum 2018;47:472–7. 10.1016/j.semarthrit.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 93.da Silva Chakr RM, Brenol JCT, Behar M, et al. Is ultrasound a better target than clinical disease activity scores in rheumatoid arthritis with fibromyalgia? A case-control study. PLoS One 2015;10:e0118620 10.1371/journal.pone.0118620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sosa J, Karina Perez S, Julia Santa Cruz M. M-DAS28, M-SDAI and M-CDAI performance in a cohort of RA patients with and without concomitant fibromyalgia. Arthritis Rheumatol 2017;69. [Google Scholar]
- 95.Tamas M, Ghib L-J, Bondor C. THU0156 Clinical assessment versus ultrasonography in patients with rheumatoid arthritis treated with biological agents - the impact of concomitant fibromyalgia. Ann Rheum Dis 2017;76:260–1. [Google Scholar]
- 96.Goossens J, Coustet B, Palazzo E, et al. Overweight and obesity affect clinical assessment of synovitis in rheumatoid arthritis: comparison of ultrasonography and clinical exam. Clin Exp Rheumatol 2019;37:49–54. [PubMed] [Google Scholar]
- 97.Ranganath VK, Duffy EL, Garg VK, et al. Obesity impacts swelling of ankle and foot joints in early rheumatoid arthritis patients. J Clin Rheumatol 2019;25:e8–11. 10.1097/RHU.0000000000000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 99.Østergaard M, Peterfy C, Conaghan P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol 2003;30:1385–6. [PubMed] [Google Scholar]
- 100.Wolfe F, Clauw DJ, Fitzcharles M-A, et al. The American College of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010;62:600–10. 10.1002/acr.20140 [DOI] [PubMed] [Google Scholar]
- 101.Bakker MF, Cavet G, Jacobs JWG, et al. Performance of a multi-biomarker score measuring rheumatoid arthritis disease activity in the camera tight control study. Ann Rheum Dis 2012;71:1692–7. 10.1136/annrheumdis-2011-200963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res 2012;64:1794–803. 10.1002/acr.21767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hirata S, Dirven L, Shen Y, et al. A multi-biomarker score measures rheumatoid arthritis disease activity in the best study. Rheumatology 2013;52:1202–7. 10.1093/rheumatology/kes362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fleischmann R, Connolly SE, Maldonado MA, et al. Brief report: estimating disease activity using Multi-Biomarker disease activity scores in rheumatoid arthritis patients treated with abatacept or adalimumab. Arthritis Rheumatol 2016;68:2083–9. 10.1002/art.39714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res 2015;67:891–7. 10.1002/acr.22583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davis JM Editorial: the Multi-Biomarker disease activity test for rheumatoid arthritis: is it a valid measure of disease activity? Arthritis Rheumatol 2016;68:2061–6. 10.1002/art.39716 [DOI] [PubMed] [Google Scholar]
- 107.Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis 2013;72:804–14. 10.1136/annrheumdis-2012-203158 [DOI] [PubMed] [Google Scholar]
- 108.Calisir C, Murat Aynaci AI, Korkmaz C. The accuracy of magnetic resonance imaging of the hands and feet in the diagnosis of early rheumatoid arthritis. Joint Bone Spine 2007;74:362–7. 10.1016/j.jbspin.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 109.Emery P, van der Heijde D, Østergaard M, et al. Exploratory analyses of the association of MRI with clinical, laboratory and radiographic findings in patients with rheumatoid arthritis. Ann Rheum Dis 2011;70:2126–30. 10.1136/ard.2011.154500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Forslind K, Johanson A, Larsson EM, et al. Magnetic resonance imaging of the fifth metatarsophalangeal joint compared with conventional radiography in patients with early rheumatoid arthritis. Scand J Rheumatol 2003;32:131–7. 10.1080/03009740310002452 [DOI] [PubMed] [Google Scholar]
- 111.Forslind K, Larsson EM, Eberhardt K, et al. Magnetic resonance imaging of the knee: a tool for prediction of joint damage in early rheumatoid arthritis? Scand J Rheumatol 2004;33:154–61. 10.1080/03009740410006862 [DOI] [PubMed] [Google Scholar]
- 112.Goupille P, Roulot B, Akoka S, et al. Magnetic resonance imaging: a valuable method for the detection of synovial inflammation in rheumatoid arthritis. J Rheumatol 2001;28:35–40. [PubMed] [Google Scholar]
- 113.Haavardsholm EA, Østergaard M, Hammer HB, et al. Monitoring anti-TNFalpha treatment in rheumatoid arthritis: responsiveness of magnetic resonance imaging and ultrasonography of the dominant wrist joint compared with conventional measures of disease activity and structural damage. Ann Rheum Dis 2009;68:1572–9. 10.1136/ard.2008.091801 [DOI] [PubMed] [Google Scholar]
- 114.Roimicher L, Lopes FPPL, de Souza SAL, et al. (99m)Tc-anti-TNF-α scintigraphy in RA: a comparison pilot study with MRI and clinical examination. Rheumatology 2011;50:2044–50. 10.1093/rheumatology/ker234 [DOI] [PubMed] [Google Scholar]
- 115.Tamai M, Kawakami A, Iwamoto N, et al. Comparative study of the detection of joint injury in early-stage rheumatoid arthritis by magnetic resonance imaging of the wrist and finger joints and physical examination. Arthritis Care Res 2011;63:436–9. 10.1002/acr.20395 [DOI] [PubMed] [Google Scholar]
- 116.Tonolli-Serabian I, Poet JL, Dufour M, et al. Magnetic resonance imaging of the wrist in rheumatoid arthritis: comparison with other inflammatory joint diseases and control subjects. Clin Rheumatol 1996;15:137–42. 10.1007/BF02230330 [DOI] [PubMed] [Google Scholar]
- 117.Wakefield RJ, Freeston JE, O'Connor P, et al. The optimal assessment of the rheumatoid arthritis hindfoot: a comparative study of clinical examination, ultrasound and high field MRI. Ann Rheum Dis 2008;67:1678–82. 10.1136/ard.2007.079947 [DOI] [PubMed] [Google Scholar]
- 118.Nagy G, Roodenrijs NMT, Welsing PMJ. EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. Manuscript in preparation. [Google Scholar]
- 119.Wolfe F, Clauw DJ, Fitzcharles M-ANN, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol 2011;38:1113–22. 10.3899/jrheum.100594 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2020-001511supp001.pdf (124.4KB, pdf)
rmdopen-2020-001511supp002.pdf (80.2KB, pdf)
rmdopen-2020-001511supp003.pdf (88.2KB, pdf)
rmdopen-2020-001511supp004.pdf (232.5KB, pdf)
rmdopen-2020-001511supp005.pdf (140.5KB, pdf)
rmdopen-2020-001511supp006.pdf (101.5KB, pdf)