Abstract
Background
Whether calcium oxalate (CaOx) deposition in kidney allografts following transplantation (Tx) adversely affects patient outcomes is uncertain, as are its associated risk factors.
Methods
We performed a retrospective cohort study of patients who had kidney allograft biopsies performed within 3 months of Tx at Brigham and Women’s Hospital and examined the association of CaOx deposition with the composite outcome of death or graft failure within 5 years.
Results
Biopsies from 67 of 346 patients (19.4%) had CaOx deposition. In a multivariable logistic regression model, higher serum creatinine [odds ratio (OR) = 1.28 per mg/dL, 95% confidence interval (CI) 1.15–1.43], longer time on dialysis (OR = 1.11 per additional year, 95% CI 1.01–1.23) and diabetes (OR = 2.26, 95% CI 1.09–4.66) were found to be independently associated with CaOx deposition. CaOx deposition was strongly associated with delayed graft function (DGF; OR = 11.31, 95% CI 5.97–21.40), and with increased hazard of the composite outcome after adjusting for black recipient race, donor type, time on dialysis before Tx, diabetes and borderline or acute rejection (hazard ratio 1.90, 95% CI 1.13–3.20).
Conclusions
CaOx deposition is common in allografts with poor function and portends worse outcomes up to 5 years after Tx. The extent to which CaOx deposition may contribute to versus result from DGF, however, cannot be determined based on our retrospective and observational data. Future studies should examine whether reducing plasma and urine oxalate prevents CaOx deposition in the newly transplanted kidney and whether this has an effect on clinical outcomes.
Keywords: biopsy, calcium oxalate, graft survival, kidney transplantation, renal insufficiency
INTRODUCTION
Oxalate is a dicarboxylic anion which is both formed endogenously as an end product of metabolism and derived from dietary sources. It is predominantly excreted by the kidney, where it can form a highly insoluble salt with calcium, and thus tends to crystallize in the setting of hyperoxaluria, leading to nephrolithiasis, and acute and chronic kidney injury [1]. Kidney injury, often referred to as oxalate nephropathy can result from tubular obstruction, direct tubular toxicity, sterile inflammation, induction of epithelial-to-mesenchymal transition and acceleration of kidney fibrosis, [2–4].
Progressive chronic kidney disease (CKD) can result from oxalate nephropathy. Indeed, in patients with primary hyperoxaluria, end-stage renal disease (ESRD) may ensue in early childhood [5]. Secondary forms of hyperoxaluria, associated with excessive intake or increased intestinal absorption of oxalate (so-called enteric hyperoxaluria), can also lead to failure of both native kidneys and kidney allografts (Figure 1) [6–9]. Following kidney transplantation (Tx), the allograft is exposed to high levels of circulating oxalate that are typical in patients with ESRD [10–13]. Acute allograft failure from oxalate deposition has been reported in cases of ESRD with previously undiagnosed primary hyperoxaluria undergoing kidney Tx [14, 15]. Whether oxalate deposition may be a more general cause of graft failure has not been well examined [16–18]. In addition, risk factors for calcium oxalate (CaOx) deposition after Tx remain inadequately studied. To address this, we studied kidney transplant recipients who underwent allograft biopsies within 3 months following kidney Tx to understand the frequency of and risk factors for CaOx deposition and its association with clinical outcomes.
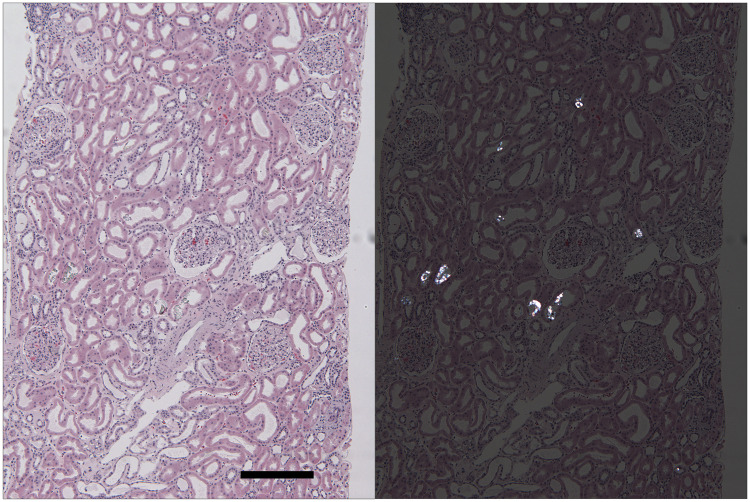
FIGURE 1.
CaOx deposition in a kidney allograft with delayed graft function at 20 days post-transplant. Left panel: this hematoxylin and eosin-stained section of kidney cortex reveals preserved kidney parenchyma with moderate distention of all tubules. Several tubules contain transparent crystals best visualized when the sections are examined under polarized light (right panel). Oxalate crystals are dissolved and disappear under the staining conditions of routinely used special stains, including PAS, Masson’s trichrome and Jones’ silver methenamine stains. (H&E; final magnification: 58×, bar = 250 µm).
MATERIALS AND METHODS
Study design, population and setting
This retrospective cohort study included kidney transplant recipients who had allograft biopsies performed at Brigham and Women’s Hospital within 3 months of Tx, between October 1999 and February 2015. Demographic, clinical and histopathological data were gathered through review of electronic medical records. For calculation of the estimated glomerular filtration rate (eGFR), we used the CKD epidemiology collaboration serum creatinine-based formula [19]. Delayed graft function (DGF) was defined as the need for renal replacement therapy within a week after Tx, and slow graft function (SGF) as failure of the serum creatinine to decrease <3 mg/dL within 5 days after Tx. The study was approved by the Partners Human Research Committee (IRB# 2007P000003) and is in accordance with the principles of the Declaration of Helsinki and the Declaration of Istanbul.
Histopathological data
Information on the presence or absence of CaOx deposition was extracted from biopsy reports. Biopsies were examined for CaOx deposition on hematoxylin and eosin-stained sections under polarized light, which was performed as part of the standard pathological examination on every biopsy. In histopathological analyses, the first CaOx-positive biopsies were compared with the first biopsies in the CaOx-negative group. Other histopathological data recorded included global glomerular sclerosis (%), interstitial fibrosis and tubular atrophy (IFTA, %), vascular sclerosis (present/absent), acute tubular injury (ATI, present/absent) and evidence of borderline or acute cellular or antibody-mediated rejection (present/absent).
Covariates
Covariates considered in the multivariable models included recipient characteristics (age, sex, race, cause of ESRD, dialysis modality and time on dialysis prior to Tx), donor characteristics (living versus deceased, related versus unrelated, demographics, comorbidities), peri-operative data (warm ischemia time, cold ischemia time, SGF, DGF, immunosuppressive medication use) and laboratory data at the time of biopsy (serum calcium, phosphorus, albumin and creatinine).
Outcomes
The primary study outcome was a composite of graft failure or death within 5 years of Tx. Each component of the composite outcome was examined separately in secondary analyses. Graft failure was defined as the need to return to chronic renal replacement therapy or undergo re-transplantation. Data from individual patients were gathered until the occurrence of death, loss to follow-up or 1 January 2016.
Statistical analysis
Descriptive statistics were summarized as mean ± standard deviation (SD) or median [interquartile range (IQR)] for continuous variables, and as percentages for categorical variables. Normally distributed continuous variables were compared using Student’s t-test. In cases where continuous variables were not normally distributed, the Wilcoxon rank sum test was used. Categorical variables were compared between groups using Chi-square or Fisher’s exact tests. For survival analyses, the follow-up period was defined from the time of Tx to the time of graft failure or death. Patients who had neither event were censored at the time they were lost to follow-up, 5 years after Tx or on 1 January 2016. We used the Kaplan–Meier method and log-rank test to compare survival between those with versus without CaOx deposition on allograft biopsies. We then used Cox proportional hazards models to evaluate the association of CaOx deposition with the primary outcome after adjusting for relevant covariates based on previous literature and univariate associations (with P < 0.2 as a threshold for consideration for inclusion in multivariable models). We verified the proportional hazards assumption and model fit by plotting survival curves and examining Schoenfeld and Cox–Snell residuals. Logistic regression models were used to identify independent risk factors for CaOx deposition. All variables included in statistical models had <5% missing data and missing values were not imputed. Statistical tests were two-sided, and P < 0.05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline characteristics, frequency of CaOx deposition and association with DGF
The study population included 346 patients who had 520 biopsies obtained within 3 months of Tx. CaOx deposition was found to be present on at least one biopsy from 67/346 patients (19.4%). Of the 67 patients with CaOx deposition on at least one biopsy within 3 months of Tx, CaOx deposition was noted on the first biopsy in 53/67, on the second biopsy in 7/67 and on the third or later biopsy in 7/67. The primary reason for biopsy was decreased graft function (342/346). A total of three biopsies were obtained for evaluation of proteinuria and one as part of a research study protocol. The majority of oxalate deposition was within tubular lumens; three biopsies had deposits within tubular epithelial cells and two of these had co-existing intraluminal deposits. A total of 28/67 patients with biopsy-proven CaOx deposition had subsequent repeat biopsies within 3 months of Tx, and 13 of these 28 patients continued to have evidence of CaOx deposition on the last biopsy available. Time zero biopsies were available in 77/346 patients (13/67 with later CaOx deposition and 64/279 without later CaOx deposition), and none showed CaOx deposition at the time of implantation.
Baseline characteristics of patients with versus without CaOx deposition on their kidney allograft biopsies are summarized in Table 1. Immunosuppression did not differ between groups, and 94% of patients were treated with a regimen including both a calcineurin inhibitor and mycophenolic acid at the time of biopsy. Individuals with CaOx deposition were more likely to be black, to have diabetes mellitus (DM), to have been treated with hemodialysis for a longer time prior to Tx, and to have received an allograft from a deceased donor. The presence of CaOx deposition was also found to be strongly associated with DGF [odds ratio (OR) = 11.31, 95% confidence interval (CI) 5.97–21.40].
Table 1.
Characteristics of patients with versus without evidence of CaOx deposition on allograft biopsies obtained within 3 months of Tx
| CaOx present (n = 67) | CaOx absent (n = 279) | P-value | |
|---|---|---|---|
| Age, mean (±SD) | 50 ± 15 | 49 ± 13 | 0.53 |
| Female (%) | 43 | 37 | 0.36 |
| Race (%) | |||
| White | 54 | 68 | <0.01 |
| Black | 38 | 20 | |
| Other | 8 | 12 | |
| Cause of ESRD (%) | |||
| DM | 27 | 16 | 0.05 |
| Hypertensive nephrosclerosis | 19 | 17 | 0.70 |
| Glomerular disease | 32 | 38 | 0.46 |
| Dialysis prior to Tx (%) | 94 | 81 | <0.01 |
| Years on dialysis (IQR)a | 4 (3–6) | 2 (1–4) | <0.01 |
| Peritoneal dialysis (%)a | 11 | 14 | 0.52 |
| Donor type (%) | |||
| Deceased | 64 | 38 | <0.01 |
| Living unrelated | 18 | 32 | |
| Living related | 18 | 30 | |
| Median hours of cold ischemia time (IQR)b | 14.1 (10.5– 18.5) | 13.7 (9.3– 18.3) | 0.36 |
| Median hours of warm ischemia time (IQR) | 0.58 (0.50– 0.75) | 0.58 (0.47– 0.67) | 0.14 |
| DGF (%) | 78 | 23 | <0.01 |
| SGF (%) | 84 | 40 | <0.01 |
| Median creatinine at biopsy, mg/dL (IQR) | 5.7 (3.4–7.4) | 2.2 (1.7–3.6) | <0.01 |
| Median eGFR at biopsy, mL/min/1.73 m2 (IQR) | 10 (7–21) | 33 (16–43) | <0.01 |
| Median number of days from Tx to biopsy (IQR) | 13 (7–27) | 17 (9–33) | 0.12 |
IQR (25th–75th percentile).
Calculated among those who received dialysis prior to transplant.
Calculated among those received an allograft from a deceased donor.
Time to death or graft failure in patients with versus without CaOx deposition in the kidney allograft
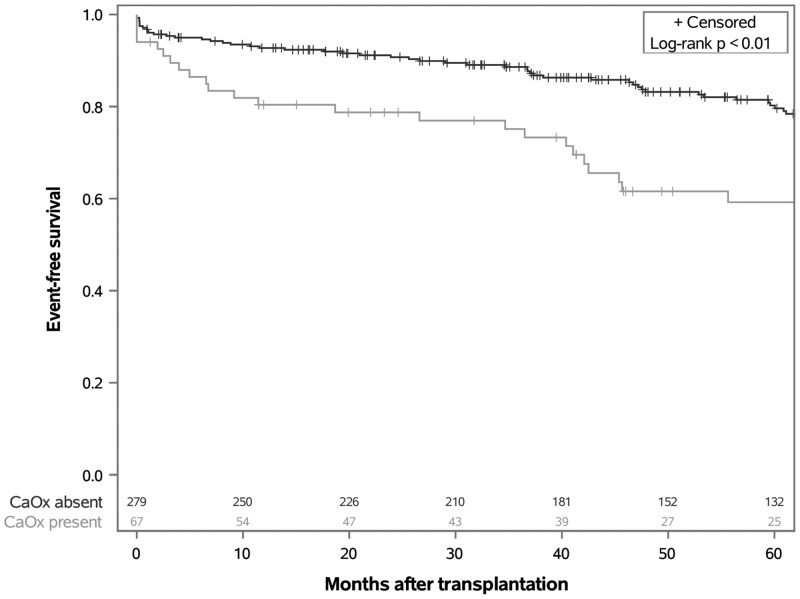
Figure 2 shows time to the primary outcome of death or graft failure, according to the presence or absence of CaOx deposition over 5 years of follow-up time. Patients with evidence of CaOx deposition were significantly more likely to reach the composite endpoint than those without such deposition (log-rank P < 0.01). A total of 70 patients reached the primary outcome. The cumulative survival rate (i.e. probability of remaining event-free) at 5 years was 59% in the CaOx-positive group and 80% in the CaOx-negative group. When the individual components of the composite outcome were analyzed separately in secondary analyses, the difference in the rate of death was not statistically significant (log-rank P = 0.33), whereas risk of graft failure was found to be significantly higher in the group with CaOx deposition (log-rank P = 0.02) (Supplementary data, Figure S1).
FIGURE 2.
Time to death or graft failure after kidney Tx among recipients with versus without CaOx deposition on early allograft biopsies.
In a Cox proportional hazards model, CaOx deposition was associated with increased hazard of the composite outcome of graft failure or death within 5 years, after adjusting for black recipient race, donor type (living versus deceased), time on dialysis prior to Tx, diabetes as the underlying cause of ESRD and histopathological findings of borderline or acute rejection [Table 2; hazard ratio (HR) = 1.90, 95% CI 1.13–3.20]. Further adjusting for ATI did not change the HR significantly, whereas by adjusting for DGF, the association between CaOx deposition and the composite primary outcome became nonsignificant, with DGF alone remaining significantly associated with the outcome (HR = 2.76, 95% CI 1.49–5.11).
Table 2.
HRs for the composite outcome of death or graft failure within 5 years of Tx among patients with versus without CaOx deposition, in an unadjusted model and a multivariable model adjusting for donor type (living versus deceased), black recipient race, borderline or acute rejection, diabetes as cause of ESRD and time on dialysis prior to Tx
| HR (95% CI) | P-value | |
|---|---|---|
| Unadjusted model | 2.42 (1.48–3.96) | <0.01 |
| Multivariable-adjusted model | 1.90 (1.13–3.20) | 0.02 |
Factors associated with CaOx deposition
Table 3 shows characteristics associated with CaOx deposition in unadjusted and multivariable-adjusted analyses. In a multivariable model, higher serum creatinine, longer time on dialysis before Tx, and DM as the primary cause of ESRD remained independently associated with CaOx deposition.
Table 3.
Characteristics associated with CaOx deposition, before and after multivariable adjustment
| Univariate analyses |
Multivariable model | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Black race | 2.47 (1.38–4.42) | <0.01 | 1.19 (0.60–2.35) | 0.62 |
| DDKT | 2.92 (1.68–5.10) | <0.01 | 1.07 (0.52–2.22) | 0.86 |
| DM | 1.86 (1.00–3.48) | 0.05 | 2.26 (1.09–4.66) | 0.03 |
| Time on dialysis (per added year) | 1.17 (1.08–1.26) | <0.01 | 1.11 (1.01–1.23) | 0.03 |
| Creatinine (per 1 mg/dL increase) | 1.35 (1.23–1.48) | <0.01 | 1.28 (1.15–1.43) | <0.01 |
| Corrected calcium (per 1 mg/dL increase) | 0.73 (0.54–0.98) | 0.04 | 0.83 (0.59–1.16) | 0.28 |
DDKT, deceased donor kidney transplant; DM as cause of ESRD.
None of the patients in our cohort had known primary hyperoxaluria. Risk factors for enteric hyperoxaluria were recorded in 2/67 patients with CaOx deposition and 6/279 patients without CaOx deposition (three gastric bypass surgery; five inflammatory bowel disease; P = 0.65). Diarrhea was commonly reported after Tx, but a statistically significant difference in its prevalence at the time of biopsy was not found between groups (18% in those with versus 11% in those without CaOx deposition, P = 0.14). Thirteen patients had a documented prior history of nephrolithiasis but, the difference between groups was not statistically significant (P = 0.14).
ATI was present in 88% of biopsies with CaOx deposition and 78% of those without CaOx deposition (P = 0.06). Vascular sclerosis or hyalinosis was present in 73% of biopsies with CaOx deposition compared with 59% of biopsies without CaOx deposition (P = 0.05). Global glomerulosclerosis, IFTA and borderline or acute rejection were not associated with CaOx deposition (Table 4).
Table 4.
Histopathological findings on kidney allograft biopsies with versus without CaOx deposition
| CaOx present | CaOx absent | P-value | |
|---|---|---|---|
| ATI present (%) | 88 | 78 | 0.06 |
| Borderline or acute rejection present (%) | 28 | 32 | 0.66 |
| Vascular sclerosis or hyalinosis present (%) | 73 | 59 | 0.05 |
| Median IFTA (IQR) | 5% (5–10%) | 5% (5–10%) | 0.53 |
| Median global glomerulosclerosis (IQR) | 2% (0–9%) | 2% (0–7%) | 0.64 |
DISCUSSION
Our study demonstrates that CaOx deposition in the kidney allograft is common among patients with early graft dysfunction. We identified several patient characteristics associated with CaOx deposition and found that the presence of CaOx deposition was associated with DGF and with worse outcomes during 5 years of follow-up.
Previous studies that have routinely examined biopsies under polarized light have reported highly variable prevalence of allograft CaOx deposition. This variation, in addition to stemming from differences in patient characteristics, is likely strongly influenced by the time frame after Tx during which biopsies were included in these analyses. In an early study by Truong et al. [16] of allograft biopsies obtained over a 7-year period in Texas, regardless of the time elapsed from Tx, the reported prevalence of CaOx deposition was 4% (present on 13 biopsies from 9 patients, out of 315 biopsies examined). In contrast, a subsequent study by Pinheiro et al. [17] in Brazil included 97 biopsies which had been obtained within 3 months of Tx and reported a prevalence of 53%. A third biopsy study by Bagnasco et al. [18] at Johns Hopkins University, reported that 9% of patients (63/680) who had allograft biopsies performed within 1 year of Tx had evidence of CaOx deposition on at least one biopsy.
Plasma oxalate levels rapidly decline following Tx, as was shown in a prospective study of 212 kidney transplant recipients, whose median plasma oxalate levels fell from 35 µmol/L prior to Tx to 9 µmol/L at 10 weeks post-operatively [11]. In lieu of other predisposing factors, the period immediately after Tx can thus be expected to carry the highest risk of CaOx deposition. Although random sampling error could have influenced our findings, the absence of CaOx deposition on repeat biopsies from several patients in our study with earlier evidence of this further suggests that CaOx deposition might be a cause of early allograft injury and not necessarily a progressive process. The reason why the prevalence of CaOx deposition in our cohort was substantially higher than in the study from Texas and lower than in the Brazilian study is not clear [16, 17]. It is notable that on average, patients in our full cohort compared with those studied by Pinheiro et al. [17] had slightly lower serum creatinine levels at the time of biopsy, and shorter time on dialysis prior to Tx, both of which we found to be associated with CaOx deposition. It could be speculated that genetic polymorphisms may partially explain this discrepancy, given the different origins of these cohorts, and it is also intriguing to consider whether unidentified differences in dietary practices or other modifiable risk factors might have contributed.
In the study by Pinheiro et al. [17], CaOx deposition was associated with significantly lower probability of graft survival at 12 years (50% compared with 79% in the group without CaOx deposition). The study by Bagnasco et al. [18] found that patients with CaOx deposition, compared with a randomly selected control group of 70 kidney allograft recipients without CaOx deposition, had a lower eGFR 1 year after Tx, but that this difference was no longer significant after 2 years. Due to limited data on longer term follow-up, however, they could not perform an analysis on the association of CaOx deposition with graft survival. In our study, CaOx deposition was found to be associated with an increased risk of the primary outcome of death or graft failure within 5 years of Tx. In secondary analyses, we found that this difference between groups was driven primarily by increased rates of graft failure in those with allograft CaOx deposition, as the difference in mortality was not statistically significant. After adjustment for several risk factors associated with poor outcomes after Tx, the presence of CaOx deposition remained independently associated with the primary outcome.
It is important to recognize that the nature of the striking association we observed between CaOx deposition and DGF is uncertain. Since the study’s underlying hypothesis is that CaOx deposition may cause acute kidney injury and could therefore contribute to DGF, this parameter can be considered on the causal pathway between the exposure of interest and the outcome. DGF was thus not included in our primary multivariable model. An alternative explanation, which cannot be excluded based on our retrospective and observational data, is that DGF increases the risk of CaOx deposition and is a confounder of the association between CaOx deposition and outcomes.
Determinants of CaOx deposition following kidney Tx have not been well studied. Pinheiro et al. [17] found an association between acute tubular necrosis and CaOx deposition, and a trend toward increased risk among recipients of allografts from deceased donors. Although Bagnasco et al. [18] subsequently reported that 88% of patients with CaOx deposits had evidence of ATI, they did not report its prevalence among their control group. In our cohort, ATI was also highly prevalent, and there was a trend toward this finding being more common among those with versus without CaOx deposition in the kidney allograft. Even though global glomerulosclerosis within the first 3 months after Tx was not associated with CaOx deposition, we found a significant association with vascular sclerosis or hyalinosis. Notably, it has been demonstrated in animal models that tubular injury may promote the formation of CaOx crystals and their adherence to tubular epithelial cells, which may suggest a mechanistic link between these findings and CaOx deposition [20, 21].
Beyond these histopathological correlates, we found in our multivariable logistic regression model that higher serum creatinine, longer time on dialysis prior to Tx and diabetes as an underlying cause of ESRD were independently associated with CaOx deposition. Our findings are consistent with a prior study that found serum creatinine and duration of dialysis therapy as predictors of plasma oxalate levels in patients undergoing kidney Tx [11]. Urinary oxalate excretion rate is higher among those with versus without diabetes [22, 23], which could account for our finding of diabetes as a risk factor for CaOx deposition following kidney Tx.
Multiple cases of native kidney and kidney allograft oxalate nephropathy have been reported in the literature in patients who had undergone gastric bypass surgery, suffered from chronic malabsorption from other causes or been exposed to a high dietary load of oxalate or its precursors, e.g. in the setting of unusual and excessive intake of star fruit, rhubarb or ascorbic acid [24–35]. Although these reports are illustrative of important pathophysiological states, the overall contribution of such risk factors to the development of oxalate nephropathy among transplant recipients is not clear. We were not able to gather data on dietary risk factors but found diagnoses typically associated with enteric oxaluria to be rarely recorded. Diarrhea was indeed common in our cohort, as would be expected in this patient population, but a significant difference in its prevalence was not found between the groups with versus without CaOx deposition on allograft biopsies. Concerns that mycophenolate could mediate enteric hyperoxaluria and oxalate nephropathy could not be examined in our study, as the overwhelming majority of patients in our cohort, both with and without CaOx deposition, were treated with this immunosuppressive agent [36].
Our study has limitations. Even though it is the largest study of CaOx deposition in kidney allograft recipients to date, the modest size of our cohort places constraints on the complexity of statistical models. Residual confounding cannot be ruled out as information on several variables known from the literature to influence patient outcomes after Tx, including certain donor characteristics, was not available. As in previous biopsy studies, measurements of plasma or urine oxalate levels were not available for analysis, as these are rarely obtained in current clinical practice prior to Tx. Lastly, our study is based on a cohort from a single tertiary medical center in the USA, and findings may not be generalizable.
In summary, our study demonstrates that CaOx deposition in the kidney allograft is common among kidney transplant recipients with early graft dysfunction, particularly among those with higher serum creatinine, longer treatment with dialysis prior to Tx, or diabetes. Furthermore, we found that the presence of CaOx deposition is associated with DGF and with worse patient outcomes up to 5 years after Tx. The causal nature of the association between CaOx and graft failure cannot be determined from our study given its retrospective and observational design. It is possible that CaOx is a marker and not a mediator of DGF, because injured tubular cells and a low urine output may predispose to oxalate deposition. Alternatively, given the increasing evidence linking oxalate to tissue injury, CaOx deposition may in some cases be a cause of DGF and lead to later allograft failure. Strategies to limit CaOx deposition in the allograft could prove beneficial for allograft survival but will need to be tested in interventional studies.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
FUNDING
R.P. is supported by an American Society of Nephrology Ben J. Lipps Research Fellowship grant. This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK103784 and by an investigator-initiated grant from Allena Pharmaceuticals. The company was not involved in the study design, data collection, data analysis or manuscript preparation.
AUTHORS’ CONTRIBUTIONS
R.P., G.M.M. and S.S.W. were responsible for the concept and design of the study. R.P. and S.S.W. were responsible for the statistical analysis and drafting the manuscript. All authors contributed to the interpretation of data and revision of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Mulay SR, Anders H-J.. Crystal nephropathies: mechanisms of crystal-induced kidney injury. Nat Rev Nephrol 2017; 13: 226–240 [DOI] [PubMed] [Google Scholar]
- 2. Knauf F, Asplin JR, Granja I.. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int 2013; 84: 895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mulay SR, Kulkarni OP, Rupanagudi KV. et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 2013; 123: 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Convento MB, Pessoa EA, Cruz E. et al. Calcium oxalate crystals and oxalate induce an epithelial-to-mesenchymal transition in the proximal tubular epithelial cells: contribution to oxalate kidney injury. Sci Rep 2017; 7: 45740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cochat P, Rumsby G.. Primary hyperoxaluria. N Engl J Med 2013; 369: 649–658 [DOI] [PubMed] [Google Scholar]
- 6. Getting JE, Gregoire JR, Phul A. et al. Oxalate nephropathy due to “Juicing”: case report and review. Am J Med 2013; 126: 768–772 [DOI] [PubMed] [Google Scholar]
- 7. Solomon LR, Nixon AC, Ogden L. et al. Orlistat-induced oxalate nephropathy: an under-recognised cause of chronic kidney disease. BMJ Case Rep 2017; 2017: pii: bcr-2016-218623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nazzal L, Puri S, Goldfarb DS.. Enteric hyperoxaluria: an important cause of end-stage kidney disease. Nephrol Dial Transplant 2016; 31: 375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang AR, Grams ME, Navaneethan SD.. Bariatric surgery and kidney-related outcomes. Kidney Int Rep 2017; 2: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Worcester EM, Fellner SK, Nakagawa Y. et al. Effect of renal transplantation on serum oxalate and urinary oxalate excretion. Nephron 1994; 67: 414–418 [DOI] [PubMed] [Google Scholar]
- 11. Elgstoen KBP, Johnsen LF, Woldseth B. et al. Plasma oxalate following kidney transplantation in patients without primary hyperoxaluria. Nephrol Dial Transplant 2010; 25: 2341–2345 [DOI] [PubMed] [Google Scholar]
- 12. McConnell KN, Rolton HA, Modi KS. et al. Plasma oxalate in patients with chronic renal failure receiving continuous ambulatory peritoneal dialysis or hemodialysis. Am J Kidney Dis 1991; 18: 441–445 [DOI] [PubMed] [Google Scholar]
- 13. Franssen CFM. Oxalate clearance by haemodialysis—a comparison of seven dialysers. Nephrol Dial Transplant 2005; 20: 1916–1921 [DOI] [PubMed] [Google Scholar]
- 14. Spasovski G, Beck BB, Blau N. et al. Late diagnosis of primary hyperoxaluria after failed kidney transplantation. Int Urol Nephrol 2010; 42: 825–829 [DOI] [PubMed] [Google Scholar]
- 15. Malakoutian T, Asgari M, Houshmand M. et al. Recurrence of primary hyperoxaluria after kidney transplantation. Iran J Kidney Dis 2011; 5: 429–433 [PubMed] [Google Scholar]
- 16. Truong LD, Yakupoglu U, Feig D. et al. Calcium oxalate deposition in renal allografts: morphologic spectrum and clinical implications. Am J Transplant 2004; 4: 1338–1344 [DOI] [PubMed] [Google Scholar]
- 17. Pinheiro HS, Saraiva Câmara NO, Osaki KS. et al. Early presence of calcium oxalate deposition in kidney graft biopsies is associated with poor long-term graft survival. Am J Transplant 2005; 5: 323–329 [DOI] [PubMed] [Google Scholar]
- 18. Bagnasco SM, Mohammed BS, Mani H. et al. Oxalate deposits in biopsies from native and transplanted kidneys, and impact on graft function. Nephrol Dial Transplant 2009; 24: 1319–1325 [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asselman M, Verhulst A, De Broe ME. et al. Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J Am Soc Nephrol 2003; 14: 3155–3166 [DOI] [PubMed] [Google Scholar]
- 21. Cao Y, Liu W, Hui L. et al. Renal tubular injury induced by ischemia promotes the formation of calcium oxalate crystals in rats with hyperoxaluria. Urolithiasis 2016; 44: 389–397 [DOI] [PubMed] [Google Scholar]
- 22. Hartman C, Friedlander JI, Moreira DM. et al. Differences in 24-h urine composition between nephrolithiasis patients with and without diabetes mellitus. BJU Int 2015; 115: 619–624 [DOI] [PubMed] [Google Scholar]
- 23. Taylor EN, Curhan GC.. Determinants of 24-hour urinary oxalate excretion. Clin J Am Soc Nephrol 2008; 3: 1453–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marques S, Santos S, Fremin K. et al. A case of oxalate nephropathy: when a single cause is not crystal clear. Am J Kidney Dis 2017; 70: 722–724 [DOI] [PubMed] [Google Scholar]
- 25. Suneja M, Kumar AB.. Secondary oxalosis induced acute kidney injury in allograft kidneys. Clin Kidney J 2013; 6: 84–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cuvelier C, Goffin E, Cosyns J-P. et al. Enteric hyperoxaluria: a hidden cause of early renal graft failure in two successive transplants: spontaneous late graft recovery. Am J Kidney Dis 2002; 40: e3.1. [DOI] [PubMed] [Google Scholar]
- 27. Albersmeyer M, Hilge R, Schröttle A. et al. Acute kidney injury after ingestion of rhubarb: secondary oxalate nephropathy in a patient with type 1 diabetes. BMC Nephrol 2012; 13: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Capolongo G, Abul-Ezz S, Moe OW. et al. Subclinical celiac disease and crystal-induced kidney disease following kidney transplant. Am J Kidney Dis 2012; 60: 662–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Troxell ML, Houghton DC, Hawkey M. et al. Enteric oxalate nephropathy in the renal allograft: an underrecognized complication of bariatric surgery. Am J Transplant 2013; 13: 501–509 [DOI] [PubMed] [Google Scholar]
- 30. Yaich S, Chaabouni Y, Charfeddine K. et al. Secondary oxalosis due to excess vitamin C intake: a cause of graft loss in a renal transplant recipient. Saudi J Kidney Dis Transpl 2014; 25: 113–116 [DOI] [PubMed] [Google Scholar]
- 31. Nankivell BJ, Murali KM.. Images in clinical medicine. Renal failure from vitamin C after transplantation. N Engl J Med 2008; 358: e4. [DOI] [PubMed] [Google Scholar]
- 32. Rankin AC, Walsh SB, Summers SA. et al. Acute oxalate nephropathy causing late renal transplant dysfunction due to enteric hyperoxaluria. Am J Transplant 2008; 8: 1755–1758 [DOI] [PubMed] [Google Scholar]
- 33. Taheri D, Gheissari A, Shaabani P. et al. Acute oxalate nephropathy following kidney transplantation: report of three cases. J Res Med Sci 2015; 20: 818–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abeysekera RA, Wijetunge S, Nanayakkara N. et al. Star fruit toxicity: a cause of both acute kidney injury and chronic kidney disease: a report of two cases. BMC Res Notes 2015; 8: 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schleich A, Fehr T, Gaspert A. et al. Unexpected deterioration of graft function after combined kidney and pancreas transplantation. Clin Kidney J 2013; 6: 228–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamidian JA, Roberts ISD, Winearls CG. et al. Acute renal failure secondary to oxalosis in a recipient of a simultaneous kidney-pancreas transplant: was mycophenolate the cause? Nephrol Dial Transplant 2008; 23: 2409–2411 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.