Abstract
Epidemiological evidence for a radiation effect on prostate cancer risk has been inconsistent and largely indicative of no or little effect. Here we studied prostate cancer incidence among males of the Life Span Study cohort of atomic bomb survivors in a follow-up from 1958 to 2009, eleven years more than was previously reported. During this period there were 851 incident cases of prostate cancer among 41,544 male subjects, doubling the total number of cases in the cohort. More than 50% of the cases were diagnosed among those who were less than 20 years of age at the time of the bombings and who were at, or near, the ages of heightened prostate cancer risks during the last decade of follow-up. In analyses of the radiation dose response using Poisson regression methods, we used a baseline-rate model that allowed for calendar period effects corresponding to the emergence of prostate-specific antigen screening in the general population as well as effects of attained age and birth cohort. The model also allowed for markedly increased baseline rates among the Adult Health Study participants between 2005 and 2009, a period during which a prostate-specific antigen test was included in Adult Health Study biennial health examinations. We found a significant linear dose response with an estimated excess relative risk (ERR) per Gy of 0.57 (95% CI: 0.21, 1.00, P = 0.001). An estimated 40 of the observed cases were attributed to radiation exposure from the bombings. There was a suggestion of the ERR decreasing with increasing age at exposure (P = 0.09). We found no indication of effects of smoking, alcohol consumption and body mass index on the baseline risk of prostate cancer. The observed dose response strengthens the evidence of a radiation effect on the risk of prostate cancer incidence in the atomic bomb survivors.
INTRODUCTION
Prostate cancer is one of the most common cancers in many Western countries but has been relatively uncommon in Japan and most Asian countries until recently (1). Apart from advancing age, ethnicity and family history, there are no universally established environmental or lifestyle risk factors for prostate cancer (2). Epidemiological studies have provided inconsistent evidence for radiation effects on prostate cancer risk. An increased risk of prostate cancer has been found after X-ray treatment for ankylosing spondylitis (3) and in a subset of nuclear workers who were internally exposed to various radionuclides (4), but with little support from subsequent studies of medically or occupationally exposed populations (5–10).
Previously published analyses of prostate cancer incidence or mortality rates in the Life Span Study (LSS) of Japanese atomic bomb survivors, with a more limited follow-up period, have consistently indicated increased risks with increasing dose, but the estimated radiation-related risks were not statistically significant (11, 12). This may have been due, at least in part, to low prostate cancer rates in Japan and the under-representation of younger adult males at the time of the bombings in this cohort (13). Recently, however, the relatively large portion of male cohort members exposed as children or adolescents have reached ages at which prostate cancer rates are increased. Combined with recently introduced prostate-specific antigen (PSA) screening, this led to a dramatic increase in prostate cancer incident cases in this cohort.
This, together with an elevated risk of prostate cancer reported among proximally exposed atomic bomb survivors in Nagasaki (14), motivated us to evaluate the radiation dose response for prostate cancer in the latest series of LSS solid cancer incidence studies (15). Since the most recent rise in prostate cancer rates in Japan has been linked to increasing use of the PSA test, we examined a possible effect of PSA screening in the radiation risk analysis.
MATERIALS AND METHODS
Life Span Study Cohort
As described in more detail elsewhere (13, 15), the LSS cohort, followed up by the Radiation Effects Research Foundation (RERF), consists of 120,321 persons, including 93,741 atomic bomb survivors in Hiroshima and Nagasaki and 26,580 city residents who were not in either city at the time of the 1945 bombings. The survivor group consists of 54,322 persons who were within 2.5 km of either hypocenter at the time of the bombings and thus exposed to relatively high doses of radiation, and 39,419 city, sex and age-matched persons who were between 2.5 and 10 km of the hypocenter and exposed to lower-to-negligible doses. Less than one half, i.e., 50,175 (42%), of the cohort are males.
Adult Health Study
The Adult Health Study (AHS) cohort is a clinical subset of the LSS cohort. This sub-cohort was created originally in 1958, drawing 19,961 persons from the LSS cohort; approximately one half of these were within 2 km of the hypocenter, one quarter were exposed between 3 and 3.5 km in Hiroshima and between 3 km and 4 km in Nagasaki, and another one quarter were not in either city at the time of the bombings. They were matched on age, sex and city (13, 16). This sub-cohort has been expanded twice, with 2,436 persons added in 1997 and 2008, and currently includes 24,358 persons (of whom 9,440 are males) (13). Surviving AHS members have been invited to biennial clinical health examinations conducted at RERF. Health examinations of the subjects who were not in either city were terminated in 1977.
As described later, the current analysis was restricted to a subset of 41,554 male LSS members (including 8,140 AHS members) aged 45 years or older with known radiation dose. Demographic and dose characteristics of the male AHS and non-AHS members considered in the current study are presented in Appendix Table A1 and described later.
PSA Screening
The PSA test was added to routine laboratory work as part of the AHS clinical examination protocol in December 2004. Participants were informed of their PSA test results; those with elevated PSA levels (4 ng/ml or higher) were advised to consult their primary-care physician or a urologist with assistance or advice from RERF. No further efforts have been made to follow individual participants for whom a PSA test was performed. The AHS program as such does not provide diagnostic services for prostate or any other cancer. As described below, all incident prostate cancers in this study were ascertained by linkage to cancer registries without knowledge of AHS PSA screening participation.
Cancer Case Ascertainment and Follow-up
Incident cancer cases in the LSS cohort have been ascertained by linkage with the Hiroshima and Nagasaki cancer registries since 1958 (12, 15, 17, 18). The current study, conducted in this framework, involved 42,910 males with dose estimates who were alive and not known to have cancer as of 1958. A total of 851 males had first primary prostate cancers (ICD-10, topography code C61) diagnosed in the cancer registry catchment areas between 1958 and 2009. As indicated elsewhere (15), these excluded prostate cancer cases diagnosed only at autopsy (n = 63) because a large number of autopsies performed under the pathology program during the 1960s and 1970s occurred more often among those who had higher radiation doses and were older. Of the 851 cases, 771 (91%) had histologically verified diagnoses; for 24 (3%), diagnoses were based solely on death certificates.
Since there were no prostate cancer cases in males younger than 45 years of age, we restricted the analysis to a subset of 41,554 males with known radiation dose and followed up after their 45th birthday. Follow-up began on each survivor’s 45th birthday and ended at the earliest date of diagnosis of first primary cancer of the prostate or other organs, date of death or December 31, 2009. Because incident cancers diagnosed outside of the cancer registry’s catchment area were not systematically ascertained, the analysis was restricted to cases with cancer diagnosed in the registry catchment area with person-years of observation adjusted for probability of residence in the catchment area using city-, sex-, age- and period-specific migration-rate estimates obtained from the AHS cohort, as described elsewhere (15, 19).
AHS Subjects in the Current Study
By design, the AHS sub-cohort is more heavily represented by high-dose-exposed individuals but covers the full range of survivor doses in the LSS cohort; 74% of AHS males with known dose were in the dose group that received >0.5 Gy while 15% were in the dose group that received <0.2 Gy (Appendix Table A1). The corresponding figures for all LSS males are 17% and 71%, respectively. The AHS subjects are distributed similarly to the LSS subjects with respect to age, city and follow-up years. A total of 6,850 AHS subjects participated in one or more of the AHS biennial health examinations with an overall participation rate of 84%. The participation rates did not differ by dose: 84%, 87% and 84% for those with dose <0.2, 0.2–0.5 and 0.5+ Gy, respectively.
Radiation Doses and Other Risk Factors
Dosimetry System 2002 (DS02) provided estimated individual organ-specific DS02 Revision 1 (DS02R1) doses received from the bombings (20, 21). We used weighted absorbed doses, to be referred to as “Gy” in this work, for the urinary bladder, located directly adjacent to the prostate, and calculated using a neutron weighting factor of 10. Estimated doses were adjusted to account for implausibly large estimates (shielded kerma >4 Gy) and random errors in dose assignments (22).
Several epidemiological studies of prostate cancer in Japan and elsewhere (23, 24) have suggested smoking, alcohol consumption and body mass index (BMI) as possible prostate cancer risk factors. We used self-reported information on these risk factors obtained from mailed questionnaire surveys conducted in the LSS between 1969 and 1991 (15, 25).
Data Organization
Data were aggregated into a person-year table stratified on attained age (8 five-year categories from 45 to 84 and one of ≥85 to <110), calendar time period (13 categories: 1958–1960, 1961–1965, 1966–1970, 1971–1975, 1976–1980, 1981–1985, 1986–1987, 1988–1990, 1991–1995, 1996–1998, 1999–2000, 2001–2004, 2005–2009), age at exposure (14 five-year categories from 0 to 69 and one category for ≥70) and DS02R1 weighted bladder dose (23 categories with dose cut points at 0, 0.005, 0.02, 0.04, 0.06, 0.08, 0.1, 0.125, 0.150, 0.175, 0.2, 0.25, 0.3, 0.5, 0.75, 1, 1.25, 1.5, 175, 2, 2.5, 3 Gy) and an indicator of high dose (unweighted gamma plus neutron shielded kerma >4 Gy).
Smoking status was characterized as unknown, never-smoker or ever-smoker with information on duration and average intensity for ever-smokers, and time since quitting for past smokers. Alcohol consumption patterns were characterized as unknown, non-drinker, drinker (current or past) and for drinkers, the number of drinks per day (unknown, none, less than 1, 1–2, 2–3, 3 or more). Body mass index [weight (kg)/height (m)2] was categorized as unknown or into four categories with cut-points of 15, 18.5, 25 and 30. The risk factors were considered to be unknown for persons who had never provided any information or prior to the first available information for questionnaire respondents. Smoking, drinking and BMI data were available for approximately 60% of the cohort members.
Analytical Models
Age-period-cohort model.
Because PSA screening was likely to be a major factor in the prostate cancer rate increase in recent years, we characterized and adjusted for effects of calendar period, attained age, and birth cohort on the baseline and excess absolute rates. Because these three factors are collinear (that is, any one can be expressed as the sum or difference of the other two), special methods are required to characterize the joint effects. We did this using a method suggested by Lockenhoff and Carstensen (26) in which one first fits a baseline rate model that includes two of the three effects, then fixes the parameters associated with these two effects and estimates parameters describing the third effect. For these analyses, the initial model for the log of baseline rates was described using log-linear quadratic spline in attained age with a knot at age 70 years and a two-knot quadratic spline in calendar year with knots at 1980 and 2000. After fitting this model, we described the birth-year-related “drift” using a quadratic spline in year of birth with a knot in 1915. The final model was fit by fixing the birth-year drift parameters and re-estimating the age and period effects.
In addition, to account for the effect of AHS PSA screening starting in December 2004, we allowed baseline rates for post-2004 AHS participants to differ from those for non-participants in fitting these models.
Risk models.
We used Poisson regression to model prostate cancer incidence rates as a function of radiation dose, city, attained age, age at exposure, birth year and other factors. We used excess relative risk (ERR) models primarily in the analysis of the association between radiation dose and incidence of prostate cancer. The ERR model can be expressed as λ0*[1 + ERR], where λ0 is the baseline cancer rate for unexposed (zero dose) individuals. The baseline rate was modeled as a function of birth year, attained age, city of exposure and location at the time of the bombings (within 10 km of the hypocenters vs. not-in-city). As described above, we also allowed for calendar-time-period effects on the baseline rates. The effects of smoking and alcohol consumption and BMI on baseline prostate cancer rates were also examined. The radiation-related ERR was modeled as ρ(d) * ε(a,e,f), where ρ(d) describes the shape of the dose response while ε(.) describes effect modification as a log-linear function of log attained age (a), age at exposure (e) and other factors (f), e.g., smoking. We considered several forms for the dose-response function, including: linear (βd); linear-quadratic (βd) + d2); a linear-threshold [β(d − dth)I(d > dth), where dth is the threshold dose] and categorical. Departure from linearity was assessed by testing = 0 in the linear-quadratic model.
We also considered excess absolute rate (EAR) models of the form λ0 + ρ(d)∈(a,e,f). In these models, attained age, period, birth cohort and age at exposure were included as radiation effect modifiers. The period and birth cohort effects on the dose response were constrained to be the same as the corresponding effects in the baseline rates while attained age and age at exposure were unconstrained effect modifiers.
Maximum-likelihood parameter estimates and 95% Wald or profile-likelihood confidence intervals (CIs) were computed based on Poisson regression methods using the AMFIT module of Epicure (27). Parameter estimates were obtained using likelihood methods, hypothesis tests, confidence intervals and significance test likelihood ratio tests. All statistical tests were two-sided and considered significant when P < 0.05.
Ethical Considerations
This study was approved by the Radiation Effects Research Foundation Human Investigation Committee. The Hiroshima and Nagasaki Prefectures approved the linkages between the LSS cohort and data from the Cancer Registries, while the Hiroshima and Nagasaki Medical Associations approved the linkages with their tumor tissue registries.
RESULTS
Baseline Cancer Rates
In Table 1, crude prostate cancer rates are shown by city, age, calendar period of diagnosis and bladder dose among the 41,554 male subjects. The rates were similar in Hiroshima and Nagasaki and increased rapidly after age 65 years, with 88% of prostate cancers diagnosed at age ≥65 years. Almost one half (49%) of the male cohort members were <20 years of age at the time of exposure, and 66% of this group were alive at the end of follow-up (compared to 34% of all LSS males). More than one half (58%) of the prostate cancers were diagnosed among survivors who were <20 years at the time of the bombing. This is a consequence of various factors including the large younger birth cohorts reaching the ages of peak prostate cancer incidence in the recent decades, during which there was increasing PSA screening and increasing age-specific prostate cancer rates among Japanese males. The crude rates increased with decreasing age at exposure as well as increasing calendar time. The rates increased monotonically with increasing bladder dose below 2 Gy.
TABLE 1.
Number of Persons, Person-Years of Follow-up, Prostate Cancer Cases and Crude Incidence Rates by City, Age at Exposure, DS02R1 Weighted Bladder Dose, Attained Age and Calendar Period of Diagnosis
| Men | Person-years | Cases | Crude ratea | |
|---|---|---|---|---|
| Total | 41,554 | 760,508 | 851 | 11.2 |
| City | ||||
| Hiroshima | 28,650 | 544,642 | 617 | 11.3 |
| Nagasaki | 12,904 | 215,866 | 234 | 10.8 |
| Age at exposure | ||||
| 0–19 | 20,367 | 367,817 | 497 | 13.5 |
| 20–39 | 8,390 | 216,844 | 221 | 10.2 |
| 40+ | 12,797 | 175,847 | 133 | 7.6 |
| Attained age | ||||
| 45–54 | 207,723 | 6 | 0.3 | |
| 55–64 | 246,306 | 99 | 4.0 | |
| 65–74 | 197,920 | 366 | 18.5 | |
| 75–84 | 88,847 | 301 | 33.9 | |
| 85+ | 19,712 | 79 | 40.1 | |
| Calendar period | ||||
| 1958–1964 | 119,048 | 27 | 2.3 | |
| 1965–1974 | 139,822 | 44 | 3.1 | |
| 1975–1984 | 153,695 | 80 | 5.2 | |
| 1985–1994 | 170,055 | 118 | 6.9 | |
| 1995–2004 | 125,998 | 319 | 25.3 | |
| 2005–2009 | 51,891 | 263 | 50.7 | |
| Bladder dose category (Gy) | ||||
| Not in either city | 10,209 | 197,177 | 190 | 9.6 |
| <0.005 | 14,088 | 250,474 | 286 | 11.4 |
| −0.1 | 10,787 | 195,936 | 211 | 10.8 |
| −0.2 | 2,087 | 38,353 | 45 | 11.7 |
| −0.5 | 2,201 | 39,806 | 52 | 13.1 |
| −1 | 1,245 | 22,572 | 33 | 14.6 |
| −2 | 714 | 12,657 | 29 | 22.9 |
| 2+ | 223 | 3,534 | 5 | 14.1 |
Per 10,000 person-years.
Table 2 summarizes the crude rates by attained age and calendar period. More than one half (61%) of the cases had occurred since the previous report, i.e., during the last 11 years of follow-up. The rates in the last decade of follow-up were markedly higher than in the earlier period, except for the oldest attained-age group. The largest relative increase in rates during the last decade occurred in the two youngest attained-age groups of 45–64 and 65–74 years. The bottom row in Table 2 presents age-adjusted period-specific rate ratios calculated relative to the earliest period (1958–1979). These show a rising trend of prostate cancer rates over the entire follow-up period, with a marked increase for the last two decades.
TABLE 2.
LSS Incident Prostate Cancer Case Counts and Crude Rates by Attained Age and Period: 1958–2009
| Age | Calendar period |
Total | ||||
|---|---|---|---|---|---|---|
| 1958–1979 | 1980–1989 | 1990–1998 | 1999–2009 | |||
| 45–64 | Casesa | 12 | 10 | 15 | 68 | 105 |
| Rate | 0.5 | 0.7 | 1.2 | 10.5 | 1.9 | |
| 65–74 | Cases | 46 | 24 | 41 | 255 | 366 |
| Rate | 4.9 | 8.4 | 13.3 | 34.2 | 16.1 | |
| 75–84 | Cases | 40 | 44 | 53 | 164 | 301 |
| Rate | 10.5 | 19.8 | 38.9 | 67.8 | 30.6 | |
| 85+ | Cases | 7 | 14 | 24 | 34 | 79 |
| Rate | 16.5 | 22.7 | 45.4 | 37.9 | 36.6 | |
| Total | Cases | 105 | 92 | 133 | 521 | 851 |
| Rate | 3.0 | 4.4 | 7.5 | 30.8 | 9.3 | |
| Period effect (RR)b | 1 | 1.9 | 3.5 | 7.8 | ||
| (Ref) | (1.4; 2.5) | (2.7; 4.5) | (6.3; 9.7) | |||
Cases per 10,000 person-years.
Attained-age adjusted risk relative to the pre-1980 period.
Using a simple age-power model with neither period nor birth cohort effects, prostate cancer rates increased rapidly with advancing age, roughly proportional to age to the 7th power peaking around age 80 and then declining slightly. Effects of period and birth cohort were both significant. The results illustrated in Fig. 1 show advancing age as a major determinant of the baseline rates (upper left-side panel) and a modest birth cohort effect (lower left-side panel). The period effect started in 1990–2000 (lower right-side panel) and was seen for all surviving birth cohorts (upper right-side panel). As suggested by the data in Table 2, the plots indicate a marked period effect reflecting, in large measure, the rapid increase in prostate cancer screening activities in Japan between 1990 and 2000.
FIG. 1.
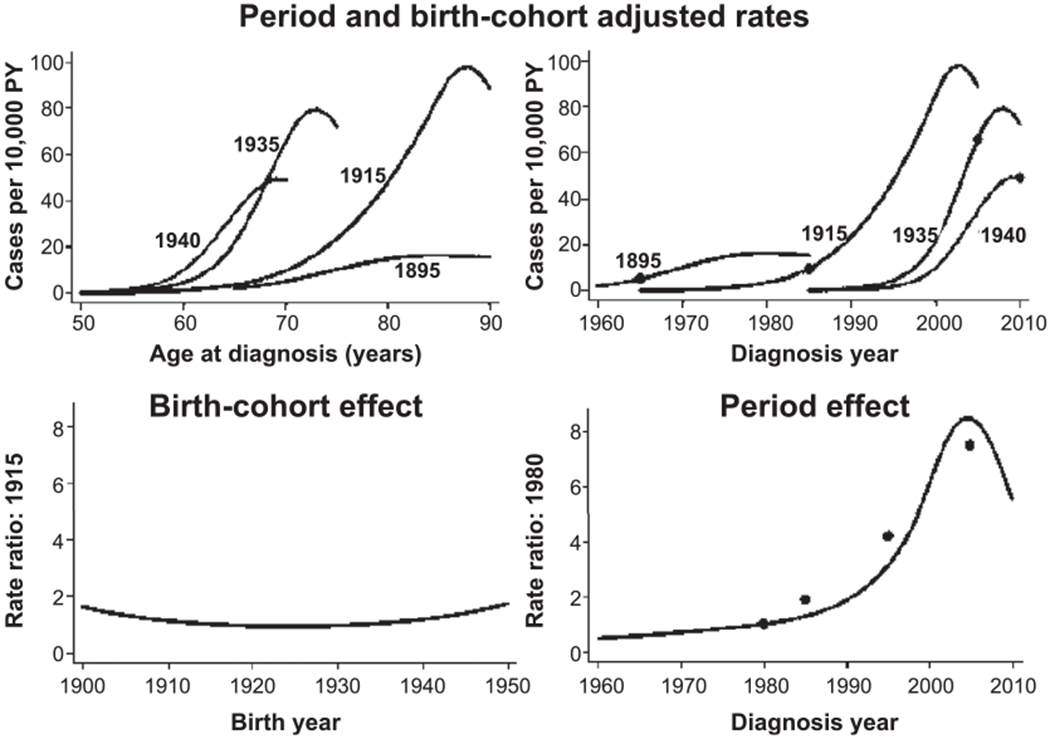
Summary of age, period and birth cohort effects on LSS prostate cancer rates. The upper panels present age-specific prostate cancer incidence rates for men born in 1895, 1915, 1935 and 1940. In the left-side panel the rates are plotted against age while in the right-side panel they are plotted against year of diagnosis. The fitted rates are the product of a (fitted) standard age curve (for someone born in 1915 and an exam in 1990) times the appropriate period and birth cohort effects. The points in the upper right-side panel indicate the risk at age 70 for the different birth cohorts. The plots in the bottom row display the birth cohort effect (left-side panel) and the fitted period effect (right-side panel). The points on the period effect plot are categorical estimates of the age-adjusted period effect (see Table 2). The same vertical scale was used for the birth cohort (rate ratio relative to birth year 1915) and period (rate ratio relative to year 1980) effects to provide a better indication of the relative magnitudes of these effects. PY = person-years.
The introduction of PSA screening in the AHS in December 2004 resulted in a marked increase in the baseline rates in AHS participants. For the period before 2005, the baseline rates for AHS and non-AHS participants were similar (P > 0.5). Between 2005 and 2009, age-specific rates among AHS participants were 2.5 times (95% CI: 1.83, 3.38) those for non-participants. For three decades before 2005, approximately 25% of prostate cancers were in the AHS and this proportion increased to 30% during the 2005–2009 period.
Lifestyle Risk Factors
We found no indication of a difference in the rates for overweight/obese (BMI ≥25 kg/m2) and underweight/normal weight (P = 0.25) nor was there an indication of a trend in prostate cancer risk with increasing BMI (P = 0.5). Rates for non-smokers were somewhat higher than those for past or current smokers or males with unknown smoking status (most of whom are likely to have been smokers), and there was no indication of a trend with pack-years (P = 0.4). Also, there was no indication of a trend in baseline rates with alcohol consumption levels (P > 0.5). Because these lifestyle factors were not significantly associated with baseline rates of prostate cancer, we did not consider any of these factors in the radiation risk analysis. Distributions of prostate cancer cases by smoking status, alcohol consumption and BMI level are presented in Appendix Table A2.
Radiation Effects
Excess relative risk.
We first used a linear dose-response model with age- and birth-cohort-adjusted baseline rates, but with no adjustment for AHS participation. With this model the estimated ERR/Gy for the current follow-up period was 0.65 (95% CI: 0.30, 1.08, P < 0.001). To allow for the temporal changes in the baseline rates, we then adjusted for both the general period effect and the effect of post-2004 AHS PSA screening. This resulted in a slightly lower, but significantly elevated ERR/Gy of 0.57 (95%: CI 0.21, 1.00, P = 0.001). While the baseline rates increased markedly during the period of AHS PSA screening, the ERR/Gy among AHS participants did not differ significantly (P > 0.5) before (0.77, 95% CI: 0.29, 1.37) and after screening (0.86, 95% CI: 0.03, 2.4). Therefore, the impact of PSA screening appeared to be primarily on the baseline rates.
In the LSS, a very large proportion of non-AHS members are in low-dose categories (92% at <0.2 Gy) while very few of them are in high-dose categories (2% at doses >0.5 Gy) (Appendix Table A1). Consequently, dose-response analysis among non-AHS members is underpowered and uninformative. The ERR/Gy estimate for non-AHS participants was −0.08 (95% CI: <−0.2, 0.60) while the estimated ERR/Gy for AHS participants was 0.79 (95% CI: 0.36, 1.33).
As described above, we found no significant effect of AHS participation on the baseline rates for prostate cancer before 2005. For sensitivity analysis, we analyzed the pre-2005 data in the full cohort, allowing for the general period effect, and found a significantly elevated ERR/Gy of 0.46 (95% CI: 0.09, 0.94). This provides evidence for the radiation effect before AHS PSA screening in this cohort.
For comparison with previously reported results (12), we applied the simple linear model with age- and birth-cohort-adjusted baseline rates to the current data with a follow-up limited to the end of 1998. This analysis involved 330 cases (Table 2), excluding 63 autopsy-only cases and one case found to be a non-case while including seven pre-1999 cases that were identified retroactively from updated cancer registry data. The estimated ERR/Gy of 0.21 (95% CI: −0.20, 0.80) was not significant but twice the previous estimate of 0.11 (90% CI: −0.10, 0.54).
There was no indication of ERR effect modification by attained age (P = 0.3) or time since exposure (P = 0.4). However, there was some suggestion that the ERR/Gy decreases with increasing age at exposure (P = 0.09).
Shape of dose response.
Figure 2 shows the fitted linear dose response over the range from 0 to 2 Gy, together with dose-category-specific estimates of the prostate cancer ERR, a smoothed dose-response curve estimated using the category-specific ERR estimates, and upper and lower pointwise 95% bounds on the smoothed curve. There was no indication of non-linearity in a linear-quadratic dose-response model (P > 0.5) and the estimated quadratic effect was essentially 0. There was no indication of a statistically significant non-zero threshold effect in the dose response (P = 0.4). The threshold estimate in a linear threshold model was 0.06 Gy (95% CI: 0 to 0.67).
FIG. 2.
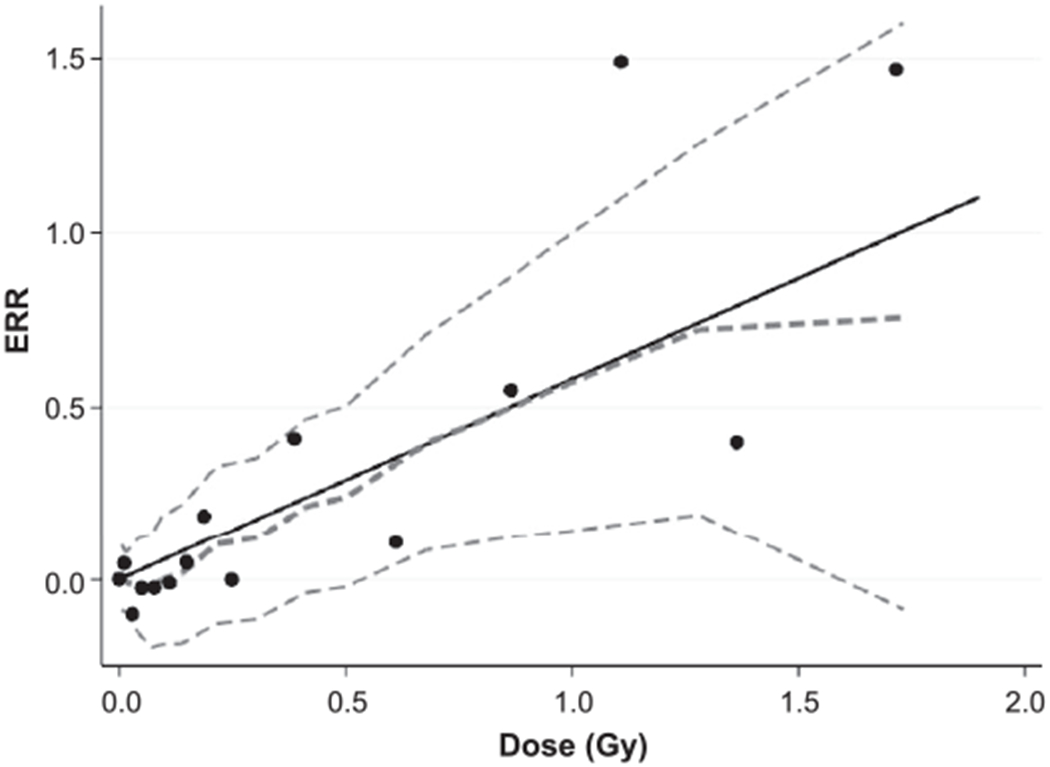
Prostate cancer excess relative risk (ERR) in relationship to weighted absorbed DS02R1 bladder dose. Shown are the fitted linear ERR dose-response function (black solid line), the ERR estimates for 16 dose categories (black points) and nonparametric smoothed estimate with pointwise 95% confidence intervals (dashed curves) over the entire dose range.
Excess absolute rates and excess cases.
The estimated EAR at age 70 for a male exposed at age 30 was 3.5 cases per 10,000 person-year-Gy (95% CI: 0.08 to 8.84, P = 0.001). This was higher than the previous estimate of 0.34 (90% CI: −0.064 to 1.6) (12).
Table 3 presents the observed and fitted baseline and radiation-associated excess cases by dose category. Overall, the estimated number of excess cases was approximately 40, accounting for almost 11% of the cases among cohort members with doses in excess of 0.005 Gy. More than one half (23) of the radiation-associated cases were diagnosed since 1999 and most (31) were diagnosed between the ages of 60 and 80 (not shown in Table 3). We estimated that six of the excess cases occurred among AHS participants after 2004.
TABLE 3.
Observed and Fitted Incident Prostate Cancer Cases:a LSS, AHS Participants and Non-participants, 1958–2009
| Dose category (Gy) | Men | Person-years | Cases | Fitted casesa |
Attributable fractionb | |
|---|---|---|---|---|---|---|
| Background | Excess | |||||
| Not in either city | 10,209 | 197,194 | 190 | 190 | 0.0 | |
| <0.005 | 14,088 | 250,479 | 286 | 274.4 | 0.1 | |
| −0.1 | 10,787 | 195,941 | 211 | 216.6 | 4.1 | 2% |
| −0.2 | 2,087 | 38,351 | 45 | 42.8 | 3.5 | 8% |
| −0.5 | 2,201 | 39,803 | 52 | 43 | 7.9 | 16% |
| −1 | 1,245 | 22,570 | 33 | 26 | 10.7 | 29% |
| −2 | 714 | 12,656 | 29 | 13.6 | 10.4 | 44% |
| 2+ | 223 | 3,534 | 5 | 3.8 | 3.4 | 48% |
| Total | 41,554 | 760,508 | 851 | 810 | 40.0 | 11% |
Estimated background and fitted excess cases based on an ERR model including age and period effects.
Fraction attributable to radiation exposure among those with doses in excess of 0.005 Gy.
DISCUSSION
Since the previously published LSS cancer incidence report (12), the number of incident prostate cancer cases in this cohort had increased from 387 to 851. We found that the rising baseline rates starting in 1990–2000 was a period effect, which affected the entire LSS cohort, corresponding to the rising trend of PSA screening in Japan (28). Furthermore, the PSA screening in AHS biennial examinations resulted in a 2.5-fold increase in the baseline rates among AHS participants after 2004. In the analysis allowing for both the general period effect and the post-2004 AHS baseline-rate increase (together with attained age and birth cohort effects), we found a significant linear dose response for prostate cancer with the estimated ERR/Gy of 0.57 (95% CI: 0.21 to 1.00). Approximately 40 of the 851 observed cases were estimated to be excess cases attributed to radiation exposure. There was a suggestion of the ERR decreasing with increasing age at exposure.
The current evidence for a radiation effect on prostate cancer is much stronger than previously found in the LSS. For the previous follow-up period ending in 1998 (12), the estimated ERR/Gy (excluding autopsy-only cases) was 0.24. With an additional six years of follow-up through 2004, before PSA screening began in the AHS, the ERR/Gy increased to 0.46 and attained statistical significance. With further follow-up through 2009 the ERR/Gy further increased to 0.57. The latest LSS mortality data showed ERR/Gy for prostate cancer increasing from 0.21 (90% CI: <−0.3 to 0.96) in 1997 to 0.33 (95% CI: NA, 1.2) in 2003; neither of these estimates were statistically significant (11, 29), but mortality data may be less powerful for analysis of risk for less fatal prostate cancer.
Potential biases introduced by PSA screening need to be considered carefully (30, 31). Because AHS participants were fully screened regardless of radiation dose (and health conditions or other factors that may influence PSA test outcomes), dose-related selection of screened participants would seem unlikely. Of specific concern in the current study was a possible effect of the markedly increased baseline rates among AHS PSA screening participants on radiation risk estimates. The data indicated that AHS PSA screening elevated both the baseline and radiation-related excess rates proportionally, but did not affect the dose response within the AHS sub-cohort. The dose response could be reliably estimated within the AHS sub-cohort because AHS subjects, though heavily weighted with high-dose survivors, represent the full range of survivor doses. However, in the full cohort analysis, failure to allow for the PSA screening effect on the AHS baseline rates would have biased the radiation risk estimate. In our analysis, therefore, we adjusted the radiation risk for both AHS PSA screening participation and the general period effect on the baseline rates.
In Japan, municipal governments began community-wide PSA screening in the 1990s. Participation rates in municipal screening have been low, approximately 20% of the targeted population, having little effect on annual cancer detection rates (0.54–1.13%) (32); they are unlikely to be influenced by survivor dose, as the survivors are generally not informed of radiation dose. However, those survivors who were close to the explosion may have participated more actively in screening. Since all proximally exposed LSS subjects who had acute radiation symptoms are included in the AHS (16), they are likely to have been screened, together with other survivors, as part of AHS examinations. Cancer screening for atomic bomb survivors was enacted in the mid-1960s in Hiroshima and Nagasaki (33), but has not included prostate cancer screening. Therefore, the impact of screening activities outside the AHS on radiation risk would appear negligible. On an individual level, increasing awareness of the PSA test may have led some to voluntarily seek a PSA test (32); it is difficult to assess the impact of such cases, the size and characteristics of which are unknown.
The study by Kondo et al. of the Nagasaki atomic bomb survivors found a significantly increased relative risk (~1.5) of prostate cancer (excluding those diagnosed by screening) for proximally compared with distally exposed survivors (14). No dose-response analysis was performed. The subjects in the Kondo study were largely young at the time of the bombings (mean exposure age of 11–14 years) and followed up during the same decade as in our study. Some of the cases that study are likely to overlap with ours.
Studies of populations having received medical radiation exposure have provided variable evidence of a radiation-related risk of prostate cancer. In early published studies of patients treated with high-dose X rays (mean, 1.41 Gy) for ankylosing spondylitis (3, 34, 35), increased prostate cancer mortality was found, but only within five years after treatment; it was noted that prostate cancer, which frequently presents with pain in the back due to direct spread or spinal secondaries, was prone to be confused with ankylosing spondylitis. In an extended follow-up of this cohort, prostate cancer mortality was elevated more than five years after treatment and there was a significant dose response with an estimated excess relative risk of 0.14 at 1 Gy (3). A follow-up study of patients treated with X rays for peptic ulcer disease (mean, 80 mGy) found no indication of increased mortality for prostate cancer (36, 37). Several follow-up studies of patients who received radiotherapy for rectal cancer (5, 38–41) reported a decreased risk of prostate cancer, but this may be explained by a cell-killing effect of high therapeutic doses.
In published occupational studies, early data from UK Atomic Energy Authority (UKAEA) employees showed an increased risk of prostate cancer (diagnosed before 1987) associated with external radiation exposure among those who had probable environmental internal exposure to one or more of several radionuclides (4); however, a further follow-up of the UKAEA workforce through 1997 presented no evidence of a continuing elevation of the risk of prostate cancer in any subset of the workers (10). More recently reported studies of nuclear-worker populations provide quantitative risk estimates (Appendix Table A3). While the NRRW-3 update (9), INWORKS (6) and Wismut German uranium miner cohorts (8) represent low-dose exposure (mean, 23–34 mGy), the Mayak population was exposed to moderately high dose (350–540 mGy) (42, 43). None of the ERR/Gy estimates in these nuclear worker studies are significantly different from zero, although the Mayak estimates tend to be higher than the others. It should be noted that nearly one half of the INWORKS data are from the NRRW-3 cohort (44), which was subsequently updated in the NRRW-3 update (9). The current LSS incidence ERR/Gy estimate (0.58) is higher than the estimates from any of these nuclear worker studies (ranging between −1.18 and 0.16). The higher ERR for the LSS may in part be related to the fact that the current LSS data are largely driven by the large proportion of the youngest birth cohort of survivors. Given the wide confidence intervals, however, the LSS risk data are not inconsistent with those of nuclear worker cohorts, with the exception of the Wismut cohort.
Most recently, a long-term follow-up study of U.S. nuclear weapons test participants has reported an increased standardized mortality ratio (1.13) for prostate cancer, but no evidence of a dose response with an ERR per 100 mGy of 0.03 (95% CI: −0.27, 0.33). Estimated mean doses were low (6 mGy for red bone marrow) and approximately 25% of the cohort subjects first participated in the test between 16 and 19 years of age (45). Similarly, an increased relative risk for prostate cancer incidence and mortality was found among UK atmospheric nuclear weapons test participants, but no dose-response analysis was performed (46). Doses were considered low (mean gamma, 9.9 mGy for those with recorded dose).
The current study suggested increased radiation-related risk of prostate cancer associated with younger exposure age. Recent LSS data on female breast and endometrial cancers suggested heightened risk associated with radiation exposure around puberty for these hormone-related cancers (25, 47). The number of prostate cancer cases in the current study was still insufficient for detailed analysis of the possible age effect.
The time-dependent impact of PSA screening complicated the analysis and interpretation of the radiation risk of prostate cancer in the LSS. We used the linear dose-response model including a population-wide period effect beginning in 1990–2000 augmented with additional PSA-associated period effects for post-2004 AHS participants and obtained a statistically significant radiation dose response with an estimated ERR/Gy of 0.57 for the full cohort for the current follow-up period. This, together with the significant dose response that existed prior to AHS PSA screening, is the strongest evidence to date of a radiation effect on prostate cancer in the LSS. The extent to which the younger birth cohort may have contributed to the recent increase in the risk will become clearer with future follow-up. Unfortunately, because of the nature of the linkage-based incidence data used in the current study, we were unable to identify screening-detected prostate cancers at individual levels. However, efforts are underway to ascertain individual screening-detected cancers to further assess the implications of PSA screening on the radiation risk estimate.
ACKNOWLEDGMENTS
We appreciate Drs. Demetrius Albanes and Amy Berrington for their helpful advice and manuscript reviews. The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan is a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the U.S. Department of Energy (DOE). This research was also funded in part through DOE award no. DE-HS0000031 to the National Academy of Sciences and contract no. HHSN261201400009C through the U.S. National Cancer Institute (NCI), with additional support from the Division of Cancer Epidemiology and Genetics in the NCI Intramural Research Program. This publication was supported by RERF Research Protocol 1-75 and 18-61.
APPENDIX
Table A1.
Distribution of Male Subjects, Person-Years by Age at Exposure, Attained Age, Calendar Period and Urinary Bladder Dose among LSS, Non-AHS and AHS Participants
| LSS |
Non-AHS |
AHS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Persons | (%) | Person-years | (%) | Persons | (%) | Person-years | (%) | Persons | (%) | Person-years | (%) | |
| Total | 41,554 | 100.0 | 760,508 | 100.0 | 33,414 | 100.0 | 602,327 | 100.0 | 8,140 | 100.0 | 158,181 | 100 |
| Age at exposure (years) | ||||||||||||
| 0–19 | 20,367 | 49.0 | 367,817 | 48.4 | 16,417 | 49.1 | 294,682 | 48.9 | 3,950 | 48.5 | 73,134 | 46.2 |
| 20–39 | 8,390 | 20.2 | 216,844 | 28.5 | 6,345 | 19.0 | 163,704 | 27.2 | 2,045 | 25.1 | 53,140 | 33.6 |
| 40+ | 12,797 | 30.8 | 175,847 | 23.1 | 10,652 | 31.9 | 143,941 | 23.9 | 2,145 | 26.4 | 31,906 | 20.2 |
| Bladder dose category (Gy) | ||||||||||||
| NIC | 10,209 | 24.6 | 197,177 | 25.9 | 8,386 | 25.1 | 160,015 | 26.6 | 1,823 | 22.4 | 37,162 | 23.5 |
| <0.005 | 14,088 | 33.9 | 250,474 | 32.9 | 11,576 | 34.6 | 200,696 | 33.3 | 2,512 | 30.9 | 49,778 | 31.5 |
| −0.1 | 10,787 | 26.0 | 195,936 | 25.8 | 9,824 | 29.4 | 178,161 | 29.6 | 963 | 11.8 | 17,775 | 11.2 |
| −0.2 | 2,087 | 5.0 | 38,353 | 5.0 | 1,616 | 4.8 | 29,520 | 4.9 | 471 | 5.8 | 8,832 | 5.6 |
| −0.5 | 2,201 | 5.3 | 39,806 | 5.2 | 1,447 | 4.3 | 25,358 | 4.2 | 754 | 9.3 | 14,448 | 9.1 |
| −1 | 1,245 | 3.0 | 22,572 | 3.0 | 416 | 1.2 | 6,459 | 1.1 | 829 | 10.2 | 16,113 | 10.2 |
| −2 | 714 | 1.7 | 12,657 | 1.7 | 115 | 0.3 | 1,674 | 0.3 | 599 | 7.4 | 10,983 | 6.9 |
| 2+ | 223 | 0.5 | 3,534 | 0.5 | 34 | 0.1 | 446 | 0.1 | 189 | 2.3 | 3,089 | 2.0 |
| Attained age (years) | ||||||||||||
| 45–54 | 207,723 | 27.3 | 163,911 | 27.2 | 43,812 | 27.7 | ||||||
| 55–64 | 246,306 | 32.4 | 194,244 | 32.2 | 52,061 | 32.9 | ||||||
| 65–74 | 197,920 | 26.0 | 157,161 | 26.1 | 40,759 | 25.8 | ||||||
| 75–84 | 88,847 | 11.7 | 70,934 | 11.8 | 17,914 | 11.3 | ||||||
| 85+ | 19,712 | 2.6 | 16,078 | 2.7 | 3,634 | 2.3 | ||||||
| Period | ||||||||||||
| 1958–1964 | 119,048 | 15.7 | 96,607 | 16.0 | 22,441 | 14.2 | ||||||
| 1965–1974 | 139,822 | 18.4 | 108,529 | 18.0 | 31,293 | 19.8 | ||||||
| 1975–1984 | 153,695 | 20.2 | 118,716 | 19.7 | 34,978 | 22.1 | ||||||
| 1985–1994 | 170,055 | 22.4 | 135,049 | 22.4 | 35,006 | 22.1 | ||||||
| 1995–2004 | 125,998 | 16.6 | 101,257 | 16.8 | 24,741 | 15.6 | ||||||
| 2005–2009 | 51,891 | 6.8 | 42,170 | 7.0 | 9,721 | 6.1 | ||||||
NIC = not in either city.
Table A2.
Distribution of Male Subjects and Prostate Cancer Cases by Smoking Intensity, Alcohol Consumption and Body Mass Index (BMI)
| Persons | Cases | Ratea | |
|---|---|---|---|
| Total | 41,554 | 851 | 11.2 |
| Smoking intensity | |||
| Unknown | 16,228 | 192 | 5.7 |
| Never-smoker | 3,601 | 117 | 19.4 |
| 1–14 CPD | 8,346 | 229 | 16.5 |
| 15–25 CPD | 9,327 | 224 | 15.1 |
| 25+ CPD | 4,052 | 89 | 14.5 |
| Alcohol consumption (g/week) | |||
| Unknown | 21,727 | 290 | 6.6 |
| None | 3,351 | 91 | 16.8 |
| 1–49 | 1,850 | 52 | 19.3 |
| 50–249 | 7,943 | 231 | 18.9 |
| 250+ | 6,683 | 187 | 18.5 |
| BMI category | |||
| Unknown | 16,481 | 203 | 8.8 |
| Underweight (<18.5) | 3,158 | 73 | 11.3 |
| Low normal (−21.4) | 9,416 | 236 | 11.9 |
| High normal (−24.9) | 9,111 | 245 | 12.6 |
| Overweight (−29.9) | 3,154 | 89 | 13.4 |
| Obese (30+) | 234 | 5 | 10.9 |
Per 10,000 person-years.
Abbreviation: CPD = cigarettes per day
Table A3.
Comparisons of ERRs for Prostate Cancer from Occupationally Exposed Populations and LSS
| Cohort | Deaths/cases | ERR/Gy | (95% CI) | Mean gamma dose (mGy) |
|---|---|---|---|---|
| Prostate cancer mortality | ||||
| NRRW-3 update (9) | 1,115 | 0.072 | (−0.63, 1.04) | 28 |
| INWORKS (6) | 1,685 | −0.11 | (−0.71, 0.67)a | 23 |
| Wismut German uranium miners (8) | 263 | −1.18 | (−2.4, 0.02) | 34 |
| Mayak (43) | 80 | 0.11 | (<, 0.63) | 354 |
| LSS | 130 | 0.33 | (NA, 1.25) | 125 |
| Prostate cancer incidence | ||||
| NRRW-3 update (9) | 3,809 | −0.268 | (−0.68, 0.24) | 28 |
| Mayak (42) | 70 | 0.16 | (−0.12. 0.73) | 540 |
| LSS | 851 | 0.59 | (0.21, 1.07) | 125 |
90% confidence interval (CI).
REFERENCES
- 1.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61: 1079–92. [DOI] [PubMed] [Google Scholar]
- 2.Tangen C, Neuhauser M, Stanford JL. Prostate cancer In: Thun M, Linet M, Cerha J, Haiman C, Schottenfeld D, editors. Cancer epidemiology and prevention, 4th ed. New York: Oxford University Press; 2018. p. 998–1018. [Google Scholar]
- 3.Weiss HA, Darby SC, Doll R. Cancer mortality following X-ray treatment for ankylosing spondylitis. Int J Cancer 1994; 59: 327–38. [DOI] [PubMed] [Google Scholar]
- 4.Rooney C, Beral V, Maconochie N, Fraser P, Davies G. Case-control study of prostatic cancer in employees of the United Kingdom Atomic Energy Authority. BMJ 1993; 307:1391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YC, Hsieh CC, Li CY, Chuang JP, Lee JC. Secondary cancers after radiation therapy for primary prostate or rectal cancer. World J Surg 2016; 40:895–905. [DOI] [PubMed] [Google Scholar]
- 6.Richardson DB, Cardis E, Daniels RD, et al. Site-specific solid cancer mortality after exposure to ionizing radiation: A cohort study of workers (INWORKS). Epidemiology 2018; 29:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR 2006 Report. Volume I, Scientific Annex A. New York: United Nations; 2008. [Google Scholar]
- 8.Walsh L, Dufey F, Tschense A, Schnelzer M, Sogl M, Kreuzer M. Prostate cancer mortality risk in relation to working underground in the Wismut cohort study of German uranium miners, 1970–2003. BMJ Open 2012; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haylock RGE, Gillies M, Hunter N, Zhang W, Phillipson M. Cancer mortality and incidence following external occupational radiation exposure: an update of the 3rd analysis of the UK national registry for radiation workers. Br J Cancer 2018; 119:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson WD, Law DV, Bromley KJ. A decline in mortality from prostate cancer in the UK Atomic Energy Authority workforce. J Radiol Prot 2007; 27:437–45. [DOI] [PubMed] [Google Scholar]
- 11.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res 2012; 177:229–43. [DOI] [PubMed] [Google Scholar]
- 12.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007; 168:1–64. [DOI] [PubMed] [Google Scholar]
- 13.Ozasa K, Cullings HM, Ohishi W, Hida A, Grant EJ. Epidemiological studies of atomic bomb radiation at the Radiation Effects Research Foundation. Int J Radiat Biol 2019; 95:879–91. [DOI] [PubMed] [Google Scholar]
- 14.Kondo H, Soda M, Mine M, Yokota K. Effects of radiation on the incidence of prostate cancer among Nagasaki atomic bomb survivors. Cancer Sci 2013; 104:1368–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M, et al. Solid cancer incidence among the Life Span Study of atomic bomb survivors: 1958–2009. Radiat Res 2017; 187:513–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beebe GW, Fujisawa H, Yamasaki M. Adult health study reference papers Selection of the sample. Characteristics of the sample. Japan: ABCC-RERF Technical Report; 1960. [Google Scholar]
- 17.Mabuchi K, Soda M, Ron E, Tokunaga M, Ochikubo S, Sugimoto S, et al. Cancer incidence in atomic bomb survivors. Part I: Use of the tumor registries in Hiroshima and Nagasaki for incidence studies. Radiat Res 1994; 137:S1–16. [PubMed] [Google Scholar]
- 18.Thompson DE, Mabuchi K, Ron E, Soda M, Tokunaga M, Ochikuboet S, et al. Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958–1987. Radiat Res 1994; 137:S17–67. [PubMed] [Google Scholar]
- 19.Sposto R, Preston DL. Correcting for catchment area nonresidency in studies based on tumor-registry data. RERF CR Japan 1993; 1–92:1–16. [Google Scholar]
- 20.Cullings HM, Grant EJ, Egbert SD, Watanabe T, Oda T, Nakamura F, et al. DS02R1: Improvements to atomic bomb survivors’ input data and implementation of Dosimetry System 2002 (DS02) and resulting changes in estimated doses. Health Phys 2017; 112:56–97. [DOI] [PubMed] [Google Scholar]
- 21.Cullings HM, Fujita S, Funamoto S, Grant EJ, Kerr GD, Preston DL. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiat Res 2006; 166:219–54. [DOI] [PubMed] [Google Scholar]
- 22.Pierce DA, Stram DO, Vaeth M. Allowing for random errors in radiation dose estimates for the atomic bomb survivor data. Radiat Res 1990; 123:275–84. [PubMed] [Google Scholar]
- 23.Fowke JH, McLerran DF, Gupta PC, He J, Shu X-O, Ramadas K, et al. Associations of body mass index, smoking, and alcohol consumption with prostate cancer mortality in the Asia Cohort Consortium. Am J Epidemiol 2015; 182:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health 2010; 100:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner AV, Preston DL, Sakata R, Sugiyama H, Berrington de Gonzalez A, French B, et al. Incidence of breast cancer in the Life Span Study of Atomic Bomb Survivors: 1958–2009. Radiat Res 2018; 190:433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lockenhoff CE, Carstensen LL. Aging, emotion, and health-related decision strategies: motivational manipulations can reduce age differences. Psychol Aging 2007; 22:134–46. [DOI] [PubMed] [Google Scholar]
- 27.Preston DL, Lubin J, Pierce DA, McConney ME, Shilinikova NS. Epicure user guide. Ottawa: Risk Sciences International; 2015. [Google Scholar]
- 28.Katanoda K, Hori M, Matsuda T, Shibata A, Nishino Y, Hattori M, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol 2015; 45:390–401. [DOI] [PubMed] [Google Scholar]
- 29.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 2003; 160:381–407. [DOI] [PubMed] [Google Scholar]
- 30.Weiss NS. Adjusting for screening history in epidemiologic studies of cancer: Why, when, and how to do it. Am J Epidemiol 2003; 157:957–61. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Closas M, Berrington de Gonzalez A. Invited commentary: Screening and the elusive etiology of prostate cancer. Am J Epidemiol 2015; 182:390–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa Y, Namiki M. Prostate-specific antigen-based population screening for prostate cancer: current status in Japan and future perspective in Asia. Asian J Androl 2015; 17:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Measures against atomic bomb survivors. Tokyo: Ministry of Health, Labor and Wefare of Japan; (https://bit.ly/355Zn1h) [Google Scholar]
- 34.Darby SC, Doll R, Gill SK, Smith PG. Long term mortality after a single treatment course with X-rays in patients treated for ankylosing spondylitis. Br J Cancer 1987; 55:179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith PG, Doll R. Mortality among patients with ankylosing spondylitis after a single treatment course with x rays. Br Med J (Clin Res Ed) 1982; 284:449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carr ZA, Kleinerman RA, Stovall M, Weinstock RM, Griem ML, Land CE. Malignant neoplasms after radiation therapy for peptic ulcer. Radiat Res 2002; 157:668–77. [DOI] [PubMed] [Google Scholar]
- 37.Griem ML, Kleinerman RA, Boice JD Jr.., Stovall M, Shefner D, Lubin JH. Cancer following radiotherapy for peptic ulcer. J Natl Cancer Inst 1994; 86:842–9. [DOI] [PubMed] [Google Scholar]
- 38.Birgisso Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol 2005; 23:6126–31. [DOI] [PubMed] [Google Scholar]
- 39.Martling A, Smedby KE, Birgisson H, Olsson H, Granath F, Ekbom A, et al. Risk of second primary cancer in patients treated with radiotherapy for rectal cancer. Br J Surg 2017; 104:278–87. [DOI] [PubMed] [Google Scholar]
- 40.Rombouts AJM, Hugen N, Elferink MAG, Feuth T, Poortmans PMP, Nagtegaal D, et al. Incidence of second tumors after treatment with or without radiation for rectal cancer. Ann Oncol 2017; 28:535–40. [DOI] [PubMed] [Google Scholar]
- 41.Warschkow R, Guller U, Cerny T, Schmied BM, Plasswilm L, Putora PM. Secondary malignancies after rectal cancer resection with and without radiation therapy: A propensity-adjusted, population-based SEER analysis. Radiother Oncol 2017; 123:139–46. [DOI] [PubMed] [Google Scholar]
- 42.Hunter N, Kuznetsova IS, Labutina EV, Harrison JD. Solid cancer incidence other than lung, liver and bone in Mayak workers: 1948–2004. Br J Cancer 2013; 109:1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sokolnikov M, Preston D, Gilbert E, Schonfeld S, Koshurnikova N. Radiation effects on mortality from solid cancers other than lung, liver, and bone cancer in the Mayak worker cohort: 1948–2008. PLoS One 2015; 10:e0117784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muirhead CR, O’Hagan JA, Haylock RG, Phillipson MA, Willcock T, Berridge GLC, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009; 100:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boice JD, Cohen SS, Mumma MT, Chen H, Golden AP, Beck HL, et al. Mortality among U.S. military participants at eight aboveground nuclear weapons test series. Int J Radiat Biol 2020; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Muirhead CR, Bingham D, Haylock RG, O’Hagan JA, Goodill AA, Berridge GLC, et al. Follow up of mortality and incidence of cancer 1952–98 in men from the UK who participated in the UK’s atmospheric nuclear weapon tests and experimental programmes. Occup Environ Med 2003; 60:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Utada M, Brenner AL, Preston DL, Cologne JB, Sakata R, Sugiyama H, et al. Radiation risks of uterine cancer in atomic bomb survivors: 1958–2009. JNCI Cancer Spectr 2019; 2:pky081. [DOI] [PMC free article] [PubMed] [Google Scholar]


